Abstract
Antibody maturation as well as memory B and plasma cell differentiation occur primarily in the germinal centers (GC). Systemic lupus erythemotosus (SLE) may develop as a result of enhanced GC activity. Previous studies have shown that the dysregulated signal transducer and activator of transcription 3 (STAT3) pathway is linked to lupus pathogenesis. However, the exact role of STAT3 in regulating SLE disease progression has not been fully understood. Here, we demonstrated that the STAT3 signaling in B cells is essential for the GC formation and maintenance as well as antibody response. Increased cell apoptosis and down-regulated Bcl-xL and Mcl-1 anti-apoptotic gene expression were found in the STAT3 deficient GC B cells. The Tfh cell response positively correlated with the GC B cells and was significantly decreased in immunized B cell STAT3-deficient mice. STAT3 deficiency also led to the defect of plasma cell differentiation. Furthermore, STAT3 deficiency in autoreactive B cells resulted in decreased autoantibody production. Results obtained from B-cell STAT3 deficient B6.MRL/lpr mice suggest that STAT3 signaling significantly contributes to the SLE pathogenesis by regulation of the GC reactivity, autoantibody production, and kidney pathology. Our findings provide new insights into the role of STAT3 signaling in the maintenance of the GC formation and GC B cell differentiation and identify STAT3 as a novel target for the treatment of SLE.
Introduction
Systemic lupus erythematosus (SLE) is a systemic autoimmune disease characterized by numerous types of autoantibody (autoAb) and multi-organ involvement (1). Autoreactive B cell activation and differentiation into Ab-secreting plasma cells play important roles in the etiology of SLE (2). Although increased understanding of the mechanisms underlying the pathogenesis of SLE has provided the foundation for novel treatments, such as B-cell depletion and B cell modulation (3, 4), the development of novel therapy for lupus has been challenging because of the heterogeneity of the disease. There is appreciable interest in developing better strategies to constrain autoAb production.
Ab maturation as well as memory B and plasma cell differentiation occur primarily in the germinal centers (GCs). GCs are unique microenvironment that has proliferative B cells undergoing class switching, somatic hypermuation (SHM), and affinity maturation. Although alternative pathways exist, GCs are the major source of long-lived Ab-secreting plasma cells and memory B cells (5–8). It has become clear that SLE may develop as a result of enhanced GC activity because the pathogenic autoAbs are high affinity, somatically mutated, and Ig-switched (2, 9, 10). Many factors involved in establishing GCs, including follicular helper T cells (Tfh), IL-21, and IL-6, also play critical roles in lupus pathogenesis (11, 12). These inflammatory cytokines are elevated in the sera of SLE patients (13, 14), and predominantly activate the signal transducer and activator of transcription 1 (STAT1) and STAT3 signaling pathways. Dysregulation of the STAT3 pathway has been implicated in lupus pathogenesis (15–17). For example, STAT3 mRNA and phosphorylation of STAT3 (pSTAT3) are increased in B cells of NZB/NZW F1 lupus mice (18). In B6.Sle1ab mice, STAT3 and ras-ERK signaling pathways are aberrantly activated in B cells (19). Active SLE patients also have abnormal GC reactions and an increased number of circulating CD27+ plasma cells (20). Therefore, inhibition of the GC process may provide a novel strategy for the successful intervention of SLE.
Despite those studies, the role of STAT3 in GC B cell response has been controversial. A previous study has demonstrated that B cell-specific STAT3-deficient mice have normal B cell development and normal levels of serum IgM, IgG, and IgA, but the T-dependent IgG response is significant lower compared with those in control mice (21). In addition, they showed that these mice displayed normal GC formation and suggested that the requirement for STAT3 in B cell response was limited to plasma cell differentiation (21). Paradoxically, GC is the major source of long-lived plasma cells. One caveat of this study is that they only examined GC response at one time point (day 12). Human subject studies with STAT3 mutated patients have demonstrated that STAT3 is required for memory B cell generation (11). In addition, human naïve and memory B cells have distinct requirements for STAT3 activation to differentiate into Ab-secreting plasma cells (22). Therefore, it is still unknown whether STAT3 signaling is critical in maintaining the GC formation and GC B cell differentiation. In the current studies, we sought to determine the role of STAT3 signaling in the maintenance of GC reaction. Furthermore, we examined how STAT3 signaling regulates autoreactive B cell activation and lupus pathogenesis using B6.MRL/lpr mice as a murine model of lupus.
Materials and Methods
Mice
C57BL/6 B-cell STAT3 KO mice (STAT3fl/flCD19Cre/+) were generated by interbreeding STAT3flox/flox CD19+/+ mice (23) (control) with STAT3+/+CD19Cre/Cre mice (Jackson Laboratory). B-cell STAT3 KO mice were also intercrossed to anti-snRNP Ig Tg mice (24) and C57BL/6 MRL.Faslpr mice (Jackson) to generate anti-snRNP Ig Tg B-cell STAT3 KO mice and B6.MRL/lpr B-cell STAT3 KO mice, respectively. All animals were maintained under specific pathogen–free conditions and handled in accordance with the protocols approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Louisville.
Immunizations and Ab detection
STAT3 control and KO mice were immunized by intraperitoneal (i.p.) injection with 200 μL human red blood cells (hRBCs) or subcutaneous injection with 50 μg ovalbumin (OVA) in complete Freund’s adjuvant (CFA, Sigma-Aldrich). For apoptotic cell immunization, anti-snRNP Ig Tg STAT3 KO and control mice (6–8 weeks old) were immunized by intravenous injection of 2×107 apoptotic thymocytes weekly for 9 weeks. The sera were collected at various time points after immunization. The levels of anti-OVA and anti-snRNP were measured by ELISA. Anti-dsDNA was measured by ELISA kit (Signosis, Sunnyvale, CA). Antinuclear antibody (ANA) was detected by indirect immunofluorescence. Briefly, serum samples were diluted 1:20 in PBS and placed onto HEp-2 cell-coated slides (Antibodies Incorporated, Davis, CA) and incubated for 1 h. Each slide was washed with PBS before addition of FITC conjugated anti-mouse IgG (eBioscience, San Diego, CA). Images were obtained using Nikon ECLIPSE Ti fluorescence microscopy.
Abs and flow cytometry
Fluochrome-labeled mAbs against CD45R/B220, CD19, CD95, GL7, CD4, CXCR5, PD1, CD40, CD80, CD86, CD138 and cytokines IL-6, IL-12 and IFN-γ were purchased from BioLegend (San Diego, CA). Single-cell suspensions were blocked in the presence of anti-CD16/CD32 at 4°C for 15 min and stained on ice with the appropriate Abs and isotype controls. For cytokine intracellular staining, cells were stimulated with PMA/ionomycin for 5 h and then stained with surface markers, fixed and permeabilized for intracellular cytokine staining including IL-6, IL-12, and IFN-γ. The samples were acquired using FACSCalibur cytometer (BD Bioscience, San Jose, CA) and analyzed using FlowJo software (Tree Star, Ashland, OR).
Immunohistochemistry and histopathological staining
To detect GC formation in the spleens of immunized mice, PNA immunohistochemistry staining was performed using biotinylated PNA (Vector Laboratories, Burlingame, CA) as described previously (25). Kidneys from B6.MRL/lpr STAT3 control and KO mice were either snap-frozen in OCT compound for cryostat sections or fixed in 10% neutral-buffered formalin. For detection of IgG and C3 deposition, cryostat sections were incubated with FITC-anti-mouse IgG and C3 for 1 h. Routine H&E staining was performed on formalin-fixed, paraffin-embedded sections of kidney.
Cell cycle and apoptosis analysis
Splenocytes were stained with anti-mouse GL7 followed by Vybrant DyeCycle Green (Life Technologies) staining at 37°C for 30 min. Phase distribution of GC B cells was analyzed by flow cytometry. For detection of GC B cells apoptosis, fresh isolated splenocytes were stained with Annexin V and 7-AAD after surface staining and analyzed by flow cytometry.
B cell isolation and in vitro stimulation
Splenic B cells were purified by negative selection using CD43 MACS beads (Miltenyi Biotech). Purified B cells (5×105 cells/well) were stimulated with 10 μg/ml LPS from Escherichia coli (O111:B4; Sigma-Aldrich) or 10 μg/ml LPS plus 10 ng/ml IL-4 (PeproTech).
Quantitative real-time PCR
Splenic GC B cells were sorted by FACSAria III. Total RNA was prepared with TriZol (Life Technologies) and RNeasy Mini kit (Qiagen, Valencia, CA). After reverse transcription into cDNA with a Reverse Transcription Kit (Bio-Rad), qPCR was then performed on Bio-Rad MyiQ single color RT-PCR detection system using SYBR Green Supermix (Bio-Rad). We normalized gene expression level to β-2 microglobulin (β-MG) housekeeping gene and represented data as fold differences by the 2−ΔΔCt method, where ΔCt=Cttarget gene-Ctβ-MG and ΔΔCt=ΔCtinduced-ΔCtreference. Primer sequences were listed in the supplemental Table S1.
Statistical analysis
Data are expressed as mean ± SEM. Statistical significance of difference was determined by the unpaired two-tailed student’s t test. Differences were considered significant for p < 0.05. Statistical analysis was performed using Prism 5.0 (GraphPad Software).
Results
STAT3 is essential for the maintenance of GC B cell response and GC formation upon particular Ag hRBC immunization
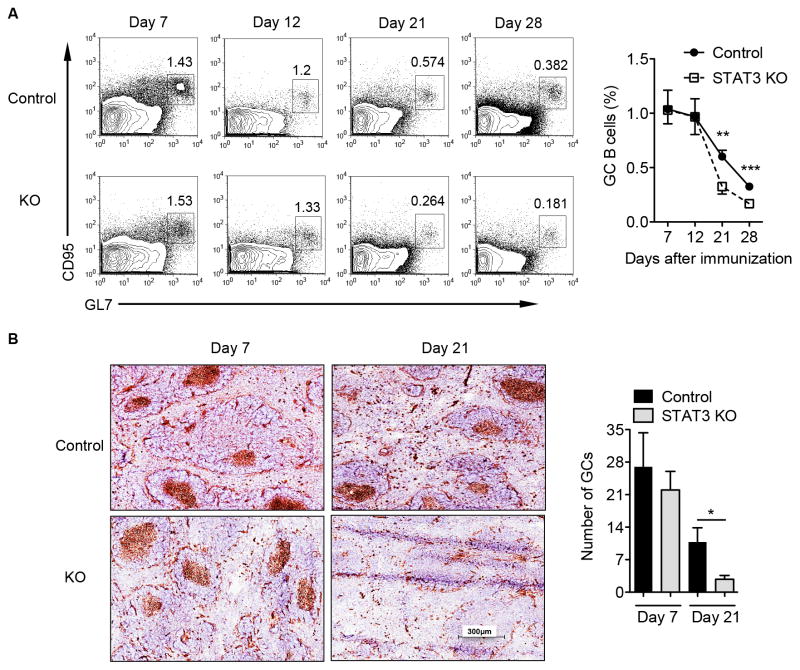
A previous study has concluded that STAT3 signaling in B cells is not essential for the GC formation and development upon immunization with hRBC (21). However, this work only examined the GC formation at day 12. It has become evident that the GC responses can persist for a long period of time (26). Furthermore, prolonged GC reaction has been shown to enhance pathogenic autoAbs production (10). Thus, we reassessed the influence of STAT3 on both the formation and maintenance of the GC B cell reaction at different timepoints. STAT3 was specifically deleted in B cells but not in non-B cell populations (Supplemental Fig. 1A). In addition, STAT3 deficiency in B cells did not alter B cell development in the bone marrow (Supplemental Fig. 1B) and different B cell subsets in the periphery (Supplemental Fig. 1C). Subsequently, B cell-STAT3 control and KO mice were immunized i.p. with hRBC. Consistent with the previous report (21), the GC B cells in the B cell STAT3 KO mice were comparable to those in control mice at days 7 and 12 after immunization (Fig. 1A). However, the GC B cells were substantially decreased in B cell-STAT3 KO mice at days 21 and 28 compared to those in control mice (Fig. 1A). Staining of splenic tissue sections revealed that the GC formation developed normally at day 7 and then rapidly disappeared at late stage (Fig. 1B). The results strongly suggest that STAT3 is critical for the maintenance, but not for the initiation of the GC reaction in this immunization protocol.
Figure 1.
STAT3 signaling in B cells is required for the GC B cell maintenance upon hRBC immunization. B cell-specific STAT3 KO and control mice were immunized i.p. with 0.2 ml of 50% v/v hRBC. (A) Splenocytes were stained with B220, CD95, and GL7 Abs and cells were gated on B220+ population. Representative dot plots are shown. Summarized data are presented as mean ± SEM and pooled from three experiments (n = 8–19 mice per group). (B) Immunohistochemical staining for peanut agglutinin (PNA, brown) of spleens from control and STAT3 KO mice obtained on days 7 and 21 after immunization. Summarized data are presented as mean ± SEM (n=2–5 mice per group). *p<0.05, **p<0.01, ***p<0.001.
STAT3 deficiency in B cells impairs GC formation in response to OVA/CFA immunization
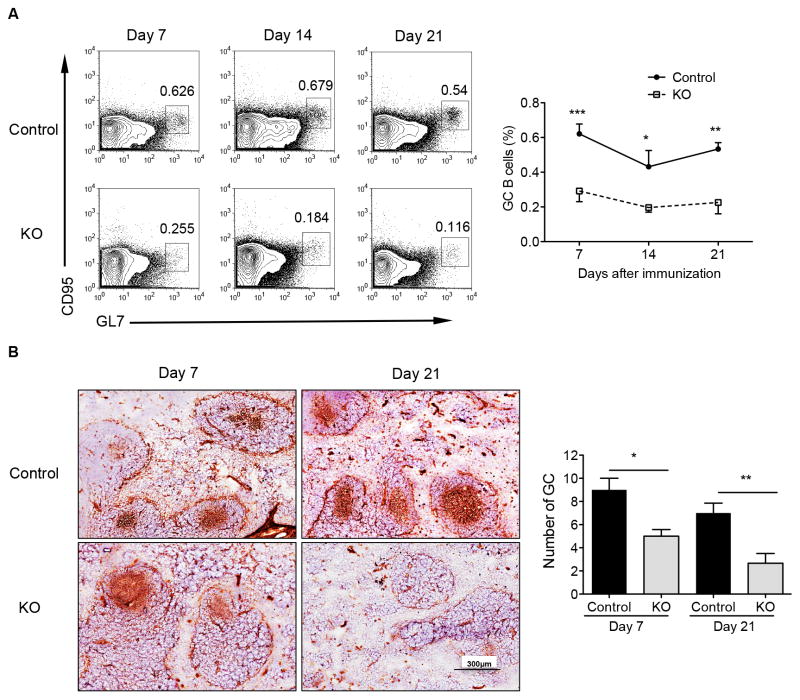
To determine whether this finding is the general phenotype in those mice, B cell STAT3 control and KO mice were immunized s.c. with TD-Ag OVA in CFA. As shown in Fig. 2A, fewer GC B cells were generated at all time points in KO mice as compared with those in control mice. Accordingly, histological analysis of spleen sections demonstrated impaired GC formation in KO mice compared to those in control mice (Fig. 2B). These data suggest that the STAT3 signaling in B cells is essential for both the initiation and maintenance of the GC formation upon TD-Ag immunization.
Figure 2.
STAT3 signaling in B cells is essential for the GC formation and GC B cell maintenance after immunization with OVA with adjuvant CFA. Control and STAT3 KO mice were immunized s.c. with 50 μg OVA in CFA. (A) Splenocytes were stained with B220, CD95, and GL7 Abs and cells were gated on B220+ population. Representative dot plots are shown. Summarized data are presented as mean ± SEM and pooled from three experiments (n = 10–17 mice per group). (B) Immunohistochemical staining for the GC marker PNA (brown) of spleens from control and STAT3 KO mice obtained on days 7 and 21 after immunization. Summarized data are presented as mean ± SEM (n=4–6 mice per group). *p<0.05, **p<0.01, ***p<0.001.
STAT3 deficiency in B cells promotes GC B cell apoptosis
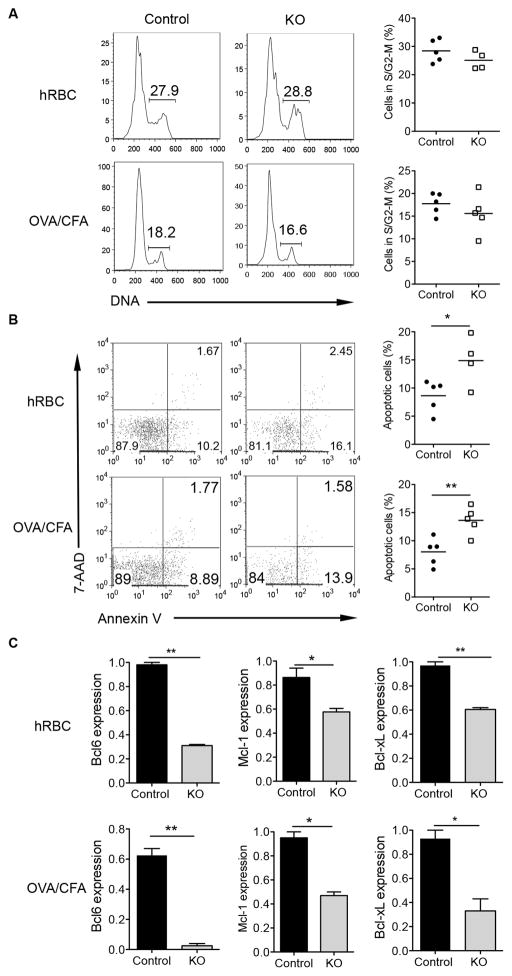
GC B cells proliferate and die quickly, and alterations in numbers of GC B cells may be due to inefficient proliferation and/or impaired cell survival (5). We thus assessed the proliferation and apoptosis of GC B cells. As shown in Fig. 3A, the fraction of STAT3 KO GC B cells in the S/G2-M phase was comparable with that of control GC B cells in both immunization protocols. However, GC B cells exhibited increased apoptosis in the absence of STAT3 (Fig. 3B). However, increased apoptosis in STAT3 KO GC B cells only occurred in the later time points of immunization but not at day 7 (data not shown).
Figure 3.
GC B cell apoptosis is increased in the absence of STAT3 signaling. Control and STAT3 KO mice were immunized with hRBC or OVA in CFA. Spleens were collected at day 21 and a single cell suspension was prepared. (A) Splenocytes were stained with anti-mouse GL7 followed by staining with Vybrant DyeCycle Green. DNA content of GC B cells was analyzed by flow cytometry. Numbers in plot indicate the percent of cells in the phase of S/G2-M. Each symbol represents an individual mouse. (B) GC B cell apoptosis was measured by staining of Annexin V and 7-AAD. Cells were gated on the GC B cells. (C) qRT-PCR for the expression of Bcl6, Mcl-1 and Bcl-xL in the GC B cells sorted from immunized mice. Data are representative of at least three independent experiments. *p<0.05, **p<0.01.
The anti-apoptotic protein Mcl-1 and Bcl-xL expression is mainly regulated by the JAK/STAT3 pathway. Previous studies have shown that Mcl-1 and Bcl-xL molecules play critical roles in the formation and persistence of GCs (27). Transcription factor Bcl6 is a transcriptional repressor that is essential for the development of GC B cells and Tfh cells (28, 29). The expression of Bcl6 in GCs is maintained by IL-21, and STAT3 can bind to Bcl6 promoter and activate its expression in cancer cells and Tfh cells (30). Therefore, we sought to determine the mRNA expression levels of Mcl-1, Bcl-xL, and Bcl6 in GC B cells. We observed the lower expression of these genes in the GC B cells from STAT3-deficient mice at day 21 after hRBC or OVA/CFA immunizations (Fig. 3C). These results suggest that STAT3 signaling in B cells is vital in maintaining the GC B cell survival by regulation of Mcl-1, Bcl-xL and Bcl6 expression.
STAT3 deficiency in B cells results in reduced frequency of Tfh cells, decreased Ab production and plasma cell differentiation
Interactions between B cells and Tfh cells are critical for the GC formation and maintenance (8, 31). A recent study demonstrated that GC B cells are required for the maintenance of Tfh cell phenotype (32). We therefore investigated whether STAT3 signaling in B cells impacts Tfh differentiation. There were significant decreases in the frequency of Tfh cells in the absence of STAT3 signaling in B cells on day 21 (Supplemental Fig. 2), which is correlated with decreased GC B cells (Figs 1 & 2).
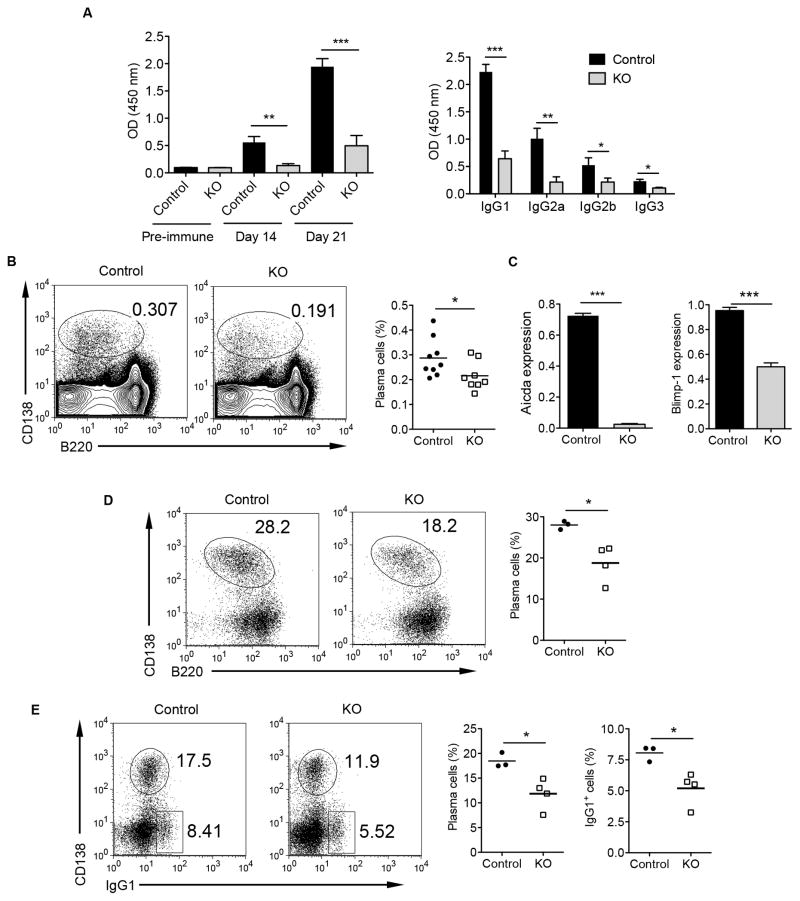
Next, we examined Ab response in OVA/CFA immunized mice. The anti-OVA IgG and isotype IgG Abs were markedly decreased in STAT3 KO mice compared to that of the control mice (Fig. 4A), suggesting that the STAT3 signaling in B cells may control humoral immunity by regulation of GC reaction and the GC B cell differentiation into plasma cells. To further support this notion, we compared CD138+ plasma cells from OVA/CFA immunized STAT3 control and KO mice. As shown in Fig. 4B, splenic plasma cells were significantly reduced in the immunized STAT3 KO mice compared with those in control mice on day 21. Plasma cell differentiation and Ig isotype as well as SHM are initiated by transcription factor Blimp1. In addition, Ig isotype and SHM are initiated by activation-induced cytidine deaminase (Aicda) (33). We thus measured the Blimp-1 and Aicda mRNA expression in GC B cells and found that Blimp-1 and Aicda mRNA expression levels were both down-regulated in STAT3 deficient GC B cells (Fig. 4C). In accordance with the impaired generation of plasma cells in vivo, STAT3 deficient B cells developed fewer CD138highB220low plasmablasts in vitro compared with control B cells in response to LPS stimulation (Fig. 4D). In addition, cultures of STAT3 deficient B cells with LPS and IL-4 generated lower numbers of plasma cells and IgG1 switched B cells (Fig. 4E) compared to control B cells. These results suggest that the impaired humoral response in STAT3 deficient mice is due to impaired GC B cell formation and maintenance leading to reduced plasma cell generation and subsequent Ab isotype switching.
Figure 4.
STAT3 signaling in B cells regulates IgG Ab production and plasma cell differentiation. (A) Serum samples from OVA/CFA immunized mice were assessed for OVA IgG and IgG isotype Ab levels were measured by ELISA. Sera were diluted at 1:10,000 or 1:100 (IgG3). (B) Flow cytometric analysis of splenic plasma cells at day 21 after immunization. Representative dot plots and summarized data (n=8–9) are shown. (C) qRT-PCR for the expression of Aicda and Blimp-1 in the sorted GC B cells from immunized mice. (D) Purified B cells from control and KO mice (n=3–4) were stimulated in vitro with LPS (10 μg/ml) for 3 days and then analyzed for CD138 and B220 expression. (E) Purified B cells were stimulated in vitro with LPS+IL-4 (10 ng/ml) and analyzed 4 days later for CD138 and IgG1 expression by flow cytometry. Summarized data on the percentages of plasma cells and IgG1+ cells are presented. Data are representative of at least two independent experiments. *p<0.05, **p<0.01, ***p<0.001.
STAT3 deficiency in autoreactive B cells decreases autoAb production and GC B cell reaction
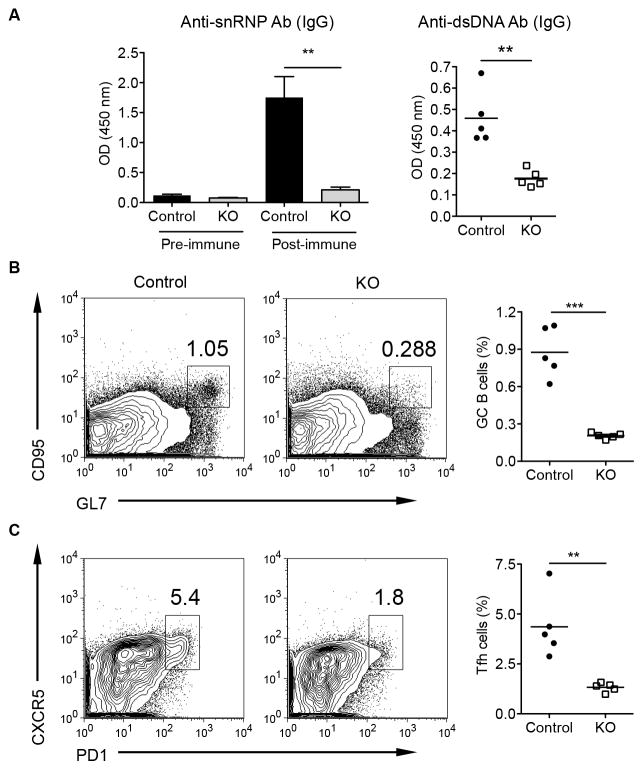
One of the hallmarks in SLE is the breakdown of autoreactive B cell tolerance with large amounts of autoAb production. We thus asked whether STAT3 signaling in B cells is essential for autoAb response. Apoptotic cells are considered to be the major source of autoAgs that can efficiently stimulate B cells (34, 35). We used anti-snRNP Ig Tg mice in which approximately 30% of B cells bind to autoAg snRNP (24). Although anti-snRNP autoreactive B cells of normal background mice do not produce autoAb spontaneously, they are not deleted in the periphery (36). The anti-snRNP Ig Tg B cell-STAT3 KO and control mice were immunized by intravenous injection of apoptotic thymocytes weekly for 9 weeks. As shown in Fig. 5A, anti-snRNP and anti-dsDNA autoAbs were significantly decreased in STAT3 KO mice as compared to those in control mice. In addition, the percentages of GC B cells and Tfh cells were also markedly decreased in STAT3 KO mice (Figs 5B and C). These results suggest that the STAT3 signaling is also critical in autoreactive B cell response and GC formation, which may contribute to the pathogenesis of lupus.
Figure 5.
Depletion of STAT3 signaling in B cells down-regulates autoAb production. Anti-snRNP Ig Tg control and STAT3 KO mice of C57Bl/6 background (n=5) were immunized by intravenous injection of 2×107 apoptotic thymocytes weekly for 9 weeks. (A) Sera were collected at day 7 after last injection, and autoantibodies anti-snRNP IgG and anti-dsDNA IgG in serum (1:10 dilution in PBS) were measured by ELISA. (B and C) Splenocytes were collected and the frequencies of the GC B cells (B, B220+ population) and Tfh cells (C, gated on CD4+ population) were analyzed by flow cytometry. Data are representative of two independent experiments. **p<0.01, ***p<0.001.
Lupus mice with B cell STAT3 deficiency have significantly reduced autoAb production and kidney pathology
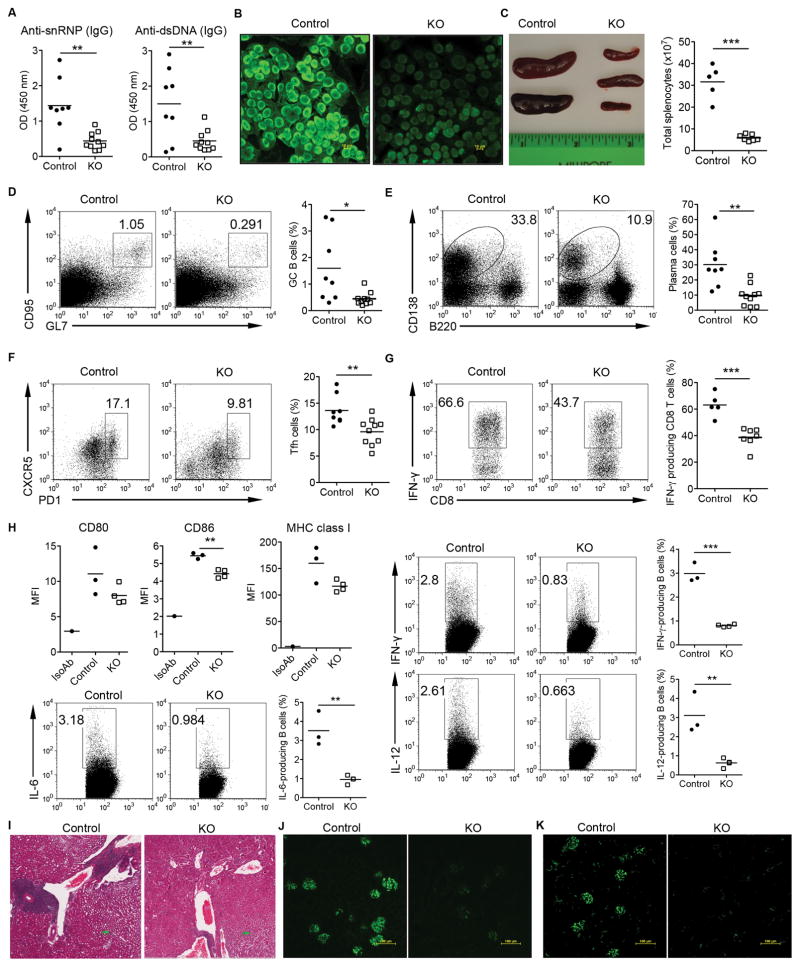
To better understand the impact of STAT3 deficiency in B cells on the pathogenesis of lupus, B6.MRL/lpr murine lupus model was used. We generated B6.MRL/lpr B cell-STAT3 KO mice and first assessed the production of autoAbs in those mice. As shown in Figs 6A and B, anti-snRNP, anti-dsDNA, and ANA autoAbs were all significantly reduced in 8 months old B6.MRL/lpr B cell-STAT3 KO mice as compared to these in control mice. Total cellularity in spleens was also drastically decreased in B6.MRL/lpr STAT3 KO mice (Fig. 6C). In addition, the percentages GC B cells (Fig. 6D) and plasma cells (Fig. E) were significantly reduced in B6.MRL/lpr B cell-STAT3 KO mice compared with control mice. In contrast, GC B cells, plasma cells, and Tfh cells were comparable in 10 wks old control and KO B6.MRL/lpr mice (Supplemental Fig. S3A).
Figure 6.
Depletion of STAT3 signaling in B cells protects B6.MRL/lpr lupus-prone mice from developing autoAbs, splenomegaly, and lupus nephritis. (A) AutoAbs of snRNP IgG (1:20 dilution) and dsDNA IgG (1:50 dilution) were measured from 8-mo-old B6. MRL/lpr control and B cell STAT3 KO mice. (B) Representative ANA staining of a control and a STAT3 KO mouse serum (1:20). (C) Representative spleens and the total cellular numbers in the spleens from control and STAT3 KO mice are shown. (D, E, and F) The frequencies of the GC B cells, CD138+ plasma cells, and Tfh were analyzed by flow cytometry. The percentages of GC B cells (gated on CD19+ population), CD138+ cells, and Tfh cells (gated on CD4+ population) are shown. (G) Splenocytes from aged control and STAT3 KO mice were stimulated with PMA/Ionomycin and then stained for intracellular IFN-γ. Representative dot plots and summarized data are shown. (H) Splenocytes from 6-mo-old control and STAT3 KO mice were stained for surface markers expression and then stimulated with PMA/Inomycin for intracellular cytokine staining. Representative dot plots and summarized data are shown. Cells were gated on Cd19+ population. (I) A representative image of H&E-stained kidney sections from control and STAT3 KO mice. Scale bars: 200 μm. (J and K) Kidney sections were probed with FITC anti-mouse IgG or anti-mouse C3 to detect IgG (J) and C3 deposition (K) in the glomeruli of aged mice. *p<0.05, **p<0.01, ***p<0.001.
Accumulation of Tfh and IFN-γ–producing T cells is a prominent feature in B6.MRL/lpr mice. We found that the frequency of Tfh was significantly decreased in KO mice (Fig. 6F). In addition, IFN-γ–producing CD8+ T cells were profoundly decreased in the STAT3 KO B6.MRL/lpr mice (Fig. 6G). To gain mechanistic insights into this regulation, surface marker expression and cytokine profiles on B cells were examined. We found that CD86 was significantly downregulated in KO B cells. MHC class I expression was also trending low. In addition, KO B cells secreted significantly lower levels of IL-6, IL-12, and IFN-γ (Fig. 6H). Since B6.MRL/lpr mice have Fas apoptosis signaling defect, we next examined whether the loss of the STAT3 in B cells is still associated with GC B cell survival mechanism. To this end, GC B cells were sorted from 6 months old control and KO B6.MRL/lpr mice. As shown in Supplemental Fig. S3B, Bcl-xL mRNA expression level was significantly lower in KO GC B cells while Mcl-1 and Bcl-6 were comparable. Finally, we examined whether the STAT3 signaling in B cells contributes to renal pathology in B6.MRL/lpr mice. As shown in Fig. 6I, inflammatory cell infiltrates were dramatically reduced in the kidneys of B6.MRL/lpr B cell-STAT3 KO mice compared with control mice. In addition, IgG (Fig. 6J) and C3 (Fig. 6K) deposition was readily observed in the glomeruli of control mice while kidneys obtained from B6.MRL/lpr B cell-STAT3 KO mice had significantly reduced levels of IgG and C3 deposition, suggesting that the STAT3 signaling in B cells plays critical roles in lupus pathogenesis.
Discussion
Upon TD-Ag stimulation, the predominant pathways of B cell responses include: 1) to differentiate into extrafollicular plasma cells and 2) to form GCs within the B cell follicle. The extrafollicular plasma cells are short-lived and produce low affinity Abs, whereas the end products of GCs are memory B cells and long-lived plasma cells which are capable of generating high-affinity Abs (8, 37). Although a previous study showed that B cell STAT3 deficient mice displayed normal GC formation upon hRBC immunization (21), we demonstrated that depletion of STAT3 in B cells resulted in fewer GCs and GC B cells, particularly in the later time points. This phenotype was demonstrated using three different immunization protocols (hRBC, OVA/CFA, and apoptotic cells). Our data also suggest that decreased IgG Ab response is due to defect in the GC B cell response and further differentiation into plasma cells. Furthermore, we showed that the GC B cells and autoAb production were also significantly decreased in autoreactive B cell STAT3-deficient mice. Results obtained from lupus-prone B6.MRL/lpr mice suggest that the STAT3 signaling in B cells contributes to the pathogenesis of lupus.
GC B cells proliferate quickly, but are prone to apoptosis because of elevated level of Fas expression (8, 29). Many factors influence the fate of GC B cells. For example, transcription factor Bcl6 is essential for the GC B cell development (28). Anti-apoptotic molecule Mcl-1 has been shown to be critical for the GC formation and GC B cell survival (27). Our studies showed that the GC B cells from immunized B cell STAT3 deficient mice had increased apoptosis with significant lower mRNA expression levels of Bcl6, Mcl-1 and Bcl-xL compared to those in control mice. This may explain why the GCs and GC B cells are significantly decreased in STAT3 KO mice. These results further suggest that the STAT3 signaling in B cells is required for the GC B cell survival. However, it appears that STAT3 signaling is not required for early GC formation. This is consistent with the finding that there is no difference of apoptosis between control and KO GC B cells at day 7 after immunization. It is worth noting that the STAT3 deficiency in B cells did not impact the initiation of the GC formation in hRBC immunized mice and lupus-prone B6.MRL/lpr mice while it is required in OVA/CFA immunized mice. Although the detailed mechanism is unknown, it may be related to different forms of Ags or administration routes.
We also found that the frequency of Tfh cells is positively correlated with GC B cells. Tfh cells represent a distinct subset of CD4+ T cells specialized in providing essential signals for B cell maturation and Ig production (31, 38). Tfh cells also promote B cell affinity maturation and isotype switching within the GC (38). Pervious studies have shown that active SLE patients have expanded circulating Tfh cells (39). On the other hand, recent data have revealed a novel role for B cells in Tfh development. GC B cells or bystander B cells via provision of Ags and ICOS-L promote Tfh cell maturation and follicular migration (32, 40). Depletion of GC B cells diminishes Tfh cells in autoimmune mice (41). Here, we observed reciprocal interactions between GC B cells and Tfh cell in immunization models as well as in lupus-prone mice. STAT3 KO B cells express low levels of CD86 and secrete significantly lower level of IL-6, both of which are critical in Tfh differentiation (42, 43). These data suggest that STAT3 signaling in B cells not only is critical for GC B cell maintenance (frequency) but also is essential for GC B cell quality for Tfh differentiation and maintenance.
Autoreactive B cell activation and autoAb production are thought to have a primary role in the pathogenesis of lupus. Although the STAT3 signaling in T cells has been demonstrated as an important regulator in Tfh cell and GC B cell differentiation (31), the role of B cell STAT3 in the pathogenesis of lupus has never been formally tested. Previous studies have demonstrated that IL-21, a STAT3 activating cytokine, is critical for the spontaneous GC formation and plasma cell accumulation observed in MRLlpr mice (44). Our study provides detailed insight into how STAT3 signaling in B cells contributes to lupus pathogenesis. Specifically, we demonstrated that spontaneous GC formation, plasma cell and Tfh cell accumulation as well as splenomegaly were abrogated in B cell-STAT3 KO mice. Spontaneous T cell activation was also impaired, particularly IFN-γ-producing CD8 T cells. This may be due to decreased CD86 expression and cytokine IL-12 and IFN-γ production by STAT3 KO B cells. More importantly, inflammatory cell infiltration and Ig and C3 deposition in kidneys were significantly reduced in B cell-STAT3 B6.MRL/lpr mice. These findings highlighted critical roles of the STAT3 signaling in B cells for activation of multiple disease-associated effector pathways in lupus-prone mice. In summary, we have delineated the essential roles of B cell-STAT3 signaling in the GC formation and maintenance as well as lupus pathogenesis. These findings also suggest that blockade of the STAT3 pathway in B cells may be a novel strategy for the treatment of B and T cell-mediated autoimmune diseases, such as SLE.
Supplementary Material
Acknowledgments
This work was supported by the NIH/NIAID AI124235, NIH/NIAID AI124081, and the National Natural Science Foundation of China 81228018. Huang-ge Zhang is supported by a Research Career Scientist Award.
References
- 1.Kotzin BL. Systemic lupus erythematosus. Cell. 1996;85:303–306. doi: 10.1016/s0092-8674(00)81108-3. [DOI] [PubMed] [Google Scholar]
- 2.Liu Z, Zou Y, Davidson A. Plasma cells in systemic lupus erythematosus: the long and short of it all. Eur J Immunol. 2011;41:588–591. doi: 10.1002/eji.201041354. [DOI] [PubMed] [Google Scholar]
- 3.Kamal A, Khamashta M. The efficacy of novel B cell biologics as the future of SLE treatment: a review. Autoimmun Rev. 2014;13:1094–1101. doi: 10.1016/j.autrev.2014.08.020. [DOI] [PubMed] [Google Scholar]
- 4.Murphy G, Lisnevskaia L, Isenberg D. Systemic lupus erythematosus and other autoimmune rheumatic diseases: challenges to treatment. Lancet. 2013;382:809–818. doi: 10.1016/S0140-6736(13)60889-2. [DOI] [PubMed] [Google Scholar]
- 5.Zotos D, Tarlinton DM. Determining germinal centre B cell fate. Trends Immunol. 2012;33:281–288. doi: 10.1016/j.it.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 6.Klein U, Dalla-Favera R. Germinal centres: role in B-cell physiology and malignancy. Nat Rev Immunol. 2008;8:22–33. doi: 10.1038/nri2217. [DOI] [PubMed] [Google Scholar]
- 7.Gatto D, Brink R. The germinal center reaction. J Allergy Clin Immunol. 2010;126:898–907. doi: 10.1016/j.jaci.2010.09.007. quiz 908–899. [DOI] [PubMed] [Google Scholar]
- 8.Victora GD, Nussenzweig MC. Germinal centers. Annu Rev Immunol. 2012;30:429–457. doi: 10.1146/annurev-immunol-020711-075032. [DOI] [PubMed] [Google Scholar]
- 9.Dorner T, Giesecke C, Lipsky PE. Mechanisms of B cell autoimmunity in SLE. Arthritis Res Ther. 2011;13:243. doi: 10.1186/ar3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang JH, New JS, Xie S, Yang P, Wu Q, Li J, Luo B, Ding Y, Druey KM, Hsu HC, Mountz JD. Extension of the germinal center stage of B cell development promotes autoantibodies in BXD2 mice. Arthritis Rheum. 2013;65:2703–2712. doi: 10.1002/art.38059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Avery DT, Deenick EK, Ma CS, Suryani S, Simpson N, Chew GY, Chan TD, Palendira U, Bustamante J, Boisson-Dupuis S, Choo S, Bleasel KE, Peake J, King C, French MA, Engelhard D, Al-Hajjar S, Al-Muhsen S, Magdorf K, Roesler J, Arkwright PD, Hissaria P, Riminton DS, Wong M, Brink R, Fulcher DA, Casanova JL, Cook MC, Tangye SG. B cell-intrinsic signaling through IL-21 receptor and STAT3 is required for establishing long-lived antibody responses in humans. J Exp Med. 2010;207:155–171. doi: 10.1084/jem.20091706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Linker-Israeli M, Deans RJ, Wallace DJ, Prehn J, Ozeri-Chen T, Klinenberg JR. Elevated levels of endogenous IL-6 in systemic lupus erythematosus. A putative role in pathogenesis. J Immunol. 1991;147:117–123. [PubMed] [Google Scholar]
- 13.Yap DY, Lai KN. Cytokines and their roles in the pathogenesis of systemic lupus erythematosus: from basics to recent advances. Journal of biomedicine & biotechnology. 2010;2010:365083. doi: 10.1155/2010/365083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakou M, Papadimitraki ED, Fanouriakis A, Bertsias GK, Choulaki C, Goulidaki N, Sidiropoulos P, Boumpas DT. Interleukin-21 is increased in active systemic lupus erythematosus patients and contributes to the generation of plasma B cells. Clinical and experimental rheumatology. 2013;31:172–179. [PubMed] [Google Scholar]
- 15.Harada T, Kyttaris V, Li Y, Juang YT, Wang Y, Tsokos GC. Increased expression of STAT3 in SLE T cells contributes to enhanced chemokine-mediated cell migration. Autoimmunity. 2007;40:1–8. doi: 10.1080/08916930601095148. [DOI] [PubMed] [Google Scholar]
- 16.Huang X, Guo Y, Bao C, Shen N. Multidimensional single cell based STAT phosphorylation profiling identifies a novel biosignature for evaluation of systemic lupus erythematosus activity. PloS one. 2011;6:e21671. doi: 10.1371/journal.pone.0021671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maeda K, Malykhin A, Teague-Weber BN, Sun XH, Farris AD, Coggeshall KM. Interleukin-6 aborts lymphopoiesis and elevates production of myeloid cells in systemic lupus erythematosus-prone B6.Sle1.Yaa animals. Blood. 2009;113:4534–4540. doi: 10.1182/blood-2008-12-192559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakou M, Bertsias G, Stagakis I, Centola M, Tassiulas I, Hatziapostolou M, Kritikos I, Goulielmos G, Boumpas DT, Iliopoulos D. Gene network analysis of bone marrow mononuclear cells reveals activation of multiple kinase pathways in human systemic lupus erythematosus. PloS one. 2010;5:e13351. doi: 10.1371/journal.pone.0013351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu K, Liang C, Liang Z, Tus K, Wakeland EK. Sle1ab mediates the aberrant activation of STAT3 and Ras-ERK signaling pathways in B lymphocytes. J Immunol. 2005;174:1630–1637. doi: 10.4049/jimmunol.174.3.1630. [DOI] [PubMed] [Google Scholar]
- 20.Jacobi AM, Odendahl M, Reiter K, Bruns A, Burmester GR, Radbruch A, Valet G, Lipsky PE, Dorner T. Correlation between circulating CD27high plasma cells and disease activity in patients with systemic lupus erythematosus. Arthritis Rheum. 2003;48:1332–1342. doi: 10.1002/art.10949. [DOI] [PubMed] [Google Scholar]
- 21.Fornek JL, Tygrett LT, Waldschmidt TJ, Poli V, Rickert RC, Kansas GS. Critical role for Stat3 in T-dependent terminal differentiation of IgG B cells. Blood. 2006;107:1085–1091. doi: 10.1182/blood-2005-07-2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deenick EK, Avery DT, Chan A, Berglund LJ, Ives ML, Moens L, Stoddard JL, Bustamante J, Boisson-Dupuis S, Tsumura M, Kobayashi M, Arkwright PD, Averbuch D, Engelhard D, Roesler J, Peake J, Wong M, Adelstein S, Choo S, Smart JM, French MA, Fulcher DA, Cook MC, Picard C, Durandy A, Klein C, Holland SM, Uzel G, Casanova JL, Ma CS, Tangye SG. Naive and memory human B cells have distinct requirements for STAT3 activation to differentiate into antibody-secreting plasma cells. J Exp Med. 2013;210:2739–2753. doi: 10.1084/jem.20130323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bolli R, Stein AB, Guo Y, Wang OL, Rokosh G, Dawn B, Molkentin JD, Sanganalmath SK, Zhu Y, Xuan YT. A murine model of inducible, cardiac-specific deletion of STAT3: its use to determine the role of STAT3 in the upregulation of cardioprotective proteins by ischemic preconditioning. J Mol Cell Cardiol. 2011;50:589–597. doi: 10.1016/j.yjmcc.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 24.Yan J, Mamula MJ. Autoreactive T cells revealed in the normal repertoire: escape from negative selection and peripheral tolerance. J Immunol. 2002;168:3188–3194. doi: 10.4049/jimmunol.168.7.3188. [DOI] [PubMed] [Google Scholar]
- 25.Xu H, Yan J, Huang Y, Chilton PM, Ding C, Schanie CL, Wang L, Ildstad ST. Costimulatory blockade of CD154-CD40 in combination with T-cell lymphodepletion results in prevention of allogeneic sensitization. Blood. 2008;111:3266–3275. doi: 10.1182/blood-2006-10-053801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Good-Jacobson KL, Song E, Anderson S, Sharpe AH, Shlomchik MJ. CD80 expression on B cells regulates murine T follicular helper development, germinal center B cell survival, and plasma cell generation. J Immunol. 2012;188:4217–4225. doi: 10.4049/jimmunol.1102885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vikstrom I, Carotta S, Luthje K, Peperzak V, Jost PJ, Glaser S, Busslinger M, Bouillet P, Strasser A, Nutt SL, Tarlinton DM. Mcl-1 is essential for germinal center formation and B cell memory. Science. 2010;330:1095–1099. doi: 10.1126/science.1191793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Basso K, Schneider C, Shen Q, Holmes AB, Setty M, Leslie C, Dalla-Favera R. BCL6 positively regulates AID and germinal center gene expression via repression of miR-155. J Exp Med. 2012;209:2455–2465. doi: 10.1084/jem.20121387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hamel KM, V, Liarski M, Clark MR. Germinal center B-cells. Autoimmunity. 2012;45:333–347. doi: 10.3109/08916934.2012.665524. [DOI] [PubMed] [Google Scholar]
- 30.Berglund LJ, Avery DT, Ma CS, Moens L, Deenick EK, Bustamante J, Boisson-Dupuis S, Wong M, Adelstein S, Arkwright PD, Bacchetta R, Bezrodnik L, Dadi H, Roifman CM, Fulcher DA, Ziegler JB, Smart JM, Kobayashi M, Picard C, Durandy A, Cook MC, Casanova JL, Uzel G, Tangye SG. IL-21 signalling via STAT3 primes human naive B cells to respond to IL-2 to enhance their differentiation into plasmablasts. Blood. 2013;122:3940–3950. doi: 10.1182/blood-2013-06-506865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ray JP, Marshall HD, Laidlaw BJ, Staron MM, Kaech SM, Craft J. Transcription factor STAT3 and type I interferons are corepressive insulators for differentiation of follicular helper and T helper 1 cells. Immunity. 2014;40:367–377. doi: 10.1016/j.immuni.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baumjohann D, Preite S, Reboldi A, Ronchi F, Ansel KM, Lanzavecchia A, Sallusto F. Persistent antigen and germinal center B cells sustain T follicular helper cell responses and phenotype. Immunity. 2013;38:596–605. doi: 10.1016/j.immuni.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 33.White CA, Pone EJ, Lam T, Tat C, Hayama KL, Li G, Zan H, Casali P. Histone deacetylase inhibitors upregulate B cell microRNAs that silence AID and Blimp-1 expression for epigenetic modulation of antibody and autoantibody responses. J Immunol. 2014;193:5933–5950. doi: 10.4049/jimmunol.1401702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qian Y, Wang H, Clarke SH. Impaired clearance of apoptotic cells induces the activation of autoreactive anti-Sm marginal zone and B-1 B cells. J Immunol. 2004;172:625–635. doi: 10.4049/jimmunol.172.1.625. [DOI] [PubMed] [Google Scholar]
- 35.Ding C, Ma Y, Chen X, Liu M, Cai Y, Hu X, Xiang D, Nath S, Zhang HG, Ye H, Powell D, Yan J. Integrin CD11b negatively regulates BCR signalling to maintain autoreactive B cell tolerance. Nature communications. 2013;4:2813. doi: 10.1038/ncomms3813. [DOI] [PubMed] [Google Scholar]
- 36.Santulli-Marotto S, Retter MW, Gee R, Mamula MJ, Clarke SH. Autoreactive B cell regulation: peripheral induction of developmental arrest by lupus-associated autoantigens. Immunity. 1998;8:209–219. doi: 10.1016/s1074-7613(00)80473-2. [DOI] [PubMed] [Google Scholar]
- 37.Shlomchik MJ, Weisel F. Germinal center selection and the development of memory B and plasma cells. Immunol Rev. 2012;247:52–63. doi: 10.1111/j.1600-065X.2012.01124.x. [DOI] [PubMed] [Google Scholar]
- 38.Craft JE. Follicular helper T cells in immunity and systemic autoimmunity. Nat Rev Rheumatol. 2012;8:337–347. doi: 10.1038/nrrheum.2012.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simpson N, Gatenby PA, Wilson A, Malik S, Fulcher DA, Tangye SG, Manku H, Vyse TJ, Roncador G, Huttley GA, Goodnow CC, Vinuesa CG, Cook MC. Expansion of circulating T cells resembling follicular helper T cells is a fixed phenotype that identifies a subset of severe systemic lupus erythematosus. Arthritis Rheum. 2010;62:234–244. doi: 10.1002/art.25032. [DOI] [PubMed] [Google Scholar]
- 40.Xu H, Li X, Liu D, Li J, Zhang X, Chen X, Hou S, Peng L, Xu C, Liu W, Zhang L, Qi H. Follicular T-helper cell recruitment governed by bystander B cells and ICOS-driven motility. Nature. 2013;496:523–527. doi: 10.1038/nature12058. [DOI] [PubMed] [Google Scholar]
- 41.Yusuf I, Stern J, McCaughtry TM, Gallagher S, Sun H, Gao C, Tedder T, Carlesso G, Carter L, Herbst R, Wang Y. Germinal Center B Cell Depletion Diminishes CD4+ Follicular T Helper Cells in Autoimmune Mice. PloS one. 2014;9:e102791. doi: 10.1371/journal.pone.0102791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Salek-Ardakani S, Choi YS, Rafii-El-Idrissi Benhnia M, Flynn R, Arens R, Shoenberger S, Crotty S, Croft M, Salek-Ardakani S. B cell-specific expression of B7–2 is required for follicular Th cell function in response to vaccinia virus. J Immunol. 2011;186:5294–5303. doi: 10.4049/jimmunol.1100406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Choi YS, Eto D, Yang JA, Lao C, Crotty S. Cutting edge: STAT1 is required for IL-6-mediated Bcl6 induction for early follicular helper cell differentiation. J Immunol. 2013;190:3049–3053. doi: 10.4049/jimmunol.1203032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rankin AL, Guay H, Herber D, Bertino SA, Duzanski TA, Carrier Y, Keegan S, Senices M, Stedman N, Ryan M, Bloom L, Medley Q, Collins M, Nickerson-Nutter C, Craft J, Young D, Dunussi-Joannopoulos K. IL-21 receptor is required for the systemic accumulation of activated B and T lymphocytes in MRL/MpJ-Fas(lpr/lpr)/J mice. J Immunol. 2012;188:1656–1667. doi: 10.4049/jimmunol.1003871. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.