Abstract
Context
Children at end of life often experience multiple complex chronic conditions, with over 50% of children reportedly having two or more conditions. These complex chronic conditions are unlikely to occur in an entirely uniform manner in children at end of life. Previous work has not fully accounted for patterns of multiple conditions when evaluating care among these children.
Objectives
To understand the clusters of complex chronic conditions present among children in the last year of life.
Methods
Participants were 1423 pediatric decedents from the 2007 to 2008 California Medicaid data. A latent class analysis was used to identify clusters of children with multiple complex chronic conditions (neurological, cardiovascular, respiratory, renal, gastrointestinal, hematologic, metabolic, congenital, cancer). Multinomial logistic regression analysis was used to examine the relationship between demographic characteristics and class membership.
Results
Four latent classes were yielded: medically fragile (31%); neurological (32%); cancer (25%); and cardiovascular (12%). Three classes were characterized by a 100% likelihood of having a complex chronic condition coupled with a low or moderate likelihood of having the other eight conditions. The four classes exhibited unique demographic profiles.
Conclusion
This analysis presented a novel way of understanding patterns of multiple complex chronic conditions among children that may inform tailored and targeted end-of-life care for different clusters.
Keywords: complex chronic conditions, latent class analysis, pediatric, children, end of life
Introduction
Children at end of life often experience multiple complex chronic conditions. From cancer to birth defects, complex chronic conditions are defined as conditions that are reasonably expected to last at least 12 months and to involve either several different organ systems or one organ system severely (1). Multiple complex chronic conditions are common, with over 50% of children with chronic conditions reporting two or more conditions (2). These complex chronic conditions are unlikely to occur in an entirely uniform manner in children at end of life (3). For example, a child with cerebral palsy may have gastrointestinal conditions with feeding difficulties and recurrent aspiration pneumonia. As a result of these unpredictable patterns of multiple complex chronic conditions, children with complex chronic conditions generally have costly, high utilization of health care services, including the emergency room (4–8).
Previous work has not fully accounted for patterns of multiple conditions when evaluating care among these children. Instead, two strategies have been used. First, Feudtner and colleagues (1, 9, 10) have used death certificate diagnoses to assign a primary diagnosis to children with complex chronic conditions. While this technique allows an understanding of the most clinically important condition that led to the child’s death, many administrative data sources are not readily linked to death certificate data, limiting the availability of this information. In addition, this technique still assigns a single diagnosis to each child, even though some conditions may occur together. An alternative strategy has been to evaluate care within categories of complex chronic conditions using diagnostic data from inpatient and outpatient administrative records, a technique that means that a child with five chronic conditions may be counted five times in each analysis (2, 10, 11). Therefore, current strategies have not effectively accounted for multiple complex chronic conditions among children.
Latent class analysis is a useful method for identifying patterns within a heterogeneous population (12, 13). Instead of creating every possible cluster, latent class analysis reduces the data into the most parsimonious set of clusters or classes. Latent class analysis has been used widely in health care research to identify patterns of community-based service use (14), foster care provision (15–18), and comorbidities (19–21).
Identification of such groupings of co-occurring conditions among children with complex chronic conditions would be highly beneficial to future studies of the underlying mechanisms of these chronic conditions and in addressing the end-of-life care needs of these children. This information may guide the design of interventions specific to multiple complex chronic conditions, which are tailored to the health needs of children at end of life. Such tailored interventions may ultimately improve the quality of end-of-life care for children and their families. For researchers, the use of latent class analysis presents a unique methodological technique to account for patterns of comorbid conditions that might be used with administrative data sources.
Therefore, using latent class analysis, we sought to understand the patterns of conditions present among children in the last year of life, using clustering of conditions as a way to identify children with similar care needs and utilization patterns. Specifically, we aimed to clarify 1) the number of classes that could parsimoniously describe patterns of complex chronic conditions, 2) the configurations of complex chronic conditions represented in each class, with the expected prevalence of each class, and 3) the demographic correlates of class membership. Although we hypothesized that some children may have one primary condition that accounts for most of their health care needs, even if other conditions are also present, we did not formulate hypotheses regarding the particular number of classes or the configuration of each class.
Conceptual Model
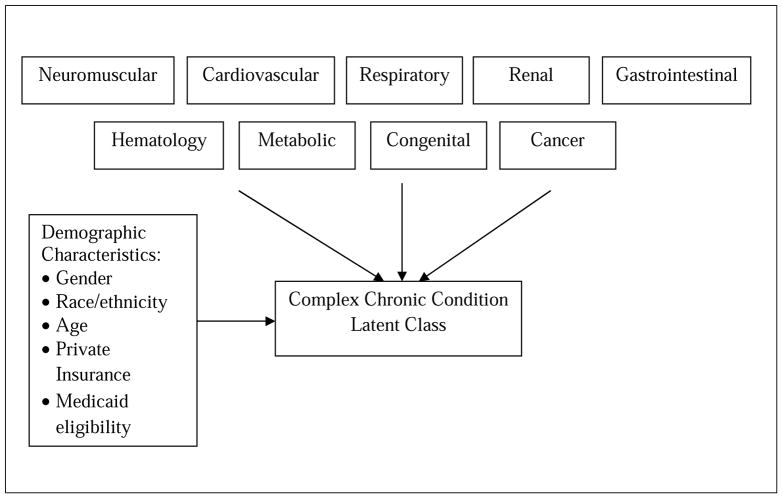
Fig. 1 depicts the conceptual relationships among the complex chronic conditions, the latent classes, and the covariate predictors for the latent class model estimated in these analyses. Our group of demographic characteristics included gender, race/ethnicity, and age, which may predispose children to certain complex chronic conditions. For example, infants may be more likely to suffer from congenital anomalies compared to older children, whereas sickle cell disease occurs among African-American children. We also included additional private insurance coverage because families with added health insurance coverage may have more resources to diagnose complex chronic conditions. Finally, we included Medicaid eligibility because disability eligibility may provide children with access to additional programs and resources in which complex chronic conditions might be diagnosed.
Figure 1.
Conceptual Model of Complex Chronic Condition Latent Class
Methods
Study Design
The study was designed as a pooled cross-sectional secondary analysis of 2007 and 2008 California Medicaid data, using latent class analysis to illustrate patterns of complex chronic conditions among children at end of life.
Participants
Participants were pediatric California Medicaid beneficiaries. Medicaid data were selected because Medicaid is one of the few publicly available data sources that includes pediatric claims data at end of life. California was selected for this study because it has the largest population of children enrolled in Medicaid of any state (22). The sampling frame was based on the inclusion criteria that children were between the ages of 0 and 20 years, died between January 1, 2007 and December 31, 2008, and were enrolled in the California Medicaid program for any part of their last year of life. Children who were not California residents and duplicate entries were excluded. The sample was further restricted to those who had at least one complex chronic condition (i.e., neurological, cardiovascular, respiratory, renal, gastrointestinal, hematologic, metabolic, congenital, cancer) based on the International Classification of Diseases, 9th revision (ICD-9) code as recommended by Feudtner and colleagues (9). Requiring the presence of one or more complex chronic conditions to be included in the analysis increased the likelihood that a reasonable number of latent classes would be yielded with adequate distributions of children in each class. Our final sample was 1423 children.
Measures
Dependent Variables
The presence of each complex chronic condition was dichotomized into yes or no. We used Feudtner’s nine categories of complex chronic conditions rather than the 30 subcategories to ensure conditional dependence where complex conditions were relatively independent of each other within a latent class (23).
Independent Variables
A set of demographic variables was created for this study from the Medicaid administrative data. Female was a binary variable (0 = male, 1 = female). Categories were created for race and ethnicity as Caucasian, African American, Hispanic, and other races (e.g., Asian American, Native American). Age was the child’s age at death and was categorized in the Medicaid data as less than one year, 1 to 5 years, 6 to 15 years, and 15 to 20 years. Whether or not the child had additional private health insurance, along with Medicaid coverage, was a measure of private insurance. Medicaid eligibility type was a binary variable (0 = no disability status, 1=disability status).
Statistical Analysis
We used a series of latent class models to examine clusters of complex chronic conditions among children at end of life (24). Latent class analysis is a method to identify discrete subgroups of similar cases within an overall population (25). The subcategories are referred to as latent classes. Latent class analysis was used because it accommodated the categorical variables, unlike factor analysis, and it generated probabilities of class membership, unlike cluster analysis.
Stage 1
In the first stage of the analysis, we used the nine complex chronic conditions as class indicators and estimated the smallest set of latent classes that best fit the data. To do so, various model fit indices were evaluated including Pearson χ2, likelihood ratio χ2, Akaike information criterion (AIC), Bayesian information criterion (BIC), Lo-Mendell-Rubin (LMR) likelihood ratio, and entropy values (26, 27). Although there is no definitive goodness-of-fit statistical test in latent class analysis, a model with one additional class is often considered an improvement with lower AIC and BIC values, a statistically significant (P<0.05) likelihood ratio test, and higher entropy scores. Anticipating specific sources of bias, we performed a sensitivity analysis in Stage 1. We were concerned that infants (<1 year) might have different classes compared to children (1 to 20 years). To assess potential difference in clusters by age, we ran the stage 1 analysis for infants separate from children. We found that, for the infants, the model indices were a very poor fit and that for children, the model indices were the same with or without infants in the model. As a result of the sensitivity analysis, we did not separate infants from children for this analysis.
Stage 2
In the second stage of the analysis, the classes within the best-fit model were described. This included estimating the prevalence of child membership in the latent classes. We also calculated the conditional item-response probabilities of each complex chronic condition into the class. Probabilities ranged from 0 (i.e., non-class membership) to 1 (i.e., perfect prediction of class membership). The conditional probabilities of complex chronic conditions within a class were used to assign descriptive labels to the classes.
Stage 3
For the final stage of the analysis, we added a set of covariate predictors to examine the relationships between demographic characteristics and membership in a given latent class. A multinomial logistic regression was conducted that regressed each latent class against the covariates, using the class with the most complex chronic conditions as the reference category. Results are reported as relative risk ratios. All analyses were conducted using Mplus version 6.11 (28) and Stata version11 (StataCorp LP, College Station, TX).
Results
In the sample, 53.83%, 46.38%, and 12.30% of the children at end of life met the criteria for having a neurological, cardiovascular, or respiratory complex chronic condition, respectively. Renal, gastrointestinal, and hematologic complex chronic condition criteria were met by 4.08%, 12.02%, and 8.85%, respectively. In addition, 10.40%, 22.87%, and 29.87% of children had metabolic, congenital, and cancer conditions. Approximately 44% of the sample had one complex chronic condition, while 56% had two or more complex chronic conditions.
Latent Class Analysis
Stage 1
The latent class model fit indices are illustrated in Table 1. As the number of classes increased, AIC and BIC decreased, indicating improved model fit. Improved fit of the model was present only up to four classes, as evidenced by the LMR probability greater than P >0.01 with five classes. The four-class model also had the highest entropy value, which also suggested that it was the best-fitting model.
Table 1.
Model Fit Indices for Latent Class Models
| Number of Classes | |||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Pearson χ2 | 1678.441 | 1241.109 | 954.449 | 649.605 | 613.916 |
| LR χ2 | 1112.544 | 836.825 | 561.228 | 474.321 | 424.913 |
| df | 499 | 490 | 480 | 471 | 462 |
| No. of parameters | 9 | 19 | 29 | 39 | 49 |
| Log likelihood | −5793.281 | −5649.319 | −5511.630 | −5462.465 | −5432.891 |
| AIC | 11604.561 | 11336.637 | 11081.327 | 11002.930 | 10963.782 |
| BIC | 11651.906 | 11436.587 | 11233.882 | 11208.090 | 11221.548 |
| LMR Testing hypothesis | ------ | 1 class vs. 2 classes | 2 classes vs. 3 classes | 3 classes vs. 4 classes | 4 classes vs. 5 classes |
| LMR probability | ------ | < .001 | < .001 | < .001 | .013 |
| Entropy | ------ | .669 | .757 | .758 | .692 |
Note: LR: Log likelihood ratio; AIC: Akaike information criterion; BIC: Bayesian information criterion; LMR: Lo-Mendell Rubin test
Stage 2
For the four-class solution, conditional item-response probabilities are presented in Table 2. A total of 31.41% of the sample was expected to belong to Class 1, which had a response pattern we have characterized as “medically fragile.” Children in Class 1 had a moderate likelihood of neurological (45%), cardiovascular (64%), respiratory (29%), gastrointestinal (24%), congenital (45%), and cancer (20%) conditions. They had a low likelihood of renal (10%), hematologic (15%), and metabolic (17%) conditions. Class 2 (31.90%) was characterized as “neurological.” These children primarily had a 100% likelihood of a neurological condition with a moderate likelihood of cardiovascular (21%) and low likelihood of respiratory (4%), metabolic (6%), congenital (16%), and cancer (1%) conditions. Class 3 (24.74%) was characterized as “cancer.” Cancer class children had a 100% likelihood of having a cancer condition with moderate likelihood of neurological (31%) and cardiovascular (27%) conditions. They also had a low likelihood of renal (1%), gastrointestinal (11%), hematologic (16%), metabolic (10%), and congenital (5%) conditions. Class 4 constituted the smallest latent class (11.95%) and was characterized as a 100% likelihood of having a cardiovascular condition with low likelihoods of having respiratory (4%), gastrointestinal (7%), hematologic (1%), metabolic (1%), and congenital (7%) conditions.
Table 2.
Four class solution: Prevalence of latent classes and conditional probabilities
| Class 1 | Class 2 | Class 3 | Class 4 | |
|---|---|---|---|---|
| 31.41% | 31.90% | 24.74% | 11.95% | |
| n=447 | n=454 | n=352 | n=170 | |
| Medically Fragile | Neurological | Cancer | Cardiovascular | |
| Neuromuscular | 0.45 | 1.00 | 0.31 | 0.00 |
| Cardiovascular | 0.64 | 0.21 | 0.27 | 1.00 |
| Respiratory | 0.29 | 0.04 | 0.00 | 0.04 |
| Renal | 0.10 | 0.00 | 0.01 | 0.00 |
| Gastrointestinal | 0.24 | 0.00 | 0.11 | 0.07 |
| Hematologic | 0.15 | 0.00 | 0.16 | 0.01 |
| Metabolic | 0.17 | 0.06 | 0.10 | 0.01 |
| Congenital | 0.45 | 0.16 | 0.05 | 0.07 |
| Cancer | 0.20 | 0.01 | 1.00 | 0.00 |
Note: CCC=complex chronic conditions
(perfect prediction of class membership = 1.00, perfect prediction of non-class membership = 0.00)
Stage 3
After the latent classes were identified, we examined the demographic correlates of class membership (Table 3). Relative to the medically fragile class, children 6 to 14 years (RRR = 5.57, P < 0.001), 15 to 20 years (RRR = 4.59, P < 0.001), and with additional private insurance (RRR = 3.47, P < 0.001) were more likely to belong to the neurological class. Children 1 to 5 years (RRR = 4.23, P < 0.001), 6 to 14 years (RRR = 28.27, P < 0.001), 15 to 20 years (RRR = 22.29, P < 0.001), and with additional private insurance (RRR = 2.65, p=P < 0.001) were more likely to belong to the cancer class. Child with income eligible for Medicaid (RRR = 0.48, P < 0.001), however, were negatively associated with cancer class. Children who were Hispanic (RRR = 1.71, P < 0.05), and 15 to 20 years (RRR = 2.00, P < 0.05) were more likely to belong to the cardiovascular class; whereas children with Medicaid eligible income (RRR = 0.18, P < 0.001) were less likely to belong to the class, relative to the medically fragile class.
Table 3.
Multinomial Regression Analysis for Demographic Characteristics Predicting Latent Classes (N=1423)
| Class 2 | Class 3 | Class 4 | |
|---|---|---|---|
| Neurological RRR(SE) | Cancer RRR(SE) | Cardiovascular RRR(SE) | |
| Female | 0.79 (0.11) | 0.91 (0.14) | 0.83 (0.16) |
| Race/Ethnicity | |||
| White | 1.41 (0.32) | 0.93 (0.23) | 0.73 (0.25) |
| Black | 1.49 (0.41) | 0.88 (0.28) | 1.27 (0.48) |
| Hispanic | 1.12 (0.20) | 1.27 (0.24) | 1.71 (0.40)* |
| Age | |||
| 1–5 years | 1.31 (0.36) | 4.23 (1.91)*** | 0.74 (0.20) |
| 6–14 years | 5.57 (1.62) *** | 28.27(13.01)*** | 1.34 (0.45) |
| 15–20 years | 4.59 (1.34)*** | 22.29 (10.22)*** | 2.00 (0.60)* |
| Private Insurance | 3.47 (0.93)*** | 2.65 (0.77)*** | 2.11 (0.83) |
| Medicaid Eligibility | 1.19 (0.21) | 0.48 (0.08)*** | 0.18 (0.04)*** |
Note. RRR = relative risk ratio. SE = standard error.
p<0.05,
p<0.01,
p<0.001
Discussion
In a Medicaid-based sample of pediatric decedents, four distinct latent classes adequately accounted for variation in co-occurrence patterns of nine clinically important complex chronic conditions. Each of these classes exhibited unique disease patterns and demographic profiles from one another. Thus, this analysis presented a novel way of understanding clusters of multiple complex chronic conditions among children that may inform tailored and targeted end-of-life care for different clusters.
Among the children in the study who had at least one complex chronic condition, three of the four classes (neurological, cancer, and cardiovascular) were characterized by 100% likelihood of having one of the complex chronic conditions coupled with a low or moderate likelihood of having the other complex chronic conditions. Latent classes with 100% or 0% probabilities have been reported before in the literature (19, 17), and suggest highly discriminative classes. Each of these three classes was typified by the presence of different complex chronic conditions. Collectively, the three classes represented 69% of the overall sample. Given that these are the top three causes of death among children, this result was expected. However, it was interesting that among children in the cancer class, they had a high prevalence of both neuromuscular and cardiovascular conditions, suggesting that these forms of problems are often complications that arise during cancer treatment. Future longitudinal analysis might reveal whether these other conditions were in fact related to treatments. This finding also has interesting research implications. Latent class analysis may be useful in identifying primary diagnoses. In several claims databases, including Medicaid, there is no reported primary diagnosis for patients. For example, each time a child has a Medicaid claims encounter, the provider can include two diagnoses in the child’s Medicaid record. In this study, children had as many as 400 different diagnoses. By using latent class analysis, our findings suggest that those children with a 100% likelihood of a complex chronic condition with few comorbidities may have a primary diagnosis for analysis purposes. The nature claims data, however, leave some unanswered questions such as did the primary diagnosis initiate the state of health? Or did this primary condition arise as a complication based on the overall health of the child? Or was the condition the leading reason for health care utilization? Future research might explore these questions with other data sources, such as the death certificate, which includes cause of death diagnosis, and/ or electronic medical records, which include diagnostic detail. Additional research is warranted in different data sets and across different populations to determine whether primary diagnoses can be consistently identified.
Another interesting finding was that over 30% of the sample participants were characterized by the likelihood of many complex chronic conditions. We conducted a post-estimation analysis to better understand the number of complex chronic conditions the group had on average and found that over 60% had three or more conditions, compared to the other classes, which commonly had two conditions. These medically fragile children had a wide range of possible combinations of complex chronic conditions, which presents unique clinical challenges for comprehensive and coordinated care. Although it is common for children with complex chronic conditions to obtain care from several specialists (29), specialists may not have access to the clinical information necessary to understand the other conditions. Care focused primarily on a single complex chronic condition may not be effectively treating the child’s overall health at end of life. However, most children with complex chronic conditions also have a pediatric primary care provider, whose role is to provide comprehensive and coordinated care for children. Future research might compare the quality of care delivered to these four classes of children by specialists and pediatricians.
Given that the sample in our study was Medicaid beneficiaries, it was surprising to find that children with additional private insurance were correlated to class membership among all the classes. It is possible that carrying private insurance along with Medicaid is an important enabling factor for families as they manage the costs of complex chronic conditions. Families with children at end of life often experience the financial hardship associated with trips to the doctor, out-of-pocket expenses, and overnight travel to hospitals (30, 31). Additional health insurance coverage is a mechanism by which some families may be able to afford the costly treatments and care associated with the child’s complex chronic condition (32–34). Additional insurance may mitigate the financial burden these families encounter by covering medical services, medical transportation, equipment and supplies, and treatments not covered or fully covered by Medicaid. Thus, this family resource may enable families to access health care.
This study had limitations that impact interpretation of the findings. The goal of the study was to assess clustering of complex chronic conditions among children at end of life in California. Thus, the findings primarily generalize to this group of children and results might be different among children who are not at end of life or reside in other states. The intention of this study was to describe patterns of multiple complex chronic conditions, and therefore, we used a pooled cross-sectional, correlational design. However, this design does not allow for causal and temporal explanations. On this note, while it is interesting that insurance coverage appears to be related to membership in each of the major classes, it is not clear whether a) the coverage led to the diagnosis, b) the diagnosis led to the coverage, or c) both were co-determined by some omitted third factor. The extent to which end-of-life diagnoses are related to insurance coverage is an important area for future study, especially given the expansion of coverage in recent years. Additionally, we may not have captured all the complex chronic conditions recorded for a child in the Medicaid records because Medicaid patients often cycle in and out of the system, leading to incomplete record keeping. Finally, the sample included pediatric decedents. The four classes identified by this analysis only pertain to children with complex chronic conditions who were ill enough to have died. This limits generalizability. Nevertheless, the study offers important insights into a subpopulation of children with complex chronic conditions in order to advance our understanding of their care at end of life.
To our knowledge, this was the first latent class analysis study of multiple complex chronic conditions in children, conditions which were purposefully selected because of their clinical significance and their tendency to co-occur in non-uniform patterns (3). The results suggest that complex chronic conditions cluster together in a unique fashion, with a majority of the children expected to have a high probability of having one of the three most common primary complex chronic conditions: neurological, cardiovascular, or cancer. Accordingly, end-of-life care for children that targets specific clusters should perhaps consider different patterns of care for different clusters.
Acknowledgments
This publication was made possible by grant no. K01NR014490 from the National Institute of Nursing Research.
The authors extend special thanks to Beth Schewe for her assistance with the manuscript.
Footnotes
Disclosures
Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institute of Nursing Research or National Institutes of Health. The authors declares no conflicts of interest with respect to the authorship and/or publication of this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Feudtner C, Christakis DA, Connell FA. Pediatric death attributable to complex chronic conditions: a population-based study of Washington state, 1980–1997. Pediatrics. 2000;106:205–209. [PubMed] [Google Scholar]
- 2.Lindley LC, Lyon ME. A profile of children with complex chronic conditions at end of life among Medicaid-beneficiaries: implications for healthcare reform. J Palliat Med. 2013;16:1388–1393. doi: 10.1089/jpm.2013.0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Himelstein B, Hilden J, Boldt A, Weissman D. Pediatric palliative care. N Engl J Med. 2004;350:1752–1762. doi: 10.1056/NEJMra030334. [DOI] [PubMed] [Google Scholar]
- 4.Feinstein JA, Feudtner C, Kempe A. Adverse drug event-related emergency department visits associated with complex chronic conditions. Pediatrics. 2014;133:e1575–e1585. doi: 10.1542/peds.2013-3060. [DOI] [PubMed] [Google Scholar]
- 5.Hudson SM, Newman SD, Hester WH, et al. Factors influencing hospital admissions and emergency department visits among children with complex chronic conditions: a qualitative study of parents’ and providers’ perspectives. Issues Compr Pediatr Nurs. 2014;37:61–80. doi: 10.3109/01460862.2013.855844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jurgens V, Spaeder MC, Pavuluri P, Waldman Z. Hospital readmissions in children with complex chronic conditions discharged from subacute care. Hosp Pediatr. 2014;4:153–158. doi: 10.1542/hpeds.2013-0094. [DOI] [PubMed] [Google Scholar]
- 7.Murtagh-Kurowski E, Byczkowski T, Grupp-Phelan JM. Comparison of emergency care delivered to children and young adults with complex chronic conditions between pediatric and general emergency departments. Acad Emerg Med. 2014;21:778–784. doi: 10.1111/acem.12412. [DOI] [PubMed] [Google Scholar]
- 8.Simons TD, Berry J, Feudtner C, et al. Children with complex chronic conditions in inpatient hospital settings in the United States. Pediatrics. 2010;126:647–655. doi: 10.1542/peds.2009-3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feudtner C, Hays R, Haynes G, et al. Deaths attributed to pediatric complex chronic conditions: national trends and implications for supportive care services. Pediatrics. 2001;107:e99–e103. doi: 10.1542/peds.107.6.e99. [DOI] [PubMed] [Google Scholar]
- 10.Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version2: Updated for ICD-10 and complex medical technology dependence transplantation. BMC Pediatrics. 2014;14:199. doi: 10.1186/1471-2431-14-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lindley LC, Shaw SL. Who are the children enrolled in hospice care? J Spec Pediatr Nurs. 2014;19:308–315. doi: 10.1111/jspn.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collins LM, Lanza ST. Latent Class and latent transition analysis: With applications in the social, behavioral, and health sciences. New York: John Wiley & Sons; 2009. [Google Scholar]
- 13.Magidson J, Vermunt JK. Latent class models for clustering: a comparison with K-means. Canadian Journal of Market Research. 2002;20:36–43. [Google Scholar]
- 14.Beeber AS, Thorpe JM, Clipp EC. Community-based service use by elders with dementia and their caregivers: a latent class analysis. Nurs Res. 2008;57:312–320. doi: 10.1097/01.NNR.0000313500.07475.eb. [DOI] [PubMed] [Google Scholar]
- 15.Cherry DJ, Orme JG. The vital few foster mother. Children and Youth Services Review. 2013;35:1625–1633. [Google Scholar]
- 16.Orme JG, Combs-Orme T. Foster parenting together: Foster parent couples. Children and Youth Services Review. 2014;36:124–132. [Google Scholar]
- 17.Orme JG, Cherry DJ, Krcek TE. Who is willing to foster children with disabilities. J Public Child Welfare. 2013;7:566–585. [Google Scholar]
- 18.Combs-Orme T, Orme JG. Fostering parenting together: assessing foster parent applicant couples. Children and Youth Services Review. 2014;36:70–80. [Google Scholar]
- 19.Leventhal AM, Huh J, Dunton GF. Clustering of modifiable biobehavioral risk factors for chronic disease in US adults: a latent class analysis. Perspect Public Health. 2014;134:331–338. doi: 10.1177/1757913913495780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nguyen RHN, Veasley C, Smolenski D. Latent class analysis of comorbidity patterns among women with generalized and localized vulvodynia: preliminary findings. J Pain Res. 2013;6:303–309. doi: 10.2147/JPR.S42940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Swartz JA. Chronic medical conditions among jail detainees in residential psychiatric treatment: a latent class analysis. J Urban Health. 2011;88:700–717. doi: 10.1007/s11524-011-9554-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaiser Family Foundation. [Accessed August 8, 2012];Distribution of Medicaid enrollees by enrollment group, FY2009. 2010 Available at: http://www.statehealthfacts.org/comparemaptable.jsp?ind=200&cat=4.
- 23.Hagenaars JM. Latent structure models with direct effects between indicators: local dependence models. Sociological Methods & Research. 1988;16:379–405. [Google Scholar]
- 24.McCutcheon AL. Latent class analysis. Newbury Park, CA: Sage Publications; 1987. [Google Scholar]
- 25.Muthen B. Latent variable hybrids: overview of old and new models. In: Hancock GR, Samuelson KM, editors. Advances in latent variable mixture models. Charlotte, NC: Information Age Publishing; 2008. pp. 1–24. [Google Scholar]
- 26.Lo Y, Mendell N, Rubin D. Testing the number of components in a normal mixture. Biometrika. 2001;88:767–778. [Google Scholar]
- 27.Nyland KL, Asparouhov T, Muthen B. Deciding on the number of classes in latent class analysis and growth mixture modeling: a Monte Carlo simulation study. Struct Equ Modeling. 2007;14:535–569. [Google Scholar]
- 28.Muthen L, Muthen B. Mplus. Los Angeles, CA: Muthen & Muthen; 2009. [Google Scholar]
- 29.Himelstein B. Palliative care for infants, children, adolescents, and their families. J Palliat Med. 2006;9:163–181. doi: 10.1089/jpm.2006.9.163. [DOI] [PubMed] [Google Scholar]
- 30.Zelcer S, Cataudella D, Cairney A, Bannister S. Palliative care of children with brain tumors: a parental perspective. Arch Pediatr Adolesc Med. 2010;164:225–230. doi: 10.1001/archpediatrics.2009.284. [DOI] [PubMed] [Google Scholar]
- 31.Michelson KN, Koogler T, Sullivan C, et al. Parental views on withdrawing life-sustaining therapies in critically ill children. Arch Pediatr Adolesc Med. 2009;163:986–992. doi: 10.1001/archpediatrics.2009.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mack JW, Chen K, Boscoe FP, et al. Underuse of hospice care by Medicaid-insured patients with stage IV lung cancer in New York and California. J Clin Oncol. 2013;31:2569–2579. doi: 10.1200/JCO.2012.45.9271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCarthy EP, Burns RB, Ngo-Metzger Q, Davis RB, Phillips RS. Hospice use among Medicare managed care and fee-for-service patients dying with cancer. JAMA. 2003;289:2238–2245. doi: 10.1001/jama.289.17.2238. [DOI] [PubMed] [Google Scholar]
- 34.Virnig BA, Fischer ES, McBean AM, Kind S. Hospice use in Medicare managed care and fee-for-service systems. Am J Manag Care. 2001;7:777–786. [PubMed] [Google Scholar]