Abstract
The transcription factor Sox2 is both necessary and sufficient for the generation of sensory regions of the inner ear. It regulates expression of the Notch ligand Jag1 in prosensory progenitors, which signal to neighboring cells to up-regulate Sox2 and sustain prosensory identity. However, the expression pattern of Sox2 in the early inner ear is very broad, suggesting that Sox2-expressing progenitors form a wide variety of cell types in addition to generating the sensory regions of the ear. We used Sox2-CreER mice to follow the fates of Sox2-expressing cells at different stages in ear development. We find that Sox2-expressing cells in the early otocyst give rise to large numbers of non-sensory structures throughout the inner ear, and that Sox2 only becomes a truly prosensory marker at embryonic day (E)11.5. Our fate map reveals the organ of Corti derives from a central domain on the medial side of the otocyst and shows that a significant amount of the organ of Corti derives from a Sox2-negative population in this region.
Keywords: Sox2, Cochlea, Otocyst, Inner Ear, Organ of Corti, Sensory
Introduction
The perception of sound and linear and angular acceleration are mediated by dedicated sensory organs in the inner ear. The specialized array of hair cells and supporting cells in each sensory organ derives from prosensory patches that are induced shortly after formation of the otocyst, and which express the transcription factor Sox2 and the Notch ligand Jag1 (Adam et al., 1998; Eddison et al., 2000; Kiernan, 2013; Kiernan et al., 2005; Lewis, 1998; Morrison et al., 1999; Morsli et al., 1998; Neves et al., 2007; Ohyama et al., 2010). Gain- and loss-of-function studies support a model in which Jag1 signals through Notch receptors to activate Sox2 expression in adjacent prosensory cells. Consequently, mutation of Sox2 or Jag1 either abolishes or greatly reduces the size of inner ear sensory patches and their derived sensory organs (Brooker et al., 2006; Kiernan et al., 2001; Kiernan et al., 2005; Kiernan et al., 2006). Ectopic expression of Sox2, Jag1, or activation of the canonical Notch signaling pathway is sufficient to induce sensory patches in non-sensory parts of the inner ear (Hartman et al., 2010; Neves et al., 2011; Neves et al., 2012; Pan et al., 2013; Pan et al., 2010).
Several lines of evidence suggest Jag1-Notch signaling serves to maintain prosensory patch identity, but may not be necessary to initiate it. Jag1 expression in the otocyst is initially regulated by canonical Wnt signaling (Jayasena et al., 2008), and the presence of Wnt-responsive elements in the Jag1 promoter suggests this regulation is direct (Estrach et al., 2006). Moreover, both Jag1 and Sox2 are initially expressed in broad domains in the developing otocyst, extending well beyond the regions that will ultimately form sensory patches (Jayasena et al., 2008; Mak et al., 2009; Morrison et al., 1999; Neves et al., 2007). These domains later refine to the prosensory patches, and it is likely that Notch signaling acts at these times to stabilize sensory patch identity (Neves et al., 2013a; Raft and Groves, 2015).
To understand the decisions that govern the production of sensory versus non-sensory epithelium in the inner ear, we used Sox2-CreER mice (Arnold et al., 2011) to follow the fate of Sox2-expressing cells between embryonic days 8–12. We find that most non-sensory regions of the inner ear derive from Sox2-expressing progenitors at early stages. Surprisingly, significant regions of the organ of Corti are not generated from Sox2-expressing regions of the inner ear at these stages. Rather, we show that the apical-basal axis of the organ of Corti can be mapped onto the interface of a Sox2+ and Sox2− region in the ventromedial face of the otocyst. Our data suggest that distinct sets of signals are necessary to restrict the initially broad patterns of Sox2 and Jag1 to prosensory patches, and then to maintain the identity of these patches as the sensory organs of the ear differentiate.
Materials And Methods
Experimental animals
Sox2-CreERT2 knock-in mice (MGI: Sox2tm1(Cre/ERT2)Hoch; Arnold et al., 2011) were provided by Dr. Konrad Hochedlinger. These mice are now available from the Jackson Laboratory (stock number 017593). Ai3 Cre reporter mice (MGI: Gt(ROSA)26Sortm3(CAG-EYFP)Hze; Madisen et al., 2010) were purchased from the Jackson Laboratory, (stock number 007903). Genotyping was performed by PCR using the following primers: Sox2-CreERT2: Cre1F (GCC TGC ATT ACC GGT CGA TGC AAC GA) and Cre1R (GTG GCA GAT GGC GCG GCA ACA CCA TT) yield a 700 bp band. Ai3: P020 (AAG GGA GCT GCA GTG GAG TA), P021 (CCG AAA ATC TGT GGG AAG TC), P102 (ACA TGG TCC TGC TGG AGT TC) P103 (GGC ATT AAA GCA GCG TAT CC), yielding a 297bp wild type band and a 212bp transgene band.
Fate Mapping of Sox2 progenitors
For fate mapping experiments, heterozygous male Sox2-CreERT2 mice were mated with homozygous Ai3 Cre reporter female mice and the presence of a vaginal plug the following morning was used to designate the day of pregnancy. A single dose of 2.5mg tamoxifen and 2.5mg progesterone (0.1ml; dissolved in peanut oil at a concentration of 25 mg/ml each) was administered to pregnant females by oral gavage at embryonic day (E)8.5, E9.5, E10.5, E11.5 or E12.5. The genotypes of embryos or newborn pups from offspring of Sox2-Cre; Ai3 crosses were determined by the presence of EYFP fluorescence and confirmed by PCR as described above. In all cases, 4 samples were analyzed with each marker for each age of tamoxifen administration. All animal experiments were approved by the Baylor College of Medicine Institutional Animal Care and Use committee.
Tissue preparation
Inner ears were collected for analysis at either E18.5 or the day of birth. After fixing with 4% paraformaldehyde for 2 hours at room temperature, the inner ears were kept in 30% sucrose in PBS for cryoprotection overnight at 4°C, then embedded in OCT and frozen. Tissue was sectioned at 8μm and sections collected as 3 serial sets. For vestibular organs, the utricle, saccule and the three ampullae were isolated separately and embedded for serial cryosectioning at 8μm. For whole mount preparations of the cochlea, the membranous cochlea duct was dissected and the lateral wall tissue, including the stria vascularis and spiral ligament, was trimmed away after immunostaining prior to mounting and photographing.
Immunohistochemistry
The primary antibodies used in this study were: anti-GFP (1:1000, chicken, Abcam), anti-Myosin 7a (1:200, rabbit, Proteus), anti-Sox10 (1:250, goat, Santa Cruz Biotechnology), anti-Jag1 (1:75, rabbit, Santa Cruz Biotechnology), anti-NeuN (1:500, mouse, Millipore), anti-GFAP (1:500, rabbit, Dako Z0334) and anti-Cre (1:150, mouse, Millipore). The secondary antibodies used in this study include Alexa Fluor 488 or Alexa Fluor 596 conjugated goat anti rabbit IgG, goat anti mouse IgG, goat anti chicken IgG or donkey anti goat IgG, respectively (1:200, Invitrogen). The immunohistochemistry procedure followed standard protocols. The sections and isolated cochlear whole mount samples were blocked with PBS containing 10% normal goat serum or 5% normal donkey serum and 0.2% Triton X-100 for one hour at room temperature, then incubated with primary antibody overnight at 4°C. After rinsing with PBS, the tissues were incubated with secondary antibody for one hour at room temperature, followed by DAPI staining (1:1000 in PBS) for 15 minutes, then mounted with Fluoromount. For 3D reconstruction of Sox2 expression in the otocyst, sections stained with Sox2 antibodies were processed for horseradish peroxidase-based visualization using a Vectastain kit and DAB.
Cell counting
EYFP/Myosin7a double positive hair cells and Myosin 7a-positive, EYFP-negative hair cells were counted separately on serial cross sections of the basal, middle and apical portions of the cochlear duct. Sets of serial sections were analyzed from each of three animals for each age. The number of sections counted for each cochlea ranged from 15 to 19 sections, covering between 360–456μm for each region. For analysis of the spiral ganglion, adjacent serial sections were stained with neuronal (NeuN) and glial (GFAP) markers. 8–10 sections were counted for each antibody on each sample, with 2–3 embryos analyzed for each age.
3D Reconstruction of Sox2-stained otocyst sections
To generate 3D models of Sox2-expressing regions in the developing E9.0, E9.25, and E9.5 otocyst, a series of anti-Sox2 stained otocyst sections processed with a Vectastain HRP kit at were imaged on a Zeiss Axio Explorer. The region of interest of each section was manually outlined and converted to binary image in Photoshop. Each set of stacked images was then aligned by ImageJ with the StackReg plugin (Thevenaz et al., 1998). Three-dimensional rendering of the otocyst was then generated by using Imaris (Bitplane).
Results
Fate mapping Sox2-expressing progenitor cells in the developing otocyst
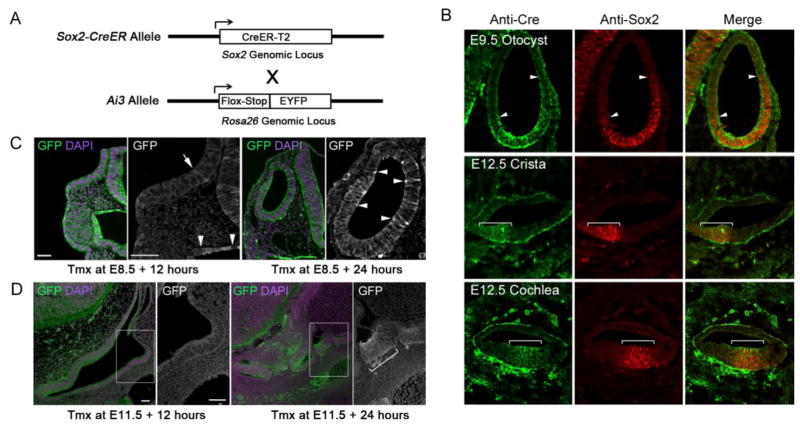
We first confirmed that the Sox2-CreERT2 knock-in mice used in our study (Arnold et al., 2011; Juuri et al., 2012; Figure 1A) faithfully reported on the normal expression of Sox2 protein in the inner ear. We sectioned E9.5 and E12.5 inner ear tissue from Sox2-CreERT2 mice and stained serial sections of the otocyst (E9.5) and the anterior crista, cochlear duct and saccule (E12.5) with antibodies to Sox2 and Cre proteins. The patterns of Sox2 and Cre immunoreactivity were indistinguishable in all sections examined (Figure 1B). The use of tamoxifen-inducible Cre recombinase for fate mapping progenitor populations is complicated in tissues such as the otocyst where gene expression patterns change rapidly. This is due to the delay between tamoxifen administration and onset of Cre-mediated recombination, and the stability of Cre recombinase (Sauer and Henderson, 1988). We attempted to estimate this delay in our Sox2-CreER experiments by analyzing the extent of recombination at 12 and 24 hours after tamoxifen administration at E8.5 and E11.5 (Figure 1C, D). We mated heterozygous male Sox2-CreERT2 mice with homozygous Ai3 Cre reporter female mice (Madisen et al., 2010; Figure 1A), and administered a single dose of 2.5mg tamoxifen and 2.5mg progesterone to the pregnant female mice by oral gavage at E8.5 or E11.5. Progesterone was administered with tamoxifen to prevent complications in late stages of pregnancy (Gridley and Groves, 2014). 12 hours after tamoxifen administration at E8.5, we saw large numbers of EYFP+ cells in the pharyngeal endoderm and neural tube, two regions that begin to express Sox2 after gastrulation (Pevny and Nicolis, 2010; Que et al., 2007). At this time, however, we saw only occasional strong EYFP+ cells in the invaginating otic pit (Figure 1C). After 24 hours, we observed significant numbers of EYFP+ cells in the otocyst (Figure 1C). We observed a similar result when tamoxifen was administered at E11.5: after 12 hours, we saw only small numbers of EYFP+ cells in the cochlear primordium or developing utricular macula (Figure 1D). However, we saw many more EYFP+ cells in both sensory organs 24 hours after the tamoxifen pulse (Figure 1D). This allowed us to estimate that the marking of Sox2-expressing otocyst progenitors by CreER commenced between 12–18 hours after administration of tamoxifen.
Figure 1.
(A) Diagram of the Sox2-CreER knock-in allele (Arnold et al., 2011). Heterozygous Sox2-CreER male mice were mated with homozygous Ai3 reporter female mice (Madisen et al., 2010). (B) Sections of E9.5 or E12.5 inner ears from Sox2-CreER mice, stained with antibodies to Cre and Sox2 protein. The co-localization of both proteins is shown with arrowheads and brackets in the otocyst (E9.5) and anterior crista and cochlea (E12.5). Note that the Cre protein is localized in the cytoplasm, as animals were not given tamoxifen. (C) Monitoring the speed of recombination in Sox2-CreER; Ai3 ROSA reporter mice. After a single dose of tamoxifen at E8.5, embryos were harvested at either 12 or 24 hours and processed for immunostaining with EYFP antibodies (green) and DAPI to label nuclei (magenta). After 12 hours, strong EYFP labeling can be seen in the neural tube, pharyngeal endoderm and epidermis ventral to the otic placode. A higher power image with brightly labeled EYFP cells in pharyngeal endoderm (arrowheads) shows a single bright EYFP cell in the invaginating otic placode (arrow). After 24 hours, more brightly labeled EYFP cells can be seen in the otocyst (arrowheads in higher power image) as well as pharyngeal endoderm and the neural tube. (D) We observed a similar delay in EYFP expression when tamoxifen was applied at E11.5 and embryos examined 12 or 24 hours later. After 12 hours, labeling in ear tissue is very weak (box shows a higher power view of the developing utricular macula), but after 12 hours, strong EYFP fluorescence is seen in both the cochlear primordium (c) and the developing utricle (box and higher power view; brackets). Scale bars = 50μm.
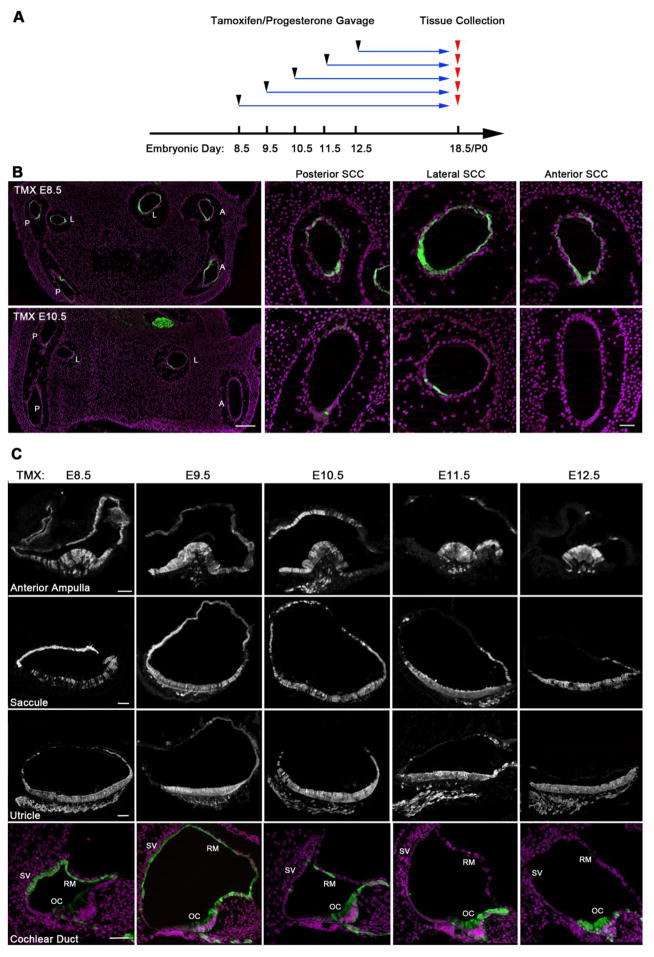
In all subsequent fate mapping experiments, we mated heterozygous male Sox2-CreERT2 mice with homozygous Ai3 Cre reporter female mice and gave tamoxifen and progesterone at ages corresponding to embryonic days 8.5–13.5 (Figure 1D). We sacrificed female mice at E18.5 or the day of birth and identified the patterns of EYFP immunoreactivity in the inner ears on sectioned material by immunostaining with antibodies to GFP and Myosin7a to identify hair cells. As expected, when mice received tamoxifen at any point between E8.5 and E13.5, we observed all sensory organs in the inner ear labeled with EYFP (Table 1). However, many non-sensory regions of the E18.5 inner ear were labeled with EYFP when tamoxifen was administered between E8.5 and E10.5 (Table 1). For example, we saw large numbers of cells in the anterior, posterior and lateral semicircular canals labeled with EYFP when tamoxifen was administered at E8.5, but only a few scattered canal cells expressed EYFP when tamoxifen was administered at E10.5 (Figure 2; Figure 2A).
Table 1.
Summary of the number and distribution of Sox2 descendants (GFP-labeled cells) in different parts of the inner ear after administration of a single pulse of tamoxifen between embryonic days 8.5–13.5 and analyzed at E18.5 or P0. The approximate proportion of GFP-labeled cells in each structure is indicated by plus signs. ++++ = 90–100% labeling, +++ = 60–90% labeling, ++ = 30–60% labeling, + = 5–30% labeling. Regions with fewer than 5% labeled cells are designated with a dash.
| Ear component | Region | Day of Tamoxifen Pulse | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| E8.5 | E9.5 | E10.5 | E11.5 | E12.5 | E13.5 | ||
| Anterior Semicircular Canal and Ampulla | Non-sensory | ++ | ++ | + | − | − | − |
| Sensory (Anterior Crista) | +++ | +++ | +++ | ++ | ++ | ++ | |
| Lateral Semicircular Canal and Ampulla | Non-sensory | ++++ | ++ | + | − | − | − |
| Sensory (Lateral Crista) | +++ | +++ | +++ | ++ | ++ | ++ | |
| Posterior Semicircular Canal and Ampulla | Non-sensory | + | ++ | + | − | − | |
| Sensory (Posterior Crista) | + | +++ | ++++ | ++++ | ++++ | ++++ | |
| Utricle | Non-sensory | +++ | ++ | + | − | − | − |
| Sensory (Utricular Macula) | +++ | ++++ | ++++ | ++++ | ++++ | ++++ | |
| Saccule | Non-sensory | ++++ | ++++ | +++ | − | − | − |
| Sensory (Saccular Macula) | + | ++++ | ++++ | ++++ | ++++ | ++++ | |
| Cochlear Duct - Reissner’s membrane and stria vascularis | Apical Turn | ++++ | ++++ | ++ | + | − | − |
| Middle Turn | ++++ | +++ | + | − | − | − | |
| Basal Turn | ++++ | +++ | + | − | − | − | |
| Cochlear Duct - Greater Epithelial Ridge | Apical Turn | + | ++++ | ++++ | ++++ | ++++ | ++++ |
| Middle Turn | + | +++ | +++ | ++++ | ++++ | ++++ | |
| Basal Turn | + | ++ | ++ | +++ | ++++ | ++++ | |
| Cochlear Duct –Organ of Corti | Apical Turn | + | +++ | +++ | +++ | ++++ | ++++ |
| Middle Turn | − | +++ | +++ | +++ | ++++ | ++++ | |
| Basal Turn | − | − | + | ++ | ++++ | ++++ | |
| Spiral Ganglion | Neurons | ++++ | ++++ | ++ | + | − | − |
| Glial Cells | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ | |
Figure 2.
(A) Experimental scheme for lineage tracing of Sox2-expressing cells. Homozygous Ai3 reporter female mice mated with heterozygous Sox2-CreER male mice were administered with tamoxifen and progesterone on embryonic day 8.5, 9.5, 10.5, 11.5, 12.5 or 13.5 before being sacrificed at embryonic day 18 or the day of birth. (B) Significant numbers of Sox2-expressing cells labeled by EYFP contribute to the non-sensory epithelium of the posterior (P), lateral (L) and anterior (A) semicircular canals (SCC) when tamoxifen is administered at E8.5, but very few labeled cells are observed at E10.5. DAPI-labeled nuclei are shown with magenta. Scale bar = 200μm (low power images on left) and 50μm (high power images). (C) Sox2-expressing cells labeled by EYFP contribute to the non-sensory epithelium of all sensory organs when labeled at E8.5, becoming restricted to sensory epithelium when labeled at E12.5. Examples are shown from the anterior ampulla, saccule, utricle and the cochlear duct. In the cochlea, the stria vascularis (SV) and Reissner’s membrane (RM) are labeled at E8.5 and 9.5, with significantly less label observed when tamoxifen is applied at later times. In contrast, the organ of Corti (OC) is only sparsely labeled at early time points (E8.5, 9.5), becoming much more strongly labeled at later times. Scale bars = 50μm.
Sox2-expressing progenitors in the early otocyst contribute to both non-sensory and sensory tissue in each inner ear end-organ
Sox2 is initially expressed in both non-sensory and prosensory progenitor cells in the early (E8.5-E9.5) otocyst but gradually becomes restricted to prosensory progenitors (Neves et al., 2007; Mak et al., 2009). We used Sox2-CreER mice to visualize this phenomenon in greater detail in each sensory end-organ. We examined serial sections taken from neonatal animals that received a single dose of tamoxifen at different ages between E8.5-E12.5 (Figure 2B). A summary of the data described in the following sections is presented in Figure 5. Animations of light sheet fluorescent microscope images of cleared specimens of neonatal inner ears from animals that received a single dose of tamoxifen at different ages between E8.5-E12.5 are presented as Supplementary Movies 1–5.
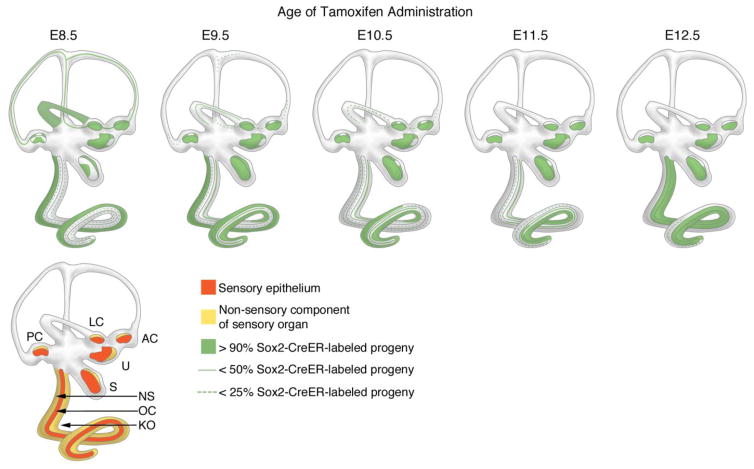
Figure 5.
Summary of lineage labeling of Sox2-expressing progenitors with Sox2-CreER; Ai3 mice. Embryonic litters, labeled with a single dose of tamoxifen between E8.5 and E12.5 were analyzed at embryonic day 18 or the day of birth. The spatial distribution of EYFP is shown in green shading. Regions of the semicircular canals or cochlear duct showing moderate labeling are indicated with green lines; regions showing sparse labeling are indicated with dashed green lines. The lower cartoon shows a summary of the sensory organs of the ear divided into their sensory components (orange) and non-sensory epithelium (yellow). PC, LC, AC: Posterior, lateral and anterior cristae. U and S: utricle and saccule. NS: Non-sensory epithelium of the cochlear duct, excluding Kölliker’s organ (KO). OC: Organ of Corti.
Ampullary organs
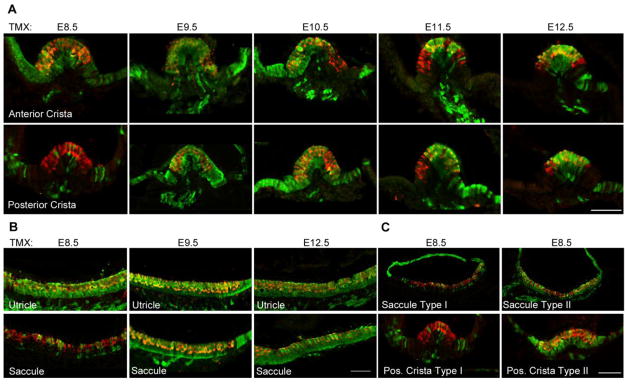
Large numbers of cells in the roof and sides of each ampulla were labeled with EYFP when we gave animals tamoxifen at E8.5, 9.5 and 10.5 (Figure 2B, Figure 3A). The anterior and lateral ampullae showed very similar patterns of widespread labeling, consistent with their derivation from a single progenitor population in the anterior region of the otocyst (Morsli et al., 1998; Wu and Kelley, 2012). The posterior ampulla had fewer numbers of labeled non-sensory cells at each age, although still over 50%. When we administered tamoxifen at E11.5, significantly fewer non-sensory cells were labeled in each ampulla, and at E12.5, only occasional EYFP+ cells could be seen in the roof of the ampullae. Non-sensory tissue on either side of each sensory crista was also strongly labeled when we gave tamoxifen between E8.5 and E10.5. At E11.5 and E12.5, only small regions of this peri-cristal epithelium were labeled (Figure 2B, Figure 3A). Hair cells and supporting cells were labeled in all cristae at all ages of tamoxifen administration. The one exception to this was the posterior crista, in which approximately half of all embryos examined had very few EYFP+ cells when tamoxifen was give at E8.5 (Figure 3C; type I). The remaining samples had similar labeling of hair cells and supporting cells as the other cristae (Figure 3C; type II). We also observed small regions of hair cells and supporting cells at the lateral edges of the cristae that were unlabeled with EYFP when tamoxifen was administered between E10.5-E12.5 (Figure 3A).
Figure 3.
(A) Contribution of Sox2-expressing cells labeled by EYFP (green) to the anterior and posterior cristae when tamoxifen is applied between E8.5 and E12.5. Strong EYFP label is seen in both the cristae and the non-sensory epithelium adjacent to the cristae when tamoxifen is applied between E8.5 and E10.5. The anterior and lateral cristae gave essentially similar results; consequently only the anterior crista is illustrated. At later times, the majority of EYFP labeling is confined to the cristae proper. Myosin7a labeling of hair cells is shown in red. Scale bar = 50μm. (B) Sox2-expressing cells labeled by EYFP mark the entire sensory maculae of the utricle at all ages when tamoxifen was administered. The saccular macula was often sparsely labeled at E8.5, but almost completely labeled at later times. Scale bar = 50μm. (C) We observed two distinct patterns of lineage labeling of the posterior crista and saccular macula when tamoxifen was applied at E8.5. In approximately half the embryos, sparse labeling was observed (Type I, images are from the same higher power images of the sparsely labeled sensory organs in A and B), whereas in the other half of embryos, almost complete labeling was observed (Type II). Scale bar = 50μm.
Otolithic organs
Almost the entire epithelium of the saccule was labeled when we gave tamoxifen between E8.5 and 10.5. At E11.5, the most dorsal region of the roof of the saccule was unlabeled by EYFP, and at E12.5, only non-sensory tissue immediately adjacent to the saccular macula was labeled with EYFP (Figure 2B). A similar trend was seen for the utricle, with many non-sensory regions labeled when we gave tamoxifen between E8.5 and E10.5. However, in this case, the most dorsal cells in the roof of the utricle were never labeled, even at the earliest ages of tamoxifen treatment, and only the utricular macula was labeled with EYFP when we gave tamoxifen at E12.5 (Figure 2B). The majority of hair cells and supporting cells in the utricular macula were labeled at all ages of tamoxifen administration (Figure 2B, Figure 3B). We observed the same pattern in the saccular macula (Figure 2B, Figure 3B), except that when we gave tamoxifen at E8.5, about half of the samples showed little EYFP labeling in the macula (Figure 3B,C; type I) with the remaining samples showing robust EYFP labeling in the macula (Figure 3B, C; type II).
Cochlear duct
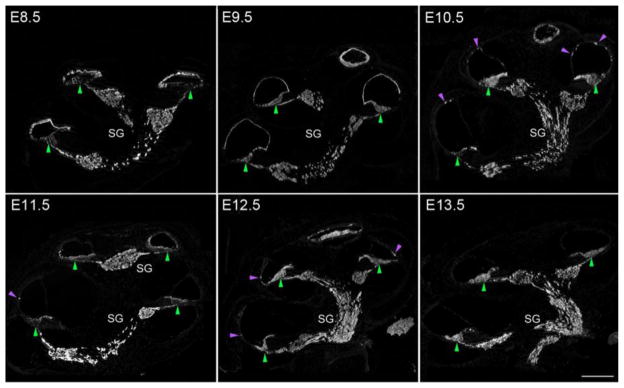
We observed EYFP labeling in most of the non-sensory regions of the cochlear epithelium when we gave tamoxifen at E8.5 and E9.5. We saw many EYFP cells in the inner and outer sulci, the epithelial layer of Reissner’s membrane and the lateral wall of the cochlear duct including the stria vascularis (Figure 2B, Figure 4). We observed no EYFP labeling in mesenchymal components of the cochlear duct such as the spiral ligament, consistent with the absence of Sox2 expression in peri-otic mesenchyme. We continued to observe significant EYFP expression in non-sensory regions of the cochlear duct when we gave tamoxifen at E10.5 and E11.5, albeit in smaller numbers of cells (Table 1). When we gave tamoxifen at E12.5, we saw EYFP labeling restricted to the organ of Corti and the adjacent Kölliker’s organ (Figure 2B, Figure 4).
Figure 4.
Dynamic EYFP labeling of Sox2-expressing cells in the cochlear duct when tamoxifen is applied between E8.5 and E13.5. Two patterns of labeling can be observed in these panels – first, non-sensory structures are labeled strongly at E8.5, with labeling gradually disappearing when single doses of tamoxifen are applied on one of the next five days. Small patches of labeled non-sensory epithelium are indicated with magenta arrowheads. Labeling of the non-sensory regions disappears from basal regions first (by E10.5), with apical regions losing labeling at later times (E12.5). Second, the organ of Corti (green arrowheads) is initially sparsely labeled, especially at the base, becoming more strongly labeled at later ages, with almost complete labeling being seen in the apex from E9.5 onwards and in the base from E12.5 onwards. Scale bar = 200μm. The spiral ganglion is labeled at all ages (see Figure 8).
The organ of Corti and Kölliker’s organ arise from a boundary of Sox2+ and Sox2− progenitors in the early ventromedial otocyst
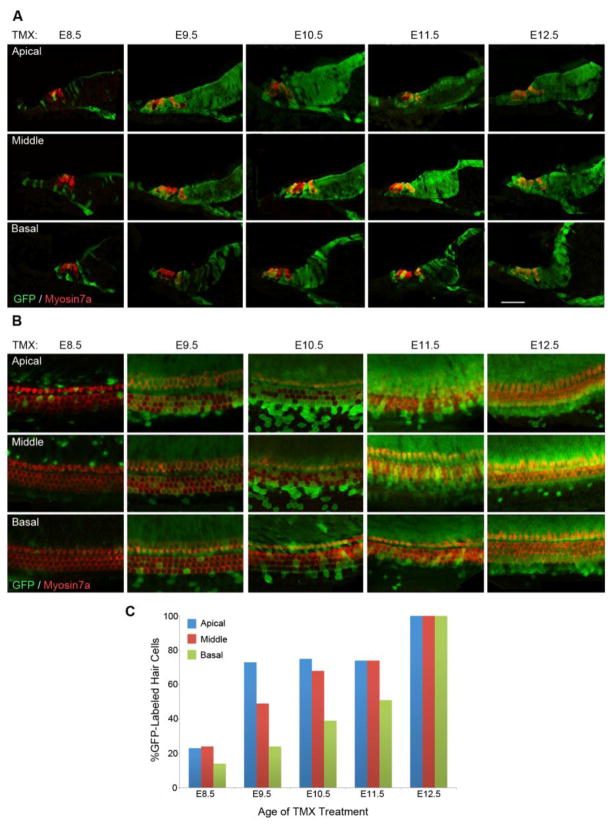
We were surprised to observe only sparse labeling of the organ of Corti and Kölliker’s organ when we gave tamoxifen at E8.5, despite robust EYFP labeling of tissue in all other regions of the cochlear duct (Figure 2B, Figure 4). These regions began to be labeled more strongly at later stages, starting in the apex and middle turns of the duct when we gave tamoxifen at E9.5, but the organ of Corti and Kölliker’s organ were still not fully labeled in the basal part of the duct until E11.5 (Figure 4). We therefore performed a detailed examination of the contribution of Sox2-expressing cells to the organ of Corti and its surrounding non-sensory tissue. We examined sections and whole mount preparations of cochleae from E18.5 embryos that received a single dose of tamoxifen between E8.5 and E12.5.
When we gave tamoxifen at E8.5, small numbers of EYFP+ supporting cells of most types (border cells, inner phalangeal cells, pillar cells, Deiters’ cells, and Hensen’s cells) were present in apical regions of the cochlea (Figure 6A), while in middle and basal turns, Claudius’ cells and Böttcher’s cells were also labeled. In apical regions, we observed more EYFP+ cells in the non-sensory cells of Kölliker’s organ than in other regions of the cochlea (Figure 6A, B). Similarly, we observed small numbers of EYFP+ inner and outer hair cells in the apical region of the cochlea, but almost no labeled hair cells in middle or basal turns (Figure 6C). When we gave tamoxifen at E9.5, almost all supporting cells and Kölliker’s organ cells were labeled with EYFP in the apical and middle turns of the cochlea, and many inner and outer hair cells were labeled. We also observed EYFP+ hair cells, supporting cells and Kölliker’s organ cells in the base of the cochlea, but at lower frequency. At E10.5 and E11.5, we saw increasing numbers of EYFP+ hair cells and supporting cells in the basal turn of the cochlea, but it was not until we gave tamoxifen at E12.5 that all turns of the cochlear duct were comparably labeled (Figure 6A–C).
Figure 6.
Dynamic EYFP labeling of Sox2-expressing cells in the organ of Corti when tamoxifen is applied between E8.5 and E12.5. (A) Representative sections are shown from apical, middle and basal turns of the cochlea from embryos produced by administering single tamoxifen pulses between E8.5 and E12.5. By E9.5, tamoxifen pulses label most hair cells and supporting cells in apex of the organ of Corti and greater epithelial ridge, but complete labeling of these structures is not seen in the base of the cochlear duct until E12.5. (B) The same experiment as shown in (A), except whole mount preparations of the cochlear duct are shown. In both cases, hair cells are labeled with Myosin7a antibodies (red). (C) Quantification of EYFP-labeled hair cells in apical, middle and basal turns when tamoxifen is applied between E8.5 and E12.5. A single representative experiment is shown, with between 50 and 125 hair cells being quantified for each apical, middle and basal turn at each time point. Scale bars in A and B = 20μm.
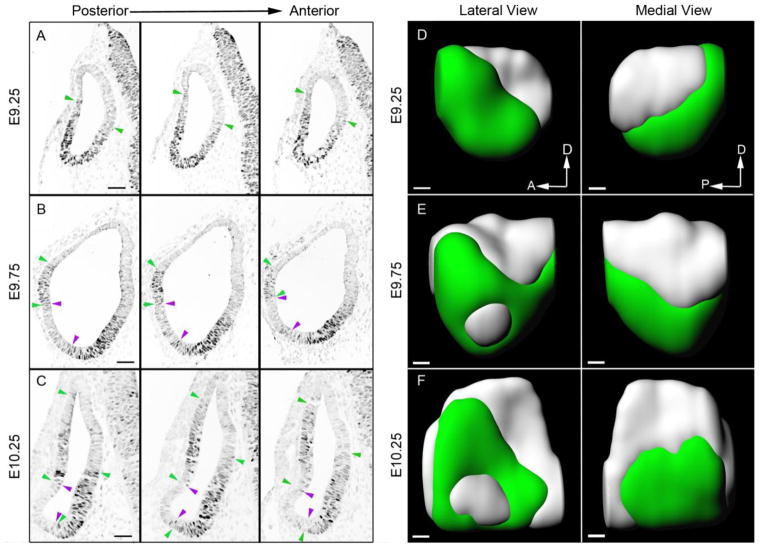
These results suggest that, unlike other sensory patches in the inner ear, many organ of Corti and Kölliker’s organ progenitors do not express Sox2 at early stages of ear development. In particular, our results suggest that these progenitors span a boundary of Sox2+ and Sox2− cells, with progenitors of the future apex of the organ of Corti and Kölliker’s organ located in a predominantly Sox2+ region, and basal progenitors located in a predominantly Sox2− region. To visualize this in more detail, we performed an analysis of Sox2 expression between E9.25 and E10.25. We sectioned a series of wild type embryos whose ages spanned the ninth and early tenth days of gestation at 8μm intervals, stained them with Sox2 antibodies and reconstructed the resulting images in three dimensions (Figure 7). Animations showing 360° rotations of each reconstructed image are shown in Supplementary Movies 6–8. The embryos came from litters collected 12 hours apart; for convenience, we refer to the three ages analyzed as E9.25, E9.75 and E10.25 to order them with respect to one another. Sox2 is expressed most strongly in the ventral and anterior regions of the E9.25 otocyst. The most anterior regions of the otocyst expressed Sox2 throughout the dorso-ventral axis, but more posterior regions of the otocyst did not express Sox2 in dorsal regions (Figure 7A, D). Sox2 expression was confined more ventrally on the medial side of the otocyst compared to the lateral side (Figure 7A, D). At E9.75, a largely Sox2-negative region appeared in the middle of the otocyst on the lateral side (Figure 7B, E) and this region expanded and continued to down-regulate Sox2 over the next 12 hours (Figure 7C, F). The medial side of the otocyst continued to express Sox2 in its ventral half at E9.75 and E10.25. Together, these data are consistent with the location of progenitors of the organ of Corti on the medial side of the otocyst, midway along the anterior-posterior axis of the otocyst, and with the roof of the cochlear duct forming from the Sox2-negative region on the ventro-lateral surface of the otocyst.
Figure 7.
Analysis of Sox2 expression in the otocyst on the ninth and tenth days of embryonic development. Three different ages of mouse embryo, denoted as E9.25, 9.75 and 10.25, were sectioned and stained with antibodies to Sox2. Panels A-C show three adjacent serial sections located approximately in the middle region along the anterior-posterior axis of the otocyst. Sox2-expressing regions are indicated between green arrowheads; Sox2-negative regions are indicated between magenta arrowheads. At the intermediate stage (E9.75), occasional Sox2-positive cells can be observed in regions that have largely down-regulated Sox2. Scale bars = 50μm. Panels D-F show three-dimensional reconstructions of the otocysts shown in A-C, viewed from the medial and lateral faces. Regions of cells expressing Sox2 are shown in green, with Sox2-negative regions shown in light grey. The most anterior and posterior sections are omitted from the three-dimensional models. Scale bars = 50μm.
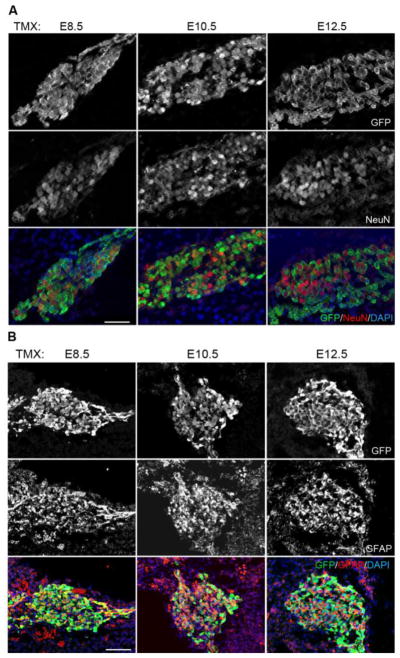
The contribution of Sox2+ neurosensory progenitors to the spiral ganglion declines by E12.5
Sox2 is expressed in the anterior and ventral neurosensory region of the otocyst that will contribute to the cochleovestibular ganglion as well as the utricular and saccular maculae (Evsen et al., 2013; Raft et al., 2007; Wu and Kelley, 2012). As neuronal progenitors express neurogenic markers such as Neurog1 and NeuroD and delaminate from the otocyst, they down-regulate Sox2. We examined the contribution of Sox2-expressing cells in the neurosensory region to the spiral ganglion in E18.5 embryos derived from litters exposed to tamoxifen at E8.5, E10.5 or E12.5 (Figure 8A). When we gave tamoxifen at E8.5, we saw almost all (96.8 +/− 2.4%) NeuN+ spiral ganglion neurons expressing EYFP. The number of EYFP-labeled neurons declined when we gave tamoxifen at E10.5 (59.9 +/− 8.8%), and by E12.5 almost no NeuN+ neurons were labeled with EYFP (Figure 8A). The EYFP+ NeuN− cells observed in the spiral ganglion are likely to be neural crest-derived glial cells. To confirm this, we also examined sections of the spiral ganglion with the glial marker GFAP. Consistent with the expression of Sox2 in both pre-migratory and migrating neural crest cells, we observed GFAP+, EYFP+ cells in the spiral ganglion at all ages examined (Figure 8B).
Figure 8.
Contribution of Sox2-expressing progenitors to the neurons and glia of the spiral ganglion. (A) A single pulse of tamoxifen at E8.5 labels almost all NeuN+ neurons in the spiral ganglion with EYFP, whereas in labeling carried out at E12.5, almost no neurons are labeled with EYFP. (B) In contrast, GFAP-expressing glia of the spiral ganglion, which are derived from Sox2-expressing neural crest progenitors, are labeled by the Sox2-CreER transgene at all stages between E8.5 and E12.5. Scale bar = 40μm.
Discussion
The role and regulation of Sox2 in the development of sensory and non-sensory regions of the ear
Sox2 is commonly cited as an archetypal stem cell marker; it is a core pluripotency gene and is found in many stem cell populations in the embryo and adult (Abdelalim et al., 2014; Arnold et al., 2011; Boyer et al., 2005; Kang and Hebert, 2012; Papanayotou et al., 2008; Taranova et al., 2006). It is expressed in all prosensory patches in the developing inner ear (Neves et al., 2013b), is necessary for their differentiation (Kiernan et al., 2005) and is sufficient to direct the formation of neuroblasts and sensory patches when ectopically expressed (Evsen et al., 2013; Neves et al., 2011; Neves et al., 2012; Pan et al., 2013). However, Sox2 is expressed quite broadly when the inner ear is first induced (Mak et al., 2009; Neves et al., 2007), and in this fate-mapping study, we show that during the first two days of ear development, Sox2-expressing cells have a wide range of fates including both sensory, non-sensory and neuronal derivatives. Surprisingly, we find that much of the organ of Corti derives from a Sox2-negative region in the dorsal part of the medial side of the otocyst between day 9 and 10, and Sox2 only becomes restricted to sensory lineages of the inner ear after embryonic day 11. This suggests that Sox2 serves additional functions early in the development of the inner ear.
It is not clear what signals are responsible for the early broad domain of Sox2 expression in the invaginating otic placode and otocyst. A variety of signals can induce Sox2 and Sox3 expression during nervous system induction, such as FGFs or the modulation of BMP and Wnt signals (Litsiou et al., 2005; Streit et al., 2000; Streit and Stern, 1999; Takemoto et al., 2006), and at least some of these signals also regulate Sox2 expression in the otic placode through their induction of Sox3 (Abello et al., 2010). It is also possible that Wnt signaling regulates Sox2 in a similar manner to its regulation of Jag1 in the otic placode (Estrach et al., 2006; Jayasena et al., 2008). Studies in which Notch signaling is blocked during early ear development show that at least some prosensory patches are induced (Daudet et al., 2007; Kiernan et al., 2006; Pan et al., 2010), suggesting that this pathway is more responsible for the maintenance of sensory fate rather than its initial induction. This suggests that as-yet unidentified signals suppress Sox2 and Jag1 expression in many parts of the ear between E8.5 and E10.5. However, in prosensory patches, an inductive process in which Jag1-Notch signaling maintains Sox2 expression and Sox2 confers competence for hair cell differentiation is sufficient to stabilize prosensory identity. Since ectopic expression of Jag1, Sox2 or an activated Notch receptor at early stages of ear development is sufficient to induce ectopic prosensory patches (Hartman et al., 2010; Neves et al., 2011; Neves et al., 2012; Neves et al., 2013b; Pan et al., 2013; Pan et al., 2010), this suggests that the inner ear epithelium exists in a transiently bistable state, in which expression of activating or inhibitory signals can switch non-sensory fates to prosensory ones and vice versa. It is possible that cells at the periphery of the prosensory patches undergo switching between Sox2 expression and Sox2 repression as the final boundaries of each prosensory patch are defined. Our observation that some hair cells and supporting cells at the lateral edges of the cristae are not lineage-labeled when tamoxifen is applied between E10.5-E12.5 provides circumstantial support for this idea, as these ages mark the restriction in Sox2 expression from the entire ampulla epithelium to the cristae.
What regions of the otocyst give rise to the organ of Corti?
The cochlear duct grows out from the ventral otocyst starting at embryonic day 10.5 (Brown et al., 2015; Li et al., 1978; Riccomagno et al., 2005; Wu and Kelley, 2012). In contrast to the vestibular sensory organs (Morsli et al., 1998; Raft and Groves, 2015; Wu and Kelley, 2012; Wu and Oh, 1996), there is a dearth of fate-mapping or gene expression data that describes the early location of progenitors of the organ of Corti (Wu and Kelley, 2012). Studies using Neurog1-CreER transgenic mice show that some cells in the antero-ventral region of the otocyst give rise to non-sensory regions of the cochlear duct, but that almost no Neurog1-expressing progenitors contribute to the organ of Corti itself (Koundakjian et al., 2007; Raft et al., 2007). Fate mapping of Wnt-responsive progenitors using a CreER mouse line driven by Lef/TCF binding sites (TOP-CreER), and more recently a Gbx2-CreER mouse show that some Wnt-responsive progenitors in dorsal parts of the medial wall of the otocyst between E9.5 and 11.5 will contribute to sensory epithelium of the cochlear duct (Brown et al., 2015; Riccomagno et al., 2005). One recent study suggested that neural tube-derived cells may contribute to the inner ear, including the cochlea, at very early stages in its development (Freyer et al., 2011). Since Sox2 is expressed strongly in the neural tube, it is possible that some of our labeled cells may derive from outside the otocyst. However, the widespread expression of Sox2 within the otocyst at these early stages (Mak et al., 2009) suggests it is more likely that our labeled cells are bona fide otocyst derivatives, especially as we see widespread labeling in regions of the ear that were unlabeled in the study by Freyer and colleagues using Pax2-Cre mice.
Our data suggest that the apical-basal axis of the organ of Corti can be mapped onto a region that spans a Sox2-expressing domain in the ventral otocyst (producing mostly apical regions of the organ of Corti) and a Sox2-negative domain located more dorsally in the otocyst that will generate mostly basal region of the organ of Corti. This region was also identified as containing cochlear progenitors in a recent study using Wnt-responsive Cre-ER mice (Brown et al., 2015; Riccomagno et al., 2005). These divisions are not absolute, however, as our data suggests there is some cell mixing between these regions, particularly among supporting cell progenitors (Figure 6A). Surprisingly, we found that far fewer hair cells were labeled at early ages in all regions of the organ of Corti by the Sox2-CreER mouse than supporting cells (Figure 6A), although we currently have no explanation for these differences.
Our Sox2-CreER transgenic mice offer a complementary fate map of the medial otocyst compared to a recently published TOP-CreER mouse study (Brown et al., 2015): between E9.0 and E10.0, Sox2 is expressed in the ventral region of the medial otocyst (Figure 7), whereas the TOP-CreER transgene that marks Wnt-responsive cells is confined to the dorsal half of the medial otocyst (Brown et al., 2015; Riccomagno et al., 2005). Fate-mapping the medial wall of the otocyst with the two mouse lines confirms that much of the organ of Corti derives from a region on either side of the dorso-ventral equator. Although the TOP-CreER mice used by Brown and colleagues gave most Cre expression in the dorsal otocyst, the fact that even the most apical regions of the cochlea were labeled in their study suggests that a low level of Wnt-responsive Cre activity might be present in the more ventral regions of the medial otocyst and that this was sufficient to completely label the apical parts of the organ of Corti (Brown et al., 2015).
We found that non-sensory regions of the cochlear duct, including Reissner’s membrane and the lateral wall, were labeled by tamoxifen pulses at E8.5 and E9.5, with labeling declining sharply after these ages in a basal-apical gradient (Figure 5). These data are consistent with the expression pattern of Sox2 in the ventral otocyst between E9.25 and 10.25 (Figure 7), where the ventrolateral region of the otocyst is initially Sox2-positive, with Sox2 being gradually down-regulated over the next 24 hours. This ventrolateral domain has been shown to express transcription factors such as Otx2 and Ecel1 (Bok et al., 2007; Lin et al., 2005; Riccomagno et al., 2002) that become restricted to Reissner’s membrane. Recent data suggest that one function of Otx2 is to repress prosensory fates in the ventrolateral region of the cochlear duct, as Otx2 conditional mutant mice develop ectopic Sox2+ prosensory regions hair cells in place of Reissner’s membrane (Vendrell et al., 2015).
Our data reinforce an emerging model suggesting that by the 11th day of gestation, the ventral portion of the mouse otocyst contains four gene expression domains which will transform into the main divisions of the cochlear duct as it grows out from the ventral otocyst. The prosensory domain derives from the ventromedial region of the otocyst and expresses markers such as Sox2 and Jag1 (Basch et al., 2011; Kiernan et al., 2005; Lewis et al., 1998; Morrison et al., 1999; Murata et al., 2006; Ohyama et al., 2010). Kölliker’s organ derives from the antero-ventral region of the otocyst and expresses Lfng and Fgf10, and also overlaps somewhat with Sox2 expression (Morsli et al., 1998; Ohyama et al., 2010; Pauley et al., 2003; Urness et al., 2015). The outer sulcus derives from the postero-ventral region of the otocyst that expresses a stripe of Bmp4 (Morsli et al., 1998; Ohyama et al., 2010; Roberts et al., 2012); and the roof of the cochlear duct, which will ultimately form Reissner’s membrane, derives from the ventro-lateral otocyst that expresses Otx2 (Bok et al., 2007; Koo et al., 2009; Lin et al., 2005; Morsli et al., 1999; Vendrell et al., 2015). As the cochlear duct grows, these four domains elongate in a manner reminiscent of toothpaste with four stripes being squeezed out of a tube. This pattern of growth preserves their respective territories along the length of the cochlear duct (Figure 9). It will be of great interest to understand how these four domains are induced and restricted to their respective regions of the otocyst, and to determine whether Sox2 has additional roles in the specification of non-sensory epithelium in addition to its role in the formation of inner ear sensory organs.
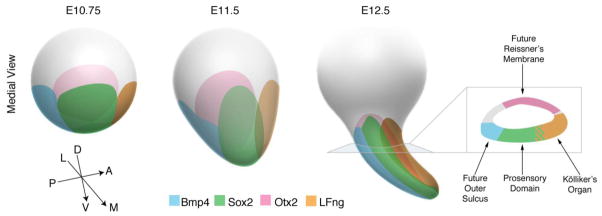
Figure 9.
Schematic summary of the development of the otocyst between E10–12 with regard to the origin of the cochlea. All views are shown from the medial side of the otocyst. At E10.75, four domains can be seen on the basis of gene expression: A medial Sox2+ domain (green; closest to the viewer) that includes most of the progenitors for the organ of Corti and Kölliker’s organ, a lateral Otx2+ domain (pink; furthest away from the viewer) that will give rise to Reissner’s membrane, an anterior LFng+ domain (brown) that will give rise to Kölliker’s organ (as well as neurons and hair cells and supporting cells of the utricle and saccule; Raft et al., 2007), and a posterior stripe of Bmp4 expression (blue) that will form the future outer sulcus. As the ventral region of the otocyst grows out to form the beginnings of the cochlear duct, all four expression domains grow out in proportion to each other, so that by E12.5, they delineate four distinct regions of the cochlear duct. N.B. At all stages, Sox2 expression overlaps partially with the LFng domain.
Supplementary Material
Animations of light sheet fluorescent microscope images of cleared specimens of neonatal inner ears from animals that received a single dose of tamoxifen at different ages between E8.5-E12.5 are presented as Supplementary Movies 1 (tamoxifen at E8.5), 2 (tamoxifen at E9.5), 3 (tamoxifen at E10.5), 4 (tamoxifen at E11.5), and 5 (tamoxifen at E12.5). A gradual restriction of EYFP fluorescence from non-sensory to sensory regions can be observed across this time range
Animations of 360° rotations of reconstructed images of Sox2 expression in the E9.25 (Movie 6), E9.75 (Movie 7) and E10.25 (Movie 8) otocysts shown in Figure 7. The elimination of Sox2 expression on the ventro-lateral side of the otocyst can be observed during this time window. Anterior and posterior axes are denoted on each movie. Each movie begins with a lateral view and rotates to show the medial view of the otocyst.
Highlights.
Sox2 is initially expressed in both sensory and non-sensory tissues
Sox2 undergoes a large-scale restriction to sensory regions over a period of 48 hours.
The ear progenitors that give rise to the organ of Corti arise from the medio-ventral region of the otocyst
The basal and apical regions of the organ of Corti span a Sox2-expressing domain
Acknowledgments
This work was supported by NIH DC006185, NIH DC013072S1 and DOD W81XWH1020116 (A.K.G.). We thank Konrad Hochedlinger for providing Sox2-CreER mice in advance of publication. We thank Hongyuan Zhang and Huiling Li for excellent technical assistance, members of the Groves lab for helpful discussions and Daisy Chung (daisychung.com) for participating in preliminary experiments on this project while an undergraduate at Rice University, and for providing the summary diagrams in Figures 5 and 9.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdelalim EM, Emara MM, Kolatkar PR. The SOX transcription factors as key players in pluripotent stem cells. Stem cells and development. 2014;23:2687–2699. doi: 10.1089/scd.2014.0297. [DOI] [PubMed] [Google Scholar]
- Abello G, Khatri S, Radosevic M, Scotting PJ, Giraldez F, Alsina B. Independent regulation of Sox3 and Lmx1b by FGF and BMP signaling influences the neurogenic and non-neurogenic domains in the chick otic placode. Dev Biol. 2010;339:166–178. doi: 10.1016/j.ydbio.2009.12.027. [DOI] [PubMed] [Google Scholar]
- Adam J, Myat A, Le Roux I, Eddison M, Henrique D, Ish-Horowicz D, Lewis J. Cell fate choices and the expression of Notch, Delta and Serrate homologues in the chick inner ear: parallels with Drosophila sense-organ development. Development. 1998;125:4645–4654. doi: 10.1242/dev.125.23.4645. [DOI] [PubMed] [Google Scholar]
- Arnold K, Sarkar A, Yram MA, Polo JM, Bronson R, Sengupta S, Seandel M, Geijsen N, Hochedlinger K. Sox2(+) adult stem and progenitor cells are important for tissue regeneration and survival of mice. Cell Stem Cell. 2011;9:317–329. doi: 10.1016/j.stem.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basch ML, Ohyama T, Segil N, Groves AK. Canonical Notch signaling is not necessary for prosensory induction in the mouse cochlea: insights from a conditional mutant of RBPjkappa. J Neurosci. 2011;31:8046–8058. doi: 10.1523/JNEUROSCI.6671-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bok J, Chang W, Wu DK. Patterning and morphogenesis of the vertebrate inner ear. Int J Dev Biol. 2007;51:521–533. doi: 10.1387/ijdb.072381jb. [DOI] [PubMed] [Google Scholar]
- Boyer LA, Lee TI, Cole MF, Johnstone SE, Levine SS, Zucker JP, Guenther MG, Kumar RM, Murray HL, Jenner RG, Gifford DK, Melton DA, Jaenisch R, Young RA. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;122:947–956. doi: 10.1016/j.cell.2005.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooker R, Hozumi K, Lewis J. Notch ligands with contrasting functions: Jagged1 and Delta1 in the mouse inner ear. Development. 2006;133:1277–1286. doi: 10.1242/dev.02284. [DOI] [PubMed] [Google Scholar]
- Brown AS, Rakowiecki SM, Li JY, Epstein DJ. The cochlear sensory epithelium derives from Wnt responsive cells in the dorsomedial otic cup. Dev Biol. 2015;399:177–187. doi: 10.1016/j.ydbio.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daudet N, Ariza-McNaughton L, Lewis J. Notch signalling is needed to maintain, but not to initiate, the formation of prosensory patches in the chick inner ear. Development. 2007;134:2369–2378. doi: 10.1242/dev.001842. [DOI] [PubMed] [Google Scholar]
- Eddison M, Le Roux I, Lewis J. Notch signaling in the development of the inner ear: lessons from Drosophila. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:11692–11699. doi: 10.1073/pnas.97.22.11692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrach S, Ambler CA, Lo Celso C, Hozumi K, Watt FM. Jagged 1 is a beta-catenin target gene required for ectopic hair follicle formation in adult epidermis. Development. 2006;133:4427–4438. doi: 10.1242/dev.02644. [DOI] [PubMed] [Google Scholar]
- Evsen L, Sugahara S, Uchikawa M, Kondoh H, Wu DK. Progression of neurogenesis in the inner ear requires inhibition of Sox2 transcription by neurogenin1 and neurod1. J Neurosci. 2013;33:3879–3890. doi: 10.1523/JNEUROSCI.4030-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freyer L, Aggarwal V, Morrow BE. Dual embryonic origin of the mammalian otic vesicle forming the inner ear. Development. 2011;138:5403–5414. doi: 10.1242/dev.069849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gridley T, Groves AK. Overview of genetic tools and techniques to study Notch signaling in mice. Methods in molecular biology. 2014;1187:47–61. doi: 10.1007/978-1-4939-1139-4_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman BH, Reh TA, Bermingham-McDonogh O. Notch signaling specifies prosensory domains via lateral induction in the developing mammalian inner ear. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:15792–15797. doi: 10.1073/pnas.1002827107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayasena CS, Ohyama T, Segil N, Groves AK. Notch signaling augments the canonical Wnt pathway to specify the size of the otic placode. Development. 2008;135:2251–2261. doi: 10.1242/dev.017905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang W, Hebert JM. A Sox2 BAC transgenic approach for targeting adult neural stem cells. PloS one. 2012;7:e49038. doi: 10.1371/journal.pone.0049038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiernan AE. Notch signaling during cell fate determination in the inner ear. Seminars in cell & developmental biology. 2013;24:470–479. doi: 10.1016/j.semcdb.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiernan AE, Ahituv N, Fuchs H, Balling R, Avraham KB, Steel KP, Hrabe de Angelis M. The Notch ligand Jagged1 is required for inner ear sensory development. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:3873–3878. doi: 10.1073/pnas.071496998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiernan AE, Pelling AL, Leung KK, Tang AS, Bell DM, Tease C, Lovell-Badge R, Steel KP, Cheah KS. Sox2 is required for sensory organ development in the mammalian inner ear. Nature. 2005;434:1031–1035. doi: 10.1038/nature03487. [DOI] [PubMed] [Google Scholar]
- Kiernan AE, Xu J, Gridley T. The Notch ligand JAG1 is required for sensory progenitor development in the mammalian inner ear. PLoS genetics. 2006;2:e4. doi: 10.1371/journal.pgen.0020004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo SK, Hill JK, Hwang CH, Lin ZS, Millen KJ, Wu DK. Lmx1a maintains proper neurogenic, sensory, and non-sensory domains in the mammalian inner ear. Dev Biol. 2009;333:14–25. doi: 10.1016/j.ydbio.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koundakjian EJ, Appler JL, Goodrich LV. Auditory neurons make stereotyped wiring decisions before maturation of their targets. J Neurosci. 2007;27:14078–14088. doi: 10.1523/JNEUROSCI.3765-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis AK, Frantz GD, Carpenter DA, de Sauvage FJ, Gao WQ. Distinct expression patterns of notch family receptors and ligands during development of the mammalian inner ear. Mechanisms of development. 1998;78:159–163. doi: 10.1016/s0925-4773(98)00165-8. [DOI] [PubMed] [Google Scholar]
- Lewis J. Notch signalling and the control of cell fate choices in vertebrates. Seminars in cell & developmental biology. 1998;9:583–589. doi: 10.1006/scdb.1998.0266. [DOI] [PubMed] [Google Scholar]
- Li CW, Van De Water TR, Ruben RJ. The fate mapping of the eleventh and twelfth day mouse otocyst: an in vitro study of the sites of origin of the embryonic inner ear sensory structures. Journal of morphology. 1978;157:249–267. doi: 10.1002/jmor.1051570302. [DOI] [PubMed] [Google Scholar]
- Lin Z, Cantos R, Patente M, Wu DK. Gbx2 is required for the morphogenesis of the mouse inner ear: a downstream candidate of hindbrain signaling. Development. 2005;132:2309–2318. doi: 10.1242/dev.01804. [DOI] [PubMed] [Google Scholar]
- Litsiou A, Hanson S, Streit A. A balance of FGF, BMP and WNT signalling positions the future placode territory in the head. Development. 2005;132:4051–4062. doi: 10.1242/dev.01964. [DOI] [PubMed] [Google Scholar]
- Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, Lein ES, Zeng H. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nature Neuroscience. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak AC, Szeto IY, Fritzsch B, Cheah KS. Differential and overlapping expression pattern of SOX2 and SOX9 in inner ear development. Gene expression patterns : GEP. 2009;9:444–453. doi: 10.1016/j.gep.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison A, Hodgetts C, Gossler A, Hrabe de Angelis M, Lewis J. Expression of Delta1 and Serrate1 (Jagged1) in the mouse inner ear. Mechanisms of development. 1999;84:169–172. doi: 10.1016/s0925-4773(99)00066-0. [DOI] [PubMed] [Google Scholar]
- Morsli H, Choo D, Ryan A, Johnson R, Wu DK. Development of the mouse inner ear and origin of its sensory organs. J Neurosci. 1998;18:3327–3335. doi: 10.1523/JNEUROSCI.18-09-03327.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morsli H, Tuorto F, Choo D, Postiglione MP, Simeone A, Wu DK. Otx1 and Otx2 activities are required for the normal development of the mouse inner ear. Development. 1999;126:2335–2343. doi: 10.1242/dev.126.11.2335. [DOI] [PubMed] [Google Scholar]
- Murata J, Tokunaga A, Okano H, Kubo T. Mapping of notch activation during cochlear development in mice: implications for determination of prosensory domain and cell fate diversification. J Comp Neurol. 2006;497:502–518. doi: 10.1002/cne.20997. [DOI] [PubMed] [Google Scholar]
- Neves J, Abello G, Petrovic J, Giraldez F. Patterning and cell fate in the inner ear: a case for Notch in the chicken embryo. Dev Growth Differ. 2013a;55:96–112. doi: 10.1111/dgd.12016. [DOI] [PubMed] [Google Scholar]
- Neves J, Kamaid A, Alsina B, Giraldez F. Differential expression of Sox2 and Sox3 in neuronal and sensory progenitors of the developing inner ear of the chick. J Comp Neurol. 2007;503:487–500. doi: 10.1002/cne.21299. [DOI] [PubMed] [Google Scholar]
- Neves J, Parada C, Chamizo M, Giraldez F. Jagged 1 regulates the restriction of Sox2 expression in the developing chicken inner ear: a mechanism for sensory organ specification. Development. 2011;138:735–744. doi: 10.1242/dev.060657. [DOI] [PubMed] [Google Scholar]
- Neves J, Uchikawa M, Bigas A, Giraldez F. The prosensory function of Sox2 in the chicken inner ear relies on the direct regulation of Atoh1. PloS one. 2012;7:e30871. doi: 10.1371/journal.pone.0030871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves J, Vachkov I, Giraldez F. Sox2 regulation of hair cell development: incoherence makes sense. Hear Res. 2013b;297:20–29. doi: 10.1016/j.heares.2012.11.003. [DOI] [PubMed] [Google Scholar]
- Ohyama T, Basch ML, Mishina Y, Lyons KM, Segil N, Groves AK. BMP signaling is necessary for patterning the sensory and nonsensory regions of the developing mammalian cochlea. J Neurosci. 2010;30:15044–15051. doi: 10.1523/JNEUROSCI.3547-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W, Jin Y, Chen J, Rottier RJ, Steel KP, Kiernan AE. Ectopic expression of activated notch or SOX2 reveals similar and unique roles in the development of the sensory cell progenitors in the mammalian inner ear. J Neurosci. 2013;33:16146–16157. doi: 10.1523/JNEUROSCI.3150-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W, Jin Y, Stanger B, Kiernan AE. Notch signaling is required for the generation of hair cells and supporting cells in the mammalian inner ear. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:15798–15803. doi: 10.1073/pnas.1003089107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papanayotou C, Mey A, Birot A-M, Saka Y, Boast S, Smith JC, Samarut J, Stern CD. A mechanism regulating the onset of Sox2 expression in the embryonic neural plate. PLoS biology. 2008:6. doi: 10.1371/journal.pbio.0060002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauley S, Wright TJ, Pirvola U, Ornitz D, Beisel K, Fritzsch B. Expression and function of FGF10 in mammalian inner ear development. Dev Dyn. 2003;227:203–215. doi: 10.1002/dvdy.10297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pevny LH, Nicolis SK. Sox2 roles in neural stem cells. The international journal of biochemistry & cell biology. 2010;42:421–424. doi: 10.1016/j.biocel.2009.08.018. [DOI] [PubMed] [Google Scholar]
- Que J, Okubo T, Goldenring JR, Nam KT, Kurotani R, Morrisey EE, Taranova O, Pevny LH, Hogan BL. Multiple dose-dependent roles for Sox2 in the patterning and differentiation of anterior foregut endoderm. Development. 2007;134:2521–2531. doi: 10.1242/dev.003855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raft S, Groves AK. Segregating neural and mechanosensory fates in the developing ear: patterning, signaling, and transcriptional control. Cell Tissue Res. 2015;359:315–332. doi: 10.1007/s00441-014-1917-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raft S, Koundakjian EJ, Quinones H, Jayasena CS, Goodrich LV, Johnson JE, Segil N, Groves AK. Cross-regulation of Ngn1 and Math1 coordinates the production of neurons and sensory hair cells during inner ear development. Development. 2007;134:4405–4415. doi: 10.1242/dev.009118. [DOI] [PubMed] [Google Scholar]
- Riccomagno MM, Martinu L, Mulheisen M, Wu DK, Epstein DJ. Specification of the mammalian cochlea is dependent on Sonic hedgehog. Genes Dev. 2002;16:2365–2378. doi: 10.1101/gad.1013302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccomagno MM, Takada S, Epstein DJ. Wnt-dependent regulation of inner ear morphogenesis is balanced by the opposing and supporting roles of Shh. Genes Dev. 2005;19:1612–1623. doi: 10.1101/gad.1303905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts KA, Abraira VE, Tucker AF, Goodrich LV, Andrews NC. Mutation of Rubie, a novel long non-coding RNA located upstream of Bmp4, causes vestibular malformation in mice. PloS one. 2012;7:e29495. doi: 10.1371/journal.pone.0029495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer B, Henderson N. Site-specific DNA recombination in mammalian cells by the Cre recombinase of bacteriophage P1. Proceedings of the National Academy of Sciences of the United States of America. 1988;85:5166–5170. doi: 10.1073/pnas.85.14.5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit A, Berliner AJ, Papanayotou C, Sirulnik A, Stern CD. Initiation of neural induction by FGF signalling before gastrulation. Nature. 2000;406:74–78. doi: 10.1038/35017617. [DOI] [PubMed] [Google Scholar]
- Streit A, Stern CD. Establishment and maintenance of the border of the neural plate in the chick: involvement of FGF and BMP activity. Mechanisms of development. 1999;82:51–66. doi: 10.1016/s0925-4773(99)00013-1. [DOI] [PubMed] [Google Scholar]
- Takemoto T, Uchikawa M, Kamachi Y, Kondoh H. Convergence of Wnt and FGF signals in the genesis of posterior neural plate through activation of the Sox2 enhancer N-1. Development. 2006;133:297–306. doi: 10.1242/dev.02196. [DOI] [PubMed] [Google Scholar]
- Taranova OV, Magness ST, Fagan BM, Wu Y, Surzenko N, Hutton SR, Pevny LH. SOX2 is a dose-dependent regulator of retinal neural progenitor competence. Genes Dev. 2006;20:1187–1202. doi: 10.1101/gad.1407906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urness LD, Wang X, Shibata S, Ohyama T, Mansour SL. Fgf10 is required for specification of non-sensory regions of the cochlear epithelium. Dev Biol. 2015;400:59–71. doi: 10.1016/j.ydbio.2015.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendrell V, Lopez-Hernandez I, Alonso MB, Feijoo-Redondo A, Abello G, Galvez H, Giraldez F, Lamonerie T, Schimmang T. Otx2 is a target of N-myc and acts as a suppressor of sensory development in the mammalian cochlea. Development. 2015 doi: 10.1242/dev.122465. [DOI] [PubMed] [Google Scholar]
- Wu DK, Kelley MW. Molecular mechanisms of inner ear development. Cold Spring Harb Perspect Biol. 2012;4:a008409. doi: 10.1101/cshperspect.a008409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu DK, Oh SH. Sensory organ generation in the chick inner ear. J Neurosci. 1996;16:6454–6462. doi: 10.1523/JNEUROSCI.16-20-06454.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Animations of light sheet fluorescent microscope images of cleared specimens of neonatal inner ears from animals that received a single dose of tamoxifen at different ages between E8.5-E12.5 are presented as Supplementary Movies 1 (tamoxifen at E8.5), 2 (tamoxifen at E9.5), 3 (tamoxifen at E10.5), 4 (tamoxifen at E11.5), and 5 (tamoxifen at E12.5). A gradual restriction of EYFP fluorescence from non-sensory to sensory regions can be observed across this time range
Animations of 360° rotations of reconstructed images of Sox2 expression in the E9.25 (Movie 6), E9.75 (Movie 7) and E10.25 (Movie 8) otocysts shown in Figure 7. The elimination of Sox2 expression on the ventro-lateral side of the otocyst can be observed during this time window. Anterior and posterior axes are denoted on each movie. Each movie begins with a lateral view and rotates to show the medial view of the otocyst.