Abstract
The ECM of the intervertebral disc and articular cartilage contain a highly organised network of collagens and proteoglycans which resist compressive forces applied to these tissues. A pathological hallmark of the intervertebral disc is the imbalance between production of anabolic and catabolic factors by the resident cells. This process is thought to be mediated by pro-inflammatory cytokines, predominantly TNF-α and IL-1β, which upregulate expression of matrix degrading enzymes such as MMPs and ADAMTSs. This imbalance ultimately results in tissue degeneration causing failure of the biomechanical function of the tissues. A similar cascade of events is thought to occur in articular cartilage during development of osteoarthritis. Within these skeletal tissues a small, cell surface heparan sulphate proteoglycan; syndecan-4 (SDC4) has been implicated in maintaining physiological functions. However in the degenerating niche of the intervertebral disc and cartilage, dysregulated activities of this molecule may exacerbate pathological changes. Studies in recent years have elucidated a role for SDC4 in mediating matrix degradation in both intervertebral discs and cartilage by controlling ADAMTS-5 function and MMP3 expression. Discourse presented in this review highlights the potential of SDC4 as possible therapeutic target in slowing the progression of ECM degradation in both degenerative disc disease and osteoarthritis.
Keywords: Intervertebral disc, cartilage, extracellular matrix, disc degeneration, syndecan-4, cytokines
1. Background
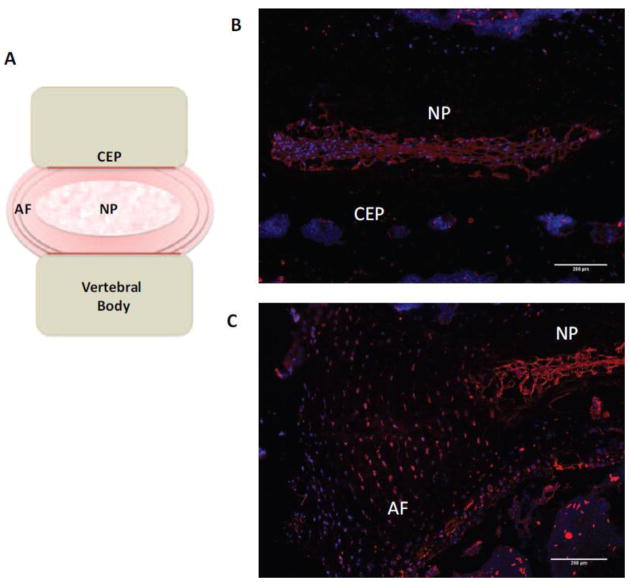
The intervertebral disc (IVD), often referred to as a hydrodynamic pad, is a specialized tissue situated between adjacent vertebrae within the spinal column, and is crucial for providing structure and function to the spine(1). One of the main functions of the IVD is to transmit mechanical load as well as permitting movement and flexibility of the spine. The IVD is composed of three main anatomical regions, the gelatinous nucleus pulposus (NP), constrained by the annulus fibrosus (AF) and cartilaginous endplates (CEP) (Figure 1A). The highly specialised composition of the extracellular matrix (ECM) within the IVD allows movement and offers resilience to compressive forces (2). Articular cartilage covers bony surfaces of articulating joints permitting smooth, frictionless movement and allowing pressure and weight-bearing activities.
Figure 1.
(A) Schematic representation of the IVD. (B, C) Immunofluorescent staining of SDC4 in murine IVD shows strong localization in nucleus pulposus (B) and inner two thirds of the annulus fibrosus (C). Scale bars; 200 μm. NP, nucleus pulposus; AF, annulus fibrosus; CEP, cartilaginous endplate.
Both IVD and articular cartilage are composed of a highly organised network of collagens and proteoglycans secreted by resident cells. High concentration of proteoglycans and their negatively charged glycosaminoglycan (GAG) side chains within the ECM results in tissue hydration, elevated tissue osmolarity and generation of a swelling pressure, resisted by collagen fibers(3). However, a distinguishing factor between the IVD and articular cartilage is the relative amounts of proteoglycans and collagens present within the tissues. The NP of the IVD has a considerably higher ratio of proteoglycans to collagens [measured as GAG/hydroxyproline mass ratio](27:1) compared to that in articular cartilage (2:1) (4). Likewise, within the IVD, type I collagen constitutes 70–80% dry weight of the AF whereas type II collagen dominates within the NP constituting 20–30% dry weight. Although types I and II are the dominant collagens, a small percentage of type VI, IX and XI are present. Similarly, articular cartilage shows regional differences in the types of collagens deposited throughout the different zones accounting for approximately two thirds of the dry weight of cartilage. Mature articular cartilage is composed of large fibril forming and fibril-associated collagens including type II (90%), type XI (3%), and type IX (1%).
Back pain and osteoarthritis (OA) are two common painful, debilitating conditions often linked to progressive degeneration of IVD and articular cartilage respectively. A pathological hallmark of both conditions is the degradation of ECM caused by an uncontrolled upregulation of matrix degrading enzymes (5–7). An early feature of IVD degeneration is the decrease in aggrecan, which is known for its ability to imbibe water. The loss of aggrecan consequently results in a decrease in osmotic swelling pressure, and an inability to withstand compressive loads (8). Similarly in cartilage, the early loss of proteoglycans and collagen type II result in the destruction of articular cartilage and irreversible progression towards OA (9, 10). Recently within these tissues, a small cell surface heparan sulphate proteoglycan (HSPG) syndecan-4 (SDC4) has been implicated in matrix homeostasis and turnover. However in the degenerating niche of the IVD and cartilage, activities of this molecule may exacerbate pathological changes. This review will address the possible functions of syndecans in health and disease of disc and cartilage.
2. Catabolism response in the disc and the cartilage mediated by NP cells and chondrocytes
Although the cell density within these tissues is sparse, it is suggested that NP cells and chondrocytes contribute to the pathogenesis of disc degeneration and OA. Degeneration is enhanced by increased proteolysis mediated by members of the MMP’s (matrix metalloproteinases) and ADAMTS (a disintegrin and metalloproteinase with thrombospondin motifs) family of enzymes and other serine proteases. These enzymes primarily target aggrecan, collagens I and II and other matrix components such as fibronectin (8, 11–16).
The roles of cytokines within IVD degeneration have been profusely studied, and their actions have been well documented over the past decade or so. Native cells of the NP in both non-degenerate and degenerate IVDs produce cytokines, in particular interleukin-1 (IL-1β) and tumour necrosis factor (TNFα) (7, 17–22). Numerous studies by Le Maitre and colleagues localised all members of the IL-1 family (IL-1α, IL-1β, IL-1RI, ICE, IL-1Ra) in IVD tissue, yet it was only the natural inhibitor of IL-1, IL-1 receptor antagonist (IL-1Ra) that did not increase with severity of degeneration (18, 23). These cytokines are known to contribute to the ECM degradation by mediating increased expression of catabolic enzymes; ADAMTS-4, ADAMTS-5, MMP-1, -2, -3, -4, -13 (6) and promote a broader inflammatory response by cells (24, 25) at the same time decreasing anabolic factors; aggrecan and collagen II (26). Stimulation of NP cells with IL-1Ra demonstrated a decreased expression of matrix degrading enzymes, as opposed to IL-1β stimulating their expression. In agreement with this study, a more recent study by Phillips et al., (2013) demonstrated the importance of IL-1 in the pathogenesis of IVD degeneration, by reporting the occurrence of spontaneous degeneration characterized by elevated levels of catabolic enzymes, loss of matrix and increased senescence in mice that had IL-1Ra removed (20). Similarly, increases in TNF-α expression have been reported within degenerate and herniated discs and treatment of NP cells with TNF-α cause increased expression of several catabolic mediators (27–29) and expression of molecules that control cell proliferation and differentiation (30). Moreover, in vitro studies have also shown that TNF-α causes irreversible changes in biophysical and mechanical properties of NP cells (31). Noteworthy, levels of TNF-α have been reported to correlate with disease severity and therefore TNF-α is considered to be an important contributing factor to IVD degeneration.
With regards to OA, IL-1β and TNF-α are both dominant mediators in the pathophysiological processes occurring during the course of inflammatory destruction of the joint. Patients with OA have an elevated level of IL-1β and TNF-α in the synovial fluid, synovial membrane, cartilage and the subchondral bone (32–34). As observed in IVDs, many studies report that both these cytokines affect the synthesis of anabolic and catabolic factors by chondrocytes; interfering with the production of aggrecan and collagen II; favouring the production of MMPs. Interestingly, while ADAMTS-4 was mildly induced by IL-1β it is expressed at a very low level compared to ADAMTS-5, suggesting a dominant role for ADAMTS-5 in OA pathogenesis (35). Although it is well established that IL-1β and TNF-α potentiate expression of matrix degrading enzymes from chondrocytes and NP cells, the signalling pathways involved in this process are complex and cell type dependent, thus warranting further investigations.
3. Syndecan-4: a molecular link between health and disease of IVD and Cartilage?
During recent years, a number of studies have uncovered a potential role for syndecans in the pathogenesis of many diseases (36) including IVD degeneration and OA. Syndecans are a small family of transmembrane core proteins consisting four members within vertebrates (SDC1 to SDC4). These proteins usually carry three to five heparan or chondroitin sulphate side chains, allowing interactions with a variety of ligands including fibroblast growth factor (FGF), vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), bone morphogenetic protein-2 (BMP-2) and Indian hedgehog (Ihh) (37).
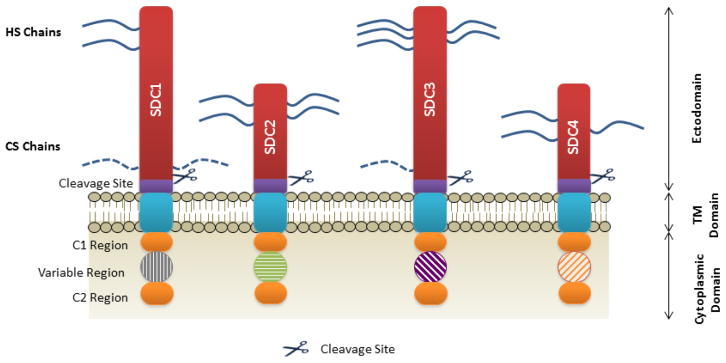
Each syndecan family member has specific spatial and temporal patterns of expression. In vivo studies revealed that each member is expressed at different times during development and on specific cell types (38). SDC1 is predominantly expressed early on during development and is present on epithelial and mesenchymal tissues; relevant to cartilage, SDC1 expression is modulated in OA (39), and is shown to promote activation of ADAMTS-4 (40). SDC2 is expressed by cells of mesenchymal origin as well as neuronal and epithelial cells. Whereas, SDC3 is most commonly linked with neuronal and musculoskeletal tissues and is shown to be involved in the process of endochondral differentiation (41), whilst SDC4 is found virtually within every cell type (38). Structurally, all members are composed of a short cytoplasmic domain containing two highly conserved regions (C1 and C2) that flank the variable region, followed by a transmembrane (TM) domain and an ectodomain (Figure 2) (42). Syndecans exhibit a modest core protein of approximately 20–45 kDa, with a short cytoplasmic domain lacking intrinsic kinase activity. This being said, syndecans are involved in cytoplasmic signalling. In regards to protein sequence homology, SDC1 and SDC3 form one subfamily, and SDC2 and SDC4 form the other (43). SDC1 and SDC3 have the ability to bind heparan and chondroitin sulphate side chains, whereas SDC2 and SDC4 are thought to exclusively bind heparan sulphate chains (Figure 2).
Figure 2. The core-domain structure of syndecans.
The extracellular domain is depicted in red and both heparan and chondroitin sulphate chains are indicated by the blue lines. Cleavage sites are indicated by purple blocks, followed by a short transmembrane domain in blue. Each syndecan has a short cytoplasmic domain in which a variable region is flanked by two highly conserved regions termed C1 and C2. Cleavage of the ectodomain can occur via numerous metalloproteinases such as MMP2, 3, 7 and 9, as well as proteases thrombin and plasmin. HS, Heparan sulphate; CS, Chondroitin sulphate; SDC, syndecan; TM, transmembrane.
The perceived function of syndecans is to modulate the activation of receptors at the cell surface through the structural features of heparan sulphate chains that are responsible for the interactions with a number of soluble factors, cell-associated molecules and ECM components. Reflective of the syndecans structural diversity, is the range of activities they are involved in; these include adhesion, migration, cytoskeletal organisation and differentiation, as well as acting as cell surface receptors (44).
Of the members of the family, SDC4 is the best characterised in terms of structure and biological activity. The intracellular domain of SDC4 is responsible for interactions with numerous cytosolic binding partners involved in the mechanotransduction and signalling. SDC4 exerts its effects via several pathways mostly dependent on the ligand. Proteolytic cleavage and subsequent shedding of the extracellular domain can occur under certain physiological conditions such as, inflammation (45, 46). The release of the extracellular domain fragments from SDC4 with intact GAG chains still permits the interactions with growth factors such as FGF (47). The native as well as shed SDC4 extracellular domain mediates the direct contact of cells with ECM proteins such as fibronectin, serving as cell attachment sites and subsequently leads to focal adhesion formation through the interaction of integrin α5β1 and extracellular fibronectin. This process results in activation of protein kinase C (PKC) pathway downstream, of SDC4 (48). Importantly, the binding of SDC4 to fibronectin is required by certain cell types for the activation of focal adhesion kinase (FAK), which stabilises focal adhesions by activating Rho and recruiting vinculin and paxillin (49, 50). Further to this, the HS side chains have been suggested to act as sensors of ECM stress (51). This phenomenon is mediated independently of integrins, and involves the MAPK pathway. Other signalling pathways induced by SDC4 signalling include RhoA, ERK1/2, Rac1 and AKT; however how these molecules signal within skeletal tissues is yet to be fully elucidated.
3.1 Syndecan-4 in Disc and Cartilage Physiology
Although very recently investigations have started focussing on the role of SDC4 in skeletal biology, evidence thus far demonstrates a very prominent role for this molecule in the early ECM biosynthesis, growth factor signalling as well in ECM degradation.
Risbud and colleagues were the first group to demonstrate SDC4 expression within tissues of the IVD (Figure 1B–C); observing differential expression by cells of the NP and AF. Immunopositivity of SDC4 was strongly associated with cells of the NP in both embryonic and mature discs. Fujita et al., (2014) using microarray analysis confirmed this observation and showed high enrichment of SDC4 amongst other syndecans in the NP. These authors showed that the expression of SDC4 in the NP cells was under the control of the transcription factor HIF-1α (52), a molecule constitutively expressed by the NP cells and regarded as their characteristic trait (53, 54). Moreover, it was demonstrated that the heparan-sulphate side chains of SDC4 were involved in controlling the expression of another critical factor, Sox9 that is involved in the regulation of aggrecan and collagen II expression. Numerous studies have also highlighted roles of multiple morphogenic factors during the development of the IVD, including FGF-2, BMP-2, sonic hedgehog (Shh), Ihh amongst others (55, 56). It is known that these molecules interact with heparan-sulphate within their environment to mediate binding to their receptors. Since NP is enriched in SDC4, it would not be unreasonable to assume a role for this HSPG in controlling bioactivity of these factors, similar to those proposed in cartilage. This possibly could be one of the mechanisms by which SDC4 controls the Sox9 levels in NP cells and may play a pivotal role in matrix homeostasis within the hypoxic IVD (52). A more recent study by Beckett et al., (2015) confirmed earlier observations by Risbud’s group and showed strong SDC4 expression in NP and AF tissues that was sensitive to aging. Moreover, SDC4 evidenced overlapping expression with fibronectin in the disc. Authors of this study conclude that SDC4 plays an important role in the development and maintenance of the annular lamellae (57). Additionally, the high levels of SDC4 associated with cell clusters in the NP of aged animals could possibly indicate a role for SDC4 in the migration and proliferation of these cells. Although the contribution of syndecans in disc has only been recently appreciated, the role of syndecans in cartilage and OA pathogenesis has been studied for some time now. All members of the syndecan family have been identified in human chondrocytes, with early studies revealing SDC4 being the most abundant at mRNA level (58); and of particular interest due to its potential role in tissue remodelling. Similarly to expression in the disc, SDC4 expression is also differentially expressed in the different zones of articular cartilage (59). It is documented that SDC4 is involved in mechanosensing whereby biomechanical stress influences its expression on cell surface, in turn causing changes to the cell morphology, movement and orientation. Therefore, it could be speculated, that SDC4 is able to regulate ECM turnover by inducing pathways involved in the assembly of focal adhesion complexes as well as responding to compressive forces exerted on these skeletal tissues.
3.2 Syndecan-4 in Pathophysiology: therapeutic target for matrix restoration?
A pathological hallmark of both IVD degeneration and OA is the degradation of the ECM. Studies within the cartilage and disc field have elucidated potential roles for SDC4 in this process (60). During IVD degeneration, ADAMTS-4 and ADAMTS-5 expression is increased causing a detrimental imbalance of anabolic and catabolic factors. This leads to the cleavage of aggrecan, and results in altered tissue biomechanical properties. Studies by Wang and colleagues (2011) demonstrated that expression of SDC4 in NP cells is elevated by inflammatory cytokines, TNF-α and IL-1β. This induction was at the transcript level and mediated by p65/RelA interaction with a conserved NF-κB binding site in the SDC4 promoter. Relevant to disc degeneration, it was shown that SDC4 selectively interacts with pro-ADAMTS-5 at the cell surface promoting its activation that is important for aggrecan degradation (27). Importantly, analysis of human NP tissues showed a strong correlation between levels of SDC4 and ADAMTS-5 with aggrecan neoepitopes, suggesting that an increase in SDC4 expression by cytokines IL-1β and TNF-α contributes to aggrecan turnover in human disease. These studies were in agreement with studies by Echtermeyer et al., (2009) who investigated the expression of SDC4 and its regulation of ADAMTS-5 in arthritic cartilage. SDC4 null mice were protected from development of post-traumatic OA and maintain a healthy level of cartilage ECM and tissue integrity. Importantly these authors report a concomitant decrease in ADAMTS-5 activity in SDC4 knockout mice, leading to the exploration of molecular mechanisms that could control this phenomenon (59). These studies identified that the control of ADAMTS-5 by SDC4, was mediated through another proteolytic enzyme; MMP-3 via the MAPK pathway (59). Interestingly in contrast to cartilage, in NP cells MMP-3 was noted to be dispensable for SDC4 dependent activation of ADAMTS-5. Expression of MMP-3 in human IVD tissues during degeneration has been studied previously, and is known to be up regulated in response to pro-inflammatory cytokines. However the question still remained as to what was involved in the activation process of this metalloproteinase. Wang et al., (2011) had previously reported role of SDC4 in the activation process of ADAMTS-5, and similarly observed a role of SDC4 in the activation of cytokine-induced expression of MMP-3(27). More recent studies by Wang and colleagues shed further light on mechanistic aspects of this regulation. Their studies clearly showed an important role of MAPK, NF-κB and a yet unknown signalling event downstream of SDC4 in controlling TNF- dependent MMP-3 expression (29).
As described earlier, a relationship between cytokines and pathology of disc degeneration and OA is well documented. A further understanding of the interplay between cytokines and SDC4 was forthcoming from recent in vitro and in vivo studies by Godmann et al., (2015) elucidating a role of SDC4 in trafficking of IL-1RI to surface of synovial fibroblasts that are known to actively contribute to pathogenesis of OA. In mice lacking SDC4, IL-1R presentation on fibroblasts was completely abolished thereby decreasing IL-1 signalling (61). Moreover, Tran and colleagues (2014) recently observed that IL-1β induced SDC4 expression was successfully supressed by CCN2 (connective tissue growth factor) treatment, via interaction with integrins αvβ3 and α5β1 (62). On the other hand, expression of CCN2, which is an important anabolic factor in the disc (63), is suppressed by both IL-1β and TNF-α thus constituting a negative feedback loop. Similarly, the importance of cytokine regulation of SDC4 is reiterated in setting of fracture healing. SDC4 knockout mice demonstrated impaired healing of fractures, yet this is alleviated by the addition of anti-TNF-α(64), yet again demonstrating a strong relationship between SDC4 expression and inflammation. Numerous studies have also shown great progress in resolving the function of SDC4 in OA and inflammatory arthritis. Echtermeyer et al., (2009) not only demonstrated that the loss of SDC4 actually reduces the progression towards an osteoarthritic phenotype in the murine PTOA model but intra-articular injection of specific anti-SDC4 antibodies can halt the disease progression (59).
4. Concluding Remarks
SDC4 plays important roles in normal physiology of IVD and cartilage through controlling growth factor signalling and matrix homeostasis. However, several studies to date clearly show that in diseased joints, SDC4 and inflammatory cytokines IL-1β and TNF-α form a positive feedback loop, wherein they control each other’s expression and/or activity (Figure 3). These exciting findings provide an avenue that needs further exploration in both IVD and cartilage to elucidate the potential of SDC4 as a therapeutic target, whereby; inhibition of SDC4 slows the progression of ECM degeneration. Since the activities of both IL-1β and TNF-α are essential to the destructive processes in these skeletal tissues, if we could find a way to dampen down catabolic responses mediated by these cytokines, these conditions may become more manageable.
Figure 3. The role of SDC4 in the degenerating nucleus pulposus.
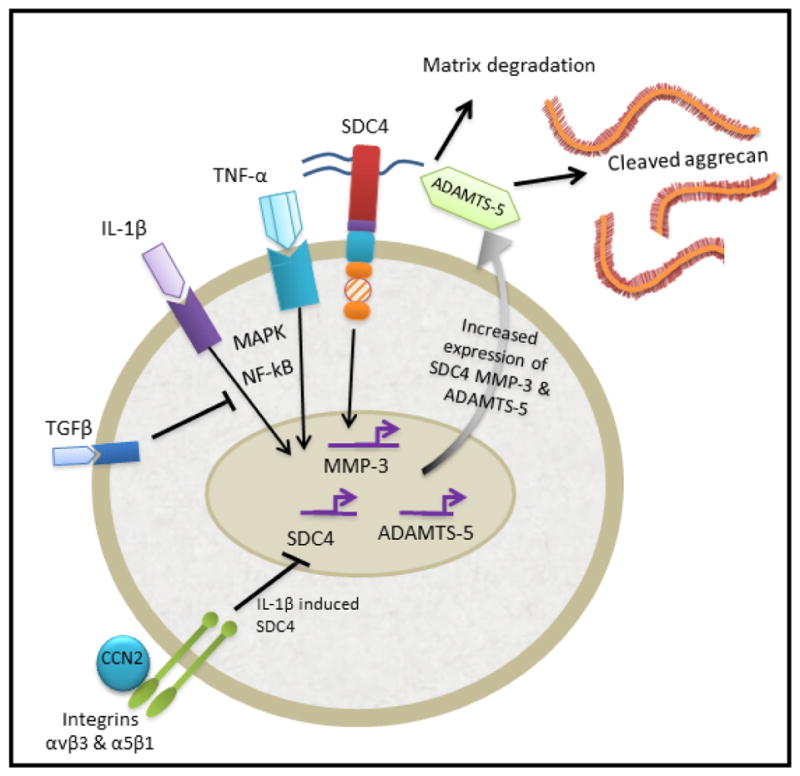
In the degenerating NP, the increase in inflammatory cytokines TNF-α and IL-1β drives the expression of catabolic enzymes MMP-3 and ADAMTS-5, and have also been shown to regulate SDC4 expression via MAPK and NF-κB signalling pathways. SDC4 selectively interacts with ADAMTS-5 at the cell surface promoting its activation and subsequent cleavage of aggrecan. SDC4 is also important in controlling TNF-dependent MMP-3 expression via MAPK and NF-κB pathways. The expression of these catabolic factors leads to the degeneration of the IVD. Likewise, IL-1β induced SDC4 is supressed by CCN2/CTGF bound to integrins αvβ3 and α5β1. Additionally, anabolic growth factor TGFβ inhibits NF-κB signalling in NP cells, supressing SDC4 and MMP-3 expression.
Highlights.
SDC4 shows enriched expression in nucleus pulposus
SDC4 plays important roles in normal physiology of IVD and cartilage
SDC4 expression is responsive to inflammatory cytokines
SDC4 controls activity of ADAMTS-5 and MMP-3
Acknowledgments
This work was supported by grants from the National Institutes of Health AR055655, AR064733 and AR050087. Authors would like to thank Hyowon Choi for providing the histological images of syndecan-4 staining in Figure 1.
Abbreviations
- ADAMTS
A disintegrin and metalloproteinase with thombospondin motif
- AF
Annulus Fibrosus
- CEP
Cartilaginous end plate
- CCN2
Connective tissue growth factor
- FAK
Focal Adhesion Kinase
- HIF
Hypoxia Inducible Factor
- HSPG
Heparan-Sulphate Proteoglycan
- Ihh
Indian Hedgehog
- IL
Interleukin
- IL-1Ra
Interleukin-1 Receptor Antagonist
- IVD
Intervertebral disc
- NP
Nucleus Pulposus
- OA
Osteoarthritis
- PKC
Protein Kinase C
- SDC
Syndecan
- Shh
Sonic Hedgehog
Footnotes
Conflict of Interest: none to declare
Competing Interest: none to declare
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Abbie LA. Binch, Email: Abbie.Binch@Jefferson.edu.
Irving M. Shapiro, Email: Irving.Shapiro@Jefferson.edu.
Makarand V. Risbud, Email: Makarand.Risbud@Jefferson.edu.
References
- 1.Adams MA, Roughley PJ. What is intervertebral disc degeneration, and what causes it? Spine. 2006;31:2151–2161. doi: 10.1097/01.brs.0000231761.73859.2c. [DOI] [PubMed] [Google Scholar]
- 2.Hutton WC, Ganey TM, Elmer WA, Kozlowska E, Ugbo JL, Doh ES, Whitesides TE., Jr Does long-term compressive loading on the intervertebral disc cause degeneration? Spine (Phila Pa 1976) 2000;25:2993–3004. doi: 10.1097/00007632-200012010-00006. [DOI] [PubMed] [Google Scholar]
- 3.Johnson ZI, Shapiro IM, Risbud MV. Extracellular osmolarity regulates matrix homeostasis in the intervertebral disc and articular cartilage: evolving role of TonEBP. Matrix Biology. 2014;40:10–16. doi: 10.1016/j.matbio.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mwale F, Roughley P, Antoniou J. Distinction between the extracellular matrix of the nucleus pulposus and hyaline cartilage: a requisite for tissue engineering of intervertebral disc. Eur Cell Mater. 2004;8:58–63. doi: 10.22203/ecm.v008a06. discussion 63–4. [DOI] [PubMed] [Google Scholar]
- 5.Haefeli M, Kalberer F, Saegesser D, Nerlich A, Boos N, Paesold G. The course of macroscopic degeneration in the human lumbar intervertebral disc. Europ Cells Mater. 2005;10:25. doi: 10.1097/01.brs.0000222032.52336.8e. [DOI] [PubMed] [Google Scholar]
- 6.Le Maitre CL, Freemont AJ, Hoyland JA. Localization of degradative enzymes and their inhibitors in the degenerate human intervertebral disc. J Pathol. 2004;204:47–54. doi: 10.1002/path.1608. [DOI] [PubMed] [Google Scholar]
- 7.Le Maitre CL, Hoyland JA, Freemont AJ. Catabolic cytokine expression in degenerate and herniated human intervertebral discs: IL-1beta and TNFalpha expression profile. Arthritis Res Ther. 2007;9:R77. doi: 10.1186/ar2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roughley PJ. Biology of intervertebral disc aging and degeneration: involvement of the extracellular matrix. Spine. 2004;29:2691–2699. doi: 10.1097/01.brs.0000146101.53784.b1. [DOI] [PubMed] [Google Scholar]
- 9.Pattoli MA, MacMaster JF, Gregor KR, Burke JR. Collagen and aggrecan degradation is blocked in interleukin-1-treated cartilage explants by an inhibitor of IkappaB kinase through suppression of metalloproteinase expression. J Pharmacol Exp Ther. 2005;315:382–388. doi: 10.1124/jpet.105.087569. [DOI] [PubMed] [Google Scholar]
- 10.Poole AR, Nelson F, Dahlberg L, Tchetina E, Kobayashi M, Yasuda T, Laverty S, Squires G, Kojima T, Wu W. Proteolysis of the collagen fibril in osteoarthritis. 2003;70:115–124. doi: 10.1042/bss0700115. [DOI] [PubMed] [Google Scholar]
- 11.Little CB, Meeker CT, Golub SB, Lawlor KE, Farmer PJ, Smith SM, Fosang AJ. Blocking aggrecanase cleavage in the aggrecan interglobular domain abrogates cartilage erosion and promotes cartilage repair. J Clin Invest. 2007;117:1627–1636. doi: 10.1172/JCI30765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malfait AM, Liu RQ, Ijiri K, Komiya S, Tortorella MD. Inhibition of ADAM-TS4 and ADAM-TS5 prevents aggrecan degradation in osteoarthritic cartilage. J Biol Chem. 2002;277:22201–22208. doi: 10.1074/jbc.M200431200. [DOI] [PubMed] [Google Scholar]
- 13.Roughley PJ, Melching LI, Heathfield TF, Pearce RH, Mort JS. The structure and degradation of aggrecan in human intervertebral disc. Eur Spine J. 2006;15(Suppl 3):S326–32. doi: 10.1007/s00586-006-0127-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song R, Tortorella MD, Malfait A, Alston JT, Yang Z, Arner EC, Griggs DW. Aggrecan degradation in human articular cartilage explants is mediated by both ADAMTS- 4 and ADAMTS- 5. Arthritis & Rheumatism. 2007;56:575–585. doi: 10.1002/art.22334. [DOI] [PubMed] [Google Scholar]
- 15.Tiaden AN, Klawitter M, Lux V, Mirsaidi A, Bahrenberg G, Glanz S, Quero L, Liebscher T, Wuertz K, Ehrmann M, Richards PJ. Detrimental role for human high temperature requirement serine protease A1 (HTRA1) in the pathogenesis of intervertebral disc (IVD) degeneration. J Biol Chem. 2012;287:21335–21345. doi: 10.1074/jbc.M112.341032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akhatib B, Onnerfjord P, Gawri R, Ouellet J, Jarzem P, Heinegard D, Mort J, Roughley P, Haglund L. Chondroadherin fragmentation mediated by the protease HTRA1 distinguishes human intervertebral disc degeneration from normal aging. J Biol Chem. 2013;288:19280–19287. doi: 10.1074/jbc.M112.443010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seguin CA, Pilliar RM, Roughley PJ, Kandel RA. Tumor necrosis factor-alpha modulates matrix production and catabolism in nucleus pulposus tissue. Spine (Phila Pa 1976) 2005;30:1940–1948. doi: 10.1097/01.brs.0000176188.40263.f9. [DOI] [PubMed] [Google Scholar]
- 18.Le Maitre CL, Freemont AJ, Hoyland JA. The role of interleukin-1 in the pathogenesis of human intervertebral disc degeneration. Arthritis Res Ther. 2005;7:R732–45. doi: 10.1186/ar1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weiler C, Nerlich AG, Bachmeier BE, Boos N. Expression and distribution of tumor necrosis factor alpha in human lumbar intervertebral discs: a study in surgical specimen and autopsy controls. Spine (Phila Pa 1976) 2005;30:44–53. doi: 10.1097/01.brs.0000149186.63457.20. discussion 54. [DOI] [PubMed] [Google Scholar]
- 20.Phillips KL, Jordan-Mahy N, Nicklin MJ, Le Maitre CL. Interleukin-1 receptor antagonist deficient mice provide insights into pathogenesis of human intervertebral disc degeneration. Ann Rheum Dis. 2013 doi: 10.1136/annrheumdis-2012-202266. [DOI] [PubMed] [Google Scholar]
- 21.Risbud MV, Shapiro IM. Role of cytokines in intervertebral disc degeneration: pain and disc content. Nat Rev Rheumatol. 2014;10:44–56. doi: 10.1038/nrrheum.2013.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Phillips KL, Cullen K, Chiverton N, Michael AL, Cole AA, Breakwell LM, Haddock G, Bunning RA, Cross AK, Le Maitre CL. Potential roles of cytokines and chemokines in human intervertebral disc degeneration: interleukin-1 is a master regulator of catabolic processes. Osteoarthritis Cartilage. 2015;23:1165–1177. doi: 10.1016/j.joca.2015.02.017. [DOI] [PubMed] [Google Scholar]
- 23.Hoyland JA, Le Maitre C, Freemont AJ. Investigation of the role of IL-1 and TNF in matrix degradation in the intervertebral disc. Rheumatology (Oxford) 2008;47:809–814. doi: 10.1093/rheumatology/ken056. [DOI] [PubMed] [Google Scholar]
- 24.Wang J, Tian Y, Phillips KL, Chiverton N, Haddock G, Bunning RA, Cross AK, Shapiro IM, Le Maitre CL, Risbud MV. Tumor necrosis factor alpha- and interleukin-1beta-dependent induction of CCL3 expression by nucleus pulposus cells promotes macrophage migration through CCR1. Arthritis Rheum. 2013;65:832–842. doi: 10.1002/art.37819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li J, Yuan W, Jiang S, Ye W, Yang H, Shapiro IM, Risbud MV. Prolyl-4-hydroxylase domain protein 2 controls NF-kappaB/p65 transactivation and enhances the catabolic effects of inflammatory cytokines on cells of the nucleus pulposus. J Biol Chem. 2015;290:7195–7207. doi: 10.1074/jbc.M114.611483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fujita N, Gogate SS, Chiba K, Toyama Y, Shapiro IM, Risbud MV. Prolyl hydroxylase 3 (PHD3) modulates catabolic effects of tumor necrosis factor-alpha (TNF-alpha) on cells of the nucleus pulposus through co-activation of nuclear factor kappaB (NF-kappaB)/p65 signaling. J Biol Chem. 2012;287:39942–39953. doi: 10.1074/jbc.M112.375964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang J, Markova D, Anderson DG, Zheng Z, Shapiro IM, Risbud MV. TNF-alpha and IL-1beta promote a disintegrin-like and metalloprotease with thrombospondin type I motif-5-mediated aggrecan degradation through syndecan-4 in intervertebral disc. J Biol Chem. 2011;286:39738–39749. doi: 10.1074/jbc.M111.264549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tian Y, Yuan W, Fujita N, Wang J, Wang H, Shapiro IM, Risbud MV. Inflammatory Cytokines Associated with Degenerative Disc Disease Control Aggrecanase-1 (ADAMTS-4) Expression in Nucleus Pulposus Cells through MAPK and NF-kappaB. Am J Pathol. 2013;182:2310–2321. doi: 10.1016/j.ajpath.2013.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang X, Wang H, Yang H, Li J, Cai Q, Shapiro IM, Risbud MV. Tumor Necrosis Factor-α–and Interleukin-1β–Dependent Matrix Metalloproteinase-3 Expression in Nucleus Pulposus Cells Requires Cooperative Signaling via Syndecan 4 and Mitogen-Activated Protein Kinase–NF-κB Axis: Implications in Inflammatory Disc Disease. The American journal of pathology. 2014;184:2560–2572. doi: 10.1016/j.ajpath.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang H, Tian Y, Wang J, Phillips KL, Binch AL, Dunn S, Cross A, Chiverton N, Zheng Z, Shapiro IM, Le Maitre CL, Risbud MV. Inflammatory Cytokines Induce NOTCH Signaling in Nucleus Pulposus Cells: IMPLICATIONS IN INTERVERTEBRAL DISC DEGENERATION. J Biol Chem. 2013;288:16761–16774. doi: 10.1074/jbc.M112.446633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maidhof R, Jacobsen T, Papatheodorou A, Chahine NO. Inflammation induces irreversible biophysical changes in isolated nucleus pulposus cells. 2014 doi: 10.1371/journal.pone.0099621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Farahat MN, Yanni G, Poston R, Panayi GS. Cytokine expression in synovial membranes of patients with rheumatoid arthritis and osteoarthritis. Ann Rheum Dis. 1993;52:870–875. doi: 10.1136/ard.52.12.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goldring MB. Osteoarthritis and cartilage: the role of cytokines. Curr Rheumatol Rep. 2000;2:459–465. doi: 10.1007/s11926-000-0021-y. [DOI] [PubMed] [Google Scholar]
- 34.Sohn DH, Sokolove J, Sharpe O, Erhart JC, Chandra PE, Lahey LJ, Lindstrom TM, Hwang I, Boyer KA, Andriacchi TP. Plasma proteins present in osteoarthritic synovial fluid can stimulate cytokine production via Toll-like receptor 4. Arthritis Res Ther. 2012;14:R7. doi: 10.1186/ar3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bau B, Gebhard PM, Haag J, Knorr T, Bartnik E, Aigner T. Relative messenger RNA expression profiling of collagenases and aggrecanases in human articular chondrocytes in vivo and in vitro. Arthritis Rhenum. 2002;46:2648–2657. doi: 10.1002/art.10531. [DOI] [PubMed] [Google Scholar]
- 36.Fears CY, Woods A. The role of syndecans in disease and wound healing. Matrix biology. 2006;25:443–456. doi: 10.1016/j.matbio.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 37.Tkachenko E, Rhodes JM, Simons M. Syndecans: new kids on the signaling block. Circ Res. 2005;96:488–500. doi: 10.1161/01.RES.0000159708.71142.c8. [DOI] [PubMed] [Google Scholar]
- 38.Kim CW, Goldberger OA, Gallo RL, Bernfield M. Members of the syndecan family of heparan sulfate proteoglycans are expressed in distinct cell-, tissue-, and development-specific patterns. Mol Biol Cell. 1994;5:797–805. doi: 10.1091/mbc.5.7.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salminen-Mankonen H, Säämänen AM, Jalkanen M, Vuorio E, Pirilä L. Syndecan-1 expression is upregulated in degenerating articular cartilage in a transgenic mouse model for osteoarthritis. Scand J Rheumatol. 2005;34:469–474. doi: 10.1080/03009740500304338. [DOI] [PubMed] [Google Scholar]
- 40.Gao G, Plaas A, Thompson VP, Jin S, Zuo F, Sandy JD. ADAMTS4 (aggrecanase-1) activation on the cell surface involves C-terminal cleavage by glycosylphosphatidyl inositol-anchored membrane type 4-matrix metalloproteinase and binding of the activated proteinase to chondroitin sulfate and heparan sulfate on syndecan-1. J Biol Chem. 2004;279:10042–10051. doi: 10.1074/jbc.M312100200. [DOI] [PubMed] [Google Scholar]
- 41.Shimazu A, Nah H, Kirsch T, Koyama E, Leatherman JL, Golden EB, Kosher RA, Pacifici M. Syndecan-3 and the control of chondrocyte proliferation during endochondral ossification. Exp Cell Res. 1996;229:126–136. doi: 10.1006/excr.1996.0350. [DOI] [PubMed] [Google Scholar]
- 42.Iozzo RV, Schaefer L. Proteoglycan form and function: A comprehensive nomenclature of proteoglycans. Matrix Biology. 2015;42:11–55. doi: 10.1016/j.matbio.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oh ES, Couchman JR. Syndecans-2 and -4; close cousins, but not identical twins. Mol Cells. 2004;17:181–187. [PubMed] [Google Scholar]
- 44.Stewart MD, Sanderson RD. Heparan sulfate in the nucleus and its control of cellular functions. Matrix Biology. 2014;35:56–59. doi: 10.1016/j.matbio.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kainulainen V, Wang H, Schick C, Bernfield M. Syndecans, heparan sulfate proteoglycans, maintain the proteolytic balance of acute wound fluids. J Biol Chem. 1998;273:11563–11569. doi: 10.1074/jbc.273.19.11563. [DOI] [PubMed] [Google Scholar]
- 46.Subramanian SV, Fitzgerald ML, Bernfield M. Regulated shedding of syndecan-1 and -4 ectodomains by thrombin and growth factor receptor activation. J Biol Chem. 1997;272:14713–14720. doi: 10.1074/jbc.272.23.14713. [DOI] [PubMed] [Google Scholar]
- 47.Elenius K, Maatta A, Salmivirta M, Jalkanen M. Growth factors induce 3T3 cells to express bFGF-binding syndecan. J Biol Chem. 1992;267:6435–6441. [PubMed] [Google Scholar]
- 48.Mostafavi-Pour Z, Askari JA, Parkinson SJ, Parker PJ, Ng TT, Humphries MJ. Integrin-specific signaling pathways controlling focal adhesion formation and cell migration. J Cell Biol. 2003;161:155–167. doi: 10.1083/jcb.200210176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Woods A, Couchman JR. Syndecan-4 and focal adhesion function. Curr Opin Cell Biol. 2001;13:578–583. doi: 10.1016/s0955-0674(00)00254-4. [DOI] [PubMed] [Google Scholar]
- 50.Wilcox-Adelman SA, Denhez F, Goetinck PF. Syndecan-4 modulates focal adhesion kinase phosphorylation. J Biol Chem. 2002;277:32970–32977. doi: 10.1074/jbc.M201283200. [DOI] [PubMed] [Google Scholar]
- 51.Moon JJ, Matsumoto M, Patel S, Lee L, Guan J, Li S. Role of cell surface heparan sulfate proteoglycans in endothelial cell migration and mechanotransduction. J Cell Physiol. 2005;203:166–176. doi: 10.1002/jcp.20220. [DOI] [PubMed] [Google Scholar]
- 52.Fujita N, Hirose Y, Tran CM, Chiba K, Miyamoto T, Toyama Y, Shapiro IM, Risbud MV. HIF-1-PHD2 axis controls expression of syndecan 4 in nucleus pulposus cells. FASEB J. 2014;28:2455–2465. doi: 10.1096/fj.13-243741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Risbud MV, Schoepflin ZR, Mwale F, Kandel RA, Grad S, Iatridis JC, Sakai D, Hoyland JA. Defining the phenotype of young healthy nucleus pulposus cells: recommendations of the Spine Research Interest Group at the 2014 annual ORS meeting. J Orthop Res. 2015;33:283–293. doi: 10.1002/jor.22789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Agrawal A, Guttapalli A, Narayan S, Albert TJ, Shapiro IM, Risbud MV. Normoxic stabilization of HIF-1alpha drives glycolytic metabolism and regulates aggrecan gene expression in nucleus pulposus cells of the rat intervertebral disk. Am J Physiol Cell Physiol. 2007;293:C621–31. doi: 10.1152/ajpcell.00538.2006. [DOI] [PubMed] [Google Scholar]
- 55.Risbud MV, Shapiro IM. Notochordal cells in the adult intervertebral disc: new perspective on an old question. Crit Rev Eukaryot Gene Expr. 2011;21:29–41. doi: 10.1615/critreveukargeneexpr.v21.i1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dahia CL, Mahoney E, Wylie C. Shh signaling from the nucleus pulposus is required for the postnatal growth and differentiation of the mouse intervertebral disc. PLoS One. 2012;7:e35944. doi: 10.1371/journal.pone.0035944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Beckett MC, Ralphs JR, Caterson B, Hayes AJ. The transmembrane heparan sulphate proteoglycan syndecan-4 is involved in establishment of the lamellar structure of the annulus fibrosus of the intervertebral disc. Eur Cell Mater. 2015;30:69–88. doi: 10.22203/ecm.v030a06. discussion 88. [DOI] [PubMed] [Google Scholar]
- 58.Grover J, Roughley PJ. Expression of cell-surface proteoglycan mRNA by human articular chondrocytes. Biochem J. 1995;309:963–968. doi: 10.1042/bj3090963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Echtermeyer F, Bertrand J, Dreier R, Meinecke I, Neugebauer K, Fuerst M, Lee YJ, Song YW, Herzog C, Theilmeier G. Syndecan-4 regulates ADAMTS-5 activation and cartilage breakdown in osteoarthritis. Nat Med. 2009;15:1072–1076. doi: 10.1038/nm.1998. [DOI] [PubMed] [Google Scholar]
- 60.Mwale F. Syndecan 4 Signaling and Intervertebral Disc Degeneration. The American journal of pathology. 2014;184:2371–2373. doi: 10.1016/j.ajpath.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 61.Godmann L, König U, Stratis A, Cromme C, Neugebauer K, Herzog C, Korb-Pap A, Niederreiter B, Dankbar B, Redlich K. A4. 22 Syndecan-4 controls interleukin (IL)-1 receptor trafficking and IL-1 signalling in chronic destructive arthritis. Ann Rheum Dis. 2015;74:A45–A46. [Google Scholar]
- 62.Tran CM, Schoepflin ZR, Markova DZ, Kepler CK, Anderson DG, Shapiro IM, Risbud MV. CCN2 suppresses catabolic effects of interleukin-1beta through alpha5beta1 and alphaVbeta3 integrins in nucleus pulposus cells: implications in intervertebral disc degeneration. J Biol Chem. 2014;289:7374–7387. doi: 10.1074/jbc.M113.526111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tran CM, Shapiro IM, Risbud MV. Molecular regulation of CCN2 in the intervertebral disc: lessons learned from other connective tissues. Matrix Biology. 2013;32:298–306. doi: 10.1016/j.matbio.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bertrand J, Stange R, Hidding H, Echtermeyer F, Nalesso G, Godmann L, Timmen M, Bruckner P, Dell’Accio F, Raschke MJ. Syndecan 4 supports bone fracture repair, but not fetal skeletal development, in mice. Arthritis & Rheumatism. 2013;65:743–752. doi: 10.1002/art.37817. [DOI] [PubMed] [Google Scholar]