Abstract
Autoantigen development is poorly understood at the atomic level. Heparin-induced thrombocytopenia (HIT) is an autoimmune thrombotic disorder caused by antibodies to an antigen composed of platelet factor 4 (PF4) and heparin or cellular glycosaminoglycans (GAGs). In solution, PF4 exists as an equilibrium among monomers, dimers and tetramers. Structural studies of these interacting components helped delineate a multi-step process involved in the pathogenesis of HIT. First, heparin binds to the ‘closed’ end of the PF4 tetramer, and stabilizes its conformation and exposing the ‘open’ end. Second, PF4 arrays along heparin/GAG chains, which approximates tetramers, forming large antigenic complexes that enhance antibody avidity. Third, pathogenic HIT antibodies bind to the ‘open’ end of stabilized PF4 tetramers to form an IgG/PF4/heparin ternary immune complex and also to propagate formation of “ultralarge immune complexes” (ULCs) that contain multiple IgG antibodies. Fourth, ULCs signal through FcγRIIA receptors, activating platelets and monocytes directly and generating thrombin, which transactivates hematopoietic and endothelial cells. A non-pathogenic anti-PF4 antibody prevents tetramer formation, binding of pathogenic antibody, platelet activation and thrombosis, providing a new approach to manage HIT. An improved understanding of the pathogenesis of HIT may lead to novel diagnostics and therapeutics for this autoimmune disease.
Keywords: Heparin-induced thrombocytopenia, autoimmunity, immune complex structure, FcγRIIA, pathogenesis
1. Introduction
We are interested in the general process of autoantigen development and defining neoantigens at the atomic level. Heparin-induced thrombocytopenia (HIT) is the most common drug-induced, antibody-mediated autoimmune thrombotic disorder. HIT is caused by IgG antibodies that bind to a complex formed between platelet factor 4 (PF4) – a host protein, and heparin or cellular glycosaminoglycans (GAGs) – host polysaccharides [1-3]. HIT may lead to recurrent thromboembolism, limb amputation and death in ~1% of patients receiving unfractionated heparin (UFH) for at least 5 days, and, less commonly, in patients who receive low-molecular-weight heparins (LMWH) and other anionic polysaccharides [4]. Circulating immune complexes composed of PF4/heparin and IgG antibodies bind to platelet and monocyte Fc receptors and promote cellular activation, leading to generation of thrombin and downstream thromboembolic events [3, 5]. Therapy is based on anticoagulants that directly or indirectly inhibit thrombin [6] with significant, but incomplete, reduction in recurrent thrombosis, no reduction in the rate of amputation or death and a significant risk of major bleeding [7].
Many if not most patients exposed to heparin develop anti-PF4 antibodies, yet few develop HIT. This raises both fundamental immunologic and clinical questions. First, how does an endogenous sugar convert a normal host protein into an autoantigen in such a high proportion of otherwise seemingly immunologically normal individuals? Second, is there a fundamental difference in epitope specificity that differentiates a small fraction of pathogenic antibodies from the almost ubiquitous anti-PF4 antibodies that form after heparin exposure but are not associated with HIT? Third, can this distinction be used to develop clinical diagnostic tools to identify pathogenic antibodies and even to intervene in formation of ULCs as a disease-specific non-anticoagulant approach to mitigate or prevent thrombosis?
To begin to address these questions, we studied a murine monoclonal antibody (KKO) to human PF4/heparin complexes that causes thrombocytopenia and thrombosis in a transgenic mouse that expresses human PF4 and platelet FcγRIIA receptors [8, 9]. KKO provides a model antibody for the study of HIT because it competes with pathogenic human HIT antibodies for binding to PF4 in vitro [10], augments formation of pathogenic ultralarge immune complexes (ULCs; see below) [11, 12], and recapitulates the salient features of HIT in vivo. Therefore, we compared the properties of KKO with an isotype matched anti-PF4 monoclonal antibody (RTO) that binds comparably to PF4 in vitro, but does not foster formation of ULCs and is not pathogenic [10].
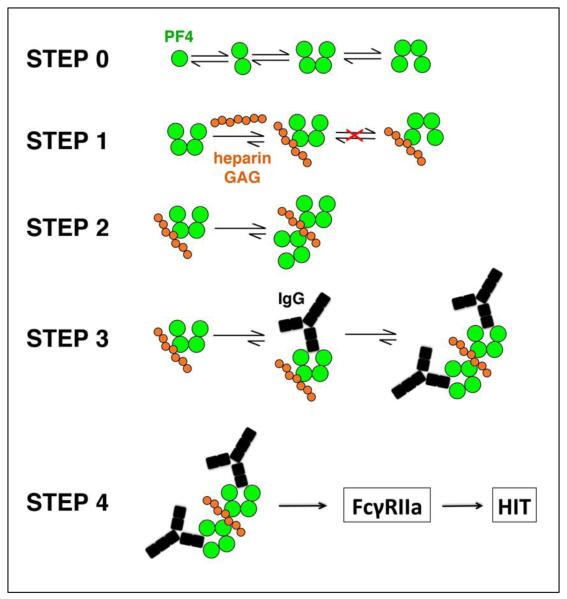
We solved the crystal structure of human PF4 in complex with a heparin-mimic pentasaccharide and crystal structures of PF4 complexed with Fab fragments derived from KKO and RTO [13]. These atomic level structural studies delineate the first three steps in a 4-step model of the pathogenesis of this autoimmune disease: Step 1: Heparin binds to the ‘closed’ end of PF4 tetramer. Heparin binding orients and stabilizes the ‘open’ end of PF4 tetramer, which contains an epitope recognized by KKO, thus increasing antibody affinity. Step 2. A heparin fragment can be “shared” by multiple PF4 tetramers. This aligns and approximates the tetramers to form a large antigenic complex, with the potential to increase antibody avidity. Step 3. Binding of multiple pathogenic antibodies propagates oligomerization forming stable ultralarge immune complexes (ULCs). Step 4. ULCs engage FcγIIA receptors on the surface of platelets and monocytes, which activates the cells, leads to expression of tissue factor and generates thrombin, which back-activates hematopoetic and endothelial cells promoting thrombosis [5, 9]. Binding of the non-HIT antibody RTO to PF4 monomer inhibits tetramerization, antigen formation and higher-order assembly into ULCs. As a consequence, RTO inhibits HIT antibody-induced platelet activation and aggregation in vitro and thrombus progression in vivo [13]. These studies provide insights into the process by which endogenous or exogenous GAGs interact with a normal human host protein, alter its structure and assembly and render the protein/GAG complex ‘antigenic’ to the mammalian immune system.
2. Pathogenesis of HIT: an autoimmune disease
2.1 Steps 1-2: Formation of PF4/heparin antigenic complexes
PF4, a host protein, becomes antigenic after complexing with heparin or GAGs. Binding of pathogenic antibodies to PF4 is markedly enhanced by heparin. In the absence of polysaccharide, apo- human [14] and bovine PF4 [15] adopt an asymmetric tetrameric conformation at the high concentrations employed for crystallization. When the N-terminal fragment of PF4 was replaced by a fragment from interleukin-8, the conformation became symmetrical and binding of heparin decreased [16]. This lead to the inference that the asymmetry of the PF4 tetramer plays a role in binding heparin [16] and several alternative models [17, 18] have been proposed to explain the formation of PF4/heparin complexes.
In order to study the formation of PF4/heparin antigenic complexes and to investigate how the polysaccharide might induce antigen formation, we solved the crystal structure of PF4 in complex with fondaparinux, a homogenous synthetic pentasaccharide heparin fragment (PDB ID: 4R9W)[13]. Fondaparinux forms complexes with PF4 as assessed by atomic force microscopy and photon correlation spectroscopy [19]. Fondaparinux induces anti-PF4/heparin autoantibodies [20-22] and occasionally causes HIT [23-26]. The crystal structure of PF4/fondaparinux reveals a potential mechanism underlying the first steps in the pathogenesis of HIT: formation of the PF4/heparin antigenic complex.
Both the apo-PF4 tetramer and PF4 in complex with fondaparinux displays a pseudosymmetry characterized by an ‘open’ end and a ‘closed’ end [13, 14]. Due to the structural asymmetry, there are only two major positively charged grooves on the surface of the tetramer each of which can accommodate one molecule of fondaparinux. These grooves are located at the ‘closed’ end of the tetramer and among monomers within each tetramer (Figure 1, step 1). Binding of fondaparinux within the groove between monomers stabilizes their self-association.
Figure 1.
Model of step-wise pathogenesis of HIT. Step 0, PF4 (green circles) released from activated platelets exists as an equilibrium among tetramers, dimers and monomers in plasma or whole blood. Tetramers may be transient and may switch between ‘open’-‘closed’ and ‘closed’-‘open’ conformations. Step 1, Heparin or GAGs (orange circles) bind to the ‘closed’ end of PF4 and stabilize the PF4 tetramers. Based on steric contraints, it is not possible to for a similar switch in conformations to occur in heparin/GAG-bound tetramers. Step 2, Binding of the first tetramer enhances the binding and approximation of additional tetramers, which eventuates in the formation of large PF4/heparin antigenic complexes. Step 3, Binding of PF4 tetramers also enhances binding of pathogenic antibodies (black) to the ‘open’ end of tetramer, forming ultralarge IgG/PF4/heparin immune complexes. Step 4, immune complexes activate platelets and monocytes though FcγRIIA’s and through expression of tissue factor, which leads to thrombin-dependent transactivation of platelets and endothelial cells.
A single fondaparinux molecule not only binds within the groove on the surface of one PF4 tetramer, it also has extensive potential interactions with a second adjacent tetramer, especially at the C-terminal helix. In this way, PF4 tetramers can cluster around a series of semi-rigid regions along the linear heparin chain (Figure 1, step 2). We suggest that the alignment and clustering of PF4 tetramers along heparin chains represent the first two steps in pathogenesis by stabilizing the epitope recognized by pathogenic HIT antibodies.
2.2 Step 3: Formation of IgG/PF4/Heparin immune complexes
After the binary PF4/heparin complex is formed, both the affinity and avidity of pathogenic HIT antibodies is enhanced leading to the formation of ULCs. To address the first part of these next steps, we next solved the crystal structure of PF4/KKOFab complex (PDB ID: 4R9Y)[13]. KKOFab binds to the PF4 tetramer by making contacts with three monomers within a single tetramer. This finding affirms that formation of tetrameric PF4 is required for optimal binding of the HIT antibody KKO [13, 27]. KKOFab binds to the ‘open’ end of the PF4 tetramer. We then used the structure of the PF4/KKOFab complex along with the heparin cluster model (step 2) to build a structural model of the KKO-Fab/PF4/heparin immune complex (Figure 1, step 3). In this ternary model, heparin binds to the ‘closed’ end of PF4, stabilizing the PF4 tetramer, clustering multiple PF4 tetramers and exposing the ‘open’ ends to recognition by HIT antibodies. In this way, heparin and HIT antibody act in concert to stabilize the ternary complex. KKO IgG might also cross-link two tetramers on separate PF4/heparin clusters. We suggest that the formation of IgG/PF4/heparin ternary complexes represents the third important step in the pathogenesis of HIT. This finding is accord with prior biochemical evidence that KKO fosters oligomerization of PF4 tetramers [11], a process that we believe is accelerated by their alignment along heparin.
This proposed structure of the ternary complex supports and helps to elucidate the basis of previous models that seek to explain how unfractionated heparin is more likely to cause HIT than low molecular weight heparins [12, 28]. Our data suggest that fondaparinux, a short fragment of heparin, is less able to stabilize inter-tetramer associations within the ternary complex than unfractionated heparin, so that the ternary complex is less likely to form, bind multiple antibodies and form ULCs [12]. This distinction helps to explain the seeming paradox that although fondaparinux/PF4 complexes are recognized by HIT antibodies through its effect on the structure of the PF4 tetramer, the pentamer rarely causes HIT and indeed is used successfully to prevent recurrent thromboembolism.
2.3 Step 4: ULCs activate platelets and induce generation of thrombin by monocytes and endothelial cells. Transactivation and formation of coated platelets
IgG/PF4/heparin ULCs activate platelets by engaging FcγRIIA receptors [9](Figure 1, step 4). This stimulates a spleen tyrosine kinase-dependent intracellular signal transduction pathway that promotes platelet activation [29] and release of procoagulant microparticles [30]. Unlike autoimmune disorders mediated by anti-platelet antibodies, the ULCs that mediate HIT also activate monocytes [3, 5, 31] leading to the expression of tissue factor and generation of thrombin [32]. Monocytes express a glycocalyx that contains various proportions of heparan sulfate, which binds PF4 more avidly than does chondroitin sulfate expressed on platelets [33, 34]. Therefore HIT antibodies may bind to monocytes with greater avidity [5] and monocytes may be activated both early in the disease process when PF4 levels begin to rise and may remain susceptible to activation by HIT antibodies after heparin has been discontinued and binding to platelets has dissipated [32]. ULCs also activate endothelium [3, 35], which promotes platelet adhesion and expression of tissue factor, but the binding and signal transduction mechanisms have not been elucidated in detail.
The combination of direct platelet activation by HIT immune complexes through FcγRIIA and transactivation by monocyte and likely endothelial cell-derived thrombin increases expression of phosphatidyl serine and binding of factor Xa to platelets. These are characteristic of highly procoagulant “coated” platelets, which helps explain clinical resistance to anti-platelet agents such as aspirin that have little impact on this subpopulation [32, 36].
3. Non-pathogenic antibody: a potential new therapy
RTO is a non-pathogenic anti-PF4 antibody that shares the same isotype with pathogenic antibody KKO but does not cause HIT in a mouse model [8, 31]. Binding of RTO to PF4 is not enhanced by heparin [8] in contrast to the pathogenic antibody KKO. RTO does not compete with KKO and shows the same Bmax as KKO by ELISA, which suggests that multiple forms of PF4 are present in the wells, making it difficult to distinguish pathogenic and non-pathogenic antibodies using bulk-equilibrium assays in clinical use [11].
We solved the crystal structure of PF4/RTOFab complex (PDB ID: 4RAU)[13]. The non-pathogenic antibody RTO binds to a PF4 monomer. The C-terminal helices of the PF4 monomer in the complex, although still packing against the β-sheet domain, are shifted approximately 60 degrees compared with the helices of a monomer within the apo- tetramer. This indicates that PF4 monomers undergo a dramatic structural rearrangement when they self-associate to form a tetramer.
Binding of RTOFab to the PF4 monomer inhibits the formation of PF4 tetramer, which is required for the formation of a stable IgG/PF4/heparin ternary immune complex. In support of this inference, RTO prevents KKO- and human HIT IgG-induced platelet activation in vitro in the presence of heparin in a dose dependent manner. RTO also inhibits KKO-induced progression of thrombosis in a murine laser microvascular injury model [13]. These data indicate that the non-pathogenic antibody RTO might provide a potential new therapeutic approach for this autoimmune disease.
4. Summary and future perspectives
In summary, our studies delineate certain features of the four steps in the pathogenesis of HIT: heparin or cell surface GAGs binds to the ‘closed’ end of PF4 tetramer, clamping the monomers together, which stabilizes the PF4 tetramer and approximating tetramers. HIT antibodies recognize the stabilized ‘open’ end of the PF4 tetramers and promote their oligomerization, forming multimolecular immune complexes that activate FcγRIIA receptors on platelets and monocytes and generate tissue factor which back-activates hematopoietic cells and endothelial cells, thereby accelerating thrombosis.
Our structural studies suggest that existing ELISA formats using immobilized wild type PF4 may not distinguish potentially pathogenic tetramer-binding antibodies from non-pathogenic non-tetramer-binding antibodies because the wells contain a mixture of monomers, dimers, tetramers and oligomers of PF4. Studies are in process to determine whether a monomer or dimer PF4 mutant incapable of forming tetramers might improve identification of clinically relevant antibodies.
A nonpathogenic antibody RTO that binds to PF4 monomers and impedes assembly of stable PF4 tetramers and blocks platelet activation and thrombosis provides a template to develop non-anticoagulant HIT-specific intervention for this serious autoimmune disorder. However, because PF4 is involved in many critical biological processes [37-42], it will be necessary to carefully analyze the effect of disrupting tetramer formation before adopting this approach.
Take-home messages.
PF4 exists as an equilibrium among tetramers, dimers and monomers; tetramers are stabilized by heparin or GAGs, forming antigenic complexes.
Alignment along heparin/GAG chains approximates PF4 tetramers forming larger antigenic complexes and increasing antibody avidity.
Pathogenic HIT antibodies bind preferentially to tetramers accelerating oligomerization of PF4/heparin complexes forming ultralarge IgG/PF4/heparin immune complexes (ULCs).
ULCs activate platelets and monocytes though FcγRIIA and mediate thrombin-dependent transactivation of platelets and endothelial cells.
A non-pathogenic anti-PF4 antibody prevents tetramer formation, binding of pathogenic antibody, platelet activation and thrombosis, providing a new approach to manage HIT.
Acknowledgments
Grant support.
This work was supported by NIH grant R01HL128895 (Z.C) and P01HL110860 (D.B.C).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Arepally GM, Ortel TL. Clinical practice. Heparin-induced thrombocytopenia. N Engl J Med. 2006;355:809–17. doi: 10.1056/NEJMcp052967. [DOI] [PubMed] [Google Scholar]
- [2].Arepally GM, Ortel TL. Heparin-induced thrombocytopenia. Annu Rev Med. 2010;61:77–90. doi: 10.1146/annurev.med.042808.171814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Arepally GM, Cines DB. Pathogenesis of heparin-induced thrombocytopenia and thrombosis. Autoimmun Rev. 2002;1:125–32. doi: 10.1016/s1568-9972(02)00031-9. [DOI] [PubMed] [Google Scholar]
- [4].Linkins L-A, Dans AL, Moores LK, Bona R, Davidson BL, Schulman S, Crowther M. Treatment and prevention of heparin-induced thrombocytopenia: antithrombotic therapy and prevention of thrombosis: American College of Chest Physicians evidence-based clinical practice guidelines. CHEST Journal. 2012;141:e495S–e530S. doi: 10.1378/chest.11-2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Rauova L, Hirsch JD, Greene TK, Zhai L, Hayes VM, Kowalska MA, Cines DB, Poncz M. Monocyte-bound PF4 in the pathogenesis of heparin-induced thrombocytopenia. Blood. 2010;116:5021–5031. doi: 10.1182/blood-2010-03-276964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Cuker A, Cines DB. How I treat heparin-induced thrombocytopenia. Blood. 2012;119:2209–18. doi: 10.1182/blood-2011-11-376293. [DOI] [PubMed] [Google Scholar]
- [7].Kang M, Alahmadi M, Sawh S, Kovacs MJ, Langner A. Lazo. Fondaparinux for the treatment of suspected heparin-induced thrombocytopenia: a propensity score-matched study. Blood. 2015;125:924–9. doi: 10.1182/blood-2014-09-599498. [DOI] [PubMed] [Google Scholar]
- [8].Arepally GM, Kamei S, Park K, Kamei K, Li Z, Liu W, Siegel D, Kisiel W, Cines DB, Poncz M. Characterization of a murine monoclonal antibody that mimics heparin-induced thrombocytopenia antibodies. Blood. 2000;95:1533–40. [PubMed] [Google Scholar]
- [9].Reilly MP, Taylor SM, Hartman NK, Arepally GM, Sachais BS, Cines DB, Poncz M, McKenzie SE. Heparin-induced thrombocytopenia/thrombosis in a transgenic mouse model requires human platelet factor 4 and platelet activation through FcgammaRIIa. Blood. 2001;98:2442–2447. doi: 10.1182/blood.v98.8.2442. [DOI] [PubMed] [Google Scholar]
- [10].Cuker A, Rux AH, Hinds JL, Dela CM, Yarovoi SV, Brown IA, Yang W, Konkle BA, Arepally GM, Watson SP, Cines DB, Sachais BS. Novel diagnostic assays for heparin-induced thrombocytopenia. Blood. 2013;121:3727–32. doi: 10.1182/blood-2013-01-479576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Sachais BS, Litvinov RI, Yarovoi SV, Rauova L, Hinds JL, Rux AH, Arepally G, Poncz M, Cuker A, Weisel JW, Cines DB. Dynamic antibody-binding properties in the pathogenesis of HIT. Blood. 2012;120:1137–42. doi: 10.1182/blood-2012-01-407262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Rauova L, Poncz M, McKenzie SE, Reilly MP, Arepally G, Weisel JW, Nagaswami C, Cines DB, Sachais BS. Ultralarge complexes of PF4 and heparin are central to the pathogenesis of heparin-induced thrombocytopenia. Blood. 2005;105:131–8. doi: 10.1182/blood-2004-04-1544. [DOI] [PubMed] [Google Scholar]
- [13].Cai Z, Yarovoi SV, Zhu Z, Rauova L, Hayes V, Lebedeva T, Liu Q, Poncz M, Arepally G, Cines DB. Atomic description of the immune complex involved in heparin-induced thrombocytopenia. Nat Commun. 2015;6:8277. doi: 10.1038/ncomms9277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Zhang X, Chen L, Bancroft DP, Lai CK, Maione TE. Crystal structure of recombinant human platelet factor 4. Biochemistry. 1994;33:8361–6. doi: 10.1021/bi00193a025. [DOI] [PubMed] [Google Scholar]
- [15].St Charles R, Walz D, Edwards B. The three-dimensional structure of bovine platelet factor 4 at 3.0-A resolution. J Biol Chem. 1989;264:2092–2099. [PubMed] [Google Scholar]
- [16].Mayo K, Roongta V, Ilyina E, Milius R, Barker S, Quinlan C, La RG, Daly TJ. NMR solution structure of the 32-kDa platelet factor 4 ELR-motif N-terminal chimera: a symmetric tetramer. Biochemistry. 1995;34:11399–409. doi: 10.1021/bi00036a012. [DOI] [PubMed] [Google Scholar]
- [17].Cowan SW, Bakshi EN, Machin KJ, Isaacs NW. Binding of heparin to human platelet factor 4. Biochem J. 1986;234:485–8. doi: 10.1042/bj2340485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Stuckey JA, Charles RS, Edwards BF. A model of the platelet factor 4 complex with heparin. Proteins. 1992;14:277–87. doi: 10.1002/prot.340140213. [DOI] [PubMed] [Google Scholar]
- [19].Greinacher A, Gopinadhan M, Günther JU, Omer-Adam MA, Strobel U, Warkentin TE, Papastavrou G, Weitschies W, Helm CA. Close approximation of two platelet factor 4 tetramers by charge neutralization forms the antigens recognized by HIT antibodies. Arterioscler Thromb Vasc Biol. 2006;26:2386–93. doi: 10.1161/01.ATV.0000238350.89477.88. [DOI] [PubMed] [Google Scholar]
- [20].Bauer KA, Eriksson BI, Lassen MR, Turpie AG. Fondaparinux compared with enoxaparin for the prevention of venous thromboembolism after elective major knee surgery. N Engl J Med. 2001;345:1305–1310. doi: 10.1056/NEJMoa011099. [DOI] [PubMed] [Google Scholar]
- [21].Turpie AG, Bauer KA, Eriksson BI, Lassen MR, Committee PSS. Postoperative fondaparinux versus postoperative enoxaparin for prevention of venous thromboembolism after elective hip-replacement surgery: a randomised double-blind trial. Lancet. 2002;359:1721–6. doi: 10.1016/S0140-6736(02)08648-8. [DOI] [PubMed] [Google Scholar]
- [22].Warkentin TE, Cook RJ, Marder VJ, Sheppard JA, Moore JC, Eriksson BI, Greinacher A, Kelton JG. Anti-platelet factor 4/heparin antibodies in orthopedic surgery patients receiving antithrombotic prophylaxis with fondaparinux or enoxaparin. Blood. 2005;106:3791–6. doi: 10.1182/blood-2005-05-1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Burch M, Cooper B. Fondaparinux-associated heparin-induced thrombocytopenia. Proc (Bayl Univ Med Cent) 2012;25:13–5. doi: 10.1080/08998280.2012.11928771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Rota E, Bazzan M, Fantino G. Fondaparinux-related thrombocytopenia in a previous low-molecular-weight heparin (LMWH)-induced heparin-induced thrombocytopenia (HIT) Thromb Haemost. 2008;99:779–81. doi: 10.1160/TH07-09-0573. [DOI] [PubMed] [Google Scholar]
- [25].Warkentin TE, Maurer BT, Aster RH. Heparin-induced thrombocytopenia associated with fondaparinux. N Engl J Med. 2007;356:2653–5. doi: 10.1056/NEJMc070346. [DOI] [PubMed] [Google Scholar]
- [26].Bhatt VR, Aryal MR, Shrestha R, Armitage JO. Fondaparinux-associated heparin-induced thrombocytopenia. Eur J Haematol. 2013;91:437–41. doi: 10.1111/ejh.12179. [DOI] [PubMed] [Google Scholar]
- [27].Amiral J, Bridey F, Wolf M, Boyer-Neumann C, Fressinaud E, Vissac A, Peynaud-Debayle E, Dreyfus M, Meyer D. Antibodies to macromolecular platelet factor 4-heparin complexes in heparin-induced thrombocytopenia: a study of 44 cases. Thromb Haemost. 1995;73:21–28. [PubMed] [Google Scholar]
- [28].Brandt S, Krauel K, Gottschalk KE, Renné T, Helm CA, Greinacher A, Block S. Characterisation of the conformational changes in platelet factor 4 induced by polyanions: towards in vitro prediction of antigenicity. Thromb Haemost. 2014;112:53–64. doi: 10.1160/TH13-08-0634. [DOI] [PubMed] [Google Scholar]
- [29].Daëron M. Fc receptor biology. Annu Rev Immunol. 1997;15:203–234. doi: 10.1146/annurev.immunol.15.1.203. [DOI] [PubMed] [Google Scholar]
- [30].Warkentin TE, Hayward C, Boshkov L, Santos AV, Sheppard J, Bode AP, Kelton JG. Sera from patients with heparin-induced thrombocytopenia generate platelet-derived microparticles with procoagulant activity: an explanation for the thrombotic complications of heparin-induced thrombocytopenia. Blood. 1994;84:3691–3699. [PubMed] [Google Scholar]
- [31].Rauova L, Zhai L, Kowalska MA, Arepally GM, Cines DB, Poncz M. Role of platelet surface PF4 antigenic complexes in heparin-induced thrombocytopenia pathogenesis: diagnostic and therapeutic implications. Blood. 2006;107:2346–53. doi: 10.1182/blood-2005-08-3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Tutwiler V, Madeeva D, Ahn HS, Andrianova I, Hayes V, Zheng XL, Cines DB, McKenzie SE, Poncz M, Rauova L. Platelet transactivation by monocytes promotes thrombosis in heparin-induced thrombocytopenia. Blood. 2016;127:464–72. doi: 10.1182/blood-2013-11-539262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ward JV, Packham MA. Characterization of the sulfated glycosaminoglycan on the surface and in the storage granules of rabbit platelets. Biochim Biophys Acta. 1979;583:196–207. doi: 10.1016/0304-4165(79)90427-6. [DOI] [PubMed] [Google Scholar]
- [34].Nader HB. Characterization of a heparan sulfate and a peculiar chondroitin 4-sulfate proteoglycan from platelets. Inhibition of the aggregation process by platelet chondroitin sulfate proteoglycan. J Biol Chem. 1991;266:10518–10523. [PubMed] [Google Scholar]
- [35].Cines DB, Tomaski A, Tannenbaum S. Immune endothelial cell injury in heparin-associated thrombocytopenia. N Engl J Med. 1987;316:581–589. doi: 10.1056/NEJM198703053161004. [DOI] [PubMed] [Google Scholar]
- [36].Batar P, Dale GL. Simultaneous engagement of thrombin and FcγRIIA receptors results in platelets expressing high levels of procoagulant proteins. J Lab Clin Med. 2001;138:393–402. doi: 10.1067/mlc.2001.120049. [DOI] [PubMed] [Google Scholar]
- [37].Maione TE, Gray GS, Petro J, Hunt AJ, Donner AL, Bauer SI, Carson HF, Sharpe RJ. Inhibition of angiogenesis by recombinant human platelet factor-4 and related peptides. Science. 1990;247:77–79. doi: 10.1126/science.1688470. [DOI] [PubMed] [Google Scholar]
- [38].Nesmelova IV, Sham Y, Dudek AZ, Van Eijk LI, Wu G, Slungaard A, Mortari F, Griffioen AW, Mayo KH. Platelet factor 4 and interleukin-8 CXC chemokine heterodimer formation modulates function at the quaternary structural level. J Biol Chem. 2005;280:4948–4958. doi: 10.1074/jbc.M405364200. [DOI] [PubMed] [Google Scholar]
- [39].El-Asrar AMA, Mohammad G, Nawaz MI, Abdelsaid M, Siddiquei MM, Alam K, Van den Eynde K, De Hertogh G, Opdenakker G, Shabrawey M. Al. The Chemokine Platelet Factor-4 Variant (PF-4var)/CXCL4L1 Inhibits Diabetes-Induced Blood-ìRetinal Barrier Breakdown. Invest Ophthalmol Vis Sci. 2015;56:1956–1964. doi: 10.1167/iovs.14-16144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Yang L, Du J, Hou J, Jiang H, Zou J. Platelet factor-4 and its p17-70 peptide inhibit myeloma proliferation and angiogenesis in vivo. BMC cancer. 2011;11:1. doi: 10.1186/1471-2407-11-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Maurer A-M, Zhou B, Han ZC. Roles of platelet factor 4 in hematopoiesis and angiogenesis. Growth Factors. 2006;24:242–252. doi: 10.1080/08977190600988225. [DOI] [PubMed] [Google Scholar]
- [42].Aidoudi S, Bikfalvi A. Interaction of PF4 (CXCL4) with the vasculature: a role in atherosclerosis and angiogenesis. Thromb Haemost. 2010;104:941. doi: 10.1160/TH10-03-0193. [DOI] [PubMed] [Google Scholar]