Abstract
The current study harnessed the variability in infants’ neural and behavioral responses as a novel method for evaluating the potential relations between motor system activation and social behavior. We used electroencephalography (EEG) to record neural activity as 7-month-old infants observed and responded to the actions of an experimenter. To determine whether motor system activation predicted subsequent imitation behavior, we assessed event-related desynchronization (ERD) at central sites during action observation as a function of subsequent behavior. Greater mu desynchronization over central sites was observed when infants subsequently reproduced the experimenter’s goal than when they did not reproduce the goal and instead selected the nongoal object. We also found that mu desynchronization during action execution predicted the infants’ later propensity to reproduce the experimenter’s goal-directed behavior. These results provide the first evidence that motor system activation predicts the imitation of other individuals’ goals during infancy.
Keywords: social cognition, imitation, infancy, neural mirroring
The ability to interpret social partners’ actions in terms of goals is fundamental to human experience and is foundational to human development (Woodward & Gerson, 2014). This ability emerges early in ontogeny; by the age of 6 months, infants attend and respond selectively to the goal structure of other individuals’ actions (Hamlin, Hallinan, & Woodward, 2008; Woodward, 1998). Despite considerable interest in infants’ action understanding and its implications for later development (Meltzoff, 1995; Wellman, Phillips, Dunphy-Lelii, & LaLonde, 2004; Woodward & Gerson, 2014), the neural mechanisms underpinning this critical social-cognitive ability are poorly understood. Activating one’s own motor system while observing someone else acting is one neural process that is hypothesized to support action understanding (Gallese, Fadiga, Fogassi, & Rizzolatti, 1996). Even so, there is active debate about whether motor system activation could facilitate action understanding in adulthood (Cook, Bird, Catmur, Press, & Heyes, 2014; Hickok, 2014) or during development (Csibra, 2008; Ferrari, Tramacere, Simpson, & Iriki, 2013; Woodward & Gerson, 2014).
One source of debate is that studies on this topic often lack independent assessments of neural activity and behaviors that index social-cognitive functions (Hickok, 2014; Woodward & Gerson, 2014). Researchers sometimes interpret differential activation of the sensorimotor system during action observation as indicating that this system functions to facilitate action understanding. However, without behavioral measurements to assess participants’ social cognition, the functional significance of this activity is unclear. Recent research with adults has addressed this issue by using transcranial magnetic stimulation (TMS). When the sensorimotor cortex is temporarily disrupted using TMS, adults are slower to visually anticipate an actor’s goal (Stadler et al., 2012) and worse at recognizing other individuals’ actions (Michael et al., 2014). These findings indicate a functional link between action understanding and motor system activation by showing that particular behavioral responses are contingent on motor system recruitment.
A growing body of research has documented that infants, like adults, recruit the motor system when viewing other individuals’ actions (e.g., Southgate, Johnson, Karoui, & Csibra, 2010). However, there is currently no evidence from infants that goes beyond simply measuring motor system responses during action observation. In part, this reflects the restricted range of experimental tools that can be used in infancy research. Even so, infants offer a vantage point on this issue that adults cannot: In adults, the perception of goal-directed action is automatic and robust (Spunt & Lieberman, 2013), but during infancy, when this ability first emerges, it may be more variable (Woodward & Gerson, 2014). Indeed, infants are variable in their behavioral responses to other individuals’ goal-directed actions, and this variation is meaningful for understanding developmental processes, both concurrently and longitudinally (Sommerville & Woodward, 2005; Wellman et al., 2004). More generally, behavioral variability, both between children and across time within the behavior of an individual child, is inherent to developmental change and can be harnessed to understand developmental mechanisms (Siegler & Shipley, 1995; Smith & Thelen, 2003). In the current study, we used infancy as a test case, examining whether developmental variability in infants’ selective behavioral response to the goal structure of an observed action reflects variation in motor system activity. We focused on a behavior we term goal imitation, that is, the propensity to reproduce the goal of an observed action.
The ability to imitate other people’s actions is critical in early development. Converging behavioral research shows that infants do not automatically copy the actions they observe. Rather, they selectively reproduce the goal-relevant aspects of the actions (e.g., Hamlin et al., 2008; Meltzoff, 1995). For example, Hamlin et al. (2008) demonstrated that when 7-month-old infants see a person grasp one of two objects, they systematically act on the same goal object (i.e., the object that the actor grasped). This response is specific to well-formed, goal-directed actions. When infants observe an actor perform an ambiguous action or observe inanimate objects move, they do not respond this way—even when these events and actions are identical in their patterns of movement and contact and entrain attention similarly (Gerson & Woodward, 2012; Mahajan & Woodward, 2009). These findings indicate that infants engage in goal imitation. Although there is open debate about the richness of infants’ understanding of other individuals’ intentions (Lou & Baillargeon, 2010; Meltzoff, 1995; Woodward & Gerson, 2014), at the very least these behavioral responses indicate that infants are sensitive to the goal structure of others’ actions.
We examined whether infants’ goal imitation is linked to recruitment of the sensorimotor system. To do so, we used the paradigm from Hamlin et al. (2008) with 7-month-old infants, while collecting electroencephalography (EEG) data. We hypothesized that recruitment of the motor system selectively predicts infants’ goal imitation. We evaluated variability in motor system recruitment by examining mu desynchronization over sensorimotor regions when the infants observed an experimenter choose between two objects. If motor system activation supports goal imitation, then within participants, we would expect mu desynchronization during action observation to be greater when an infant subsequently imitated the experimenter’s goal-directed behavior (i.e., goal response) than when an infant subsequently generated an action directed toward the other object (i.e., nongoal response). Further, we predicted that between-participants variability in the robustness of infants’ motor system activity during action execution would also relate to their tendency to imitate an observed goal-directed action.
Method
Participants
Thirty-six full-term 7-month-old infants from a metropolitan area (15 female; mean age = 7 months 8 days, range = 6 months 17 days–8 months 5 days) were included in the final sample of the study. An additional 27 infants were tested but excluded from the final sample (n = 11 females) because of technical error (n = 1) or because they became distressed shortly after application of the EEG electrode net (n = 7) or they did not meet the inclusion criteria established for the experiment (n = 19; see the Supplemental Material available online for further details on the inclusion criteria). This rate of data loss is similar to that of other EEG studies with infants (e.g., Saby, Marshall, & Meltzoff, 2012).
Procedure
Each infant sat on his or her parent’s lap in front of a black-curtained puppet stage (99 cm wide × 61 cm deep × 89 cm tall), where the infant observed reaching actions performed by an experimenter who acted as the presenter. Each testing session was recorded on video. First, the infant was fitted with a 128-channel EEG net. Once the net was in place, the infant was familiarized with each of the 12 toys that would be used during the procedure. During this familiarization period, the infant observed the presenter place a single toy on a tray and slide the tray within the infant’s reach. The infant was then given an opportunity to pick up the toy and play with it. Then, another experimenter came out from behind the stage and took the toy from the infant so that the next toy could be presented. This was continued until all 12 toys had been presented. The infants in our study engaged in coordinated visual and tactile contact with the toys on 94% of the familiarization trials, and on average, the mean duration of their reaches toward the toys was 1,452 ms. These trials provided an opportunity for us to assess EEG during action execution.
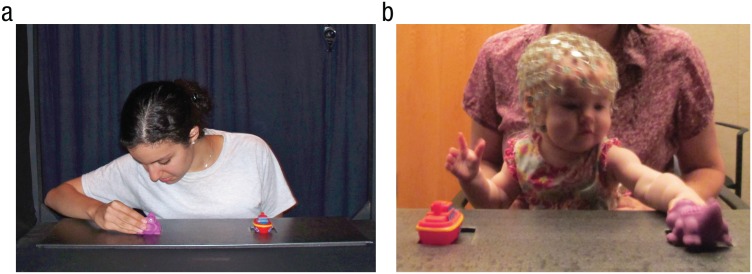
Following familiarization, a suspended curtain came down to hide the stage from the infant, and the presenter set up a tray with two toys in front of her. When the curtain came up, the presenter first ensured that the infant saw both toys. Then, the presenter drew the infant’s attention to center by saying “Hi!” (i.e., so that the infant attended to the presenter). Next, the presenter turned her head toward one of the toys and said, “Look.” After a brief pause, the presenter reached toward and grasped the toy (see Fig. 1a). The presenter reached with her right hand when reaching for the toy on her right and with her left hand when reaching for the toy on her left. On average, the duration of the presenter’s reach was 292 ms. After grasping the toy, the presenter released her grip, put her hands to her sides, and drew the infant’s attention to center by making eye contact and again saying “Hi!” Once the infant looked at the presenter, the presenter pushed the tray of toys toward the infant so that it was within the infant’s reach, and the infant was allowed to select one of the toys (see Fig. 1b). That is, the infant could select either the same toy that the presenter had selected (goal response) or the toy that the presenter had not selected (nongoal response). Trials on which the infant did not make a clear selection of one of the toys were excluded from subsequent analyses.
Fig. 1.
Depiction of the experimental setup. In the action-observation phase (a), infants observed the presenter as she selected one of two toys. Subsequently (b), the infants were given an opportunity to select one of the toys themselves.
This procedure was repeated for a total of 12 trials. The presenter alternated between reaching to the toy on the left and reaching to the toy on the right. Because there were six unique pairs of toys, after the sixth pair was presented, the presenter repeated the sequence. Each infant saw the pairs of toys presented in one of four random orders. The side that the experimenter reached to first (right or left) and which toy in each pair was the goal were counterbalanced within each random order.
Behavior coding
We identified the first toy that an infant coordinated visual and manual contact toward on a given trial as the infant’s selection. We coded the first toy that each infant touched because some infants were not always able to remove the toys from the table.1 Responses were coded by two independent coders, who agreed on 93% of the trials (κ = .87). When there were disagreements, a third coder decided which toy the infant coordinated visual and manual contact toward. This coding was completed off-line by coders who were unaware of the presenter’s actions. A trial on which the infant touched a toy without looking at it was marked as a mistrial and excluded from further analyses (5% of trials were excluded for this reason). To determine the proportion of goal-response trials, we divided the number of trials on which an infant produced a goal response by the total number of trials on which that infant generated a codable response of either kind (goal or nongoal).
To ensure that the infants were attending to the presenter as she chose between the toys, an independent observer coded (off-line) how long each infant looked at each event, using a digital coding program (Mangold, 2010). A second independent observer coded the trials of 25% of the infants, and the two coders were in agreement on 97% of these trials. Trials on which the infant was not attending to the presenter were excluded from analysis (3% of trials).
Motor system activation
Our measure of motor system activation was event-related desynchronization (ERD) of the mu rhythm (within the alpha frequency band, 6–9 Hz) over central sites during both action execution and action observation (Marshall & Meltzoff, 2011; Pfurtscheller & Aranibar, 1979). ERD refers to instances when there is less EEG power in the frequency band of interest during a test event as compared with a baseline period (Pfurtscheller & Aranibar, 1979). Prior research has demonstrated that power in the mu frequency range (i.e., 8–13 Hz for adults) is reduced over central electrode sites when adults produce actions and when they observe other people’s goal-directed actions (Arnstein, Cui, Maurits, & Gazzola, 2011; Pfurtscheller & Aranibar, 1979). This pattern is similarly found in infants—although in a lower frequency range (i.e., 6–9 Hz; Cuevas, Cannon, Yoo, & Fox, 2014; Marshall & Meltzoff, 2011; Southgate et al., 2010). This reduction in power over central sites is thought to reflect sensorimotor cortical activity. Evidence in support of this claim comes from simultaneous functional MRI and EEG recordings showing that during action observation and execution, the mu rhythm is correlated with activation of several cortical areas of the mirror neuron system in adults (Arnstein et al., 2011). As has been demonstrated in adults (e.g., Cannon et al., 2014), mu desynchronization over central sites in infants may be modulated by prior action experience (Cannon et al., 2016; Saby et al., 2012; van Elk, van Schie, Hunnius, Vesper, & Bekkering, 2008). Specifically, the more experience infants have producing an action, the stronger their mu-desynchronization response when they observe someone else performing this same action (Cannon et al., 2016; van Elk et al., 2008).
EEG collection and processing
EEG was recorded using a 128-channel HydroCel Geodesic Sensor Net and sampled at 500 Hz via EGI software (Net Station Version 4.5.1; Electrical Geodesics, Inc., Eugene, OR). Impedance values for all EEG channels were below 100 kΩ at the start of data acquisition. All processing of the data was completed off-line in MATLAB (Release 2013a; The MathWorks, Natick, MA). Continuous data from the entire recording session were first baseline-corrected and forward/reverse Butterworth-filtered (pass band: 1–50 Hz; stop band: 0.1–59 Hz; 3-dB ripple; 10-dB attenuation from pass to stop band). By default, we excluded from analysis a set of 31 channels on the outermost ring of the sensor array, which lie furthest down on the head and nearest to the face and eyes, as they are heavily prone to artifact in infancy research. The continuous data were then artifact-edited using a thresholding procedure that removed high-amplitude waveforms associated with egregious movement artifact. The procedure was applied as follows: First, the continuous data were broken into adjacent 250-ms epochs. Epochs for which 5 or more channels exceeded a threshold of 250 µV were removed from the record, and the time stamp of all such discontinuities was recorded. Individual channels that exceeded the threshold on more than 10% of all epochs were deemed bad, and their data were interpolated (spherical spline) from the set of channels for all epochs. Data for channels that were not deemed bad for all epochs but that exceeded the threshold in individual epochs that had not been dropped were interpolated from the set of good subthreshold channels.
The resulting data were then average-referenced and decomposed into independent components using the fastica algorithm developed by Hyvärinen (1999). Components related to eye movement and net displacement over the front of the head were rejected using a twofold criterion. First, rejected components had to have their greatest loading magnitude at one of seven channels located over the most anterior part of the head (closest to the eyes). Second, rejected components had to have their greatest spectral power outside a band of interest from 4 through 16 Hz. This second criterion ensured that we rejected only those frontally dominant components with EEG that peaked either in the 0- to 4-Hz delta band (e.g., components related to blink or saccade waveforms) or above 16 Hz (e.g., components related to high-frequency broadband muscle artifact). This procedure resulted in an average of 13 independent components being rejected for each participant. Artifact-cleaned EEG data were then reconstructed in channel space from the remaining set of good components.
Next, we segmented the EEG data into intervals surrounding our events of interest. Two independent coders created markers for segmentation by viewing each video off-line, frame by frame, and identifying these events.
The test event in the familiarization phase was the time when the infant first touched a toy. For 87% of the trials, the two coders agreed within three frames on the time of the first touch. EEG data were taken from the 1-s window ranging from 1,000 ms prior to the touch through the touch. The baseline event for this action-execution test event corresponded to the first movement of the tray toward the infant (i.e., ~3,000 to 2,000 ms before the infant touched the toy). For this marker, the two coders agreed within three frames on 98% of the trials. We took EEG data from a 1-s window starting at this event.
The test event during the action-observation phase was when the presenter first touched the toy with her hand. For 90% of the trials, the two coders agreed on the time of the first touch. We took EEG data from the 1-s window ranging from 1,000 ms prior to the touch through the touch. If the infant produced an overt movement during the time when the presenter was reaching, the trial was eliminated from further analyses. On average, 1.08 trials per infant were excluded for this reason. The baseline event for this action-observation test event corresponded to the lifting of the curtain that began each observation trial. We took EEG data from the 1-s window ranging from 3,000 ms to 2,000 ms prior to that event.
Any trials for which our artifact-editing routine resulted in a discontinuity in any of these intervals were excluded from analysis. For each trial, data from both the baseline and the test intervals were Fourier-transformed, and an ERD score was then computed as 10 times the log (base 10) ratio of power during the test interval to power during the baseline interval (i.e., as the decibel difference). Resultant ERD scores were averaged across the 6- through 9-Hz band. Thus, negative scores indicate desynchronization, and positive scores indicate synchronization, of band-specific EEG during the execution or observation of the grasp relative to baseline. Finally, for topographic comparison across the head, band-averaged ERD scores were averaged over groups of channels corresponding to left and right central channels (C3: 93, 103, 104, 105, 111; C4: 29, 30, 36, 41, 42), parietal channels (P3: 85, 86, 91, 92, 97, 98; P4: 47, 51, 52, 53, 59, 60), frontal channels (F3: 3, 4, 117, 118, 123, 124; F4: 19, 20, 23, 24, 27, 28), and occipital channels (O1: 82, 83, 84, 89, 76; O2: 66, 69, 70, 71, 74). Additionally, prior to data analysis, we excluded average ERD values that were more than 2.5 standard deviations above the group mean for both the action-observation phase (n = 2 excluded; frontal site: n = 1; parietal site: n = 1) and the action-execution phase (n = 3 excluded; frontal site: n = 1; central site: n = 2).
Results
Preliminary analysis of behavioral responses
Preliminary analyses indicated that, on average, the infants in our sample selected the same object as the experimenter (i.e., generated a goal response) on 49% of the trials (SD = 18%). This naturally occurring variability in goal-response behavior permitted us to compare neural activity when the infants subsequently reproduced the presenter’s goal-directed action and when they did not. Although the goal-response rate in this study was lower than has been previously reported, this is likely due to the extended duration of the testing session, which was required to collect sufficient EEG data. (See the Supplemental Material for more details.)
Neural activity as a predictor of goal imitation
The focal analysis concerned relations between neural response and behavior. First, we examined whether within-participants variation in sensorimotor activation during the action-observation phase predicted whether the subsequent response would be goal based. Next, we examined whether between-participants variation in sensorimotor activation during action execution predicted later propensity to reproduce the observed goal-directed actions. Each of these analyses revealed that sensorimotor system activation was related to goal imitation.
Within-participants analyses
We first assessed whether mu desynchronization during action observation predicted infants’ subsequent behavioral responses. These analyses included only trials on which the infants attended to the presenter’s actions during the observation phase and then launched a clear motor response, selecting one of the two toys. On average, the infants in our sample provided 10.89 artifact-free trials (SD = 1.43, range = 6–12), 5.28 trials followed by goal responses (SD = 1.88) and 5.61 trials followed by nongoal responses (SD = 2.32). The infants attended to the presenter’s actions an average of 97% of the time (SD = 5%), and attention did not differ between trials with goal and nongoal responses, t(33) = −0.17, p < .864. Preliminary analyses demonstrated that there were no effects of hemisphere on desynchronization at central sites (p > .138), so we collapsed across hemisphere for subsequent analyses (see the Supplemental Material for topographic maps of the EEG data).
We evaluated neural activity over central sites during the action-observation phase of each trial and binned this activity according to the infant’s subsequent behavioral response on that trial (i.e., goal response vs. nongoal response). We ran a repeated measures analysis of variance (ANOVA) on mean ERD values with trial type (goal response vs. nongoal response) as a within-participants factor. Results indicated that there was a significant main effect of trial type, F(1, 35) = 4.54, p < .040, ηp2 = .12; infants showed greater mu desynchronization on trials on which they subsequently produced the goal response (M = −0.48, SD = 1.04) than on trials on which they produced a nongoal response (M = 0.16, SD = 1.56; see Fig. 2). One-sample t tests indicated that desynchronization was significantly different from zero (suggesting significant change from a resting baseline period) prior to goal responses, t(35) = −2.78, p < .009, and was not different from zero prior to nongoal responses, t(35) = 0.62, p < .539.
Fig. 2.
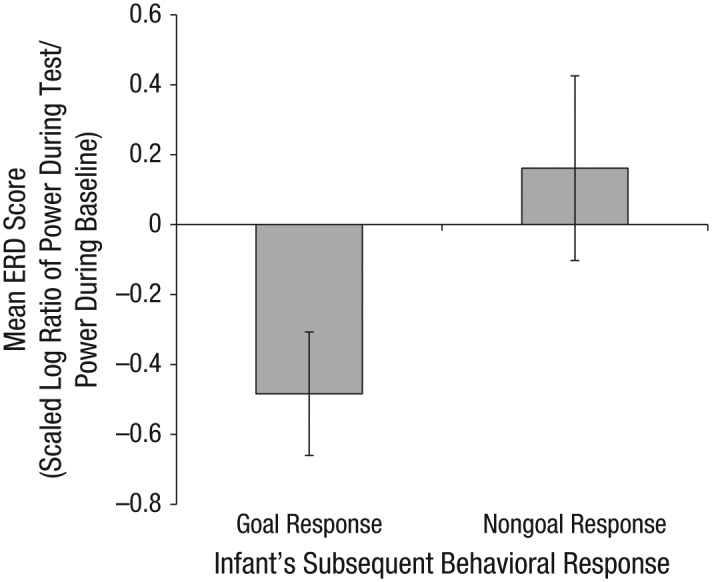
Mean event-related desynchronization (ERD) scores in the mu frequency range (6–9 Hz) over central sites during action observation, for trials with goal responses and trials with nongoal responses. The ERD score for a trial was calculated as 10 times the log ratio of power during the test interval to power during the baseline interval. Error bars indicate ±1 SE.
To determine whether this power reduction preceding a goal response was unique to central sites, we ran identical repeated measures ANOVAs on mean ERD values for frontal, parietal, and occipital sites. We found no effects at frontal sites (goal response: M = −0.21, SD = 1.14; nongoal response: M = 0.21, SD = 1.58; ps > .21). However, at parietal sites, there was a significant main effect of trial type, F(1, 34) = 7.72, p < .009, ηp2 = .19; there was more power during action observation relative to baseline prior to a nongoal response (M = 0.31, SD = .1.08) than prior to a goal response (M = −0.18, SD = 0.76). One-sample t tests indicated that parietal desynchronization prior to goal responses was not significantly different from zero, t(34) = −1.43, p < .163. Additionally, the difference between power during the test interval and baseline power was not significantly different from zero on trials with nongoal responses, t(35) = 1.73, p < .093. Thus, although the desynchronization scores for parietal sites differed significantly between trials with goal responses and trials with nongoal responses, power during action observation was not significantly different from baseline levels in either trial type. At occipital sites, we also found a significant main effect of trial type, F(1, 35) = 4.87, p < .034, ηp2 = .12; there was significantly more desynchronization prior to goal responses (M = −0.66, SD = 1.36) compared with nongoal responses (M = −0.03, SD = 1.18). Activation of occipital regions during action observation was significantly different from baseline activation for trials with goal responses, t(35) = −2.93, p < .006, but not for trials with nongoal responses, p < .866. Thus, similar patterns of neural activation were found at occipital sites and central sites. This result is consistent with prior reports that mu desynchronization over central sites is accompanied by desynchronization at occipital sites during action observation (e.g., Marshall, Bouquet, Shipley, & Young, 2009).
Given that sensorimotor regions and occipital regions were both recruited to a greater extent before the infants generated a goal response than before they generated a nongoal response, we next evaluated whether the neural response at central sites differed from the neural response at occipital sites during action observation. We examined correlations between occipital ERD and central ERD on trials with goal responses and trials with nongoal responses. We found no relation between activity at central sites and activity at occipital sites for either trial type (goal response: p > .235; nongoal response: p > .622). (See the Supplemental Material for further discussion of the relations between central- and occipital-site activity.)
To further examine the relative contribution of activity at central and occipital sites, we tested whether neural activity at either site uniquely predicted imitation of goal-directed behavior. We conducted a stepwise multiple regression to determine whether average central ERD scores or average occipital ERD scores during action observation predicted the most unique variance in the proportion of goal responses generated by an infant. In the first step, the predictor with the highest predictive value was entered. This predictor was ERD at central sites, which was significantly related to goal-response behavior, F(1, 35) = 4.17, p < .049. The multiple correlation coefficient was .33, indicating that 11% of the variance in the proportion of trials on which an infant reproduced the goal of the presenter could be accounted for by average central-site ERD. Average occipital ERD (t = 0.70, p = .486) did not enter into the regression equation at Step 2 because occipital ERD did not uniquely predict any of the remaining variance in the proportion of goal responses generated by an infant. Thus, sensorimotor activity predicted more unique variance in imitative behavior.
Between-participants analyses
To further assess the relations between the sensorimotor system and goal imitation, we next examined between-participants variation in neural activity during action execution. Previous research has demonstrated that infants exhibit substantial individual differences in the mu-ERD response during both action execution and action observation (e.g., Marshall, Saby, & Meltzoff, 2013). In particular, new research suggests that differences in motor development may be linked to the variability in the mu-ERD response during the observation of action (Cannon et al., 2016). Given that variations in motor experience are related to the robustness of the neural response during action observation, it seems possible that the mu-ERD response might reflect variation in motor skill. We reasoned that early in development, variation in the mu-ERD response during action execution could reflect differences in the maturity of the motor system (a follow-up analysis in the Supplemental Material provides initial evidence for this claim). Thus, examining neural activity during action execution could provide further evidence that general developments in the sensorimotor system are linked to goal imitation. We therefore tested whether variation in the neural response during action execution in the familiarization phase predicted the infants’ subsequent propensity to reproduce the observed goal-directed actions. On average, we obtained artifact-free data for 10.2 (SD = 1.75) familiarization trials per infant (range = 5–12).
We conducted a stepwise multiple regression to determine whether average ERD values at each site (frontal, central, parietal, and occipital) during action execution was necessary to predict the proportion of goal responses generated by an infant. Average central ERD predicted the most unique variance and was therefore entered into the regression equation in Step 1. Central ERD was significantly related to goal-response behavior, F(1, 32) = 8.12, p < .008. The multiple correlation coefficient was .46, indicating that 21% of the variance in the proportion of trials on which the infants produced goal responses could be accounted for by action-execution ERD values at central sites. ERD values at occipital (t = 1.61, p = .118), frontal (t = −0.76, p = .452), and parietal (t = −0.52, p = .605) sites did not enter into the equation in Step 2 of the analysis because ERD at these sites did not uniquely predict any of the remaining variance in the proportion of goal responses generated. Thus, desynchronization at central sites during action execution uniquely predicted the infants’ behavioral responses to observed actions (see Fig. 3). (See the Supplemental Material for further analyses.)
Fig. 3.
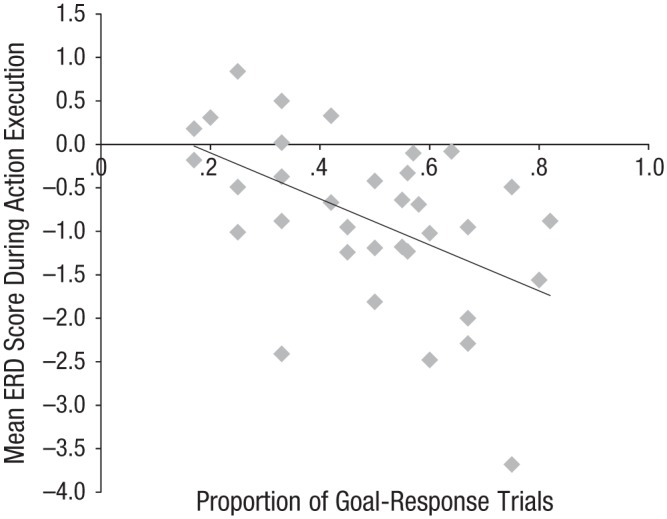
Scatterplot (with best-fitting regression line) demonstrating the relation between mean event-related desynchronization (ERD) at central sites during action execution and the proportion of trials on which each infant produced goal responses.
Discussion
Responding selectively to other people’s goal-directed actions is central to social interaction. In the current study, we made use of spontaneous variation in infants’ abilities as a natural case for exploring contingent relations between neural activity and social behavior. By integrating a behavioral measure of goal sensitivity with EEG, we were able to test whether infants’ motor system activation as they observed an action was selectively linked to their goal-based behavioral responses. On each trial, infants observed (and were attentive to) an adult who performed a well-formed goal-directed action. On each trial, they also generated a behavioral response to that action, choosing either the goal or the nongoal object. Nevertheless, we found that activation of the motor system during observation was selective to trials on which the infants reproduced the observed action. That is, motor system activation (and visual system activity) during the infants’ observation of a goal-directed action predicted whether they subsequently chose the presenter’s prior goal rather than the nongoal toy.
Because we included only those trials on which the infants attended to the actions of the presenter and then produced a clear behavioral response, we conclude that this differential neural activity reflected variations in the infants’ sensitivity to the presenter’s goal during observation rather than variations in attentiveness, engagement, or motor preparation. Furthermore, ERD over central sites during action execution predicted the infants’ later behavioral propensity to produce goal-based behavioral responses. Our findings provide evidence that the neural response during action observation is linked to the neural systems recruited for producing goal-directed actions. These findings demonstrate a link between the motor system and social behavior at the level of individual trials and at the level of variation across infants. Thus, these findings provide novel evidence that motor system activation predicts goal-based behavioral responses during infancy.
These results with infants are consonant with findings from adults in indicating that certain social-cognitive functions are contingent on motor system activation. In adults, aspects of social perception, including visual anticipation of actions (Stadler et al., 2012) and inference of actions from context (Michael et al., 2014), are reduced, but not eliminated, by TMS to the motor cortex. In our task, the infants were not required to anticipate the outcome of an action nor to make an inference about an action from context. Nevertheless, performance in our task, as in the tasks used with adults, depended on the infants’ ability to respond to other people’s goal-directed actions. Furthermore, our findings with infants suggest a starker contrast than has been observed in adults, in that there was no detectable mu desynchronization on trials on which the infants attended to the presenter’s actions but chose the nongoal toy. In adults, mu desynchronization occurs robustly during the observation of goal-directed actions. The variation in this response in infants is consistent with the variation that typically occurs when skills are new in development (e.g., Siegler & Shipley, 1995), and with behavioral findings showing that variability in behavioral measures of goal perception correlates with motor expertise during periods of developmental change (e.g., Sommerville & Woodward, 2005). Together, these findings indicate that studying early development provides a unique lens for characterizing processes that in adulthood may show little variability.
Findings from adult neuroimaging studies have shown that mature action understanding involves a network of regions (Arnstein et al., 2011; de Lange, Spronk, Willems, Toni, & Bekkering, 2008), and this raises questions about the extent to which broader neural networks are involved in infants’ responses to other people’s actions. Our finding of significant desynchronization over occipital regions before goal responses, but not before nongoal responses, is in line with the idea that a network of regions is activated during action processing. Given that the mu frequency band overlaps with the alpha frequency band and occipital alpha is linked to visual attention (Pfurtscheller, 2003), it is possible that our findings reflect engagement of visual processing or selective attention in perceiving and responding to the presenter’s actions. To examine this possibility, we assessed the infants’ visual attention behavior and their neural response over visual attention regions. We found that the link between sensorimotor system response and the infants’ goal-imitation behavior was independent of variations in global attention. We also found that occipital ERD scores were not correlated with central ERD scores and that central ERD scores during action observation accounted for the most variance in goal-response behavior. Furthermore, results in the between-participants analyses demonstrated that sensorimotor activity during action execution uniquely predicted the infants’ tendency to imitate goal-directed behavior, and there was no evident relation between imitation behavior and occipital ERD. Thus, there is converging evidence that visual processing cannot solely account for our findings.
Even so, the attention network may interact with the motor system in functionally interesting ways during the perception of other individuals’ actions. In adults, the motor system response is modulated by changes in attention (Johansen-Berg & Matthews, 2002). Indeed, action observation likely involves specialized, fine-grained patterns of visual attention. For example, skilled action production requires tightly coordinated shifts in visual attention, and adults (Flanagan & Johansson, 2003) and infants (Rosander & von Hofsten, 2011) both exhibit similar patterns of attention when observing others’ actions and when acting themselves. Future research is needed to determine how attention may modulate changes in the motor system response in infancy.
In adults, activation of the parietal cortex has been associated with goal processing, particularly for events involving animated stimuli (e.g., Hamilton & Grafton, 2006; see Southgate, Begus, Lloyd-Fox, di Gangi, & Hamilton, 2014, for similar findings in infants). Although we did not find desynchronization at parietal sites, we cannot conclude from this that the parietal attention network plays an insignificant role in infants’ action processing. Although there is suggestive evidence that mu desynchronization over central sites reflects motor system activity in infants (see Marshall & Meltzoff, 2011), little is known about how activity across different brain regions relates to scalp EEG in infants. Further work is needed, at both behavioral and neural levels, to understand relations among different behavioral indicators of goal perception and the neural networks that support them during early development.
We found that the strength of the ERD in mu rhythm as infants reached for objects predicted their propensity to act selectively with respect to another person’s goals. Other studies have shown that the magnitude of the ERD when infants observe actions correlates with their motor skill and experience (Cannon et al., 2016; de Klerk, Johnson, Heyes, & Southgate, 2014; Marshall et al., 2013; van Elk et al., 2008). Thus, foundational developments in motor skill during infancy may have cascading effects on social behavior. Understanding the neural bases that underlie the development of skilled, attention-driven motor control will be critical for understanding social behavior during typical development and may shed new light on developmental impairments in social cognition.
In summary, the current study provides novel evidence that motor system activation is selectively associated with infants’ responses to other individuals’ goal-directed actions. In addition, our findings demonstrate that a developmental perspective offers insight into the functional role that the motor system plays in this foundational social-cognitive ability. This work raises new questions about the development of the neural systems that underlie skilled action production and action perception, and it provides a methodological approach—using naturally occurring variability during early development—to address these questions.
Supplementary Material
Although this is not an exact replication of the actor’s behavior (the actor grasped the toy), it is a reproduction of the goal structure of the action. Each of the infants’ goal responses mirrored the goal structure of the action that they observed—the actor coordinated visual and manual contact toward one toy and then the infant did the same.
Footnotes
Action Editor: Wendy Berry Mendes served as action editor for this article.
Declaration of Conflicting Interests: The authors declared that they had no conflicts of interest with respect to their authorship or the publication of this article.
Funding: This research was supported by Eunice Kennedy Shriver National Institute of Child Health and Human Development Grant P01- HD064653, awarded to A, L. Woodward, N. A. Fox, and P. F. Ferrari.
Supplemental Material: Additional supporting information can be found at http://pss.sagepub.com/content/by/supplemental-data
References
- Arnstein D., Cui F., Maurits N. M., Gazzola V. (2011). µ-suppression during action observation and execution correlates with BOLD in dorsal premotor, inferior parietal and SI cortices. Journal of Neuroscience, 31, 14243–14249. doi: 10.1523/JNEUROSCI.0963-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon E. N., Simpson E. A., Fox N. A., Vanderwert R. E., Woodward A. L., Ferrari P. F. (2016). Relations between infants’ emerging reach-grasp competence and event-related desynchronization in EEG. Developmental Science, 19, 50–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon E. N., Yoo K. H., Vanderwert R. E., Ferrari P. F., Woodward A. L., Fox N. A. (2014). Action experience, more than observation, influences mu rhythm desynchronization. PLoS ONE, 9(3), Article e92002. doi: 10.1371/journal.pone.0092002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook G., Bird G., Catmur C., Press C., Heyes C. (2014). Mirror neurons: From origin to function. Behavioral & Brain Sciences, 37, 177–241. doi: 10.1017/S0140525X13000903 [DOI] [PubMed] [Google Scholar]
- Csibra G. (2008). Action mirroring and action understanding: An alternative account. In Haggard P., Rossetti Y., Kawato M. (Eds.), Attention and performance XXII: Sensorimotor foundations of higher cognition (pp. 435–459). Oxford, England: Oxford University Press. [Google Scholar]
- Cuevas K., Cannon E. N., Yoo K., Fox N. A. (2014). The infant EEG mu rhythm: Methodological considerations and best practices. Developmental Review, 34, 26–43. doi: 10.1016/j.dr.2013.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Klerk C. C. J. M., Johnson M. H., Heyes C. M., Southgate V. (2014). Baby steps: Investigating the development of perceptual–motor couplings in infancy. Developmental Science, 18, 270–280. doi: 10.1111/desc.12226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lange F. P., Spronk M., Willems R. M., Toni I., Bekkering H. (2008). Complementary systems for understanding action intentions. Current Biology, 18, 454–457. doi: 10.1016/j.cub.2008.02.057 [DOI] [PubMed] [Google Scholar]
- Ferrari P., Tramacere A., Simpson E. A., Iriki A. (2013). Mirror neurons through the lens of epigenetics. Trends in Cognitive Sciences, 17, 450–457. doi: 10.1016/j.tics.2013.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan J. R., Johansson R. S. (2003). Action plans used in action observation. Letters to Nature, 630, 1–3. doi: 10.1038/nature01861 [DOI] [PubMed] [Google Scholar]
- Gallese V., Fadiga L., Fogassi L., Rizzolatti G. (1996). Action recognition in premotor cortex. Brain, 119, 593–609. doi: 10.1093/brain/119.2.593 [DOI] [PubMed] [Google Scholar]
- Gerson S. A., Woodward A. L. (2012). A claw is like my hand: Comparison supports goal analysis in infants. Cognition, 122, 181–192. doi: 10.1016/j.cognition.2011.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton A., Grafton S. T. (2006). Goal representation in human anterior intraparietal sulcus. Journal of Neuroscience, 26, 1133–1137. doi: 10.1523/JNEUROSCI.4551-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlin J. K., Hallinan E. V., Woodward A. L. (2008). Do as I do: 7-month-old infants selectively reproduce others’ goals. Developmental Science, 11, 487–494. doi: 10.1111/j.1467-7687.2008.00694.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok G. (2014). The myth of mirror neurons: The real neuroscience of communication and cognition. New York, NY: W. W. Norton. [Google Scholar]
- Hyvärinen A. (1999). Fast and robust fixed-point algorithms for independent component analysis. IEEE Transactions on Neural Networks, 10, 626–634. doi: 10.1109/72.761722 [DOI] [PubMed] [Google Scholar]
- Johansen-Berg H., Matthews P. M. (2002). Attention to movement modulates activity in sensori-motor areas including primary motor cortex. Experimental Brain Research, 142, 13–24. doi: 10.1007/s00221-001-0905-8 [DOI] [PubMed] [Google Scholar]
- Lou Y., Baillargeon R. (2010). Toward a mentalistic account of early psychological reasoning. Current Directions in Psychological Science, 19, 301–307. doi: 10.1177/0963721410386679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan N., Woodward A. L. (2009). Seven-month-old infants selectively reproduce the goals of animate but not inanimate agents. Infancy, 14, 667–679. doi: 10.1080/15250000903265184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangold. (2010). INTERACT quick start manual V2.4. Arnstorf, Germany: Author. [Google Scholar]
- Marshall P. J., Bouquet C. A., Shipley T. F., Young T. (2009). Effects of brief imitative experience on EEG desynchronization during action observation. Neuropsychologia, 47, 2100–2106. doi: 10.1016/j.neuropsychologia.2009.03.022 [DOI] [PubMed] [Google Scholar]
- Marshall P. J., Meltzoff A. N. (2011). Motor system activation systems: Exploring the EEG mu rhythm in human infancy. Developmental Cognitive Neuroscience, 1, 110–123. doi: 10.1016/j.dcn.2010.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall P. J., Saby J. N., Meltzoff A. N. (2013). Infant brain responses to object weight: Exploring goal-directed actions and self-experience. Infancy, 18, 942–960. doi: 10.1111/infa.12012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzoff A. N. (1995). Understanding the intentions of others: Re-enactment of intentional acts by 18-month-old children. Developmental Psychology, 31, 838–850. doi: 10.1037/0012-1649.31.5.838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael J., Sandberg K., Skewes J., Wolf T., Blicher J., Overgaard M., Frith C. D. (2014). Continuous theta-burst stimulation demonstrates a causal role of premotor homunculus in action understanding. Psychological Science, 25, 963–972. doi: 10.1177/0956797613520608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfurtscheller G. (2003). Induced oscillations in the alpha band: Functional meaning. Epilepsia, 44, 2–8. doi: 10.1111/j.0013-9580.2003.12001.x [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G., Aranibar A. (1979). Evaluation of event-related desynchronization (ERD) preceding and following voluntary self-paced movement. Electroencephalography and Clinical Neurophysiology, 46, 138–146. doi: 10.1016/0013-4694(79)90063-4 [DOI] [PubMed] [Google Scholar]
- Rosander K., von Hofsten C. (2011). Predictive gaze shifts elicited during observed and performed actions in 10-month-old infants and adults. Neuropsychologia, 49, 2911–2917. doi: 10.1016/j.neuropsychologia.2011.06.018 [DOI] [PubMed] [Google Scholar]
- Saby J. N., Marshall P. J., Meltzoff A. N. (2012). Neural correlates of being imitated: An EEG study in preverbal infants. Social Neuroscience, 6, 650–661. doi: 10.1080/17470919.2012.691429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegler R. S., Shipley C. (1995). Variation, selection, and cognitive change. In Simon T., Halford G. (Eds.), Developing cognitive competence: New approaches to process modeling (pp. 31–76). Hillsdale, NJ: Erlbaum. [Google Scholar]
- Smith L. B., Thelen E. (2003). Development as a dynamic system. Trends in Cognitive Sciences, 7, 343–348. [DOI] [PubMed] [Google Scholar]
- Sommerville J. A., Woodward A. L. (2005). Pulling out the intentional structure of human action: The relation between action production and processing in infancy. Cognition, 95, 1–30. doi: 10.1016/j.cognition.2003.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southgate V., Begus K., Lloyd-Fox S., di Gangi V., Hamilton A. (2014). Goal representation in the infant brain. NeuroImage, 85, 294–301. doi: 10.1016/j.neuroimage.2013.08.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southgate V., Johnson M. H., Karoui I. E., Csibra G. (2010). Motor system activation reveals infants’ on-line prediction of others’ goals. Psychological Science, 21, 355–359. doi: 10.1177/0956797610362058 [DOI] [PubMed] [Google Scholar]
- Spunt R. P., Lieberman M. D. (2013). The busy social brain: Evidence for automaticity and control in the neural systems supporting social cognition and action understanding. Psychological Science, 24, 80–86. doi: 10.1177/0956797612450884 [DOI] [PubMed] [Google Scholar]
- Stadler W., Ott D. V. M., Springer A., Schubotz R. I., Schutz-Bosbach S., Prinz W. (2012). Repetitive TMS suggests a role of the human dorsal premotor cortex in action prediction. Frontiers in Human Neuroscience, 6, Article 20. doi: 10.3389/fnhum.2012.00020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Elk M., van Schie H. T., Hunnius S., Vesper C., Bekkering H. (2008). You’ll never crawl alone: Neurophysiological evidence for experience-dependent motor resonance in infancy. NeuroImage, 43, 808–814. doi: 10.1016/j.neuroimage.2008.07.057 [DOI] [PubMed] [Google Scholar]
- Wellman H. M., Phillips A. T., Dunphy-Lelii S., LaLonde N. (2004). Infant social attention predicts preschool social cognition. Developmental Science, 7, 283–288. doi: 10.1111/j.1467-7687.2004.00347.x [DOI] [PubMed] [Google Scholar]
- Woodward A. L. (1998). Infants selectively encode the goal of an actor’s reach. Cognition, 69, 1–34. doi: 10.1016/S0010-0277(98)00058-4 [DOI] [PubMed] [Google Scholar]
- Woodward A. L., Gerson S. A. (2014). Mirroring and the development of action understanding. Philosophical Transactions of the Royal Society B: Biological Sciences, 369, Article 20130181. doi: 10.1098/rstb.2013.0181 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.