SUMMARY
Background
While numerous studies have shown associations between neighborhood quality and chronic disease outcomes, such correlations are potentially confounded by selection of different types of people into different neighborhood environments. We sought to identify the causal effects of neighborhood deprivation on type 2 diabetes risk, by comparing refugees to Sweden who were actively dispersed by government policy to low-, moderate-, or high-deprivation neighborhoods.
Methods
We studied national register data on N=61,386 refugees who arrived in Sweden at age 25–50 during 1987–1991, a period of quasi-random dispersal of refugees to Swedish neighborhoods varying in poverty, unemployment levels, schooling, and social welfare participation. Individuals in our sample were assigned to one of 4,833 neighborhoods, categorized as high-deprivation” (≥1 SD above the mean), “moderate deprivation” (within 1 SD of the mean), or “low-deprivation” (≥1 SD below the mean). The primary outcome was diagnosis with type 2 diabetes measured through 2010. We used multivariate logistic and linear regressions to assess the effects of neighborhood deprivation on diabetes risk, controlling for potential confounders affecting neighborhood assignment and assessing effects of cumulative exposure to different neighborhood conditions.
Findings
Being assigned to an area deemed high-deprivation versus low-deprivation was associated with an increased risk of diabetes among refugees (OR 1·22, 95% CI: 1·07, 1·38; p=0·001). In analyses that included fixed effects for assigned municipality, the increased diabetes risk was estimated to be 0·85 percentage points (−0·030, 1·728; p=0·058). Neighborhood effects grew over time, such that 5 years of additional exposure to high-deprivation versus low-deprivation neighborhoods was associated with a 9% increase in diabetes risk.
Interpretation
This study leverages a natural experiment to show that neighborhood deprivation increased the risk of diabetes among refugees to Sweden. This finding has heightened significance in the context of the current refugee crisis in Europe.
Funding
U.S. National Heart, Lung, and Blood Institute, U.S. National Center for Advancing Translational Sciences, U.S. National Institute on Minority Health and Health Disparities, Swedish Research Council.
INTRODUCTION
Studies have documented that adverse neighborhood socioeconomic characteristics are associated with worse chronic disease outcomes.3 Of note, several studies have shown a strong association between disadvantaged neighborhood environment and the incidence of insulin resistance and type 2 diabetes (T2D).4–7 Hypothesized mediating pathways include reduced employment and income opportunities resulting in the purchase of cheaper unhealthy food, reduced psychosocial resources resulting in higher chronic stress, and poorer food availability and walkability.8 Unfortunately, most existing studies on neighborhoods and health have been correlational and unable to account for several methodological challenges: selection bias, in that less healthy individuals may move to more disadvantaged neighborhoods; confounding by unobserved individual factors such as family socioeconomic status; and an inability to disentangle individual- and area-level factors.9–11 The only randomized trial was the Moving to Opportunity study, which found that individuals randomized to receive a housing voucher in five U.S. cities had small but significant improvements in obesity and T2D.1 Moving to Opportunity has been criticized on methodological grounds, such as residual selection bias and limited generalizability.12, 13
In this study, we overcome persistent challenges in the neighborhood effects literature by means of a novel quasi-experiment. We take advantage of a policy in Sweden during 1985–1994 that assigned incoming refugees in a quasi-random fashion to neighborhoods throughout the country. The policy’s goal was to ease labor market conditions in heavily settled areas and promote better integration of refugees. The dispersal policy was most strictly applied from 1987 through 1991,14 creating a unique natural experiment that enables us to examine how neighborhood environment affects the onset of T2D. As a result of the dispersal policy, refugees were spread more evenly across the country during this period (Figure 1). Several studies have examined the impact of European refugee dispersal policies on labor markets, crime, and educational outcomes.14–16 Only one has examined health, finding that neighborhood income inequality was not associated with risk of hospitalization.2 A significant contribution of the present study is the follow-up period of more than 20 years, longer than existing experimental or quasi-experimental studies of neighborhoods and health.
Figure 1. Percent immigrants by municipality in the year before and after the study period.
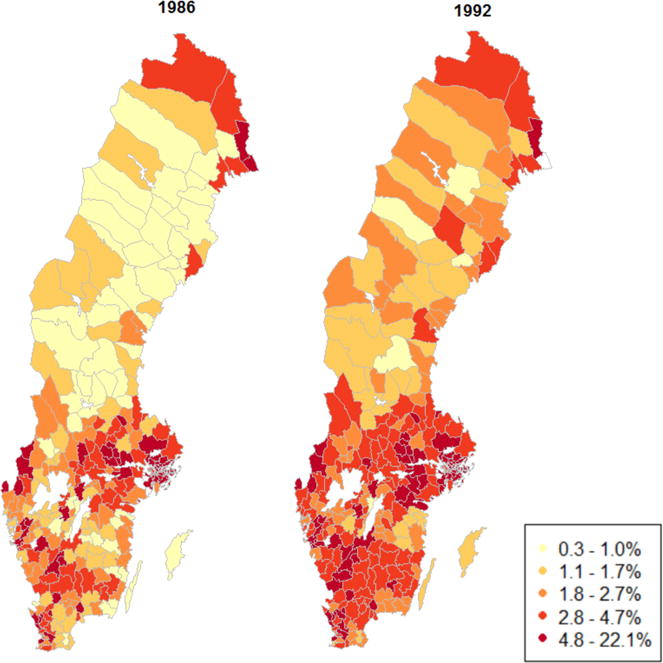
Note: Data from Statistics Sweden. Municipalities were classified into quintiles at baseline in order to create categories. The policy was strictly enforced during 1987–1991.
Neighborhood effects are particularly important for vulnerable populations such as immigrants. Studies have consistently shown that immigrants to Sweden, as elsewhere, have an elevated risk of diabetes and mortality compared to the native population and higher incident diabetes following migration from a lower-income country.6, 17, 18 Moreover, incidence may be higher among refugees than among immigrants more generally.19 In recent years, the European Union has experienced the largest inflow of immigrants and refugees since the end of the Second World War. The current wave of immigration includes a majority of individuals from the Middle East and North Africa, where T2D prevalence is the highest of any region worldwide: 11% among adults aged 20–79 in 2013.20 While policymakers have focused on the economic, political, and cultural implications of absorbing a large number of immigrants, the mass migration also has ramifications for the health and welfare of the newly settled immigrants. This study therefore has the potential to provide critical insights into the design of community-level interventions to influence the long-term health of refugees in Europe, testing the hypothesis that neighborhood deprivation affects the development of T2D.
METHODS
Data sources
We drew on data from multiple national registers on the entire Swedish population, including immigrants (Table 1). We linked the data sets using personal identification numbers assigned to all permanent residents in Sweden. Individual-level socio-demographic characteristics and aggregated statistics representing the neighborhood-level variables, described below, were available in the Total Population Register. Health outcome data, including the date of each health encounter, were available in the inpatient register (1987–2010), outpatient register (2001–2010), and prescription drug register (2005–2010). These data included roughly 11·8 million individuals and were more than 99% complete for all Swedish residents.
Table 1.
Data sources
| Data source | Years | Variables |
|---|---|---|
| Total Population Register | 1987–1991 | Age, sex, marital status, family size, region of residence, year of arrival, region of origin, neighborhood deprivation |
| Inpatient hospital register | 1987–2010 | Diagnostic codes and dates |
| Outpatient register | 2001–2010 | Diagnostic codes and dates |
| Prescription drug register | 2005–2010 | Prescriptions filled of insulin (analogs) and antidiabetic agents |
Note: Inpatient register data prior to 2001 were only used to exclude pre-existing cases of type 2 diabetes.
Sample selection
Our analytic sample consisted of immigrants who 1) obtained a Swedish residence permit during 1987–1991, 2) originated from a refugee-sending country, and 3) were age 25–50 at the time of entry into Sweden. In this manuscript, we refer to immigrants as any foreign-born person and refugees as the subset of immigrants who were forced to flee from persecution, entitled to protections under international law. We excluded persons belonging to a household with an adult already residing in Sweden, since they were likely to have resettled with family members and, consequently, were not assigned to a residence by a placement officer. A total of 61,386 individuals met the selection criteria.
Summary of Swedish dispersal policy
During 1985–1994, the Swedish Immigration Board assigned refugees arriving in Sweden to neighborhoods across the country. By 1989, 277 of 284 municipalities were participating in the program. All refugees, except those reuniting with family members living in Sweden or those having the financial resources to support themselves, were subjected to the dispersal policy. Our study focuses on the period 1987–1991 when the dispersal policy was strictly followed. From 1987–1991, about 90% of incoming refugees were assigned to an initial municipality according to official reports, indicating high participation in the placement program.14 During this time period, Sweden experienced a large influx of refugees (Figure 2). The Middle East was the most common sending region for refugees in our sample (Table 2), resembling immigrants entering during the current wave of immigration and making this study relevant to today’s refugee crisis.21
Figure 2. Number of new refugees in Sweden by year, 1951–2014.
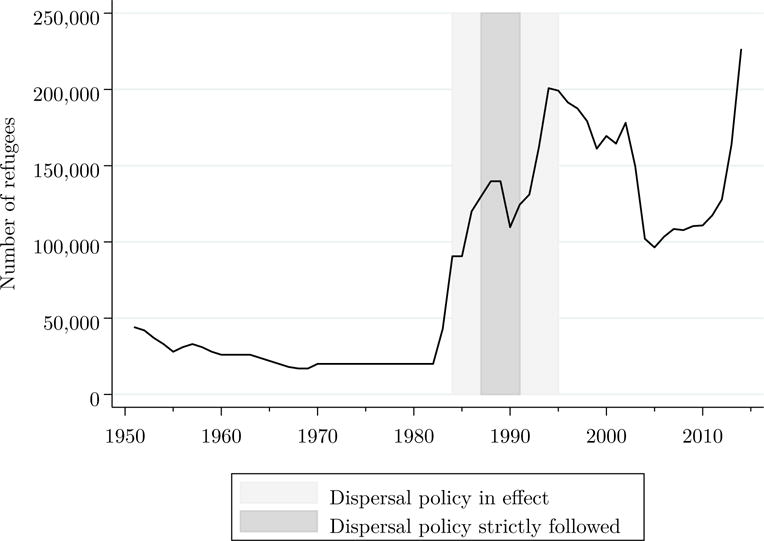
Note: Data from the United Nations High Commissioner for Refugees (UNHCR) Population Statistics Database, available online at http://popstats.unhcr.org and accessed on October 10, 2015. The counts include refugees, asylum seekers, internally displaced persons, and stateless persons.
Table 2.
Baseline characteristics, overall and by level of neighborhood deprivation
| Population
|
Percentage by deprivation
|
||||
|---|---|---|---|---|---|
| N | % | Low | Moderate | High | |
| Age (years) | |||||
| 25–29 | 23,925 | 39·0 | 37·8 | 39·0 | 39·2 |
| 30–34 | 17,351 | 28·3 | 28·8 | 28·2 | 28·2 |
| 35–39 | 10,724 | 17·5 | 17·5 | 17·8 | 17·2 |
| 40–44 | 5,721 | 9·3 | 9·9 | 9·4 | 9·1 |
| 45–50 | 3,665 | 6·0 | 6·0 | 5·6 | 6·3 |
| Male | 28,686 | 46·7 | 48·6 | 52·9 | 54·4 |
| Educational attainment | |||||
| ≤ 9 years | 15,640 | 25·5 | 17·9 | 25·2 | 27·0 |
| 10–12 years | 5,519 | 9·0 | 8·0 | 9·7 | 8·5 |
| > 12 years | 14,582 | 23·8 | 29·5 | 24·6 | 21·9 |
| Unknown | 25,645 | 41·8 | 44·6 | 40·5 | 42·6 |
| Married/cohabitating | 45,601 | 74·3 | 72·2 | 73·5 | 75·4 |
| Family size (no. of children) | |||||
| 0 | 10,347 | 16·9 | 21·9 | 17·6 | 15·3 |
| 1 | 10,060 | 16·4 | 20·3 | 16·3 | 15·8 |
| 2 | 18,553 | 30·2 | 32·2 | 30·1 | 30·1 |
| 3 | 11,247 | 18·3 | 13·4 | 18·3 | 19·1 |
| ≥4 | 11,179 | 18·2 | 12·2 | 17·8 | 19·7 |
| Region of residence | |||||
| Southern Sweden, large cities† | 35,992 | 58·6 | 73·5 | 53·1 | 61·4 |
| Southern Sweden, other | 19,130 | 31·2 | 15·7 | 33·2 | 31·8 |
| Northern Sweden | 6,264 | 10·2 | 10·8 | 13·6 | 6·8 |
| Year of arrival | |||||
| 1987 | 9,462 | 15·4 | 21·9 | 17·6 | 15·3 |
| 1988 | 11,436 | 18·6 | 20·3 | 16·3 | 15·8 |
| 1989 | 14,806 | 24·1 | 32·2 | 30·1 | 30·1 |
| 1990 | 13,120 | 21·4 | 13·4 | 18·3 | 19·1 |
| 1991 | 12,562 | 20·5 | 12·2 | 17·8 | 19·7 |
| Region of origin | |||||
| Iran | 13,456 | 21·9 | 18·0 | 22·3 | 22·2 |
| Middle East/Northern Africa | 14,199 | 23·1 | 16·1 | 20·3 | 27·1 |
| Other Africa | 5,954 | 9·7 | 9·7 | 9·9 | 9·5 |
| Asia | 7,596 | 12·4 | 20·1 | 12·4 | 11·0 |
| Eastern Europe | 11,783 | 19·2 | 20·9 | 19·8 | 18·3 |
| Latin America | 8,398 | 13·7 | 15·2 | 15·2 | 11·9 |
|
| |||||
| Number of observations | 61,386 | 4,815 | 27,786 | 28,785 | |
| Cumulative diabetes events | 4,553 | 281 | 1,994 | 2,278 | |
| Cumulative diabetes prevalence | 7·4% | 5·8% | 7·2% | 7·9% | |
Large cities are Stockholm, Gothenburg, and Malmo.
The Immigration Board assigned refugees to one of the refugee centers distributed nationwide, where individuals awaited a response on whether a residential permit had been approved. The typical duration spent in the refugee centers was 3 to 12 months.22 Once a refugee received a residential permit, placement officers assigned the person to an apartment. Placement officers did not have any direct interaction with refugees, meaning that any selection bias must be on the observed characteristics available to the officers in the application: refugees’ language, formal schooling, and family size.14 After refugees were placed in an initial residence, the Immigration Board offered Swedish language and training courses and social welfare support, lasting for about 18 months depending on municipality and year. There were no restrictions on mobility if refugees found a residence independently, and receipt of welfare was not conditional on remaining in the assigned residence. Therefore, our study design is akin to a randomized encouragement design in which participants are randomly encouraged to be exposed to a given neighborhood, and represents a lower bound on the health effects of neighborhood deprivation. In this study, our focus is on estimating the effect of initial quasi-random neighborhood placement. We therefore do not estimate the subsequent effects of relocation, as these are likely subject to selection bias.
From 1987–1991, a thriving housing market in Sweden made it difficult for incoming refugees to find an apartment in a desirable area.14 Consequently, placement officers based their assignment even more strictly on housing availability. Refugees had limited ability to influence their initial place of residence during this period. In this study, we therefore assume that refugee placement during this narrower time frame of 1987–1991 was essentially random, conditional on observed factors available to placement officers, all of which are available in Swedish register data. This is a strategy employed in and supported by prior research.14, 15, 23 In essence, there were no unobserved factors that could have influenced neighborhood placement, overcoming the challenge of confounding present in the existing literature on neighborhood effects.
Study variables
Health outcome: type 2 diabetes
The primary outcome measure was diagnosis with T2D during 2002–2010, the years in which outcome data were available. Cases were identified in the inpatient and outpatient registers from 2002–2010 according to ICD-10 codes E11–E14 and in the prescription drug register from 2005–2010 according to individuals who were prescribed or filled a prescription of insulin, insulin analogs, or oral antidiabetic agents (Anatomical Therapeutic Chemical codes A10B and A10X). The prescription drug register alone has been shown to capture 90% of all diabetic cases in Sweden.24, 25 Validation studies have also found Sweden’s clinical registers to have broad coverage and high quality.26
We excluded individuals diagnosed with other types of diabetes, including type 1 diabetes (ICD-10 codes E8–E10). To capture only incident disease, we used inpatient register data dating back to 1987 to exclude individuals who were diagnosed with any form of diabetes (ICD-10 codes E8–E14) within five years of arrival in Sweden, as these individuals likely had pre-existing clinical or pre-clinical disease. For example, if a refugee arrived in 1987, he or she would not be considered an incident case if diagnosed with diabetes during 1987–1992.
Neighborhood deprivation measures
We defined neighborhoods on the basis of small-area market statistics (SAMS) that use boundaries determined by homogeneous building types (e.g., high-rise buildings). SAMS have been a common neighborhood definition in prior studies in Sweden.27, 28 The average population in each SAMS was about 2,000 residents in Stockholm and 1,000 residents elsewhere in Sweden.15 After excluding areas with fewer than 50 residents because of unstable statistical estimates, our analysis included individuals who were initially assigned to 4,833 different SAMS.
We created a summary measure to characterize neighborhood deprivation in 1987, following the approach adopted by prior Swedish studies.6, 29 Specifically, we used a principal components analysis that included four variables for all residents aged 25–64 in each SAMS, each measured in percentages in the year of initial placement: low education status (<10 years of formal schooling), low income (<50% of individual median income from all sources), unemployment (not employed, excluding full-time students, military, and retirees), and social welfare assistance. We calculated a Z-score for each SAMS, weighted by the coefficients on the eigenvectors. We then classified neighborhoods into three categories, established in prior studies: low (≥1 standard deviation (SD) below the mean), moderate (within 1 SD of the mean), and high (≥1 SD above the mean).29 We also considered a second measure that trichotomizes the component score into tertiles to achieve an equal number of individuals from the study population in each category, resulting in similar findings (results available upon request).
Covariates
To improve the validity and precision of our estimates, our analyses adjusted for several individual characteristics measured in the year of initial placement. These included: 5-year age categories (e.g., 25–29, 30–34), sex, educational attainment (≤9 years, 10–12 years, >12 years), marital status (married/cohabitating versus not), region of initial placement (large cities in southern Sweden, other areas in southern Sweden, northern Sweden), family size (0, 1, 2, 3, ≥4 children), and region of origin (Iran, Middle East/North Africa, other Africa, Asia, Eastern Europe, Latin America). We also included indicator variables for year of arrival to adjust for secular trends.
Statistical analyses
We assumed that initial neighborhood assignment within a municipality was quasi-random, conditional on all observed factors available to placement officers: refugees’ language, formal schooling, and family size. This assumption is not directly testable, although studies have found increased dispersal of refugees during the policy period, no evidence of sorting by ethnic group, and covariate balance by neighborhood type.14, 15, 23 We also investigated the balance of covariates by neighborhood deprivation level as a guide to the validity of this assumption.
We next conducted unadjusted logistic regressions to determine the association of neighborhood deprivation with diabetes risk. We then adjusted for individual-level baseline characteristics described in the Covariates section above, including the factors that may have affected the initial placement assignment by officials.
We then conducted linear probability models (ordinary least squares, OLS) to more precisely estimate the causal effect of neighborhood deprivation on diabetes risk. To do so, we included fixed effects (i.e., indicator variables) for assigned municipality, which adjusted for all time-invariant factors at the municipality level. Thus, we identified the neighborhood effects using socioeconomic variation across neighborhoods within a municipality. In other words, we considered the difference in outcomes for refugees placed in a given municipality who were assigned to a high-deprivation neighborhood as opposed to a low-deprivation neighborhood. We chose a fixed effects approach over a multilevel modeling approach, because we are substantively interested in identifying a causal effect, and multilevel models do not perform as well as fixed effects models in controlling for omitted variables bias.30
Finally, to analyze how the effects of exposure to neighborhood deprivation accumulate over time, we estimated the covariate-adjusted OLS models for outcomes reported in each year from 2002–2010.
In order to account for correlated outcomes among individuals assigned to the same municipality, robust standard errors in all models were clustered by municipality (N = 288).
The analyses used an intent-to-treat approach that included all refugees who arrived in Sweden from 1987–1991. Individuals who emigrated from Sweden prior to follow-up were classified as non-cases. We obtain similar results when we excluded emigrants from our analysis (results available upon request).
Role of the funding source
The sponsor of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had final responsibility for the decision to submit for publication.
RESULTS
Descriptive statistics
Table 2 displays the characteristics of the sample at the time of arrival. A large fraction of refugees in the sample were initially assigned to deprived neighborhoods: 45% to a moderate-deprivation area and 47% to a high-deprivation area. This reflects that housing was most readily available in more deprived areas during the arrival period. About two-thirds of the sample was under age 35. About three-quarters were married, and 83% had children. Most were settled in the three largest cities. The refugees arrived from a diverse set of geographic regions, with almost half from Iran and the Middle East or North Africa.
The last three columns of Table 2 show the balance of baseline characteristics by neighborhood deprivation level. The placement officers only had information about refugees’ language, formal schooling, and family size, so we would expect these variables might vary by deprivation level, and we are able to adjust for these observed characteristics in our analyses. Most baseline characteristics do not vary greatly by neighborhood deprivation level. This supports our assumption that initial neighborhood assignment within a municipality is quasi-random conditional on observed variables. Any other imbalance in unobserved factors should be due to random chance, as in a randomized study.
The cumulative prevalence of T2D at follow-up is 7·4% in our sample. The last row of Table 2 demonstrates a deprivation-diabetes gradient, with cumulative prevalence of diabetes increasing by deprivation level. By comparison, overall diabetes prevalence in Sweden is estimated at 4–6%.31, 32
Association between neighborhood deprivation and diabetes risk
In order to further analyze the association between T2D prevalence and neighborhood deprivation, we estimate two sets of logistic regression models (Figure 3). In unadjusted models (top panel), being assigned to a moderate-deprivation or high-deprivation SAMS increased the odds of T2D diagnosis by 25% or 39%, relative to a low-deprivation SAMS. In adjusted models controlling for the covariates listed in Table 2 (bottom panel), there remains an increase, though somewhat attenuated: 15% for moderate-deprivation and 22% for high-deprivation areas.
Figure 3. Odds ratios of diabetes risk by deprivation level.
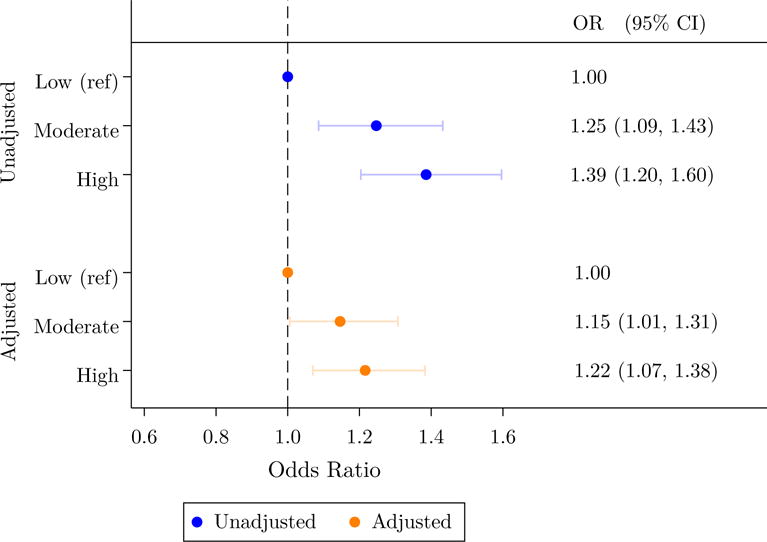
Note: N = 61,386. Odds ratios are derived from logistic regression models. The adjusted model includes 5-year age categories, sex, educational attainment, marital status, region of initial placement, family size, region of origin, and year of arrival. Parentheses indicate 95% confidence intervals, with robust standard errors clustered by municipality.
Neighborhood effects on diabetes risk
In the second half of the analysis, we estimated linear probability models with fixed effects for initial municipality (Table 3). As described above, the municipality fixed effects accounted for any sorting that occurred in which refugees were assigned to different municipal refugee centers while awaiting residential permits. Diabetes risk increased for refugees initially assigned to a high-deprivation vs. low-deprivation area by 0·8–1·7 percentage points, depending on adjustment for covariates. This translates to a 15–30% increase in diabetes risk. Coefficients for moderate deprivation were not statistically significant in these models, although they did suggest higher diabetes risk than in low-deprivation areas. The coefficients were less precisely estimated because of added parameters for the 288 municipality fixed effects.
Table 3.
Effect of neighborhood deprivation on type 2 diabetes risk
| Model 1 (Unadjusted) | Model 2 (Adjusted) | |
|---|---|---|
| Deprivation level (ref = Low) | ||
| Moderate | 0·724 (−0·14,1·593) |
0·437 (−0·420, 1·295) |
| High | 1·734*** (0·846, 2·622) |
0·849* (−0·030, 1·728) |
|
| ||
| Initial municipality fixed effects | Yes | Yes |
| Control variables | No | Yes |
p<0·10
p<0·05
p<0·01
Note: N = 61,386. Linear probability models of neighborhood deprivation on diabetes. Coefficients are expressed as percentage point changes in diabetes risk. Model 2 adjusts for 5-year age categories, sex, educational attainment, marital status, region of initial placement, family size, region of origin, and year of arrival. 95% confidence intervals are shown in parentheses.
Cumulative effects
Diabetes risk accumulated over time, rather than occurring immediately after arrival (Figure 4). Five years of additional exposure to high-deprivation versus low-deprivation neighborhoods was associated with a 9% increase in diabetes risk.
Figure 4. Cumulative effect of neighborhood deprivation on diabetes risk over time.
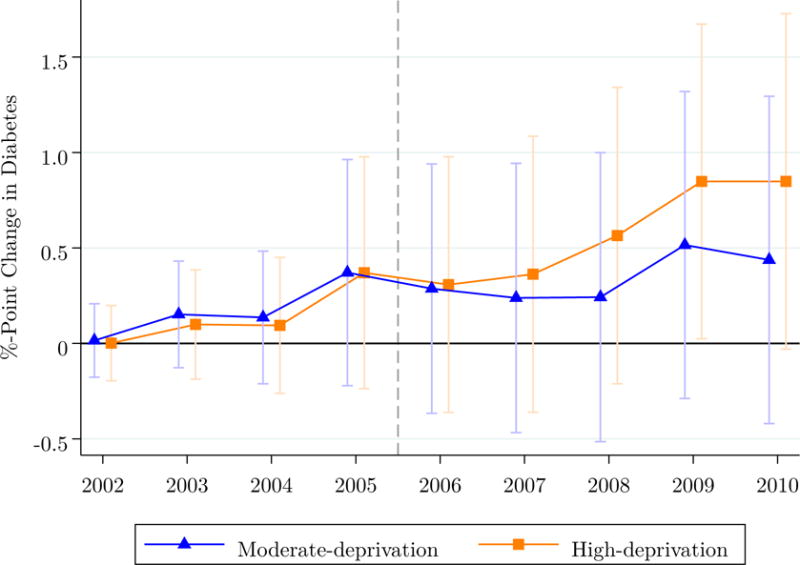
Note: N = 61,386. This figure plots the marginal effects of neighborhood deprivation relative to low-deprivation areas, derived from separate yearly regression models corresponding to Model 2 in Table 3. The dotted line indicates the point at which the prescription drug register became available for identifying diabetes cases.
DISCUSSION
In this study, we tracked the effects of neighborhood deprivation on refugees’ diabetes risk two decades after arrival in Sweden, using comprehensive national register data. The results indicate that refugees initially assigned to highly deprived neighborhoods experienced an increased risk of T2D of 1·7 percentage points. Covariate adjustment attenuated the magnitude of the association to 0·8 percentage points. This represents a relative increase of 15–30%. The accumulation of this effect over time is consistent with hypothesized mediating pathways, such as chronic stress, employment and income opportunities, and food environment, all of which may take years to influence health. Moreover, the results suggest a gradient, such that high-deprivation areas have worse outcomes than moderate-deprivation areas. In the absence of the policy, greater numbers of refugees would have continued to settle in areas with higher proportions of immigrants, which have higher deprivation on average. Therefore, this policy likely reduced the cumulative prevalence of diabetes among refugees in this sample.
A strength of our study design is that we leverage a natural policy experiment. Our estimates are not subject to selection bias and unobserved confounding that have plagued the neighborhood effects literature. One prior study using traditional correlational methods estimated the association of neighborhoods with diabetes risk among the full population in Sweden, with odds ratios of 1·28 (95% CI: 1·23,1·35) and 1·67 (95% CI: 1·57,1·77) for moderate- and high-deprivation areas, respectively.6 The corresponding estimates in our study were 1·15 (95% CI: 1·01,1·31) and 1·22 (1·07,1·38). Although the study populations differ, this comparison highlights the importance of gathering additional experimental and quasi-experimental evidence on neighborhood effects to reduce potential bias.
Our estimates are likely to be a lower bound on the true effects of neighborhoods on diabetes risk. The dispersal policy did not restrict mobility away from initial neighborhood assignment. If refugees moved to wealthier areas, it could undo some or all of the negative effects of being assigned to a deprived neighborhood. In this respect, the dispersal policy is analogous to a randomized encouragement design in which participants are randomly encouraged or not encouraged to be exposed to a deprived neighborhood, a situation in which nonadherence can play a role.33 In our study, about half of participants left their assigned municipality after 10 years. This likely weakened the estimated effect size. Yet, even in the presence of high relocation rates, our study nevertheless demonstrated a long-term impact on refugee health. Possible mechanisms include poor employment opportunities in the initial assigned area that altered housing and income trajectories. In our sample, initial neighborhood deprivation was highly correlated with the deprivation of neighborhood residence after 5, 10, and 15 years (Supp. Table S2), suggesting that initial assignment had long-term consequences on residential decisions and may be a mediating pathway through which initial neighborhood placement affected diabetes risk.
This study has several limitations. First, register data do not indicate which immigrants were refugees that would have been subject to this policy. Instead, we followed prior research in which the sample was identified based on the year and region of migration to Sweden.2, 14 Government statistics show that most immigrants to Sweden during the study period were refugees (46.5%) or those moving to rejoin family members (i.e., “tied movers”) (47.7%), compared to labor immigrants (0.6%), guest students (2.4%), or adopted children (2.7%). Adopted children and many students would not meet our age inclusion criterion, and we exclude tied movers. Consequently, a large majority of our sample consisted of refugees, and misclassification is likely to be limited. Second, Sweden provided relatively comprehensive social welfare assistance to refugees. It is possible that neighborhoods exert a stronger effect in settings where social support is more limited. It is striking that even in a context renowned for its strong public safety net, we still observed large neighborhood effects. As a result, however, our analyses may not generalize to other waves of immigrants or to contexts beyond Sweden. Our estimates may also not generalize to areas with fewer than 50 residents, as these were excluded from our sample due to unstable estimates. In addition, we do not have data on the length of time that refugees spent in refugee centers before being assigned housing by a placement officer; it is possible that those with longer wait times were more likely to find housing independently, which could bias the results. As mentioned previously, however, compliance with the policy was above 90%. Further, we do not adjust for neighborhood-level differences in access to care, the presence of which would bias our estimates toward the null if health care utilization is worse in more deprived neighborhoods. However, this bias is likely negligible, as studies have found that access to care was no worse for immigrants from refugee-sending countries during the study period.34 Finally, we rely only on the inpatient register to exclude pre-existing cases of T2D during the first years after immigration, as the outpatient register was not established until 2001; this may miss less severe cases. As long as these cases are randomly distributed across neighborhoods, as we would expect, this will not bias our estimates.
Our study has direct relevance to the ongoing wave of immigration to Europe. Due to the historically high numbers of incoming refugees combined with already high unemployment rates, the new entrants are encountering less hospitable environments. Our findings indicate that decisions that affect the settlement and integration of immigrants can have long-term consequences for the health of the new arrivals. Refugees are among the most vulnerable populations in any society, and as such deserve special attention from governments in crafting policies that protect and promote their health. Further investigation of the pathways through which neighborhoods affect the risk of diabetes and other diseases may shed light on how best to buffer immigrants against the consequences of neighborhood deprivation. Future studies should also consider the impacts of these factors on other outcomes, such as mental health, which may be adversely affected by dispersal policies of this nature.
Supplementary Material
RESEARCH IN CONTEXT.
Evidence before this study
We searched the PubMed database for articles about neighborhood characteristics and type 2 diabetes. Our search terms included “diabetes” and “neighborhoods” or “neighbourhoods.” We restricted the search to articles published in English prior to November 31, 2015. We identified more than a dozen observational studies that investigated the relationship between neighborhood characteristics and the prevalence or incidence of type 2 diabetes. Most found an association between increased diabetes risk and various measures of neighborhood deprivation. However, we found only one randomized trial, the Moving to Opportunity experiment.1 It found that women whose family received a voucher to relocate to a low-poverty neighborhood had a 4-percentage-point reduction in having glycated hemoglobin ≥6.5% at follow-up 10–15 years later. We did not find any quasi-experimental evidence on the topic. In our search of the broader literature on neighborhood health effects, we found no other randomized trials and one quasi-experimental study, which found no relationship between neighborhood-level income inequality and hospitalization risk.2
Added value of this study
In this study, we took advantage of a unique quasi-experiment to assess the causal relationship between neighborhood deprivation and type 2 diabetes risk. To our knowledge, it is the first quasi-experiment involving neighborhood deprivation and any health outcome, building on existing correlational and experimental evidence. Our follow-up period of more than 20 years is longer than Moving to Opportunity and the majority of existing observational studies of neighborhoods and type 2 diabetes. Our results suggest that exposure to neighborhood deprivation increased diabetes risk in our sample.
Implications of all the available evidence
In combination with the existing evidence, our study indicates that the association between neighborhood deprivation and type 2 diabetes risk is not driven solely by selection of families into neighborhoods or other confounding. Neighborhood environments exert a causal influence on diabetes risk, which accumulates over time. Policy efforts to reduce area-level socioeconomic disparities may contribute to lowering the risk of type 2 diabetes. The focus of our study is the effects of neighborhoods on refugees who arrived in Sweden 25–30 years ago. While policymakers should be cautious about generalizability, these findings nevertheless have critical implications for the unprecedented current wave of migrants to Europe.
Acknowledgments
This work was supported by the Stanford Clinical and Translational Science Award to Spectrum (UL1-TR-001085). Dr. White was supported by a National Heart, Lung, and Blood Institute (NHLBI) training grant to the Stanford Prevention Research Center of Stanford University (T32-HL-7034). Dr. Hamad was supported by a KL2 Mentored Career Development Award of the Stanford Clinical and Translational Science Award to Spectrum (NIH KL2-TR-001083). Drs. Li, Ohlsson, J. Sundquist, and K. Sundquist were supported by the NHLBI (R01-HL-16381). Dr. K. Sundquist was also supported by the Swedish Research Council. Dr. Basu was supported by the NHLBI (K08-HL-121056) and the National Institute on Minority Health and Health Disparities (DP2-MD-010478). The opinions expressed are those of the investigators and do not necessarily reflect the views of the funding agencies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
JW, RH, and SB conceived of the study. JW, RH, SB, JS, and KS designed the study. XL performed the data analysis. JW, RH, SB, JS, and KS contributed to interpretation of the data. JW wrote the initial manuscript, and RH, SB, HO, JS, and KS provided comments on the draft.
Conflicts of interest
We declare that we have no conflicts of interest.
References
- 1.Ludwig J, Sanbonmatsu L, Gennetian L, Adam E, Duncan GJ, Katz LF, et al. Neighborhoods, obesity, and diabetes: A randomized social experiment. New England Journal of Medicine. 2011;365(16):1509–19. doi: 10.1056/NEJMsa1103216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grönqvist H, Johansson P, Niknami S. Income inequality and health: Lessons from a refugee residential assignment program. Journal of Health Economics. 2012;31(4):617–29. doi: 10.1016/j.jhealeco.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 3.Diez Roux AV, Mair C. Neighborhoods and health. Annals of the New York Academy of Sciences. 2010;1186(1):125–45. doi: 10.1111/j.1749-6632.2009.05333.x. [DOI] [PubMed] [Google Scholar]
- 4.Diez Roux AV, Jacobs DR, Kiefe CI. Neighborhood Characteristics and Components of the Insulin Resistance Syndrome in Young Adults The Coronary Artery Risk Development in Young Adults (CARDIA) Study. Diabetes Care. 2002;25(11):1976–82. doi: 10.2337/diacare.25.11.1976. [DOI] [PubMed] [Google Scholar]
- 5.Laraia BA, Karter AJ, Warton EM, Schillinger D, Moffet HH, Adler N. Place matters: neighborhood deprivation and cardiometabolic risk factors in the Diabetes Study of Northern California (DISTANCE) Social Science & Medicine. 2012;74(7):1082–90. doi: 10.1016/j.socscimed.2011.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mezuk B, Chaikiat Å, Li X, Sundquist J, Kendler KS, Sundquist K. Depression, neighborhood deprivation and risk of type 2 diabetes. Health Geographies of Voluntarism. 2013;23:63–9. doi: 10.1016/j.healthplace.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christine PJ, Auchincloss AH, Bertoni AG, et al. Longitudinal associations between neighborhood physical and social environments and incident type 2 diabetes mellitus: The Multi-Ethnic Study of Atherosclerosis (MESA) JAMA Internal Medicine. 2015;175(8):1311–20. doi: 10.1001/jamainternmed.2015.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adler NE, Newman K. Socioeconomic disparities in health: Pathways and policies. Health Affairs. 2002;21(2):60–76. doi: 10.1377/hlthaff.21.2.60. [DOI] [PubMed] [Google Scholar]
- 9.Oakes JM. The (mis)estimation of neighborhood effects: causal inference for a practicable social epidemiology. Social Science & Medicine. 2004;58(10):1929–52. doi: 10.1016/j.socscimed.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 10.Diez Roux AV. Estimating neighborhood health effects: the challenges of causal inference in a complex world. Social Science & Medicine. 2004;58(10):1953–60. doi: 10.1016/S0277-9536(03)00414-3. [DOI] [PubMed] [Google Scholar]
- 11.Manski CF. Identification of Endogenous Social Effects: The Reflection Problem. The Review of Economic Studies. 1993;60(3):531–42. [Google Scholar]
- 12.Clampet-Lundquist S, Massey DS. Neighborhood Effects on Economic Self-Sufficiency: A Reconsideration of the Moving to Opportunity Experiment. American Journal of Sociology. 2008;114(1):107–43. [Google Scholar]
- 13.Sampson RJ. Moving to Inequality: Neighborhood Effects and Experiments Meet Social Structure. American Journal of Sociology. 2008;114(1):189–231. doi: 10.1086/589843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edin P-A, Fredriksson P, Åslund O. Ethnic Enclaves and the Economic Success of Immigrants: Evidence from a Natural Experiment. The Quarterly Journal of Economics. 2003;118(1):329–57. [Google Scholar]
- 15.Åslund O, Edin P-A, Fredriksson P, Grönqvist H. Peers, Neighborhoods, and Immigrant Student Achievement: Evidence from a Placement Policy. American Economic Journal: Applied Economics. 2011;3(2):67–95. [Google Scholar]
- 16.Damm AP. Neighborhood quality and labor market outcomes: Evidence from quasi-random neighborhood assignment of immigrants. Journal of Urban Economics. 2014;79(0):139–66. [Google Scholar]
- 17.Rostila M, Fritzell J. Mortality differentials by immigrant groups in Sweden: The contribution of socioeconomic position. American Journal of Public Health. 2014;104(4):686–95. doi: 10.2105/AJPH.2013.301613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kandula NR, Diez-Roux AV, Chan C, Daviglus ML, Jackson SA, Ni H, et al. Association of Acculturation Levels and Prevalence of Diabetes in the Multi-Ethnic Study of Atherosclerosis (MESA) Diabetes Care. 2008;31(8):1621–8. doi: 10.2337/dc07-2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Norredam M, Agyemang C, Hoejbjerg Hansen OK, Petersen JH, Byberg S, Krasnik A, et al. Duration of residence and disease occurrence among refugees and family reunited immigrants: test of the ‘healthy migrant effect’ hypothesis. Tropical Medicine & International Health. 2014;19(8):958–67. doi: 10.1111/tmi.12340. [DOI] [PubMed] [Google Scholar]
- 20.Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Research and Clinical Practice. 2014;103(2):137–49. doi: 10.1016/j.diabres.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 21.Swedish Migration Board. Statistics. 2015 [cited 2015 November 2, 2015] Available from: http://www.migrationsverket.se/English/About-the-Migration-Agency/Facts-and-statistics-/Statistics.html.
- 22.Rooth DO. Ph D thesis. Lund University; 1999. Refugee immigrants in Sweden. [Google Scholar]
- 23.Edin P-A, Fredriksson P, Åslund O. Settlement policies and the economic success of immigrants. Journal of Population Economics. 2004;17(1):133–55. [Google Scholar]
- 24.Jansson SPO, Fall K, Brus O, Magnuson A, Wändell P, Östgren CJ, et al. Prevalence and incidence of diabetes mellitus: a nationwide population-based pharmaco-epidemiological study in Sweden. Diabetic Medicine. 2015;32(10):1319–28. doi: 10.1111/dme.12716. [DOI] [PubMed] [Google Scholar]
- 25.Rawshani A, Landin-Olsson M, Svensson A-M, Nyström L, Arnqvist H, Bolinder J, et al. The incidence of diabetes among 0–34 year olds in Sweden: new data and better methods. Diabetologia. 2014;57(7):1375–81. doi: 10.1007/s00125-014-3225-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ludvigsson J, Andersson E, Ekbom A, Feychting M, Kim J-L, Reuterwall C, et al. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11(1):450. doi: 10.1186/1471-2458-11-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sundquist K, Chaikiat Å, León VR, Johansson S-E, Sundquist J. Country of birth, socioeconomic factors, and risk factor control in patients with type 2 diabetes: a Swedish study from 25 primary health-care centres. Diabetes/Metabolism Research and Reviews. 2011;27(3):244–54. doi: 10.1002/dmrr.1161. [DOI] [PubMed] [Google Scholar]
- 28.Sundquist K, Winkleby M, Ahlén H, Johansson S-E. Neighborhood Socioeconomic Environment and Incidence of Coronary Heart Disease: A Follow-up Study of 25,319 Women and Men in Sweden. American Journal of Epidemiology. 2004;159(7):655–62. doi: 10.1093/aje/kwh096. [DOI] [PubMed] [Google Scholar]
- 29.Winkleby M, Sundquist K, Cubbin C. Inequities in CHD Incidence and Case Fatality by Neighborhood Deprivation. American Journal of Preventive Medicine. 2007;32(2):97–106. doi: 10.1016/j.amepre.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wooldridge JM. Econometric analysis of cross section and panel data. MIT press; 2010. [Google Scholar]
- 31.Wiréhn A-BE, Karlsson HM, Carstensen JM. Estimating disease prevalence using a population-based administrative healthcare database. Scandinavian Journal of Public Health. 2007;35(4):424–31. doi: 10.1080/14034940701195230. [DOI] [PubMed] [Google Scholar]
- 32.Carlsson A, Wandell P, Osby U, Zarrinkoub R, Wettermark B, Ljunggren G. High prevalence of diagnosis of diabetes, depression, anxiety, hypertension, asthma and COPD in the total population of Stockholm, Sweden - a challenge for public health. BMC Public Health. 2013;13(1):670. doi: 10.1186/1471-2458-13-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hirano K, Imbens GW, Rubin DB, Zhou XH. Assessing the effect of an influenza vaccine in an encouragement design. Biostatistics. 2000;1(1):69–88. doi: 10.1093/biostatistics/1.1.69. [DOI] [PubMed] [Google Scholar]
- 34.Hjern A, Haglund B, Persson G, Roen M. Is there equity in access to health services for ethnic minorities in Sweden? European Journal of Public Health. 2001;11(2):147–52. doi: 10.1093/eurpub/11.2.147. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


