Abstract
We have discovered that 3,4-dimethoxy-N-[(2,2-dimethyl-2H-chromen-6-yl)methyl]-N-phenylbenzenesulfonamide, a novel small molecule HIF-1 pathway inhibitor, can antagonize tumor growth in animal models of cancer, but the treatment necessitates its delivery in a formulation, due to poor water solubility (<15 μg/mL; pH 7.4), evidencing that the chemotype needs further exploration of its amenability to additional chemical modifications for ultimate optimization of function and pharmacology.
As a first step towards this goal we investigated the structure–activity relationships of 15 lipophilic 2, 2-dimethyl-2H-chromene based arylsulfonamide analogs of 3,4-dimethoxy-N-[(2,2-dimethyl-2H-chromen-6-yl)methyl]-N-phenylbenzenesulfonamide to find out strategies of modification. A 3,4-dimethoxybenzenesulfonyl group in region 1 showed the strongest inhibition among five arylsulfonyl groups tested. The presence of propan-2-amine in region 2 conferred the strongest inhibitory effect of the compound on HIF-1 activated transcription in a reporter assay. These findings are important as they help define the structural motifs where the 3,4-dimethoxy-N-[(2,2-dimethyl-2H-chromen-6-yl)methyl]-N-phenylbenzenesulfonamide can be chemically modified to improve its pharmacological properties towards development as a cancer therapeutic.
Keywords: Hypoxia inducible factor-1; Small molecule HIF-1 transcription factor inhibitors; Tumor hypoxia; 3,4-Dimethoxy-N-[(2,2-dimethyl-2H-chromen-6-yl)methyl]-N-phenylbenzenesulfonamide; Structure–activity relationships; Anti-cancer drugs; Brain cancer
1. Introduction
Fast tumor cell proliferation in the context of a dysfunctional vasculature generates a hypoxic microenvironment in solid tumors, which hinders the efficacy of chemo- and radiotherapies. Therefore, developing novel therapeutics to target hypoxic tumor is an active area of investigation.1–3 Tumor cells respond to the hypoxic stress by activating the hypoxia inducible factor (HIF) family of transcription factors, which bind to DNA sequences called hypoxia response elements (HREs) in target genes that encode proteins regulating a variety of biological functions, including erythropoiesis, angiogenesis, glycolysis, and metastasis. Hypoxia inducible factors (HIF-1 and HIF-2), are heterodimers consisting of an oxygen-sensitive subunit (HIF-1α or -2α), and a constitutively expressed subunit HIF-1β. HIF-α subunits are stabilized under hypoxic conditions, while they undergo proteasomal degradation in an oxygenated environment.4
The best-studied family member in cancer is HIF-1. High expression of HIF-1α in solid tumors has been associated with treatment failure and mortality,5 which prompted the development of HIF-1 inhibitors as anti-cancer drugs, either alone or in combination with chemo- or radiotherapy.6–16
We previously screened a library of 10,000 small molecules, containing 2,2-dimethyl-2H-chromene as a structural motif,17,18 for inhibition of HIF activity using a cell-based reporter assay.14,15,19–22 The inhibition of HIF-1 transcriptional activation under hypoxia was measured by the activity of a genetically engineered reporter gene, which was stably transfected in a human glioma cell line. The library screening with this bioassay revealed 3,4-dimethoxy-N-[(2,2-dimethyl-2H-chromen-6-yl)methyl]-N-phenylbenzenesulfonamide (6e, Fig. 1), which showed strong inhibition of reporter activity under hypoxia.19–21 Further testing of 6e revealed that it has potent anti-cancer properties in animal models for brain cancer (glioblastoma), eye cancer (uveal melanoma) and pancreatic cancer (manuscripts in preparation). However, the treatment necessitated delivery of 6e in a formulation, due to poor water solubility (<15 μg/mL; pH7.4), suggesting that further pharmacological optimization is necessary before this family of compounds can be translated to the patient. This structural optimization requires the exploration of chemical routes for modification of the compounds.
Figure 1.
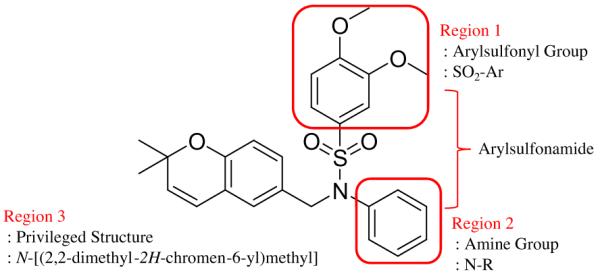
Chemical structure of 3,4-dimethoxy-N-[(2,2-dimethyl-2H-chromen-6-yl)methyl]-N-phenylbenzenesulfonamide (6e) and design of N-[(2,2-dimethyl-2H-chromen-6-yl)methyl]-N-R-arylsulfonamides. Regions were defined according to Ref. 19.
As the first step, we synthesized a series of lipophilic analogs of 6e and compared their activities. We focused on lipophilic congeners that contain a privileged structural motif, 2,2-dimethyl-2H-chromene group, and systematically diversified the arylsulfonamides. The goal was to discover effect of variations in sulfonamide groups on hypoxia-induced reporter activity, which would provide useful information for further modification. The inhibition of HIF-1 transcriptional activity under hypoxia by each compound was measured independently in an HRE-luciferase reporter cell-based assay in a dose-dependent manner. Finally, to probe the molecular mechanisms underlying the biological action of the inhibitors, we investigated the HIF-1α protein levels under hypoxia in human glioblastoma cells in the presence of analogs of 6e by a Western blot analysis.
2. Results and discussion
2.1. Design of analogs of 3,4-dimethoxy-N-[(2,2-dimethyl-2H-chromen-6-yl)methyl]-N-phenylbenzenesulfonamide
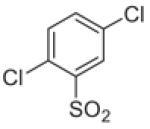
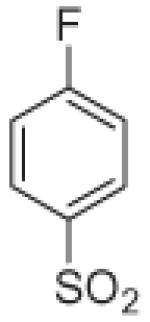
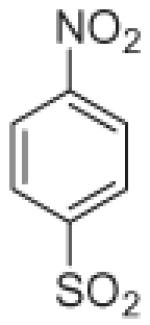
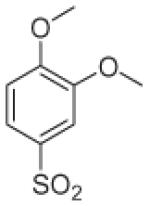
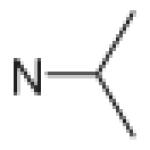
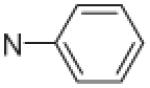
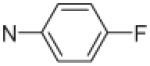
Fifteen N-[(2,2-dimethyl-2H-chromen-6-yl)methyl]-N-R-arylsulfonamides were designed, based on the structure of 6e. Analogs of 6e were designed with subtle variations with the hope that they would not change its presumable molecular pose. The 2,2-dimethyl-2H-chromene motif (region 3) was preserved in all compounds as a privileged structure, while the arylsulfonamide group was diversified to investigate structure–activity relationships (Fig. 1). Propan-2-amine (5a–5e), aniline (6a–6e) and 4-fluoroaniline (7a–7e) were chosen as alternate amines in region 2. This strategy allowed us to investigate the effect of substituting aniline with an alkylamine or a functionalized aniline on the inhibition of hypoxia/HIF-activated reporter expression. 2-Methylpropan-1-amine in region 2 produced five potent analogs in our previous study,19 so we further investigated propan-2-amine in region 2 to see if the potency would be preserved with minor chain length change. 4-Fluoroaniline was selected due to small size and the electron-withdrawing property of fluorine; the latter would also give an insight into the potential behavior of future compounds with a heteroarylaniline such as pyridin-2-amine and pyridin-4-amine in region 2. Benzenesulfonyl (5a, 6a, 7a), 2,5-dichlorobenzenesulfonyl (5b, 6b, 7b), 4-fluorobenzenesulfonyl (5c, 6c, 7c), 4-nitrobenzenesulfonyl (5d, 6d, 7d), and 3,4-dimethoxybenzenesulfonyl (5e, 6e, 7e) groups were used to investigate the effect of substituents on a benzene ring of the arylsulfonyl group in region 1 (Table 1).
Table 1.
Structures of 15 analogs of 6e
| Region 2 (N–R) | Region 1 (SO2-Ar) |
||||
|---|---|---|---|---|---|
|
|
|
|
|
|
|
5a | 5b | 5c | 5d | 5e |
|
6a | 6b | 6c | 6d | 6e |
|
7a | 7b | 7c | 7d | 7e |
Propan-2-amine (5), aniline (6), and 4-fluoroaniline (7) in region 2, were combined with benzenesulfonyl (a), 2,5-dichlorobenzenesulfonyl (b), 4-fluorobenzenesulfonyl (c), 4-nitrobenzenesulfonyl (d), and 3,4-dimethoxybenzenesulfonyl (e) group in region 1 to generate 15 different arylsulfonamide analogs. Alterations in region 1 are shown horizontally, while alterations in region 2 are shown vertically.
2.2. Syntheses of analogs of 6e
Fifteen analogs of 6e, N-[(2,2-dimethyl-2H-chromen-6-yl)-methyl]-N-R-arylsulfonamides, were synthesized from N-[(2,2-dimethyl-2H-chromen-6-yl)methyl]amine and commercially available arylsulfonyl chlorides (Scheme 1).
Scheme 1.
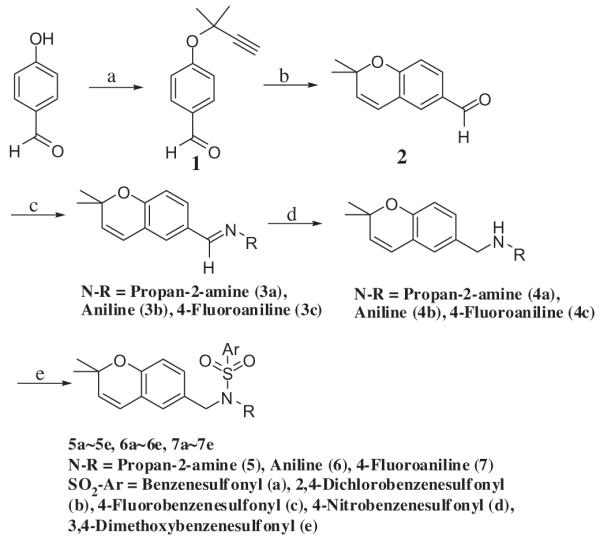
Synthesis of 15 analogs of 6e. Reagents and conditions: (a) 3-chloro-3-methyl-1-butyne, aq NaOH, DMF, 60 °C, 8 h; (b) N-methyl-2-pyrrolidone, reflux, 8 h (15% in two steps); (c) amine (propan-2-amine, aniline, 4-fluoroaniline), p-toluenesulfonic acid monohydrate, methylene chloride, reflux, overnight; (d) DIBAL, toluene, overnight (89% in two steps); (e) arylsulfonyl chloride, triethylamine, methylene chloride, reflux, overnight (12%–71%).
4-[(2-Methylbut-3-yn-2-yl)oxy]benzaldehyde (1) was synthesized from an alkylation of 4-hydroxybenzaldehyde with 3-chloro-3-methyl-1-butyne using sodium hydroxide as a base in aqueous dimethylformamide (DMF) (water/DMF (v/v) = 8:5). 4-Hydroxybenzaldehyde was used in excess, since it was the less expensive reagent, and easily eliminated from the crude reaction mixture in diethyl ether by washing with aqueous potassium hydroxide solution and then performing column chromatography. 2,2-Dimethyl-2H-chromene-6-carbaldehyde (2) was formed from 1 by Claisen cyclization in refluxing N-methyl-2-pyrrolidone (NMP). NMP was selected as a Claisen cyclization solvent due to its high boiling point (202 °C), and ease of removal from a diethyl ether solution by washing with water.23–25 N-[(2,2-dimethyl-2H-chromen-6-yl)methyl]propan-2-amine (4a) was synthesized from 2 and propan-2-amine by imine formation, and then reduction with diisobutylaluminum hydride (DIBAL). The final sulfonamide, N-[(2,2-dimethyl-2H-chromen-6-yl)methyl]-N-(propan-2-yl)benzenesulfonamide (5a), was synthesized by combining 4a with a benzenesulfonyl chloride in the presence of triethylamine. All other sulfonamides were synthesized in the same manner. The reaction yields were not optimized.
2.3. HRE-mediated luciferase reporter assays
We measured the inhibition of HIF-1 transcriptional activity under hypoxia by analogs of 6e using a cell-based reporter assay. A hypoxia/HIF-responsive luciferase gene reporter plasmid driven by a mini cytomegalovirus (CMV) gene promoter and an enhancer element made of 6 tandem repeats of the hypoxia response element (HRE) of the vascular endothelial growth factor gene (VEGF) was stably transfected in LN229 human glioblastoma cells to generate LN229-V6R cells (Fig. 2).19,27 LN229-V6R cells were cultured in an oxygenated ambient air environment (21% O2, normoxia) for 24 h, the analogs of 6e added and after 1 hour, the cell culture flasks transferred to a hypoxic cell culture incubator (1% O2) for 24 h, after which a cell extract was prepared. Hypoxia induced the intracellular stabilization of the HIF-1α subunit, HIF transcriptional complex assembly, binding to the HRE on genomic DNA in the nucleus and transcription of the luciferase reporter gene. To measure the amount of luciferase enzyme synthesized in the cells following HIF activation in the presence or absence of inhibitors, we used a chemiluminescence assay as we previously described.14,15 Briefly, the amount of luciferase present in the cell extracts was quantified by exposing it to luciferine substrate in vitro and measuring the light emitted in the reaction with a luminometer. Analogs of 6e were diluted serially to measure reporter inhibition in a dose-dependent fashion at concentrations of 0.5, 1, 2, 5, 10 and 25 μM. Three independent measurements for each concentration of each compound were performed and the experiment was repeated twice independently to obtain six measurements in total. The average percent of remaining luciferase activity in extracts from treated cells versus that found in extracts from untreated hypoxic cells as a control was plotted for each compound in a dose-dependent fashion. The IC50 values of each compound were obtained by fitting the data of luciferase activities and concentrations by a four-parameter nonlinear regression model, in which the IC50 value was one of the parameters (Table 2). The statistical analysis was conducted in R statistical software, as we previously described.19
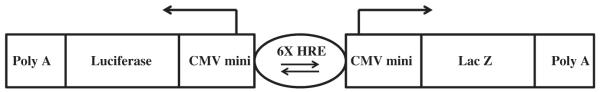
Figure 2.
pBI-GL HRE V6R plasmid structure. The pBI-GL HRE V6R plasmid was generated from the mammalian expression vector pBI-GL (TET) and is redrawn here from Figure 1 in Ref. 29. The arrows indicate the direction of transcription of both genes (luciferase and LacZ) that are under the control of the 6 copies of the VEGF gene HREs.
Table 2.
IC50 values of HIF reporter gene inhibition under hypoxia for 15 N-[(2,2-dimethyl-2H-chromen-6-yl)methyl]-N-R-arylsulfonamides
| Compound name |
IC50 (μM) |
Number of repeats |
Standard error |
95% Confidence interval |
|---|---|---|---|---|
| 5a | 2.2 | 6 | 0.7 | 0.7, 3.6 |
| 5b | 16.8 | 6 | 9.0 | −0.9, 34.5 |
| 5c | 3.8 | 6 | 0.8 | 2.2, 5.4 |
| 5d | 3.0 | 6 | 1.0 | 1.1, 4.9 |
| 5e | 1.5 | 6 | 0.3 | 0.9, 2.1 |
| 6a | 2.7 | 6 | 0.6 | 1.6, 3.9 |
| 6b | 3.3 | 6 | 0.6 | 2.1, 4.6 |
| 6c | 4.8 | 6 | 1.0 | 2.9, 6.7 |
| 6d | 4.2 | 6 | 1.0 | 2.1, 6.3 |
| 6e a | 1.6 | 6 | 0.2 | 1.2, 1.9 |
| 7a | 7.2 | 6 | 1.9 | 3.5, 10.8 |
| 7b | 8.0 | 6 | 2.2 | 3.7, 12.4 |
| 7c | 6.6 | 6 | 1.9 | 2.9, 10.4 |
| 7d | 4.8 | 6 | 1.8 | 1.3, 8.3 |
| 7e | 3.2 | 6 | 0.7 | 1.8, 4.6 |
We previously reported the IC50 values for 6e in the ~0.6 μM range (n = 42).19,21 In the current experimental set the IC50 value of 6e (1.6 μM) was larger than the previous values; this variation might be related to the particular batch of cells used and the smaller number of repeats (n = 6). Because this compound was used as an internal standard we used the latter value for comparison with the other compounds tested under the same conditions.
Fifteen analogs of 6e showed inhibition of HIF-1 transcriptional activity under hypoxia in a dose-dependent fashion with IC50 values ranging from 1.5 to 17 μM. Among the three amines tested for region 2, propan-2-amine showed the strongest inhibitory activity when combined with four arylsulfonyl groups: benzenesulfonyl (5a), 4-fluorobenzenesulfonyl (5c), 4-nitrobenzenesulfonyl (5d) and 3,4-dimethoxybenzenesulfonyl (5e). Among five sulfonyl groups tested for region 1, the 3,4-dimethoxybenzenesulfonyl group showed the strongest HIF-1 inhibitions when combined with all three amines: propan-2-amine (5e), aniline (6e) and 4-fluoroaniline (7e).
2.4. HIF-1α Western blot analyses
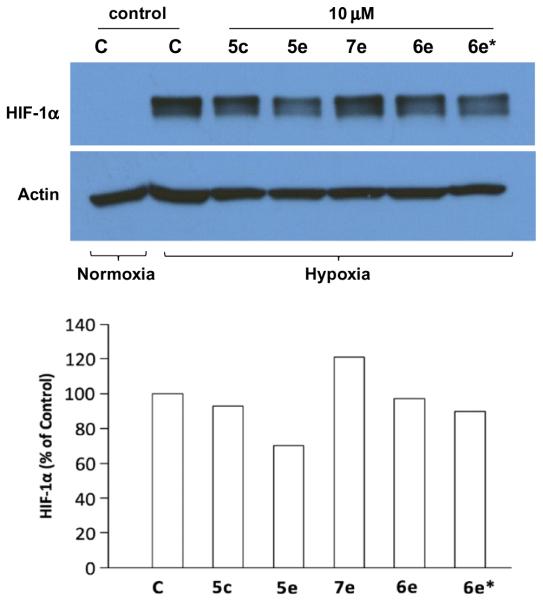
The activity of the HIF transcription factor is tightly regulated under normoxia. In the presence of oxygen, the HIF-1α subunit is constitutively hydroxylated at proline −402 and −564 by oxygen-dependent prolyl hydroxylases (PHDs), which triggers the interaction of HIF-1α with the VHL tumor suppressor protein, an E3 ubiquitin-protein ligase that targets it for proteosomal degradation.4,5,28 Under hypoxic conditions, as occurs in solid tumors, the HIF-1α subunit becomes stabilized due to lack of PHD binding, binds to HIF-1β and a stable HIF transcriptional complex is formed in the nucleus with co-factors p300/CBP.28–30 To determine whether analogs of 6e may block HIF activity by preventing HIF-1α protein accumulation under hypoxia or destabilizing the protein, HIF-1α protein levels under hypoxia were measured by Western blotting in the presence of analogs of 6e (Fig. 3). 5e, 6e, and 7e showed the strongest HIF-1 inhibition for each amine series in region 2 (less than 5% remaining activity at 10 μM), and 5c contains a 4-fluorobenzenesulfonyl group in region 1 that could predict the behavior of electron-withdrawing heteroarylsulfonyl groups (e.g. pyridine-4-sulfonyl group). HIF-1α Western blot analyses were performed on cell extracts from LN229-V6R cells incubated with 10 μM of the compounds under hypoxia for 24 h. A modest 30% reduction in the level of HIF-1α protein was observed in extracts from hypoxic cells treated with 5e, whereas little change was observed for 5c, 6e, and 7e treated cells. The batch-to-batch variation between 6e and 6e* was minimal as well. While this modest reduction in HIF-1α protein level induced by 5e might contribute to the strong inhibition of HIF transcriptional activity, it is unlikely to be the primary cause, because at 10 μM 5e almost entirely abolished HIF-1 activity in the luciferase reporter assay (less than 5% remaining activity, see Supplementary data 4). Overall, the western blot analysis showed that the compounds had little activity on the synthesis and stability of HIF-1α.
Figure 3.
Protein immunoblot (Western blot) analysis of HIF-1α expression under hypoxia. 6e* is previously synthesized 6e 19. Densitometry of the Western blot was processed by ImageJ 1.44p (Wayne Rasband, National Institutes of Health, USA, http://imagej.nih.gov/ij), and band intensity expressed as percent of untreated control cells under hypoxia normalized for β-actin.
3. Conclusions
Fifteen analogs of 6e, N-[(2,2-dimethyl-2H-chromen-6-yl)-methyl]-N-R-arylsulfonamides, were synthesized to possess 2,2-dimethyl-2H-chromene as a privileged structural motif, and a sulfonamide group composed of three different amines and five different sulfonyl groups. The inhibition of HIF-1 transcriptional activity mediated by the 15 analogs was measured in LN229-V6R, a human glioblastoma cell line containing a hypoxia/HIF activated luciferase reporter gene. All 15 N-[(2,2-dimethyl-2H-chromen-6-yl)methyl]-N-R-arylsulfonamides inhibited the reporter gene expression under hypoxia in a dose-dependent fashion with IC50 values in the range of 1.5–17 μM. The substitution of propan-2-amine in region 2 of 6e, showed the strongest inhibition among three tested amines in the sulfonamide group. The presence of a 3,4-dimethoxybenzenesulfonyl group in region 1 was most potent in inhibiting reporter activity among the five sulfonyl groups tested in the sulfonamide group.
To probe the molecular mechanisms underlying the inhibition of HIF transcriptional activity by the compounds, we measured HIF-1α protein levels under hypoxia. A modest 30% decrease in HIF-1α protein level was observed with 5e and little decrease with 5c, 6e, and 7e in comparison to the almost complete abrogation in transcriptional activity of the reporter at 10 μM. This result indicates that the mechanism of HIF inhibition by the analogs of 6e is not by preventing accumulation of HIF-1α protein or destabilizing it under hypoxia. Inhibition of HIF activity may occur at any step in its activation cascade, in which hypoxia-stabilized cytoplasmic HIF-1α translocates to the nucleus where it binds to HIF-1β and p300/CBP to form a stable HIF transcriptional complex, which can bind to the HREs of target genes and activate their transcription. Our ongoing investigations with 6e are focused on the hypothesis that 6e might bind the CH1 domain of the p300/CBP co-factors and thereby antagonize their interaction with the C-terminal activation domain of HIF-1α.
In summary, N-[(2,2-dimethyl-2H-chromen-6-yl)methyl]-N-R-arylsulfonamides are novel small molecules that inhibit the function of an important transcription factor necessary for cancer cell growth under hypoxia. These compounds have molecular weights around 400 g/mole, and can be synthesized in 4–5 steps with reasonable yields. The structure–activity relationship studies in this Letter, demonstrated that we could modify 3,4-dimethoxy-N-[(2,2-dimethyl-2H-chromen-6-yl)methyl]-N-phenylbenzenesulfonamide (6e), using either of two strategies. One is to introduce hydrophilic amines in region 2 with a 3,4-dimethoxybenzenesulfonyl group in region 1, which is suggested from results with 5e, 6e, and 7e. In this strategy, simple electron withdrawing heteroaryl amines, such as pyridine-2-amine, pyridine-4-amine, might decrease HIF-1 pathway inhibition, if they behave the same way as 4-fluoroaniline. The other strategy is to introduce hydrophilic arylsulfonyl groups in region 1 with a propan-2-amine group in region 2 as suggested from results with 5a, 5c, 5d, and 5e. In a follow-up study, we plan on using the latter strategy to introduce heteroarylsulfonyl groups in region 1 with simple alkyl groups in region 2. Since the molecules can be diversified at the last step of a synthesis, this approach is more advantageous than the former strategy.
4. Experimental
4.1. Chemistry
Glassware was oven-dried (200 °C) overnight and cooled under argon, and all reactions were performed under argon. All reagents were purchased from commercial sources. Column chromatography was performed with Silica Gel 60 (particle size 40–63 μm) with less than 4 psi of argon pressure. TLC was performed with TLC Silica gel 60 F254 and visualized by UV (254 nm) and staining with 10% (w/w) of phosphomolybdic acid hydrate solution in ethanol.
NMR spectroscopy was recorded by Varian INOVA 400, Mercury 300 and VNMRS 400 spectrometers. Mass spectroscopy was obtained by JEOL JMS-SX102/SX102A/E. HPLC was measured with a Waters 1525 Binary HPLC pump coupled with a Waters 2487 Dual λ Absorbance Detector. HPLC data was processed by Waters Breeze GPC Software. Two HPLC systems were used to measure the purity of each analog by % area of a UV absorbance peak at 254 nm: WAT045905 (Symmetry C18, 5 μm, 4.6 × 150 mm), 70:30:0.1 = methanol/water/triethylamine, 2 mL/min of a flow rate; WAT011695 (Nova-Pak C18, 4 μm, 3.9 × 300 mm), 70:30:0.1 = 90% ethanol, 5% methanol, 5% isopropanol/water/triethylamine (5a–5d, 6a–6d, and 7a–7d) or 60:40:0.1 = 90% ethanol, 5% methanol, 5% isopropanol: water: triethylamine (5e, 6e, 7e), 0.8 mL/min of a flow rate. The peaks after 3.5 min were considered as peaks of compounds. As an eluent, we used a mixture of 95% ethanol, 5% methanol, and 5% isopropanol instead of 100% of ethanol due to an institutional regulation.
4.1.1. 4-[(2-Methylbut-3-yn-2-yl)oxy]benzaldehyde (1)
A solution of 2.4 g (20 mmole) of 4-hydroxybenzaldehyde and 1 g (10 mmole) of 3-chloro-3-methyl-1-butyne in 8 mL of DMF, was mixed with 5 mL of 4 N aqueous sodium hydroxide solution. The bi-phase mixture was stirred vigorously at 60 °C for 8 h. After cooling, 20 mL of water was added to the reaction mixture, which was extracted with 30 mL of diethyl ether three times. The combined organic layer was washed with 40 mL of 1 N aqueous sodium hydroxide solution and brine. The organic portion was then dried with magnesium sulfate and concentrated in vacuo. A pale yellow viscous liquid was produced as a crude product. A small portion of the crude product was purified by column chromatography using ethyl acetate/hexane (1:8) as an eluent, and the rest of the crude product was used in the next reaction.
1H NMR (CDCl3): δ 1.73 (s, 6H), 2.67 (s, 1H), 7.34 (d, J = 8.8 Hz, 1H), 7.83 (d, J = 8.8 Hz 1H), 9.91 (s, 1H).
4.1.2. 2,2-Dimethyl-2H-chromene-6-carbaldehyde (2)
A crude 1 from the previous reaction was dissolved in 20 mL of N-methyl-2-pyrrolidone, NMP (bp 202 °C), and refluxed for 8 h. After cooling, a small portion of the crude mixture was extracted with diethyl ether to confirm complete conversion to the desired product by NMR. Forty milliliters of water was added to the reaction mixture, and then extracted with 60 mL of diethyl ether three times. The combined organic layer was washed with brine, dried with magnesium sulfate, and then concentrated in vacuo. The crude product was purified by column chromatography using ethyl acetate/hexane (1:8) as an eluent, which afforded 565 mg (3 mmole) of 2,2-dimethyl-2H-chromen-6-carboxaldehyde as a pale-yellow viscous liquid (15% in two steps, based on 20 mmole of 4-hydroxybezaldehyde).
1H NMR (CDCl3): δ 1.46 (s, 6H), 5.68 (d, J = 9.9 Hz, 1H), 6.36 (d, J = 9.9 Hz, 1H), 6.85 (d, J = 8.05 Hz, 1H), 7.50 (d, J = 2.37 Hz, 1H), 7.63 (dd, J = 8.52, 1.89 Hz, 1H), 9.83 (s, 1H).
HRMS m/z M+ calcd 189.09101, found 189.09071.
4.1.3. Imine synthesis (3a, 3b, 3c)
A mixture of 102 mg (0.54 mmole) of 2, 20 mg of p-toluenesul fonic acid monohydrate and 1 mL of propan-2-amine (3a) (0.1 mL of aniline (3b) or 0.1 mL of 4-fluoroaniline (3c)) in 2 mL of methylene chloride, was refluxed overnight. After cooling, a small portion of the crude product was concentrated in vacuo to confirm complete conversion to the desired imine by NMR. The indication was the disappearance of the aldehyde peak (9.83 ppm) and the appearance of the imine peak (8.18 ppm). After confirming the complete conversion to an imine, the crude mixture was concentrated in vacuo and then, used for the next reaction without purification.
4.1.3.1. 1-(2,2-Dimethyl-2H-chromen-6-yl)-N-(propan-2-yl)methanimine (3a)
1H NMR (CDCl3): δ 1.24 (d, J = 6.15 Hz, 6H), 1.43 (s, 6H), 3.48 (septet, J = 6.1 Hz, 1H), 5.63 (d, J = 5.6 Hz, 1H), 6.35 (d, J = 10 Hz, 1H), 6.78 (d, J = 8.2 Hz, 1H), 7.39 (dd, J = 8.2, 2.1 Hz, 1H), 7.45 (d, J = 1.76 Hz, 1H), 8.18 (s, 1H).
13C NMR (CDCl3): δ 24.4, 24.5, 28.3, 61.7, 77.0, 77.3, 77.5, 116.5, 121.5, 122.2, 122.3, 125.6, 125.6, 129.6, 130.0, 130.0, 131.3, 155.3, 157.9, 158.0.
4.1.3.2. 1-(2,2-Dimethyl-2H-chromen-6-yl)-N-phenylmethanimine (3b)
1H NMR (CDCl3): δ 1.49 (s, 6H), 5.69 (d, J = 9.67 Hz, 1H), 6.41 (d, J = 9.67 Hz, 1H), 6.70–7.61 (m, 8H), 8.35 (s, 1H).
4.1.3.3. 1-(2,2-Dimethyl-2H-chromen-6-yl)-N-(4-fluorophenyl)methanimine (3c)
1H NMR (CDCl3): δ 1.48 (s, 6H), 5.69 (d, J = 9.96 Hz, 1H), 6.40 (d, J = 9.96, 1H), 6.60–7.62 (m, 8H), 8.32 (s, 1H).
4.1.4. Amine synthesis (4a, 4b, 4c)
The crude 3a from the previous reaction was dissolved in 3 mL of toluene, to which 1 mL (1 mmole) of diisobutylaluminum hydride (1 M solution in toluene) was added slowly. The reaction mixture was stirred overnight and poured slowly into 10 mL of 1 N aqueous hydrochloric acid solution with stirring. The mixture was extracted with 20 mL of ethyl acetate three times. The combined organic layer was washed with brine, dried with magnesium sulfate, and then concentrated in vacuo. N-[(2,2-dimethyl-2H-chromen-6-yl)methyl]methanamine was purified by column chromatography using triethylamine/methanol/methylene chloride (1:3:100) as an eluent to afford 111 mg (0.5 mmole) of a pure product (4a) (89% in two steps). N-[(2,2-Dimethyl-2H-chromen-6-yl)methyl]aniline (4b) (85% in two steps) and N-[(2,2-dimethyl-2H-chromen-6-yl)methyl]-4-fluoroaniline (4c) (80% in two steps) were synthesized by the same method.
4.1.4.1. N-[(2,2-Dimethyl-2H-chromen-6-yl)methyl]propan-2-amine (4a)
1H NMR (CDCl3): δ 1.10 (d, J = 6.35 Hz, 6H), 1.42 (s, 6H), 2.86 (septet, J = 6.35 Hz, 1H), 3.67(s, 3H), 5.60(d, J = 9.85 Hz, 1H), 6.31(d, J = 9.85 Hz, 1H), 6.72(d, J = 8.26 Hz, 1H), 6.95(d, J = 2.22 Hz, 1H), 7.04(dd, J = 7.94, 1.9 Hz, 1H).
HRMS m/z M+ calcd 232.16959, found 232.17035.
4.1.4.2. N-[(2,2-Dimethyl-2H-chromen-6-yl)methyl]aniline (4b)
1H NMR (CDCl3): δ 1.44 (s, 6H), 4.21 (s, 2H), 5.63 (d, J = 9.67 Hz, 1H), 6.31 (d, J = 9.67 Hz), 6.64–6.77 (m, 4H), 7.00–7.22 (m, 4H).
HRMS m/z M+ calcd 266.15394, found 266.15385.
4.1.4.3. N-[(2,2-Dimethyl-2H-chromen-6-yl)methyl]-4-fluoroaniline (4c)
1H NMR (CDCl3): δ 1.44 (s, 6H), 4.16 (s, 2H), 6.31 (d, J = 9.96 Hz, 1H), 6.54–6.61 (m, 2H), 6.76 (d, J = 8.21 Hz, 1H), 6.85–7.12 (m, 4H).
HRMS m/z M+ calcd 284.14452, found 284.14434.
4.1.5. Sulfonamide synthesis (5a–5e, 6a–6e, 7a–7e)
A mixture of 22 mg (0.093 mmole) of 4a, 0.1 mL (164 mg, 0.93 mmole, 10 equiv) of benzenesulfonyl chloride, and 1 mL of triethylamine in 2 mL of methylene chloride was refluxed overnight. After cooling, the reaction mixture was concentrated in vacuo, and purified by column chromatography using ethyl acetate/hexane (1:6) as an eluent to afford 9 mg (0.024 mmole) of N-[(2,2-dimethyl-2H-chromen-6-yl)methyl]-N-(propan-2-yl)benzenesulfon-amide (5a, 26%). All 15 sulfonamides were synthesized by the same method.
4.1.5.1. N-[(2,2-Dimethyl-2H-chromen-6-yl)methyl]-N-(propan-2-yl)benzenesulfonamide (5a)
Yield: 26%, a column chromatography eluent: ethyl acetate/hexane (1:6).
1H NMR (CDCl3): δ 0.97 (d, J = 7.03 Hz, 6H), 1.43 (s, 6H), 4.12–4.19 (m, 1H), 4.30 (s, 2H), 5.61 (d, J = 9.67 Hz, 1H), 6.30 (d, J = 9.96 Hz, 1H), 6.70 (d, J = 8.20 Hz, 1H), 7.02 (s, 1H), 7.08 (d, J = 8.20 Hz, 1H), 7.46–7.56 (m, 3H), 7.79–7.82 (m, 2H).
HRMS m/z (M+Na)+ calcd 394.14474, found 394.14566.
HPLC (1) RT = 10.246 min, Purity = 97%.
HPLC (2) RT = 5.614 min, Purity = 91%.
4.1.5.2. 2,5-Dichloro-N-[(2,2-dimethyl-2H-chromen-6-yl)methyl]-N-(propan-2-yl)benzenesulfonamide (5b)
Yield: 12%, a column chromatography eluent: ethyl acetate/hexane (1:6).
1H NMR (CDCl3): δ 1.16 (d, J = 6.74 Hz, 6H), 1.41 (s, 6H), 4.23–4.28 (m, 1H), 4.39 (s, 2H), 5.58 (d, J = 9.96 Hz, 1H), 6.22 (d, J = 9.96 Hz, 1H), 6.60 (d, J = 8.20 Hz, 1H), 6.87 (d, J = 2.05 Hz, 1H), 6.99 (d, J = 8.20 Hz, 1H), 7.38 (d, J = 8.50 Hz, 1H), 7.54–7.63 (m, 2H).
HRMS m/z (M+Na)+ calcd 496.05114, found 496.05160.
HPLC (1) RT = 19.756 min, Purity = 96%.
HPLC (2) RT = 7.325 min, Purity = 96%.
4.1.5.3. N-[(2,2-Dimethyl-2H-chromen-6-yl)methyl]-4-fluoro-N-(propan-2-yl)benzenesulfonamide (5c)
Yield: 17%, a column chromatography eluent: ethyl acetate/hexane (1:6).
1H NMR (CDCl3): δ 1.12 (d, J = 7.03 Hz, 6H), 1.55 (s, 6H), 4.17–4.32 (m, 1H), 4.40 (s, 2H), 5.74 (d, J = 9.67 Hz, 1H), 6.41 (d, J = 9.67 Hz, 1H), 6.82 (d, J = 8.20 Hz, 1H), 7.11 (s, 1H), 7.18 (d, J = 8.50 Hz, 1H), 7.27 (t, J = 8.50 Hz, 2H), 7.88–7.93 (m, 2H).
HRMS m/z (M+Na)+ calcd 412.13532, found 412.13553.
HPLC (1) RT = 9.684 min, Purity = 99%.
HPLC (2) RT = 5.019 min, Purity = 99%.
4.1.5.4. N-[(2,2-Dimethyl-2H-chromen-6-yl)methyl]-4-nitro-N-(propan-2-yl)benzenesulfonamide (5d)
Yield: 26%, a column chromatography eluent: ethyl acetate/hexane (1:6).
1H NMR (CDCl3): δ 1.074 (d, J = 6.74 Hz, 6H), 1.43 (s, 6H), 4.21–4.26 (m, 1H), 4.31 (s, 2H), 5.62 (d, J = 9.96 Hz, 1H), 6.24 (d, J = 9.96 Hz, 1H), 6.68 (d, J = 8.20 Hz, 1H), 6.91 (s, 1H), 7.04 (d, J = 8.20 Hz, 1H), 7.88 (d, J = 8.79 Hz, 2H), 8.28 (d, J = 8.50 Hz, 2H).
HRMS m/z (M+Na)+ calcd 439.12982, found 439.13048.
HPLC (1) RT = 9.791 min, Purity = 99%.
HPLC (2) RT = 4.976 min, Purity = 99%.
4.1.5.5. 3,4-Dimethoxy-N-[(2,2-dimethyl-2H-chromen-6-yl)methyl]-N-(propan-2-yl)benzenesulfonamide (5e)
Yield: 45%, a column chromatography eluent: ethyl acetate/hexane (1:3).
1H NMR (CDCl3): δ 1.05 (d, J = 6.74 Hz, 6H), 1.47 (s, 6H), 3.94 (s, 3H), 3.99 (s, 3H), 4.14–4.24 (m, 1H), 4.33 (s, 2H), 5.66 (d, J = 9.67 Hz, 1H), 6.35 (d, J = 9.67 Hz, 1H), 6.76 (d, J = 8.20 Hz, 1H), 6.96 (d, J = 8.50 Hz, 1H), 7.07 (s, 1H), 7.144 (dd, J = 8.20, 2.05 Hz, 1H), 7.28(d, J = 2.05 Hz, 1H), 7.47 (dd, J = 8.50, 2.05 Hz, 1H).
HRMS m/z (M+Na)+ calcd 454.16587, found 454.16570.
HPLC (1) RT = 6.042 min, Purity = 98%.
HPLC (2) RT = 6.367 min, Purity = 94%.
4.1.5.6. N-[(2,2-Dimethyl-2H-chromen-6-yl)methyl]-N-phenylbenzenesulfonamide (6a)
Yield: 71%, a column chromatography eluent: ethyl acetate/hexane (1:6).
1H NMR (CDCl3): δ 1.39 (s, 6H), 4.63 (s, 2H), 5.57 (d, J = 9.67 Hz, 1H), 6.23 (d, J = 9.96 Hz, 1H), 6.59 (d, J = 8.79 Hz, 1H), 6.87–6.98 (m, 4H), 7.21–7.23 (m, 3H), 7.47–7.68 (m, 5H).
HRMS m/z (M+Na)+ calcd 428.12909, found 428.12915.
HPLC (1) RT = 8.910 min, Purity = 93%.
HPLC (2) RT = 4.974 min, Purity = 97%.
4.1.5.7. 2,5-Dichloro-N-[(2,2-dimethyl-2H-chromen-6-yl)methyl]-N-phenylbenzenesulfonamide (6b)
Yield: 28%, a column chromatography eluent: ethyl acetate/hexane (1:6).
1H NMR (CDCl3): δ 1.41 (s, 6H), 4.91 (s, 2H), 5.59 (d, J = 9.96 Hz, 1H), 6.26 (d, J = 9.96 Hz, 1H), 6.63 (d, J = 8.79 Hz, 1H), 6.88–7.49 (m, 9H), 7.82 (d, J = 2.05 Hz, 1H).
HRMS m/z (M+Na)+ calcd 496.05114, found 496.05160.
HPLC (1) RT = 22.911 min, Purity = 99%.
HPLC (2) RT = 4.985 min, Purity = 99%.
4.1.5.8. N-[(2,2-Dimethyl-2H-chromen-6-yl)methyl]-4-fluoro-N-phenylbenzenesulfonamide (6c)
Yield: 62%, a column chromatography eluent: ethyl acetate/hexane (1:6).
1H NMR (CDCl3): δ 1.39 (s, 6H), 4.62 (s, 2H), 5.58 (d, J = 9.67 Hz, 1H), 6.23 (d, J = 9.67 Hz, 1H), 6.60 (d, J = 8.50 Hz, 1H), 6.86–7.24 (m, 9H), 7.66 (dd, J = 8.79, 5.27 Hz, 2H).
HRMS m/z (M+Na)+ calcd 446.11967, found 446.11967.
HPLC (1) RT = 9.330 min, Purity = 98%.
HPLC (2) RT = 5.164 min, Purity = 98%.
4.1.5.9. N-[(2,2-Dimethyl-2H-chromen-6-yl)methyl]-4-nitro-N-phenylbenzenesulfonamide (6d)
Yield: 23%, a column chromatography eluent: ethyl acetate/hexane (1:6).
1H NMR (CDCl3): δ 1.45 (s, 6H), 4.72 (s, 2H), 5.65 (d, J = 9.96 Hz, 1H), 6.28 (d, J = 9.96 Hz, 1H), 6.67 (d, J = 7.91 Hz, 1H), 6.91–7.02 (m, 4H), 7.30–7.33 (m, 3H), 7.88 (d, J = 9.08 Hz, 2H), 8.38 (d, J = 8.79 Hz, 2H).
HRMS m/z (M+Na)+ calcd 450.12494 found 450.12551.
HPLC (1) RT = 10.179 min, Purity = 98%.
HPLC (2) RT = 5.202 min, Purity = 97%.
4.1.5.10. 3,4-Dimethoxy-N-[(2,2-dimethyl-2H-chromen-6-yl)methyl]-N-phenylbenzenesulfonamide (6e)
Yield: 31%, a column chromatography eluent: ethyl acetate/hexane (1:3).
1H NMR (CDCl3): δ 1.39 (s, 6H), 3.80 (s, 3H), 3.97 (s, 3H), 4.57 (s, 2H), 5.58 (d, J = 9.96 Hz, 1H), 6.23 (d, J = 9.67 Hz, 1H), 6.60 (d, J = 7.91 Hz, 1H), 6.85–6.95 (m, 8H), 7.00 (d, J = 2.05 Hz, 1H), 7.32 (dd, J = 8.50, 2.05 Hz, 1H).
HRMS m/z (M+Na)+ calcd 488.15022 found 488.15029.
HPLC (1) RT = 7.372 min, Purity = 99%.
HPLC (2) RT = 7.730 min, Purity = 97%.
4.1.5.11. N-[(2,2-Dimethyl-2H-chromen-6-yl)methyl]-N-(4-fluorophenyl)benzenesulfonamide (7a)
Yield: 61%, a column chromatography eluent: ethyl acetate/hexane (1:6).
1H NMR (CDCl3): δ 1.39 (s, 6H), 4.59 (s, 2H), 5.58 (d, J = 9.67 Hz, 1H), 6.23 (d, J = 9.67 Hz, 1H), 6.60 (d, J = 8.50 Hz, 1H), 6.84–6.90 (m, 6H), 7.51–7.68 (m, 5H).
HRMS m/z (M+Na)+ calcd 446.11967, found 446.11995.
HPLC (1) RT = 8.941 min, Purity = 96%.
HPLC (2) RT = 5.082 min, Purity = 88%.
4.1.5.12. 2,5-Dichloro-N-[(2,2-dimethyl-2H-chromen-6-yl)methyl]-N-(4-fluorophenyl)benzenesulfonamide (7b)
Yield: 22%, a column chromatography eluent: ethyl acetate/hexane (1:6).
1H NMR (CDCl3): δ 1.44 (s, 6H), 4.16 (s, 2H), 5.63 (d, J = 9.96 Hz, 1H), 6.31 (d, J = 9.96 Hz, 1H), 6.58 (dd, J = 9.08, 4.40 Hz, 2H), 6.75 (d, J = 8.21 Hz, 1H), 6.89 (t, J = 8.79 Hz, 2H), 6.98 (d, J = 2.05 Hz, 1H), 7.10 (dd, J=7.91, 1.76, 1H).
HRMS m/z (M+Na)+ calcd 518.38138, found 518.38149.
HPLC (1) RT = 8.951 min, Purity = 98%.
HPLC (2) RT = 5.723 min, Purity = 91%.
4.1.5.13. N-[(2,2-Dimethyl-2H-chromen-6-yl)methyl]-4-fluoro-N-(4-fluorophenyl)benzenesulfonamide (7c)
Yield: 54%, a column chromatography eluent: ethyl acetate/hexane (1:6).
1H NMR (CDCl3): δ 1.39 (s, 6H), 4.59 (s, 2H), 5.59 (d, J = J = 9.96 Hz, 1H), 6.23 (d, J = 9.96 Hz, 1H), 6.61 (d, J = 7.91 Hz, 1H), 6.84–6.92 (m, 6H), 7.18 (t, J = 8.50 Hz, 2H), 7.67 (dd, J = 8.79, 4.98 Hz, 2H).
HRMS m/z (M+Na)+ calcd 464.11024, found 464.11058.
HPLC (1) RT = 10.205 min, Purity = 91%.
HPLC (2) RT = 5.102 min, Purity = 99%.
4.1.5.14. N-[(2,2-Dimethyl-2H-chromen-6-yl)methyl]-N-(4-fluorophenyl)-4-nitrobenzenesulfonamide (7d)
Yield: 33%, a column chromatography eluent: ethyl acetate/hexane (1:6).
1H NMR (CDCl3): δ 1.40 (s, 6H), 4.63 (s, 2H), 5.60 (d, J = 9.96 Hz, 1H), 6.23 (d, J = 9.67 Hz, 1H), 6.62 (d, J = 8.21 Hz, 1H), 6.82––6.97 (m, 6H), 7.83 (d, J = 8.79 Hz, 2H), 8.34 (d, J = 9.08 Hz, 2H).
HRMS m/z (M+Na)+ calcd 469.12280, found 469.12403.
HPLC (1) RT = 10.882 min, Purity = 91%.
HPLC (2) RT = 5.325 min, Purity = 95%.
4.1.5.15. 3,4-Dimethoxy-N-[(2,2-dimethyl-2H-chromen-6-yl)methyl]-N-(4-fluorophenyl)benzenesulfonamide (7e)
Yield: 34%, a column chromatography eluent: ethyl acetate/hexane (1:3).
1H NMR (CDCl3): δ 1.37 (s, 6H), 3.74 (s, 3H), 3.95 (s, 3H), 4.59 (s, 2H), 5.55 (d, J = 9.96 Hz, 1H), 6.21 (d, J = 9.96 Hz, 1H), 6.58 (d, J = 9.08 Hz, 1H), 6.86–7.01 (m, 6H), 7.19–7.34 (m, 5H).
HRMS m/z (M+Na)+ calcd 506.14079, found 506.14080.
HPLC (1) RT = 6.565 min, Purity = 99%.
HPLC (2) RT = 7.219 min, Purity = 95%.
4.2. Biology
4.2.1. Generation of hypoxia/HIF reporter cell line (LN229-V6R cells)
The LN229-V6R cell line was constructed by stably transfecting LN229 human glioblastoma cells28 with a vector, pBI-GL VEGF HRE V6R, which was engineered by modifying the commercial pBI-GL (TET) mammalian expression vector, so as to replace the tet-responsive elements driving reporter gene expression with 6 tandem copies of the VEGF gene HRE.26 pBI-GL VEGF HRE V6R contains luciferase and lacZ reporter genes under a bi-directional hypoxia/HIF-responsive promoter. These cells synthesize luciferase and β-galactosidase under hypoxic (1% oxygen) conditions and can be used as a bioassay for the detection of HIF transcription inhibitors.19 The LN229-V6R cells were cultured in DMEM medium supplemented with 10% fetal bovine serum and penicillin/streptomycin at 37 °C under normoxic (21% O2, 5% CO2, 74% N2) or hypoxic (1% O2, 5% CO2, 74% N2) conditions.
4.2.2. Assays to measure HIF-1 transcriptional activity using HRE-driven luciferase reporter gene expression (luciferase assays)
LN229-V6R cells were seeded in 48-well plates (3 × 104 cells per well in 300 μL DMEM medium) and cultured for 24 h under normoxia before addition of new medium with chemical compounds. The synthesized compounds were dissolved in 100% of DMSO at 10 mM and a 100 μM solution of each compound was prepared in complete DMEM with 1% DMSO to prepare the compounds for cell treatment. The desired concentrations of N-[(2,2-dimethyl-2H-chromen-6-yl)methyl]-N-R-arylsulfonamides were prepared by serial dilutions in culture medium with 1% DMSO, and 300 μL was distributed to each well in triplicate for each concentration and compound. The 48-well plates were incubated at 37 °C under normoxia for one hour, then transferred to hypoxic conditions in a CO2 incubator for 24 h. The cells were washed with PBS and then lysed with lysis buffer (50 μL/well) that was prepared by a 1/5 dilution of a passive lysis buffer 5× (Promega, E194A) in distilled water. 20 μL of the protein suspension from each well was mixed with 25 μL of luciferase assay substrate and luminescence was measured using a 20/20n luminometer (Turner BioSystems Inc.).
4.2.3. Western blot analysis to determine HIF-1α protein levels
LN229-V6R cells (2 × 105) plated in 2 mL of complete DMEM culture media in 35 mm dishes were cultured under normoxia for 24 h, and then the media were removed by vacuum suction. Ten micromolars of the compounds (5c, 5e, 6e, and 7e) in culture medium with 1% of DMSO were added to each dish. Dishes were incubated under normoxia for one hour, and then transferred into a hypoxic incubator at 37 °C for 24 h. The cells were lysed with 1 × loading buffer (62.5 mM Tris–HCl (pH 6.8 at 25 °C), 2% (w/v) SDS, 50 μM 2-mercaptoethanol, 10% glycerol, 0.01% bromophenol blue) buffer, and the proteins were separated by electrophoresis in a 12.5% Tris–HCl polyacrylamide gel, before transfer onto a nitro-cellulose membrane.14,15 The membranes were immunoblotted using primary antibodies against HIF-1α (1:500 dilution, BD biosciences, San Diego, CA) and β-actin (1:3000 dilution, Santa Cruz Biotechnologies, Santa Cruz, CA). The secondary horseradish peroxide-conjugated antibodies were added and horseradish peroxide activities were measured by enhanced chemiluminescence as previously described.15 Densitometry of the Western blot was processed by ImageJ 1.44p. (Wayne Rasband, National Institutes of Health, USA, http://imagej.nih.gov/ij)
Supplementary Material
Acknowledgements
This research was supported by the National Cancer Institute Grants P50 CA 128301-01A1 Emory Molecular and Translational Imaging Research Center (EMTIC)/In vivo Cellular and Molecular Imaging Centers (ICMIC) (to Goodman, Hu, Meltzer), P50 CA 128301-01A1 Pilot Project #2 (to J.M.), the P30 CA 138292 Cancer Center grant (to the Winship Cancer Institute; W Curran, PI), R01 CA116804 and CA86335 (to E.G.V.M.), and the V Foundation, and Max Cure/Samuel Waxman cancer research foundation grants (to E.G.V.M.). Data analyses were performed in part through use of the Biostatistics and Bioinformatics shared Resource of the Winship Cancer Institute of Emory University.
Abbreviations
- HIF-1
hypoxia inducible factor-1
- HREs
hypoxia response elements
- HRE-AP assay
hypoxia response element-mediated alkaline phosphatase assay
- HRE-Luc assay
hypoxia response element-mediated luciferase assay
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.bmc.2012.04.064.
References and notes
- 1.Post DE, Devi NS, Li Z, Brat DJ, Kaur B, Nicholson A, Olson JJ, Zhang Z, Van Meir EG. Clin. Cancer Res. 2004;10:8603. doi: 10.1158/1078-0432.CCR-04-1432. [DOI] [PubMed] [Google Scholar]
- 2.Kim H-S, Peng G-Y, Hicks JM, Weiss HL, Van Meir EG, Brenner MK, Yotnda P. Mol. Ther. 2008;16:599. doi: 10.1038/sj.mt.6300391. [DOI] [PubMed] [Google Scholar]
- 3.Post DE, Sandberg EM, Devi SN, Brat DJ, Xu Z, Tighiouart M, Van Meir EG. Cancer Res. 2007;67:6872. doi: 10.1158/0008-5472.CAN-06-3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harris AL. Nat. Rev. Cancer. 2002;2:38. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- 5.Kaur B, Khwaja FW, Severson EA, Matheny SL, Brat DJ, Van Meir EG. Neuro-Oncology. 2005;7:134. doi: 10.1215/S1152851704001115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jensen RL, Ragel BT, Whang K, Gillespie D. J. Neuro-Oncol. 2006;78:233. doi: 10.1007/s11060-005-9103-z. [DOI] [PubMed] [Google Scholar]
- 7.Kung AL, Wang S, Klco JM, Kaelin WG, Jr., Livingston DM. Nat. Med. 2006;6:1335. doi: 10.1038/82146. [DOI] [PubMed] [Google Scholar]
- 8.Rapisarda A, Hollingshead M, Uranchimeg B, Bonomi CA, Borgel SD, Carter JP, Gehrs B, Raffeld M, Kinders RJ, Parchment R, Anver MR, Shoemaker RH, Melillo G. Mol. Cancer Ther. 1867;2009:8. doi: 10.1158/1535-7163.MCT-09-0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gillespie DL, Whang K, Ragel BT, Flynn JR, Kelly DA, Jensen RL. Cancer Ther. 2007;13:2441. doi: 10.1158/1078-0432.CCR-06-2692. [DOI] [PubMed] [Google Scholar]
- 10.Li L, Lin X, Shoemaker AR, Albert DH, Fesik SW, Shen Y. Cancer Ther. 2006;12:4747. doi: 10.1158/1078-0432.CCR-05-2842. [DOI] [PubMed] [Google Scholar]
- 11.Semenza GL. Oncogene. 2010;29:625. doi: 10.1038/onc.2009.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Belozerov VE, Van Meir EG. Anti-Cancer Drugs. 2005;16:901. doi: 10.1097/01.cad.0000180116.85912.69. [DOI] [PubMed] [Google Scholar]
- 13.Belozerov V, Van Meir EG. Curr. Opin. Invest. Drugs. 2006;7:1067. [PubMed] [Google Scholar]
- 14.Narita T, Yin S, Gelin CF, Moreno CS, Yepes M, Nicolaou KC, Van Meir EG. Clin. Cancer Res. 2009;15:6128. doi: 10.1158/1078-0432.CCR-08-3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tan C, de Noronha RG, Roecker AJ, Pyrzynska B, Khwaja F, Zhang Z, Zhang H, Teng Q, Nicholson AC, Giannakakou P, Zhou W, Olson JJ, Pereira MM, Nicolaou KC, Van Meir EG. Cancer Res. 2005;65:605. [PubMed] [Google Scholar]
- 16.Welsh S, Williams R, Kirkpatrick L, Pain-Murrieta G, Powis G. Mol. Cancer Ther. 2004;3:233. [PubMed] [Google Scholar]
- 17.Nicolaou KC, Pfefferkorn JA, Roecker AJ, Cao G-Q, Barluenga S, Mitchell HJ. J. Am. Chem. Soc. 2000;122:9939. [Google Scholar]
- 18.Nicolaou KC, Pfefferkorn JA, Mitchell HJ, Roecker AJ, Barluenga S, Cao G-Q, Affleck RL, Lillig JE. J. Am. Chem. Soc. 2000;122:9954. [Google Scholar]
- 19.Tan C, de Noronha RG, Devi NS, Jabbar AA, Kaluz S, Liu Y, Reid-Mooring S, Nicolaou KC, Wang B, Van Meir EG. Bioorg. Med. Chem. Lett. 2011;21:5528. doi: 10.1016/j.bmcl.2011.06.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Meir EG, Nicolaou KC. 033 286 U.S. Patent. 2005
- 21.Mooring SR, Jin H, Devi NS, Jabbar AA, Kaluz S, Liu Y, Van Meir EG, Wang B. J. Med. Chem. 2011;54:8471. doi: 10.1021/jm201018g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Meir EG, Chalet T, Roecker A, Nicolaou KC. 009,548 US patent. 2004 Mar 29;
- 23.Smith LR, Mahoney N, Molyneux RJ. J. Nat. Prod. 2003;66:169. doi: 10.1021/np020415t. [DOI] [PubMed] [Google Scholar]
- 24.Prado S, Janin YL, Saint-Joanis B, Brodin P, Michel S, Koch M, Cole ST, Tillequin F, Bost P-E. Bioorg. Med. Chem. 2007;15:2177. doi: 10.1016/j.bmc.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 25.Grubbs AW, Artman GD, III, Williams RM. Tetrahedron Lett. 2005;46:9013. [Google Scholar]
- 26.Ishii N, Maier D, Merlo A, Tada M, Sawamura Y, Diserens A-C, Van Meir EG. Brain Pathol. 1999;9:469. doi: 10.1111/j.1750-3639.1999.tb00536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Post DE, Van Meir EG. Gene Ther. 1801;2001:8. doi: 10.1038/sj.gt.3301605. [DOI] [PubMed] [Google Scholar]
- 28.Semenza GL. Nat. Rev. Cancer. 2003;3:721. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 29.Stebbins CE, Kaelin WG, Jr., Pavletich NP. Science. 1999;284:455. doi: 10.1126/science.284.5413.455. [DOI] [PubMed] [Google Scholar]
- 30.Kamura T, Koepp DM, Conrad MN, Skowyra D, Moreland RJ, Iliopoulos O, Lane WS, Kaelin WG, Jr., Elledge SJ, Conaway RC, et al. Science. 1999;284:657. doi: 10.1126/science.284.5414.657. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.