Abstract
Purpose
Sepsis remains an unresolved clinical problem with high hospital mortality. Despite intensive research over decades, no treatments for sepsis have become available. Here we explore the role of adenosine triphosphate (ATP) in the pathophysiology of sepsis. ATP is not only a universal energy carrier but it also acts as an extracellular signaling molecule that regulates immune functions. ATP stimulates a large family of purinergic receptors found on the cell surface of virtually all mammalian cells. In severe sepsis and septic shock, ATP released in large amounts into the extracellular space acts as a “danger signal”. In this review, we focus on the roles of ATP as a key regulator of immune cell function and as a disruptive signal that contributes to immune dysfunction in sepsis.
Methods
We summarized the current understanding of the pathophysiology of sepsis with special emphasis on the emerging role of systemic ATP as a disruptive force that promotes morbidity and mortality in sepsis.
Findings
Over the last two decades, the discovery that regulated ATP release and purinergic signaling represent a novel regulatory mechanism in immune cell physiology has opened up new possibilities to treat sepsis. Immune cells respond to stimulation with the release of cellular ATP, which regulates cell functions in autocrine and paracrine fashions. In sepsis, large amounts of systemic ATP produced by tissue damage and inflammation disrupt these regulatory purinergic signaling mechanisms, leading to immune dysfunction that promotes pathophysiological processes involved in sepsis.
Implications
The knowledge of these ATP-dependent signaling processes is likely to reveal exciting new avenues to treat the unresolved clinical problem of sepsis.
Keywords: purinergic signaling, sepsis, ATP, adenosine
INTRODUCTION
Sepsis is a life-threatening condition characterized by severe systemic infection and systemic inflammation that cause tissue damage and organ dysfunction.1,2 Despite substantial progress in the management of sepsis patients, sepsis remains a major public health problem that affects millions of patients worldwide every year.3,4 Severe sepsis and septic shock are among the leading causes of death in intensive care units with a mortality rate as high as 40%.5,6 In addition, the incidence of sepsis is further rising due to the increased use of immunosuppressive drugs, the widespread use of antibiotics, the emergence of drug-resistant pathogens, and the aging of our society.7 Over the last decades, the growing knowledge about the pathophysiology of sepsis has yielded a considerable number of potential drug targets and the development of new therapies to treat sepsis. However, all of these approaches have failed in clinical trials.6,8 As a result, there are still no specific pharmacological agents available for the treatment of sepsis and new directions for more effective treatment strategies are urgently needed.
Over the last few decades, a number of important discoveries have demonstrated that adenosine triphosphate (ATP) plays an essential role as an extracellular signaling molecule.9 The extracellular concentration of ATP increases under conditions that are associated with severe sepsis and septic shock, such as inflammation, ischemia, and hypoxia.10 Increased extracellular ATP is frequently considered a “danger signal” that triggers pro-inflammatory responses, particularly of the innate immune system, and thereby contributes to systemic inflammation and secondary organ damage in sepsis.11 In this brief review, we focus on how ATP and purinergic signaling regulate immune cell responses in sepsis.
Pathophysiology and treatment of sepsis
According to the current concept, sepsis arises from an overwhelming inflammatory host response to invading pathogens. In 1991, a consensus conference further classified sepsis as severe sepsis (sepsis associated with organ dysfunction) and septic shock (severe sepsis associated with the need for vasopressors following adequate fluid resuscitation). In addition, the terms systemic inflammatory response syndrome (SIRS), multiple organ dysfunction syndrome (MODS), and multiple organ failure (MOF) were defined because inflammatory diseases of non-infectious origin, including severe trauma, burns, pancreatitis or ischemia-reperfusion injuries overlap with the pathophysiology of sepsis.12 The criteria defining SIRS and sepsis have been questioned recently as being not sensitive and specific enough and it was suggested to use the term sepsis only if there is evidence of organ dysfunction or organ failure.2,6
Systemic inflammation is initiated by pattern recognition receptors such as Toll-like receptors (TLR) and NOD-like receptors (NLR) that are expressed by innate immune cells. These receptors are activated by pathogen-associated molecular patterns (PAMPs), like endotoxin, but also damage-associated molecular patterns (DAMPS) or “alarmins” that are released from injured host tissue and include a diverse group of molecules such as high-mobility group B 1 (HMGB1), uric acid, or chromosomal DNA.13 Activation of these receptors induces the immediate recruitment and activation of neutrophils and macrophages to initiate bacterial clearance and tissue repair. In sepsis, excessive activation of these pathways leads to the massive release of pro-inflammatory cytokines, activation of the coagulation cascade, endothelial dysfunction, hemodynamic failure, and finally multiple organ dysfunction and death.14 Numerous clinical trials in the past two decades focused on blocking this hyper-inflammatory response. Approaches including corticosteroid treatment and targeting of various mediators of inflammation such as tumor necrosis factor (TNF)-α, interleukin (IL)-1β, complement factor C5a, and endotoxin have failed in clinical trials.6,8,14,15 These disappointing results were attributed to difficulties with timing of intervention, the dosage of experimental drugs, and species differences between in vivo animal studies and human patients.6 However, less attention has been given to the fact that the initial hyper-inflammatory state in sepsis is offset by an anti-inflammatory response and that sepsis is associated with immunosuppression that reduces the ability of the host to clear infections. Anti-inflammatory treatment strategies exacerbate this immunosuppressed state and likely further increase the susceptibility of sepsis patients to nosocomial infections.14,16-18 Because specific pharmacological agents for sepsis are not available, the treatment of sepsis patients is limited to the use of antibiotics and supportive measures to improve hemodynamics and microcirculation.6,7
Hypertonic saline resuscitation has been studied as a potential strategy to reduce collateral tissue damage due to excessive neutrophil activation in trauma patients.19 In addition to its beneficial effects on hemodynamic functions, blood viscosity, and capillary blood flow, hypertonic saline resuscitation can suppress excessive neutrophil activation.20-23 It was shown that hypertonic saline regulates immune cell functions by inducing the release of cellular ATP into the extracellular environment.24 In the early 1980s, Chaudry and colleagues reported beneficial effects of ATP-MgCl2 infusions in experimental models of ischemia25, hemorrhagic shock26, and sepsis27,28. However, the underlying mechanisms were not well understood. Although it was unclear the extent to which ATP, MgCl2 or the combination of both were responsible for the observed beneficial effects of ATP-MgCl2, it was clear that ATP-MgCl2 infusion improved microcirculation due to its vasodilatory effect and restored cellular ATP, which improved organ blood flow and ameliorated energy metabolism in ischemic tissues.29
Since then, our understanding of the actions and fate of extracellular ATP has grown considerably and a large family of purinergic receptors that recognize ATP and related nucleotides has been identified.9,30,31 We now know that purinergic signaling regulates the functions of virtually all immune cell subtypes and it has become increasingly clear that this complex purinergic signaling system is altered in inflammation, tissue injury, and sepsis.32 Purinergic signaling has therefore come into focus as a potential new therapeutic target in the treatment of sepsis and septic shock.
ATP release and signaling through purinergic receptors
More than 40 years ago, Burnstock and coworkers first proposed the concept of purinergic neurotransmission through controlled ATP release from intact cells.33 Since then, numerous discoveries have exposed ATP and related molecules such as ADP, UTP, UDP, and adenosine as important signaling molecules that regulate many physiological processes, including immune cell responses.11,30,32,34 Immune cells respond to stimulation with the release of ATP through various mechanisms. Neutrophils release ATP through connexin 43 hemichannels or pannexin-1 (panx1) channels in response to formyl peptide receptor (FPR) stimulation.35,36 Panx1 was also reported to facilitate the release of ATP from macrophages following stimulation with LPS37 (Yang 2015) and from T cells following T cell receptor stimulation38,39 or exposure to osmotic stress40. In addition, vesicular transport also contributes to the release of ATP from T cells.41 The release of ATP is critical for the initiation of a signaling cascade that regulates immune cell responses. The purinergic receptor family comprises 19 known subtypes that recognize ATP, ADP, adenosine and similar nucleotides.31 These receptors can be categorized into three main groups: P2X, P2Y, and P1 receptors. P2X receptors (P2X1-7) are ATP-gated ion channels that facilitate the influx of extracellular cations, for example calcium. P2Y (P2Y1, 2, 4, 6, 11-14) receptors are G-protein coupled receptors (GPCRs) that recognize various nucleotides including ATP, ADP, UTP and UDP. The four P1 or adenosine receptors are also GPCRs. A1 and A3 adenosine receptors couple to Gi or Gq/11 proteins and often promote cell activation, while A2a and A2b receptors couple to stimulatory Gs proteins that increase intracellular cyclic AMP (cAMP) and typically inhibit many cell functions.32
Termination of ATP and adenosine signaling
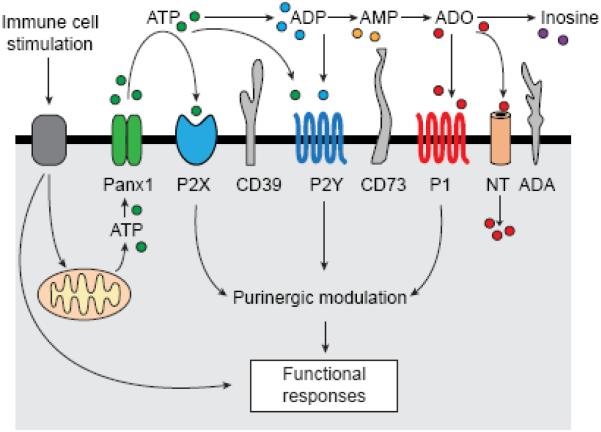
P2 receptor signaling is terminated by the conversion of ATP and ADP in the extracellular compartment to AMP and adenosine. Several groups of membrane-bound ectonucleotidases have been identified that differ with regard to their structures, substrate preferences, and cell-specific expression patterns.42,43 Among the most widely studied ectonucleotidases are ectonucleosid-triphosphate diphosphohydrolase (ENTPD)-1, also known as CD39, which catalyzes the conversion of ATP and ADP to AMP, and ecto-5’-nucleotidase (CD73), which generates adenosine from AMP. Termination of ATP signaling is closely linked to the formation of adenosine. Adenosine often suppresses inflammatory cell responses. Particularly A2a receptors were shown to be part of a negative feedback mechanism that limits local and systemic inflammation.44 The balance between ATP and adenosine signaling must be tightly controlled to prevent both P2 receptor-induced inflammatory damage as well as adenosine-dependent immunosuppression. Extracellular adenosine is metabolized by adenosine deaminase, which converts adenosine to inosine, or by adenosine kinase, which phosphorylates adenosine back to AMP.42 In addition, adenosine can be removed from the extracellular space by cellular reuptake through nucleoside transporters.32 Taken together, ATP release from stimulated immune cells, conversion of ATP to adenosine, and autocrine activation of different purinergic receptor subtypes can enhance or block immune cell functions by positive or negative feedback mechanisms that tightly control immune responses (Figure 1). Disturbances of these autocrine purinergic signaling processes may contribute to inflammatory tissue damage and immunosuppression.
Figure 1. Elements of the autocrine purinergic signaling mechanisms in immune cells.
Stimulation of specific surface receptors of immune cells that recognize pathogens, antigens, cytokines, or chemokines triggers mitochondrial ATP formation and ATP release through pannexin-1 (panx1) channels. ATP in the extracellular space can stimulate P2X or P2Y receptors. Ectonucleotidases such as CD39 or CD73 catalyze the stepwise hydrolysis of ATP to ADP (the ligand of certain P2Y receptors), AMP, and adenosine, which is the ligand of P1 (adenosine) receptors. Adenosine is removed by nucleoside transporters (NT) that facilitate the cellular uptake of adenosine or by adenosine deaminase (ADA) that converts adenosine to inosine.
Regulation of immune cells by purinergic signaling
Purinergic regulation of neutrophils
Neutrophils have a central role in host defense. Impaired neutrophil function renders the host defenseless against microbial invaders, while excessive activation causes injury to host organs. ATP and adenosine have long been known to regulate neutrophil functions, such as oxidative burst, phagocytosis, adherence, and chemotaxis.30,45,46 In recent years, autocrine purinergic signaling mechanisms have been identified that substantially advanced or understanding of the mechanisms by which ATP and adenosine regulate these cell functions. In response to chemotactic stimuli, neutrophils release cellular ATP through panx1 channels.36 The released ATP and autocrine activation of purinergic receptors is essential for chemotactic gradient recognition, cell polarization, and directed migration to the site of infection.47 Danger receptors like formyl-peptide receptors (FPRs) together with purinergic molecules such as panx1, P2Y2, and A3 adenosine receptors form a stimulatory complex at the leading edge of polarized neutrophils that triggers FPR-induced ATP release and P2Y2 and A3 receptor-dependent calcium and MAPK signaling and thus amplifies the intracellular signals that generate functional responses to chemotactic stimuli.36 Inhibition of autocrine purinergic signaling by blocking ATP release or P2Y2 receptors, but also interfering with these signaling mechanisms by adding excessive exogenous ATP blocks chemotaxis.47 CD39, CD73, and alkaline phosphatase generate adenosine that stimulates A2a and A3 adenosine receptors.36,48 While A3 receptors accumulate at the leading edge during cell polarization where they promote cell migration, A2a receptors block chemotactic responses at the back of cells.48 P2Y2, A3, and A2a receptors form together a “pull-push” mechanism that induces and maintains a polarized cell shape and offers a molecular framework for the widely anticipated but poorly defined “local excitation global inhibition” (LEGI) model of chemotaxis.48-50 In addition to the GPCR-type purinergic receptors mentioned above, P2X1 receptors are also involved in the regulation of neutrophil chemotaxis through Rho kinase activation.51
Recent discoveries point to an essential role for mitochondria in neutrophil activation, namely by producing the ATP that fuels the purinergic signaling processes involved in cell activation.52 Mitochondrial activation and ATP synthesis at the front of polarized neutrophils is augmented by mTOR signaling, while stimulation of A2a receptors at the back of cells triggers intracellular cAMP production, which inhibits mTOR signaling, mitochondrial ATP synthesis, and neutrophil activation.53 The balance of these signaling networks is essential for proper neutrophil function and an effective host immune defense. Elevated ATP levels in the plasma of sepsis patients interfere with the autocrine purinergic signaling system that regulates neutrophil function. As a result, neutrophils are excessively activated and attack host tissues but are unable to mount coordinate immune responses.54
Purinergic regulation of monocytes, macrophages, and dendritic cells
Similar to neutrophils, macrophages and dendritic cells also require autocrine purinergic signaling for the regulation of chemotaxis. In macrophages, these purinergic feedback loops involve P2Y2, P2Y12, A2a, A2b, and A3 adenosine receptors.55 P2Y2 receptors regulate chemotaxis of immature dendritic cells.56 It was suggested that large amounts of ATP are released from apoptotic cells via panx1 and that this ATP acts as a “find-me” signal to attract macrophages to inflammatory sites and promote the clearance of dead or dying cells.57,58 A direct chemotactic effect of ATP was however questioned by others and it was proposed that ATP promotes non-directed migration instead.59 The release of pro-inflammatory cytokines such as TNFα, IL-1β, and IL-18 by monocytes and macrophages contributes to tissue injury and it has been recognized some time ago that LPS triggers the release of IL-1β from monocytes, macrophages, and dendritic cells in a P2X7 receptor-dependent manner.60-62 There is compelling evidence that P2X7 receptors play a major role in the antibacterial and inflammatory responses of macrophages.10 P2X7 receptors were particularly implicated in the elimination of intracellular bacteria and parasites.63 In addition, it was discovered that P2X7 receptors have a pro-inflammatory role by activating the NOD-like receptor (NLR) mediated inflammasome assembly.64 Inflammasomes are multimeric complexes that regulate the activity of caspase-1, proteolysis of pro-IL-1β and pro-IL-18, and the release of the active forms of these proinflammatory cytokines.65 The activation of the NLRP3 inflammasome requires external ATP and stimulation of P2X7 receptors.66 The exact mechanisms by which P2X7 receptors and NLRP3 activation are connected have not been completely elucidated, however, P2X7-induced K+ efflux has been shown to play a role.67,68 P2X7 receptors have a low affinity for ATP and are activated only in the presence of high extracellular ATP concentrations found at sites of tissue injury. In addition to the stimulatory role of exogenous ATP, recent reports indicate that inflammasome activation and release of inflammatory cytokines following stimulation with LPS may also involve the release of endogenous ATP as an initial event and autocrine stimulation of purinergic receptors.37,69,70 While ATP promotes proinflammatory cell responses in monocytes and macrophages, adenosine contributes to the termination of cell activation. Both A2a and A2b receptors were reported to regulate the release of cytokines such as TNFα, IL-10, and IL-12 from monocytes and macrophages.71-73
Purinergic regulation of T cells
The majority of patients suffering from severe sepsis survive the initial hyper-inflammatory state because of improved clinical management. However, many of these patients develop a state of severe immunosuppression that renders them susceptible to nosocomial infections that are associated with poor outcome and death.16-18 T cell suppression is a hallmark of this immunosuppressive state. However, the exact mechanisms leading to T cell suppression are not well defined. Several lines of evidence indicate that purinergic signaling controls T cells in many different ways. Recently, it was proposed that autocrine purinergic signaling facilitates the signal amplification required for antigen recognition by T cells.32 Antigen recognition involves the formation of an immune synapse between T cells and antigen-presenting cells. Following T cell receptor stimulation, ATP is released through panx1 channels or by vesicular release.38,41,74,75 The released ATP promotes calcium influx through P2X1, P2X4, and P2X7 receptors that can function as calcium channels.38,39,75,76 P2X4 and P2X7 receptors are also involved in the activation of unconventional γδ T cells.77,78 Several components of purinergic signaling, including panx1 channels, P2X1 and P2X4 receptors, accumulate at the immune synapse, suggesting the formation of a powerful purinergic signaling complex.39 Interestingly, mitochondria are part of this complex and they translocate to the immune synapse where they deliver the ATP that stimulates P2X1 and P2X4 receptors and thereby fuel the autocrine purinergic signaling mechanisms in the synaptic cleft.79,80 The role of P2X7 receptors in T cells is somewhat ambiguous. It was shown that they promote T cell activation and proliferation38,39,75,76, however, P2X7 receptors can also induce the lysis and apoptosis of T cells.81,82 These opposing actions of P2X7 receptors are thought to be dependent on the extracellular ATP concentration.83 Numerous studies have shown that adenosine and A2a receptors increase cAMP in T cells, resulting in the suppression of T cell functions.84-86 Different T cell subtypes express different sets of ectonucleotidase isoforms that catalyze the breakdown of ATP to adenosine. Particularly regulatory T cells (Treg) were shown to express high levels of CD39 and CD73, which favors the generation of adenosine from extracellular ATP. Adenosine-mediated suppression of effector cells plays a central role in the inhibitory action of Tregs.87-88
ATP as a danger signal
ATP can be released from cells as a consequence of cell damage and other forms of cell stress such as hypoxia, mechanical, or osmotic stress. Besides the controlled release of ATP via membrane channels like panx1, cell necrosis can lead to the uncontrolled release of large amounts of ATP.10,32 Intracellular ATP levels in the cytoplasm reach millimolar concentrations, whereas normal plasma ATP levels are in the low nanomolar range. Local extracellular ATP levels at sites of cell or tissue damage can therefore rise significantly. It was suggested that ATP acts as a danger molecule or “alarmin”.89 In that role, ATP attracts immune cells to sites of tissue damage and contributes to the activation and amplification of immune responses needed to repair damaged tissues.11,57,58 In that context, particularly attention has been given to P2X7 receptors because the extracellular ATP levels in the microenvironment of dead or dying cells are sufficiently high to induce P2X7 receptor mediated NLRP3 inflammasome activation, which results in the production of the inflammatory cytokines IL-1β and IL-18.64,66,68,90 Recent studies with mice lacking P2X7 receptors indicated that P2X7 receptors have an important role in the outcome of experimental sepsis.91,92 Csóka and colleagues reported that P2X7 receptors on macrophages are crucial for controlling bacterial killing and inflammation and increase survival independently from inflammasome activation.91 However, another recent study showed that mortality and inflammation in response to cecal ligation and puncture (CLP)-induced sepsis were attenuated in the absence of P2X7 receptors.92 These divergent results illustrate the complexity of purinergic signaling in sepsis and indicate that targeting of a single purinergic receptor is unlikely to yield successful therapeutic strategies to treat sepsis.
Systemic ATP in sepsis
Trauma and inflammatory organ injury in septic shock cause severe cell damage, cell lysis, and necrotic cell death. The massive leakage of intracellular nucleotides into the extracellular space results in elevated systemic plasma ATP levels in septic shock.54,91 In an experimental sepsis model, mice subjected to CLP showed a 4- to 6-fold increase in plasma ATP, ADP, and AMP concentrations in the first 8 h after CLP when compared to sham-treated control mice. The increase in plasma ATP levels correlated with neutrophil activation as assessed by CD11b expression.54 Neutrophils have a pivotal role in host defense by killing and eliminating invading bacteria. However, when excessively activated neutrophils can also cause significant collateral host tissue damage. In SIRS and sepsis, uncontrolled neutrophil activation promotes the development of multiple organ dysfunction and failure.93,94 Inhibition of excessive neutrophil activation has therefore been viewed as a desirable strategy to reduce organ damage and improve outcome in sepsis.95 However, this strategy weakens the host’s defenses against bacterial pathogens. Systemic plasma ATP in sepsis reaches low micromolar concentrations54,91, which is well within the range of the ATP concentrations that activate P2Y2 receptors of neutrophils. Addition of ATP to neutrophils migrating in a chemotactic gradient field impairs gradient sensing and directed migration while increasing random motility and the production of oxygen radicals.47 Most likely, this is due to the interference of exogenous ATP with the intrinsic autocrine purinergic signaling mechanisms that regulate chemotaxis. Importantly, neutrophil chemotaxis and migration to the site of infection is impaired in severe sepsis, resulting in increased bacterial load and mortality.96 The mechanisms responsible for this loss of protective neutrophil function are not clear, however increased systemic ATP levels may be responsible by obscuring the endogenous purinergic guidance system of neutrophils. This is supported by the recent finding that treatment of mice with apyrase, an enzyme that catalyzes the breakdown of ATP, attenuated systemic inflammation and improved survival in experimental endotoxemia and polymicrobial sepsis.97-98 Interestingly, the general P2 receptor blocker suramin imitated some of the effects of apyrase treatment and reduced markers of inflammation, however failed to decrease morbidity and mortality.54,97 Furthermore, release of endogenous ATP was shown to be important for bacterial killing.91 These findings underscore the importance of autocrine purinergic signaling mechanisms as regulators of cell activation and immune function and support the concept that disruption of these mechanisms by systemic ATP contributes to inflammatory tissue damage and impaired bacterial clearance in sepsis.
Adenosine in sepsis
Adenosine plays a central role in suppressing immune responses. Like ATP, extracellular adenosine concentrations rise rapidly in response to systemic inflammation and tissue damage.99,100 For septic shock patients, a 10-fold increase in plasma adenosine concentrations was reported.99 This increase was explained by decreased enzymatic activities of adenosine deaminase and adenosine kinases and by an increase in the activity of adenosine-producing CD73 under hypoxic conditions101 and in human experimental endotoxemia102. The immunosuppressive effects of adenosine are mainly ascribed to A2a receptors on immune cells. Inflammatory mediators and endotoxin quickly up-regulate the expression of A2a and A2b receptors.11 Using A2a receptor knock-out mice, it was shown that A2a receptor activation significantly attenuates tissue damage in systemic inflammation.44 However, while dampening of excessive immune cell activation by A2a or A2b receptors may be beneficial in the hyperdynamic initial phase of sepsis and endotoxemia44,103,104, the same receptors can induce immunosuppression. In accordance, inhibition of A2a receptor signaling increased survival in a chronic model of polymicrobial sepsis by improving bacterial clearance, decreasing IL-10 release and preserving lymphocyte function.105 Conversely, A1 and A3 adenosine receptors were suggested to have beneficial effects in sepsis.106,107
Pharmacological targeting of purinergic signaling in sepsis
The profound effects of purinergic signaling on immune cells open up opportunities for novel treatments for sepsis and systemic inflammation. Pharmacological strategies to increase the tissue protective function of adenosine could include drugs that increase the enzymatic breakdown of ATP or that inhibit the enzymatic degradation or uptake of adenosine. There are several subtype-specific adenosine receptor agonists and antagonists available.108 However, due to the ubiquitous expression of purinergic receptors, undesirable side effects in non-target systems are to be expected. Such side effects could be cardiovascular depressive effects caused by A1 or A3 agonists.109 The number of specific agonists or antagonists for the 15 different P2 receptor subtypes is comparatively limited. The complexity of purinergic signaling and the pathophysiology of sepsis, however, raise concerns about the feasibility of therapeutic approaches that focus on modulating a single P1 or P2 receptor subtype.
Given the detrimental effects of systemic ATP in sepsis, strategies aimed at the restoration of normal ATP levels would seem more promising. Possible approaches could involve inhibition of ATP release mechanisms or enhancement of enzymatic ATP breakdown. Recently, two phase IIa clinical trials showed that kidney function improved in critically ill patients with sepsis-associated acute kidney injury who were treated with alkaline phosphatase. The beneficial effects were ascribed to the dephosphorylation of LPS and of ATP that is released by inflamed and hypoxic tissues.110 These results demonstrate the therapeutic potential of targeting external ATP in sepsis. Growing knowledge about the complex paracrine and autocrine purinergic signaling mechanisms that regulate immune cells, but also virtually all other physiological systems associated with sepsis will be needed to widen our understanding and help with the development of effective and targeted treatments for sepsis.
Acknowledgements
This work was supported in part by grants from the National Institutes of Health, GM-51477, GM-60475, AI-080582, and T32GM103702 (W.G.J.), and from the German Research Foundation (DFG), LE-3209/1-1 (C.L.). We thank Laura Staudenmaier for her valuable support in preparing the figure.
Abbreviations
- ADP
adenosine diphosphate
- AMP
adenosine monophosphate
- ATP
adenosine triphosphate
- CLP
cecal ligation and puncture
- DAMPs
damage-associated molecular patterns
- FPR
formyl-peptide receptor
- GPCR
G protein coupled receptor
- IL
interleukin
- mTOR
mammalian target of rapamycin
- NLR
nucleotide-binding oligomerization domain (NOD) like receptors
- panx1
pannexin-1
- PAMPs
pathogen-associated molecular patterns
- SIRS
systemic inflammatory response syndrome
- TLR
Toll-like receptor
- TNF-α
tumor necrosis factor α
- UDP
uridine diphosphate
- UTP
uridine triphosphate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: none
REFERENCES
- 1.Czura CJ. "Merinoff symposium 2010: sepsis"-speaking with one voice. Mol Med. 2011;17:2–3. doi: 10.2119/molmed.2010.00001.commentary. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vincent JL, Opal SM, Marshall JC, Tracey KJ. Sepsis definitions: time for change. Lancet. 2013;381:774–775. doi: 10.1016/S0140-6736(12)61815-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu V, Escobar GJ, Greene JD, et al. Hospital deaths in patients with sepsis from 2 independent cohorts. JAMA. 2014;312:90–92. doi: 10.1001/jama.2014.5804. [DOI] [PubMed] [Google Scholar]
- 4.Fleischmann C, Scherag A, Adhikari NKJ, et al. International Forum of Acute Care Trialists. Assessment of global incidence and mortality of hospital-treated sepsis: current estimates and limitations. Am J Respir Crit Care Med. 2016;193:259–272. doi: 10.1164/rccm.201504-0781OC. [DOI] [PubMed] [Google Scholar]
- 5.Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369:840–851. doi: 10.1056/NEJMra1208623. [DOI] [PubMed] [Google Scholar]
- 6.Cohen J, Vincent JL, Adhikari NK, et al. Sepsis: a roadmap for future research. Lancet Infect Dis. 2015;15:581–614. doi: 10.1016/S1473-3099(15)70112-X. [DOI] [PubMed] [Google Scholar]
- 7.Gupta RG, Hartigan SM, Kashiouris MG, et al. Early goal-directed resuscitation of patients with septic shock: current evidence and future directions. Crit Care. 2015;19:286. doi: 10.1186/s13054-015-1011-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wenzel RP, Edmond MB. Septic shock--evaluating another failed treatment. N Engl J Med. 2012;366:2122–2124. doi: 10.1056/NEJMe1203412. [DOI] [PubMed] [Google Scholar]
- 9.Burnstock G, Fredholm BB, North RA, Verkhratsky A. The birth and postnatal development of purinergic signalling. Acta Physiol. 2010;199:93–147. doi: 10.1111/j.1748-1716.2010.02114.x. [DOI] [PubMed] [Google Scholar]
- 10.Idzko M, Ferrari D, Eltzschig HK. Nucleotide signalling during inflammation. Nature. 2014;509:310–307. doi: 10.1038/nature13085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bours MJ, Swennen EL, Di Virgilio F, et al. Adenosine 5'-triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation. Pharmacol Ther. 2006;112:358–404. doi: 10.1016/j.pharmthera.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 12.Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 13.Nathan C, Ding A. Nonresolving inflammation. Cell. 2010;140:871–882. doi: 10.1016/j.cell.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 14.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 15.Opal SM, Glück T. Endotoxin as a drug target. Crit Care Med. 2003;31(1 Suppl):S57–S64. doi: 10.1097/00003246-200301001-00009. [DOI] [PubMed] [Google Scholar]
- 16.Boomer JS, To K, Chang KC, et al. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA. 2011;306:2594–2605. doi: 10.1001/jama.2011.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ward PA. Immunosuppression in sepsis. JAMA. 2011;306:2618–2619. doi: 10.1001/jama.2011.1831. [DOI] [PubMed] [Google Scholar]
- 18.Hotchkiss RS, Monneret G, Payen D. Immunosuppression in sepsis: a novel understanding of the disorder and a new therapeutic approach. Lancet Infect Dis. 2013;13:260–268. doi: 10.1016/S1473-3099(13)70001-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bulger EM, Hoyt DB. Hypertonic resuscitation after severe injury: is it of benefit? Adv Surg. 2012;46:73–85. doi: 10.1016/j.yasu.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 20.van Haren FM, Sleigh J, Boerma EC, et al. Hypertonic fluid administration in patients with septic shock: a prospective randomized controlled pilot study. Shock. 2012;37:268–275. doi: 10.1097/SHK.0b013e31823f152f. [DOI] [PubMed] [Google Scholar]
- 21.Junger WG, Hoyt DB, Davis RE, et al. Hypertonicity regulates the function of human neutrophils by modulating chemoattractant receptor signaling and activating mitogen-activated protein kinase p38. J Clin Invest. 1998;101:2768–2779. doi: 10.1172/JCI1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Orlic T, Loomis WH, Shreve A, et al. Hypertonicity increases cAMP in PMN and blocks oxidative burst by PKA-dependent and -independent mechanisms. Am J Physiol Cell Physiol. 2002;282:C1261–C1269. doi: 10.1152/ajpcell.00479.2001. [DOI] [PubMed] [Google Scholar]
- 23.Chen Y, Bao Y, Zhang J, et al. Inhibition of neutrophils by hypertonic saline involves pannexin-1, CD39, CD73, and other ectonucleotidases. Shock. 2015;44:221–227. doi: 10.1097/SHK.0000000000000402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Y, Shukla A, Namiki S, et al. A putative osmoreceptor system that controls neutrophil function through the release of ATP, its conversion to adenosine, and activation of A2 adenosine and P2 receptors. J Leukoc Biol. 2004;76:245–253. doi: 10.1189/jlb.0204066. [DOI] [PubMed] [Google Scholar]
- 25.Chaudry IH. ATP-MgCl2 and liver blood flow following shock and ischemia. Prog Clin Biol Res. 1989;299:19–31. [PubMed] [Google Scholar]
- 26.Chaudry IH. Use of ATP following shock and ischemia. Ann N Y Acad Sci. 1990;603:130–141. doi: 10.1111/j.1749-6632.1990.tb37667.x. [DOI] [PubMed] [Google Scholar]
- 27.Chaudry IH. Cellular energetics and ATP-MgCl2 therapy in sepsis. Am J Emerg Med. 1984;2:38–44. doi: 10.1016/0735-6757(84)90108-6. [DOI] [PubMed] [Google Scholar]
- 28.Wang P, Ba ZF, Cioffi WG, et al. Salutary effects of ATP-MgCl2 on the depressed endothelium-dependent relaxation during hyperdynamic sepsis. Crit Care Med. 1999;27:959–964. doi: 10.1097/00003246-199905000-00035. [DOI] [PubMed] [Google Scholar]
- 29.Harkema JM, Chaudry IH. Magnesium-adenosine triphosphate in the treatment of shock, ischemia, and sepsis. Crit Care Med. 1992;20:263–275. doi: 10.1097/00003246-199202000-00015. [DOI] [PubMed] [Google Scholar]
- 30.Burnstock G, Boeynaems JM. Purinergic signalling and immune cells. Purinergic Signal. 2014;10:529–564. doi: 10.1007/s11302-014-9427-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- 32.Junger WG. Immune cell regulation by autocrine purinergic signalling. Nat Rev Immunol. 2011;11:201–212. doi: 10.1038/nri2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abbracchio MP, Burnstock G, Verkhratsky A, Zimmermann H. Purinergic signaling in the nervous system: an overview. Trends Neurosci. 2009;32:19–29. doi: 10.1016/j.tins.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 34.Eltzschig HK, Sitkovsky MV, Robson SC. Purinergic signaling during inflammation. N Engl J Med. 2012;367:2322–2333. doi: 10.1056/NEJMra1205750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eltzschig HK, Eckle T, Mager A, et al. ATP release from activated neutrophils occurs via connexin 43 and modulates adenosine-dependent endothelial cell function. Circ Res. 2006;99:1100–1108. doi: 10.1161/01.RES.0000250174.31269.70. [DOI] [PubMed] [Google Scholar]
- 36.Chen Y, Yao Y, Sumi Y, et al. Purinergic signaling: a fundamental mechanism in neutrophil activation. Sci Signal. 2010;3:ra45. doi: 10.1126/scisignal.2000549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang D, He Y, Muñoz-Planillo R, Liu Q, Núñez G. Caspase-11 requires the pannexin-1 channel and the purinergic P2X7 pore to mediate pyroptosis and endotoxic shock. Immunity. 2015;43:923–932. doi: 10.1016/j.immuni.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schenk U, Westendorf AM, Radaelli E, et al. Purinergic control of T cell activation by ATP released through pannexin-1 hemichannels. Sci Signal. 2008;1:ra6. doi: 10.1126/scisignal.1160583. [DOI] [PubMed] [Google Scholar]
- 39.Woehrle T, Yip L, Elkhal A, et al. Pannexin-1 hemichannel-mediated ATP release together with P2X1 and P2X4 receptors regulate T-cell activation at the immune synapse. Blood. 2010;116:3475–3484. doi: 10.1182/blood-2010-04-277707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Woehrle T, Yip L, Manohar M, et al. Hypertonic stress regulates T cell function via pannexin-1 hemichannels and P2X receptors. J Leukoc Biol. 2010;88:1181–1189. doi: 10.1189/jlb.0410211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tokunaga A, Tsukimoto M, Harada H, et al. Involvement of SLC17A9-dependent vesicular exocytosis in the mechanism of ATP release during T cell activation. J Biol Chem. 2010;285:17406–17416. doi: 10.1074/jbc.M110.112417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yegutkin GG. Nucleotide- and nucleoside-converting ectoenzymes: Important modulators of purinergic signalling cascade. Biochim Biophys Acta. 2008;1783:673–694. doi: 10.1016/j.bbamcr.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 43.Zimmermann H. Extracellular metabolism of ATP and other nucleotides. Naunyn Schmiedebergs Arch Pharmacol. 2000;362:299–309. doi: 10.1007/s002100000309. [DOI] [PubMed] [Google Scholar]
- 44.Ohta A, Sitkovsky M. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature. 2001;414:916–920. doi: 10.1038/414916a. [DOI] [PubMed] [Google Scholar]
- 45.Cronstein BN. Adenosine, an endogenous anti-inflammatory agent. J Appl Physiol. 1994;76:5–13. doi: 10.1152/jappl.1994.76.1.5. [DOI] [PubMed] [Google Scholar]
- 46.Fredholm BB. Purines and neutrophil leukocytes. Gen Pharmacol. 1997;28:345–350. doi: 10.1016/s0306-3623(96)00169-3. [DOI] [PubMed] [Google Scholar]
- 47.Chen Y, Corriden R, Inoue Y, et al. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science. 2006;314:1792–1795. doi: 10.1126/science.1132559. [DOI] [PubMed] [Google Scholar]
- 48.Bao Y, Chen Y, Ledderose C, et al. Pannexin 1 channels link chemoattractant receptor signaling to local excitation and global inhibition responses at the front and back of polarized neutrophils. J Biol Chem. 2013;288:22650–22657. doi: 10.1074/jbc.M113.476283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu J, Wang F, Van Keymeulen A, et al. Divergent signals and cytoskeletal assemblies regulate self-organizing polarity in neutrophils. Cell. 2003;114:201–214. doi: 10.1016/s0092-8674(03)00555-5. [DOI] [PubMed] [Google Scholar]
- 50.Wang F. The signaling mechanisms underlying cell polarity and chemotaxis. Cold Spring Harb Perspect Biol. 2009;1:a002980. doi: 10.1101/cshperspect.a002980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lecut C, Frederix K, Johnson DM, et al. P2X1 ion channels promote neutrophil chemotaxis through Rho kinase activation. J Immunol. 2009;183:2801–2809. doi: 10.4049/jimmunol.0804007. [DOI] [PubMed] [Google Scholar]
- 52.Bao Y, Ledderose C, Seier T, et al. Mitochondria regulate neutrophil activation by generating ATP for autocrine purinergic signaling. J Biol Chem. 201;289:26794–26803. doi: 10.1074/jbc.M114.572495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bao Y, Ledderose C, Graf AF, et al. mTOR and differential activation of mitochondria orchestrate neutrophil chemotaxis. J Cell Biol. 2015;210:1153–1164. doi: 10.1083/jcb.201503066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sumi Y, Woehrle T, Chen Y, et al. Plasma ATP is required for neutrophil activation in a mouse sepsis model. Shock. 2014;42:142–147. doi: 10.1097/SHK.0000000000000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kronlage M, Song J, Sorokin L, et al. Autocrine purinergic receptor signaling is essential for macrophage chemotaxis. Sci Signal. 2010;3:ra55. doi: 10.1126/scisignal.2000588. [DOI] [PubMed] [Google Scholar]
- 56.Müller T, Robaye B, Vieira RP, et al. The purinergic receptor P2Y2 receptor mediates chemotaxis of dendritic cells and eosinophils in allergic lung inflammation. Allergy. 2010;65:1545–1553. doi: 10.1111/j.1398-9995.2010.02426.x. [DOI] [PubMed] [Google Scholar]
- 57.Elliott MR, Chekeni FB, Trampont PC, et al. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature. 2009;461:282–286. doi: 10.1038/nature08296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chekeni FB, Elliott MR, Sandilos JK, et al. Pannexin 1 channels mediate 'find-me' signal release and membrane permeability during apoptosis. Nature. 2010;467:863–867. doi: 10.1038/nature09413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kaufmann A, Musset B, Limberg SH, et al. "Host tissue damage" signal ATP promotes non-directional migration and negatively regulates toll-like receptor signaling in human monocytes. J Biol Chem. 2005;280:32459–32467. doi: 10.1074/jbc.M505301200. [DOI] [PubMed] [Google Scholar]
- 60.Ferrari D, Gorini S, Callegari G, la Sala A. Shaping immune responses through the activation of dendritic cells' P2 receptors. Purinergic Signal. 2007;3:99–107. doi: 10.1007/s11302-006-9024-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Grahames CB, Michel AD, Chessell IP, Humphrey PP. Pharmacological characterization of ATP- and LPS-induced IL-1beta release in human monocytes. Br J Pharmacol. 1999;127:1915–1921. doi: 10.1038/sj.bjp.0702732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mutini C, Falzoni S, Ferrari D, et al. Mouse dendritic cells express the P2X7 purinergic receptor: characterization and possible participation in antigen presentation. J Immunol. 1999;163:1958–1965. [PubMed] [Google Scholar]
- 63.Coutinho-Silva R, Ojcius DM. Role of extracellular nucleotides in the immune response against intracellular bacteria and protozoan parasites. Microbes Infect. 2012;14:1271–1277. doi: 10.1016/j.micinf.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Di Virgilio F. Liaisons dangereuses: P2X(7) and the inflammasome. Trends Pharmacol Sci. 2007;28:465–472. doi: 10.1016/j.tips.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 65.Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Annu Rev Immunol. 2009;27:229–265. doi: 10.1146/annurev.immunol.021908.132715. [DOI] [PubMed] [Google Scholar]
- 66.Qu Y, Ramachandra L, Mohr S, et al. P2X7 receptor-stimulated secretion of MHC class II-containing exosomes requires the ASC/NLRP3 inflammasome but is independent of caspase-1. J Immunol. 2009;182:5052–5062. doi: 10.4049/jimmunol.0802968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kahlenberg JM, Dubyak GR. Mechanisms of caspase-1 activation by P2X7 receptor-mediated K+ release. Am J Physiol Cell Physiol. 2004;286:C1100–C1108. doi: 10.1152/ajpcell.00494.2003. [DOI] [PubMed] [Google Scholar]
- 68.Qu Y, Franchi L, Nunez G, Dubyak GR. Nonclassical IL-1 beta secretion stimulated by P2X7 receptors is dependent on inflammasome activation and correlated with exosome release in murine macrophages. J Immunol. 2007;179:1913–1925. doi: 10.4049/jimmunol.179.3.1913. [DOI] [PubMed] [Google Scholar]
- 69.Piccini A, Carta S, Tassi S, et al. ATP is released by monocytes stimulated with pathogen-sensing receptor ligands and induces IL-1beta and IL-18 secretion in an autocrine way. Proc Natl Acad Sci U S A. 2008;105:8067–8072. doi: 10.1073/pnas.0709684105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Asgari E, Le Friec G, Yamamoto H, et al. C3a modulates IL-1β secretion in human monocytes by regulating ATP efflux and subsequent NLRP3 inflammasome activation. Blood. 2013;122:3473–3481. doi: 10.1182/blood-2013-05-502229. [DOI] [PubMed] [Google Scholar]
- 71.Link AA, Kino T, Worth JA, et al. Ligand-activation of the adenosine A2a receptors inhibits IL-12 production by human monocytes. J Immunol. 2000;164:436–442. doi: 10.4049/jimmunol.164.1.436. [DOI] [PubMed] [Google Scholar]
- 72.Németh ZH, Lutz CS, Csóka B, et al. Adenosine augments IL-10 production by macrophages through an A2B receptor-mediated posttranscriptional mechanism. J Immunol. 2005;175:8260–8270. doi: 10.4049/jimmunol.175.12.8260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kreckler LM, Wan TC, Ge ZD, Auchampach JA. Adenosine inhibits tumor necrosis factor-alpha release from mouse peritoneal macrophages via A2A and A2B but not the A3 adenosine receptor. J Pharmacol Exp Ther. 2006;317:172–180. doi: 10.1124/jpet.105.096016. [DOI] [PubMed] [Google Scholar]
- 74.Filippini A, Taffs RE, Sitkovsky MV. Extracellular ATP in T-lymphocyte activation: possible role in effector functions. Proc Natl Acad Sci U S A. 1990;87:8267–8271. doi: 10.1073/pnas.87.21.8267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yip L, Woehrle T, Corriden R, et al. Autocrine regulation of T-cell activation by ATP release and P2X7 receptors. FASEB J. 2009;23:1685–1693. doi: 10.1096/fj.08-126458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Baricordi OR, Ferrari D, Melchiorri L, et al. An ATP-activated channel is involved in mitogenic stimulation of human T lymphocytes. Blood. 1996;87:682–690. [PubMed] [Google Scholar]
- 77.Manohar M, Hirsh MI, Chen Y, et al. ATP release and autocrine signaling through P2X4 receptors regulate γδ T cell activation. J Leukoc Biol. 2012;92:787–794. doi: 10.1189/jlb.0312121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Frascoli M, Marcandalli J, Schenk U, Grassi F. Purinergic P2X7 receptor drives T cell lineage choice and shapes peripheral γδ cells. J Immunol. 2012;189:174–180. doi: 10.4049/jimmunol.1101582. [DOI] [PubMed] [Google Scholar]
- 79.Ledderose C, Bao Y, Lidicky M, et al. Mitochondria are gate-keepers of T cell function by producing the ATP that drives purinergic signaling. J Biol Chem. 2014;289:25936–25945. doi: 10.1074/jbc.M114.575308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ledderose C, Bao Y, Ledderose S, et al. Mitochondrial dysfunction, depleted purinergic signaling, and defective T cell vigilance and immune defense. J Infect Dis. 2016;213:456–464. doi: 10.1093/infdis/jiv373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Apasov SG, Koshiba M, Chused TM, Sitkovsky MV. Effects of extracellular ATP and adenosine on different thymocyte subsets: possible role of ATP-gated channels and G protein-coupled purinergic receptor. J Immunol. 1997;158(11):5095–5105. [PubMed] [Google Scholar]
- 82.Lépine S, Le Stunff H, Lakatos B, et al. ATP-induced apoptosis of thymocytes is mediated by activation of P2 X 7 receptor and involves de novo ceramide synthesis and mitochondria. Biochim Biophys Acta. 2006;1761:73–82. doi: 10.1016/j.bbalip.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 83.Trabanelli S, Ocadlíková D, Gulinelli S, et al. Extracellular ATP exerts opposite effects on activated and regulatory CD4+ T cells via purinergic P2 receptor activation. J Immunol. 2012;189:1303–1310. doi: 10.4049/jimmunol.1103800. [DOI] [PubMed] [Google Scholar]
- 84.Fredholm BB, Sandberg G, Ernström U. Cyclic AMP in freshly prepared thymocyte suspensions, Evidence for stimulation by endogenous adenosine. Biochem Pharmacol. 1978;27:2675–2682. doi: 10.1016/0006-2952(78)90041-2. [DOI] [PubMed] [Google Scholar]
- 85.Huang S, Apasov S, Koshiba M, Sitkovsky M. Role of A2a extracellular adenosine receptor-mediated signaling in adenosine-mediated inhibition of T-cell activation and expansion. Blood. 1997;90:1600–1610. [PubMed] [Google Scholar]
- 86.Lappas CM, Rieger JM, Linden J. A2A adenosine receptor induction inhibits IFN-gamma production in murine CD4+ T cells. J Immunol. 2005;174:1073–1080. doi: 10.4049/jimmunol.174.2.1073. [DOI] [PubMed] [Google Scholar]
- 87.Deaglio S, Dwyer KM, Gao W, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Antonioli L, Pacher P, Vizi ES, Haskó G. CD39 and CD73 in immunity and inflammation. Trends Mol Med. 2013;19:355–367. doi: 10.1016/j.molmed.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Oppenheim JJ, Yang D. Alarmins: chemotactic activators of immune responses. Curr Opin Immunol. 2005;17:359–365. doi: 10.1016/j.coi.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 90.Chen Q, Jin Y, Zhang K, et al. Alarmin HNP-1 promotes pyroptosis and IL-1β release through different roles of NLRP3 inflammasome via P2X7 in LPS-primed macrophages. Innate Immun. 2014;20:290–300. doi: 10.1177/1753425913490575. [DOI] [PubMed] [Google Scholar]
- 91.Csóka B, Németh ZH, Törő G, et al. Extracellular ATP protects against sepsis through macrophage P2X7 purinergic receptors by enhancing intracellular bacterial killing. FASEB J. 2015;29:3626–3637. doi: 10.1096/fj.15-272450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Santana PT, Benjamim CF, Martinez CG, et al. The P2X7 receptor contributes to the development of the exacerbated inflammatory response associated with sepsis. J Innate Immun. 2015;7:417–427. doi: 10.1159/000371388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Grommes J, Soehnlein O. Contribution of neutrophils to acute lung injury. Mol Med. 2011;17:293–307. doi: 10.2119/molmed.2010.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Perl M, Hohmann C, Denk S, et al. Role of activated neutrophils in chest trauma-induced septic acute lung injury. Shock. 2012;38:98–106. doi: 10.1097/SHK.0b013e318254be6a. [DOI] [PubMed] [Google Scholar]
- 95.Lewis SM, Khan N, Beale R, et al. Depletion of blood neutrophils from patients with sepsis: treatment for the future? Int Immunopharmacol. 2013;17:1226–1232. doi: 10.1016/j.intimp.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 96.Alves-Filho JC, de Freitas A, Spiller F, et al. The role of neutrophils in severe sepsis. Shock. 2008;30(Suppl 1):3–9. doi: 10.1097/SHK.0b013e3181818466. [DOI] [PubMed] [Google Scholar]
- 97.Cauwels A, Rogge E, Vandendriessche B, et al. Extracellular ATP drives systemic inflammation, tissue damage and mortality. Cell Death Dis. 2014;5:e1102. doi: 10.1038/cddis.2014.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Csóka B, Németh ZH, Törő G, et al. CD39 improves survival in microbial sepsis by attenuating systemic inflammation. FASEB J. 2015;29:25–36. doi: 10.1096/fj.14-253567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Martin C, Leone M, Viviand X, et al. High adenosine plasma concentration as a prognostic index for outcome in patients with septic shock. Crit Care Med. 2000;28:3198–3202. doi: 10.1097/00003246-200009000-00014. [DOI] [PubMed] [Google Scholar]
- 100.Ramakers BP, Riksen NP, van den Broek P, et al. Circulating adenosine increases during human experimental endotoxemia but blockade of its receptor does not influence the immune response and subsequent organ injury. Crit Care. 2011;15:R3. doi: 10.1186/cc9400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kobayashi S, Zimmermann H, Millhorn DE. Chronic hypoxia enhances adenosine release in rat PC12 cells by altering adenosine metabolism and membrane transport. J Neurochem. 2000;74:621–632. doi: 10.1046/j.1471-4159.2000.740621.x. [DOI] [PubMed] [Google Scholar]
- 102.Ramakers BP, Wever KE, Kox M, et al. How systemic inflammation modulates adenosine metabolism and adenosine receptor expression in humans in vivo. Crit Care Med. 2012;40:2609–2616. doi: 10.1097/CCM.0b013e318259205b. [DOI] [PubMed] [Google Scholar]
- 103.Sullivan GW, Fang G, Linden J, Scheld WM. A2A adenosine receptor activation improves survival in mouse models of endotoxemia and sepsis. J Infect Dis. 2004;189:1897–1904. doi: 10.1086/386311. [DOI] [PubMed] [Google Scholar]
- 104.Schingnitz U, Hartmann K, Macmanus CF, et al. Signaling through the A2B adenosine receptor dampens endotoxin-induced acute lung injury. J Immunol. 2010;184:5271–5279. doi: 10.4049/jimmunol.0903035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Németh ZH, Csóka B, Wilmanski J, et al. Adenosine A2A receptor inactivation increases survival in polymicrobial sepsis. J Immunol. 2006;176:5616–5626. doi: 10.4049/jimmunol.176.9.5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gallos G, Ruyle TD, Emala CW, Lee HT. A1 adenosine receptor knockout mice exhibit increased mortality, renal dysfunction, and hepatic injury in murine septic peritonitis. Am J Physiol Renal Physiol. 2005;289:F369–F376. doi: 10.1152/ajprenal.00470.2004. [DOI] [PubMed] [Google Scholar]
- 107.Lee HT, Kim M, Joo JD, et al. A3 adenosine receptor activation decreases mortality and renal and hepatic injury in murine septic peritonitis. Am J Physiol Regul Integr Comp Physiol. 2006;291:R959–R969. doi: 10.1152/ajpregu.00034.2006. [DOI] [PubMed] [Google Scholar]
- 108.Haskó G, Linden J, Cronstein B, Pacher P. Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nat Rev Drug Discov. 2008;7:759–770. doi: 10.1038/nrd2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Law WR. Adenosine receptors in the response to sepsis: what do receptor-specific knockouts tell us? Am J Physiol Regul Integr Comp Physiol. 2006;291:R957–R958. doi: 10.1152/ajpregu.00412.2006. [DOI] [PubMed] [Google Scholar]
- 110.Peters E, Heemskerk S, Masereeuw R, Pickkers P. Alkaline phosphatase: a possible treatment for sepsis-associated acute kidney injury in critically ill patients. Am J Kidney Dis. 2014;63:1038–1048. doi: 10.1053/j.ajkd.2013.11.027. [DOI] [PubMed] [Google Scholar]