Abstract
It is now clear that recognition of nascent tumors by the immune system is critical for survival of the host against cancer. During cancer immunoediting, the ability of the tumor to escape immune recognition is important for tumor development. The immune system recognizes tumors via the presence of classical antigens and also by conserved innate mechanisms. One of these mechanisms is the NKG2D receptor that recognizes ligands whose expression is induced by cell transformation. Here we show that in NKG2D receptor deficient mice, increasing numbers of B cells begin to express NKG2D ligands as they age. Their absence in wild-type mice suggests that these cells are normally cleared by NKG2D expressing cells. NKG2D deficient mice and mice constitutively expressing NKG2D ligands had increased incidence of B cell tumors, confirming that the inability to clear NKG2D ligand expressing cells was important in tumor suppression and that NKG2D ligand expression is a marker of nascent tumors. Supporting a role for NKG2D ligand expression in controlling the progression of early stage B cell lymphomas in humans, we found higher expression of a miRNA that inhibits human NKG2D ligand expression in tumor cells from low-grade compared with high-grade follicular lymphoma patients.
Introduction
NKG2D is an NK cell activating receptor expressed on all NK cells and subsets of T cells in both mouse and human (1–7). NKG2D binds to a wide variety of self-ligands, all of which are related to MHC class I in sequence. In human, the ligands are MICA and MICB and the RAET1 family member molecules (ULBP1–6) (1, 8–10). In mouse, the ligands are the RAE1 (RAE1α−ε), H60 (H60a–c), and MULT-1 proteins (11–16). These ligands are generally absent from most normal tissue; rather, their expression is induced under conditions of cellular stress, such as viral infection, transformation, or DNA damage (17). Tumors of various origin express NKG2D ligands, which facilitates NK-mediated lysis of the tumor cells in vitro and allows for rejection of transplantable tumors in vivo (1, 12, 14).
The role of NKG2D in tumor immunosurveillance was previously tested using a prostate cancer model, a B cell lymphoma model, and a carcinogen-induced sarcoma model. In the TRAMP prostate cancer model, the absence of NKG2D resulted in enhanced tumor growth and more aggressive tumors (18). Similarly, the occurrence of B cell lymphomas was accelerated in the absence of NKG2D (18). In contrast, there was no effect on the occurrence of carcinogen-induced sarcomas (18). However, an experimental murine sarcoma line induced in an immune-deficient mouse expressed variable levels of NKG2D ligands and the high-ligand expressing cells were eliminated when they were transferred into a wild-type host (19). In addition, treatment of mice with a neutralizing NKG2D antibody enhanced the sensitivity of mice to carcinogen-induced fibrosarcomas (20).
Evidence from both mouse and human studies suggest tumors actively evade NKG2D recognition. Experimental murine tumors often lose NKG2D ligand expression as they progress (18, 19, 21). Patients with certain types of cancer exhibit high serum levels of circulating NKG2D ligands (22–24), presumably released from the tumors, resulting in a down-regulation of NKG2D expression on NK cells and T cells, which ultimately blocks NKG2D-mediated recognition of tumors (24).
The cancer immunoediting hypothesis posits that a tumor is either eliminated by the immune system, exists in a state of equilibrium with the immune system, or acquires properties that allow the tumor to grow and escape recognition by the immune system (25). Given that NKG2D ligands are induced by cellular transformation (26), its role in cancer immunoediting is likely to be in the first two phases, elimination and equilibrium. Because the transgenic models of cancer used in earlier studies enforce the development of tumors, it is difficult to parse the natural role of NKG2D using these models.
Mice and humans are naturally prone to the development of B cell lymphoma as they age (27). Here we studied the natural history of spontaneously-arising, non-transgene-driven B cell lymphomas in the context of NKG2D. In mice lacking NKG2D expression, NKG2D ligand expression was clearly evident on splenic B cells as mice aged with increasing levels correlated with ageing. Increasing expression of NKG2D ligands also correlated with development of B cell lymphoma. Even in mice lacking overt tumor masses, we could easily detect multiple lymphoid aggregates in multiple organs in mice lacking NKG2D expression, suggesting nascent B cell lymphoma. These findings suggest that NKG2D ligand expression is an early marker of B cell transformation, allowing for recognition and elimination by NKG2D-expressing NK and T cells. The absence of NKG2D ligands on the B cell lymphomas that finally arise in wild-type mice suggests that loss of NKG2D ligands is a requirement for tumor escape from the immune system. The human relevance of the NGK2D receptor-ligand axis was supported by our findings suggesting that NKG2D ligands are expressed at a higher level on low-grade compared with high-grade human follicular lymphomas.
Materials and Methods
Mice
All mice were housed under specific-pathogen-free conditions in the Washington University School of Medicine or University of Kansas Medical Center animal facilities in accordance with institutional guidelines. The generation of the RAE1ε transgenic (C57BL/6/129) and Klrk1−/− (C57BL/6) mice were previously described (28, 29). The Klrk1−/− mice were bred to RAE1ε transgenic heterozygous mice to generate wild-type and Klrk1−/− littermates on the same genetic background. The OT-I transgenic and the γC−/− mice were purchased from Jackson Laboratory. Rag-1−/− mice were purchased from Taconic. The Rag-1−/− and γC−/− mice were bred together in the Washington University School of Medicine animal facilities.
T cell adoptive transfer
NKG2D-expressing CTL were generated in vitro by culturing splenocytes and lymph node cells from OT-I TCR transgenic mice in 6-well plates (2.5 × 107 cells/well) with 1 μM OVA peptide (SIINFEKL) (provided by P. Allen, Washington University School of Medicine) for 5 days. Live cells were harvested with Ficoll-hypaque (GE Healthcare) and labeled with 1 μM CFSE (Invitrogen) and injected i.v. (107 cells/mouse). The cells were analyzed 24 hours later for the expression of NKG2D by staining with an NKG2D-specific antibody (BD Biosciences) followed by flow cytometry.
Tissue Sectioning and Staining
Tissues were fixed in 10% formalin and 5 μm sections were cut and stained with hematoxylin and eosin by the Developmental Biology Histology and Microscopy Core at the Washington University School of Medicine.
B cell clonality analysis
Genomic DNA was purified from the indicated organs using the Gentra Puregene Tissue Kit (Qiagen), and the region surrounding the CDR3 of the IgH locus was PCR amplified using degenerate primers located within the framework regions (30). The PCR amplicons were gel extracted, and prepped into a library, indexed and sequenced using the Illumina HiSeq 2500 platform to generate 101bp paired end reads by the Genome Technology Access Center at Washington University School of Medicine. The paired-end reads were assembled using PEAR to reconstruct the complete sequence for each read (31). The successfully merged reads were analyzed using IMGT/HighV-QUEST for V, D, and J usage and CDR3 length (32). Unique reads were defined as having a specific V, specific J and CDR3 length. Clonality was assessed by determining the number of unique reads required to reach 5% of the total number of productive reads. The R script utilized in the assessment of clonality is available upon request.
Flow cytometric analysis of tumors
Monoclonal antibodies specific for RAE1 (pan-specific), MULT-1, H60a, and a rat IgG2a control antibody were purchased from R&D Systems. Monoclonal antibodies specific for B220, CD19, NK1.1 and CD3ε were purchased from BD Biosciences. Single cell suspensions generated from tumors or spleens were stained with these antibodies and analyzed with a FACSCalibur (BD Biosciences).
Tumor transplantation studies
Single cell suspensions were generated from tumor masses harvested from RAE1ε transgenic or wild-type mice. 106 cells were then injected i.v. into 2 month-old Rag1−/−/γC−/− mice. In some experiments, the recipient mice received NKG2D-specific, RAE1ε-specific, or control monoclonal antibodies (R&D Research) (200μg/mouse i.p.) at the time of tumor transplant and every 3 days thereafter. Three weeks after tumor transplantation, spleens, livers, lungs, kidneys, and pancreata were harvested. The splenocytes were stained with B220- and CD19- or NKG2D-specific antibodies (BD Biosciences) and analyzed by flow cytometry. The other organs were fixed in 10% formalin.
Human NKG2D ligand mRNA and miRNA analysis
Fresh excisional biopsy specimens were collected from adult patients with a diagnosis of untreated follicular lymphoma and who consented to the Washington University lymphoma tissue collection protocol. Single cell suspensions of B cells were obtained by positive magnetic cell sorting of CD19+ cells (Miltenyi Biotec). Normal B cells were obtained from tonsils or peripheral blood. Tonsillar B cells were obtained by positive magnetic cell sorting of CD19+ cells from tonsils removed from children undergoing elective tonsillectomy (Children’s Hospital, Washington University School of Medicine) with approval from the Washington University School of Medicine Human Studies Committee Board. Peripheral blood B cells were purified by negative sorting from blood drawn from volunteers by Volunteers for Heath at Washington University School of Medicine. Total RNA was purified from the cells and mRNA and miRNA expression measured with Human Exon 1.0 ST arrays and GeneChip miRNA arrays (Affymetrix), respectively. Data have been deposited in NCBI’s Gene Expression Omnibus under accession number GSE62246 (http://www.ncbi.nlm.nih.gov/gds?term=200062246).
Human MICA/B protein expression analysis
Samples from the same patients used for the RNA analyses were fixed, paraffin-embedded, and sectioned. The sections were subjected to antigen retrieval with Antigen Retrieval Reagent-Basic (R&D Systems), and stained with a MICA/B-specific or control mouse IgG2a antibody (R&D Systems) followed by anti-mouse HRP-DAB (R&D Systems).
Results
Enhanced spontaneous B cell lymphoma development in mice deficient in NKG2D expression
Using a mouse that we described previously, we generated mice with constitutive, ubiquitous expression of the murine NKG2D ligand RAE1ε (28). RAE1ε was detectable on cells from all tissues examined in the transgenic animals, as well as on all peripheral blood mononuclear cells (PBMC). Similar to other NKG2D ligand transgenic mice (33, 34), this resulted in down-regulation of NKG2D expression on both endogenous and adoptively transferred cells (Fig. 1a).
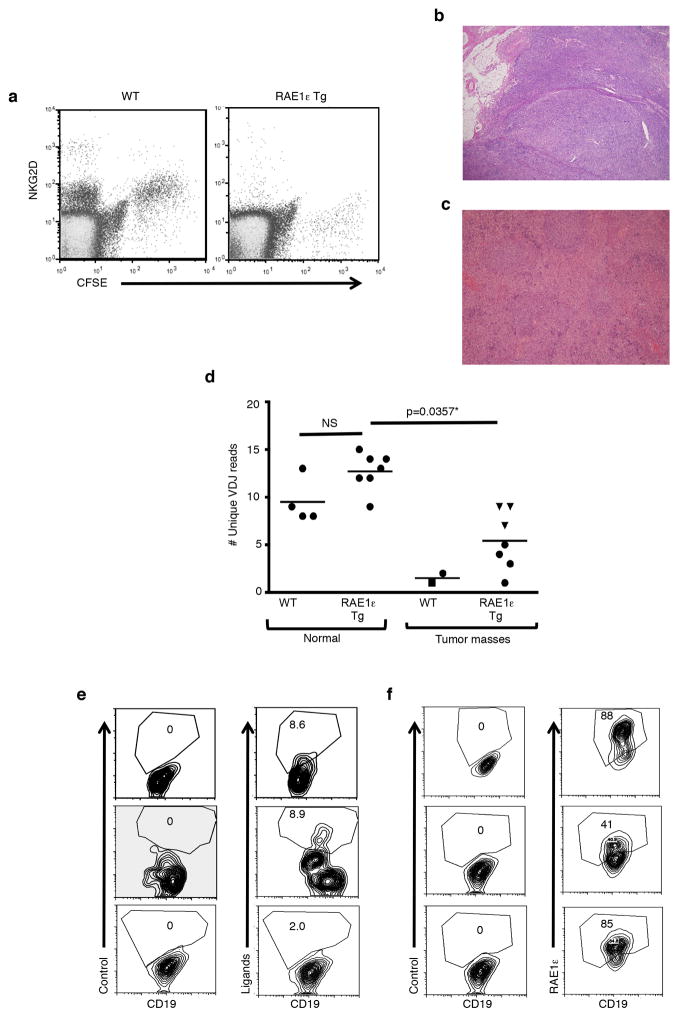
Figure 1. Down-regulation of NKG2D expression and enhanced spontaneous B cell lymphoma in RAE1ε transgenic mice.
(a) CFSE-labeled OT-I CTL (NKG2D+) were adoptively transferred into a RAE1ε transgenic (RAE1ε Tg) and wild-type mouse. Twenty-four hours later NKG2D expression in the spleens of the recipient mice were analyzed. (b) Tumor mass from RAE1ε transgenic mouse. (c) Tumor mass from wild-type mouse. (d) The number of unique VDJ reads required to reach 5% of total sequencing reads from B cells present in normal spleens of 3 month-old mice or tumor masses from the spleen (circle), lymph node (triangle), or liver (square) of 10–24 month-old RAE1ε (n=7) transgenic mice or >24 month-old wild-type mice (n=2) with a single data point per mouse. (e) NKG2D ligand expression on lymphomas from three WT mice measured by combined staining with antibodies specific for RAE1, H60, and MULT-1. The number shown is the percentage of CD19+ cells with NKG2D ligand staining above control antibody staining. (f) RAE1ε expression on lymphomas from three RAE1ε transgenic mice measured by staining with antibody specific for RAE1ε. The number shown is the percentage of CD19+ cells with RAE1ε staining above control antibody staining. Scale bar: 1mm. *two-tailed Mann-Whitney test. NS: Not statistically different.
Given the evidence for NKG2D-mediated immunity in tumor immunosurveillance (18–24, 33), we tested the hypothesis whether loss of NKG2D expression would make the RAE1ε transgenic mice more susceptible to naturally arising tumors. Therefore, we analyzed RAE1ε transgenic and non-transgenic littermates as they aged for evidence of tumor development. A significant percentage of the RAE1ε transgenic mice (28%, n=39) developed large masses in the mesenteric lymph nodes, spleen, liver or kidney between 10 and 24 months of age (Fig. 1b and d). In contrast, no wild-type mice (0%, n=32) from the same breeding colony developed tumor masses before 24 months of age. Histological analysis classified these masses as follicular or diffuse large cell B cell lymphoma (Fig. 1b). VDJ sequencing of the immunoglobulin heavy chain (IgH) confirmed the increased clonality of B cells present in the tumor masses (Fig. 1d). These lymphomas were slow growing (indolent) and did not result in mortality. As known previously (27), we also found that subclinical B cell lymphomas arise naturally in wild-type mice but we detected such tumors only when mice were older than 24 months (Fig. 1c and d). While tumors from wild-type mice expressed low levels of NKG2D ligands (Fig. 1e), tumors from RAE1ε transgenic animals, expressed much higher levels of the transgene-driven RAE1ε (Fig. 1f). These results demonstrate that downregulation of NKG2D leads to an increased susceptibility to B cell lymphomas.
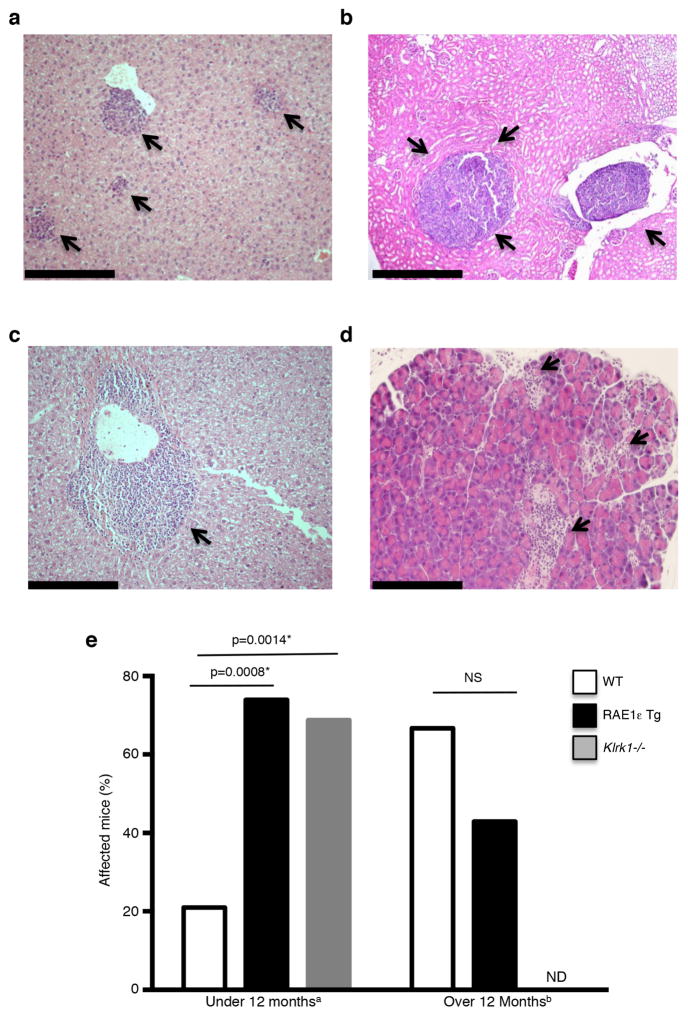
Upon necropsy of RAE1ε transgenic mice, histological analysis revealed that most mice had lymphoid aggregates in the liver, lungs, kidneys and pancreas, even when no overt tumor mass was present (Fig. 2). In transgenic mice younger than 12 months (median age 11.5 months), 73.9% had lymphoid aggregates (n=23) compared to 21% of wild-type mice (n=19; p<0.008) (median age 10 months) (Fig. 2e). After 12 months of age (medians 23 and 24 months; n=14 and n=9), the frequency of lymphoid aggregates was similar between transgenic and control mice (66.7 vs. 42.9; p=0.2752 ) (Fig. 2e). The increased incidence of both overt lymphoma masses and lymphoid aggregates in younger transgenic mice suggests that the aggregates represented nascent, low-grade lymphomas. We confirmed that this phenotype was not due to an artifact of the transgene by also analyzing NKG2D knockout mice (Klrk1−/−) (29). Compared to wild-type mice, Klrk1−/− and RAE1ε transgenic mice exhibited a similar increased frequency of lymphoid aggregates at 10 months of age (68.75%) (Fig. 2e). The similar phenotype between RAE1ε transgene expression and NKG2D knockout confirmed that the phenotype of increased lymphoid growth is due to NKG2D downregulation.
Figure 2. Enhanced accumulation of lymphoid aggregates in tissues of mice deficient in NKG2D expression.
(a–d) Lymphoid aggregates (arrows) present in the (a) liver of a RAE1ε transgenic mouse, (b) kidney of a RAE1ε transgenic mouse, (c) liver of a wild-type mouse and (d) pancreas of a Klrk1−/− mouse. (e) Percentage of WT, RAE1ε Tg or Klrk1−/− mice with lymphoid aggregates in peripheral organs without an overt tumor mass present. Scale bar: 1mm. aMedian age: WT 10 months (n=19), RAE1ε 11.5 months (n=23), Klrk1−/− 10 months (n=16); bMedian age: RAE1ε 23 months (n=9), WT 24 months (n=14); ND: Not determined. NS: Not statistically significant. *Log-rank test.
NKG2D ligand expression on splenic B cells correlates with the presence of lymphoid aggregates
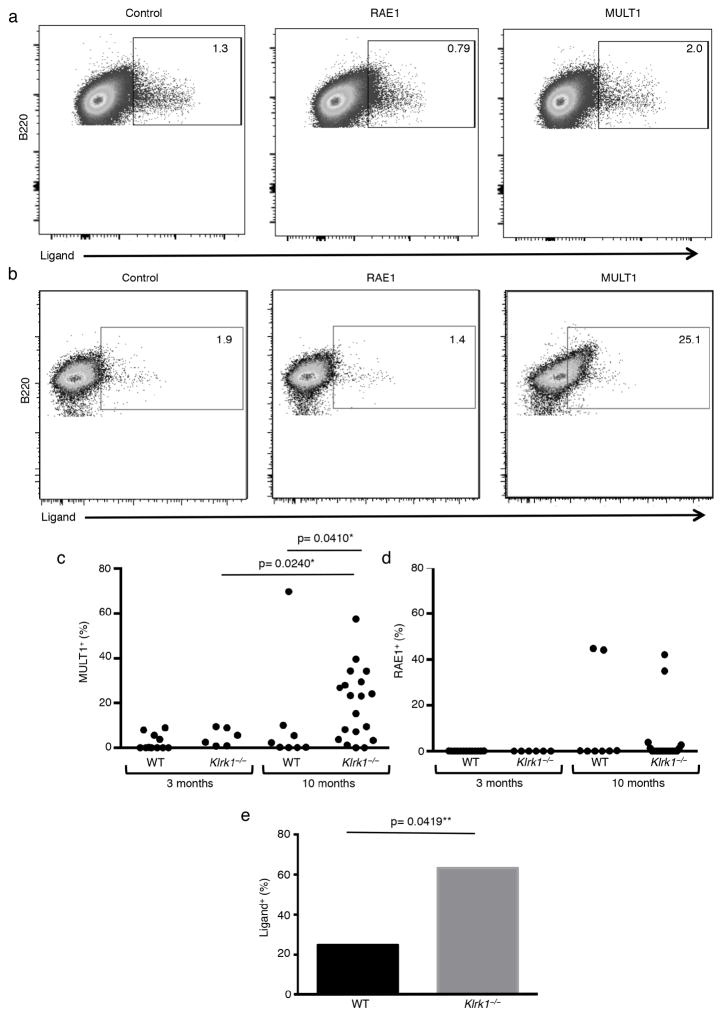
Current models suggest that NKG2D ligand expression is triggered by cell transformation (35). If NKG2D marks the early B cell transformation process, tumor outgrowth should occur only after tumor cells are selected for decreased NKG2D ligand expression. Analysis of splenic B cells from 3 month-old wild-type mice for NKG2D ligand expression showed little expression. Mice at 10 months of age, however, showed the presence of NKG2D ligands on a significant fraction of mice on splenic B cells (Fig. 3). This, to our knowledge, is the first to show a gradual increase in NKG2D ligand expression as mice age and suggests that B cells induce NKG2D ligand expression during the process of transformation. Strikingly, analysis of NKG2D-deficient mice showed that the percentage of mice with NKG2D ligand-expressing B cells (63%) was much greater at 10-months of age in comparison to wild-type animals of the same age (25%) (Fig. 3e). Importantly, NKG2D ligand expression also correlated with the fraction of mice with tissue-specific lymphoid aggregates (Fig. 2e). Since NKG2D ligand expression was absent in tumors from wild-type mice, this suggests that downregulation of NKG2D ligand expression was necessary to allow for tumor progression.
Figure 3. Increased NKG2D ligand expression on splenic B cells in NKG2D knockout mice.
(a and b) Representative flow cytometry plots of NKG2D ligand expression on splenic B cells from a 10 month-old (a) wild-type or (b) Klrk1−/− mouse. (c and d) Percentage of splenic B220+ cells expressing (c) MULT-1 (d) or RAE1 from 3 month-old WT (n= 10) and Klrk1−/− (n=6) or 10 month-old WT (n=8) and Klrk1−/− (n=19), mice. (e) Percentage of 10 month-old WT and Klrk1−/− mice with antibody staining for at least one NKG2D ligand on a greater proportion of splenic B cells than the average staining observed on 3 month-old WT mice. *One-tailed Mann-Whitney test, **Log-rank test.
Lymphomas from wild-type, but not RAE1ε transgenic mice, are transplantable into lymphodeficient hosts
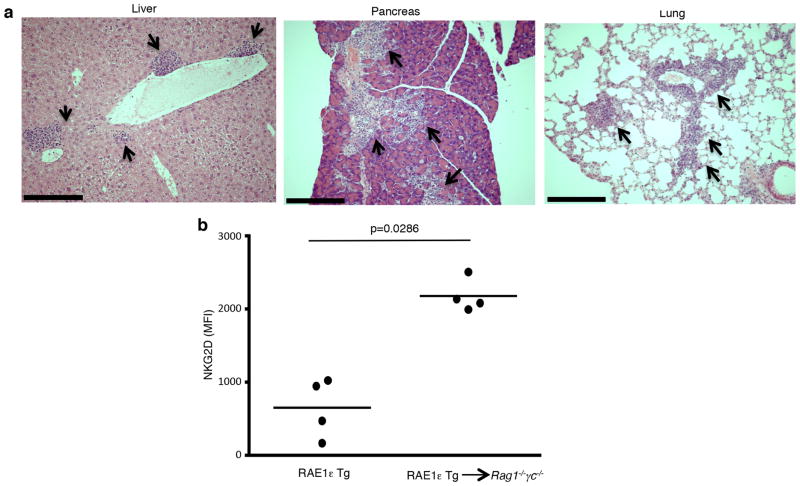
To confirm that the immune system suppresses the expansion of B lymphoma cells expressing NKG2D ligand, we transferred tumors from wild-type or transgenic mice into lymphocyte-deficient hosts. We reasoned that if tumors were eliminated by NKG2D recognition, tumors from both RAE1ε transgenic and wild-type mice should behave similarly when transferred into mice lacking lymphocytes. Single cell suspensions were generated from tumor masses harvested from transgenic or wild-type mice and injected i.v. into Rag1−/−γc−/− mice. Three weeks later, organs were harvested from the recipient mice and analyzed for the presence of tumor cells. When tumors from wild-type mice were injected, lymphoid aggregates were found in the liver, lung and pancreas in 60% of the recipient mice (n=5) (Fig. 4a), suggesting outgrowth of the transferred tumor cells. Contrary to our expectation, injection of tumors from RAE1ε transgenic mice resulted in significantly fewer (17%; n=6; p<0.05 log-rank test) recipient mice with lymphoid aggregates present in peripheral organs.
Figure 4. Lymphomas from wild-type, but not RAE1ε transgenic, mice are transplantable into lymphodeficient hosts.
(a) Lymphoid cells (arrows) present in the peripheral organs of a Rag1−/−γc−/− recipient mouse 3 weeks following transplantation of a single cell suspension from a lymphoma that developed in a WT mouse. (b) NKG2D expression on DX5+ splenocytes from 3 month-old RAE1ε transgenic mice or adoptive transfer Rag1−/−γc−/− recipient mice 9 days after transfer of RAE1ε transgenic splenocytes. Scale bar: 1mm. *One-tailed Mann-Whitney test.
Since the Rag1−/−γc−/− recipient mouse lack all lymphocytes, we hypothesized that NK and T cells that were present in the tumor masses from RAE1ε transgenic mice could explain the difference between the transfer of wild-type versus transgenic tumors. We hypothesized that in the case of the RAE1ε transgenic mice, transferring lymphocytes into a host without ubiquitous RAE1ε expression would allow for NKG2D up-regulation and tumor recognition and rejection. We first confirmed that similar numbers of T cells and NK cells were present in our single cell tumor preparations from wild-type and transgenic mice (data not shown). Consistent with our hypothesis, we found that NKG2D was re-expressed on NK cells following transfer of RAE1ε transgenic splenocytes into Rag1−/−γc−/− mice (Fig. 4b).
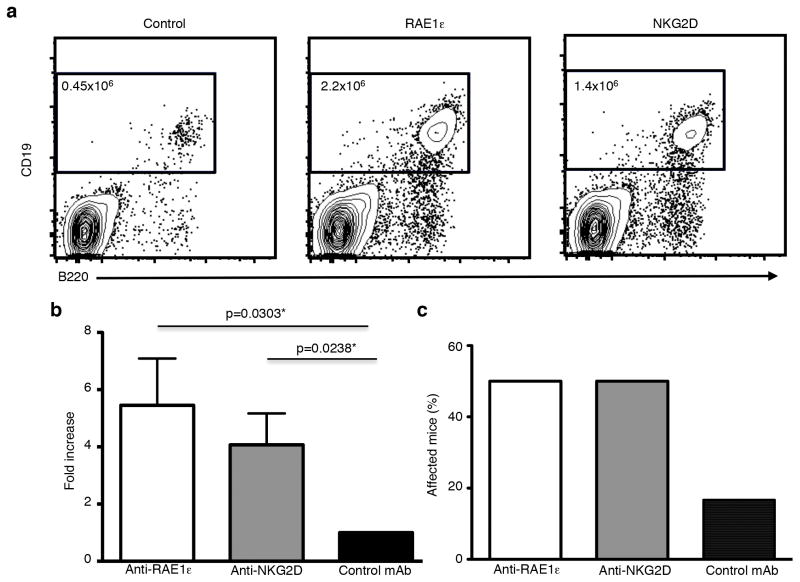
Confirming the veracity of our hypothesis, injection of RAE1ε- or NKG2D-blocking antibodies allowed for enhanced outgrowth of RAE1-ε expressing tumors, as shown by increased numbers of splenic B cells in recipient mice (Fig. 5a and b), as well as the presence of multiple lymphoid aggregates in tissues in over 50% of the host animals (Fig. 5c). Thus, NKG2D re-expression on lymphocytes was responsible for suppressing the growth of RAE1-expressing lymphoma cells after transfer.
Figure 5. Inhibition of RAE1ε-NKG2D interaction allows for the transplantation of RAE1ε transgenic lymphoma cells into lymphodeficient hosts.
(a) Number of B cells present in the spleens of Rag1−/−γc−/− recipient mice 3 weeks after transplantation of single cell suspensions generated from tumor masses from RAE1ε+ transgenic mice and injection of blocking monoclonal antibodies (mAbs) against RAE1ε, NKG2D, or a control antibody. The numbers shown are the total number of B220+CD19+ B cells present in each spleen. (b) The fold increase in the number of B cells present in the spleens of recipient mice that received blocking mAbs against RAE1ε or NKG2D compared with those that received a control mAb (n=6). (c) The percentage of Rag1−/−γc−/− recipient mice with lymphoid cells present in peripheral organs 3 weeks following transplantation of single cell suspensions generated from tumor masses from RAE1ε+ transgenic mice and injection of blocking monoclonal antibodies (mAbs) against RAE1ε, NKG2D, or a control antibody (n=6). *One-tailed Mann-Whitney test.
NKG2D ligand expression correlates with tumor grade in human follicular lymphoma
To determine whether NKG2D ligand expression may also be involved during the development of human B cell lymphomas, we compared NKG2D ligand expression by RNA microarray on low-grade (grade 1 or 2) versus high-grade (grade 3) follicular B cell tumors. No significant differences in the mRNA expression level of NKG2D ligands was observed.
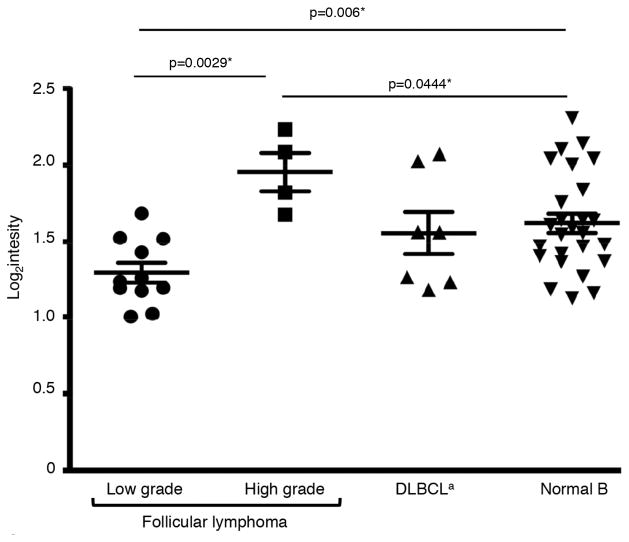
We considered that NKG2D ligand expression might be post-transcriptionally regulated by endogenous microRNAs (miRNAs) (36–40). We compared the expression level of 11 miRNAs known to regulate NKG2D ligand expression. We found that expression of one miRNA, miR-93 (37, 40), was decreased in low-grade follicular lymphoma cells compared with normal B cells (p=0.006) (Fig. 6). Expression of miR-93 was also elevated in high-grade follicular lymphoma cells compared to wild-type (p=0.0444) or low-grade follicular lymphoma cells (p=0.0029). Supporting that this change in miR-93 was specific to follicular lymphoma, no change was observed in its expression in diffuse large B cell lymphoma (DLBCL) cells, a form of aggressive non-Hodgkin’s lymphoma. We also noted a trend toward lower expression of a second miRNA implicated in suppressing NKG2D ligand expression, miR-106b (37, 40), in low-grade follicular lymphoma, compared with wild-type B cells, but this difference did not reach statistical significance likely due to our small sample size (p=0.0583, data not shown). These results suggest that cancer immunoediting may involve the upregulation of miRNAs that suppress NKG2D ligand expression.
Figure 6. Altered expression of miRNAs that target MICA and MICB in human follicular lymphoma cells.
Expression of miR-93 in follicular lymphoma cells, DLBCL cells and normal B cells. aDiffuse large B cell lymphoma. *Two-tailed Mann-Whitney test.
Discussion
In this study we analyzed the role of NKG2D in immunosurveillance of naturally arising B cell lymphomas. We found that mice with constitutive, ubiquitous expression of the murine NKG2D ligand RAE1ε had significantly enhanced outgrowth of spontaneous B cell lymphomas compared with wild-type mice, most likely due to ligand induced down-regulation of NKG2D expression on the surface of cells. Further, our data suggest that the NKG2D receptor is involved in controlling the outgrowth of sub-clinical, pre- or low-grade lymphoma in wild-type mice. This was evidenced by increased incidence of lymphoid aggregates in the peripheral tissues of RAE1ε transgenic and NKG2D-deficient mice in the absence of large tumor masses. The presence of the lymphoid aggregates correlated with NKG2D ligand expression on splenic B cells, which we hypothesize may serve as an early neoplastic marker.
The results of two previous studies utilizing the Eμ–myc transgenic mouse model suggested a role for NKG2D in immunosurveillance of B cell lymphomas (18, 41). However, because tumors are induced by forced expression of an oncogene in this model, immune escape is swift and tumors develop in the majority of wild-type mice. This suggests tumor growth quickly surpasses the ability of NKG2D to inhibit tumor progression in this model. This is further suggested by the finding that although NKG2D ligands were detected on lymphomas that developed in Eμ–myc mice, the expression level was not affected by the absence of NKG2D; loss of NKG2D ligand expression was not required for tumor escape in this model. By contrast, in the current study, tumors developed more slowly, allowing us to interrogate the interplay between tumor development and NKG2D-mediated recognition at earlier stages of the disease.
The cancer immunoediting hypothesis posits that tumors arise in three phases in immune-competent hosts (25). The first phase occurs when a nascent tumor is eliminated by the immune system. The second phase is equilibrium, where the immune system controls but fails to eliminate the tumor. In the third phase, changes in the tumor allow it to escape immune recognition and development into a full-blown malignant tumor. Our work is consistent with NKG2D playing a role in both the elimination and equilibrium phase of B cell tumors. We found that NKG2D ligands were induced on B cells as mice age. The greater frequency of ligand expression on B cells from NKG2D knockout mice suggests that ligand-expressing B cells are actively removed in wild-type mice. It is well established that NKG2D ligands induce recognition and killing by CTLs and NK cells. Our inability to transfer tumors from RAE1ε transgenic mice into Rag1−/−γc−/− mice supports the idea that the loss of NKG2D ligands by immunoediting is required for tumor growth and escape. Our findings suggest that B cell tumors are maintained in equilibrium by NKG2D ligand expression as mice age. We predict that a loss of this check-balance relationship between cytotoxic immune cells and pre-neoplastic B cells is a critical factor in their progression to overt lymphoma.
It is remarkable that NKG2D ligands were expressed on a significant proportion of splenic B cells in older mice. NKG2D ligand expression is induced by cell stress, including processes involved in transformation. The abundant ligand expression suggests that B cells in aging mice are experiencing cellular or genotoxic stress. The germinal center reaction involves genotoxic stress and could potentially explain NKG2D ligand expression in B cells (42) and it is known that DNA damage can induce the upregulation of NKG2D ligand expression (26). However, since DNA damage repair is part of the normal physiology of B cells and ligand expression is not detected in young mice, the mechanism is likely more complicated.
The detection of NKG2D ligand-expressing B cells in the spleen coincided with the presence of lymphoid aggregates in tissues. This suggests that the NKG2D ligand-expressing cells are pre-neoplastic cells, and that the constitutive expression of NKG2D ligands is potentially an early marker of B cell transformation. Interestingly, the majority of B cells expressed MULT1 but not RAE1. MULT1 was similarly observed to be the dominant ligand expressed in B cell lymphoma from Eμ-myc transgenic mice (18). This suggests that there are differential requirements for the induction of different NKG2D ligand families, and that the processes involved in the transformation of B cells favor the expression of MULT1.
Taken together, our data with the RAE1ε transgenic and NKG2D-deficient mice suggest that the increased lymphoma incidence in the transgenic mice is primarily due to an inability to respond through NKG2D. However, it is possible that there are defects in the RAE1ε transgenic mice in addition to low NKG2D expression that contribute to the defect in immunosurveillance in these mice. The results of two previous studies suggest that sustained NKG2D engagement impairs NKG2D-independent functions of NK cells (33, 43). However, a third study found NKG2D-independent functions of NK cells to be intact in transgenic mice with ubiquitous RAE1 expression (44). In addition, we demonstrated that NKG2D ligand expression decreases MHC class I surface expression (28).
It will be important to determine whether our results are relevant to human follicular lymphomas. As shown in figure 6, we found decreased expression of a miRNA, miR-93, that is known to control protein expression of the human NKG2D ligands MICA and MICB in low-grade tumors (grade 1 or 2), compared to high-grade lymphomas (grade 3). A second miRNA, miR-103b, trended towards lower levels in low-grade tumors but likely due to our small sample, the difference was not statistically significant. A larger sample set will allow for a more comprehensive statistical analysis of this miRNA. We stained a small number of tumor samples for expression of NKG2D ligands comparing high-grade and low-grade tumor samples and found a trend for higher protein expression in the low-grade tumor samples compared to the high-grade tumors (data not shown). It will be important to test this in a larger group of tumor samples.
Follicular lymphoma is thought to develop slowly, with low-grade tumor present for many years prior to aggressive tumor growth. Our data suggest NKG2D engagement on immune cells by NKG2D ligand expressed on lymphoma cells may be critical to controlling the outgrowth of the low-grade tumors. It will be important to perform future comprehensive studies of NKG2D ligand protein expression in follicular lymphoma patient samples to determine whether the expression of NKG2D ligands on low-grade lymphomas has therapeutic potential.
Footnotes
This work was supported by the American Cancer Society (IRG-58-010-55) (MM), the HHMI (A.S.S.), and the NIH (CA156690) (J.E.P. and E.M.O.).
References
- 1.Bauer S, Groh V, Wu J, Steinle A, Phillips JH, Lanier LL, Spies T. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285:727–729. doi: 10.1126/science.285.5428.727. [DOI] [PubMed] [Google Scholar]
- 2.Wu J, Song Y, Bakker AB, Bauer S, Spies T, Lanier LL, Phillips JH. An activating immunoreceptor complex formed by NKG2D and DAP10. Science. 1999;285:730–732. doi: 10.1126/science.285.5428.730. [DOI] [PubMed] [Google Scholar]
- 3.Dai Z, Turtle CJ, Booth GC, Riddell SR, Gooley TA, Stevens AM, Spies T, Groh V. Normally occurring NKG2D+CD4+ T cells are immunosuppressive and inversely correlated with disease activity in juvenile-onset lupus. J Exp Med. 2009;206:793–805. doi: 10.1084/jem.20081648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Groh V, Bruhl A, El-Gabalawy H, Nelson JL, Spies T. Stimulation of T cell autoreactivity by anomalous expression of NKG2D and its MIC ligands in rheumatoid arthritis. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:9452–9457. doi: 10.1073/pnas.1632807100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Groh V, Smythe K, Dai Z, Spies T. Fas-ligand-mediated paracrine T cell regulation by the receptor NKG2D in tumor immunity. Nat Immunol. 2006;7:755–762. doi: 10.1038/ni1350. [DOI] [PubMed] [Google Scholar]
- 6.Jamieson AM, Diefenbach A, McMahon CW, Xiong N, Carlyle JR, Raulet DH. The role of the NKG2D immunoreceptor in immune cell activation and natural killing. Immunity. 2002;17:19–29. doi: 10.1016/s1074-7613(02)00333-3. [DOI] [PubMed] [Google Scholar]
- 7.Hyka-Nouspikel N, Lucian L, Murphy E, McClanahan T, Phillips JH. DAP10 deficiency breaks the immune tolerance against transplantable syngeneic melanoma. Journal of immunology. 2007;179:3763–3771. doi: 10.4049/jimmunol.179.6.3763. [DOI] [PubMed] [Google Scholar]
- 8.Bacon L, Eagle RA, Meyer M, Easom N, Young NT, Trowsdale J. Two human ULBP/RAET1 molecules with transmembrane regions are ligands for NKG2D. Journal of immunology. 2004;173:1078–1084. doi: 10.4049/jimmunol.173.2.1078. [DOI] [PubMed] [Google Scholar]
- 9.Chalupny NJ, Sutherland CL, Lawrence WA, Rein-Weston A, Cosman D. ULBP4 is a novel ligand for human NKG2D. Biochem Biophys Res Commun. 2003;305:129–135. doi: 10.1016/s0006-291x(03)00714-9. [DOI] [PubMed] [Google Scholar]
- 10.Cosman D, Mullberg J, Sutherland CL, Chin W, Armitage R, Fanslow W, Kubin M, Chalupny NJ. ULBPs, novel MHC class I-related molecules, bind to CMV glycoprotein UL16 and stimulate NK cytotoxicity through the NKG2D receptor. Immunity. 2001;14:123–133. doi: 10.1016/s1074-7613(01)00095-4. [DOI] [PubMed] [Google Scholar]
- 11.Carayannopoulos LN, Naidenko OV, Fremont DH, Yokoyama WM. Cutting edge: murine UL16-binding protein-like transcript 1: a newly described transcript encoding a high-affinity ligand for murine NKG2D. J Immunol. 2002;169:4079–4083. doi: 10.4049/jimmunol.169.8.4079. [DOI] [PubMed] [Google Scholar]
- 12.Cerwenka A, Bakker AB, McClanahan T, Wagner J, Wu J, Phillips JH, Lanier LL. Retinoic acid early inducible genes define a ligand family for the activating NKG2D receptor in mice. Immunity. 2000;12:721–727. doi: 10.1016/s1074-7613(00)80222-8. [DOI] [PubMed] [Google Scholar]
- 13.Diefenbach A, Hsia JK, Hsiung MY, Raulet DH. A novel ligand for the NKG2D receptor activates NK cells and macrophages and induces tumor immunity. European journal of immunology. 2003;33:381–391. doi: 10.1002/immu.200310012. [DOI] [PubMed] [Google Scholar]
- 14.Diefenbach A, Jamieson AM, Liu SD, Shastri N, Raulet DH. Ligands for the murine NKG2D receptor: expression by tumor cells and activation of NK cells and macrophages. Nat Immunol. 2000;1:119–126. doi: 10.1038/77793. [DOI] [PubMed] [Google Scholar]
- 15.Takada A, Yoshida S, Kajikawa M, Miyatake Y, Tomaru U, Sakai M, Chiba H, Maenaka K, Kohda D, Fugo K, Kasahara M. Two novel NKG2D ligands of the mouse H60 family with differential expression patterns and binding affinities to NKG2D. Journal of immunology. 2008;180:1678–1685. doi: 10.4049/jimmunol.180.3.1678. [DOI] [PubMed] [Google Scholar]
- 16.Whang MI, Guerra N, Raulet DH. Costimulation of dendritic epidermal gammadelta T cells by a new NKG2D ligand expressed specifically in the skin. Journal of immunology. 2009;182:4557–4564. doi: 10.4049/jimmunol.0802439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Champsaur M, Lanier LL. Effect of NKG2D ligand expression on host immune responses. Immunol Rev. 2010;235:267–285. doi: 10.1111/j.0105-2896.2010.00893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guerra N, Tan YX, Joncker NT, Choy A, Gallardo F, Xiong N, Knoblaugh S, Cado D, Greenberg NM, Raulet DH. NKG2D-deficient mice are defective in tumor surveillance in models of spontaneous malignancy. Immunity. 2008;28:571–580. doi: 10.1016/j.immuni.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Sullivan T, Dunn GP, Lacoursiere DY, Schreiber RD, Bui JD. Cancer Immunoediting of the NK Group 2D Ligand H60a. Journal of immunology. 2011;187:3538–3545. doi: 10.4049/jimmunol.1100413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smyth MJ, Swann J, Cretney E, Zerafa N, Yokoyama WM, Hayakawa Y. NKG2D function protects the host from tumor initiation. J Exp Med. 2005;202:583–588. doi: 10.1084/jem.20050994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brenner CD, King S, Przewoznik M, Wolters I, Adam C, Bornkamm GW, Busch DH, Rocken M, Mocikat R. Requirements for control of B-cell lymphoma by NK cells. European journal of immunology. 2011;40:494–504. doi: 10.1002/eji.200939937. [DOI] [PubMed] [Google Scholar]
- 22.Salih HR, Antropius H, Gieseke F, Lutz SZ, Kanz L, Rammensee HG, Steinle A. Functional expression and release of ligands for the activating immunoreceptor NKG2D in leukemia. Blood. 2003;102:1389–1396. doi: 10.1182/blood-2003-01-0019. [DOI] [PubMed] [Google Scholar]
- 23.Hilpert J, Grosse-Hovest L, Grunebach F, Buechele C, Nuebling T, Raum T, Steinle A, Salih HR. Comprehensive analysis of NKG2D ligand expression and release in leukemia: implications for NKG2D-mediated NK cell responses. Journal of immunology. 2012;189:1360–1371. doi: 10.4049/jimmunol.1200796. [DOI] [PubMed] [Google Scholar]
- 24.Groh V, Wu J, Yee C, Spies T. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature. 2002;419:734–738. doi: 10.1038/nature01112. [DOI] [PubMed] [Google Scholar]
- 25.Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annu Rev Immunol. 2011;29:235–271. doi: 10.1146/annurev-immunol-031210-101324. [DOI] [PubMed] [Google Scholar]
- 26.Raulet DH, Gasser S, Gowen BG, Deng W, Jung H. Regulation of Ligands for the NKG2D Activating Receptor. Annu Rev Immunol. 2013;31:413–441. doi: 10.1146/annurev-immunol-032712-095951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ward JM. Lymphomas and leukemias in mice. Exp Toxicol Pathol. 2006;57:377–381. doi: 10.1016/j.etp.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 28.Cheney EE, Wise EL, Bui JD, Schreiber RD, Carayannopoulos LN, Spitzer D, Zafirova B, Polic B, Shaw AS, Markiewicz MA. A dual function of NKG2D ligands in NK-cell activation. European journal of immunology. 2012;42:2452–2458. doi: 10.1002/eji.201141849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zafirova B, Mandaric S, Antulov R, Krmpotic A, Jonsson H, Yokoyama WM, Jonjic S, Polic B. Altered NK cell development and enhanced NK cell-mediated resistance to mouse cytomegalovirus in NKG2D-deficient mice. Immunity. 2009;31:270–282. doi: 10.1016/j.immuni.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arnaout R, Lee W, Cahill P, Honan T, Sparrow T, Weiand M, Nusbaum C, Rajewsky K, Koralov SB. High-resolution description of antibody heavy-chain repertoires in humans. PloS one. 2011;6:e22365. doi: 10.1371/journal.pone.0022365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang J, Kobert K, Flouri T, Stamatakis A. PEAR: a fast and accurate Illumina Paired-End reAd mergeR. Bioinformatics. 2014;30:614–620. doi: 10.1093/bioinformatics/btt593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lefranc MP, Giudicelli V, Ginestoux C, Jabado-Michaloud J, Folch G, Bellahcene F, Wu Y, Gemrot E, Brochet X, Lane J, Regnier L, Ehrenmann F, Lefranc G, Duroux P. IMGT, the international ImMunoGeneTics information system. Nucleic Acids Res. 2009;37:D1006–1012. doi: 10.1093/nar/gkn838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oppenheim DE, Roberts SJ, Clarke SL, Filler R, Lewis JM, Tigelaar RE, Girardi M, Hayday AC. Sustained localized expression of ligand for the activating NKG2D receptor impairs natural cytotoxicity in vivo and reduces tumor immunosurveillance. Nat Immunol. 2005;6:928–937. doi: 10.1038/ni1239. [DOI] [PubMed] [Google Scholar]
- 34.Wiemann K, Mittrucker HW, Feger U, Welte SA, Yokoyama WM, Spies T, Rammensee HG, Steinle A. Systemic NKG2D down-regulation impairs NK and CD8 T cell responses in vivo. Journal of immunology. 2005;175:720–729. doi: 10.4049/jimmunol.175.2.720. [DOI] [PubMed] [Google Scholar]
- 35.Le Bert N, Gasser S. Advances in NKG2D ligand recognition and responses by NK cells. Immunology and cell biology. 2014;92:230–236. doi: 10.1038/icb.2013.111. [DOI] [PubMed] [Google Scholar]
- 36.Stern-Ginossar N, Mandelboim O. An integrated view of the regulation of NKG2D ligands. Immunology. 2009;128:1–6. doi: 10.1111/j.1365-2567.2009.03147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stern-Ginossar N, Gur C, Biton M, Horwitz E, Elboim M, Stanietsky N, Mandelboim M, Mandelboim O. Human microRNAs regulate stress-induced immune responses mediated by the receptor NKG2D. Nat Immunol. 2008;9:1065–1073. doi: 10.1038/ni.1642. [DOI] [PubMed] [Google Scholar]
- 38.Tsukerman P, Stern-Ginossar N, Gur C, Glasner A, Nachmani D, Bauman Y, Yamin R, Vitenshtein A, Stanietsky N, Bar-Mag T, Lankry D, Mandelboim O. MiR-10b downregulates the stress-induced cell surface molecule MICB, a critical ligand for cancer cell recognition by natural killer cells. Cancer Res. 2012;72:5463–5472. doi: 10.1158/0008-5472.CAN-11-2671. [DOI] [PubMed] [Google Scholar]
- 39.Heinemann A, Zhao F, Pechlivanis S, Eberle J, Steinle A, Diederichs S, Schadendorf D, Paschen A. Tumor suppressive microRNAs miR-34a/c control cancer cell expression of ULBP2, a stress-induced ligand of the natural killer cell receptor NKG2D. Cancer Res. 2012;72:460–471. doi: 10.1158/0008-5472.CAN-11-1977. [DOI] [PubMed] [Google Scholar]
- 40.Yadav D, Ngolab J, Lim RS, Krishnamurthy S, Bui JD. Cutting edge: down-regulation of MHC class I-related chain A on tumor cells by IFN-gamma-induced microRNA. Journal of immunology. 2009;182:39–43. doi: 10.4049/jimmunol.182.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Unni AM, Bondar T, Medzhitov R. Intrinsic sensor of oncogenic transformation induces a signal for innate immunosurveillance. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:1686–1691. doi: 10.1073/pnas.0701675105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mechtcheriakova D, Svoboda M, Meshcheryakova A, Jensen-Jarolim E. Activation-induced cytidine deaminase (AID) linking immunity, chronic inflammation, and cancer. Cancer Immunol Immunother. 2012;61:1591–1598. doi: 10.1007/s00262-012-1255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Coudert JD, Scarpellino L, Gros F, Vivier E, Held W. Sustained NKG2D engagement induces cross-tolerance of multiple distinct NK cell activation pathways. Blood. 2008;111:3571–3578. doi: 10.1182/blood-2007-07-100057. [DOI] [PubMed] [Google Scholar]
- 44.Champsaur M, Beilke JN, Ogasawara K, Koszinowski UH, Jonjic S, Lanier LL. Intact NKG2D-independent function of NK cells chronically stimulated with the NKG2D ligand Rae-1. Journal of immunology. 185:157–165. doi: 10.4049/jimmunol.1000397. [DOI] [PMC free article] [PubMed] [Google Scholar]