Abstract
Human milk glycans (HMGs) are prebiotics, pathogen receptor decoys, and regulators of host physiology and immune responses. Mechanistically, human lectins (glycan-binding proteins, hGBPs) expressed by dendritic cells (DC) are of major interest, as these cells directly contact HMGs. To explore such interactions, we screened many C-type lectins and Siglecs expressed by DC for glycan binding on microarrays presenting over 200 HMGs. Unexpectedly, DC-SIGN showed robust binding to many HMGs, whereas other C-type lectins failed to bind, and Siglecs-5 and -9 showed weak binding to a few glycans. By contrast, most hGBPs bound to multiple glycans on other microarrays lacking HMGs. An α-linked fucose residue was characteristic of HMGs bound by DC-SIGN. Binding of DC-SIGN to the simple HMGs 2′-fucosyllactose (2′-FL) and 3-fucosyllactose (3-FL) was confirmed by flow cytometry to beads conjugated with 2′-FL or 3-FL, as well as the ability of the free glycans to inhibit DC-SIGN binding. 2′-FL had an IC50 of ~1 mM for DC-SIGN, which is within the physiological concentration of 2′-FL in human milk. These results demonstrate that DC-SIGN among the many hGBPs expressed by DC binds to α-fucosylated HMGs, and suggest that such interactions may be important in influencing immune responses in the developing infant.
Keywords: DC-SIGN, Glycan-Binding Proteins, Glycan Microarrays, Glycan Recognition, Human Milk Glycans, Lectins
Introduction
Carbohydrates are the most abundant class of biomolecules in human milk. The majority of this total carbohydrate (~70g/L) is lactose, a major source of energy for infants, and the remainder (5–20g/L) consists of non-digestible, larger-sized glycans that are derived from lactose [1–3]. These human milk glycans (HMGs) have been classically defined as prebiotics and receptor decoys that are predicted to prevent infection by blocking pathogen adherence to the infant epithelium [4, 5]. However, HMGs may have functions beyond interactions with microbes, as more recent studies suggest that HMGs may regulate multiple physiological functions in infants, including gene expression and immune and allergic responses [6, 7]. HMGs also regulate gut motility [8] and enhance learning and memory [9], suggesting their role in neuronal responses and cognition. However, the mechanisms underlying these physiological functions of HMGs are still unclear.
Human lectins (glycan-binding proteins, hGBPs) play numerous roles in physiology and immunity, including regulation of gene expression and immune responses, pathogen sensing, cell-cell interactions, and tissue homing [10–12]. The glycan specificities of many hGBPs have been explored by multiple techniques, but the most powerful new approach has utilized glycan microarrays in which hundreds of structurally defined glycan ligands are displayed on a single slide, as developed by the Consortium for Functional Glycomics (CFG) (http://www.functionalglycomics.org/glycomics/publicdata/primaryscreen.jsp). These studies have shown that each hGBP has a restricted specificity, even within a given hGBP family [13]. The binding of different hGBPs to specific glycan determinants allows different hGBPs to regulate specific physiological functions.
There have been some recent studies broadly examining hGBP glycan specificity toward HMGs [14–16], and such general screening suggests that some HMGs may be recognized by specific hGBP. By extension, we hypothesized that HMGs might serve as general ligands for many hGBP, which could be important in modulating the hGBP downstream effector or signaling functions. The purpose of our study was to identify hGBP that bind HMGs, investigate glycan determinant specificity and the extent of the human milk metaglycome bound, and determine if binding occurs at physiologically relevant concentrations.
To address these questions, we focused on those hGBPs expressed by dendritic cells (DCs), since such cells may directly contact HMGs in the developing infant intestine via dendrite extension through the intestinal epithelium [17, 18]. We screened members of the C-type lectin [19] and I-type lectin [11, 20] families for binding to a human milk shotgun glycan microarray as well as defined glycan microarrays. The results of this study showed that from this large set of hGBPs, only Dendritic Cell-Specific Intercellular Adhesion Molecule-3-Grabbing Non-integrin (DC-SIGN) was a major binder of HMGs, with multiple α-linked fucose-containing glycans bound on an array consisting of about 250 purified HMG structures. This binding of specific HMGs by DC-SIGN suggest that DC-SIGN may serve as an HMG receptor, which may have implications in infant immunity, physiology, and development.
Materials and methods
Preparation and Screening of Microarrays
All of the recombinant hGBP used in this study were purchased from R&D Systems (Minneapolis, MN) and are shown in Table 1, which includes information on the amino acid sequences, fusion tags, and catalog numbers used. The proteins were checked for activity by their binding to one or more glycans in various glycan microarrays. The human milk shotgun glycan microarray version 2 (HM-SGM-v2), consisting of 247 purified HMGs structures and 13 controls, has been previously described [21]. The defined HMGs microarray, consisting of simple, defined HMGs structures, was generated as described previously [22]. The recombinant hGBP were screened on the Consortium for Functional Glycomics (CFG) glycan microarray version 5.1, HM-SGM-v2, and defined HMGs microarray as previously described [23]. 5μg/ml of Alexa Fluor 488-labeled anti-human IgG (Molecular Probes, Eugene, OR) or Alexa Fluor 488-labeled anti-pentaHis antibody (Qiagen, Valencia, CA) antibodies were used for detection of recombinant hGBP carrying an Fc fusion tag or 6–9x Histidine tag, respectively. As a control we screened 10μg/ml DC-SIGN binding on the HM-SGM-v2, in which Ca2+ was omitted from the binding buffer and replaced with 0.2mM EDTA to confirm Ca2+-dependent binding. For HMG inhibition experiments, the recombinant hGBP was preincubated with free HMG or 2-ethyl-N(aminoethyl)benzamide (AEAB)-derivatized HMG containing an “open-ring” reducing end or glycosylamide-glycyl-N-aminoethyl 2-aminobenzamide (GGAEAB)-derivatized HMG containing a “closed-ring” reducing end, generated as previously described [24, 25] for 1 hour prior to screening of the defined HMG microarrays. Detection was performed using 5μg/ml Alexa Fluor 633-labeled anti-human IgG (Molecular Probes). Rank and average rank calculations of the microarray data was performed as previously described [26]. The microarray data was manually examined for binding motifs and, for the CFG microarray data, was further analyzed with Glycopattern (https://glycopattern.emory.edu) [27] to define the CFG glycan microarray binding motif.
Table 1.
Recombinant Human Glycan-Binding Proteins Used in this Studya
| Name | Lectin Family | Genbank Accession Number | Amino Acid Sequence | Protein Fusion Tag | R & D Catalog Number |
|---|---|---|---|---|---|
| DC-SIGN | C-Type Lectin | Q9NNX6 | Lys62 - Ala404 | N-terminal MD-Human IgG1 Fc-IEGR fusion tag | 161-DC-050 |
| Langerin | C-Type Lectin | Q9UJ71 | Tyr64 - Pro328 | N-terminal 9x His tag | 2088-LN-050 |
| Dectin-2 | C-Type Lectin | Q6EIG7 | Thr46 - Leu209 | N-terminal 6x His tag | 3114-DC-050 |
| MGL (CLEC10A) | C-Type Lectin | Q8IUN9 | Gln61 - His316 | N-terminal 6x His tag | 4888-CL-050 |
| Siglec-1 | I-Type Lectin | Q9BZZ2 | Ser20 - Gln1641 | C-terminal 6x His tag | 5197-SL-050 |
| Siglec-5 | I-Type Lectin | O15389 | Glu17 - Thr434 | C-terminal IEGRID-Human IgG1 Fc-fusion tag | 1072-SL-050 |
| Siglec-7 | I-Type Lectin | Q9Y286 | Gln19 - Gly357 | C-terminal DIEGRMD- Human IgG1 Fc fusion tag | 1138-SL-050 |
| Siglec-9 | I-Type Lectin | Q9Y336 | Gln18 - Gly348 | C-terminal DIEGRMD- Human IgG1 Fc fusion tag | 1139-SL-050 |
| Siglec-10 | I-Type Lectin | Q96LC7 | Met17 - Thr546 | C-terminal IEGRMD- Human IgG1 Fc fusion tag | 2130-SL-050 |
All recombinant proteins were purchased from R&D Biosystems and were expressed from a mouse myeloma cell line, NS0-derived
Preparation and Screening of HMGs Microarray for MAGS
A panel of HMGs samples bound by DC-SIGN were printed on separate Nexterion N-hydroxysuccinimide (NHS) H slides (Schott AG, Mainz, Germany) and screened with lectins, antibodies, and DC-SIGN at three different concentrations of each sample. Slide printing and sample screening were performed as previously described [23]. The anti-SLea antibody was purchased from Abcam. All of the other lectins, antibodies, and glycosidases used for MAGS, as well as the concentration(s) and glycosidase treatment procedures, are the same as described in a previous study [21]. Multi-dimensional mass spectrometry on HMG-9, HMG-19, and HMG-36 was performed as previously described [28].
Preparation of HMGs-Derivatized Beads and Flow Cytometry Assessment of Binding
HMGs were first derivatized with AEAB [13] by reductive amination as previously described [24]. The HMGs were then coupled to 1.00μm diameter PolyBead® Carboxylate Microspheres using the PolyLink Protein Coupling Kit (PolySciences Inc., Warrington, PA) as follows. Beads (200μl) were pelleted by gentle centrifugation at 500–1000’g for 5 minutes and resuspended in 160μl of PolyLink coupling buffer. Twenty μl of 200mg/ml freshly prepared EDC and 20μl of freshly prepared sulfo-NHS (Thermo Scientific) were then added and the reaction incubated at room temperature with gentle rotation for 30 minutes. The beads were then washed twice with 250μl PolyLink Wash/Storage Buffer and then resuspended in 1mM glycan-AEAB in 100mM sodium phosphate pH 8.5. The reaction was incubated at room temperature with gentle mixing for 1–2 hours. The beads were washed three times with PolyLink Wash/Storage Buffer and stored at 4ºC in the same buffer until use.
For measurement of DC-SIGN binding to the glycan-derivatized beads, lacto-N-tetraose (LNT)-, 2′-fucosyllactose (2′-FL)-, and 3-fucosyllactose (3-FL)-derivatized beads were incubated for 1 hour with 5μg/ml of recombinant human DC-SIGN at room temperature, washed three times with PBS, and then incubated for 1 hour with 2μg/ml of Alexa Fluor 633-labeled goat anti-human IgG. As a negative control, 2′-FL-derivatized beads were incubated with secondary antibody only (no DC-SIGN). All samples were analyzed by flow cytometry with a BD FACSCalibur with the 633nm laser. 10,000 events were counted, and the FL-4 filter was used for detection. The data was analyzed using FlowJo. Gating was assigned in FlowJo by running the beads alone vs. buffer alone on a forward- vs. side-scatter plot, with >99% of the events falling in the gated area for all samples.
Results
Binding of hGBPs to the Human Milk Shotgun Glycan Microarray
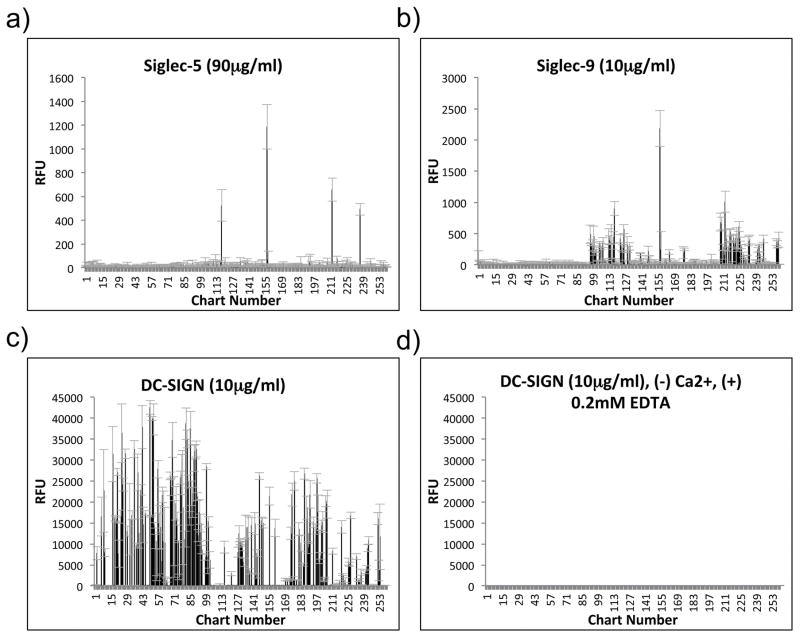
A set of eight recombinant hGBPs was tested for binding to HMGs, and this set included C-type lectins and sialic acid–binding, immunoglobulin-like lectin (Siglec) members of the I-type lectin family (refer to Table 1 for all of the hGBP used in this study). These hGBP were selected based on their stability, availability, and known expression by dendritic cells (DCs) [10, 29–31]. The C-type lectins and Siglecs were screened on a human milk shotgun glycan microarray consisting of 247 HMGs structures purified from human milk as well as 13 control glycans. This microarray was termed the HM-SGM-v2 [21]. However, only three of these hGBP, DC-SIGN, Siglec-5, and Siglec-9 showed binding to the HM-SGM-v2 (Fig. 1; also refer to Supplementary File 1 for the data for all concentrations of all hGBP screened). The binding of Langerin was considered inconclusive because high concentrations of protein were needed and the signal:noise ratio was poor (Supplementary File 1). All other hGBP showed no evidence of binding to the HM-SGM-v2, although most bound to other glycan microarrays.
Fig. 1. HM-SGM-v2 Data for Siglec-5, Siglec-9, and DC-SIGN.
Siglec-5, Siglec-9, and DC-SIGN were screened on the HM-SGM-v2 at multiple concentrations. The results for 90μg/ml Siglec-5 (a), 10μg/ml Siglec-9 (b), and 10μg/ml DC-SIGN with (c) or without (d) Ca2+ are shown; Alexa Fluor 488-labeled anti-human IgG was used for detection. Refer to Supplementary File 1 for the results at all concentrations screened. For DC-SIGN without Ca2+ (d), Ca2+ was omitted from the binding buffer and 0.2mM EDTA was added.
DC-SIGN bound to many glycans on the HM-SGM-v2 (Fig. 1c), specifically all of the glycans containing at least one α-linked fucose residue based on the calculated composition from matrix-assisted laser desorption/ionization-time of flight mass spectrometry (MALDI-TOF MS) molecular mass measurements [21]. This binding was specific in that all binding required Ca2+ even at the highest DC-SIGN concentration used (Fig. 1d). The large number of glycans bound by DC-SIGN on the HM-SGM-v2 necessitated further examination of these bound structures in order to define the HMGs determinant recognized by DC-SIGN. To this end, a Metadata-Assisted Glycan Sequencing (MAGS) approach was used [32], where a number of structures bound by DC-SIGN were printed on a separate microarray and screened with lectins and antibodies that have defined binding to a variety of glycan determinants including α-fucosylated structures, terminal β1-3-linked or β1-4-linked galactose, α2-6-linked sialic acid, Lewis epitopes, and Blood Group H Type 1 or Type 2 (Supplementary File 2). DC-SIGN was also screened on this microarray and confirmed to bind all of the printed structures (Supplementary File 2).
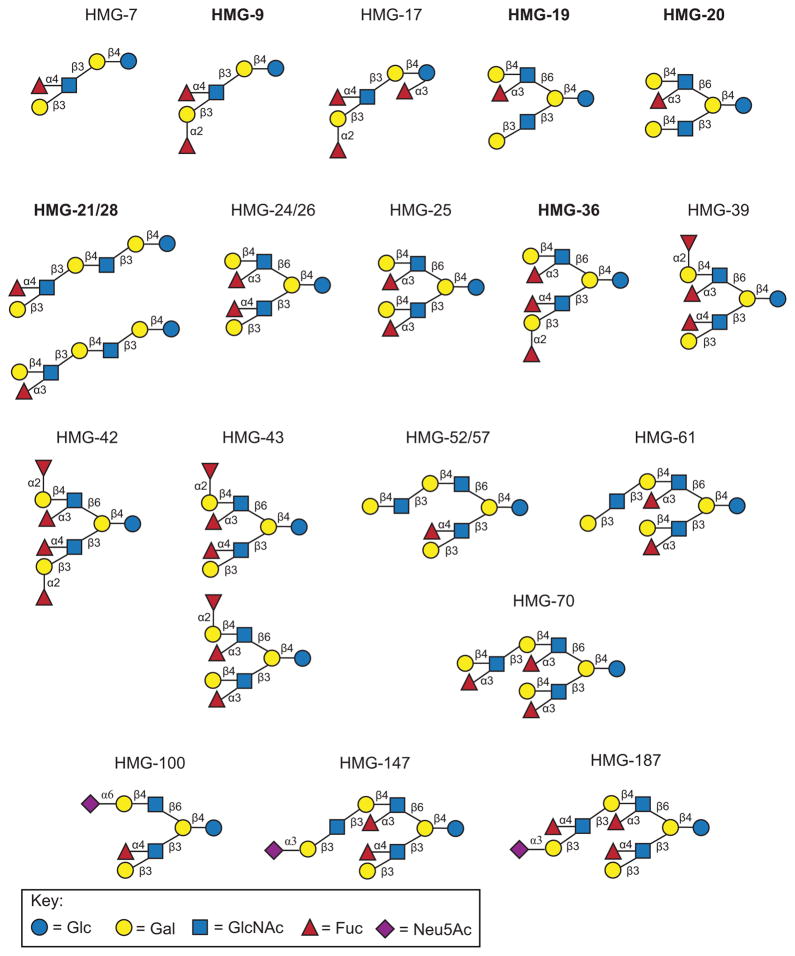
Based on this MAGS data and mass spectrometry sequencing data for some structures [33], proposed structures for the HMGs bound by DC-SIGN are shown in Figure 2. The key feature of all these structures is the presence of α-linked fucose, specifically terminal Lewis a (Galβ1-3(Fucα1-4)GlcNAcβ-), terminal Lewis b (Fucα1-2Galβ1-3(Fucα1-4)GlcNAcβ-), terminal Lewis y (Fucα1-2Galβ1-4(Fucα1-3)GlcNAcβ-) and/or a terminal Lewis x (Galβ1-4(Fucα1-3)GlcNAcβ-) determinant. Not all fucosylated HMGs were bound though. For example, HMO-8 and HMO-29 contained one fucose while HMO-37 and HMO-80 contained 2 fucoses but were not bound. The fucosylated glycan determinant present in these four structures was likely Blood Group H Type 1 (H1) since the anti-H1 antibody but none of the Lewis antibodies bound these four structures. Additionally, HMO-23, -31, -41, -47, -48, and -49, containing 1 or 2 fucoses, were also not bound and contain only an internal Lewis x determinant (or, in the case of HMO-31, internal Lewis x as the major structures) [21, 28]. Therefore, the Lewis x is a binding determinant of DC-SIGN only when present at the non-reducing end of HMGs. The binding of DC-SIGN to HMGs containing terminal Lewis glycan determinants but not Blood Group H determinants on HMGs also corroborates previous studies on the glycan specificity of DC-SIGN [34, 35]. Additionally, 2′-fucosyllactose (2′-FL, HMO-3) was also weakly bound on the HM-SGM-v2 (average rank = 11), a ligand not seen in previous studies. Overall, these results suggest that DC-SIGN recognizes α-fucosylated HMGs containing Lewis glycan determinants at the non-reducing end as well as 2′-fucosyllactose, and the high abundance of these structures and determinants in the HMGs metaglycome explains why DC-SIGN binds robustly to the HM-SGM-v2.
Fig. 2. Proposed Structures of HMGs Bound by DC-SIGN on the HM-SGM-v2.
A portion of the HM-SGM-v2 structures bound by DC-SIGN were printed on a separate microarray and interrogated by metadata-assisted glycan sequencing (MAGS), where multiple lectins and antibodies specific for particular glycan determinants were screened. Proposed structures for these HMGs samples are shown. HMG samples in bold-face font were further analyzed by multi-dimensional mass spectrometry (MSn) to more accurately determine the structures(s) within these samples; HMG-20, -21, and -28 were previously sequenced by MSn [21, 28] and HMGs-9, -19, and -36 were also by sequenced by MSn in a more recent manuscript [33]. Refer to Supplementary File 2 for the lectin and antibody screening data.
Siglec-5 bound weakly to four sialylated HMGs: HMO-157, HMO-213, HMO-118, and HMO-237 (Fig. 1a). However, this binding required a high Siglec-5 concentration of 90μg/ml and the signal was detectable but weak. While the structures of these four HMGs have not been completely defined, HMO-157, HMO-213, and HMO-237 were bound by GM35 monoclonal antibody [21], which we have shown to bind to the sialyl Lewis a (SLea) determinant (Neu5Acα2-3Galβ1-3(Fucα1-4)GlcNAcbeta;-) and so-called “sialyl Lewis c” (SLec) (Neu5Acα2-3Galbeta;1-3GlcNAcbeta;) determinant [36]. These data suggest that Siglec-5 binds to a restricted set of HMGs structures containing the SLec determinant, but the reason that Siglec-5 bound to only a restricted subset of all structures containing this determinant is unclear, since several other glycans on the array also were bound by GM35 but not Siglec-5. HMO-118 is likely to be a mixture that contains 3′-sialyllactose (3′-SL) based on its predicted composition; however, 3′-SL itself was not bound on the defined HMGs microarray by Siglec-5 (described in more detail below), suggesting trace glycans within HMO-118 may have contributed to binding. Siglec-9 bound an extensive number of HMGs, all of which are sialylated, although three of the four HMGs bound by Siglec-5 (HMO-157, HMO-213, and HMO-118) were consistently the strongest Siglec-9 binders as well (Fig. 1b). However, the binding of Siglec-9 was only weakly dose-dependent (Supplementary File 1) and oddly depended on reducing end derivatization of the glycans, as discussed below. Thus, the results indicate that DC-SIGN robustly recognizes a number of α-fucosylated HMGs, whereas Siglec binding is weak and may not be significant. In regard to Siglec binding the significance was further tested below.
Binding of hGBPs to the CFG Glycan Microarray
To confirm that all hGBPs were active, they were concurrently screened on the microarray from the Consortium for Functional Glycomics (CFG). Most hGBP tested on the CFG microarray showed binding to at least two glycans on that microarray (Supplementary File 3). However, the CFG microarray data for Siglec-1 was deemed relatively inconclusive as no specific candidate glycans were identified. Many of the hGBPs that did not bind to the HM-SGM-v2 bound glycan determinants on the CFG microarray that were not found on the HM-SGM-v2, verifying hGBP activity. For example, Dectin-2 is specific for mannan structures containing the motif Manα1-2Manα1-6(Manα1-3)Manα- or Manα1-2Manα1-6Manα1-6(Manα1-3)Manα-, although Dectin-2 also weakly bound Man8-9GlcNAc2 N-glycan structures. A common feature of all structures bound by macrophage galactose/N-acetylgalactosamine-type lectin (MGL, CLEC10A) was the presence of GalNAc, particularly at the reducing and/or non-reducing end, although not all these GalNAc-containing structures were bound. Early studies suggested that Blood Group A and B antigens and enzymes with Blood Group A and B activity may be present in human milk (reviewed in [37]), but this has not been confirmed in more recent studies [38]. Indeed, we have screened the HM-SGM-v2 microarray with an antibody recognizing Blood Group A Types 1–3 determinants and saw no binding of this antibody (data not shown), confirming more recent studies that Blood Group A (and most likely Blood Group B) determinants are not present at detectable amounts on HMGs. Thus, since mannose and GalNAc are not found on free HMGs, it is logical that Dectin-2 and MGL-1 did not bind the HMGs microarrays.
For the Siglecs, most showed a broad binding pattern on the CFG glycan microarray but no binding to the few HMGs present on the CFG microarray. Siglec-5 bound some but not all complex N-glycans containing terminal β1-3-linked galactose; beyond that, Siglec-5 did not bind to a common motif. In contrast to the HM-SGM-v2, we did not observe Siglec-5 binding to sialylated HMGs on the CFG microarray, including a lack of binding to 3′-SL and all the non-HMG glycans containing the sialyl Lec determinant. Siglec-5 also did not bind a microarray consisting of defined HMGs structures (as described below), suggesting that Siglec-5 may not bind well HMGs and thus was possibly binding trace contaminants on the HM-SGM-v2 or only binds to only specific glycan presentations such as glycans with specific linkers. Siglec-7 bound to a variety of sialylated structures, the strongest of which was sialyl Lewis x containing 6-O-sulfated GlcNAc; some N-glycan structures and α2-8-sialylated structures were also bound. However, no motifs found on HMGs were bound by Siglec-7. Siglec-10 showed a very broad binding pattern, including binding to both sialylated and non-siaylated glycans. The biological and biochemical significance of the Siglec-10 binding to non-sialylated glycans is currently unclear, but we believe that the binding may have been artificially induced by the presentation and/or aglycone component (refer to the Discussion section for more information). Langerin not only strongly bound mannan and high mannose N-glycan structures (Man6-9GlcNAc2) but also lactose that was 6-O-sulfated on the galactose, but neither determinant is found on HMGs. Additionally, Langerin bound weakly to glycans containing terminal β-linked GlcNAc, Blood Group H Types 1 and 2, Blood Group A and B Type 2, Lewis y, and other sulfated glycan determinants. These results are in good agreement with previous glycan microarray results for Langerin [39]. Although Type 1 Blood Group H and Lewis y are found on some HMGs, Langerin binding to the HMGs microarrays was inconclusive (Supplementary File 1).
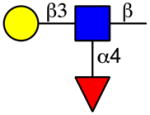
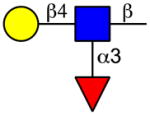
The screening of DC-SIGN on the CFG microarray revealed three major motifs (Table 2 and Supplementary File 3). The first motif was terminal α1-2-linked mannose on mannan backbones, including high-mannose N-glycans, although the mannans containing α-linked mannose at the reducing end were bound slightly stronger than the high mannose N-glycans. The second motif was the Lewis a determinant, including Lewis b structures. The third motif was the Lewis x determinant at the non-reducing end of glycan structures, which also included Lewis y structures. Notably, the Lewis a and non-reducing end Lewis x determinants were also the major HMG binding determinants revealed by the HM-SGM-v2 screening (Table 2). Sialyl Lewis a and especially sialyl Lewis x structures were typically poorly bound by DC-SIGN, although some sialylated, fucosylated HMGs were bound on the HM-SGM-v2 whose structures remain to be determined (Fig. 1 and Supplementary File 1). Glycans containing Blood Group H Type 1 and Type 2 determinants, as well as 2′-FL, were poorly bound by DC-SIGN on the CFG microarray, a finding also seen in previous studies [35]. This further suggests that α1-2 fucosylated glycan structures are lower affinity than Lewis a- and Lewis x-containing structures. Binding motifs for Siglec-9 on the CFG microarray were sialyl Lewis x on N-glycans as well as 3′- or 6′-sialyllactosamine (Neu5Acα2-3/6Galβ1-4GlcNAcβ-) that was 6-O-sulfated on the GlcNAc, but binding was slightly stronger to 6-O-sulfo-sialyl Lewis x as previously seen on this array (refer to the CFG website, http://www.functionalglycomics.org/glycomics/publicdata/primaryscreen.jsp); importantly, these motifs are not found on HMGs. Siglec-9 also weakly bound 3′-sialyllactosamine and 6′-siayllactosamine as well as the sialyl Lewis x tetrasaccharide (Neu5Acα2-3Galβ1-4(Fucα1-3)GlcNAc), although the HMGs 3′-SL and 6′-sialyllactose (6′-SL) were poorly if at all bound. Siglec-9 binding to the CFG microarray was also poorly dose-dependent, as seen when Siglec-9 was screened on the defined HMGs microarray, suggesting that the binding may not be specific. Therefore, only DC-SIGN was concluded to be a strong HMG receptor, while Siglec-5, Siglec-9, and Langerin are likely poor HMGs receptors.
Table 2.
DC-SIGN CFG Glycan Microarray Binding Motifs.
| Glycan Motif | Glycan Determinant Present |
|---|---|
|
|
α1–2 Mannose |
|
Lewis a |
|
Lewis x |
Binding of hGBPs to a Defined HMG Microarray
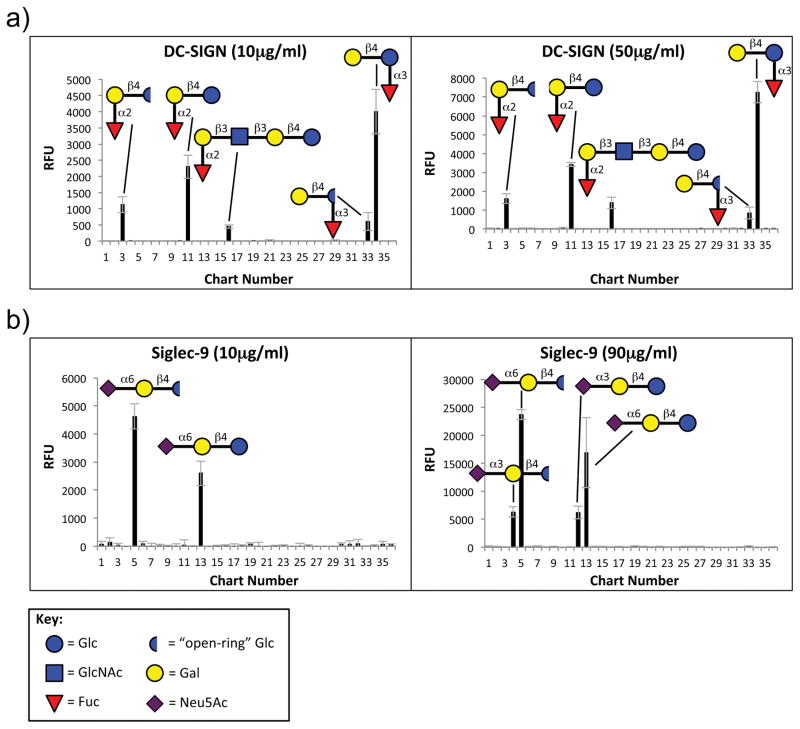
To further investigate the binding of hGBP to HMGs, the hGBP were also screened on a microarray consisting of a selection of chemically defined HMGs-related glycans and galactooligosaccharides (GOS) that are commonly used or under experimental testing as supplements in infant formula. This microarray was termed the “defined HMG microarray”. As expected from the HM-SGM-v2 screenings, DC-SIGN and Siglec-9 bound structures on the defined HMG microarray (Fig. 3) while all other hGBP showed no binding (refer to Supplementary File 4 for the defined HMG glycan microarray data at all concentrations of all hGBP screened). In contrast to the HM-SGM-v2 data, Siglec-5 at 90μg/ml did not bind to the defined HMG microarray even though HMO-118 (likely 3′-SL) was bound by Siglec-5 on the HM-SGM-v2. This suggests that Siglec-5 binds 3′-SL with low affinity and HMO-118 may contain trace contaminants that improved Siglec-5 binding. Siglec-9 bound to both 3′-SL and 6′-SL.
Fig. 3. Defined HMGs Microarray Screening Data for DC-SIGN and Siglec-9.
10μg/ml and 50μg/ml DC-SIGN (a) and 10μg/ml and 90μg/ml Siglec-9 (b) were screened on the defined HMGs microarray, and Alexa Fluor 488-labeled anti-human IgG was used for detection. Graphs on the left show the lower concentrations and the right graphs show the higher concentrations. Refer to Supplementary File 4 for the raw data for these screenings.
DC-SIGN bound the fucosylated HMGs 2′-fucosyllactose (2′-FL) and 3-fucosyllactose (3-FL), while very weak binding was seen towards the Blood Group H Type 1-containing glycan LNFPI. It should be noted that 2′-FL (HMO-3) but not 3-FL (HMO-2) was bound on the HM-SGM-v2, although 2′-FL was weakly bound relative to Lewis a and Lewis x structures (average rank = 11; Supplementary Figure 1). However, DC-SIGN binding to 3-FL was highly dependent on maintaining the ring-structure of the reducing end glucose because DC-SIGN poorly bound to reductively aminated 3-FL (Fig. 3 and Supplementary File 4), which was the only ring-form of glycans on the HM-SGM-v2. Thus, the actual strength of binding to 3-FL was likely underestimated on the HM-SGM-v2. In contrast to the HMG microarray results, 2′-FL was not bound by DC-SIGN on the CFG glycan microarray (Chart ID 77, rank < 10; Supplementary Figure 3). The reason for this non-binding on the CFG glycan microarray, but may have to do with differences in the linker or other presentation issues vs. the HMG microarrays. 3-FL was absent from the CFG glycan microarray. This suggests that 2′-FL and 3-FL are weaker ligands than structures containing terminal Lewis a or Lewis x determinants, although more studies are needed to confirm this observation. Overall, these results suggest that 2′-FL and 3-FL are also ligands for DC-SIGN. Despite their potentially lower binding strength than Lewis a and Lewis x-containing HMGs, 2′-FL and 3-FL are much more abundant than these Lewis a- and Lewis x-containing HMG structures in human milk, with concentrations ranging from about 0.5–5mM for these two HMGs [2]. The overall results of this experiment show that Siglec-9 and DC-SIGN, but not Siglec-5, may also bind to simple, defined HMGs structures.
Binding of hGBPs to the Beads Derivatized with Human Milk Glycans
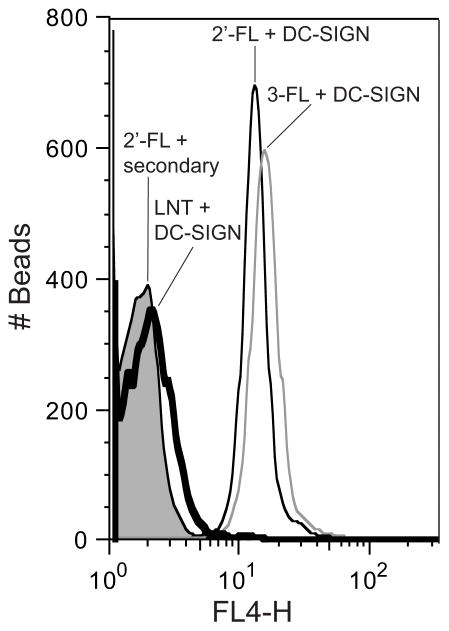
To confirm the binding of DC-SIGN to the defined HMGs 2′-FL and 3-FL in a different format, polystyrene beads were derivatized with 2′-FL, 3-FL, or LNT and binding of DC-SIGN to these derivatized beads was measured by flow cytometry (Fig. 4). As expected, DC-SIGN bound to the 2′-FL- and 3-FL-derivatized beads but not the LNT-derivatized beads (non-fucosylated HMGs control), which further confirmed the binding of DC-SIGN to fucosylated HMGs.
Fig. 4. DC-SIGN Binding to HMGs-Derivatized Microspheres.
DC-SIGN was incubated with microspheres (beads) derivatized with 2′-FL, 3-FL, or LNT. Alexa Fluor 633-labeled anti-human IgG was used for detection of recombinant DC-SIGN. 2′-FL microspheres incubated with the Alexa Fluor 633-labeled anti-human IgG alone was used as the negative control. All samples were analyzed by Flow Cytometry with a 633nm laser and FL-4 filter for detection. Histograms of DC-SIGN binding to LNT beads (thick line), 2′-FL beads (thin black line), and 3-FL beads (thin grey line), as well as secondary antibody alone binding to 2′-FL beads (filled line), are shown.
HMG Inhibition of hGBPs Binding
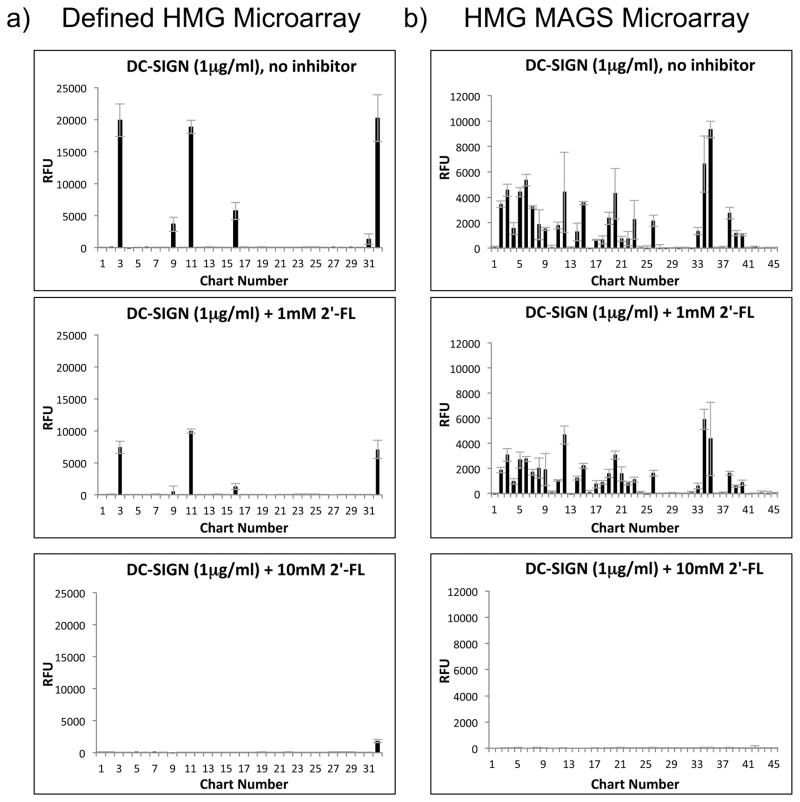
Experiments using glycan microarrays and beads are useful for defining glycan specificity and potential binding of hGBP to HMGs. However, the glycan microarray screenings themselves have a few important limitations. Specifically, the glycans on the microarray are synthetically derivatized with a bifunctional linker at the reducing end and presented in a solid-phase format, which is in contrast to HMGs that occur as free, reducing glycans in human milk. To confirm that DC-SIGN and Siglec-9 can also bind to free, underivatized HMGs in solution, DC-SIGN and Siglec-9 were screened on the defined HMGs microarray in the presence or absence of various concentrations of 2′-FL and 6′-SL, respectively; lactose was used as a negative control for non-specific HMGs inhibition. DC-SIGN binding to both the defined HMGs microarray (Fig. 5a) and the MAGS array (Fig. 5b) was inhibited by 2′-FL (refer to Supplementary File 5 for the total data for DC-SIGN inhibition) in a dose-dependent manner and with an approximate IC50 of 1mM for 2′-FL, confirming that DC-SIGN specifically binds to natural 2′-FL and in solution. Lactose (1–10mM) caused little or no inhibition of DC-SIGN binding to the defined HMGs microarray (Supplementary File 5), confirming that the presence of α-linked fucose is required for DC-SIGN binding. The data also confirm binding to all of the HMGs on the HM-SGM-v2 and defined HMGs microarrays was specific.
Fig. 5. Inhibition of DC-SIGN Binding to HMGs Microarrays with Free, Underivatized HMGs.
1μg/ml DC-SIGN was preincubated with or without 0.1, 1, or 10mM of free, underivatized 2′-FL and then screened on the defined HMGs microarray (a) or the HMGs MAGS microarray (b) described in Supplementary File 2. The results for 0mM 2′FL (no inhibitor), 1mM 2′-FL, and 10mM 2′-FL on both microarrays are shown; refer to Supplementary File 5 for the results of all other screenings, including 0.1mM 2′-FL.
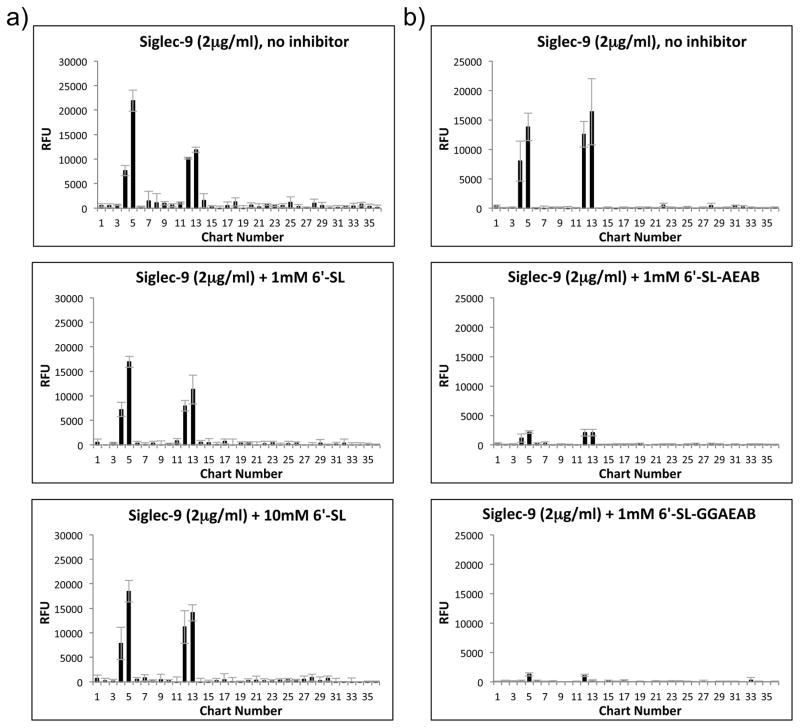
On the other hand, Siglec-9 binding to the defined HMGs microarray was not inhibited by even 10mM 6′-SL (Fig. 6a), although binding could be inhibited by 1mM 6′-SL derivatized with the AEAB linker at the reducing end (Fig. 6b; also see Supplementary File 6 for the total data for Siglec-9 inhibition). Therefore, Siglec-9 did not appear to bind the natural form of 6′-SL (and likely 3′-SL), only the chemically derivatized version; this suggests that Siglec-9 binding to the defined HMGs microarray only occurs because of this HMGs derivatization. The solution Kd of Siglec-9 for free 6′-SL and 3′-SL was determined to be >10mM, which is likely not physiologically relevant.
Fig. 6. Inhibition of Siglec-9 Binding to Defined HMGs Microarray with Free, Underivatized HMGs and Free, Derivatized 6′-Sialyllactose.
2μg/ml Siglec-9 was preincubated with 1mM or 10mM free, underivatized 6′-SL or no inhibitor and screened on the defined HMGs microarray (a). 2μg/ml Siglec-9 was preincubated with free 1mM 6′-SL-AEAB (Neu5Acα2-6Galβ1-4Glcitol-AEAB) or 6′-SL-GGAEAB (Neu5Acα2-6Galβ1-4Glc-GGAEAB) [25] or no inhibitor and screened on the defined HMGs microarray (b). Parts a and b of this figure were performed on separate slides but on the same day and at the same time. Refer to Supplementary File 6 for all other free inhibition of Siglec-9 binding to the defined HMGs microarray.
Discussion
A major finding of this study is that DC-SIGN is the only hGBP tested that showed specific binding to HMGs and binding was most robust toward α-fucosylated glycans. A striking observation was the proportion of HMGs bound by DC-SIGN. About half of the HMG structures on the HM-SGM-v2 were bound by 10μg/ml DC-SIGN (Fig. 1, Supplementary File 1), suggesting that DC-SIGN binds to nearly half of the structures in the HMG metaglycome. The strongest binding was towards HMGs containing a Lewis glycan determinant at the non-reducing end (Figs. 1, 2, 3, Supplementary Files 1, 2, and 3). Potentially weaker but likely physiologically significant binding of DC-SIGN to 2′-fucosyllactose and 3-fucosyllactose was also observed (Figs. 1, 3, 5, Supplementary Files 1, 4, 5). HMGs containing only internal Lewis x or Blood Group H Type 1 were poorly, if at all, bound by DC-SIGN. Therefore, DC-SIGN appears to be a receptor for specific fucosylated HMGs.
The approximate IC50 of DC-SIGN for 2′-FL inhibition of binding to the glycan microarray was 1mM (Fig. 5). Given the typical concentration of 1–5mM (0.5–2.5g/L) 2′-FL in Secretor-positive human milk [2], this suggests that the binding is within the physiological range. Taking into account that DC-SIGN also binds half of the total HMGs metaglycome in secretor- and Lewis-positive individuals (Fig. 1) as well as glycoproteins in human milk such as bile salt-stimulated lipase [40] and MUC1 [17], the actual concentration of DC-SIGN glycan determinants in human milk is probably much higher (~5–10mM), suggesting that DC-SIGN may be close to ligand saturation when exposed to human milk (assuming an average Kd of 1mM). Total HMGs have been previously shown to block DC-SIGN binding to HIV virions [41], further suggesting that some HMGs are DC-SIGN ligands and can block DC-SIGN functions.
Human intestinal dendritic cells express DC-SIGN [42], and DC-SIGN expression is known to occur on cells (likely dendritic cells) in infant GI tract tissue [17]. DC can extend their dendrites from the lamina propria into the intestinal lumen to “sample” microbes [18]. Since HMGs are not significantly digested by the human repertoire of digestive mechanisms and enzymes in the GI tract [43, 44], DC-SIGN on dendritic cells may be exposed to and bind HMGs to near saturable levels in the small intestine of breast-fed infants. DC-SIGN is also known to modulate immune responses, though this binding is not yet known to be a direct stimulator of gene expression [10]. However, it is possible that the interaction of DC-SIGN with HMGs may cause changes in the DC-SIGN-mediated modulation of immune responses and may also help mechanistically explain how HMGs promote changes in gene expression and immune responses [6]. Notwithstanding, how such interactions occur and if the HMGs act as agonists or antagonists of DC-SIGN activity is still not fully understood. Interestingly, about 20% of individuals lack the Secretor enzyme responsible for producing α1-2-fucosylated HMGs and 2′-FL, about 10% of individuals lack the Lewis enzyme responsible for producing α1-4-fucosylated HMGs (Lewis a structures), and ~1% of individuals lack both enzymes [45]. Thus, milk from secretor-negative and/or Lewis-negative individuals may not be capable or interacting as well with DC-SIGN as milk from Secretor- and Lewis-positive mothers, although this might be at least partially compensated by the increased 3-FL concentration in non-secretor vs. secretor human milk as 3-FL is also a DC-SIGN ligand (Fig. 3, 5, Supplementary File 3, 5). The physiological consequence of lacking the Secretor and/or Lewis enzyme on DC-SIGN binding in vivo are thus unclear.
Unexpectedly, given the fact that sialic acid is a common residue in HMGs, the only Siglecs tested that showed some binding to HMGs were Siglecs-5 and -9 (Fig. 1), consistent with the possibility that glycan recognition by Siglecs is complex and the presence of sialic acid is necessary but not sufficient in most cases. Siglec-5 binding was weak and only occurred at high Siglec-5 concentrations, while Siglec-9 binding was stronger but binding to the free, underivatized HMGs 6′-SL was still weak (Fig. 6). Instead, Siglec-9 bound strongly to 6′-SL derivatized at the reducing end with an aglycone linker, AEAB and especially GGAEAB. This finding suggests that the aglycone component and/or multivalent presentation may be an important factor in Siglec-9 and other Siglecs for binding glycoconjugate ligands, or that specific sialylated glycans yet to be identified are strong ligands for Siglecs. This finding of the potential importance the aglycone in Siglec binding may also explain why the binding of Siglecs to the CFG microarray in this study has a generally weak, broad binding pattern. This result may be due to differences in glycan presentation, which may have positively or negatively affected by the presence of specific aglycone linker units. Thus, the weak, broad binding pattern of Siglecs to the CFG microarrays was likely because of non-preferential glycan presentation and/or aglycone components as opposed to poor Siglec activity or the recombinant Siglec construct used. Future studies in our lab are aimed at understanding the functional importance of aglycone components, especially natural aglycone components such as lipids and peptides, in Siglec binding. This future study may also unravel why some Siglecs, especially Siglec-10, bind to a few non-sialylated glycans.
The glycan presentation in multivalent forms may be most important for Siglec binding, as prior studies showed that Siglec-1, -3, -5, -7, and -9 all bound 3′-SL and 6′SL-derviatized beads with μM affinity constants by surface plasmon resonance [15]. The multivalent presentation and/or aglycone bead component may contribute to this strong binding, since an IC50 of ~1mM was calculated for free 6′-SL inhibition [15], which was ~100–1000-fold higher than for the Kd for 6′-SL beads. Siglec-5 binds 3′-SL and 6′-SL with a Kd of 2–4μM but to free 3′-SL and 6′-SL with a Kd of ~8mM [46], which is high relative to the concentrations of these two sialylated glycans in human milk. Based on these current and previous findings, we conclude that the binding of dendritic cell-expressed Siglecs to free, underivatized HMGs is weak and likely non-physiological.. We speculate that this low affinity binding is due to the lack of an aglycone component on and/or multivalent presentation of HMGs, which normally exist as free, reducing glycan structures in solution. These findings also stress the importance of using other methodologies besides glycan microarrays to confirm binding of samples to HMGs, which naturally exist as free, underivatized structures in solution.
In addition to C-type lectins and Siglecs used in this study, other hGBP have been screened on the HMGs microarrays. These include galectins, most of which showed binding and, in some cases, robust binding to neutral HMGs [22]. Preliminary HMGs microarray screenings of the three human selectins (P-, E-, and L-selectin), which are known to bind sialyl Lewis x and sialyl Lewis a determinants in solution with relatively low affinity [47, 48], were negative. This suggests that human selectins are poor HMGs receptors, consistent with previous studies showing that, although selectins may bind HMGs, the interaction and effects are weak [49–51]. Preliminary screenings with Siglec-11 also revealed no binding of these hGBP to the HMGs microarrays. Future studies are aimed at examining other receptors that bind glycoconjugates, including Toll-like receptors and cytokine receptors.
This study adds DC-SIGN to the list of hGBP that may act as HMG receptors. Given the physiological concentration of HMGs binding to DC-SIGN and galectins, as well as the anatomical localization and expression patterns of these hGBP, these interactions may be important mechanisms underlying the known HMGs functions of regulating gene expression and immune responses [6]. Therefore, future studies to understand the interactions of these hGBP with HMGs and subsequent physiological effects are currently underway.
Supplementary Material
This file, containing seven worksheets, shows the average RFU data as well as graphs of binding data for 100μg/ml Langerin, 100μg/ml MGL-1, 90μg/ml Siglec-1, 90μg/ml Siglec-5, 90μg/ml Siglec-7, and 10μg/ml and 50μg/ml Siglec-9 binding to the HM-SGM-v2 on separate worksheets. Additionally, the data for 0.1μg/ml, 1.0μg/ml, and 10μg/ml DC-SIGN, as well as DC-SIGN screened in a buffer lacking Ca2+ as well as 0.2mM EDTA as a control for non-specific interactions, is included on a single worksheet. The data for Dectin-2, which showed no binding to the HM-SGM-v2, is not shown.
This file includes two worksheets. The first worksheet contains a summary of the average RFU results for the screening of anti-glycan antibodies and lectins on the MAGS array to identify glycan determinants present on the HMGs samples printed. The RFU’s in red and black text indicate positive and negative binding, respectively, by the lectin or antibody. The second worksheet includes the average RFU data as well as graphs of binding data for 0.5μg/ml, 1.0μg/ml, 2.0μg/ml, and 5.0μg/ml DC-SIGN to the MAGS array. Additionally, the results for 1.0μg/ml DC-SIGN binding after the array had been treated with neuraminidase are also shown.
This eight-worksheet file contains the average RFU data and graphs of binding data for 10μg/ml DC-SIGN, 10μg/ml Langerin, 50μg/ml Dectin-2, 10μg/ml MGL-1, 20μg/ml Siglec-1, 20μg/ml Siglec-5, 90μg/ml Siglec-7, and 10μg/ml and 90μg/ml Siglec-9 on the glycan microarray from the Consortium for Functional Glycomics (CFG).
This file contains two worksheets. The worksheets contain the average RFU binding data and graphs of binding data for 10μg/ml and 50μg/ml DC-SIGN, and 10μg/ml and 90μg/ml Siglec-9, respectively, on the defined HMGs microarray. All of the other hGBP screened showed no binding to the microarray (data not shown).
This file contains two worksheets. The first worksheet includes the average RFU data and graphs of binding data of 1.0μg/ml DC-SIGN screened on the defined HMGs microarray in the presence or absence of 0.1mM, 1.0mM, or 10mM 2′-FL or lactose. The second worksheet includes the average RFU data and graphs of binding data of 1.0μg/ml DC-SIGN screened on the MAGS array in the presence or absence of 1.0mM or 10mM 2′-FL.
This file, containing two worksheets, shows the results of 2.0μg/ml Siglec-9 binding to the defined HMGs microarray in the presence of free HMGs or HMGs derivatives including 1mM and 10mM 6′-SL, 1mM and 10mM lactose, 1mM 6′-SL-AEAB, and 1mM 6′-SL-GGAEAB. The two worksheets show the data for two different slides, although these two slides were concurrently screened in the same experiment.
Acknowledgments
We thank Jamie Heimburg-Molinaro for critical review of the manuscript and Sandra Cummings and Hong Ju for technical assistance.
Funding Information
This work was support by NIH Grants P41GM103694 to RDC and a Grant from Abbott Nutrition, Columbus, OH.
Abbreviations list
- 2′-FL
2′-fucosyllactose
- 3-FL
3-fucosyllactose
- 3′-SL
3′-sialyllactose
- 6′-SL
6′-sialyllactose
- AEAB
N-aminoethyl 2-aminobenzamide
- CFG
Consortium for Functional Glycomics
- DC
dendritic cell
- DC-SIGN
Dendritic Cell-Specific Intercellular Adhesion Molecule-3-Grabbing Non-integrin
- Fuc
L-fucose
- Gal
D-galactose
- Glc
D-glucose
- GBP
glycan-binding protein
- GalNAc
D-N-acetylgalactosamine
- GGAEAB
glycosylamide-glycyl-N-aminoethyl 2-aminobenzamide
- GI
gastrointestinal
- GlcNAc
D-N-acetylglucosamine
- GOS
galactooligosaccharides
- H1
Blood Group H Type 1
- hGBP
human glycan-binding protein
- HMGs
human milk glycans
- HM-SGM-v2
human milk shotgun glycan microarray version 2
- LNFPI
lacto-N-fucopentaose I
- LNnT
lacto-N-neotetraose
- LNT
lacto-N-tetraose
- MAGS
Metadata-Assisted Glycan Sequencing
- MALDI TOF MS
matrix-assisted laser desorption/ionization-time of flight mass spectrometry
- MGL
macrophage galactose/N-acetylgalactosamine-type lectin
- MSn
multi-dimensional mass spectrometry
- Neu5Ac
5-N-acetylneuraminic acid
- NHS
N-hydroxysuccinimide
- Siglec
sialic acid–binding, immunoglobulin-like lectin
- SLea
sialyl Lewis a
Footnotes
Declarations of Interest
R.D.C. and D.F.S. are consultants for Abbott Nutrition. G.D.M. and R.H.B. are employees of Abbott. The other authors declare that they have no conflict of interest in the work reported.
Six Supplementary Files (.xlsx files) are included in this manuscript.
Author Contribution Statement
AJN, YY, GDM, RHB, DFS, and RDC proposed and designed experiments. YY performed experiments. AJN, YY, DFS, and RDC analyzed data. YL, GDM, and RHB provided critical reagents and support. AJN and RDC organized data and wrote manuscript with review, comments, and contributions from all authors.
References
- 1.Kunz C, Rudloff S, Baier W, Klein N, Strobel S. Oligosaccharides in human milk: structural, functional, and metabolic aspects. Annu Rev Nutr. 2000;20:699–722. doi: 10.1146/annurev.nutr.20.1.699. [DOI] [PubMed] [Google Scholar]
- 2.Urashima T, Asakuma S, Leo F, Fukuda K, Messer M, Oftedal OT. The predominance of type I oligosaccharides is a feature specific to human breast milk. Adv Nutr. 2012;3:473S–482S. doi: 10.3945/an.111.001412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Newburg DS. Glycobiology of human milk. Biochemistry (Mosc) 2013;78:771–785. doi: 10.1134/S0006297913070092. [DOI] [PubMed] [Google Scholar]
- 4.Newburg DS, Ruiz-Palacios GM, Morrow AL. Human milk glycans protect infants against enteric pathogens. Annu Rev Nutr. 2005;25:37–58. doi: 10.1146/annurev.nutr.25.050304.092553. [DOI] [PubMed] [Google Scholar]
- 5.Zivkovic AM, German JB, Lebrilla CB, Mills DA. Human milk glycobiome and its impact on the infant gastrointestinal microbiota. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4653–4658. doi: 10.1073/pnas.1000083107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bode L. Human milk oligosaccharides: every baby needs a sugar mama. Glycobiology. 2012;22:1147–1162. doi: 10.1093/glycob/cws074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castillo-Courtade L, Han S, Lee S, Mian FM, Buck R, Forsythe P. Attenuation of food allergy symptoms following treatment with human milk oligosaccharides in a mouse model. Allergy. 2015;70:1091–1102. doi: 10.1111/all.12650. [DOI] [PubMed] [Google Scholar]
- 8.Bienenstock J, Buck RH, Linke H, Forsythe P, Stanisz AM, Kunze WA. Fucosylated but not sialylated milk oligosaccharides diminish colon motor contractions. PLoS One. 2013;8:e76236. doi: 10.1371/journal.pone.0076236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vazquez E, Barranco A, Ramirez M, Gruart A, Delgado-Garcia JM, Martinez-Lara E, Blanco S, Martin MJ, Castanys E, Buck R, Prieto P, Rueda R. Effects of a human milk oligosaccharide, 2′-fucosyllactose, on hippocampal long-term potentiation and learning capabilities in rodents. J Nutr Biochem. 2015;26:455–465. doi: 10.1016/j.jnutbio.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 10.Geijtenbeek TB, Gringhuis SI. Signalling through C-type lectin receptors: shaping immune responses. Nat Rev Immunol. 2009;9:465–479. doi: 10.1038/nri2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crocker PR, Paulson JC, Varki A. Siglecs and their roles in the immune system. Nat Rev Immunol. 2007;7:255–266. doi: 10.1038/nri2056. [DOI] [PubMed] [Google Scholar]
- 12.McEver RP. Adhesive interactions of leukocytes, platelets, and the vessel wall during hemostasis and inflammation. Thromb Haemost. 2001;86:746–756. [PubMed] [Google Scholar]
- 13.Song X, Xia B, Stowell SR, Lasanajak Y, Smith DF, Cummings RD. Novel fluorescent glycan microarray strategy reveals ligands for galectins. Chem Biol. 2009;16:36–47. doi: 10.1016/j.chembiol.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo Y, Feinberg H, Conroy E, Mitchell DA, Alvarez R, Blixt O, Taylor ME, Weis WI, Drickamer K. Structural basis for distinct ligand-binding and targeting properties of the receptors DC-SIGN and DC-SIGNR. Nat Struct Mol Biol. 2004;11:591–598. doi: 10.1038/nsmb784. [DOI] [PubMed] [Google Scholar]
- 15.Zou Z, Chastain A, Moir S, Ford J, Trandem K, Martinelli E, Cicala C, Crocker P, Arthos J, Sun PD. Siglecs facilitate HIV-1 infection of macrophages through adhesion with viral sialic acids. PLoS One. 2011;6:e24559. doi: 10.1371/journal.pone.0024559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brinkman-Van der Linden EC, Varki A. New aspects of siglec binding specificities, including the significance of fucosylation and of the sialyl-Tn epitope. Sialic acid-binding immunoglobulin superfamily lectins. J Biol Chem. 2000;275:8625–8632. doi: 10.1074/jbc.275.12.8625. [DOI] [PubMed] [Google Scholar]
- 17.Koning N, Kessen SF, Van Der Voorn JP, Appelmelk BJ, Jeurink PV, Knippels LM, Garssen J, Van Kooyk Y. Human Milk Blocks DC-SIGN-Pathogen Interaction via MUC1. Front Immunol. 2015;6:112. doi: 10.3389/fimmu.2015.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, Bonasio R, Granucci F, Kraehenbuhl JP, Ricciardi-Castagnoli P. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol. 2001;2:361–367. doi: 10.1038/86373. [DOI] [PubMed] [Google Scholar]
- 19.Cummings RD, McEver RP. C-type Lectins. In: Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME, editors. Essentials of Glycobiology. Cold Spring Harbor (NY): 2009. [PubMed] [Google Scholar]
- 20.Varki A, Crocker PR. I-type Lectins. In: Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME, editors. Essentials of Glycobiology. Cold Spring Harbor (NY): 2009. [PubMed] [Google Scholar]
- 21.Yu Y, Lasanajak Y, Song X, Hu L, Ramani S, Mickum ML, Ashline DJ, Prasad BV, Estes MK, Reinhold VN, Cummings RD, Smith DF. Human milk contains novel glycans that are potential decoy receptors for neonatal rotaviruses. Mol Cell Proteomics. 2014;13:2944–2960. doi: 10.1074/mcp.M114.039875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noll AJ, Gourdine JP, Yu Y, Lasanajak Y, Smith DF, Cummings RD. Galectins are human milk glycan receptors. Glycobiology. 2016 doi: 10.1093/glycob/cww002. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heimburg-Molinaro J, Song X, Smith DF, Cummings RD. Preparation and analysis of glycan microarrays. Curr Protoc Protein Sci. 2011;64:12.10.11–12.10.29. doi: 10.1002/0471140864.ps1210s64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Song X, Heimburg-Molinaro J, Smith DF, Cummings RD. Derivatization of free natural glycans for incorporation onto glycan arrays: derivatizing glycans on the microscale for microarray and other applications (ms# CP-10–0194) Curr Protoc Chem Biol. 2011;3:53–63. doi: 10.1002/9780470559277.ch100194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song X, Lasanajak Y, Xia B, Smith DF, Cummings RD. Fluorescent glycosylamides produced by microscale derivatization of free glycans for natural glycan microarrays. ACS Chem Biol. 2009;4:741–750. doi: 10.1021/cb900067h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith DF, Song X, Cummings RD. Use of glycan microarrays to explore specificity of glycan-binding proteins. Methods Enzymol. 2010;480:417–444. doi: 10.1016/S0076-6879(10)80033-3. [DOI] [PubMed] [Google Scholar]
- 27.Agravat SB, Saltz JH, Cummings RD, Smith DF. GlycoPattern: a web platform for glycan array mining. Bioinformatics. 2014;30:3417–3418. doi: 10.1093/bioinformatics/btu559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ashline DJ, Yu Y, Lasanajak Y, Song X, Hu L, Ramani S, Prasad BV, Estes MK, Cummings RD, Smith DF, Reinhold VN. Structural characterization by MSn of human milk glycans recognized by human rotaviruses. Mol Cell Proteomics. 2014;13:2961–2974. doi: 10.1074/mcp.M114.039925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lock K, Zhang J, Lu J, Lee SH, Crocker PR. Expression of CD33-related siglecs on human mononuclear phagocytes, monocyte-derived dendritic cells and plasmacytoid dendritic cells. Immunobiology. 2004;209:199–207. doi: 10.1016/j.imbio.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 30.Izquierdo-Useros N, Lorizate M, Puertas MC, Rodriguez-Plata MT, Zangger N, Erikson E, Pino M, Erkizia I, Glass B, Clotet B, Keppler OT, Telenti A, Krausslich HG, Martinez-Picado J. Siglec-1 is a novel dendritic cell receptor that mediates HIV-1 trans-infection through recognition of viral membrane gangliosides. PLoS Biol. 2012;10:e1001448. doi: 10.1371/journal.pbio.1001448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li N, Zhang W, Wan T, Zhang J, Chen T, Yu Y, Wang J, Cao X. Cloning and characterization of Siglec-10, a novel sialic acid binding member of the Ig superfamily, from human dendritic cells. J Biol Chem. 2001;276:28106–28112. doi: 10.1074/jbc.M100467200. [DOI] [PubMed] [Google Scholar]
- 32.Smith DF, Cummings RD. Application of microarrays for deciphering the structure and function of the human glycome. Mol Cell Proteomics. 2013;12:902–912. doi: 10.1074/mcp.R112.027110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Agravat SB, Song X, Rojsajjakul T, Cummings RD, Smith DF. Computational Approaches to Define a Human Milk Metaglycome. Bioinformatics. 2016 doi: 10.1093/bioinformatics/btw048. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Appelmelk BJ, van Die I, van Vliet SJ, Vandenbroucke-Grauls CM, Geijtenbeek TB, van Kooyk Y. Cutting edge: carbohydrate profiling identifies new pathogens that interact with dendritic cell-specific ICAM-3-grabbing nonintegrin on dendritic cells. J Immunol. 2003;170:1635–1639. doi: 10.4049/jimmunol.170.4.1635. [DOI] [PubMed] [Google Scholar]
- 35.van Die I, van Vliet SJ, Nyame AK, Cummings RD, Bank CM, Appelmelk B, Geijtenbeek TB, van Kooyk Y. The dendritic cell-specific C-type lectin DC-SIGN is a receptor for Schistosoma mansoni egg antigens and recognizes the glycan antigen Lewis x. Glycobiology. 2003;13:471–478. doi: 10.1093/glycob/cwg052. [DOI] [PubMed] [Google Scholar]
- 36.Brazil JC, Liu R, Sumagin R, Kolegraff KN, Nusrat A, Cummings RD, Parkos CA, Louis NA. alpha3/4 Fucosyltransferase 3-dependent synthesis of Sialyl Lewis A on CD44 variant containing exon 6 mediates polymorphonuclear leukocyte detachment from intestinal epithelium during transepithelial migration. J Immunol. 2013;191:4804–4817. doi: 10.4049/jimmunol.1301307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kobata A. Structures and application of oligosaccharides in human milk. Proc Jpn Acad Ser B Phys Biol Sci. 2010;86:731–747. doi: 10.2183/pjab.86.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jiang X, Huang P, Zhong W, Tan M, Farkas T, Morrow AL, Newburg DS, Ruiz-Palacios GM, Pickering LK. Human milk contains elements that block binding of noroviruses to human histo-blood group antigens in saliva. J Infect Dis. 2004;190:1850–1859. doi: 10.1086/425159. [DOI] [PubMed] [Google Scholar]
- 39.Holla A, Skerra A. Comparative analysis reveals selective recognition of glycans by the dendritic cell receptors DC-SIGN and Langerin. Protein Eng Des Sel. 2011;24:659–669. doi: 10.1093/protein/gzr016. [DOI] [PubMed] [Google Scholar]
- 40.Naarding MA, Dirac AM, Ludwig IS, Speijer D, Lindquist S, Vestman EL, Stax MJ, Geijtenbeek TB, Pollakis G, Hernell O, Paxton WA. Bile salt-stimulated lipase from human milk binds DC-SIGN and inhibits human immunodeficiency virus type 1 transfer to CD4+ T cells. Antimicrob Agents Chemother. 2006;50:3367–3374. doi: 10.1128/AAC.00593-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hong P, Ninonuevo MR, Lee B, Lebrilla C, Bode L. Human milk oligosaccharides reduce HIV-1-gp120 binding to dendritic cell-specific ICAM3-grabbing non-integrin (DC-SIGN) Br J Nutr. 2009;101:482–486. doi: 10.1017/s0007114508025804. [DOI] [PubMed] [Google Scholar]
- 42.Jameson B, Baribaud F, Pohlmann S, Ghavimi D, Mortari F, Doms RW, Iwasaki A. Expression of DC-SIGN by dendritic cells of intestinal and genital mucosae in humans and rhesus macaques. J Virol. 2002;76:1866–1875. doi: 10.1128/JVI.76.4.1866-1875.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Engfer MB, Stahl B, Finke B, Sawatzki G, Daniel H. Human milk oligosaccharides are resistant to enzymatic hydrolysis in the upper gastrointestinal tract. Am J Clin Nutr. 2000;71:1589–1596. doi: 10.1093/ajcn/71.6.1589. [DOI] [PubMed] [Google Scholar]
- 44.Gnoth MJ, Kunz C, Kinne-Saffran E, Rudloff S. Human milk oligosaccharides are minimally digested in vitro. J Nutr. 2000;130:3014–3020. doi: 10.1093/jn/130.12.3014. [DOI] [PubMed] [Google Scholar]
- 45.Thurl S, Henker J, Siegel M, Tovar K, Sawatzki G. Detection of four human milk groups with respect to Lewis blood group dependent oligosaccharides. Glycoconj J. 1997;14:795–799. doi: 10.1023/a:1018529703106. [DOI] [PubMed] [Google Scholar]
- 46.Zhuravleva MA, Trandem K, Sun PD. Structural implications of Siglec-5-mediated sialoglycan recognition. J Mol Biol. 2008;375:437–447. doi: 10.1016/j.jmb.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Poppe L, Brown GS, Philo JS, Nikrad PV, Shah BH. Conformation of sLex Tetrasaccharide, Free in Solution and Bound to E-, P-, and L-Selectin. Journal of the American Chemical Society. 1997;119:1727–1736. [Google Scholar]
- 48.Nelson RM, Dolich S, Aruffo A, Cecconi O, Bevilacqua MP. Higher-affinity oligosaccharide ligands for E-selectin. J Clin Invest. 1993;91:1157–1166. doi: 10.1172/JCI116275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bode L, Kunz C, Muhly-Reinholz M, Mayer K, Seeger W, Rudloff S. Inhibition of monocyte, lymphocyte, and neutrophil adhesion to endothelial cells by human milk oligosaccharides. Thromb Haemost. 2004;92:1402–1410. doi: 10.1160/TH04-01-0055. [DOI] [PubMed] [Google Scholar]
- 50.Bode L, Rudloff S, Kunz C, Strobel S, Klein N. Human milk oligosaccharides reduce platelet-neutrophil complex formation leading to a decrease in neutrophil beta 2 integrin expression. J Leukoc Biol. 2004;76:820–826. doi: 10.1189/jlb.0304198. [DOI] [PubMed] [Google Scholar]
- 51.Schumacher G, Bendas G, Stahl B, Beermann C. Human milk oligosaccharides affect P-selectin binding capacities: in vitro investigation. Nutrition. 2006;22:620–627. doi: 10.1016/j.nut.2005.12.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This file, containing seven worksheets, shows the average RFU data as well as graphs of binding data for 100μg/ml Langerin, 100μg/ml MGL-1, 90μg/ml Siglec-1, 90μg/ml Siglec-5, 90μg/ml Siglec-7, and 10μg/ml and 50μg/ml Siglec-9 binding to the HM-SGM-v2 on separate worksheets. Additionally, the data for 0.1μg/ml, 1.0μg/ml, and 10μg/ml DC-SIGN, as well as DC-SIGN screened in a buffer lacking Ca2+ as well as 0.2mM EDTA as a control for non-specific interactions, is included on a single worksheet. The data for Dectin-2, which showed no binding to the HM-SGM-v2, is not shown.
This file includes two worksheets. The first worksheet contains a summary of the average RFU results for the screening of anti-glycan antibodies and lectins on the MAGS array to identify glycan determinants present on the HMGs samples printed. The RFU’s in red and black text indicate positive and negative binding, respectively, by the lectin or antibody. The second worksheet includes the average RFU data as well as graphs of binding data for 0.5μg/ml, 1.0μg/ml, 2.0μg/ml, and 5.0μg/ml DC-SIGN to the MAGS array. Additionally, the results for 1.0μg/ml DC-SIGN binding after the array had been treated with neuraminidase are also shown.
This eight-worksheet file contains the average RFU data and graphs of binding data for 10μg/ml DC-SIGN, 10μg/ml Langerin, 50μg/ml Dectin-2, 10μg/ml MGL-1, 20μg/ml Siglec-1, 20μg/ml Siglec-5, 90μg/ml Siglec-7, and 10μg/ml and 90μg/ml Siglec-9 on the glycan microarray from the Consortium for Functional Glycomics (CFG).
This file contains two worksheets. The worksheets contain the average RFU binding data and graphs of binding data for 10μg/ml and 50μg/ml DC-SIGN, and 10μg/ml and 90μg/ml Siglec-9, respectively, on the defined HMGs microarray. All of the other hGBP screened showed no binding to the microarray (data not shown).
This file contains two worksheets. The first worksheet includes the average RFU data and graphs of binding data of 1.0μg/ml DC-SIGN screened on the defined HMGs microarray in the presence or absence of 0.1mM, 1.0mM, or 10mM 2′-FL or lactose. The second worksheet includes the average RFU data and graphs of binding data of 1.0μg/ml DC-SIGN screened on the MAGS array in the presence or absence of 1.0mM or 10mM 2′-FL.
This file, containing two worksheets, shows the results of 2.0μg/ml Siglec-9 binding to the defined HMGs microarray in the presence of free HMGs or HMGs derivatives including 1mM and 10mM 6′-SL, 1mM and 10mM lactose, 1mM 6′-SL-AEAB, and 1mM 6′-SL-GGAEAB. The two worksheets show the data for two different slides, although these two slides were concurrently screened in the same experiment.