Abstract
Mechanistic insight into how adaptive immune responses are modified along the self-nonself continuum may offer more effective opportunities to treat autoimmune disease, cancer and other sterile inflammatory disorders. Recent genetic studies in the KRN mouse model of rheumatoid arthritis demonstrate that the immunomodulatory molecule IDO2 modifies responses to self antigens, however, the mechanisms involved are obscure. In this study, we show that IDO2 exerts a critical function in B cells to support the generation of autoimmunity. In experiments with IDO2-deficient mice, adoptive transplant experiments demonstrated that IDO2 expression in B cells was both necessary and sufficient to support robust arthritis development. IDO2 function in B cells was contingent on a cognate, antigen-specific interaction to exert its immunomodulatory effects on arthritis development. We confirmed a similar requirement in an established model of contact hypersensitivity, where IDO2-expresing B cells are required for a robust inflammatory response. Mechanistic investigations showed that IDO2 deficient B cells lacked the ability to upregulate the co-stimulatory marker CD40, suggesting IDO2 acts at the T:B cell interface to modulate the potency of T cell help needed to promote autoantibody production. Overall, our findings revealed that IDO2 expression by B cells modulates autoimmune responses by supporting the cross-talk between autoreactive T and B cells.
Introduction
Autoimmune diseases such as rheumatoid arthritis and lupus that are generally poorly managed clinically pose a growing challenge in developed countries. At present, there is little understanding of the pathogenic etiology of autoimmune disease, nor the modifier pathways which may affect the course and kinetics of its clinical development or severity. At present, major efforts focus on whole-genome genetic and epigenetic screens to elucidate etiologic drivers, but there has been less attention on novel principles of immunomodulation that may function as disease modifiers. Such efforts may be useful in illuminating questions about individual variations in the kinetics and severity of disease development, as well as offering new therapeutic directions to attenuate disease.
The indoleamine 2,3-dioxygenases IDO1 and IDO2 catabolize tryptophan (Trp) and various Trp related compounds which modify inflammatory state and immune tolerance. These two enzymes resulted from an ancient gene duplication of an ancestral IDO with relatively low tryptophan catalytic activity (1). The immunoregulatory properties of IDO were first revealed in pharmacological studies of an IDO pathway inhibitor which suggested a critical role in maintaining maternal-fetal tolerance through a T cell-dependent mechanism (2). Subsequently, numerous pharmacological and genetic studies linked the IDO pathway to immune escape in cancer (e.g. 3, 4, 5) and as a contributor to autoimmunity (e.g. 6, 7, 8). IDO1, the better characterized of the two enzymes, modulates the immune system primarily through alterations in T regulatory cell populations, an effect likely mediated via a population of IDO1-expressing dendritic cells (DCs) (e.g. 9). In addition, a role for IDO1 in B cells in regulating T-independent responses has recently been reported (10). Mechanistically, IDO1 signals through the GCN2 and mTOR-mediated stress response pathways in response to Trp depletion (11–13). IDO2, a low-efficiency Trp-catabolizing enzyme, was only recently directly connected to immunomodulation (14–16) and less is known about the cellular and molecular mechanisms through which it influences immunity, though it is clear that IDO2 does not simply serve a redundant function to IDO1 (15). IDO2 expression is more restricted than IDO1, with high expression levels limited to liver, kidney, and cerebral cortex (17). IDO2 is also expressed in antigen-presenting cells, particularly DCs (16), as well as macrophages and B cells (15). Notably, the relative contributions of IDO1 and IDO2 to various immunological phenomena are somewhat convoluted given that many published studies inhibit IDO through the use of the small molecule inhibitor 1-methyltryptophan (1MT), which influences both IDO1 and IDO2 (5). In some reports, blocking IDO with 1MT was observed to exacerbate autoimmune disease (6, 18, 19), while in other reports, it was found to alleviate disease (8, 20). While the basis for these conflicting observations is unclear, they highlight the importance of genetic knockouts rather than nonspecific small molecule inhibitors in isolating the inflammatory roles played by the IDO enzymes in different disease settings.
Recently, we created an IDO2-deficient (ko) mouse (15) to isolate the immunologic contributions of the two IDO enzymes. Using these mice, we have defined a critical role for IDO2 distinct from IDO1 in mediating inflammation in murine models of contact hypersensensitivity (CHS) and autoimmune arthritis (14, 15). Despite the clear role of IDO2 in modulating autoimmune and inflammatory responses, little is known about the mechanism by which it acts. Initial studies using the KRN model of arthritis demonstrated a reduction in autoreactive T and B cell responses, resulting in attenuated joint inflammation in IDO2-deficient mice (14). While there was a pronounced defect in T helper cells, reciprocal adoptive transfer experiments demonstrated that the effect of IDO2 was extrinsic to T cells. In this study, we define the cellular mechanism through which IDO2 mediates inflammatory autoimmunity, demonstrating that IDO2 acts directly in B cells to drive inflammation in models of arthritis and CHS.
Materials and Methods
Mice
KRN TCR tg (21) and IDO2 ko (15) mice on a C57BL/6 background have been described. C57BL/6 IDO2 wild-type (wt) and ko mice lacking the TCR alpha chain (TCR ko) and carrying a single copy of the MHC Class II I-Ag7 allele (TCR ko B6.g7/b and TCR ko IDO2 ko B6.g7/b) were generated as recipient mice for adoptive transfer of KRN T cells. Mice must carry both an I-Ag7 and an I-Ab allele to prevent rejection of the injected KRN T, which are I-Ab. T cell donor mice were IDO2 ko KRN TCR tg (IDO2 ko KRN B6) carrying 2 copies of the I-Ab allele. B cell donor mice for “add-back” experiments were wt or IDO2 ko I-Ag7/b, wt or IDO2 ko I-Ab/b, GPI-specific B cell transgenic mk147 I-Ag7/b, or hen-egg lysozyme-specific B cell transgenic MD4 I-Ag7/b C57BL/6. Additionally, C57BL/6 Rag ko mice were crossed with NOD Rag ko mice to generate B6xNOD Rag ko I-Ag7/b recipients for adoptive transfers. For contact hypersensitivity experiments, IDO2 wt and ko BALB/c mice were used as both B cell donor and recipient mice. All mice were bred and housed under specific pathogen free conditions in the animal facility at the Lankenau Institute for Medical Research. Studies were performed in accordance with National Institutes of Health and Association for Assessment and Accreditation of Laboratory Animal Care guidelines with approval from the LIMR Institutional Animal Care and Use Committee.
Adoptive Transfers
Spleen and lymph node tissue from C57BL/6 mice was harvested and passed through a 70μm nylon strainer to generate a single-cell suspension. CD4+ T cells from KRN TCR tg (KRN B6) or IDO2 ko KRN TCR tg (IDO2 ko KRN B6) mice were purified by positive selection with anti-CD4 mouse MACS microbeads (Miltyeni Biotec). For T cell purification, elutant was purified over a second column to achieve higher purity (~90%). Following purification, 3.5x105 CD4+ T cells were adoptively transferred i.v. into TCR ko B6.g7 or TCR ko IDO2 ko B6.g7 hosts. For B cell add-back experiments, B cells from spleens of IDO2 wt or ko I-Ag7/b were purified by anti-CD43 negative selection with MACS beads (Miltenyi). B cell purity was routinely ~97%. 0.5–10x106 B cells were transferred with T cells. Arthritis was measured as described below. Mice were sacrificed after 2 weeks.
To purify subpopulations of B cells, spleens were harvested from IDO2 wt or ko I-Ag7/b donor mice and B cells isolated by negative selection with anti-CD43 microbeads (Miltenyi). Following purification, B cells were stained with appropriate surface markers, either B220 and CD11c for isolation of the pDC-like population, or B220, CD21, and CD23 for isolation of marginal zone and follicular B cells. Stained populations were sorted with a BD FACSAria III flow sorter. Following isolation, 5x105 of the appropriate population of B cells was co-injected with 3.5x105 KRN T cells into TCR ko IDO2 ko B6.g7 hosts. Arthritis was measured as described below. Mice were sacrificed after 2 weeks.
Arthritis incidence
The two rear ankles of experimental mice were measured starting at the day of adoptive transfer. Measurement of ankle thickness was made above the footpad axially across the ankle joint using a Fowler Metric Pocket Thickness Gauge. Ankle thickness was rounded off to the nearest 0.05mm. Change in ankle thickness was defined as (measured ankle thickness) - (thickness prior to adoptive transfer of B and T cells).
Contact Hypersensitivity
IDO2 wt or ko BALB/c recipient mice were sensitized with 3% oxazolone (Sigma) in 100% ethanol on their shaved abdomen (100μl) and hind footpads (10 μl each) 5 days prior to the start of the experiment. Three days later, IDO2 wt or ko B cell donor mice were sensitized using the same protocol. After 24 hours, spleen and inguinal lymph nodes were harvested from donor mice, B cells isolated with Pan-B cell bead kit (Miltenyi Biotec), and 5x105 B cells transferred into each recipient mouse. 24h following B cell transfer (5 days after initial sensitization), recipient mice were elicited with 20μl of 1% oxazolone in 100% ethanol, or 100% ethanol alone as a control, 10μl on each side of the ears. After 24h, ear thickness was measured using a dial gauge (Fowler, A&M Industrial Supply, Rahway, NJ USA) and harvested ears were fixed in 10% buffered formalin, embedded in paraffin, sectioned, and stained with H&E. Histology sections were imaged using a Zeiss Axioplan microscope wth a Zeiss Plan-Apochromat x10/0.32 objective and Zeiss AxioCam HRC camera using AxioVision 4..1 software. The images were then processed using Adobe Photoshop CS2 software. Trials performed in multiple mice were replicated at least 3 times.
In vitro cell cultures
For in vitro experiments, B cells were harvested from spleens of C57BL/6 mice and purified by negative selection using anti-CD43 microbeads (Miltenyi Biotech). Purified B cells were cultured at 2x106 cells/ml in IMDM + 10% FCS + 5μM 2-mercaptoethanol, 2mM glutamax (Gibco), and 50μg/ml gentamycin. Stimuli and cytokines were added to a final concentration of: LPS (Sigma, 25 μg/ml), anti-CD40 (eBioscience, 2μg/ml), anti-IgM F(ab′)2 (Jackson Immunoresearch, 20μg/ml), CpG (ODN 1826, Invivogen, 1μM), IL-4 (eBioscience, 50ng/ml), IL-6 (eBioscience, 10ng/ml), IL-21 (eBioscience, 100ng/ml), and IFNγ (Peprotech, 500 U/μl).
IDO2 RNA Expression
Spleen tissue from C57BL/6 mice was harvested and passed through a 70μm nylon strainer to generate a single-cell suspension. RNA was extracted with Trizol (Invitrogen) and first strand cDNA synthesized using oligo-dT primer (Promega GoScript). IDO2 expression was measured by real time PCR using Taqman for detection (ABsolute qPCR ROX Mix, ThermoFisher) or SYBR Green (Sigma-Aldrich). Expression of target gene IDO2 was determined relative to β-2-microglobulin (β2M) and calculated as 2^−(CtTarget gene –Ctb2M) as primers had similar efficiencies. Primers for Taqman mouse IDO2 and β2M were from Life Technologies. SYBR Green primers for IDO2 were 5′-GCCCAGAGCTCCGTGCTTCAT-3′ and 5′-TGGGAAGGCGGCATGTAGTCC-3′ and for β2M 5′-CTCGGTGACCCTGGTCTTTC-3′ and 5′-TTGAGGGGTTTTCTGGATAGCA-3′.
Flow cytometric analysis of B cell costimulatory markers
IDO2 wt and ko C57BL/6 spleen cells were harvested and stimulated for 72h with anti-IgM F(ab′)2 (Jackson Immunoresearch, 20μg/ml) or anti-CD40 (eBioscience, 2μg/ml) + IL-21 (eBioscience, 100ng/ml) in IMDM + 10% FCS + 5μM 2-mercaptoethanol, 2mM glutamax (Gibco), and 50μg/ml gentamycin. Levels of the following costimulatory markers were directly measured by flow cytometry on a BD FACSCanto with subsequent analysis using FlowJo Software (TreeStar): CD40 (eBioscience), CD80 (eBioscience), CD86 (eBioscience), ICOSL (BioLegend), IL-21R (BD Biosciences), OX40L (BioLegend), PDL1 (BioLegend), and MHC Class II (eBioscience). A minimum of 3 replicate mice were analyzed per condition.
Statistical Analysis
Statistical significance was determined using one way-ANOVAs followed by comparison of means with Tukey’s post-hoc multiple comparison correction or Kruskal-Wallis non-parametric ANOVA with Dunn’s multiple comparison correction as appropriate using Prism6 (GraphPad Software, Inc).
Results
IDO2 expression in B cells is necessary and sufficient to support the development of autoimmune arthritis
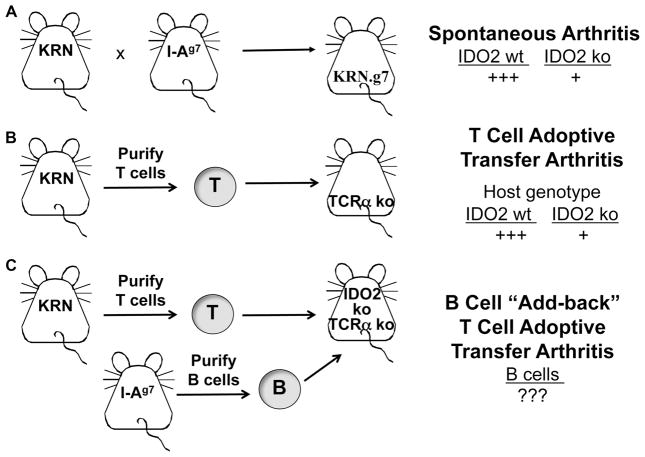
The KRN T cell transgenic (tg) mouse model of autoimmune arthritis (21) is a tractable model system that allows us to distinguish the specific contribution of IDO2 to the autoimmune response. In this model, the KRN T cell recognizes the ubiquitous autoantigen glucose-6-phosphate isomerase (GPI), a glycolytic enzyme, in the context of the MHC Class II molecule I-Ag7 (22). Unlike other models of arthritis, no adjuvants are required for disease induction. Although it is a T cell tg model, arthritis is induced by the production of pathogenic autoantibodies resulting from the activation of endogenous B cells. KRN mice can be used to model arthritis in multiple ways. First, arthritis can occur spontaneously, by breeding the KRN T cell tg to a mouse carrying I-Ag7 (Figure 1A) (21). The resulting KRN.g7 mice develop robust arthritis in the presence of wt IDO2, but a greatly attenuated joint inflammatory response in the absence of IDO2 (IDO2 ko KRN.g7) (14, 23). Arthritis can also be induced by adoptively transferring KRN T cells into a T cell-deficient mouse expressing I-Ag7 (Figure 1B) (24). Transfer of KRN T cells results in a robust arthritic response in recipient mice expressing wt IDO2. Recapitulating what is seen in the spontaneous model, arthritis is greatly reduced in IDO2 ko hosts (14). To define the cell-specific role of IDO2, in this paper, we expand the T cell adoptive transfer model by performing “add-back” of different B cell types to IDO2 ko T-cell deficient hosts (Figure 1C). Because the host has endogenous IDO2 ko B cells, these are termed “add-back” experiments to clarify that additional B cells are being added to an existing milieu.
Figure 1. IDO2 modulates arthritis development in the KRN model system.
A. Arthritis develops spontaneously when mice carrying the GPI-specific T cell tg KRN are crossed to mice with the MHC Class II molecule I-Ag7. IDO2 ko mice develop arthritis later and with a reduced severity compared to IDO2 wt (14). B. Arthritis can also be induced by adoptive transfer of KRN T cells into T-cell deficient (TCR ko) hosts. Recapitulating what is seen in the spontaneous model, arthritis in IDO2 ko hosts is reduced compared to IDO2 wt (14). C. The contribution of various cell types can be determined by the addition (“add-back”) of different cells to the T cell adoptive transfer model. Here, we will specifically examine the contribution IDO2 in B cells by coinjecting B cells of different genotypes with IDO2 ko KRN T cells into an IDO2 ko host.
Previously, we have shown that IDO2 is crucial for the B and T cell-dependent initiation phase responsible for production of autoantibodies, whereas IDO2 is dispensable for the downstream T and B cell-independent effector phase of disease (14, 25). Reduced differentiated T helper subsets, coupled with a decrease in serum autoantibody titers and autoantibody secreting cells in IDO2 ko arthritic mice suggested that IDO2 may function at the interface between a T cell and an antigen-presenting cell (APC) (14). Reciprocal adoptive transfer experiments demonstrated conclusively that this defect is not intrinsic to the T cell, thereby implicating a role for IDO2 in APCs (14).
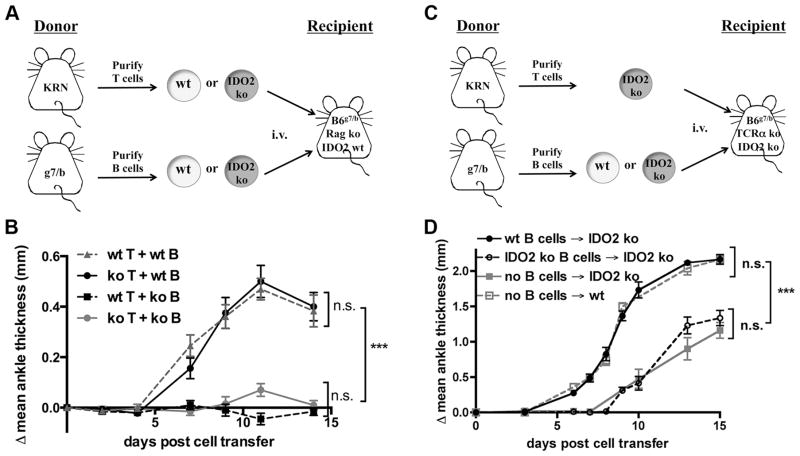
IDO2 expression has been reported in several types of APCs, including DCs, macrophages and B cells (15–17), and we hypothesized that IDO2 exerted its critical function in one or more of these cell types to influence the T cell response. To separate the action of IDO2 in innate vs. adaptive immune cells, we performed a series of adoptive transfers to induce autoimmune arthritis in Rag-deficient hosts. Here, IDO2 wt or ko KRN T cells and IDO2 wt or ko B cells were transferred into Rag ko C57BL/6xNOD recipient mice (Figure 2A). Recipient mice carried one copy of the MHC Class II allele I-Ag7 from the NOD parent. Components of the innate immune system were derived from the recipient mouse and were thus wt for the IDO2 allele. We found that arthritis developed only in mice that received wt B cells, regardless of whether wt or IDO2 ko KRN T cells were transferred (Figure 2B). Mice that received IDO2 ko B cells did not develop arthritis, not even the reduced level of joint inflammation previously described in the spontaneous IDO2 ko arthritis model (14). It is unclear why arthritis was absent; however, inflammation levels in general were lower in the Rag ko recipient mice than in spontaneous arthritis, suggesting some IDO2-independent contribution of endogenous B cells to the sustained autoimmune response present in the spontaneous model. In the Rag ko recipient mice, all components of the immune system, including DCs and macrophages from the host and transferred T cells, were wt with the exception of transferred IDO2 ko B cells, demonstrating that wt IDO2 in B cells is necessary for arthritis development.
Figure 2. IDO2 is both necessary and sufficient for arthritis.
A, B. wt B cells are necessary for arthritis induction in Rag-deficient hosts. A. Schematic of adoptive transfer strategy. 3.5x105 wt or IDO2 ko KRN CD4+ T cells and 1.5x107 wt or IDO2 ko I-Ag7/b B cells were adoptively transferred into C57BL/6 x NOD Rag ko host mice and arthritis followed for 14 days. B. Arthritis is present only when IDO2 wt B cells are transferred (wt T + wt B, n=9; ko T + wt B, n=9). No arthritis develops when IDO2 ko B cells are transferred (wt T + ko B, n=5; koT + ko B, n=5). Data pooled from four independent experiments. C, D. wt B cells are sufficient to restore the wt arthritic response in IDO2 ko host mice. C. Schematic of adoptive transfer strategy. D. Arthritis was induced by the adoptive transfer of 3.5x105 purified CD4+ IDO2 ko KRN T cells into wt (n=7) or IDO2 ko (n=7) TCR ko I-Ag7/b hosts alone or in conjunction with the add-back of 1x107 B cells from wt (n=7) or IDO2 ko I-Ag7/b (n=5) mice into IDO2 ko TCR ko C57BL/6 (I-Ag7/b) hosts. Data pooled from 2 independent experiments. Rear ankles were measured as an indication of arthritis and represented as the mean change in ankle thickness relative to day 0 ± SEM. Arthritis was followed for 14 days. *** p<0.001, n.s.=not significant.
While the experiments in the Rag ko hosts established that IDO2 expression in B cells is necessary for arthritis, they did not address whether IDO2 expression in B cells is sufficient for the complete response, or if IDO2 expression in additional cell types is needed to induce robust arthritis. To test this, we performed a series of B cell “add-back” adoptive transfers into TCR ko IDO2 ko hosts (Figure 2C), examining whether addition of IDO2 wt B cells is sufficient to restore the arthritic response to levels seen in wt hosts. Note that in contrast to the Rag ko experiments (Figure 2A, 2B), here, the host mouse and all of its immune components are IDO2 deficient. Recipient mice lack T cells but harbor an intact IDO2 ko innate immune system as well as endogenous IDO2 ko B cells. This allowed us to separate IDO2’s role specifically in B cells from that in other cell types, as well as from other critical non-IDO2 functions of B cells (e.g. establishment of proper lymphoid architecture) (26). For this series of experiments, purified IDO2 ko KRN T cells were adoptively transferred into TCR ko IDO2 ko mice. Concurrent with the T cell transfer, either wt or IDO2 ko purified B cells were transferred to the recipients so that the only source of IDO2 would be from the transferred IDO2 wt B cells; all other immune cells, including the endogenous population of B cells, being IDO2 ko. Addition of wt B cells was sufficient to restore the wt arthritic response (Figure 2D). This outcome was not simply the result of the presence of additional B cells, as addition of IDO2 ko B cells did not restore arthritis above the attenuated level observed without the transfer of additional B cells (Figure 2D), recapitulating the reduced joint inflammatory response previously described in IDO2 ko KRN.g7 mice (14). Together, these data demonstrate IDO2 expression in B cells is both necessary and sufficient in the KRN model to support robust arthritis development.
IDO2 function in B cells is contingent upon a cognate and antigen-specific interaction
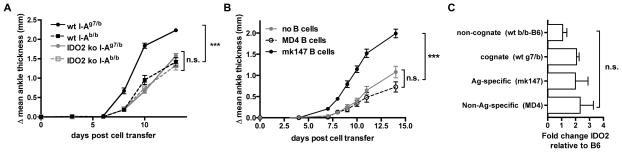
B cells have been shown to play both Ag-specific and non-specific (bystander) roles in the activation and recruitment of T helper cells (27, 28). To determine which of these roles requires a contribution from IDO2, we first examined whether a cognate interaction between wt B cells and other cell types is required for robust arthritis. As described, in the model of arthritis used here, KRN TCR tg T cells bind the autoantigen GPI in the context of the MHC Class II molecule I-Ag7. To test whether the B cells in our add-back experiments were directly involved in this TCR/MHC interaction, we transferred B cells purified from C57BL/6 mice that either could (I-Ag7/b, cognate) or could not (I-Ab/b, non-cognate) present Ag to KRN T cells. Only wt B cells expressing I-Ag7 were able to restore arthritis to wt severity, suggesting that IDO2 is required for efficient Ag presentation (Figure 3A).
Figure 3. IDO2 must be expressed in a cognate, antigen-specific B cell.
Arthritis was measured following induction by 3.5x105 purified CD4+ IDO2 ko KRN T cells and the add-back of B cells as described into TCR ko IDO2 ko I-Ag7/b C57BL/6 recipients. A. IDO2 expression only restores wt arthritis severity when expressed in a cognate B cell. 1x107 of one of the following B cells were added: Cognate (I-Ag7/b) wt (n=9) or IDO2 ko (n=5) or non-cognate (I-Ab/b) wt (n=10) or IDO2 ko (n=5). Data pooled from two independent experiments. B. IDO2 must be expressed in antigen-specific B cells for a complete arthritic response. 1x107 of one of the following B cell types added: GPI antigen-specific (mk147 I-Ag7/b) B cells (n=10), non-antigen specific (HEL-specific MD4 I-Ag7/b) B cells (n=10), or no B cells (n=10). Data pooled from two independent experiments. Rear ankles were measured as an indication of arthritis and represented as the mean change in ankle thickness relative to day 0 ± SEM. Arthritis was followed for 14 days. C. Initial IDO2 expression does not differ between transferred cell types. B cells were isolated from spleens from a pool of 2 mice of each genotype and IDO2 expression measured by real-time PCR. Experiment was repeated 3 times. *** p<0.001, n.s.=not significant.
To determine if IDO2-expressing B cells need to be antigen specific, we utilized B cell receptor tg models with defined antigen specificities as a source of IDO2-expressing B cells in the add-back strategy described above. Purified B cells from anti-GPI BCR tg mice (mk147 I-Ag7/b (29)) were used as a source of Ag-specific B cells and anti-hen egg lysozyme BCR tg mice (MD4 I-Ag7/b (30)) as a source of non-Ag-specific B cells. GPI antigen specificity in the IDO2 wt B cells was required for the complete arthritic response as only the mk147 B cells were able to restore arthritis in an IDO2 ko host to wt levels (Figure 3B). To rule out the restored arthritis response being due to differential IDO2 expression in the transferred B cells, we confirmed that the level of IDO2 in the transferred cells did not differ between the different donor B cell types prior to adoptive transfer (Figure 3C). Together, these data suggest that IDO2 is acting in a pathway that directly affects the antigen-presentation function of B cells to an interacting T cell.
Adoptive transfer of IDO2-expressing marginal zone, but not CD11c+B220+ cells, is sufficient to restore arthritis in IDO2–deficient hosts
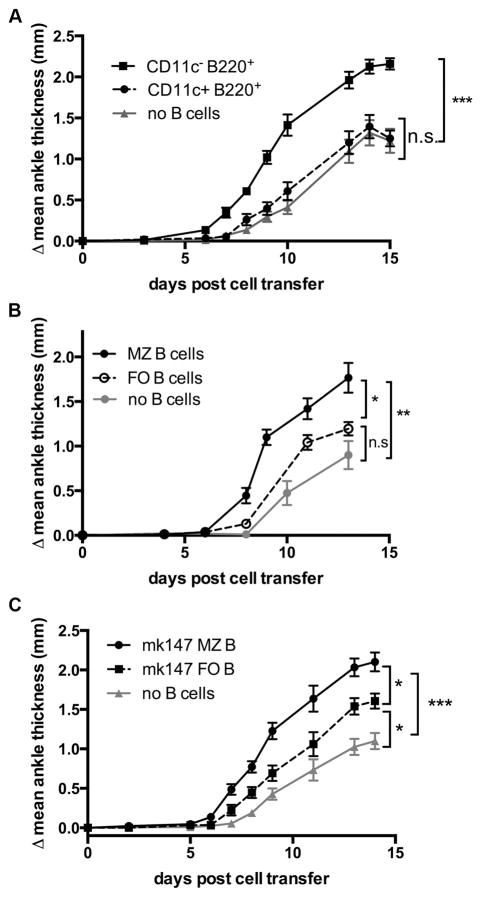
Mature B cells can be divided into subsets, each with distinct phenotypic and functional characteristics. To further examine the role of B cells in arthritis, B cell subsets were compared for their ability to restore wt arthritis in the add-back adoptive transfer model described above. There has been extensive interest in the population of B220+CD11c+ cells, which have characteristics of both B cells and DCs and have been linked to immune regulation. This population, often considered a subset of pDCs though its exact lineage remains obscured, contains a high proportion of IDO1+ cells that have been reported to contribute to immune escape in cancer through the activation of Tregs. (31–35). Age-associated B cells are also B220+CD11c+ and have been shown to be important mediators of spontaneous autoimmune responses in lupus-prone mice and human RA and SLE patients (36–38). To examine whether this subset contributes to IDO2-mediated arthritis development, B220+CD11c+ cells were sorted and compared to B220+CD11c− cells in a B cell add-back adoptive transfer approach. B220+CD11c− cells restored wt arthritis in IDO2 ko recipients, while the mice that received the B220+CD11c+ population exhibited an attenuated arthritic response that was not significantly different than the control IDO2 ko recipients that did not receive B cells (Figure 4A). Thus, while the B220+CD11c+ population has a significant role in the interplay between IDO1 function and T cell regulation, it does not appear to be important for IDO2-mediated B cell function in autoimmunity.
Figure 4. IDO2 activity is found within specific B cell subsets.
A. IDO2 function is important in B220+CD11c− cells. Arthritis was induced by adoptive transfer of 3.5x105 IDO2 ko KRN T cells and 5x105 B220+CD11c+ or B220+CD11c− cells into 8w old TCR ko IDO2 ko I-Ag7/b donors and arthritis followed by measurement of rear ankle thickness. n=8 mice per group. Data pooled from two independent experiments. B, C. IDO2 in MZ B cells is critical for robust arthritis. Arthritis was induced by T cell transfer as above along with 5x105 sorted IDO2 wt MZ (B220+CD21highCD23low) or FO (B220+CD21intCD23int) B cells into 8w old TCR ko IDO2 ko I-Ag7/b donors (n=6 per group) and compared to mice that received T cells only (n=7). MZ B cells fully restore wt arthritis while FO B cells do not. Data pooled from two independent experiments. C. To ensure this is not because the MZ contains more autoreactive B cells, the experiment was repeated by sorting MZ and FO GPI-reactive mk147 tg B cells. MZ cells again restored wt arthritis better than did FO B cells. n=9 per group. Data pooled from two independent experiments. *=p<0.05, **=p<0.01, ***=p<0.001, n.s.=not significant.
Marginal zone (MZ) B cells also contribute directly to autoimmunity, a phenomenon particularly well-documented in the KRN model. MZ B cells (B220+CD21highCD23low), a rare, non-circulating B cell population confined to the splenic marginal zone and separated from the splenic follicle by the marginal sinus, are important for the anti-GPI autoantibody response. These cells are spontaneously activated and contain a high proportion of anti-GPI B cells (29). In contrast, follicular (FO) B cells (B220+CD21intCD23int) are, in general, antigenically ignorant to GPI. To assess the relevance of IDO2 expression in these two distinct B cell subtypes, sort-purified populations of IDO2 wt MZ and FO B cells were co-injected with IDO2 ko KRN T cells into TCR ko IDO2 ko recipient mice. Transfer of MZ B cells alone generated a stronger arthritic response than the no B cell control. In contrast, arthritis in mice with FO B cells added back was reduced compared to the MZ B cell add-backs and not significantly different than the control (Figure 4B). This may be because GPI-specific MZ B cells are effectively “pre-activated”, with higher basal levels of activation markers including CD69, CD80, and CD86, leading to a rapid anti-GPI response (29). Alternatively, due to the requirement for IDO2 in Ag-specific B cells, the observed differential in arthritic responses could be due to a higher proportion of GPI-reactive cells in the MZ compartment. To control for GPI-specificity, MZ and FO B cells were sorted from mk147 anti-GPI B cell receptor tg mice (29). This allowed us to compare the effect of MZ vs. FO B cells that all recognize the GPI autoantigen. Both MZ and FO B cells from mk147 transgenic mice were able to restore wt arthritis in IDO2 ko recipients (Figure 4C), indicating that both populations can contribute to the IDO2-mediated pro-inflammatory effect. However, similar to non-tg B cells, the mk147 tg MZ B cells elicited a stronger arthritic response compared to the mk147 tg FO B cells, demonstrating that even when controlling for autoantigen specificity by using tg B cells, IDO2 is particularly important in the marginal zone B cell population.
Adoptive transfer of IDO2-expressing B cells is sufficient to induce wt contact hypersensitivity (CHS) responses in IDO2-deficient hosts
To determine if IDO2 expression in B cells is required to drive inflammation outside the context of the KRN model, we used a second model of inflammation shown to be IDO2-dependent, the CHS model. Previous studies have shown that IDO2 ko mice exhibit significantly reduced ear swelling and associated levels of inflammatory (IL-6, TNFα, IFNγ), chemotactic (MCP1/CCL2), and hematopoietic (GM-CSF, G-CSF) cytokines in response to hapten challenge (15). Although considered a classic model of T cell immunity, the CHS response has been shown to also require the involvement of anti-hapten B cells and antibodies (39, 40). This allowed us to test whether IDO2 mediates inflammation in CHS responses through the same B cell-specific mechanism we described for arthritis.
The role of IDO2 was examined using a B-cell add-back experiment (Figure 5A). Recipient and donor IDO2 wt and ko mice were first sensitized with oxazolone. Total B cells were isolated from donors and subsequently adoptively transferred to recipient mice. Mice were challenged with oxazalone on the ears and swelling measured after 24h. Control transfers showed a robust CHS response when IDO2 wt B cells were transferred into wt recipients and a significantly reduced response when IDO2 ko B cells were transferred to ko recipients. As in the arthritis model, adoptive transfer of IDO2 wt but not ko B cells restored a robust CHS response in IDO2 ko recipients (Figure 5B). The increased swelling in the hosts with transferred wt B cells was confirmed by histology (Figure 5C). The role of IDO2 in B cells is thus not limited to autoimmunity or to the KRN model, but is a general phenomenon contributing to inflammatory immune responses in two distinct model systems.
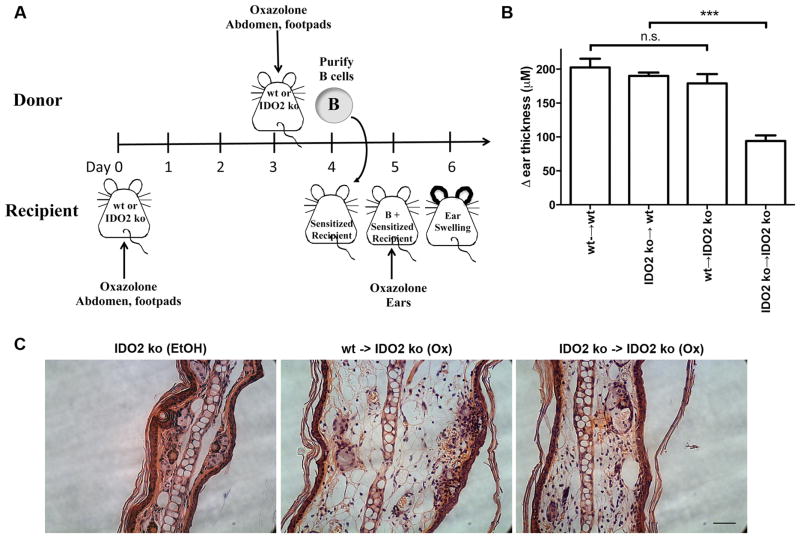
Figure 5. IDO2 wt B cells restore wt contact hypersensitivity response in IDO2-deficient hosts.
A. IDO2 wt or ko BALB/c recipient mice were sensitized to oxazalone on the abdomen and footpads. After 4 days, B cells from IDO2 wt or ko mice that had been sensitized 24 hours to oxazolone were adoptively transferred into the sensitized hosts. 24h after B cell transfer, a CHS response was elicited by painting of oxazolone on the ears of recipient mice and swelling measured relative to ear thickness prior to transfer. B. Control transfers of wt B cells into wt hosts (n=10) and IDO2 ko B cells into IDO2 ko hosts (n=14) confirm the reduction in CHS response in IDO2 ko mice. Add-back of IDO2 ko B cells into wt hosts (n=5) does not alter CHS response. Importantly, add-back of wt B cells into IDO2 ko hosts (n=9) fully restores contact hypersensitivity response to levels seen in wt hosts. Data pooled from four independent experiments. C. Histological examination confirms a greater CHS response in wt B cell add-backs. Swelling in ears of IDO2 ko mice with transferred wt B cells is greater than in mice with transferred IDO2 ko B cells. Ears are compared to ethanol treated controls. Representative sections from n=3 mice per group. Scale bar, 50μm. *** p<0.001, n.s.=not significant.
IDO2 is upregulated in B cells in response to both T-dependent and T-independent stimuli
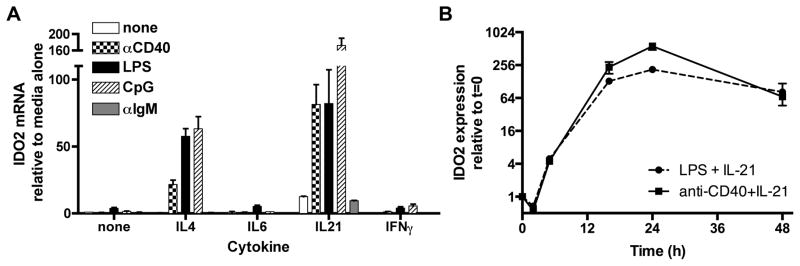
Having established that it is both necessary and sufficient for IDO2 to be expressed in B cells in order promote arthritis and CHS, we proceeded to investigate the pathways responsible for regulating the expression of IDO2 in B cells. Since IDO2 ko mice have diminished T cell responses, we first tested whether B cells might upregulate IDO2 in response to T cell-mediated costimulatory molecules and cytokines. The interaction between B and T cells required to produce an antibody response is complex and multidirectional, requiring not only the direct interaction of a TCR with an MHC Class II molecule, but also a wide variety of costimulatory molecules including ICOS/ICOSL, CD40L/CD40, and PDL1/PD1, as well as cytokines such as IL-4 and IL-21. We sought to mimic this interaction through in vitro stimulation of B cells and subsequent examination of the effect of various stimuli on the expression of IDO2 mRNA. To mimic T cell help via costimulation, purified B cells were cultured with anti-CD40 (Figure 6A). Alone, this stimulus has no effect on IDO2 message. Since cytokine signals are critical for effective T:B cell crosstalk, several cytokines were subsequently tested in concert with anti-CD40. IL-4, IL-6, and IL-21 are all downregulated in IDO2 knockout arthritic mice, suggesting a connection between these signals and IDO2 (14). These cytokines, especially IL-4 and IL-21, are intricately involved in the interaction between B and T follicular helper (TFH) cells (41). Interferon gamma (IFNγ) induces IDO1 expression (42, 43), thus we investigated whether it would also upregulate IDO2. 48h in vitro stimulation of purified B cells with anti-CD40 upregulated IDO2 expression only in conjunction with IL-4 or IL-21, but not IL-6 or IFNγ (Figure 6A).
Figure 6. IDO2 is upregulated in B cells in response to both T-dependent and T-independent stimuli.
A. Purified B cells were cultured at 2x106 cells/ml for 48h with no stimulus, anti-CD40, LPS, CpG, or anti-IgM alone or plus IL-4, IL-6, IL-21, or IFNγ. All stimuli except anti-IgM upregulated IDO2 expression when cultured with the cytokines IL-4 or IL-21. IL-6 or IFNγ did not upregulate IDO2, nor did any cytokine in conjunction with anti-IgM. IL-21 alone slightly induced IDO2 mRNA production B. Cells were stimulated with anti-CD40 + IL-21 or LPS + IL-21 and IDO2 expression measured at 2, 5, 15, 24, and 48h. Data is pooled from a minimum of two independent in vitro experiments.
Next, we investigated whether T-independent, polyclonal stimuli can upregulate IDO2 in B cells. LPS, a potent immune stimulating PAMP produced by gram-negative bacteria, signals in B cells and other APCs through TLR4, a surface-expressed TLR. LPS induced low levels of IDO2 message in purified B cells, but upregulated IDO2 message dramatically when IL-4 or IL-21 was added (Figure 6A). CpG ODN is an agonist of TLR9, a TLR expressed in endosomal compartments of several immune cell types, including B cells. Like anti-CD40, CpG only upregulated IDO2 in conjunction with IL-4 or IL-21, suggesting IDO2 functions upstream or independently of IFNγ and IL-6 production. Neither LPS nor CpG alone had a significant effect on IDO2 message, indicating that T-independent stimuli also require additional signaling in the for IDO2 upregulation. Thus, both signal 2 and an appropriate cytokine signal influence IDO2 transcription.
B cell signaling is initiated by the binding of the B cell receptor with a cognate antigen (signal 1). This can be mimicked by anti-IgM, a stimulus that binds the BCR receptor and initiates the BCR signaling cascade. We tested whether anti-IgM alone or in concert with cytokine signals could upregulate IDO2. Surprisingly, anti-IgM did not upregulate IDO2 expression in B cells under any conditions tested (Figure 6A). The increase in IDO2 seen in the anti-IgM + IL-21 condition was not significantly different than the slight upregulation in IDO2 seen with IL-21 alone (Figure 6A). Thus, IDO2 is not directly induced by stimulation of the B cell receptor (anti-IgM, signal 1), but requires a T-independent or T-dependent stimulus (signal 2) plus a cytokine (signal 3).
Previously, we showed that the IDO pathway only needed to be inhibited during the onset of the autoimmune response to provide protection against arthritis (8), suggesting that IDO2 may be expressed transiently in B cells during their activation. To determine the kinetics of IDO2 expression, B cells were stimulated with anti-CD40 + IL-21 or LPS + IL-21, the two conditions which produced the most dramatic upregulation of IDO2. IDO2 induction occurs rapidly, with IDO2 message detectable within the first 5 hours of culture. Expression peaked at approximately 24 hours in culture and was sustained over the next 2 days (Figure 6B). No significant differences in the timing of induction were seen with LPS + IL-21 vs. anti-CD40 + IL-21. Taken together, these data demonstrate that IDO2 expression in B cells is quickly and dramatically upregulated in response to specific costimulatory and cytokine signals and remains elevated for several days.
IDO2 modulates a subset of B cell costimulatory markers in vitro
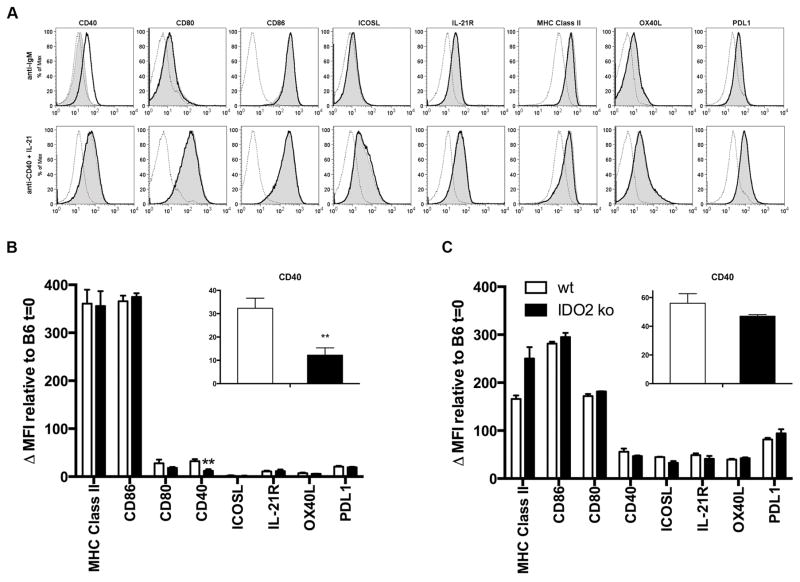
The upregulation of IDO2 by anti-CD40 plus cytokines suggests that IDO2 expression is associated with T cell help and the crosstalk between B and T cells necessary for a robust B cell response. We thus sought to determine whether IDO2-deficient B cells have an intrinsic defect either in antigen presentation or costimulation affecting this interaction. Given the idea that IDO2 may be affecting T:B collaboration, we assessed the expression of B cell costimulatory markers in IDO2 ko compared to wt B cells in response to in vitro stimulation. Cells were stimulated with either anti-IgM alone or anti-CD40 + IL-21 and levels of B cell costimulatory markers directly measured by flow cytometry (Figure 7). When stimulated with anti-IgM alone, wt B cells upregulate costimulatory molecules needed for collaboration with T cells, including CD40, the B7 markers (CD80/86), ICOSL, IL-21R, OX40L, PDL1, and the MHC Class II molecule itself (Figure 7A, B). IDO2 ko B cells also upregulate CD80, CD86, ICOSL, IL-21R, OX40L, PDL1, and MHC Class II in response to anti-IgM stimulation. In contrast, CD40 levels are reduced on IDO2 ko B cells in response to anti-IgM by ~2.5 fold at 72h in culture (Figure 7B). This differential effect of anti-IgM signaling on IDO2 ko B cells as compared to their wt counterparts indicates that IDO2 ko B cells may have a defective ability to elicit T cell help after initial encounter with antigen (signal 1) due to a reduction in the CD40 costimulatory molecule. To determine if IDO2 ko B cells are defective in their ability to upregulate costimulatory markers in response to other stimuli, IDO2 wt and ko splenocytes were cultured with anti-CD40 + IL-21, culture conditions that mimic signals derived from T cell help. No differences between IDO2 ko and wt cells were seen under these conditions for any of the markers tested (Figure 7C). This suggests that there is not a global defect in the ability of IDO2 ko B cells to upregulate costimulatory markers. These data demonstrate that IDO2 ko B cells can indeed become activated, express costimulatory molecules, and act as antigen-presenting cells when sufficient signals are received, as seen when anti-CD40 and IL-21 are supplied, but suggests that the lack of IDO2 compromises the activation process under other conditions, including anti-IgM stimulation, that may be relevant to the deficiencies observed in IDO2 ko B cells in vivo.
Figure 7. IDO2 modulates a subset of B cell costimulatory markers in vitro.
A. wt and IDO2 ko splenocytes were cultured for 72h with anti-IgM or anti-CD40 + IL-21. Costimulatory molecules were upregulated relative to unstimulated C57BL/6 cells. Experiment was repeated 3 times with a representative experiment shown. dotted line = naïve B6, bold line = IDO2 wt at 72h, shaded = IDO2 ko at 72h. B. CD40 expression is significantly reduced in IDO2 ko B cells (n=6) relative to IDO2 wt (n=6). No differences were seen for any other measured marker. C. No differences in costimulatory markers were seen when IDO2 wt (n=3) or IDO2 ko (n=3) B cells were cultured 72h with anti-CD40 + IL-21. Data pooled from a minimum of two independent experiments. **=p<0.01. Not specified=not significant.
Discussion
Novel immune modulatory mechanisms that modify the severity of autoimmune disorders may help illuminate their pathogenicity and promote insights into their clinical management. The increasingly apparent role of IDO2 as an immune modulator has led to important questions regarding its mechanism of action, particularly with regard to its better-studied family member IDO1. Little is known about the enzymatic, cellular and molecular functions of IDO2, but there is evidence of differences at each level (44). IDO2 is expressed in dendritic cells (15, 16), suggesting it acts like IDO1 to modulate the consequences of antigen presentation, but the precise consequences of IDO2 expression in this setting have yet to be determined. Our previous work has suggested a role for IDO2, but not IDO1, in mediating B and T cell responses in the KRN model of autoimmune arthritis, as evidenced by reduced autoantibody production, number of antibody secreting cells, and differentiated T helper cell populations in IDO2 ko arthritic mice (14). Initial experiments also conclusively demonstrated this effect to be T cell extrinsic, suggesting that IDO2 may be involved in antigen presentation to T cells. Our work here confirms and extends IDO2’s role in autoimmune arthritis by defining its critical function in B cells, one that is consistent with feedback control on T cell help needed for autoantibody generation. Notably, in vitro studies revealed a reduction in levels of the costimulatory marker CD40 on B cells. Given this result, along with the previously identified reduction of TFH-associated cytokines IL-4 and IL-21 in IDO2 ko arthritic mice (14), we propose here that IDO2 influences the effects of antigen presentation to T cells by modifying the costimulatory crosstalk between B and T cells.
Previously, our lab reported that IDO2 ko mice exhibit deficient autoreactive T and B cell responses mediating arthritis and that these defects are extrinsic to T cells (14). In our current study, we define the B cell as being the critical IDO2-expressing cell necessary for arthritis development. In addition to the secretion of pathogenic autoantibodies, B cells have many important functions during the onset and progression of immune responses (23, 45–48). In particular, they are highly potent APCs for specific antigens recognized and internalized through the BCR (49, 50), a role that is important in T cell activation. B cells can also act in a bystander role, a function not dependent on a cognate, antigen interaction with the TCR. Through a series of adoptive transfer experiments, we found that IDO2 must function in cognate antigen-specific B cells to mediate a robust arthritic response, suggesting a direct role for IDO2 in mediating the collaboration between T and B cells.
Mature B cells can be separated into subsets, based on their phenotypic and functional characteristics. IDO2 was found to exert particularly dramatic effects in MZ B cells. Adoptive transfer of IDO2 expressing MZ B cells into IDO2 ko recipients significantly increases arthritis development compared to transfer of conventional follicular B cells. This effect also seems to be dependent on the B cell specificity, with autoantigen-specific mk147 tg MZ B cells exhibiting a particularly dramatic effect on arthritis development. Interestingly, the antibodies produced by the mk147 tg B cells are IgM and generally do not contribute directly to disease pathogenesis, which requires anti-GPI IgG autoantibodies (22, 29). The fact that mk147 tg B cells are able to restore wt levels of arthritis suggests that these cells elicit T cell help, with these T cells then inducing endogenous IDO2 ko B cells to produce the pathogenic anti-GPI IgG, further supporting the hypothesis that IDO2 plays an important role in the cross-talk between autoreactive B and T cells.
In the CHS model, B cells also act at the initiation of the autoimmune response, though the relevant B cell population may differ from what is observed in the KRN model. Unlike the arthritis model, where we demonstrate that classical B-2 B cell populations are critical for the arthritic response, it is the B-1 B cell population that has been identified as important for initiation of the CHS elicitation phase response (39). In fact, B-2 B cells, including MZ B cells, may be suppressive under some conditions (51). In CHS, B-1 B cells generate IgM within 24h following hapten challenge (52) and mediate the CHS response through a complement-dependent mechanism allowing for T cell recruitment (53). The role of IDO2 in this process remains to be discovered, but there are several important parallels with the KRN model, particularly in the role of B cells in antigen presentation and T cell recruitment. In addition, as in the KRN model, the CHS model demonstrates a unique and separate role for IDO2 as compared to IDO1 (15).
In vitro data gives us insight into both upstream regulators and downstream effectors of IDO2. We found that signal 1 by itself, as triggered by anti-IgM, is insufficient to upregulate IDO2, yet IDO2 ko B cells nevertheless responded differently to this stimulus by generating a less robust costimulatory response. A mimic of T cell help, anti-CD40, also did not upregulate IDO2 on its own, but did so in concert with the TFH associated cytokines IL-4 or IL-21. The T-independent stimuli LPS and CpG, which with IL-4 or IL-21 also upregulated IDO2, act through Toll-like receptors to promote B cell activation. Specifically, TLR4 (LPS) and TLR9 (CpG) engage MyD88 to initiate a downstream signaling cascade that can activate the MAPK and NF-κB pathways, leading to the production of cytokines and various effector molecules (54, 55). TLR9 has been linked with autoimmunity both in the context of autoantibody production and maintenance of tolerance (56, 57). CpG is also a potent inducer of IFNγ and IL-6 (58). IDO2 is likely upstream of both of these cytokines as neither IFNγ nor IL-6 affected IDO2 expression in any context measured.
Studies with autoimmune mice highlight the differences in immune regulatory functions between IDO1 and IDO2 and the importance of targeting the two molecules individually in a therapeutic context. Existing small molecule inhibitors, particularly 1MT, likely do not directly affect IDO2 itself, but rather influence the IDO pathway downstream of tryptophan catabolism (5). IDO1 cannot be understood wholly as an anti-inflammatory or immunosuppressive factor, but it clearly influences production of Treg populations and in that sense acts to promote immunological tolerance, as illustrated in models of maternal-fetal tolerance and tumoral immune evasion (5). IDO2, in contrast, appears to have a more pro-inflammatory role in immune modulation, as evidenced by the exacerbated arthritis and CHS responses seen in IDO2 wt vs ko mice (14, 15). B220+CD11c+ cells have been shown to be an important cell type for IDO1-mediated inhibition of T cell function (31–35). Like IDO1, IDO2 appears to affect T cell help indirectly, as shown in the present study through its expression in B cells. Importantly, IDO2 wt B220+CD11c+ cells, did not restore wt arthritis when adoptively transferred into IDO2 ko hosts, further clarifying the distinctive roles of IDO1 and IDO2 in mediating immune responses. Since these two immune modulatory enzymes have non-overlapping and, in some contexts, contrasting immunoregulatory functions, it is critical that specific inhibitors be developed to target them selectively.
In considering the medicinal import that IDO2 may offer for selective treatment of autoimmunity, we have noted previously that while IDO2 ko mice exhibit an attenuated response to an autoantigen, they respond similarly to wt mice after immunization with a model neoantigen (14). Similarly, in this study, while we see strong, IDO2-dependent differences in B-cell mediated inflammatory responses in vivo, these differences can be overcome in vitro when appropriate external stimuli and cytokines are provided, as in the case of anti-CD40 + IL-21. This suggests that IDO2 ko B cells are not intrinsically defective but that in vivo they are not receiving appropriate or sufficient stimuli to trigger pathogenic autoantibody production. This was also mimicked in our in vitro cultures stimulated with signal 1 (anti-IgM) alone. We hypothesize that adjuvants necessary for immunizations with model antigens provide signals that overcome the loss of IDO2, similar to what is seen with our in vitro data with anti-CD40 + IL-21 stimulated cells. This further supports the idea that IDO2 is important in the initiation phase of immune responses, as adjuvant compounds can effectively bypass some of the initial signals that are necessary for a spontaneous response.
In summary, our work demonstrates that IDO2 functions as a modifier in B cells to control pathogenic inflammation and autoimmunity. The identification of the relevant cell type for the action of IDO2 in modulating immune responses is critical for determining the molecular mechanism by which IDO2 can exert its apparent selective effects on pathogenic inflammatory phenomena. Additionally, this finding may offer potential medical import with regard to IDO2 targeting strategies for autoimmunity, akin to those similarly leveraged for IDO1 inhibitors in cancer.
Acknowledgments
This work was supported in part by a grant from the Lupus Research Institute, Inc. (L.M.-N.), NIH grants R01 AR057847 (L.M.-N.) and R21 CA159337 (G.C.P.) and by support from the Zuckerman Family Autoimmune Disorder Research Fund at Lankenau Medical Center, the Lankenau Medical Center Foundation, and Main Line Health.
Abbreviations used in this article
- 1MT
1-methyltryptophan
- APC
antigen-presenting cell
- CHS
contact hypersensitivity
- DC
dendritic cell
- FO
follicular
- GPI
glucose-6-phosphate isomerase
- ko
knockout
- MZ
marginal zone
- TFH
T follicular helper
- tg
transgene
- Trp
tryptophan
- wt
wild-type
References
- 1.Yuasa HJ, Mizuno K, Ball HJ. Low efficiency IDO2 enzymes are conserved in lower vertebrates, whereas higher efficiency IDO1 enzymes are dispensable. FEBS J. 2015;282:2735–2745. doi: 10.1111/febs.13316. [DOI] [PubMed] [Google Scholar]
- 2.Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B, Brown C, Mellor AL. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281:1191–1193. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- 3.Prendergast GC. Immune escape as a fundamental trait of cancer: focus on IDO. Oncogene. 2008;27:3889–3900. doi: 10.1038/onc.2008.35. [DOI] [PubMed] [Google Scholar]
- 4.Prendergast GC, Chang MY, Mandik-Nayak L, Metz R, Muller AJ. Indoleamine 2,3-dioxygenase as a modifier of pathogenic inflammation in cancer and other inflammation-associated diseases. Curr Med Chem. 2011;18:2257–2262. doi: 10.2174/092986711795656072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prendergast GC, Smith C, Thomas S, Mandik-Nayak L, Laury-Kleintop L, Metz R, Muller AJ. Indoleamine 2,3-dioxygenase pathways of pathogenic inflammation and immune escape in cancer. Cancer Immunol Immunother. 2014;63:721–735. doi: 10.1007/s00262-014-1549-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Szanto S, Koreny T, Mikecz K, Glant TT, Szekanecz Z, Varga J. Inhibition of indoleamine 2,3-dioxygenase-mediated tryptophan catabolism accelerates collagen-induced arthritis in mice. Arthritis research & therapy. 2007;9:R50. doi: 10.1186/ar2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fallarino F, Volpi C, Zelante T, Vacca C, Calvitti M, Fioretti MC, Puccetti P, Romani L, Grohmann U. IDO mediates TLR9-driven protection from experimental autoimmune diabetes. J Immunol. 2009;183:6303–6312. doi: 10.4049/jimmunol.0901577. [DOI] [PubMed] [Google Scholar]
- 8.Scott GN, DuHadaway J, Pigott E, Ridge N, Prendergast GC, Muller AJ, Mandik-Nayak L. The immunoregulatory enzyme IDO paradoxically drives B cell-mediated autoimmunity. J Immunol. 2009;182:7509–7517. doi: 10.4049/jimmunol.0804328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nature reviews. 2004;4:762–774. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 10.Shinde R, Shimoda M, Chaudhary K, Liu H, Mohamed E, Bradley J, Kandala S, Li X, Liu K, McGaha TL. B Cell-Intrinsic IDO1 Regulates Humoral Immunity to T Cell-Independent Antigens. J Immunol. 2015;195:2374–2382. doi: 10.4049/jimmunol.1402854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Metz R, Rust S, Duhadaway JB, Mautino MR, Munn DH, Vahanian NN, Link CJ, Prendergast GC. IDO inhibits a tryptophan sufficiency signal that stimulates mTOR: A novel IDO effector pathway targeted by D-1-methyl-tryptophan. Oncoimmunology. 2012;1:1460–1468. doi: 10.4161/onci.21716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ravishankar B, Liu H, Shinde R, Chaudhary K, Xiao W, Bradley J, Koritzinsky M, Madaio MP, McGaha TL. The amino acid sensor GCN2 inhibits inflammatory responses to apoptotic cells promoting tolerance and suppressing systemic autoimmunity. Proc Natl Acad Sci U S A. 2015;112:10774–10779. doi: 10.1073/pnas.1504276112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Munn DH, Sharma MD, Baban B, Harding HP, Zhang Y, Ron D, Mellor AL. GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3-dioxygenase. Immunity. 2005;22:633–642. doi: 10.1016/j.immuni.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 14.Merlo LM, Pigott E, Duhadaway JB, Grabler S, Metz R, Prendergast GC, Mandik-Nayak L. IDO2 Is a Critical Mediator of Autoantibody Production and Inflammatory Pathogenesis in a Mouse Model of Autoimmune Arthritis. J Immunol. 2014;192:2082–2090. doi: 10.4049/jimmunol.1303012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Metz R, Smith C, DuHadaway JB, Chandler P, Baban B, Merlo LM, Pigott E, Keough MP, Rust S, Mellor AL, Mandik-Nayak L, Muller AJ, Prendergast GC. IDO2 is critical for IDO1-mediated T-cell regulation and exerts a non-redundant function in inflammation. Int Immunol. 2014;26:357–367. doi: 10.1093/intimm/dxt073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trabanelli S, Ocadlikova D, Ciciarello M, Salvestrini V, Lecciso M, Jandus C, Metz R, Evangelisti C, Laury-Kleintop L, Romero P, Prendergast GC, Curti A, Lemoli RM. The SOCS3-independent expression of IDO2 supports the homeostatic generation of T regulatory cells by human dendritic cells. J Immunol. 2014;192:1231–1240. doi: 10.4049/jimmunol.1300720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Metz R, Duhadaway JB, Kamasani U, Laury-Kleintop L, Muller AJ, Prendergast GC. Novel tryptophan catabolic enzyme IDO2 is the preferred biochemical target of the antitumor indoleamine 2,3-dioxygenase inhibitory compound D-1-methyl-tryptophan. Cancer Res. 2007;67:7082–7087. doi: 10.1158/0008-5472.CAN-07-1872. [DOI] [PubMed] [Google Scholar]
- 18.Criado G, Simelyte E, Inglis JJ, Essex D, Williams RO. Indoleamine 2,3 dioxygenase-mediated tryptophan catabolism regulates accumulation of Th1/Th17 cells in the joint in collagen-induced arthritis. Arthritis Rheum. 2009;60:1342–1351. doi: 10.1002/art.24446. [DOI] [PubMed] [Google Scholar]
- 19.Sakurai K, Zou JP, Tschetter JR, Ward JM, Shearer GM. Effect of indoleamine 2,3-dioxygenase on induction of experimental autoimmune encephalomyelitis. J Neuroimmunol. 2002;129:186–196. doi: 10.1016/s0165-5728(02)00176-5. [DOI] [PubMed] [Google Scholar]
- 20.Xu H, Oriss TB, Fei M, Henry AC, Melgert BN, Chen L, Mellor AL, Munn DH, Irvin CG, Ray P, Ray A. Indoleamine 2,3-dioxygenase in lung dendritic cells promotes Th2 responses and allergic inflammation. Proc Natl Acad Sci U S A. 2008;105:6690–6695. doi: 10.1073/pnas.0708809105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kouskoff V, Korganow AS, Duchatelle V, Degott C, Benoist C, Mathis D. Organ-specific disease provoked by systemic autoimmunity. Cell. 1996;87:811–822. doi: 10.1016/s0092-8674(00)81989-3. [DOI] [PubMed] [Google Scholar]
- 22.Matsumoto I, Staub A, Benoist C, Mathis D. Arthritis provoked by linked T and B cell recognition of a glycolytic enzyme. Science. 1999;286:1732–1735. doi: 10.1126/science.286.5445.1732. [DOI] [PubMed] [Google Scholar]
- 23.Anolik JH. B cell biology: implications for treatment of systemic lupus erythematosus. Lupus. 2013;22:342–349. doi: 10.1177/0961203312471576. [DOI] [PubMed] [Google Scholar]
- 24.LaBranche TP, Hickman-Brecks CL, Meyer DM, Storer CE, Jesson MI, Shevlin KM, Happa FA, Barve RA, Weiss DJ, Minnerly JC, Racz JL, Allen PM. Characterization of the KRN cell transfer model of rheumatoid arthritis (KRN-CTM), a chronic yet synchronized version of the K/BxN mouse. Am J Pathol. 2010;177:1388–1396. doi: 10.2353/ajpath.2010.100195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Korganow AS, Ji H, Mangialaio S, Duchatelle V, Pelanda R, Martin T, Degott C, Kikutani H, Rajewsky K, Pasquali JL, Benoist C, Mathis D. From systemic T cell self-reactivity to organ-specific autoimmune disease via immunoglobulins. Immunity. 1999;10:451–461. doi: 10.1016/s1074-7613(00)80045-x. [DOI] [PubMed] [Google Scholar]
- 26.Chaplin DD, Fu Y. Cytokine regulation of secondary lymphoid organ development. Curr Opin Immunol. 1998;10:289–297. doi: 10.1016/s0952-7915(98)80167-2. [DOI] [PubMed] [Google Scholar]
- 27.Xu H, Li X, Liu D, Li J, Zhang X, Chen X, Hou S, Peng L, Xu C, Liu W, Zhang L, Qi H. Follicular T-helper cell recruitment governed by bystander B cells and ICOS-driven motility. Nature. 2013;496:523–527. doi: 10.1038/nature12058. [DOI] [PubMed] [Google Scholar]
- 28.Qi H, Cannons JL, Klauschen F, Schwartzberg PL, Germain RN. SAP-controlled T-B cell interactions underlie germinal centre formation. Nature. 2008;455:764–769. doi: 10.1038/nature07345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mandik-Nayak L, Racz J, Sleckman BP, Allen PM. Autoreactive marginal zone B cells are spontaneously activated but lymph node B cells require T cell help. J Exp Med. 2006;203:1985–1998. doi: 10.1084/jem.20060701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goodnow CC, Crosbie J, Adelstein S, Lavoie TB, Smith-Gill SJ, Brink RA, Pritchard-Briscoe H, Wotherspoon JS, Loblay RH, Raphael K, et al. Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. Nature. 1988;334:676–682. doi: 10.1038/334676a0. [DOI] [PubMed] [Google Scholar]
- 31.Mellor AL, Baban B, Chandler P, Marshall B, Jhaver K, Hansen A, Koni PA, Iwashima M, Munn DH. Cutting edge: induced indoleamine 2,3 dioxygenase expression in dendritic cell subsets suppresses T cell clonal expansion. J Immunol. 2003;171:1652–1655. doi: 10.4049/jimmunol.171.4.1652. [DOI] [PubMed] [Google Scholar]
- 32.Baban B, Hansen AM, Chandler PR, Manlapat A, Bingaman A, Kahler DJ, Munn DH, Mellor AL. A minor population of splenic dendritic cells expressing CD19 mediates IDO-dependent T cell suppression via type I IFN signaling following B7 ligation. Int Immunol. 2005;17:909–919. doi: 10.1093/intimm/dxh271. [DOI] [PubMed] [Google Scholar]
- 33.Sharma MD, Baban B, Chandler P, Hou DY, Singh N, Yagita H, Azuma M, Blazar BR, Mellor AL, Munn DH. Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes directly activate mature Tregs via indoleamine 2,3-dioxygenase. J Clin Invest. 2007;117:2570–2582. doi: 10.1172/JCI31911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muller AJ, Sharma MD, Chandler PR, Duhadaway JB, Everhart ME, Johnson BA, 3rd, Kahler DJ, Pihkala J, Soler AP, Munn DH, Prendergast GC, Mellor AL. Chronic inflammation that facilitates tumor progression creates local immune suppression by inducing indoleamine 2,3 dioxygenase. Proc Natl Acad Sci U S A. 2008;105:17073–17078. doi: 10.1073/pnas.0806173105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Munn DH, Sharma MD, Hou D, Baban B, Lee JR, Antonia SJ, Messina JL, Chandler P, Koni PA, Mellor AL. Expression of indoleamine 2,3-dioxygenase by plasmacytoid dendritic cells in tumor-draining lymph nodes. J Clin Invest. 2004;114:280–290. doi: 10.1172/JCI21583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rubtsov AV, Rubtsova K, Fischer A, Meehan RT, Gillis JZ, Kappler JW, Marrack P. Toll-like receptor 7 (TLR7)-driven accumulation of a novel CD11c(+) B-cell population is important for the development of autoimmunity. Blood. 2011;118:1305–1315. doi: 10.1182/blood-2011-01-331462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rubtsov AV, Rubtsova K, Kappler JW, Marrack P. TLR7 drives accumulation of ABCs and autoantibody production in autoimmune-prone mice. Immunol Res. 2013;55:210–216. doi: 10.1007/s12026-012-8365-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rubtsov AV, Rubtsova K, Kappler JW, Jacobelli J, Friedman RS, Marrack P. CD11c-Expressing B Cells Are Located at the T Cell/B Cell Border in Spleen and Are Potent APCs. J Immunol. 2015;195:71–79. doi: 10.4049/jimmunol.1500055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Szczepanik M, Akahira-Azuma M, Bryniarski K, Tsuji RF, Kawikova I, Ptak W, Kiener C, Campos RA, Askenase PW. B-1 B cells mediate required early T cell recruitment to elicit protein-induced delayed-type hypersensitivity. J Immunol. 2003;171:6225–6235. doi: 10.4049/jimmunol.171.11.6225. [DOI] [PubMed] [Google Scholar]
- 40.Tsuji RF, Szczepanik M, Kawikova I, Paliwal V, Campos RA, Itakura A, Akahira-Azuma M, Baumgarth N, Herzenberg LA, Askenase PW. B cell-dependent T cell responses: IgM antibodies are required to elicit contact sensitivity. J Exp Med. 2002;196:1277–1290. doi: 10.1084/jem.20020649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Crotty S. Follicular helper CD4 T cells (TFH) Annu Rev Immunol. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 42.Taylor MW, Feng GS. Relationship between interferon-gamma, indoleamine 2,3-dioxygenase, and tryptophan catabolism. FASEB J. 1991;5:2516–2522. [PubMed] [Google Scholar]
- 43.Munn DH, Shafizadeh E, Attwood JT, Bondarev I, Pashine A, Mellor AL. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J Exp Med. 1999;189:1363–1372. doi: 10.1084/jem.189.9.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prendergast GC, Metz R, Muller AJ, Merlo LM, Mandik-Nayak L. IDO2 in Immunomodulation and Autoimmune Disease. Front Immunol. 2014;5:585. doi: 10.3389/fimmu.2014.00585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lund FE. Cytokine-producing B lymphocytes-key regulators of immunity. Curr Opin Immunol. 2008;20:332–338. doi: 10.1016/j.coi.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luu VP, Vazquez MI, Zlotnik A. B cells participate in tolerance and autoimmunity through cytokine production. Autoimmunity. 2014;47:1–12. doi: 10.3109/08916934.2013.856006. [DOI] [PubMed] [Google Scholar]
- 47.O’Neill SK, Shlomchik MJ, Glant TT, Cao Y, Doodes PD, Finnegan A. Antigen-specific B cells are required as APCs and autoantibody-producing cells for induction of severe autoimmune arthritis. J Immunol. 2005;174:3781–3788. doi: 10.4049/jimmunol.174.6.3781. [DOI] [PubMed] [Google Scholar]
- 48.Youinou P, Hillion S, Jamin C, Pers JO, Saraux A, Renaudineau Y. B lymphocytes on the front line of autoimmunity. Autoimmun Rev. 2006;5:215–221. doi: 10.1016/j.autrev.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 49.Lanzavecchia A. Antigen-specific interaction between T and B cells. Nature. 1985;314:537–539. doi: 10.1038/314537a0. [DOI] [PubMed] [Google Scholar]
- 50.Chen X, Jensen PE. The role of B lymphocytes as antigen-presenting cells. Arch Immunol Ther Exp (Warsz) 2008;56:77–83. doi: 10.1007/s00005-008-0014-5. [DOI] [PubMed] [Google Scholar]
- 51.Watanabe R, Fujimoto M, Ishiura N, Kuwano Y, Nakashima H, Yazawa N, Okochi H, Sato S, Tedder TF, Tamaki K. CD19 expression in B cells is important for suppression of contact hypersensitivity. Am J Pathol. 2007;171:560–570. doi: 10.2353/ajpath.2007.061279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Itakura A, Szczepanik M, Campos RA, Paliwal V, Majewska M, Matsuda H, Takatsu K, Askenase PW. An hour after immunization peritoneal B-1 cells are activated to migrate to lymphoid organs where within 1 day they produce IgM antibodies that initiate elicitation of contact sensitivity. J Immunol. 2005;175:7170–7178. doi: 10.4049/jimmunol.175.11.7170. [DOI] [PubMed] [Google Scholar]
- 53.Tsuji RF, Kawikova I, Ramabhadran R, Akahira-Azuma M, Taub D, Hugli TE, Gerard C, Askenase PW. Early local generation of C5a initiates the elicitation of contact sensitivity by leading to early T cell recruitment. J Immunol. 2000;165:1588–1598. doi: 10.4049/jimmunol.165.3.1588. [DOI] [PubMed] [Google Scholar]
- 54.Kawai T, Akira S. Signaling to NF-kappaB by Toll-like receptors. Trends Mol Med. 2007;13:460–469. doi: 10.1016/j.molmed.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 55.Brown J, Wang H, Hajishengallis GN, Martin M. TLR-signaling networks: an integration of adaptor molecules, kinases, and cross-talk. J Dent Res. 2011;90:417–427. doi: 10.1177/0022034510381264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fischer M, Ehlers M. Toll-like receptors in autoimmunity. Ann N Y Acad Sci. 2008;1143:21–34. doi: 10.1196/annals.1443.012. [DOI] [PubMed] [Google Scholar]
- 57.Sharma S, Fitzgerald KA, Cancro MP, Marshak-Rothstein A. Nucleic Acid-Sensing Receptors: Rheostats of Autoimmunity and Autoinflammation. J Immunol. 2015;195:3507–3512. doi: 10.4049/jimmunol.1500964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Klinman DM, Yi AK, Beaucage SL, Conover J, Krieg AM. CpG motifs present in bacteria DNA rapidly induce lymphocytes to secrete interleukin 6, interleukin 12, and interferon gamma. Proc Natl Acad Sci U S A. 1996;93:2879–2883. doi: 10.1073/pnas.93.7.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]