Abstract
Context
Intensity-modulated radiation therapy (IMRT), three-dimensional conformal radiation therapy (3DCRT), and proton-beam therapy (PBT) are chemoradiotherapy modalities for treating locally advanced non-small cell lung cancer (NSCLC). Although therapy is carefully planned to maximize treatment benefit while minimizing risk for adverse side effects, most patients develop radiation-induced symptom burden.
Objectives
To demonstrate the M. D. Anderson Symptom Inventory (MDASI)’s ability to detect fine differences in symptom development among these modalities.
Methods
This was a longitudinal observational study. Patients with unresectable primary or recurrent NSCLC (n=82) underwent either 3DCRT, IMRT, or PBT. Patients rated MDASI symptoms weekly for up to 12 weeks. We used mixed-effect modeling to estimate development of symptoms and functional interference.
Results
The PBT group received a significantly higher radiation target dose than did the IMRT and 3DCRT groups (P<0.001). Fatigue was the most severe symptom over time for all groups. Controlling for patient and clinical factors (age, sex, race, cancer stage, performance status, body mass index, previous cancer therapy, total radiation dose), we found that pain, as a major esophagitis-related symptom, increased more during therapy (P=0.019) and decreased more after (P=0.013) therapy in the 3DCRT and IMRT groups than in the PBT group. Compared with the PBT group, the 3DCRT and IMRT groups reported greater decrease in systemic symptoms (fatigue, drowsiness, lack of appetite, disturbed sleep) after therapy (P=0.016).
Conclusion
Patients receiving PBT reported significantly less severe symptoms than did patients receiving IMRT or 3DCRT. These results should be confirmed in a randomized study with comparable tumor burden among therapies.
Keywords: symptom, patient-reported outcomes, proton-beam therapy, MDASI, NSCLC
Introduction
Concurrent chemoradiation therapy (CXRT), a standard treatment option for patients with locally advanced non-small cell lung cancer (NSCLC),1-3 is often associated with acute side effects from the radiation and/or chemotherapy, including both systemic symptoms (e.g., fatigue, sadness, distress, disturbed sleep, drowsiness, lack of appetite) and localized symptoms from radiation-induced toxicities such as esophagitis or pneumonitis (e.g., coughing, sore throat, pain).4,5 A systematic review revealed that treatment-related toxicities (mainly acute grade 3 or 4 esophagitis and hematological issues such as neutropenia and anemia) from CXRT were more severe than the toxicities from radiotherapy alone or from sequential CXRT.2 High symptom burden contributes to the patient’s general distress during the course of treatment.6 Therefore, minimizing symptoms and their effects on a patient’s functional status, especially during aggressive cancer therapy, is an important treatment goal.7
Over the past decade, photon radiotherapy modalities such as three-dimensional conformal radiation therapy (3DCRT) and intensity-modulated radiotherapy (IMRT) have been widely adopted for the treatment of NSCLC.2,8 In addition, other radiotherapy modalities for treating NSCLC, such as proton-beam therapy (PBT), are emerging.9 PBT provides a substantial dosimetric benefit compared with conventional photon radiotherapies like 3DCRT or IMRT, because protons deliver essentially no dose beyond the end of the range and a much-reduced dose proximal to the target volume.10 This improved dose localization from PBT permits administration of a high radiation dose to the tumor while minimizing exposure of the surrounding normal tissues.
Although toxicity data for various modes of radiation, with or without concurrent chemotherapy, are becoming available,9,11,12 there is a paucity of empirical research into the severity of the multiple symptoms experienced by patients with NSCLC undergoing this type of aggressive cancer treatment. Having a sensitive symptom-assessment tool for capturing accurate symptom profiles is critical for establishing the patient’s experience of various advanced therapies, especially as new technologies arise, and for managing symptoms effectively. In a study of NSCLC patients who underwent either 3DCRT or IMRT,6 a validated multisymptom assessment tool, the M. D. Anderson Symptom Inventory (MDASI),13 was used to describe the longitudinal development of esophagitis-related pain and systemic symptoms (fatigue, drowsiness, lack of appetite, disturbed sleep) that arose during treatment. The longitudinal study presented here compared the symptom profiles of patients with NSCLC undergoing PBT versus conventional IMRT or 3DCRT therapy. We hypothesized that the technologies with better normal tissue protection (PBT or IMRT) would cause significantly less severe symptom burden.
Methods
Participants
Patients being seen in the Department of Radiation Oncology at The University of Texas M. D. Anderson Cancer Center in Houston, Texas from 2002–2010 were approached to participate in the study. Eligible patients had a pathological diagnosis of locally advanced, unresectable primary or recurrent NSCLC, were scheduled for 3DCRT, IMRT, or PBT, and were at least 18 years old. Patients were enrolled prior to therapy under two separate protocols that were prospective, observational, patient-reported outcome (PRO)-based studies of the symptomatic effects of either PBT in patients with a recurrent tumor after surgery and/or chemotherapy, or photon radiotherapy (3DCRT or IMRT) in patients with nonoperable NSCLC, in each case with concurrent chemotherapy. PBT was delivered with passively-scattered protons, the 3DCRT analogue of photons. The chemotherapy regimen (standard platinum/taxane-based doublets) was consistent for all patients regardless of radiation modality. Both protocols used the same study schema for PRO measurement.
The studies were approved by the M. D. Anderson Institutional Review Board. All participants gave written informed consent.
Patient-Reported Outcomes Tool: The MDASI
The MDASI is a PRO assessment tool validated for use in the cancer population.13 The severity of 13 common cancer-related symptoms (pain, fatigue, nausea, vomiting, dry mouth, shortness of breath, lack of appetite, difficulty remembering, drowsiness, disturbed sleep, sadness, distress, numbness/tingling) during the previous 24 hours is assessed on a 0–10 numerical rating scale, with 0 being “not present” and 10 being “as bad as you can imagine.” The additional symptoms “coughing” and “sore throat” were added to the MDASI in the 3DCRT/IMRT protocol, and “mouth or throat sores” was added in the PBT protocol. The MDASI also contains six items that describe how much symptoms have interfered with various aspects of the patient’s life (general activity, mood, walking ability, normal work, relations with other people, and enjoyment of life) during the past 24 hours. These interference items also are assessed on a 0–10 scale, with 0 being “does not interfere” and 10 being “completely interferes.” The MDASI was administered before the start of CXRT (baseline) and then weekly during and after therapy for up to 12 weeks.
Statistical Analysis
The symptoms targeted in the current analysis were the most severe MDASI symptoms reported in previous studies of patients with NSCLC undergoing CXRT. We operationally defined the four most severe symptoms (fatigue, lack of appetite, disturbed sleep, and drowsiness) as the “systemic symptoms” component,6 whereas pain is a “local” symptom that is more relevant to esophagitis. An interference composite score was computed from the mean scores of the six individual interference items to present the functional burden of cancer-related symptoms. Differences in patient and clinical variables between radiotherapy groups were examined by Chi-square test (for categorical variables) and analysis of variance (for continuous variables). P-values < 0.05 were considered statistically significant.
For systemic symptoms and pain, the effects of treatment technique on change in symptom severity during the seven weeks from before the commencement of CXRT to the end of treatment were examined by mixed-effect growth-curve models with a random subject effect and linear splines to approximate the change over time. Similar modeling was applied to demonstrate symptom changes during the five weeks post-CXRT (weeks 7–12 from CXRT start). To actively address potential confounding issues in this nonrandomized study, we included age, sex, race, cancer stage, Eastern Cooperative Oncology Group performance status, body mass index, previous cancer therapy, and total radiation dose in all models. The interpretation focuses on the average weekly rate of change in symptom severity along the 0–10 scale. The interactive effects of time and CXRT technique, and among the three types of CXRT, were estimated using multivariate analysis.
Results
Patient and Treatment Characteristics
Patient demographics and cancer therapy data are shown in Table 1. In all, 22 patients receiving 3DCRT, 34 patients receiving IMRT, and 26 patients receiving PBT were enrolled. Platinum/taxane-based chemotherapy regimens were administered concurrently with radiotherapy for all patients. There were no significant differences in patient demographics, disease stage, or type of chemotherapy agent used among the three radiotherapy groups. Fewer patients in the IMRT group had previously undergone chemotherapy, and more patients in the PBT group had previously undergone surgery. All patients completed their planned therapy.
Table 1.
Patient Characteristics
| Treatment Technique | Pa | ||||||
|---|---|---|---|---|---|---|---|
| 3DCRT (n = 22) | IMRT (n = 34) | PBT (n = 26) | |||||
| Age | 0.634 | ||||||
| Mean (SD) | 63.6 (9.0) | 65.7 (6.7) | 65.0 (9.2) | ||||
| Median (range) | 63.7 (42.5–77.9) | 65.6 (48.1–77.8) | 65.5 (43.0–79.0) | ||||
| BMI (kg/m2) | 0.606 | ||||||
| Mean (SD) | 26.7 (6.0) | 28.2 (5.8) | 28.1 (4.6) | ||||
| Median (range) | 25.1 (19.6–45.2) | 27.0 (19.7–42.2) | 27.3 (20.5–38.9) | ||||
| Total tumor dose (Gy RBE) | < 0.0001 | ||||||
| Mean (SD) | 62.1 (3.3) | 64.7 (6.4) | 70.6 (5.7) | ||||
| Median (range) | 63.0 (50.4–70.0) | 63.0 (41.4–70.0) | 74.0 (54.0–74.0) | ||||
| n | % | n | % | n | % | ||
| Sex | 0.846 | ||||||
| Male | 10 | 45.45 | 17 | 50.00 | 14 | 53.85 | |
| Female | 12 | 54.55 | 17 | 50.00 | 12 | 46.15 | |
| Race | 0.920 | ||||||
| Non-Hispanic white | 19 | 86.36 | 28 | 82.35 | 22 | 84.62 | |
| Other | 3 | 13.64 | 6 | 17.65 | 4 | 15.38 | |
| Marital status | 0.893 | ||||||
| Married | 15 | 68.18 | 25 | 73.53 | 18 | 69.23 | |
| Unmarried | 7 | 31.82 | 9 | 26.47 | 8 | 30.77 | |
| Education | 0.216 | ||||||
| College or higher | 10 | 45.45 | 15 | 44.12 | 17 | 65.38 | |
| High school or lower | 12 | 54.55 | 19 | 55.88 | 9 | 34.62 | |
| Cancer stage | 0.108 | ||||||
| I/II | 2 | 9.09 | 10 | 29.41 | 8 | 34.78 | |
| III | 20 | 90.91 | 24 | 70.59 | 15 | 65.22 | |
| ECOG PS | 0.023 | ||||||
| 0–1 | 18 | 81.82 | 31 | 93.94 | 24 | 100.00 | |
| 2–3 | 4 | 18.18 | 2 | 6.06 | 0 | 0 | |
| Previous chemotherapy | 0.036 | ||||||
| No | 9 | 40.91 | 25 | 73.53 | 13 | 50.00 | |
| Yes | 13 | 59.09 | 9 | 26.47 | 13 | 50.00 | |
| Previous surgery | < 0.001 | ||||||
| No | 20 | 90.91 | 33 | 97.06 | 15 | 57.69 | |
| Yes | 2 | 9.09 | 1 | 2.94 | 11 | 42.31 | |
3DCRT = three-dimensional conformal radiation therapy; BMI = body mass index; ECOG PS = Eastern Cooperative Oncology Group performance status; IMRT = intensity-modulated radiotherapy; PBT = proton-beam therapy; RBE = relative biological effectiveness; SD = standard deviation.
Chi-square test for categorical variables and analysis of variance for continuous variables.
A significantly higher radiation dose was received by the PBT group compared with the 3DCRT group (P < 0.001) or IMRT group (P = 0.002) (Table 1).
All of the patients contributed symptom data at baseline; three patients (3.7%) withdrew from the study before CXRT completion at week 7. The random missing-data rate ranged from 4.8% to 11.0% for the MDASI symptom items during CXRT, stemming primarily from administrative error.
White blood cell (WBC) count and hemoglobin levels were not significantly different at baseline and decreased during CXRT in all three radiotherapy groups. At the end of CXRT, there was no significant difference in hemoglobin levels between the three groups; patients who received 3DCRT had a lower WBC count (2.88×103/μL ± 1.74×103/μL) than did patients in the PBT group (4.94×103/μL ± 2.61×103/μL; P < 0.023), but no significant difference in WBC count was found between the 3DCRT group and the IMRT group (3.07×103/μL ± 0.99×103/μL).
Longitudinal Patterns of Symptom Development by Radiotherapy Type
Fatigue was consistently the most severe MDASI symptom throughout the course of CXRT for all three radiotherapy modalities. Other most severe symptoms in terms of mean severity were drowsiness, pain, disturbed sleep, lack of appetite, and sore throat. Pre-CXRT, no significant differences between groups were noted for any symptom. Table 2 presents the mixed-modeling results of weekly dynamic increase in severity during and after CXRT for these individual most severe symptoms. Each model was adjusted for patient and clinical factors. During CXRT, patients receiving 3DCRT or IMRT reported significant increases over time in pain, sore throat, and lack of appetite (all P < 0.001), fatigue (P = 0.019 and P < 0.001, respectively) and drowsiness (P = 0.016 and P < 0.001, respectively). For patients receiving PBT, only pain increased significantly (P = 0.024). Post-CXRT, patients receiving IMRT reported significant decreases in pain, sore throat, fatigue, drowsiness, and lack of appetite (all P < 0.05). The 3DCRT patients reported significantly decreased sore throat, lack of appetite, and disturbed sleep (all P < 0.05). PBT patients reported no significant change in any symptom.
Table 2.
Mixed Modeling of Weekly Change of Individual Symptom Burden Among Three Groups by Type of CXRT
| 3DCRT (151 observations)a | IMRT (240 observations)a | PBT (144 observations)a | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Est | SE | P | Est | SE | P | Est | SE | P | |
| During CXRT (weeks 1–7) | |||||||||
| Pain | 0.43 | 0.09 | < 0.001 | 0.44 | 0.08 | < 0.001 | 0.20 | 0.08 | 0.024 |
| Sore throat | 0.65 | 0.10 | < 0.001 | 0.50 | 0.08 | < 0.001 | 0.10b | 0.06 | 0.097 |
| Fatigue | 0.22 | 0.08 | 0.019 | 0.41 | 0.07 | < 0.001 | 0.16 | 0.10 | 0.132 |
| Drowsiness | 0.30 | 0.11 | 0.016 | 0.32 | 0.06 | < 0.001 | 0.22 | 0.10 | 0.050 |
| Lack of appetite | 0.48 | 0.12 | < 0.001 | 0.36 | 0.08 | < 0.001 | 0.16 | 0.11 | 0.151 |
| Disturbed sleep | 0.15 | 0.12 | 0.249 | 0.06 | 0.08 | 0.409 | 0.02 | 0.11 | 0.826 |
| Post-CXRT (weeks 7–12) | |||||||||
| Pain | –0.14 | 0.14 | 0.308 | –0.40 | 0.13 | 0.005 | –0.10 | 0.10 | 0.341 |
| Sore throat | –0.54 | 0.15 | 0.001 | –0.37 | 0.12 | 0.004 | –0.06 | 0.07 | 0.409 |
| Fatigue | –0.14 | 0.08 | 0.093 | –0.44 | 0.11 | < 0.001 | 0.19 | 0.12 | 0.117 |
| Drowsiness | –0.11 | 0.11 | 0.352 | –0.31 | 0.10 | 0.003 | –0.06 | 0.11 | 0.599 |
| Lack of appetite | –0.48 | 0.14 | 0.002 | –0.28 | 0.13 | 0.037 | –0.13 | 0.13 | 0.317 |
| Disturbed sleep | –0.30 | 0.13 | 0.030 | –0.04 | 0.12 | 0.728 | –0.03 | 0.12 | 0.796 |
3DCRT = three-dimensional conformal radiation therapy; CXRT = concurrent chemoradiation therapy; Est = estimate of the effect of independent variables (or factors) on symptoms; IMRT = intensity-modulated radiotherapy; PBT = proton-beam therapy; SE = standard error.
Age, sex, race, cancer stage, Eastern Cooperative Oncology Group performance status, body mass index, total radiation dose, previous chemotherapy, and previous surgery were included as covariates in all models. Estimate value represents the average weekly change in symptom score on the MDASI’s 0–10 scale, with a positive score indicating increased severity.
This item was worded as “mouth or throat sores” in the PBT group.
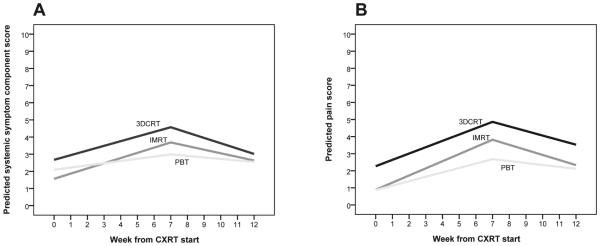
The mean severities of the systemic symptoms component score and pain for all three radiotherapy groups during and after CXRT are presented in Fig. 1. Before CXRT (at baseline), the differences between groups were not significant for the mean symptom severity component score.
Fig. 1.
Weekly change in mean severity of (A) systemic symptoms and (B) pain during CXRT. 3DCRT = three-dimensional conformal radiation therapy; CXRT = concurrent chemoradiation therapy; IMRT = intensity-modulated radiotherapy; PBT = proton-beam therapy.
Differences in Symptom Outcomes by Radiotherapy Modality
Adjusted for patient and clinical factors, mixed modeling (Table 3) demonstrated the estimation (est) of differences in symptom change for the component scores and pain among the three groups, by multivariate analysis. During CXRT, the trend toward lower severity for the systemic symptoms component score in the PBT group versus the 3DCRT group or IMRT group was not statistically significant (est = 0.11, P = 0.307). After CXRT, both 3DCRT and IMRT patients experienced a more rapid decrease in systemic symptoms, compared with PBT patients (all P < 0.05).
Table 3.
Mixed Modeling of Weekly Change in Major Symptom Burden Among Three Groups During and After CXRT (785 Observations)a
| Effect | Est | SE | Pr > |t| |
|---|---|---|---|
| Systemic symptoms (fatigue, lack of appetite, disturbed sleep, drowsiness) | |||
| Weeks 0–7 | |||
| IMRT vs. PBT | 0.12 | 0.13 | 0.338 |
| 3DCRT vs. PBT | 0.10 | 0.12 | 0.394 |
| IMRT vs. 3DCRT | –0.02 | 0.12 | 0.840 |
| 3DCRT/IMRT vs. PBT | 0.11 | 0.11 | 0.307 |
| Weeks 7–12 | |||
| IMRT vs. PBT | –0.35 | 0.16 | 0.026 |
| 3DCRT vs. PBT | –0.31 | 0.15 | 0.032 |
| IMRT vs. 3DCRT | 0.04 | 0.13 | 0.775 |
| 3DCRT/IMRT vs. PBT | –0.33 | 0.14 | 0.016 |
| Total radiation dose (Gy) | –0.10 | 0.04 | 0.007 |
| Pain | |||
| Weeks 0–7 | |||
| IMRT vs. PBT | 0.24 | 0.10 | 0.014 |
| 3DCRT vs. PBT | 0.17 | 0.11 | 0.118 |
| IMRT vs. 3DCRT | 0.08 | 0.09 | 0.409 |
| 3DCRT/IMRT vs. PBT | 0.21 | 0.09 | 0.019 |
| Weeks 7–12 | |||
| 3DCRT vs. PBT | –0.38 | 0.21 | 0.070 |
| IMRT vs. PBT | –0.51 | 0.20 | 0.010 |
| IMRT vs. 3DCRT | –0.13 | 0.17 | 0.452 |
| 3DCRT/IMRT vs. PBT | –0.46 | 0.19 | 0.013 |
| Total radiation dose (Gy) | –0.09 | 0.04 | 0.027 |
3DCRT = three-dimensional conformal radiation therapy; CXRT = concurrent chemoradiation therapy; Est = estimate of the effect of independent variables (or factors) on symptoms; IMRT = intensity-modulated radiotherapy; PBT = proton-beam therapy; SE = standard error.
Age, sex, race, cancer stage, Eastern Cooperative Oncology Group performance status, body mass index, total radiation dose, previous chemotherapy, and previous surgery were included in all models.
Compared with the PBT group, the IMRT patients had a more rapid increase in pain during CXRT (P = 0.014). No significant difference was found for change in pain between the 3DCRT and IMRT groups (P = 0.409). The average weekly increase in pain was significantly greater in the 3DCRT and IMRT groups than in the PBT group during CXRT (est = 0.21, P = 0.019), and decrease in pain was greater post-CXRT (est = −0.46, P = 0.013). The model was also adjusted for patient and clinical factors.
Cumulative Effects on Interference
Patient-reported symptom interference increased significantly in the 3DCRT (P < 0.001), IMRT (P < 0.001), and PBT (P = 0.016) groups. As expected, systemic symptoms contributed significantly to interference (est = 0.62, P < 0.001), but pain, as a local symptom from esophagitis, did not (est = 0.04, P = 0.206).
Discussion
In this longitudinal study, we examined the patient-reported symptom burden produced by three different modes of concurrent CXRT for NSCLC: photon radiotherapies (3DCRT and IMRT) and proton-beam therapy (PBT). Although the three modes shared a similar symptom-development pattern over the course of CXRT, comparisons between modes indicated a significant difference in the emergence of acute esophagitis-related pain, which was more severe for the 3DCRT and IMRT groups than for the PBT group. These results support our hypothesis that less severe symptom burden would be associated with a radiotherapy technology with enhanced normal-tissue protection.11,14
Previous studies in patients with NSCLC undergoing CXRT identified two treatment-related symptom clusters in the acute phase of the therapy: an esophagitis symptoms component (pain and sore throat)15 and a systemic symptoms component comprising the most severe symptoms reported by this patient cohort (fatigue, lack of appetite, disturbed sleep, and drowsiness).6 In the latter study, the systemic symptoms component was shown to have a steady increase in severity that was correlated with increasing CXRT dose, reflecting the body’s overall response to radiation damage to tissue (both the tumor and normal structures) plus systemic damage from concurrently administered chemotherapy.16,17 Because systemic symptoms have a strong impact upon daily functioning,18 examining new therapies with potential for reduced symptom burden is of high interest. In the current study, the systemic symptoms fatigue, drowsiness, disturbed sleep, and lack of appetite worsened significantly in the 3DCRT and IMRT groups but not in the PBT group, despite the 15% higher tumor radiation dose and an additional week of treatment for PBT. These results are indicative of the symptom benefit associated with PBT.
In terms of tumor burden and impact from prior therapy, all patients had locally advanced NSCLC and were therefore generally comparable among CXRT modalities, although 3DCRT patients (many of whom had undergone previous chemotherapy) and IMRT patients had nonresectable disease, whereas PBT patients (many of whom had undergone surgery) had recurrent disease. Nonetheless, we adjusted the models for cancer stage and prior cancer therapies as important factors. Because ours was a nonrandomized study, we remain interested in results from randomized studies among these three treatment groups that address the benefits of better normal tissue protection. The results from a Phase II study of high-dose proton therapy with CXRT for stage III NSCLC19 showed improved toxicity profiles, supporting our notion that sparing normal tissue reduces the symptomatic effects of therapy.
Results from the current study also suggest that accumulated effects over time from concurrent CXRT could substantially affect a patient’s daily functioning, As expected, systemic symptoms contributed significantly to interference (est = 0.62, P < 0.001).
The MDASI, a brief multisymptom assessment tool, can be completed frequently with minimal effort by cancer patients. Such feasibility is especially important for longitudinal studies in which repeated measures are required. Because this longitudinal study was not a randomized trial, we used mixed modeling to handle the change in PRO data over time and included both patient and clinical variables in the modeling to proactively address potential confounding issues.
Our study had certain limitations. First, this was a nonrandomized prospective study with convenience samples. The significantly higher radiation dose and trend toward lower symptom burden that we observed in the PBT group should be carefully examined in a randomized study of the three treatment modalities in patients with similar disease and prior treatment status, along with potentially related differences in damage to normal tissue, with or without passive scattering technology. Second, the wording on the additional MDASI item “sore throat” used in the 3DCRT and IMRT groups did not match the additional item “mouth or throat sores” used in the PBT group; therefore, we did not include this comparison result among groups in this report. Although treatment-induced mouth sores were not expected in the PBT group, the same wording should be tested and compared among the three radiotherapy groups in a future study. Third, we did not have sufficient data to analyze dose-distribution patterns (because of missing dose volume histogram data from the 3DCRT group), and clinician-rated toxicity data (especially lower-grade toxicity ratings),20 or tumor size and location, which would have allowed us to review fine differences in symptom burden by radiation dose to lung tissue and their relationship to clinically rated acute toxicities. In a future study with a longer study period, PRO measures might further clarify the fine differences in post-CXRT symptom burden related to lung damage, as well as grade 2 or higher radiation pneumonitis, produced by each of the three radiotherapy types. Incorporation of other widely used PRO-based symptom assessment tools, such as the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-Core 30 or the Functional Assessment of Cancer Therapy-Lung, would facilitate comparison of results with those of other published studies.
The longitudinal symptom profile findings from this study demonstrate methods for accurately monitoring patients during and after treatment that might lead to a better consensus about symptom burden during standard and advanced cancer care with CXRT. Clinically, this study provides evidence for both patients and clinicians as to when to expect the greatest symptomatic impact from treatment, and it could inform their choice of therapy; in particular, our results may provide justification for choosing proton therapy.21 Methodologically, this study provides evidence for PRO-based symptom clusters that are relevant to and affected by specific cancer therapies.22
Acknowledgments
This study is supported by grants from the National Cancer Institute of the National Institutes of Health: NCI R21 CA132109 to Dr. Wang; NCI R01 CA026582 to Dr. Cleeland; NCI P01 CA021239 to Drs. Delaney and Mohan (co-PIs); and M. D. Anderson Cancer Center Support Grant NCI P30 CA016672. The funding agency played no role in study design, in the collection, analysis and interpretation of data, in the writing of the report, or in the decision to submit the article for publication. The MDASI is patented and licensed to The University of Texas M. D. Anderson Cancer Center and Dr. Cleeland.
The authors acknowledge the assistance of Maria Sanchez, LVN (data collection), Gary Mobley, MA (data management), and Jeanie Woodruff, BS, ELS (editorial support).
Footnotes
The findings of this study were presented in part at the American Society of Clinical Oncology 2013 Annual Conference, June 1, 2013, Chicago, IL.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors report no other conflicts of interest in this work.
References
- 1.Akamatsu H, Mori K, Naito T, et al. Progression-free survival at 2 years is a reliable surrogate marker for the 5-year survival rate in patients with locally advanced non-small cell lung cancer treated with chemoradiotherapy. BMC Cancer. 2014;14:18. doi: 10.1186/1471-2407-14-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feliciano J, Feigenberg S, Mehta M. Chemoradiation for definitive, preoperative, or postoperative therapy of locally advanced non-small cell lung cancer. Cancer J. 2013;19:222–230. doi: 10.1097/PPO.0b013e318293238d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 4.Hickok JT, Morrow GR, Roscoe JA, Mustian K, Okunieff P. Occurrence, severity, and longitudinal course of twelve common symptoms in 1129 consecutive patients during radiotherapy for cancer. J Pain Symptom Manage. 2005;30:433–442. doi: 10.1016/j.jpainsymman.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 5.Wang XS, Shi Q, Williams LA, et al. Inflammatory cytokines are associated with the development of symptom burden in patients with NSCLC undergoing concurrent chemoradiation therapy. Brain Behav Immun. 2010;24:968–974. doi: 10.1016/j.bbi.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang XS, Fairclough DL, Liao Z, et al. Longitudinal study of the relationship between chemoradiation therapy for non-small-cell lung cancer and patient symptoms. J Clin Oncol. 2006;24:4485–4491. doi: 10.1200/JCO.2006.07.1126. [DOI] [PubMed] [Google Scholar]
- 7.Cleeland CS. Cancer-related symptoms. Semin Radiat Oncol. 2000;10:175–190. doi: 10.1053/srao.2000.6590. [DOI] [PubMed] [Google Scholar]
- 8.Grutters JP, Kessels AG, Pijls-Johannesma M, et al. Comparison of the effectiveness of radiotherapy with photons, protons and carbon-ions for non-small cell lung cancer: a meta-analysis. Radiother Oncol. 2010;95:32–40. doi: 10.1016/j.radonc.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 9.McAvoy SA, Ciura KT, Rineer JM, et al. Feasibility of proton beam therapy for reirradiation of locoregionally recurrent non-small cell lung cancer. Radiother Oncol. 2013;109:38–44. doi: 10.1016/j.radonc.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 10.Gomez DR, Chang JY. Accelerated dose escalation with proton beam therapy for non-small cell lung cancer. J Thorac Dis. 2014;6:348–355. doi: 10.3978/j.issn.2072-1439.2013.11.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gomez DR, Tucker SL, Martel MK, et al. Predictors of high-grade esophagitis after definitive three-dimensional conformal therapy, intensity-modulated radiation therapy, or proton beam therapy for non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2012;84:1010–1016. doi: 10.1016/j.ijrobp.2012.01.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oshiro Y, Mizumoto M, Okumura T, et al. Results of proton beam therapy without concurrent chemotherapy for patients with unresectable stage III non-small cell lung cancer. J Thorac Oncol. 2012;7:370–375. doi: 10.1097/JTO.0b013e31823c485f. [DOI] [PubMed] [Google Scholar]
- 13.Cleeland CS, Mendoza TR, Wang XS, et al. Assessing symptom distress in cancer patients: the M. D. Anderson Symptom Inventory. Cancer. 2000;89:1634–1646. doi: 10.1002/1097-0142(20001001)89:7<1634::aid-cncr29>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 14.Tucker SL, Mohan R, Liengsawangwong R, Martel MK, Liao Z. Predicting pneumonitis risk: a dosimetric alternative to mean lung dose. Int J Radiat Oncol Biol Phys. 2013;85:522–527. doi: 10.1016/j.ijrobp.2012.03.052. [DOI] [PubMed] [Google Scholar]
- 15.Bradley J, Deasy JO, Bentzen S, El-Naqa I. Dosimetric correlates for acute esophagitis in patients treated with radiotherapy for lung carcinoma. Int J Radiat Oncol Biol Phys. 2004;58:1106–1113. doi: 10.1016/j.ijrobp.2003.09.080. [DOI] [PubMed] [Google Scholar]
- 16.Fournel P, Robinet G, Thomas P, et al. Randomized phase III trial of sequential chemoradiotherapy compared with concurrent chemoradiotherapy in locally advanced non-small-cell lung cancer: Groupe Lyon-Saint-Etienne d'Oncologie Thoracique-Groupe Francais de Pneumo-Cancerologie NPC 95-01 Study. J Clin Oncol. 2005;23:5910–5917. doi: 10.1200/JCO.2005.03.070. [DOI] [PubMed] [Google Scholar]
- 17.Liu HH, Wang X, Dong L, et al. Feasibility of sparing lung and other thoracic structures with intensity-modulated radiotherapy for non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2004;58:1268–1279. doi: 10.1016/j.ijrobp.2003.09.085. [DOI] [PubMed] [Google Scholar]
- 18.Patrick DL, Ferketich SL, Frame PS, et al. National Institutes of Health State-of-the-Science Conference Statement: Symptom management in cancer: pain, depression, and fatigue, July 15-17, 2002. J Natl Cancer Inst. 2003;95:1110–1117. doi: 10.1093/jnci/djg014. [DOI] [PubMed] [Google Scholar]
- 19.Chang JY, Komaki R, Lu C, et al. Phase 2 study of high-dose proton therapy with concurrent chemotherapy for unresectable stage III nonsmall cell lung cancer. Cancer. 2011;117:4707–4713. doi: 10.1002/cncr.26080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC) Int J Radiat Oncol Biol Phys. 1995;31:1341–1346. doi: 10.1016/0360-3016(95)00060-C. [DOI] [PubMed] [Google Scholar]
- 21.Pijls-Johannesma M, Grutters JP, Verhaegen F, Lambin P, De Ruysscher D. Do we have enough evidence to implement particle therapy as standard treatment in lung cancer? A systematic literature review. Oncologist. 2010;15:93–103. doi: 10.1634/theoncologist.2009-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barsevick AM, Whitmer K, Nail LM, Beck SL, Dudley WN. Symptom cluster research: conceptual, design, measurement, and analysis issues. J Pain Symptom Manage. 2006;31:85–95. doi: 10.1016/j.jpainsymman.2005.05.015. [DOI] [PubMed] [Google Scholar]