Abstract
Background
Human metapneumovirus (HMPV) is a leading cause of acute respiratory tract infection (ARTI) in young children. Our objectives were to define HMPV epidemiology and circulating strains and determine markers of severe disease in Jordanian children.
Methods
We conducted a prospective study March 16, 2010-March 31, 2013 using quantitative RT-PCR to determine the frequency of HMPV infection among children <2 years old admitted with fever and/or acute respiratory illness to a major government hospital in Amman, Jordan.
Results
HMPV was present in 273/3168 (8.6%) of children presenting with ARTI. HMPV A2, B1, and B2, but not A1, were detected during the 3-year period. HMPV-infected children were older and more likely to be diagnosed with bronchopneumonia than HMPV-negative children. HMPV-infected children with lower respiratory tract infection (LRTI) had higher rates of cough and shortness of breath than children with LRTI infected with other or no identifiable viruses. Symptoms and severity were not different between children with HMPV only compared with HMPV co-infection. Children with HMPV subgroup A infection were more likely to require supplemental oxygen. In a multivariate analysis, HMPV subgroup A and age <6 months were independently associated with supplemental oxygen requirement.
Conclusions
HMPV is a leading cause of acute respiratory tract disease in Jordanian children <2 years old. HMPV A and young age were associated with severe disease. Ninety percent of HMPV-infected hospitalized children were full-term and otherwise healthy, in contrast to high-income nations; thus, factors contributing to disease severity likely vary depending on geographic and resource differences.
Keywords: human metapneumovirus, acute respiratory tract infection, Jordan, Middle East, severity
INTRODUCTION
Human metapneumovirus (HMPV) is a leading cause of acute respiratory tract infection, and the incidence of hospitalization is highest in young children.1 Prevalence varies from year-to-year, and the four subgroups (A1, A2, B1, and B2) circulate unpredictably, even within similar geographic regions.2–4 HMPV reports from the Middle East have primarily consisted of retrospective or single-year prospective studies; therefore, the true prevalence and characteristics of HMPV in Middle Eastern children are not well described.5–7 Given the emergence of Middle Eastern Respiratory Syndrome (MERS) virus in this region, prospective surveillance studies are important for understanding the etiology and epidemiology of viral respiratory illnesses.8–10
No vaccines or therapeutics are currently licensed for HMPV, but recent studies have identified potential vaccine candidates and monoclonal antibodies to prevent and treat severe disease.11, 12 Therefore, recognizing at-risk populations and markers of disease severity could help target the use of these agents. Reports of such populations have been conflicting and were conducted mostly in high-income countries. Hospitalization in HMPV-infected children has been linked to age <6 months, viral load, and household crowding.13, 14 Female sex, prematurity, and presence of a chronic medical condition were also associated with severe disease.14, 15 Approximately 40% of US children hospitalized with HMPV have underlying medical conditions,1 and viral load is not consistently a predictor of severe disease.16, 17 Whether one HMPV subgroup causes more severe disease also remains undetermined, with conflicting data.18, 14, 19, 20, 21 Viral co-infections in children are common and may increase the likelihood of hospitalization.22–24 The relationship between single HMPV infection and co-infection has not been well described. In a small cohort of intensive care unit (ICU) patients with HMPV, children with multiple viral pathogens did not have increased mortality.15 Some studies reported that children with both HMPV and respiratory syncytial virus (RSV) had more severe disease,25, 26 but this relationship has not been upheld.27 A major limitation of these studies is sample size and retrospective design. We conducted a large prospective cohort study to identify the epidemiology of HMPV in Middle Eastern children and identify markers of severe HMPV disease.
MATERIALS and METHODS
Surveillance
We prospectively conducted year-round viral surveillance in children <2 years old hospitalized at Al-Bashir Hospital, a large referral-based government hospital in Amman, Jordan. Enrollment occurred 5 days/week Sunday-Thursday from March 16, 2010 to March 31, 2013. Eligible children were <2 years old, admitted to the hospital within 48 hours of enrollment, and had to have fever and/or respiratory symptoms with one or more of the following admission diagnoses: acute respiratory illness, apnea, asthma exacerbation, bronchiolitis, bronchopneumonia, croup, cystic fibrosis exacerbation, febrile seizure, fever without localizing signs, pharyngitis, pneumonia, pneumonitis, pertussis, pertussis-like cough, respiratory distress, rule-out sepsis, or upper respiratory infection. Exclusion criteria were children ≥2 years, not enrolled within 48 hours, children with fever and neutropenia, and newborns who never left the hospital. Subjects could be included more than once during the study period for a new illness. The Institutional Review Boards of Vanderbilt University, Jordan University, and the Jordanian Ministry of Health approved the study. Written consent and clinical/demographic data were obtained from parents/guardians. Missing data and length of stay were obtained from medical records.
Classification
To better understand the role of HMPV in pediatric lower respiratory tract infection (LRTI), we identified a sub-cohort of children. The LRTI cohort consisted of children with an admission diagnosis of asthma, bronchiolitis, bronchopneumonia, pneumonia, respiratory distress, or wheezing; or clinical signs of retractions or accessory muscle use; or wheezing on examination. We also characterized the group of children who did not present with lower respiratory tract disease.
Viral testing
Nasal and throat swabs were collected, placed into lysis buffer, aliquoted, and frozen at −80°C. Specimen aliquots were shipped to Vanderbilt for viral testing. Nucleic acids were extracted on a MagMax-96 express automated instrument (ABI) using the MagMax 96 Viral RNA kit (ABI). Individual quantitative RT-PCR reactions were run on a Step One Plus (ABI) using the AgPath-ID RT-PCR kit (ABI). Primers and dual-labeled probes were used to detect HMPV,28 respiratory syncytial virus (RSV),29 rhinovirus (HRV),30 influenza A and B,31 adenovirus,29 and parainfluenza viruses 1, 2, 3 (PIV1–3) (unpublished data). Specimens testing positive for HMPV were genotyped by RT-PCR to amplify a 227nt portion of the N gene. Primers were: for 5′-CTCTTCAAGGGATTCACC-3′ and rev 5′-TGGCATARCTTTCTGCTG-3′. Amplicons were PCR purified, directly sequenced, aligned with N sequences from prototype subgroup strains32 and GenBank using MacVector 12.0 (MacVector), and subgroup genotypes assigned. The evolutionary history was inferred by using the Maximum Likelihood method based on the Tamura-Nei model33 using MEGA6.34
Statistical Methods
Descriptive statistics are reported as total number and percentage or median and interquartile range (IQR). Categorical variables were compared between groups using χ2 test or Fisher’s exact test. Continuous variables were compared by Mann-Whitney U test or Kruskal-Wallis test with adjustment for multiple comparisons. Significant and near significant variables (P<0.10) from univariate analysis were used in a multivariate logistic regression model. A type I error rate of a=0.05 was used throughout, unless otherwise specified. Statistical analysis was carried out in IBM SPSS Statistics (version 22.0) and R (version 3.1.1).35
We used a Bayesian hierarchical model to derive population prevalence estimates for each of the three years of the study.36 Al-Bashir hospital admissions data were filtered to exclude children not residing in Amman. Estimates of the <2-year-old Jordanian population were obtained from the World Bank database and the proportion residing in Amman from the 2012 national census.37 These values were used in a binomial model to estimate the population of children <2 years old in Amman in 2010–2012. The market share for Al-Bashir hospital was modeled as a random variable and the probability of enrollment set to 71.4% (5 of 7 days) and used in a binomial model. Prevalence was given diffuse beta(1,5) priors for all models. Models were fitted using Markov chain Monte Carlo methods38 in PyMC 2.3 software.39 Models were checked for convergence using the Gelman-Rubin diagnostic36 and for goodness-of-fit using posterior predictive checks.40
RESULTS
From March 16, 2010 to March 31, 2013, 3,175 children were enrolled. Seven children were excluded: 4 were referred for meningitis, and 3 were ≥2 years at enrollment. None of the excluded children were positive for HMPV. Therefore, 3,168 children were included.
Hospitalization Rates
The rates of HMPV-associated hospitalization for children <2 years varied for each study year: 0.7, 3.1, and 1.2 per 1000 children for years 1, 2, and 3, respectively. Rates were higher in children <6 months each study year (1.5, 7.2, and 1.9 per 1000 children) compared to the older age groups, 6–11 months (0.9, 2.9, and 1.9 per 1000 children) and 12–23 months (0.4, 1.2, and 0.5 per 1000 children).
Characteristics of HMPV infected children
Of the 3,168 children included in the study, 273 (8.6%) children were positive for HMPV (Table 1). HMPV-infected children were significantly older than children without HMPV (Table 2). Both groups had a slight male predominance. More HMPV-infected children were diagnosed clinically with bronchopneumonia compared with the HMPV-negative group. Bronchiolitis and pneumonia were common diagnoses in both groups. Asthma was rarely diagnosed, although this is a difficult diagnosis to make in children <2 years. Compared with the HMPV-negative group, significantly fewer HMPV-infected children were diagnosed with rule-out sepsis. A second virus was identified in 145 (53.1%) HMPV-positive children, most commonly HRV or RSV (Table 1).
Table 1.
Viruses detected during acute respiratory tract infection admissions.
| Virus | Number of specimens (%) |
|---|---|
| RSV | 1397 (44.1) |
| Rhinovirus | 1238 (39.1) |
| Adenovirus | 475 (15.0) |
| HMPV | 273 (8.6) |
| PIV 3 | 128 (4.0) |
| Influenza A | 72 (2.3) |
| PIV 1 | 34 (1.1) |
| Influenza B | 29 (0.9) |
| Influenza C | 19 (0.6) |
| PIV 2 | 13 (0.4) |
| HMPV co-infections with another virus: | Number of specimens |
| RSV | 71 |
| Rhinovirus | 72 |
| Adenovirus | 40 |
| PIV 3 | 2 |
| Influenza A | 6 |
| PIV 1 | 2 |
| Influenza B | 2 |
| Influenza C | 3 |
| PIV 2 | 0 |
Total specimens=3,168
Table 2.
Characteristics of HMPV-infected children.
| HMPV positive (%) |
HMPV negative (%) |
P value | |
|---|---|---|---|
| Age, median [IQR] | 5.8 [2.6–10.0] | 3.4 [1.6–8.4] | <0.001 |
| Gender, male | 157 (57.5) | 1755 (60.6) | 0.31 |
| Admission diagnosis | |||
| Asthma | 14 (5.1) | 132 (4.6) | 0.67 |
| Bronchiolitis | 56 (20.5) | 491 (17.0) | 0.14 |
| Bronchopneumonia | 123 (45.1) | 897 (31.0) | <0.001 |
| Pneumonia | 37 (13.6) | 357 (12.3) | 0.56 |
| Rule-out sepsis | 34 (12.5) | 866 (29.9) | <0.001 |
| Chronic medical conditions | 25 (9.2) | 296 (10.2) | 0.59 |
| Gestational age, median, weeks [IQR] | 40 [38–40] | 40 [38–40] | 0.68 |
| Number of siblings, median [IQR] | 2 [1–3] | 2 [1–3]a | 0.76 |
| Daycare | 5 (1.8) | 45 (1.6)a | 0.43 |
| Smoke exposure | 212 (77.7) | 2213 (76.4) | 0.65 |
| Antibiotics prior to hospitalization | 142 (52%) | 1144 (39.5%) | <0.01 |
| Antibiotics during hospitalizations | 253/271 (93.4%) | 2638/2876 (91.7%) | 0.35 |
Total patients: HMPV positive (273), HMPV negative (2895); p<0.01 is significant n=2894
Few HMPV-positive children (9.2%) had underlying medical conditions. Most children in the cohort were full-term and had a normal birth weight. Forty-four (16%) of HMPV infected children were premature (<37 weeks), and 10 (3.7%) were very preterm (<32 weeks). Few children attended daycare; most had exposure to a household smoker. These findings were not significantly different between children with and without HMPV infection.
Seasonal circulation of HMPV
HMPV circulated during the winter-spring months with a February to April peak (Figure 1). We were able to genotype 215 specimens (78.8%), revealing seasonal strain variation. In spring 2010, 5/13 (38%) were A2 with 8/13 (62%) B2. In 2010–2011, 60/64 (94%) were A2 and 2/64 (3%) each B1 and B2. In 2011–2012, 51/106 (48%) were A2, 4/106 (4%) B1, and 51/106 (48%) B2. In 2012–2013, 1/32 (3%) was A2, 5/32 (16%) B1, and 26/32 (81%) B2. No A1 was identified during this period. Sequencing of the N region demonstrated marked similarity of strains within subgroups (see Figure, Supplemental Digital Content 1).
Figure 1.
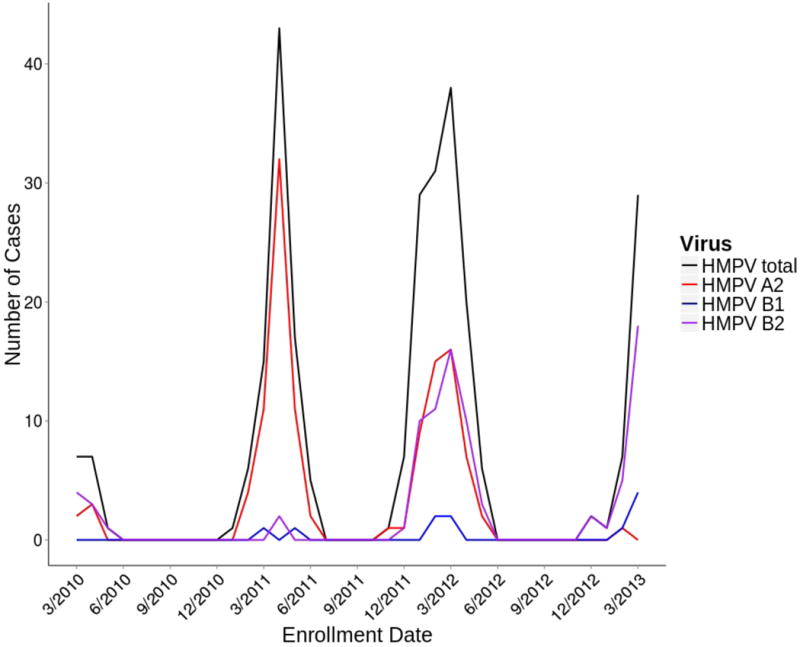
HMPV circulates predominately during the winter-spring months. A2 was the major subgroup during 2010–2011. A2 and B2 co-circulated during 2011–2012 while B2 was the dominant subgroup during 2012–2013.
Characteristics of children with HMPV LRTI
In order to characterize the features of HMPV-associated LRTI, we excluded children who did not present with LRTI. Of the initial 3,169 children, 2,263 (71.4%) met criteria for LRTI. 268 (11.8%) had no virus identified on PCR, 242 (10.7%) were HMPV-positive, and 1753 (77.5%) were positive for a virus other than HMPV (Table 3).
Table 3.
Characteristics of HMPV infected children with LRTI.
| HMPV positive (%) |
Other virus positive (%) |
Virus negative (%) |
P value | |
|---|---|---|---|---|
| Symptoms | ||||
| Cough | 234 (96.7) | 1627 (92.8) | 205 (76.5) | <0.001 |
| Diarrhea | 13 (5.4) | 108 (6.2) | 17 (6.3) | 0.88 |
| Fever | 144 (59.5) | 914 (52.1) | 142 (53) | 0.10 |
| Poor appetite | 33 (13.6) | 296 (16.9) | 47 (17.5) | 0.41 |
| Rhinorrhea | 3 (1.2) | 35 (2) | 6 (2.2) | 0.68 |
| Shortness of breath | 192 (79.3) | 1317 (75.1) | 175 (65.3) | <0.001 |
| Vomiting | 30 (12.4) | 216 (12.3) | 41 (15.3) | 0.39 |
| Physical examination | ||||
| Retractions | 33 (13.6) | 213 (12.2) | 35 (13.1) | 0.76 |
| Wheezing | 190 (78.8)a | 1349 (77.0)b | 216 (80.9)c | 0.32 |
| Abnormal chest X-ray | 214 (89.9)d | 1536 (89.6)e | 222 (84.4)f | 0.04 |
| Required oxygen | 73 (30.4)g | 653 (37.7)h | 100 (37.6)i | 0.09 |
| Days on oxygen [IQR] | 2 [1–4] | 2 [1–5]j | 2 [1–3] | 0.003 |
| Required ventilation | 7 (2.9)g | 72 (4.2)k | 15 (5.6)i | 0.31 |
| Days on ventilator [IQR] | 3 [1–4] | 2 [1–5] | 1 [1–3] | 0.15 |
| Required ICU | 11 (4.6) | 159 (9.2) | 40 (15.0) | <0.001 |
| Length of stay | 4 (3–6)g | 4 (3–7)l | 4 (2–7)m | 0.09 |
| Deceased | 2 (0.8)g | 12 (0.7)n | 7 (2.7)o | 0.002 |
Total patients: HMPV positive (242), other virus positive (1753), virus negative (268); p<0.01 is significant
n=241,
n=1753,
n=267,
n=238,
n=1715,
n=263,
n=240,
n=1733,
n=266,
n=652,
n=1732,
n=1731,
n=265,
n=1734,
n=264
Cough (96.7%) and shortness of breath (79.3%) were common in children with HMPV and LRTI. Approximately 60% of children with HMPV had fever. Gastrointestinal symptoms were less frequent.
238/242 (98.3%) HMPV LRTI had a chest X-ray, and most were abnormal. Approximately 30% of HMPV LRTI children required supplemental oxygen (median, 2 days). Seven (2.9%) children required ventilation, and 2 (0.8%) children died. Median length of stay for children with HMPV was 4 days. Fewer children with HMPV required intensive care unit (ICU) stay compared with virus negative and other virus positive children; however, no significant difference in overall lengths of stay was noted.
Of the 242 children with HMPV LRTI, 127 (52.5%) were co-infected with another viral pathogen (Table 1). Children with HMPV co-infections and LRTI did not have a statistically significant difference in their symptomatology, chest X-ray findings, need for supplementary oxygen, or need for mechanical ventilation compared to HMPV-only infection. Ten (7.9%) children with HMPV co-infections and LRTI were admitted to the ICU compared with 1 child with isolated HMPV infection (0.9%), P=0.009.
We were able to genotype 192 (79%) of 242 specimens from HMPV LRTI children. Symptoms were not significantly different between the two subgroups (Table 4). However, 38 (35.5%) HMPV A children required supplemental oxygen compared with 18 (21.4%) HMPV B children, P=0.03. No significant difference in days on oxygen, need for ventilation, or need for ICU care was noted. Children with HMPV B were more likely to have viral co-infection. Length of stay was not different between children infected with HMPV A versus B (not shown).
Table 4.
Characteristics of patients infected with HMPV A and B.
| HMPV A (%) | HMPV B (%) | P value | |
|---|---|---|---|
| Ever on oxygen | 38 (35.5) | 18 (21.4)a | 0.03 |
| Ever on vent | 4 (3.7) | 0 (0)a | 0.13 |
| Deceased | 1 (0.9) | 1 (1.2) a | 1.00 |
| ICU | 5 (4.7) | 2 (2.4) | 0.47 |
| Co-infection | 44 (41.1) | 51 (60.0) | 0.009 |
Total patients: HMPV A (107), HMPV B (85)
n=84
We evaluated the characteristics of HMPV LRTI children on oxygen and in the ICU. Seventy-three children required oxygen (Table 5). Forty-two (57.5%) were male and 66 (90.4%) had no past medical history. The median gestational age was 40 weeks. Viral load, smoke exposure, and daycare were not significantly different between those who did and did not require supplemental oxygen. Children on oxygen were younger than children not on oxygen (3.9 vs. 7.0 months, P<0.001). Supplemental oxygen was required by 41/155 (26.5%) breastfed children compared with 32/85 (37.6%) of not breastfed children (P=0.07). Although HMPV co-infection overall was not significantly associated with oxygen requirement, HMPV-RSV co-infection was associated with oxygen use. Supplemental oxygen was required by 26/65 (40.0%) children with both HMPV and RSV compared with 47/175 (26.9%) with HMPV but not RSV, P=0.049.
Table 5.
Univariate and multivariate analysis of factors associated with oxygen use in HMPV LRTI.
| Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|
| Oxygen (%) |
No oxygen (%) |
P value | Adjusted OR (95% CI) |
P value | |
| Gender, male | 42 (57.5) | 94 (56.3) | 0.86 | ||
| No past medical history | 66 (90.4) | 149 (89.2) | 0.78 | ||
| Daycare | 1 (1.4) | 4 (2.4) | 1.00 | ||
| HMPV and RSV positive | 26 (35.6) | 39 (23.4) | 0.049 | 1.59 (0.73–3.45) |
0.24 |
| HMPV A | 38 (67.9)a | 69 (51.1)b | 0.03 | 2.17 (1.07–4.40) |
0.03 |
| Breastfeeding | 41 (56.2) | 114 (68.3) | 0.07 | 0.48 (0.24–0.96) |
0.04 |
| Smoke exposure | 60 (82.2) | 125 (74.9) | 0.21 | ||
| Median age, months [IQR] | 3.9 [1.8–7.6] |
7.0 [4.5–12.2] |
<0.001 | ||
| Age <6 months | 2.70 (1.37–5.32) |
0.004 | |||
| Viral load, log10 copies/μL [IQR] | 3.1 [2.1–4.3] |
3.3 [2.3–4.5] |
0.15 | ||
Total patients: oxygen (73), no oxygen (167)
Abbreviations: OR, odds ratio; CI, confidence interval
n=56,
n=135
Of the 11 children with HMPV LRTI in the ICU, 7 (64%) were male. The median age was 1.4 months. Two (18%) children had underlying medical conditions (Down syndrome with cardiac disease and Down syndrome alone). Nine (82%) children were co-infected with RSV. ICU patients had a significantly lower median HMPV viral load (2.1 log10 copies/μL) compared with non-ICU patients (3.3 log10 copies/μL), P=0.01. Breastfeeding was not significantly protective.
Since few children required ICU care stay, we used supplemental oxygen requirement as a surrogate for HMPV severe disease. In a multivariate model (Table 5), we included factors that were significant or neared significance in univariate analysis: age, HMPV subgroup, co-detection of RSV, and breastfeeding. When adjusting for other factors, age <6 months and HMPV A were independent risk factors of severe disease while breastfeeding was protective. Co-infection with RSV and HMPV was not significantly associated with oxygen requirement in the model.
HMPV infected children without LRTI
Of the original 273 children with HMPV infection, 31 (11.4%) did not meet criteria for LRTI. 18 were diagnosed with rule-out sepsis, and the remainder were diagnosed with paroxysmal/pertussis-like cough/pertussis (8), upper respiratory infection (2), croup (1), febrile seizure (1), or upper respiratory infection and vasculitis (1). The median age was 2.1 months, younger than the LRTI cohort. 13 (42%) were infected with only HMPV. Of the 30 children with data available, 8 (27%) were on oxygen for a median of 3 days, and 4 (13%) were in the ICU. The median LOS was 5 days. None of these patients died.
DISCUSSION
Our study provides one of the largest prospective cohorts of HMPV disease in young children, including data from the Middle East. Similar to studies in other regions, HMPV caused a significant burden of disease in children, and rates of HMPV infection in children <6 months were higher than United States cohorts.1 HMPV-positive children were older than HMPV-negative children. However, chronic medical conditions and prematurity were much less common in our hospitalized HMPV-positive cohort compared with other studies.1, 14, 41 Low daycare rates and high smoke exposure were also dissimilar compared with other large studies.41, 42 Since hospital admissions are free for children <6 years old in Jordan, this may explain the high percentage of healthy children being admitted. With around 40% of all children <6 months of age, the threshold to admit younger children may be lower than high-income countries. Thus, our cohort of young Jordanian children is unique in that many previously described risk factors for viral LRTI are present at lower rates, and the majority of children hospitalized were full-term and otherwise healthy.
Circulating subgroups identified from Jordanian children are similar to recent studies, with predominance of A2, B1, and B2 with yearly variability.2 A1 was not detected, and this genotype has not been recently identified in other geographic locations.43 In contrast, A2 has been the predominant subgroup in other recent studies worldwide.43–45 Although we only sequenced part of the N gene, there was little variability within subgroups, suggesting the predominance of few circulating strains.
Viral respiratory co-infection in young children is common. In children with multiple viral infections, it can be difficult to discern which virus is contributing to symptoms or whether interplay between the two viruses occurs. In children with LRTI, we detected no differences in symptoms or disease severity other than ICU stay in children with HMPV single versus co-infection. In univariate analysis, HMPV-RSV co-infection was associated with an increased risk for supplemental oxygen, but this association did not reach significance in multivariate analysis. A previous pilot study in this region found a trend toward increased oxygen usage and ICU admission in HMPV-RSV co-infection.5 Since children with RSV are typically younger than children with HMPV,46 age may be a confounding factor in previous reports of HMPV-RSV causing more severe disease.
Symptomatology between HMPV A and B was similar. However, children with HMPV A were more likely to require supplemental oxygen, suggesting that HMPV A may cause more severe disease. Other studies have suggested that genotype B was associated with a higher clinical score19 or disease severity.14 However, our study included a considerably larger number of subjects. Significantly more HMPV B children were co-infected with other viruses, which may be related to the years that HMPV B was circulating. Further research is needed to validate our findings.
Approximately 30% of children in our cohort required supplemental oxygen, which we used as a surrogate for severity. In a multivariate regression model, HMPV A independently predicted oxygen requirement, but co-infection with RSV did not. Previously established factors such as young age were independently associated with disease severity. However, prematurity, daycare, and smoke exposure were not associated with severe disease, and breastfeeding was not protective. Prematurity and daycare are underrepresented factors in this population, highlighting that factors contributing to severe disease differ substantially between geographic and developing regions. We did not find an association with viral load and disease severity, which corroborates some studies.16, 17 Interestingly, viral load was significantly lower in the ICU group, the majority of whom (81%) were co-infected with RSV. Young age and RSV co-infection may have contributed to severity in the ICU subjects, since HMPV viral load was lower than non-ICU patients. Unlike other studies, female gender and past medical history were not associated with severe disease.15
We identified a unique group of children with HMPV infection and no LRTI symptoms at admission. Approximately 25% developed an oxygen requirement during hospitalization, suggesting that upper respiratory disease may have progressed to the lower tract. These children were notably younger than the LRTI cohort. Despite the lack of LRTI symptoms on admission, 13% required ICU care and the overall median length of stay was 5 days. Fever without respiratory symptoms is a recognized clinical syndrome in young children with other respiratory viruses, particularly influenza.47, 48 Therefore, young children with fever should be screened for HMPV infection as they may present without classic LRTI symptoms but develop severe disease. Recognition of HMPV could avoid unnecessary antibiotics in these children, and they could benefit from an antiviral or vaccine in the future.
Our study has strengths and limitations. This is a prospective viral surveillance study over a three-year study period, and it is one of the largest cohorts of children infected with HMPV. We only enrolled children at one large, referral hospital in Amman, Jordan andmay have selected for sicker children. Data not obtained from parents was gleaned from chart review andwas dependent on provider accuracy. Admission diagnosis was based on physician discretion. We tested for 10 common respiratory viruses, but we did not test for parainfluenza 4, coronavirus, or human bocavirus, which have been implicated in LRTI.49, 50 There were few blood cultures and thus limited data on bacterial co-infection, which could contribute to disease severity. However, neither child who died had cultures consistent with bacterial infection (not shown). Although HMPV is rarely found in asymptomatic children,1 we did not have a matched healthy cohort to determine the role of HMPV in causing symptomatology. Lastly, we were not able to genotype ~20% of specimens.
In addition to the unique geographic location, our population differs from many studies evaluating HMPV disease severity in that the majority of children were not premature, had no significant past medical history, and did not attend daycare, but had smoke exposure. Thus, the epidemiology of previously established risk factors in this population are different than other cohorts where these factors are often predictors of respiratory virus severity. Previous studies have used hospitalization, clinical severity scores, and ICU mortality as markers of disease severity.13–15 Since our population did not include outpatient children with HMPV and few children required ICU care, we used supplemental oxygen as a surrogate for severe LRTI. In a large population of previously healthy children, with HMPV subgroups A2, B1, and B2 circulating over a 3 year period, HMPV A and young age were independently associated with oxygen use. Our study highlights that the epidemiology of established risk factors are unique to specific populations. Pediatricians should be aware of local epidemiology and risk factors and recognize that young children and children with HMPV A may be at higher risk for severe disease and benefit from future development of antivirals and monoclonal antibodies.
Acknowledgments
We wish to acknowledge our research staff who enrolled these subjects: Hanan Amin, Amani Altaber, Hana’a Khalaf, Isra’a Kharbat, Darin Yasin, and Shireen Issa.
Funding Source: This work was supported by the UBS Optimus Foundation; National Institutes of Health: R01AI085062 and T32HD060554; and the CTSA award UL1TR000445 from the National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
References
- 1.Edwards KM, Zhu Y, Griffin MR, et al. Burden of human metapneumovirus infection in young children. N Engl J Med. 2013;368:633–643. doi: 10.1056/NEJMoa1204630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williams JV, Wang CK, Yang CF, et al. The role of human metapneumovirus in upper respiratory tract infections in children: a 20-year experience. J Infect Dis. 2006;193:387–395. doi: 10.1086/499274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Embarek Mohamed MS, Reiche J, Jacobsen S, et al. Molecular analysis of human metapneumovirus detected in patients with lower respiratory tract infection in upper egypt. Int J Microbiol. 2014;2014:290793. doi: 10.1155/2014/290793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aberle JH, Aberle SW, Redlberger-Fritz M, Sandhofer MJ, Popow-Kraupp T. Human metapneumovirus subgroup changes and seasonality during epidemics. Pediatr Infect Dis J. 2010;29:1016–1018. doi: 10.1097/INF.0b013e3181e3331a. [DOI] [PubMed] [Google Scholar]
- 5.Ali SA, Williams JV, Chen Q, et al. Human metapneumovirus in hospitalized children in Amman, Jordan. J Med Virol. 2010;82:1012–1016. doi: 10.1002/jmv.21768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Sonboli N, Hart CA, Al-Aeryani A, et al. Respiratory syncytial virus and human metapneumovirus in children with acute respiratory infections in Yemen. Pediatr Infect Dis J. 2005;24:734–736. doi: 10.1097/01.inf.0000172937.80719.7f. [DOI] [PubMed] [Google Scholar]
- 7.Shatizadeh Malekshahi S, Mokhtari Azad T, Shahmahmoodi S, Yavarian J, Rezaei F, Naseri M. First report of respiratory syncytial virus and human metapneumovirus co-infection in a 2-year-old kawasaki patient in iran. Iran J Public Health. 2010;39:140–142. [PMC free article] [PubMed] [Google Scholar]
- 8.Al-Abdallat MM, Payne DC, Alqasrawi S, et al. Hospital-associated outbreak of Middle East respiratory syndrome coronavirus: a serologic, epidemiologic, and clinical description. Clin Infect Dis. 2014;59:1225–1233. doi: 10.1093/cid/ciu359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Memish ZA, Al-Tawfiq JA, Assiri A, et al. Middle East respiratory syndrome coronavirus disease in children. Pediatr Infect Dis J. 2014;33:904–906. doi: 10.1097/INF.0000000000000325. [DOI] [PubMed] [Google Scholar]
- 10.Khuri-Bulos N, Payne DC, Lu X, et al. Middle East respiratory syndrome coronavirus not detected in children hospitalized with acute respiratory illness in Amman, Jordan, March 2010 to September 2012. Clin Microbiol Infect. 2014;20:678–682. doi: 10.1111/1469-0691.12438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Talaat KR, Karron RA, Thumar B, et al. Experimental infection of adults with recombinant wild-type human metapneumovirus. J Infect Dis. 2013;208:1669–1678. doi: 10.1093/infdis/jit356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schuster JE, Cox RG, Hastings AK, et al. A broadly neutralizing human monoclonal antibody exhibits in vivo efficacy against both human metapneumovirus and respiratory syncytial virus. J Infect Dis. 2015;211:216–225. doi: 10.1093/infdis/jiu307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roussy JF, Carbonneau J, Ouakki M, et al. Human metapneumovirus viral load is an important risk factor for disease severity in young children. J Clin Virol. 2014;60:133–140. doi: 10.1016/j.jcv.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 14.Papenburg J, Hamelin ME, Ouhoummane N, et al. Comparison of risk factors for human metapneumovirus and respiratory syncytial virus disease severity in young children. J Infect Dis. 2012;206:178–189. doi: 10.1093/infdis/jis333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spaeder MC, Custer JW, Bembea MM, Aganga DO, Song X, Scafidi S. A multicenter outcomes analysis of children with severe viral respiratory infection due to human metapneumovirus. Pediatr Crit Care Med. 2013;14:268–272. doi: 10.1097/PCC.0b013e3182720fc7. [DOI] [PubMed] [Google Scholar]
- 16.Fuller JA, Njenga MK, Bigogo G, et al. Association of the CT values of real-time PCR of viral upper respiratory tract infection with clinical severity, Kenya. J Med Virol. 2013;85:924–932. doi: 10.1002/jmv.23455. [DOI] [PubMed] [Google Scholar]
- 17.Peng D, Zhao X, Liu E, et al. Analysis of viral load in children infected with human metapneumovirus. Iran J Pediatr. 2010;20:393–400. [PMC free article] [PubMed] [Google Scholar]
- 18.Vicente D, Montes M, Cilla G, Perez-Yarza EG, Perez-Trallero E. Differences in clinical severity between genotype A and genotype B human metapneumovirus infection in children. Clin Infect Dis. 2006;42:e111–113. doi: 10.1086/504378. [DOI] [PubMed] [Google Scholar]
- 19.Pitoiset C, Darniot M, Huet F, Aho SL, Pothier P, Manoha C. Human metapneumovirus genotypes and severity of disease in young children (n = 100) during a 7-year study in Dijon hospital, France. J Med Virol. 2010;82:1782–1789. doi: 10.1002/jmv.21884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bosis S, Esposito S, Osterhaus AD, et al. Association between high nasopharyngeal viral load and disease severity in children with human metapneumovirus infection. J Clin Virol. 2008;42:286–290. doi: 10.1016/j.jcv.2008.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agapov E, Sumino KC, Gaudreault-Keener M, Storch GA, Holtzman MJ. Genetic variability of human metapneumovirus infection: evidence of a shift in viral genotype without a change in illness. J Infect Dis. 2006;193:396–403. doi: 10.1086/499310. [DOI] [PubMed] [Google Scholar]
- 22.Hara M, Takao S, Shimazu Y, Nishimura T. Three-year study of viral etiology and features of febrile respiratory tract infections in Japanese pediatric outpatients. Pediatr Infect Dis J. 2014;33:687–692. doi: 10.1097/INF.0000000000000227. [DOI] [PubMed] [Google Scholar]
- 23.Kouni S, Karakitsos P, Chranioti A, Theodoridou M, Chrousos G, Michos A. Evaluation of viral co-infections in hospitalized and non-hospitalized children with respiratory infections using microarrays. Clin Microbiol Infect. 2013;19:772–777. doi: 10.1111/1469-0691.12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.da Silva ER, Pitrez MC, Arruda E, et al. Severe lower respiratory tract infection in infants and toddlers from a non-affluent population: viral etiology and co-detection as risk factors. BMC Infect Dis. 2013;13:41. doi: 10.1186/1471-2334-13-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Semple MG, Cowell A, Dove W, et al. Dual infection of infants by human metapneumovirus and human respiratory syncytial virus is strongly associated with severe bronchiolitis. J Infect Dis. 2005;191:382–386. doi: 10.1086/426457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greensill J, McNamara PS, Dove W, Flanagan B, Smyth RL, Hart CA. Human metapneumovirus in severe respiratory syncytial virus bronchiolitis. Emerg Infect Dis. 2003;9:372–375. doi: 10.3201/eid0903.020289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lazar I, Weibel C, Dziura J, Ferguson D, Landry ML, Kahn JS. Human metapneumovirus and severity of respiratory syncytial virus disease. Emerg Infect Dis. 2004;10:1318–1320. doi: 10.3201/eid1007.030983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klemenc J, Asad Ali S, Johnson M, et al. Real-time reverse transcriptase PCR assay for improved detection of human metapneumovirus. J Clin Virol. 2012;54:371–375. doi: 10.1016/j.jcv.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kodani M, Yang G, Conklin LM, et al. Application of TaqMan low-density arrays for simultaneous detection of multiple respiratory pathogens. J Clin Microbiol. 2011;49:2175–2182. doi: 10.1128/JCM.02270-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu X, Holloway B, Dare RK, et al. Real-time reverse transcription-PCR assay for comprehensive detection of human rhinoviruses. J Clin Microbiol. 2008;46:533–539. doi: 10.1128/JCM.01739-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Talbot HK, Poehling KA, Williams JV, et al. Influenza in older adults: impact of vaccination of school children. Vaccine. 2009;27:1923–1927. doi: 10.1016/j.vaccine.2009.01.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Piyaratna R, Tollefson SJ, Williams JV. Genomic analysis of four human metapneumovirus prototypes. Virus Res. 2011;160:200–205. doi: 10.1016/j.virusres.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tamura K, Nei M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Biol Evol. 1993;10:512–526. doi: 10.1093/oxfordjournals.molbev.a040023. [DOI] [PubMed] [Google Scholar]
- 34.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2011. [Google Scholar]
- 36.Gelman A, Rubin DB. Inference from iterative simulation using multiple sequences. Statistical Science. 1992;7:457–472. [Google Scholar]
- 37.The World Bank. Population, total 2015. Available at: http://data.worldbank.org/indicator/SP.POP.TOTL. Accessed June 15, 2015.
- 38.Brooks SP, Catchpole EA, Morgan BJT. Bayesian Animal Survival Estimation. Statistical Science. 2000;15:357–376. [Google Scholar]
- 39.Patil A, Huard D, Fonnesbeck CJ. PyMC: Bayesian Stochastic Modelling in Python. J Stat Softw. 2010;35:1–81. [PMC free article] [PubMed] [Google Scholar]
- 40.Gelman A. Bayesian data analysis. Third. Boca Raton: CRC Press; 2014. [Google Scholar]
- 41.Mullins JA, Erdman DD, Weinberg GA, et al. Human metapneumovirus infection among children hospitalized with acute respiratory illness. Emerg Infect Dis. 2004;10:700–705. doi: 10.3201/eid1004.030555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.von Linstow ML, Hogh M, Nordbo SA, Eugen-Olsen J, Koch A, Hogh B. A community study of clinical traits and risk factors for human metapneumovirus and respiratory syncytial virus infection during the first year of life. Eur J Pediatr. 2008;167:1125–1133. doi: 10.1007/s00431-007-0643-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Neemuchwala A, Duvvuri VR, Marchand-Austin A, Li A, Gubbay JB. Human metapneumovirus prevalence and molecular epidemiology in respiratory outbreaks in Ontario, Canada. J Med Virol. 2015;87:269–274. doi: 10.1002/jmv.24024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xiao NG, Zhang B, Xie ZP, et al. Prevalence of human metapneumovirus in children with acute lower respiratory infection in Changsha, China. J Med Virol. 2013;85:546–553. doi: 10.1002/jmv.23501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wei HY, Tsao KC, Huang CG, Huang YC, Lin TY. Clinical features of different genotypes/genogroups of human metapneumovirus in hospitalized children. J Microbiol Immunol Infect. 2013;46:352–357. doi: 10.1016/j.jmii.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 46.Wolf DG, Greenberg D, Kalkstein D, et al. Comparison of human metapneumovirus, respiratory syncytial virus and influenza A virus lower respiratory tract infections in hospitalized young children. Pediatr Infect Dis J. 2006;25:320–324. doi: 10.1097/01.inf.0000207395.80657.cf. [DOI] [PubMed] [Google Scholar]
- 47.Iwane MK, Edwards KM, Szilagyi PG, et al. Population-based surveillance for hospitalizations associated with respiratory syncytial virus, influenza virus, and parainfluenza viruses among young children. Pediatrics. 2004;113:1758–1764. doi: 10.1542/peds.113.6.1758. [DOI] [PubMed] [Google Scholar]
- 48.Poehling KA, Edwards KM, Weinberg GA, et al. The underrecognized burden of influenza in young children. N Engl J Med. 2006;355:31–40. doi: 10.1056/NEJMoa054869. [DOI] [PubMed] [Google Scholar]
- 49.Frost HM, Robinson CC, Dominguez SR. Epidemiology and clinical presentation of parainfluenza type 4 in children: a 3-year comparative study to parainfluenza types 1–3. J Infect Dis. 2014;209:695–702. doi: 10.1093/infdis/jit552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Choi EH, Lee HJ, Kim SJ, et al. The association of newly identified respiratory viruses with lower respiratory tract infections in Korean children, 2000–2005. Clin Infect Dis. 2006;43:585–592. doi: 10.1086/506350. [DOI] [PMC free article] [PubMed] [Google Scholar]


