Abstract
In vertebrates and invertebrates biomineralization is controlled by the cell and the proteins they produce. A large number of these proteins are intrinsically disordered, gaining some secondary structure when they interact with their binding partners. These partners include the component ions of the mineral being deposited, the crystals themselves, the template on which the initial crystals form, and other intrinsically disordered proteins and peptides. This review speculates why intrinsically disordered proteins are so important for biomineralization, providing illustrations from the SIBLING (small integrin binding N-glycosylated) proteins and their peptides. It is concluded that the flexible structure, and the ability of the intrinsically disordered proteins to bind to a multitude of surfaces is crucial, but details on the precise-interactions, energetics and kinetics of binding remain to be determined.
Keywords: Biomineralization, Intrinsically Disordered Proteins, Phosphophoryn, Hydroxyapatite
1. Introduction
1.1 Composition of Mineralized Tissues
Biomineralization occurs in a variety of organisms, from unicellular bacteria containing gold deposits [1] to diatoms [2] having silicon based mineral deposits, shells with calcium carbonate phases (aragonite, calcite and occasionally vaterite as well as amorphous calcium carbonate) [3], to hierarchical structures in vertebrate bones and teeth containing the calcium phosphate, hydroxyapatite (HA)[4]. Common features of these mineralized structures are (i) formation on an organic matrix, produced by the cells of that organism. The organic matrix within each organism or within their tissues, extra- or intra-cellular, plays a role in the mineralization process. This is well-known due to the observation that the removal or modification of the matrix, or any given component within the matrix, alters its properties and thus the manner in which the mineral is formed (Table 1). (ii) An additional feature of the matrix proteins regulating mineralization is that a large percentage of them, greater than that within the general protein data base [5], are intrinsically disordered proteins (IDPs). In this review, we suggest the reasons IDPs1 are essential for the biomineralization process and provide examples to illustrate its importance.
Table 1.
Examples of Alterations in Matrix Composition that Affect the Mineralization Process
| Species | Type of mineral | Treatment of Matrix | Change in Mineral |
|---|---|---|---|
| Magnetic Bacteria7 | Magnetite (Fe3O4) | Deletion of operon | Irregular crystals |
| Avian Egg shell8 | Calcite | Incubation in Dermatan sulfate | Delays Formation |
| Abalone shell9 | Aragonite/Calcite | Perlucin variants | Modulate rate of precipitation |
| Diatoms10 | Silicate | Composite of silk and silaffin | Promotes mineralization |
| Mice11 | Hydroxyapatite | Knockout or overexpression of ameloblastin | Decreased enamel formation |
| Mice12 | Hydroxyapatite | Knockout of phospho-1 | Smaller crystals |
| Vertebrate13 | Hydroxyapatite | Dephosphorylation of osteopontin | No inhibition of growth |
| Vertebrate14 | Hydroxyapatite | Dephosphorylation of all phosphoproteins | Decreases extent of mineralization |
| Zebra-fish15 | Hydroxyapatite | Mutation in BMP1 (enzyme) | Defective mineralization |
1.2 Overview of the Process of Mineralization
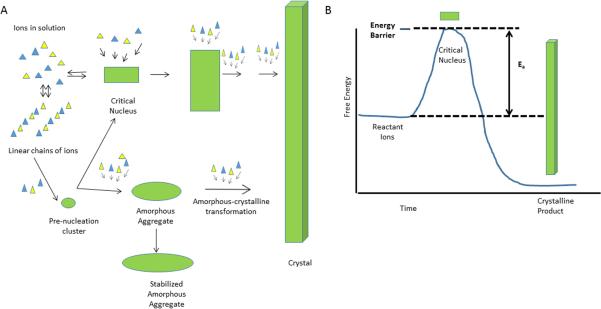
Mineral deposition is a physical-chemical process that, in living organisms, is regulated by cells and by the intra- and extra-cellular matrices they produce. In purely chemical terms, a mineral will not precipitate unless its solubility is exceeded, either by having an increase in the concentration of its component ions, a change in temperature or pressure, or the addition of a surface that lowers the activation energy for the initial mineral formation (Figure 1). Precursor phases such as pre-nucleation clusters [6] and amorphous calcium phosphate can form in solution, reducing the activation energy barrier. Within the organism, the cells and vascular system provide the necessary increases in the concentration of component ions for initial crystal formation (critical nucleus). Intra- or extra-cellular matrices may also provide surfaces that facilitate this initial mineral deposition. Inhibitors of mineralization protect the matrix or extracellular matrix from being mineralized, when mineral is neither needed nor wanted, and are modified or removed by enzymes produced by the cell. This entire process is highly complex, even in the simplest organism, yet common themes exist; the elevation of local concentrations of precipitating ions and the regulation of the process by the extracellular matrix.
Figure 1.
Physical Chemistry of Mineralization. A) Schematic illustrating how ions in solution can either form linear chains of ions, critical nucleus (the classical concept of nucleation) or unstructured (amorphous) clusters. The critical nucleus continues to accumulate ions and develops into a crystal. The clusters may also associate to form a “critical nucleus”. Proteins can interact with any of these forms to facilitate or inhibit the next step. B) The major expenditure of energy is in the formation of the critical nucleus as illustrated by this plot of activation energy vs. time.
1.3 Protein Regulation of Mineralization
According to classic nucleation theory, deposition of crystals requires the formation of nuclei which support the growth and proliferation of mineral crystals. Nucleation can take place, de novo, when solution ionic concentrations are increased and ions collide with sufficient energy to form a critical nucleus. This critical nucleus has the same structure, only a few unit cells in size, as does the final crystal. Once a critical nucleus is formed, less energy is required for the addition of ions to the nucleus, thus allowing for growth or branching which facilitates the spread of the crystals. Pre-nucleation clusters or foreign surfaces can resemble the critical nucleus or the aggregates of these pre-nucleation clusters might themselves transform into crystals which will then grow, facilitating mineral deposition. It is believed that in the process of biomineralization, the matrix has a structure resembling that of the crystal phase being formed, and epitaxial nucleation takes place upon that surface. We know that if a protein can bind to the mineral acting as an epitaxial nucleator, it also could coat the mineral surface and regulate the size or shape of that mineral. This phenomenon has been seen recently in HA binding studies using peptides [16] and proteins [13]. It is also likely that the functions of proteins that control mineralization in multicellular organisms are redundant, both because the pattern of mineralization may differ in different tissues throughout the organism, and because mineralization is so important for life itself. In more complex organisms, a template, such as collagen for bone, demonstrated by atomic-level molecular modeling [17] or the self-assembled matrix of amelogenin nano-spheres during enamel formation [18] may interact with the other proteins that regulate the mineralization process. It is here, that the IDPs become important.
2. Intrinsically Disordered Proteins (IDPs)
IDPs are proteins whose structures are highly variable [19, 20], and do not assume typical secondary structures (α-helix, β-sheet, etc.). Some of these proteins have a total random coil-structure; others, have intrinsically disordered regions. IDPs are often hydrophilic, having multiple amino acid repeats, often with either very negatively or positively charged domains. IDPs gain more folded-features when post-translationally modified and/or when bound to their numerous partners (Figure 2). Such partners may also have diverse structures and functions. IDPs account for ~30% of the human proteome [21]. The study of IDPs has increased extensively in recent years because these proteins are associated with major diseases such as schizophrenia and Alzheimer's disease, among others [22]. In vertebrate bio-mineralization, with the exception of collagen which does, in fact, have disordered teleopeptides [23], small leucine rich proteoglycans and some enzymes, the majority of proteins associated with HA formation and growth, recently reviewed [24,5], are IDPs. These IDPs may stabilize the ions or ion clusters, the critical nucleus, provide epitaxial sites for initial mineral deposition, and/or stabilize the crystal. The flexible nature of these structures enables them to perform these and many other functions. The observation that unstructured proteins were involved in mineralization was first reported in 1977 in a circular dichroism (CD) study of phosphophoryn (DPP), in the presence of Ca2+, which, with the addition of this ion, changed from a random linear chain to a β-pleated sheet [25]. Phosphophoryn, also known as DPP or DMP2, is the product of the cleavage of DSPP into DPP and dentin sialoprotein (DSP). DSPP is the most abundant protein in dentin [26] and is also expressed in enamel and in bone. Absence of or mutations in DSPP, result in dentin abnormalities, discussed below.
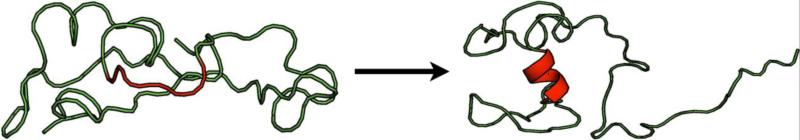
Figure 2.
Intrinsically disordered proteins or regions of proteins (as shown here by the unstructured ribbon) can take on increased secondary structure when interacting with their partners. Here, a domain of the retinal cGMP phosphodiesterase was predicted by molecular dynamics (10 ns) to form an alpha helix (red) on interaction with cGMP. Note that not all of the protein folds on this interaction. The NMR structure for this interaction was reported elsewhere [35].
In 1994, Evans and Chan using P31 and H1 –NMR, showed the folding of DPP was dependent on pH and reported the presence of hinge regions that allowed the charged flexible regions to interact [27]. Their model predicted the most compact (greatest folding) at low pH. Fisher in 2001 also used NMR to show that osteopontin (OPN) and bone sialoprotein (BSP) also had flexible (random coil or disordered) structures [28]. OPN, BSP, DSPP and matrix extracellular phosphor glycoprotein (MEPE), dentin matrix protein-1 (DMP1) are all part of the SIBLING protein gene family, where SIBLING indicates small integrin-binding ligand N-linked glycoproteins. They are all found on human chromosome 4, mouse chromosome 5, and have many sequence similarities [29]. They all include repeat sequences with groups capable of phosphorylation and the presence of multiple aspartate or glutamate repeats. Our group used vibrational spectroscopy to show the transition from random coils in OPN and DMP1 to slightly more structured forms in the presence of HA, calcium ions and collagen [30.31]. One of the features of the flexible structures of IDPs is that post-translational modifications (phosphorylation, glycation, acetylation, sulfation, cleavage) can be facilitated by virtue of an open chain [32]. In fact, post-translational modifications were shown to have a preference for disordered regions in a large number of proteins with known NMR-structures [33]. The post-translational modified structures will also fold differently when they bind to their partners. The importance of post-translational modification (cleavage and phosphorylation) for mineralization was suggested in these studies, since the non-phosphorylated proteins did not interact with HA or Ca2+ [34]. Thus IDPs, by having variable post-translational modifications, can regulate their interactions with mineral phases and modulate the mineralization process A second key aspect of the flexible structure is that different parts of the IDP may fold on interaction with different partners. This could allow the IDP to fold one region to stabilize the amorphous cluster and leave another region unfolded to interact with growth factors, the nascent crystal, or other noncollagenous proteins.
IDPs are also involved in the formation of mineral in the invertebrate species. Calcium carbonate formation in shells, for example, is regulated by IDPs. One shell protein, AP7, first shown to form supramolecular-assemblies and nucleate single crystals of aragonite [36] is also involved in the regulation of calcite growth [37]. The C-RING-like sequence is an important site for AP7 self-association and mineral nucleation; self-assembling, to form protein phases that congregate amorphous calcium carbonate nanoparticles, into hybrid, protein–mineral supramolecular networks, and create nano-porosities on the surfaces of the calcite that forms. Chang et al. [37] speculated that the repeated deposition of AP7 on the calcite surface allows for the creation of these nano-porosities. The ability of IDPs to interact with and regulate more than one mineral phase is another important theme that will occur throughout this discussion. Thus, it is likely that where multiple crystalline phases may form, a pre-cursor or well crystallized phase interaction with different proteins can stabilize and direct the nature of the phase that is formed and the way in which it grows.
Another IDP associated with calcium carbonate formation, SM50, the most abundant protein in sea urchin spicules, stabilizes amorphous calcium carbonate [38]. Sea urchins spicules are believed to form by first depositing a hydrated amorphous calcium carbonate onto the forming spicule surface, which dehydrates and crystallizes to form calcite [38]. SM50 has different domains that are important for calcium binding; directing proper growth of the mineral and directing the development of mineralized spicules [39]. Studies of SM50's conformational changes during these processes were reported by the Evan's group [40] who noted an unusual 20-residue proline-asparagine-containing repeat sequences, which were investigated by CD dichroism and other methods, showing the repeat motif adopts an extended “twist” structure, consisting of turn- and coil-like regions. They suggested that this domain acts either as a mineral-binding domain, a protein-protein docking domain, or as an internal “molecular spacer” for the SM50 protein during spicule formation. Proof for these roles is yet to be determined. Variation in conformation and function, however, is a common theme among the IDPs involved in mineralization.
A third example of an IDP involved in calcium carbonate deposition is Star-maker, a protein made in otoliths (ear organs) of fish. This protein, similar to many of the IDPs involved in calcium phosphate formation, is highly phosphorylated via post-translational modifications; and like the examples discussed below, folds to become more compact on phosphorylation [41,42]. Calcium binding also causes a condensation of the molecule, leading to the appearance of a secondary structure (loss of random coil) and the consequent decrease in hydrodynamic radius.
2.1 HA formation in Enamel: Role of IDPs
Enamel formation, a complex process in which large mineral (HA) crystals are formed as the organic matrix is removed, is similarly dependent on IDPs. Janet Moradian-Oldak and her collaborators John Evans and Wendy Shaw investigated the secondary structure of amelogenin and ameloblastin, two major enamel proteins. Both were shown to be IDPs [43,44], their binding partners were other IDPs (enamelin and tuftelin) and HA. Enamel formation does not occur on a collagen matrix; rather it forms under the direction of the previously mentioned IDPs. The enamel forming cells (ameloblasts), produce several IDPs: amelogenin [45], ameloblastin [46], and enamelin [47,48]. These IDPs are eventually cleaved into smaller fragments by enzymes, e.g., enamelysin (matrix metalloproteinase 20, Mmp-20) and kallikrein 4 [49]. The ways in which these proteins guide the formation of HA in enamel is unknown. There are, however, some important clues from recent studies. In the teeth of mice that produce a truncated form of ameloblastin, enamel fails to appear [50,51]. These mice also have reduced expression of amelogenin with no apparent change in the levels of several other ameloblast-produced proteins [52,53] indicating that there must be some interaction between amelogenin and ameloblastin during the mineralization process. Studying mouse ameloblasts at different stages of development, Moradian Oldak [54] reported amelogenin and ameloblastin co-localized near the secretory face of ameloblasts at the earliest stages of their formation; with maturation, ameloblastin was lost from the enamel surface. Additionally, they found using circular dichroism, that amelogenin and ameloblastin could form stable complexes. C-terminal polypeptides of ameloblastin were cleaved into smaller peptides and lost from the extracellular matrix [55] maturation is complete. This implies that the interactions of these IDPs may facilitate specific cleavage. The observation that the amelogenin protein in some patients with amelogenesis imperfecta formed aggregates supports the concept that having an IDP-like flexible structure is germane to the mineralization process [56]. Further, Wendy Shaw's group used NMR spectroscopy to examine the WT mouse and mutant amelogenins to confirm the pre-mature self-assembly of the mutant proteins [57]. Other studies show how the amelogenin structure is modified during its interaction with HA, phospholipids, other enamel proteins [58] and even collagen (remember that amelogenin does not interact with collagen in situ) [59] suggesting that during the complex interactions leading to HA formation other conformational changes are occurring.
Cryo-TEM investigations on the initial formation of enamel crystals have provided high resolution insight into the way an IDP, amelogenin, interacts with pre-nucleation clusters. In calcium phosphate solution, the presence of amelogenin stabilized small particles, aggregates with two or three particles and larger aggregates (pre-nucleation clusters). While needle shaped particles of hydroxyapatite were eventually formed, the amorphous aggregates were stabilized by amelogenin, leading to the statement that amelogenin inhibited (retarded) mineralization [60,61]. It is of interest to note that atomistic modeling of prenucleation clusters for calcium carbonate suggested that they also consisted of chains of cations and anions [62]; the formation of such chains could also be true of the calcium phosphate prenucleation clusters.
2.2 Pathologic Calcification: Role of IDPs
Calcifications occurring in unwanted places – the aorta, salivary glands and soft tissues, are all associated with IDPs. To illustrate, salivary stones occur when mucins, such as statherin, an un-folded protein [63], are defective [64]. Statherin, a salivary IDP that lubricates enamel, binds bacteria, and regulates the amount of calcium and phosphate to which enamel is exposed, is involved in prevention of dental calculus formation. Statherin, which also controls the nucleation and growth HA in saliva, contains an N-terminus with small acidic motif (DSpSpEE) that is also found in SIBLING proteins [65]. When solubilized, statherin has a random coil conformation. When bound to HA, it takes on a more alpha-helical structure exposing a bacterial binding site [63], leading to bacterial entrapment.
Another example is the role of the IDP, DMP1, in preventing kidney and cardiovascular calcification. The double knockout mouse lacking both DMP1 and Klotho (a kidney transmembrane protein that regulates phosphate levels), develops kidney and cardiovascular calcifications associated with inability to manage high phosphate levels in the absence of DMP1 [66]. A third example is provided by a collagen-disease. The hinge region of type VII collagen, associated with dystrophic epidermolysis bullosa, is also intrinsically disordered [67]. Additionally, the SIBLING proteins, all IDPs, are also associated with vascular [68,69] and other soft tissue calcifications [70]. Since their genes are expressed in cells associated with these deposits they cannot have accumulated simply due to their affinities for HA or collagen, rather, they must have some function in the deposition of these unwanted calcifications.
2.3 Abundance of IDPs in Mineralization
Structure predictions demonstrate that almost all proteins associated with mineralization in the Swiss Protein Database are IDPs [5]. Many of these proteins share the common repeat sequence: aspartate-serine-serine (DSS) or glutamate-serine-serine (ESS), where the serine is often phosphorylated. An example of such proteins we and others have associated with the mineralization process, are the so-called SIBLING proteins: osteopontin [30,71,72,73], dentin matrix protein 1 [33,74,75], matrix extracellular phosphoglycoprotein [76], bone sialoprotein [77,78] and the cleaved products of dentin sialophosphoprotein, dentin phosphophoryn [79] and dentin sialoprotein [80]. It should be noted in this discussion that enamelin, originally considered a SIBLING, lacks cell binding activity in some species and thus is no longer considered a SIBLING protein [28]. Much like sibling children, they share common heritage (genes) and some structural homology, often appearing to play different roles in regulating biomineralization in various states of health and disease of dentin and bone (Table 2). All bind to and regulate cell differentiation via different factors [81]. They all have numerous repeat sequences, most are charged. To illustrate, we developed a PERL-based script to identify all possible triplet amino acids repeat sequences in human SIBLING proteins (Table 3). For the sake of simplification “N” represents glutamic acid (E) or aspartate (D), “P” represents serine (S), Threonine (T) and Tyrosine (Y), with high probabilities of being phosphorylated based on NETPHOS software. We also include SPARC (osteonectin), a protein involved with mineralization (linked in some ways to osteoporosis) that has some IDP regions.
TABLE 2.
SIBLING Proteins and their Roles in Vertebrate Mineralization
| Protein | Associated Disease | Properties of KO | Effect on HA formation |
|---|---|---|---|
| Bone sialoprotein | ? | Impaired bone turnover ; higher bone mass82 Embryonic delayed mineralization83 |
Promotes (nucleates) mineralization84 |
| Dentin matrix protein 1 | Dentinogenesis Imperfecta III Autosomal recessive form of hypophosphatemic rickets |
Dentin hypomineralization in maturation85 Reduced dspp levels.86 |
Full protein inhibits74 Peptides promote33 |
| DSPP | Dentin dysplasia | Hypomineralized dentin87,88 Bone composition varies with age89 |
DPP inhibits HA formation at low concentration and promotes it at high71; DSP has little effect80 |
| Enamelin | Hypoplastic amelogenesis imperfecta | Impaired enamel mineralization90 | Inhibited seeded HA growth91 |
| MEPE | Oncogenic osteomalacia | Increased bone formation and bone mass92 | Full protein nucleates; peptides inhibit93 |
| Osteopontin | Vascular calcification | Reduced fracture toughness without change in bone mass941; Increased mineral content and decreased crystallinity95 | Full protein inhibits96 Fragments- variable effects16 |
Table 3.
Simplified Triplet Repeat Count
| NNN | NPP | NNP | PNN | PPN | PPP | |
|---|---|---|---|---|---|---|
| DSPP | 9 | 167 | 5 | 170 | 3 | 11 |
| DMP1 | 14 | 7 | 15 | 11 | 11 | 6 |
| BSP | 17 | 8 | 10 | 7 | 2 | 4 |
| MEPE | 5 | 3 | 5 | 1 | ||
| OPN | 7 | 6 | 3 | 9 | 5 | |
| SPARC | 2 | 1 | 3 | 1 |
“N” represents glutamic acid (E) or aspartate (D) and “P” represents serine (S), Threonine (T) and Tyrosine (Y) with high probabilities of being phosphorylated (NETPHOS).
The SIBLINGs all bind to HA, however characterizing adsorption of proteins to solid surfaces is challenging. The energy controlling the absorption of proteins comes from intermolecular forces, such as Coulombic, van der Waals, Lewis acid-base, and hydrophobic interactions. Temperature, pH, ionic strength, protein concentration, protein polarizability, and surface polarizability can influence these forces. Together, they control the conformational entropy of the protein structure and the motilities of the protein over solid surfaces [97,98,99]. The proteins more frequently studied are large globular proteins, such as enzymes [99,100], which show some conformational changes, decreases in secondary structure or loss of three dimensional structure upon surface adsorption [101,102,103,104,105]. Globular proteins show a loss of native structural stability upon adsorption to solid interfaces due to increased entropy as proteins are absorbed to a solid surface. These conformational rearrangements in the native structure are thought to affect the protein functions [102, 106]. In contrast, IDPs are charged proteins, thus they can control their interfacial absorption onto solid surfaces [106]. Experiments, in silico, suggest that intrinsically disordered peptides absorbed to a surface with a complementary pattern form a well-defined structure (α-helix) indicating that a specific surface can stabilize the structure of an IDP peptide. The effect of a complementary surface pattern surface on protein structure in vitro was demonstrated by Wendy Shaw's group. They used a peptide from Leucine-Rich Amelogenin Protein (LRAP) to analyze the changes in the secondary structure when this peptide was absorbed to HA and carbonated HA (CAP). The structure of LRAP peptide absorbed to CAP was consistent with an α-helix, whereas when bound to HA the structure corresponded more to a random coil [107]. We studied DPP peptides by FTIR spectroscopy and found the phosphorylated peptides in solution in the presence of HA formed α-helical structures and lost their random coil characteristics [108]. Perhaps, through evolution, the most efficient strategy found by nature for protein-surface interactions, was using IDPs to bind to solid surfaces and in this way control the nucleation, growth and morphology of biominerals while still being able to interact with other partners. IDPs do not have a structure to lose on binding and thus can form more favorable secondary structures on binding.
2.4 Self-Assembly of IDPs
IDPs frequently undergo self-assembly. The extended conformation of IDPs facilitates the intermolecular interactions between them and promotes formation of supramolecular architecture [109]. This is illustrated in some of the mineralized tissues discussed above. Silaffins, for example, are protein constituents of biosilica, playing an active role in silica formation. These small proteins with numerous phosphoserine repeats self-assemble via electrostatic interactions; a prerequisite for biosilica formation [110,111]. SM50, from the mineralized larval of sea urchin spicules, contains C-type lectin and IDPs repeat domains. The C-terminal region of the IDP domain containing an asparagine and proline (Asn- and Pro)-rich region, with glycine (Gly)- and glutamine (Gln)-rich repeats mid-sequence is implicated in self- assembly. Based on analytical ultracentrifugation, the glycine-rich region oligomerizes to form large protein assemblies. The proline-rich region also shows a self-assembly due to the hydrophobic interactions between pyrolidine rings and hydrogen bonding interactions between the Asn residues [39,112].
In enamel, amelogenin conformational changes upon self-assembly differ from those observed with interactions with calcium phosphate mineral [113]. In vitro studies with amelogenin reported a disorder-to-order transition during the self-assembly process. The amelogenin molecules at pH 7.2 spontaneously self-assemble into oligomers and nanospheres. These subsequently bind together to form micro-ribbons [58]. Beniash et al suggest that this amelogenin self-assembly is attributable to formation of intramolecular hydrogen bonds between extended unordered regions of the protein, leading to the formation of tightly bound intrafibrillar β-strands [88]. As a final example, two peptides from dentin matrix protein 1 cleavage can self-assemble in vitro to form micro-fibrils. This was suggested to be crucial for HA mineral nucleation [59]. Self-assembly is thus another way that the IDP proteins can regulate the process of biomineralization.
3. Potential Mechanisms of IDP Action: Biomineralization
There must be precise reasons why so many IDP proteins are associated with mineralization. It is our suggestion that among these reasons are: i) the energetics of initial mineral deposition in the presence of IDP is more favorable. Namely, the driving force (free energy) needed to bind IDP to either collagen, precursor mineral phase or HA, whichever initiates crystal deposition, is lower, due to the change in entropy Δ S, when an unstructured protein is bound and folded (transiently), is smaller than when a structured protein is bound (Δ G = Δ H –TΔS). This concept may be debated, however, due to the relative order of the IDP vs. the dis-order of water upon IDP-partner binding in aqueous media. ii) An open structure gives an IDP the ability to bind to more than one partner and present different surfaces facilitating the regulation of mineralization. iii) An open structure facilitates post translational modifications, which can alter IDP net charge by phosphorylation and glycosylation, etc., providing for a greater structural variability, opening the door to new ligands, enabling the IDP to act as either nucleators, inhibitors, or both. It is likely that different combinations of each of the above exist in situ.
IDPs in general have multiple functions. Biomineralization-related IDPs, with their flexible chains, have several possible functions, which, depending on the above-proposed mechanisms, may include [114]:
-
(a)
Stabilization of calcium phosphate nano-clusters or amorphous calcium phosphate, ACP. For example, unstructured chains of the milk proteins casein [64] and the SIBLING protein osteopontin [34] are known to stabilize ACP. A recent neutron spectroscopy, small-angle X-ray and neutron scattering of osteopontin-calcium phosphate interactions, confirmed that the 1-149 fragment of osteopontin maintained its flexible linear chain structure in solution, stabilizing ACP particles [115]. Additionally, stabilization of calcium-carbonate nanoparticles and creation of nano-porosities within them is due to the IDP mollusk protein, AP7 [37], discussed above, implying this could be a common IDP –mineral regulation mechanism. As reviewed elsewhere [116] the initial mineral that forms, be it an amorphous liquid, or pre-nucleation chains or clusters, may exhibit polyamorphism. One or more amorphous phases that are formed may be stabilized by IDPs, in some cases facilitating the transformation to a crystalline phase and in others providing a means for remaining in an amorphous state. Some of these amorphous structures have short range order [117] and may be considered proto-crystalline [116]. For amorphous calcium carbonate, this short range order is only noted in the presence of additives, such as poly-aspartic acid. Since the amorphous structures aggregate to form prenucleation clusters, this suggests that the prenucleation clusters also have short-range order, and this is likely to be associated with the presence of IDPs, which in turn could determine whether there is an amorphous to crystalline transformation and what phases are formed.
-
(b)
Enabling formation of supramolecular structures, as discussed above and as seen for i) enamel and ameloblastin [53], ii) complexes of collagen, IDP and mineral, or iii) peptide-amphiphiles being developed for bone regeneration [118]. Ameloblastin self-associates into long (10-100 nm) ribbon-like structures averaging 18 and 0.34 nm, in width and thickness [44]. How this regulates enamel mineralization is under investigation but it important to remember that mineral formation does not occur when ameloblastin is absent [51].
-
(c)
Allowing for variations in post-translational processes, as exists for the various SIBLING proteins of tooth and bone. This is illustrated by the SIBLING proteins, which undergo extensive and variable post translational modification in different tissues, facilitating their interactions with their binding partners (cell surface integrins, HA, Ca2+ and collagen). When phosphorylated, they are all highly acidic. The extent of phosphorylation of these proteins and their peptides affects their ability to bind to HA and to regulate mineralization [119]. Modeling of the most frequently appearing repeat peptide in DPP [108] by molecular dynamics simulations, showed the phosphorylated peptide bound with greater affinity and a more organized structure to the surface of hydroxyapatite (Figure 3A,B). The change to a more-ordered structure after phosphorylation and interactions with either calcium ions or hydroxyapatite was verified by circular dichroism, small angle scattering and vibrational spectroscopy (Figure 4). Based on modeling, the phosphorylated peptide folded differently when associated with a model of type I collagen than did the non-phosphorylated peptide (Figure 5). These changes in conformation associated with post-translational modifications, could facilitate nucleation and growth of mineral crystals upon and within the collagen matrix.
-
(d)
Regulating protein expression within cells. DMP1, for one, regulates circulating phosphate levels by controlling bone expression of the hormone FGF23 [66]. IDPs assume many different functions because their “unfolded” structure presents with a multitude of surfaces which enables them to interact with a diverse set of enzymes, cell membranes, and other partners [120]. The overall question, however remains, as to why the IDPs are both associated with and required for biomineralization. It might be argued, that IDPs arose by gene multiplication and are incidental to the mineralization process. (DSPP, for example, is a duplication product of DMP1 [121]). The observation that IDPs are so ubiquitous during mineralization argues against this. IDPs are known to be “promiscuous” [109] with multiple binding partners. This promiscuity most likely is due to the multiple surfaces presented by the IDPs or the low energy requirement associated with their role in mineralization. Thus, it is not surprising that DMP1 can interact with cell membrane receptors (e.g. αvβ3 integrin [122] to regulate signaling [123], FGF23 expression [66], bind to HA crystals so as to regulate HA growth [34], interact with itself [124] and with collagen [125] to modulate dentin and bone mineralization. The ASARM (acidic serine aspartate-rich motif) peptide released from MEPE, DPP and DMP1 has roles in regulation, vascularization, soft-tissue calcification, osteoclastogenesis, mechanotransduction, fat energy metabolism, along with regulation of mineralization [126]. As illustrated in Figure 4, IDPs also interact with HA, as reported by our group, for DPP [79] and OPN [13] and by the Clarkson's group for DSP and DPP interacting with enamel crystals [127].
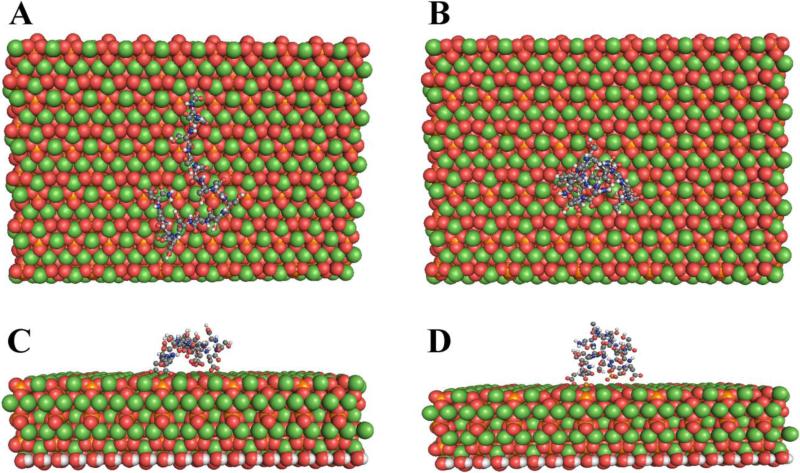
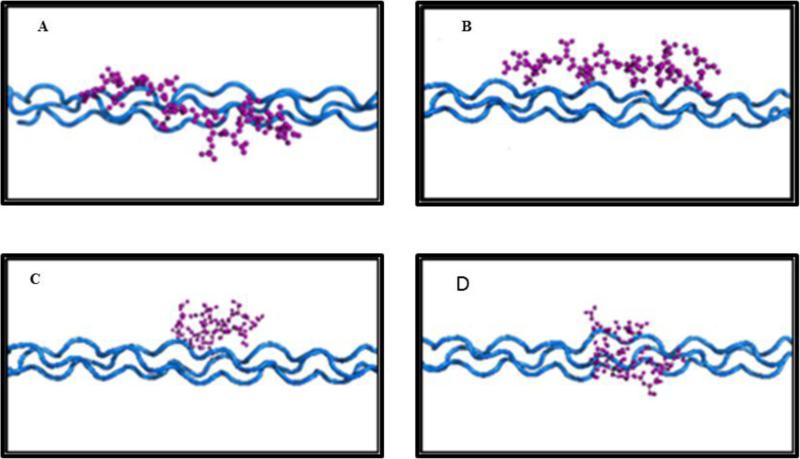
Figure 3.
Molecular Dynamics Simulation: The most commonly repeated peptide of Dentin phosphophoryn (P5 Ace-SSDSSDSSDSSDSSD-NH2 and P5P Ace-SSDpSpSDpSpSDpSpSDpSpSD -NH2) binds in a more extended form to the 100 surface of hydroxyapatite when phosphorylated (A,C) than when unphosphorylated (B,D). A and B show the mineral surface, B and D are viewed perpendicular to the surface. In the mineral model red circles are oxygen (O) atoms, orange circles phosphorus (P) atoms, white circles hydrogen (H) atoms and green circles calcium (Ca) atoms. Water is not shown but was included in the model. For details of the modeling procedures see Villarreal-Ramirez et al., 2014 [108]).
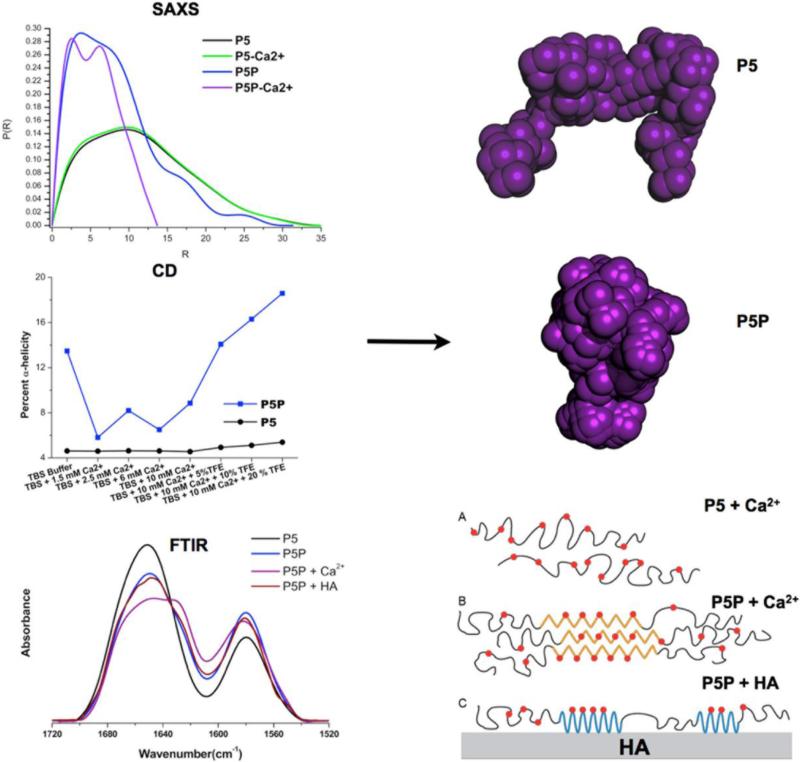
Figure 4.
In Situ Validation of Molecular Dynamics Predictions: The peptide detailed in figure 3 was studied by a variety of techniques to confirm the predictions from Molecular Dynamics. Small-angle/wide-angle X-ray scattering (SAXS/WAXS) confirmed the formation of a more structured molecule for P5P in the presence of calcium ions. This was also confirmed by circular dichroism (CD). Fourier transform infrared analysis (FTIR) combined with curve-fitting showed the peptides increased their secondary structure when bound to the HA surface; with phosphorylation (P5P) causing a shift to more helical structures. The ab initio dummy atom models (purple models) on the right were generated with the DAMMIN algorithm with SAXS data. In the lower right corner a cartoon illustrates the conformational changes suggested by these studies.
Figure 5.
Molecular Dynamics Simulation of P5 and P5P interactions with a typical type I collagen peptide structure: Collagen binding was predicted using a rigid body docking model. P5P interacts more with fibrillar collagen (A/B) than P5 (C/D). Two different orientations are shown to illustrate the differences in binding. The backbone of the collagen peptide is shown in blue ribbons. The P5 (A-C) and P5P (B-D) peptides are represented as colored balls and sticks in purple. A-B) viewed from the side; C-D) viewed from above.
To date, in addition to the studies mentioned above, atomistic modeling has suggested ways in which IDPs regulate biomineralization. For example, based on modeling of different domains of the bone sialoprotein structure, first reports found ser-136 had to be phosphorylated for HA nucleation to occur [128]. In contrast, another study reported there was no domain that was involved in nucleation [129]. Using a larger portion of the molecule for modeling, it was later suggested that a cationic loop region promoted nucleation by attracting Ca2+ ions, with the loop's flexibility allowing for rapid self-assembly with phosphate ions [130], rather than providing a regular template for crystallization. The flexible nature of the osteopontin structure when bound to the HA surface, was also predicted by molecular dynamics [120]; the phosphorylation of peptides modified binding in these studies [131], as in studies of interaction with calcium oxalates [132]. These data led the Hunter group to suggest that adsorption of acidic proteins to Ca2+-rich crystal faces of minerals was governed by electrostatic phenomena and facilitated by the conformational flexibility of the chain [131]. These and other atomistic modeling studies suggest the importance of the flexible conformation of the IDPs for interaction with mineral crystals and with collagen [133]. The best demonstration of the importance of IDPs function in the mineralization process comes from studies of animals and people in which IDP has been manipulated, by nature or by genetic techniques. The effect of mutations in biomineralization-related IDPs on bones and teeth are discussed below.
4. Relationship of IDPs to Bone and Tooth Diseases
In both animal models and human samples, defects in IDPs, have been associated with tooth and bone diseases. Defects in DMP1, for example, are associated with Dentinogenesis Imperfecta type III and autosomal recessive hypophosphatemic rickets [134,135]. Although the observed phenotype may be linked to low phosphate levels and inappropriate FGF23 expression in those with aberrant DMP1, these condition were the first directly linked to a defect in an IDP, and thus deserves mention. Dentinal dysplasias, in general, are relatively rare diseases. When present, they have major effects on the patient's lifestyle, altering the craniofacial complex and exacerbating tooth decay. They have been associated with mutations in collagen in cases of osteogenesis imperfecta (OI) [136]. Defects in the human DSPP gene are the major cause of dentin disorders identified to date [137,138,139]. Mutations in DSPP are associated with five different types of inherited dentin defects – dentinogenesis imperfecta (DGI) types I, II, III and dentinal dysplasias (DD) types I and II. DGI has an incidence of 1/6000 to 1/8000 and DD an incidence of 1/100,000 [137]. In DGI and DI, the weakened dentin can lead to loss of enamel as it shears off the surface. DGI type I is associated with osteogenesis imperfecta and hence a defect in the underlying collagen matrix [140]. To date, all other types of DGI and DD have been associated with mutations in DSPP. It should be noted, that with the exception of the abnormal collagen in DGI type I, all of the other forms are associated with abnormalities in an IDP. Due to the collagen anomaly we suggest that the IDPs similarly contribute to the phenotype in DGI type I as the IDPs bind to specific sites on the collagen matrix. These sites may be altered in DG I or in OI, thereby altering the structure of the environment in which mineral crystals form. Thus, for most types of OI studied to date, the matrices tend to be hyper-mineralized (due to decreased collagen content) and perhaps normal amounts of HA, albeit with smaller HA crystals because the IDPs that regulate their growth are also altered in structure [141,142,143,144,145,146].
Enamel malformations are also associated with DSPP mutations, although enamel mineralization is not dependent on DSPP [147]. In the Dspp knockout (KO) mice, the teeth are hypo-mineralized [148] and similar to humans with DSPP mutations, the mice develop periodontal disease [149,150]. The dentin phenotype of the Dspp KO mouse mimics that of DGI type III, with widened pulp spaces and predentin and the presence of hypomineralized areas within the circumpulpal dentin [148]. A key study by von Marschall et al [151] suggested that the dentinal dysplasia phenotype is likely associated with the retention of the mutant DSPP in odontoblasts, i.e. a failure to “traffic” the mature DSPP protein out of the cell. Thus if the protein, independent of its mutation, remains trapped in the cell and cannot interact with collagen so as to regulate the mineralization process, the hypo-mineralized phenotype persists. This is likely to be true of other IDPs where mutations could lead to their inability to exit the Golgae and hence form the required complexes needed to initiate and facilitate mineral deposition.
DSPP is also expressed in bone, but patients with DSPP mutations have not been reported to have bone abnormalities. This is probably due to the low level of expression of DSPP in bone, redundant functions of the protein or differences in the processing of DSPP in bone and dentin [121, 139]. It should be noted that immature Dspp KO mice, contrasted with patients with DSPP mutations, have hypomineralized bones [89]. It is not known whether this is corrected as the mice age. In vitro TEM data suggested that DSPP could induce highly oriented intrafibrillar HA mineralization along the collagen fibril axis of self-assembled collagen gels [125].
Osteogenesis imperfecta (OI) is caused by abnormalities in at least 17 genes, all concerned with type I collagen synthesis, secretion, alignment and mineralization [136, 152,153]. The phenotypic variability of OI in different families and within the same family, with the identical mutations [154], may be attributable to unknown effects on other genes or to similarly unknown interactions with collagen during development. As noted above, interactions of many of the IDPs can be mapped to distinct sites on the type I collagen matrix [155]. It has been theorized that alterations in these sites can affect the binding of these noncollagenous IDPs [155]. This is supported by the previously reported lower amount of various IDPs in different forms of OI [153,154]. Related to the OI story, the serpins, proteins which regulate protease activities, are IDPs, and serpin F1 mutations are associated with type V OI [153]. Another possible example of defective IDPs leading to bone disease is osteoporosis which has been associated with defects in osteonectin [156], which, as noted above has IDP regions.
Osteopontin deficiency results in dystrophic calcification in humans [94] and in mice [157]. Studies from our laboratory in 2002 [158] showed that the osteopontin KO animal had greater amounts of mineral in mature areas of the bone than did control mice and mineral crystal size and perfection throughout all bones of the KO mice was significantly increased. The OPN KO animals had a 30% decrease in fracture toughness, and significant reductions in their elastic modulus [94]. In a solution [71] and in ectopic sites, osteopontin is an inhibitor of the formation and growth of HA crystals [157]. FGF23 deficiency leads to renal phosphate wasting, elevated circulating phosphate levels and surprisingly, rickets (hypomineralization). FGF23 KO mice over-express osteopontin [159] which is thought to be the origin of the rickets. Taken together, these data show that osteopontin needs to be flexible in order to i) be phosphorylated and dephosphorylated, ii) to interact with collagen (to facilitate mineralization), (iii) to interact with crystals (regulating their growth ad matrix crack population). Raine's syndrome, a rare condition with an incidence of 1/1,000,000, is due to a mutation in FAM20C the Golgi protein kinase that phosphorylates most secreted phosphoproteins including SIBLING proteins [160,161]. The osteosclerosis characteristic of this skeletal dysplasia provides clinical support for the importance of controlled phosphorylation of IDPs for proper mineralization [162,163].
5. Conclusions
This review has presented evidence that a large number of proteins associated with biomineralization are intrinsically disordered. We also summarized data showing that these proteins are involved in the process of biomineralization, and in some cases, have provided proof that these proteins are required for appropriate mineralization. It is demonstrated that having a flexible structure is important for allowing these proteins to take on a variety of forms in order to interact with their partners. To prove our theory of why IDPs are needed (lowering activation energy), kinetic experiments need to be conducted; but at this point the first parts of our concept on why IDPs are so important, have been validated.
HIGHLIGHTS.
A preponderance of the noncollagenous proteins associated with biomineralization are intrinsically disordered (IDPs).
IDPs regulate the mineralization process through interactions with ion clusters, pre-nucleation clusters, amorphous phases and crystalline phases.
Examples are provided from shells, enamel, dentin, bones and pathologic calcifications.
Acknowledgments
Our work as presented in this paper was acquired under NIH grants DE04141 and DE022716. Dr Villarreal-Ramírez acknowledges financial support received from DGAPA-CONACYT and programa UNAM-DGAPA-PAPIIT IA207216. The PERL-based script and SIBLINGs sequences analysis was carried out by Dr. Luis Fernando Lozano-Aguirre Beltran. We appreciate the editorial assistance of Dr. Judah Gerstein.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
ABBREVIATION; BSP-bone sialoprotein; CAP – carbonate hydroxyapatite; CD- circular dichroism; DD- dentinal dysplasia; DGI- dentinogenesis imperfecta; DMP1- dentin matrix protein 1; DPP- phosphophoryn; DSP – dentin sialophosphoprotein; DSPP – dentin sialophosphoprotein gene; HA – hydroxyapatite; IDP – intrinsically disordered protein; KO- knockout; MEPE-matrix extracellular phosphoglycoprotein; OI- osteogenesis imperfecta; OPN- osteopontin; SIBLING – small ligand binding N-glycoslated.
Conflict of Interest
The authors report no conflict of interest.
References
- 1.Reith F, Etschmann B, Grosse C, Moors H, Benotmane MA, Monsieurs P, Grass G, Doonan C, Vogt S, Lai B, Martinez-Criado G, George GN, Nies DH, Mergeay M, Pring A, Southam G, Brugger J. Mechanisms of gold biomineralization in the bacterium Cupriavidus metallidurans. Proc. Natl. Acad. Sci. U. S. A. 2001;106:17757–17762. doi: 10.1073/pnas.0904583106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sumper M, Brunner E, Lehmann G. Biomineralization in diatoms: characterization of novel polyamines associated with silica. FEBS Lett. 2005;579:3765–3769. doi: 10.1016/j.febslet.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 3.DeVol RT, Sun CY, Marcus MA, Coppersmith SN, Myneni SC, Gilbert PU. Nanoscale Transforming Mineral Phases in Fresh Nacre. J. Am. Chem. Soc. 2015;137:13325–13333. doi: 10.1021/jacs.5b07931. [DOI] [PubMed] [Google Scholar]
- 4.Paris O, Zizak I, Lichtenegger H, Roschger P, Klaushofer K, Fratzl P. Analysis of the hierarchical structure of biological tissues by scanning X-ray scattering using a micro-beam. Cell Mol. Biol. (Noisy-le-grand) 2000;46:993–1004. [PubMed] [Google Scholar]
- 5.Kalmar L, Homola D, Varga G, Tompa P. Structural disorder in proteins brings order to crystal growth in biomineralization. Bone. 2012;51:528–534. doi: 10.1016/j.bone.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 6.Dey A, Bomans PH, Müller FA, Will J, Frederik PM, de With G, Sommerdijk NA. The role of prenucleation clusters in surface-induced calcium phosphate crystallization. Nat. Mater. 2010;9:1010–1014. doi: 10.1038/nmat2900. [DOI] [PubMed] [Google Scholar]
- 7.Lohsse A, Ullrich S, Katzmann E, Borg S, Wanner G, Richter M, Voigt B, Schweder T, Schüler D. Functional analysis of the magnetosome island in Magnetospirillum gryphiswaldense: the mamAB operon is sufficient for magnetite biomineralization. PLOS One. 2011;6:e25561. doi: 10.1371/journal.pone.0025561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fernandez MS, Passalacqua K, Arias JI, Arias JL. Partial biomimetic reconstitution of avian eggshell formation. J. Struct. Biol. 2004;148:1–10. doi: 10.1016/j.jsb.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 9.Dodenhof T, Dietz F, Franken S, Grunwald I, Kelm S. Splice variants of perlucin from Haliotis laevigata modulate the crystallisation of CaCO3. PLOS One. 2014;9:e97126. doi: 10.1371/journal.pone.0097126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Asharani PV, Keupp K, Semler O, Wang W, Li Y, Thiele H, Yigit G, Pohl E, Becker J, Frommolt P, Sonntag C, Altmüller J, Zimmermann K, Greenspan DS, Akarsu NA, Netzer C, Schönau E, Wirth R, Hammerschmidt M, Nürnberg P, Wollnik B, Carney TJ. Attenuated BMP1 function compromises osteogenesis, leading to bone fragility in humans and zebrafish. Am. J. Hum. Genet. 2012;90:661–674. doi: 10.1016/j.ajhg.2012.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teepe JD, Schmitz JE, Hu Y, Yamada Y, Fajardo RJ, Smith CE, Chun YH. Correlation of ameloblastin with enamel mineral content. Connect. Tissue Res. 55 Suppl. 2014;1:38–42. doi: 10.3109/03008207.2014.923871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodriguez-Florez N, Garcia-Tunon E, Mukadam Q, Saiz E, Oldknow KJ, Farquharson C, Millán JL, Boyde A, Shefelbine SJ. An investigation of the mineral in ductile and brittle cortical mouse bone. Journal of Bone and Mineral Research. 2015;30:786–795. doi: 10.1002/jbmr.2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boskey AL, Christensen B, Taleb H, Sørensen ES. Post-translational modification of osteopontin: effects on in vitro hydroxyapatite formation and growth. Biochem. Biophys. Res. Commun. 2012;419:333–338. doi: 10.1016/j.bbrc.2012.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Endo A, Glimcher MJ. The effect of complexing phosphoproteins to decalcified collagen on in vitro calcification. Connective Tissue Research. 1989;21:179–90. doi: 10.3109/03008208909050008. [DOI] [PubMed] [Google Scholar]
- 15.Zhou S, Huang W, Belton DJ, Simmons LO, Perry CC, Wang X, Kaplan DL. Control of silicification by genetically engineered fusion proteins: silk-silica binding peptides. Acta Biomat. 2015;15:173–180. doi: 10.1016/j.actbio.2014.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gungormus M, Fong H, Kim IW, Evans JS, Tamerler C, Sarikaya M. Regulation of in vitro calcium phosphate mineralization by combinatorially selected hydroxyapatite-binding peptides. Biomacromolecules. 2008;9:966–873. doi: 10.1021/bm701037x. [DOI] [PubMed] [Google Scholar]
- 17.Xu Z, Yang Y, Zhao W, Wang Z, Landis WJ, Cui Q, Sahai N. Molecular mechanisms for intrafibrillar collagen mineralization in skeletal tissues. Biomaterials. 2015;39:59–66. doi: 10.1016/j.biomaterials.2014.10.048. [DOI] [PubMed] [Google Scholar]
- 18.Bromley KM, Kiss AS, Lokappa SB, Lakshminarayanan R, Fan D, Ndao M, Moradian-Oldak J. Dissecting amelogenin protein nanospheres: characterization of metastable oligomers. J. Biol. Chem. 2011;286:34643–34653. doi: 10.1074/jbc.M111.250928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wojtas M, Dobryszycki P, Ozyhar A. Seto Jong., editor. Intrinsically Disordered Proteins in Biomineralization. Advanced Topics in Biomineralization. 2012 www.Intechopen.com. published 2012-02-17.
- 20.van der Lee R, Buljan M, Lang B, Weatheritt RJ, Daughdrill GW, Dunker AK, Fuxreiter M, Gough J, Gsponer J, Jones DT, Kim PM, Kriwacki RW, Oldfield CJ, Pappu RV, Tompa P, Uversky VN, Wright PE, Babu MM. Classification of intrinsically disordered regions and proteins. Chem. Rev. 2014;114:6589–6631. doi: 10.1021/cr400525m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Theillet FX, Binolfi A, Frembgen-Kesner T, Hingorani K, Sarkar M, Kyne C, Li C, Crowley PB, Gierasch L, Pielak GJ, Elcock AH, Gershenson A, Selenko P. Physicochemical properties of cells and their effects on intrinsically disordered proteins (IDPs). Chem. Rev. 2014;114:6661–6714. doi: 10.1021/cr400695p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uversky VN, Oldfield CJ, Midic U, Xie H, Xue B, Vucetic S, Iakoucheva LM, Obradovic Z, Dunker AK. Unfoldomics of human diseases: linking protein intrinsic disorder with diseases. BMC Genomics. 2009;10(Suppl 1):S7. doi: 10.1186/1471-2164-10-S1-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Orgel JP, Irving TC, Miller A, Wess TJ. Microfibrillar structure of type I collagen in situ. Proc. Natl. Acad. Sci. U. S .A. 2006;103:9001–9005. doi: 10.1073/pnas.0502718103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Habchi J, Tompa P, Longhi S, Uversky VN. Introducing protein intrinsic disorder. Chem. Reviews. 2014;114:6561–6588. doi: 10.1021/cr400514h. [DOI] [PubMed] [Google Scholar]
- 25.Lee SL, Veis A, Glonek T. Dentin phosphoprotein: an extracellular calcium-binding protein. Biochemistry. 1977;16:2971–2979. doi: 10.1021/bi00632a026. [DOI] [PubMed] [Google Scholar]
- 26.Prasad M, Butler WT, Qin C. Dentin sialophosphoprotein in biomineralization. Connect. Tissue Res. 2010;51:404–417. doi: 10.3109/03008200903329789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Evans JS, Chan SI. Phosphophoryn, a biomineralization template protein: pH-dependent protein folding experiments. Biopolymers. 1994;34:507–527. doi: 10.1002/bip.360340407. [DOI] [PubMed] [Google Scholar]
- 28.Fisher LW, Torchia DA, Fohr B, Young MF, Fedarko NS. Flexible structures of SIBLING proteins, bone sialoprotein, and osteopontin. Biochem. Biophys. Res. Commun. 2001;280:460–465. doi: 10.1006/bbrc.2000.4146. [DOI] [PubMed] [Google Scholar]
- 29.Fisher LW. DMP1 and DSPP: Evidence for Duplication and Convergent Evolution of Two SIBLING Proteins. Cells, Tissues, Organs. 2011;194:113–118. doi: 10.1159/000324254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gericke A, Qin C, Spevak L, Fujimoto Y, Butler WT, Sørensen ES, Boskey AL. Importance of phosphorylation for osteopontin regulation of biomineralization. Calcif. Tissue Int. 2005;77:45–54. doi: 10.1007/s00223-004-1288-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gericke A, Qin C, Sun Y, Redfern R, Redfern D, Fujimoto Y, Taleb H, Butler WT, Boskey AL. Different forms of DMP1 play distinct roles in mineralization. J. Dent. Res. 2010;89:355–359. doi: 10.1177/0022034510363250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fuxreiter M, Tompa P. Fuzzy complexes: a more stochastic view of protein function. Adv. Exp. Med. Biol. 2012;725:1–14. doi: 10.1007/978-1-4614-0659-4_1. [DOI] [PubMed] [Google Scholar]
- 33.Gao J, Xu D. Correlation between posttranslational modification and intrinsic disorder in protein. Pac. Symp. Biocomput. 2012:94–103. [PMC free article] [PubMed] [Google Scholar]
- 34.Tartaix PH, Doulaverakis M, George A, Fisher LW, Butler WT, Qin C, Salih E, Tan M, Fujimoto Y, Spevak L, Boskey AL. In vitro effects of dentin matrix protein-1 on hydroxyapatite formation provide insights into in vivo functions. J. Biol. Chem. 2004;279:18115–18120. doi: 10.1074/jbc.M314114200. [DOI] [PubMed] [Google Scholar]
- 35.Song J, Guo L-W, Muradov H, Artemyev NO, Ruoho AE, Markley JL. Intrinsically disordered γ-subunit of cGMP phosphodiesterase encodes functionally relevant transient secondary and tertiary structure. P.N.A.S. 2008;105:1505–1510. doi: 10.1073/pnas.0709558105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Amos FF, Ndao M, Ponce CB, Evans JS. A C-RING-like domain participates in protein self-assembly and mineral nucleation. Biochemistry. 2011;50:8880–8887. doi: 10.1021/bi201346d. [DOI] [PubMed] [Google Scholar]
- 37.Chang EP, Russ JA, Verch A, Kröger R, Estroff LA, Evans JS. The Intrinsically Disordered C-RING Biomineralization Protein, AP7, Creates Protein Phases That Introduce Nanopatterning and Nanoporosities into Mineral Crystals. Biochemistry. 2014;53:4317–4319. doi: 10.1021/bi500664w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gong YU, Killian CE, Olson IC, Appathurai NP, Amasino AL, Martin MC, Holt LJ, Wilt FH, Gilbert PU. Phase transitions in biogenic amorphous calcium carbonate. Proc. Natl. Acad. Sci. U. S. A. 2012;109:6088–6093. doi: 10.1073/pnas.1118085109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rao A, Seto J, Berg JK, Kreft SG, Scheffner M, Cölfen H. Roles of larval sea urchin spicule SM50 domains in organic matrix self-assembly and calcium carbonate mineralization. J. Struct. Biol. 2013;183:205–215. doi: 10.1016/j.jsb.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 40.Zhang B, Xu G, Evans JS. Model peptide studies of sequence repeats derived from the intracrystalline biomineralization protein, SM50. II. Pro, Asn-rich tandem repeats. Biopolymers. 2000;54:464–475. doi: 10.1002/1097-0282(200011)54:6<464::AID-BIP90>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 41.Różycka M, Wojtas M, Jakób M, Stigloher C, Grzeszkowiak M, Mazur M, Ożyhar A. Intrinsically disordered and pliable Starmaker-like protein from medaka (Oryzias latipes) controls the formation of calcium carbonate crystals. PLoS One. 2014;9:e114308. doi: 10.1371/journal.pone.0114308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wojtas M, Hołubowicz R, Poznar M, Maciejewska M, Ożyhar A, Dobryszycki P. Calcium Ion Binding Properties and the Effect of Phosphorylation on the Intrinsically Disordered Starmaker Protein. Biochemistry. 2015;54:6525–6534. doi: 10.1021/acs.biochem.5b00933. [DOI] [PubMed] [Google Scholar]
- 43.Delak K, Harcup C, Lakshminarayanan R, Sun Z, Fan YW, Moradian-Oldak J, Evans JS. The tooth enamel protein, porcine amelogenin, is an intrinsically disordered protein with an extended molecular configuration in the monomeric form. Biochemistry. 2009;48:2272–2281. doi: 10.1021/bi802175a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wald T, Osickova A, Sulc M, Benada O, Semeradtova A, Rezabkova L, Veverka V, Bednarova L, Maly J, Macek P, Sebo P, Slaby I, Vondrasek J, Osicka R. Intrinsically disordered enamel matrix protein ameloblastin forms ribbon-like supramolecular structures via an N-terminal segment encoded by exon 5. J. Biol. Chem. 2013;288:22333–22345. doi: 10.1074/jbc.M113.456012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Snead ML, Lau EC, Zeichner-David M, Fincham AG, Woo SLC, Slavkin HC. DNA-sequence for cloned cDNA for murine amelogenin reveal the amino-acid sequence for enamel-specific protein. Biochem. Biophys. Res. Commun. 1985;129:812–818. doi: 10.1016/0006-291x(85)91964-3. [DOI] [PubMed] [Google Scholar]
- 46.Krebsbach PH, Lee SK, Matsuki Y, Kozak CA, Yamada RM, Yamada Y. Full-length sequence, localization, and chromosomal mapping of ameloblastin - a novel tooth-specific gene. J. Biol. Chem. 1996;271:4431–4435. doi: 10.1074/jbc.271.8.4431. [DOI] [PubMed] [Google Scholar]
- 47.Hu JCC, Sun XL, Zhang CH, Simmer JP. A comparison of enamelin and amelogenin expression in developing mouse molars. Eur. J. Oral Sci. 2001;109:125–132. doi: 10.1034/j.1600-0722.2001.00998.x. [DOI] [PubMed] [Google Scholar]
- 48.Hu JC, Yamakoshi Y. Enamelin and autosomal-dominant amelogenesis imperfecta. Crit. Rev. Oral Biol. Med. 2003;14:387–398. doi: 10.1177/154411130301400602. [DOI] [PubMed] [Google Scholar]
- 49.Bartlett JD, Simmer JP. Proteinases in developing dental enamel. Crit. Rev. Oral Biol. Med. 1999;10:425–441. doi: 10.1177/10454411990100040101. [DOI] [PubMed] [Google Scholar]
- 50.Smith CE, Wazen R, Hu Y, Zalzal SF, Nanci A, Simmer JP, Hu JC. Consequences for enamel development and mineralization resulting from loss of function of ameloblastin or enamelin. Eur. J. Oral Sci. 2009;117:485–497. doi: 10.1111/j.1600-0722.2009.00666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wazen RM, Moffatt P, Zalzal SF, Yamada Y, Nanci A. A mouse model expressing a truncated form of ameloblastin exhibits dental and junctional epithelium defects. Matrix Biol. 2009;28:292–303. doi: 10.1016/j.matbio.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fukumoto S, Yamada A, Nonaka K, Yamada Y. Essential roles of ameloblastin in maintaining ameloblast differentiation and enamel formation. Cells Tissues Organs. 2005;181:189–195. doi: 10.1159/000091380. [DOI] [PubMed] [Google Scholar]
- 53.Zalzal SF, Smith CE, Nanci A. Ameloblastin and amelogenin share a common secretory pathway and are co-secreted during enamel formation. Matrix Biol. 2008;27:352–359. doi: 10.1016/j.matbio.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 54.Mazumder P, Prajapati S, Lokappa SB, Gallon V, Moradian-Oldak J. Analysis of co-assembly and co-localization of ameloblastin and amelogenin. Front. Physiol. 2014;5:274. doi: 10.3389/fphys.2014.00274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Uchida T, Murakami C, Dohi N, Wakida K, Satoda T, Takahashi O. Synthesis, secretion, degradation, and fate of ameloblastin during the matrix formation stage of the rat incisor as shown by immunocytochemistry and immunochemistry using region-specific antibodies. J. Histochem. Cytochem. 1997;45:1329–1340. doi: 10.1177/002215549704501002. [DOI] [PubMed] [Google Scholar]
- 56.Lakshminarayanan R, Bromley KM, Lei YP, Snead ML, Moradian-Oldak J. Perturbed amelogenin secondary structure leads to uncontrolled aggregation in amelogenesis imperfecta mutant proteins. J. Biol. Chem. 2010;285:40593–40603. doi: 10.1074/jbc.M110.131136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Buchko GW, Lin G, Tarasevich BJ, Shaw WJ. A solution NMR investigation into the impaired self-assembly properties of two murine amelogenins containing the point mutations T21→I or P41→T. Arch. Biochem. Biophys. 2013;537:217–224. doi: 10.1016/j.abb.2013.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ruan Q, Moradian-Oldak J. Amelogenin and Enamel Biomimetics. J. Mater. Chem. B. Mater. Biol. Med. 2015;3:3112–3129. doi: 10.1039/C5TB00163C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Deshpande AS, Fang PA, Simmer JP, Margolis HC, Beniash E. Amelogenin-collagen interactions regulate calcium phosphate mineralization in vitro. J. Biol. Chem. 2010;285:19277–19287. doi: 10.1074/jbc.M109.079939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fang PA, Conway JF, Margolis HC, Simmer JP, Beniash E. Hierarchical self-assembly of amelogenin and the regulation of biomineralization at the nanoscale. Proc. Natl. Acad. Sci. U. S. A. 2011;108:14097–14102. doi: 10.1073/pnas.1106228108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fang PA, Margolis HC, Conway JF, Simmer JP, Beniash E. CryoTEM study of effects of phosphorylation on the hierarchical assembly of porcine amelogenin and its regulation of mineralization in vitro. J. Struct. Biol. 2013;183:250–257. doi: 10.1016/j.jsb.2013.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Demichelis R, Raiteri P, Gale JD, Quigley D, Gebauer D. Stable prenucleation mineral clusters are liquid-like ionic polymers. Nat. Commun. 2011;2:590. doi: 10.1038/ncomms1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Goobes G, Goobes R, Schueler-Furman O, Baker D, Stayton PS, Drobny GP. Folding of the C-terminal bacterial binding domain in statherin upon adsorption onto hydroxyapatite crystals. Proc. Natl. Acad. Sci. U.S.A. 2006;103:16083–16088. doi: 10.1073/pnas.0607193103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Holt C. Unfolded phosphopolypeptides enable soft and hard tissues to coexist in the same organism with relative ease. Curr. Opin. Struct. Biol. 2013;23:420–425. doi: 10.1016/j.sbi.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 65.Stayton PS, Drobny GP, Shaw WJ, Long JR, Gilbert M. Molecular Recognition at the Protein-Hydroxyapatite Interface. Crit. Rev. Oral Biol. Med. 2003;14:370–376. doi: 10.1177/154411130301400507. [DOI] [PubMed] [Google Scholar]
- 66.Rangiani A, Cao Z, Sun Y, Lu Y, Gao T, Yuan B, Rodgers A, Qin C, Kuro-O M, Feng JQ. Protective Roles of DMP1 in High Phosphate Homeostasis. PLoS ONE. 2012;7:e42329. doi: 10.1371/journal.pone.0042329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wegener H, Paulsen H, Seeger K. The cysteine-rich region of type VII collagen is a cystine knot with a new topology. J. Biol. Chem. 2014;289:4861–4869. doi: 10.1074/jbc.M113.531327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Murshed M, McKee MD. Molecular determinants of extracellular matrix mineralization in bone and blood vessels. Curr. Opi. Nephrol. Hypertens. 2010;19:359–365. doi: 10.1097/MNH.0b013e3283393a2b. [DOI] [PubMed] [Google Scholar]
- 69.Wolak T. Osteopontin - a multi-modal marker and mediator in atherosclerotic vascular disease. Atherosclerosis. 2014;236:327–337. doi: 10.1016/j.atherosclerosis.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 70.Urganus AL, Zhao YD, Pachman LM. Juvenile dermatomyositis calcifications selectively displayed markers of bone formation. Arthritis Rheum. 2009;61:501–508. doi: 10.1002/art.24391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Boskey AL, Maresca M, Ullrich W, Doty SB, Butler WT, Prince CW. Osteopontin-hydroxyapatite interactions in vitro: inhibition of hydroxyapatite formation and growth in a gelatin-gel. Bone Miner. 1993;22:147–159. doi: 10.1016/s0169-6009(08)80225-5. [DOI] [PubMed] [Google Scholar]
- 72.Hunter GK, Kyle CL, Goldberg HA. Modulation of crystal formation by bone phosphoproteins: structural specificity of the osteopontin-mediated inhibition of hydroxyapatite formation. Biochem. J. 1994;300:723–728. doi: 10.1042/bj3000723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.de Bruyn JR, Goiko M, Mozaffari M, Bator D, Dauphinee RL, Liao Y, Flemming RL, Bramble MS, Hunter GK, Goldberg HA. Dynamic light scattering study of inhibition of nucleation and growth of hydroxyapatite crystals by osteopontin. PLoS One. 2013;8:e56764. doi: 10.1371/journal.pone.0056764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.He G, Dahl T, Veis A, George A. Nucleation of apatite crystals in vitro byself-assembled dentin matrix protein 1. Nat. Mater. 2003;2:552–558. doi: 10.1038/nmat945. [DOI] [PubMed] [Google Scholar]
- 75.He G, Dahl T, Veis A, George A. Dentin matrix protein 1 initiates hydroxyapatite formation in vitro. Connect .Tissue Res . 2003;44(Suppl 1):240–245. [PubMed] [Google Scholar]
- 76.Boskey AL, Chiang P, Fermanis A, Brown J, Taleb H, David V, Rowe PS. MEPE's diverse effects on mineralization. Calcif. Tissue Int. 2010;86:42–46. doi: 10.1007/s00223-009-9313-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hunter GK, Goldberg HA. Nucleation of hydroxyapatite by bone sialoprotein. Proc. Natl. Acad. Sci. U. S. A. 1993;90:8562–8565. doi: 10.1073/pnas.90.18.8562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hunter GK, Goldberg HA. Modulation of crystal formation by bone phosphoproteins: role of glutamic acid-rich sequences in the nucleation of hydroxyapatite by bone sialoprotein. Biochem. J. 1994;302:175–179. doi: 10.1042/bj3020175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Boskey AL, Maresca M, Doty S, Sabsay B, Veis A. Concentration-dependent effects of dentin phosphophoryn in the regulation of in vitro hydroxyapatite formation and growth. Bone Miner. 1990;11:55–65. doi: 10.1016/0169-6009(90)90015-8. [DOI] [PubMed] [Google Scholar]
- 80.Boskey A, Spevak L, Tan M, Doty SB, Butler WT. Dentin sialoprotein (DSP) has limited effects on in vitro apatite formation and growth. Calcif. Tissue Int. 2000;67:472–4788. doi: 10.1007/s002230001169. [DOI] [PubMed] [Google Scholar]
- 81.Jain A, Karadag A, Fohr B, Fisher LW, Fedarko NS. Three SIBLINGs (small integrin-binding ligand, N-linked glycoproteins) enhance factor H's cofactor activity enabling MCP-like cellular evasion of complement-mediated attack. J. Biol. Chem. 2002;277:13700–13708. doi: 10.1074/jbc.M110757200. [DOI] [PubMed] [Google Scholar]
- 82.Bouleftour W, Boudiffa M, Wade-Gueye NM, Bouët G, Cardelli M, Laroche N, Vanden-Bossche A, Thomas M, Bonnelye E, Aubin JE, Vico L, Lafage-Proust MH, Malaval L. Skeletal development of mice lacking bone sialoprotein (BSP)--impairment of long bone growth and progressive establishment of high trabecular bone mass. PLoS One. 2014;9:e95144. doi: 10.1371/journal.pone.0095144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Holm E, Aubin JE, Hunter GK, Beier F, Goldberg HA. Loss of bone sialoprotein leads to impaired endochondral bone development and mineralization. Bone. 2015;71:145–154. doi: 10.1016/j.bone.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 84.Baht GS, Hunter GK, Goldberg HA. Bone sialoprotein-collagen interaction promotes hydroxyapatite nucleation. Matrix Biol. 2008;27:600–608. doi: 10.1016/j.matbio.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 85.Ye L, MacDougall M, Zhang S, Xie Y, Zhang J, Li Z, Lu Y, Mishina Y, Feng JQ. Deletion of dentin matrix protein-1 leads to a partial failure of maturation of predentin into dentin, hypomineralization, and expanded cavities of pulp and root canal during postnatal tooth development. J. Biol. Chem. 2004;279:19141–19148. doi: 10.1074/jbc.M400490200. [DOI] [PubMed] [Google Scholar]
- 86.Negri AL. Hereditary hypophosphatemias: new genes in the bone-kidney axis. Nephrology (Carlton) 2007;12:317–320. doi: 10.1111/j.1440-1797.2007.00824.x. [DOI] [PubMed] [Google Scholar]
- 87.Napierala D, Sun Y, Maciejewska I, Bertin TK, Dawson B, D'Souza R, Qin C, Lee B. Transcriptional repression of the Dspp gene leads to dentinogenesis imperfecta phenotype in Col1a1-Trps1 transgenic mice. J. Bone Miner. Res. 2012;27:735–1745. doi: 10.1002/jbmr.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fang PA, Verdelis K, Yang X, Lukashova L, Boskey AL, Beniash E. Ultrastructural organization of dentin in mice lacking dentin sialo-phosphoprotein. Connect Tissue Res.55 Suppl. 2014;1:92–96. doi: 10.3109/03008207.2014.923861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Verdelis K, Ling Y, Sreenath T, Haruyama N, MacDougall M, van der Meulen MC, Lukashova L, Spevak L, Kulkarni AB, Boskey AL. DSPP effects on in vivo bone mineralization. Bone. 2008;43:983–990. doi: 10.1016/j.bone.2008.08.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hu JC, Hu Y, Lu Y, Smith CE, Lertlam R, Wright JT, Suggs C, McKee MD, Beniash E, Kabir ME, Simmer JP. Enamelin is critical for ameloblast integrity and enamel ultrastructure formation. PLoS One. 2014;9:e89303. doi: 10.1371/journal.pone.0089303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Doi Y, Eanes ED, Shimokawa H, Termine JD. Inhibition of seeded growth of enamel apatite crystals by amel16ogenin and enamelin proteins in vitro. J. Dent. Res. 1984;63:98–105. doi: 10.1177/00220345840630021801. [DOI] [PubMed] [Google Scholar]
- 92.Gowen LC, Petersen DN, Mansolf AL, Qi H, Stock JL, Tkalcevic GT, Simmons HA, Crawford DT, Chidsey-Frink KL, Ke HZ, McNeish JD, Brown TA. Targeted disruption of the osteoblast/osteocyte factor 45 gene (OF45) results in increased bone formation and bone mass. J. Biol. Chem. 2003;278:1998–2007. doi: 10.1074/jbc.M203250200. [DOI] [PubMed] [Google Scholar]
- 93.Boskey AL, Chiang P, Fermanis A, Brown J, Taleb H, David V, Rowe PS. MEPE's diverse effects on mineralization. Calcif. Tissue Int. 2010;86:42–46. doi: 10.1007/s00223-009-9313-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Thurner PJ, Chen CG, Ionova-Martin S, Sun L, Harman A, Porter A, Ager JW, 3rd, Ritchie RO, Alliston T. Osteopontin deficiency increases bone fragility but preserves bone mass. Bone. 2010;46:1564–1573. doi: 10.1016/j.bone.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Boskey AL, Spevak L, Paschalis E, Doty SB, McKee MD. Osteopontin deficiency increases mineral content and mineral crystallinity in mouse bone. Calcif. Tissue Int. 2002;71:145–154. doi: 10.1007/s00223-001-1121-z. [DOI] [PubMed] [Google Scholar]
- 96.Boskey AL, Maresca M, Ullrich W, Doty SB, Butler WT, Prince CW. Osteopontin-hydroxyapatite interactions in vitro: inhibition of hydroxyapatite formation and growth in a gelatin-gel. Bone Miner. 1993;22:147–159. doi: 10.1016/s0169-6009(08)80225-5. [DOI] [PubMed] [Google Scholar]
- 97.Norde W. Protein adsorption at solid surfaces: A thermodynamic approach. Pure & Appl. Chem. 1994;66:491–496. [Google Scholar]
- 98.Bhakta SA, Evans E, Benavidez TE, Garcia CD. Protein adsorption onto nanomaterials for the development of biosensors and analytical devices: A review. Anal. Chim. Acta. 2015;872:7–25. doi: 10.1016/j.aca.2014.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rabe M, Verdes D, Seeger S. Understanding protein adsorption phenomena at solid surfaces. Adv. Colloid Interface Sci. 2011;162:87–106. doi: 10.1016/j.cis.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 100.Wahlgren M, Arnebrant T. Protein adsorption to solid surfaces. Trends in biotechnology. 1991;9:201–208. doi: 10.1016/0167-7799(91)90064-o. [DOI] [PubMed] [Google Scholar]
- 101.Billsten P, Wahlgren M, Arnebrant T, McGuire J, Elwing H. Structural Changes of T4 Lysozyme upon Adsorption to Silica Nanoparticles Measured by Circular Dichroism. J. Colloid Interface Sci. 1995;175:77–82. [Google Scholar]
- 102.Giacomelli CE, Norde W. The Adsorption–Desorption Cycle. Reversibility of the BSA– Silica System. J. Colloid Interface Sci. 2001;233:234–240. doi: 10.1006/jcis.2000.7219. [DOI] [PubMed] [Google Scholar]
- 103.Barbucci R, Magnani A. Conformation of human plasma proteins at polymer surfaces: the effectiveness of surface heparinization. Biomaterials. 1994;15:955–962. doi: 10.1016/0142-9612(94)90075-2. [DOI] [PubMed] [Google Scholar]
- 104.Santore MM, Wertz CF. Protein Spreading Kinetics at Liquid−Solid Interfaces via an Adsorption Probe Method†. Langmuir. 2005;21:10172–10178. doi: 10.1021/la051059s. [DOI] [PubMed] [Google Scholar]
- 105.Sethuraman A, Belfort G. Protein Structural Perturbation and Aggregation on Homogeneous Surfaces. Biophys. J . 2005;88:1322–1333. doi: 10.1529/biophysj.104.051797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hlady V, Buijs J. Protein adsorption on solid surfaces. Curr. Opin. Biotech. 1996;7:72–77. doi: 10.1016/s0958-1669(96)80098-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lu J-x., Burton SD, Xu YS, Buchko GW, Shaw WJ. The flexible structure of the K24S28 region of Leucine-Rich Amelogenin Protein (LRAP) bound to apatites as a function of surface type, calcium, mutation, and ionic strength. Front. Physiol. 2014;5:86. doi: 10.3389/fphys.2014.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Villarreal-Ramirez E, Garduño-Juarez R, Gericke A, Boskey A. The role of phosphorylation in dentin phosphoprotein peptide absorption to hydroxyapatite surfaces: a molecular dynamics study. Connect. Tissue Res. 55 Suppl. 2014;1:134–137. doi: 10.3109/03008207.2014.923870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Uversky VN. Unusual biophysics of intrinsically disordered proteins. Biochem. Biophys. Acta. 2013;18344:932–951. doi: 10.1016/j.bbapap.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 110.Kröger N, Lorenz S, Brunner E, Sumper M. Self-assembly of highly phosphorylated silaffins and their function in biosilica morphogenesis. Science. 2002;298:584–586. doi: 10.1126/science.1076221. [DOI] [PubMed] [Google Scholar]
- 111.Lechner C, Becker C. Silaffins in Silica Biomineralization and Biomimetic Silica Precipitation. Marine Drugs. 2015;13:5297–5333. doi: 10.3390/md13085297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gilbert PUPA, Wilt F. Molecular Aspects of Biomineralization of the Echinoderm Endoskeleton. In: Müller WEG, editor. Molecular Biomineralization. Vol. 52. Springer; Berlin Heidelberg: 2011. pp. 199–223.pp. 19 [DOI] [PubMed] [Google Scholar]
- 113.Beniash E, Simmer JP, Margolis HC. Structural Changes in Amelogenin upon Self-assembly and Mineral Interactions. J. Dent. Res. 2012;91:967–972. doi: 10.1177/0022034512457371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Uversky VN. Functional roles of transiently and intrinsically disordered regions within protons. FEBS. J. 2015;282:1182–1189. doi: 10.1111/febs.13202. [DOI] [PubMed] [Google Scholar]
- 115.Lenton S, Seydel T, Nylander T, Holt C, Hartlein M, Teixeira S, Zaccai G. Dynamic footprint of sequestration in the molecular fluctuations of osteopontin. J. R. Soc. Interface. 2015;12:20150506. doi: 10.1098/rsif.2015.0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cartwright JH, Checa AG, Gale JD, Gebauer D, Sainz-Díaz CI. Calcium carbonate polyamorphism and its role in biomineralization: how many amorphous calcium carbonates are there? Angew. Chem. Int. Ed. Engl. 2012;51:11960–11970. doi: 10.1002/anie.201203125. [DOI] [PubMed] [Google Scholar]
- 117.Gebauer D, Gunawidjaja PN, Ko JY, Bacsik Z, Aziz B, Liu L, Hu Y, Bergström L, Tai CW, Sham TK, Edén M, Hedin N. Proto-calcite and proto-vaterite in amorphous calcium carbonates. Angew. Chem. Int. Ed. 2010;49:8889–8891. doi: 10.1002/anie.201003220. [DOI] [PubMed] [Google Scholar]
- 118.Park YS, Lee JY, Suh JS, Jin YM, Yu Y, Kim HY, Park YJ, Chung CP, Jo I. Selective osteogenesis by a synthetic mineral inducing peptide for the treatment of osteoporosis. Biomaterials. 2014;35:9747–9754. doi: 10.1016/j.biomaterials.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 119.Veis A, Wu CB. Phosphorylation of the proteins of the extracellular matrix of mineralized tissues by casein kinase-like activity. Crit. Rev. oral Biol. 1997;8:360–79. doi: 10.1177/10454411970080040101. [DOI] [PubMed] [Google Scholar]
- 120.Hunter GK, O'Young J, Grohe B, Karttunen M, Goldberg HA. The flexible polyelectrolyte hypothesis of protein-biomineral interaction. Langmuir. 2010;26:18639–18646. doi: 10.1021/la100401r. [DOI] [PubMed] [Google Scholar]
- 121.Suzuki S, Haruyama N, Nishimura F, Kulkarni AB. Dentin sialophosphoprotein and dentin matrix protein-1: Two highly phosphorylated proteins in mineralized tissues. Arch. Oral Biol. 2012;57:1165–1175. doi: 10.1016/j.archoralbio.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wu H, Teng PN, Jayaraman T, Onishi S, Li J, Bannon L, Huang H, Close J, Sfeir C. Dentin matrix protein 1 (DMP1) signals via cell surface integrin. J. Biol. Chem. 2011;286:29462–29469. doi: 10.1074/jbc.M110.194746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Eapen A, Ramachandran A, Pratap J, George A. Activation of the ERK1/2 mitogen-activated protein kinase cascade by dentin matrix protein 1 promotes osteoblast differentiation. Cells Tissues Organs. 2011;194:255–260. doi: 10.1159/000324258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.He G, Gajjeraman S, Schultz D, Cookson D, Qin C, Butler WT, Hao J, George A. Spatially and temporally controlled biomineralization is facilitated by interaction between self-assembled dentin matrix protein 1 and calcium phosphate nuclei in solution. Biochemistry. 2005;44:16140–16148. doi: 10.1021/bi051045l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Deshpande AS, Fang PA, Zhang X, Jayaraman T, Sfeir C, Beniash E. Primary structure and phosphorylation of dentin matrix protein 1 (DMP1) and dentin phosphophoryn (DPP) uniquely determine their role in biomineralization. Biomacromolecules. 2011;12:2933–2945. doi: 10.1021/bm2005214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rowe PS. Regulation of bone-renal mineral and energy metabolism: the PHEX, FGF23, DMP1, MEPE ASARM pathway. Crit. Rev. Eukaryot. Gene Expr. 2012;22:61–86. doi: 10.1615/critreveukargeneexpr.v22.i1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wallwork ML, Kirkham J, Chen H, Chang SX, Robinson C, Smith DA, Clarkson BH. Binding of dentin noncollagenous matrix proteins to biological mineral crystals: an atomic force microscopy study. Calcif. Tissue Int. 2002;71:249–255. doi: 10.1007/s00223-001-1011-4. [DOI] [PubMed] [Google Scholar]
- 128.Baht GS, O'Young J, Borovina A, Chen H, Tye CE, Karttunen M, Lajoie GA, Hunter GK, Goldberg HA. Phosphorylation of Ser136 is critical for potent bone sialoprotein-mediated nucleation of hydroxyapatite crystals. Biochem. J. 2010;428:385–395. doi: 10.1042/BJ20091864. [DOI] [PubMed] [Google Scholar]
- 129.Yang Y, Cui Q, Sahai N. How does bone sialoprotein promote the nucleation of hydroxyapatite? A molecular dynamics study using model peptides of different conformations. Langmuir. 2010;26:9848–9859. doi: 10.1021/la100192z. [DOI] [PubMed] [Google Scholar]
- 130.Vincent K, Durrant MC. A structural and functional model for human bone sialoprotein. J. Mol. Graph. Model. 2013;39:108–117. doi: 10.1016/j.jmgm.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 131.Azzopardi PV, O'Young J, Lajoie G, Karttunen M, Goldberg HA, Hunter GK. Roles of electrostatics and conformation in protein-crystal interactions. PLoS One. 2010;5:e9330. doi: 10.1371/journal.pone.0009330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.O'Young J, Chirico S, Al Tarhuni N, Grohe B, Karttunen M, Goldberg HA, Hunter GK. Phosphorylation of osteopontin peptides mediates adsorption to and incorporation into calcium oxalate crystals. Cells Tissues Organs. 2009;189:51–55. doi: 10.1159/000151724. [DOI] [PubMed] [Google Scholar]
- 133.Tao J, Battle KC, Pan H, Salter EA, Chien YC, Wierzbicki A, De Yoreo JJ. Energetic basis for the molecular-scale organization of bone. Proc. Natl. Acad. Sci. U. S. A. 2015;112:326–331. doi: 10.1073/pnas.1404481112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ye L, MacDougall M, Zhang S, Xie Y, Zhang J, Li Z, Lu Y, Mishina Y, Feng JQ. Deletion of dentin matrix protein-1 leads to a partial failure of maturation of predentin into dentin, hypomineralization, and expanded cavities of pulp and root canal during postnatal tooth development. J. Biol. Chem. 2004;279:19141–19148. doi: 10.1074/jbc.M400490200. [DOI] [PubMed] [Google Scholar]
- 135.Negri AL. Hereditary hypophosphatemias: new genes in the bone-kidney axis. Nephrology (Carlton) 2007;12:317–220. doi: 10.1111/j.1440-1797.2007.00824.x. [DOI] [PubMed] [Google Scholar]
- 136.Marini JC, Forlino A, Cabral WA, Barnes AM, San Antonio JD, Milgrom S, Hyland JC, Körkkö J, Prockop DJ, De Paepe A, Coucke P, Symoens S, Glorieux FH, Roughley PJ, Lund AM, Kuurila-Svahn K, Hartikka H, Cohn DH, Krakow D, Mottes M, Schwarze U, Chen D, Yang K, Kuslich C, Troendle J, Dalgleish R, Byers PH. Consortium for osteogenesis imperfecta mutations in the helical domain of type I collagen: regions rich in lethal mutations align with collagen binding sites for integrins and proteoglycans. Hum. Mutat. 2007;28:209–221. doi: 10.1002/humu.20429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Barron MJ, McDonnell ST, Mackie I, Dixon MJ. Hereditary dentine disorders: dentinogenesis imperfecta and dentine dysplasia. Orphanet. J. Rare Dis. 2008;3:31. doi: 10.1186/1750-1172-3-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Takagi Y, Veis A, Sauk JJ. Relation of mineralization defects in collagen matrices to noncollagenous protein components. Identification of a molecular defect in dentinogenesis imperfecta. Clin. Orthop. Relat. Res. 1983;176:282–90. [PubMed] [Google Scholar]
- 139.Thotakura SR, Mah T, Srinivasan R, Takagi Y, Veis A, George A. The noncollagenous dentin matrix proteins are involved in dentinogenesis imperfecta type II (DGI-II). J. Dent. Res. 2000;79:835–839. doi: 10.1177/00220345000790030901. [DOI] [PubMed] [Google Scholar]
- 140.Di Lullo GA, Sweeney SM, Korkko J, Ala-Kokko L, San Antonio JD. Mapping the ligand-binding sites and disease-associated mutations on the most abundant protein in the human, type I collagen. J. Biol. Chem. 2002;277:4223–42. doi: 10.1074/jbc.M110709200. [DOI] [PubMed] [Google Scholar]
- 141.Brenner RE, Vetter U, Stöss H, Müller PK, Teller WM. Defective collagen fibril formation and mineralization in osteogenesis imperfecta with congenital joint contractures (Bruck syndrome). Eur. J. Pediatr. 1993;152:505–508. doi: 10.1007/BF01955060. [DOI] [PubMed] [Google Scholar]
- 142.Fisher LW, Eanes ED, Denholm LJ, Heywood BR, Termine JD. Two bovine models of osteogenesis imperfecta exhibit decreased apatite crystal size. Calcif. Tissue Int. 1987;40:282–285. doi: 10.1007/BF02555262. [DOI] [PubMed] [Google Scholar]
- 143.Fratzl P, Paris O, Klaushofer K, Landis WJ. Bone mineralization in an osteogenesis imperfecta mouse model studied by small-angle x-ray scattering. J. Clin. Invest. 1996;97:396–402. doi: 10.1172/JCI118428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Imbert L, Aurégan JC, Pernelle K, Hoc T. Mechanical and mineral properties of osteogenesis imperfecta human bones at the tissue level. Bone. 2014;65:18–24. doi: 10.1016/j.bone.2014.04.030. [DOI] [PubMed] [Google Scholar]
- 145.Vanleene M, Porter A, Guillot PV, Boyde A, Oyen M, Shefelbine S. Ultra-structural defects cause low bone matrix stiffness despite high mineralization in osteogenesis imperfecta mice. Bone. 2012;50:1317–1323. doi: 10.1016/j.bone.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Vetter U, Eanes ED, Kopp JB, Termine JD, Robey PG. Changes in apatite crystal size in bones of patients with osteogenesis imperfecta. Calcif. Tissue Int. 1991;49:248–250. doi: 10.1007/BF02556213. [DOI] [PubMed] [Google Scholar]
- 147.Wang SK, Chan HC, Rajderkar S, Milkovich RN, Uston KA, Kim JW, Simmer JP, Hu JC. Enamel malformations associated with a defined dentin sialophosphoprotein mutation in two families. Eur. J. Oral Sci. 119 Suppl. 2011;1:158–167. doi: 10.1111/j.1600-0722.2011.00874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sreenath T, Thyagarajan T, Hall B, Longenecker G, D'Souza R, Hong S, Wright JT, MacDougall M, Sauk JB, Kulkarni AB. Dentin sialophosphoprotein knockout mouse teeth display widened predentin zone and develop defective dentin mineralization similar to human dentinogenesis imperfecta type III. J. Biol. Chem. 2003;278:24874–24880. doi: 10.1074/jbc.M303908200. [DOI] [PubMed] [Google Scholar]
- 149.Gibson MP, Jani P, Liu Y, Wang X, Lu Y, Feng JQ, Qin C. Failure to process dentin sialophosphoprotein into fragments leads to periodontal defects in mice. Eur. J. Oral Sci. 2013;121:545–550. doi: 10.1111/eos.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Gibson MP, Zhu Q, Liu Q, D'Souza RN, Feng JQ, Qin C. Loss of dentin sialophosphoprotein leads to periodontal diseases in mice. J. Periodontal .Res. 2013;48:221–227. doi: 10.1111/j.1600-0765.2012.01523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.von Marschall Z, Mok S, Phillips MD, McKnight DA, Fisher LW. Rough endoplasmic reticulum trafficking errors by different classes of mutant dentin sialophosphoprotein (DSPP) cause dominant negative effects in both dentinogenesis imperfecta and dentin dysplasia by entrapping normal DSPP. J. Bone Miner. Res. 2012;27:1309–1321. doi: 10.1002/jbmr.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Fedarko NS, Vetter U, Robey PG. The bone cell biology of osteogenesis imperfecta. Connect. Tissue Res. 1995;31:269–273. doi: 10.3109/03008209509010821. [DOI] [PubMed] [Google Scholar]
- 153.Forlino A, Cabral WA, Barnes AM, Marini JC. New perspectives on osteogenesis imperfecta. Nat. Rev. Endocrinol. 2011;7:540–557. doi: 10.1038/nrendo.2011.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Fitzgerald J, Holden P, Wright H, Wilmot B, Hata A, Steiner RD, Basel D. Phenotypic Variability In Individuals With Type V Osteogenesis Imperfecta With Identical Ifitm5 Mutations. J. Rare Disorders. 2013;1:37–42. [PMC free article] [PubMed] [Google Scholar]
- 155.Sweeney SM, Orgel JP, Fertala A, McAuliffe JD, Turner KR, Di Lullo GA, Chen S, Antipova O, Perumal S, Ala-Kokko L, Forlino A, Cabral WA, Barnes AM, Marini JC, San Antonio JD. Candidate cell and matrix interaction domains on the collagen fibril, the predominant protein of vertebrates. J. Biol. Chem. 2008;283:21187–21197. doi: 10.1074/jbc.M709319200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Dole NS, Kapinas K, Kessler CB, Yee SP, Adams DJ, Pereira RC, Delany AM. A single nucleotide polymorphism in osteonectin 3′ untranslated region regulates bone volume and is targeted by miR-433. J. Bone Miner. Res. 2015;30:723–732. doi: 10.1002/jbmr.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Steitz SA, Speer MY, McKee MD, Liaw L, Almeida M, Yang H, Giachelli CM. Osteopontin inhibits mineral deposition and promotes regression of ectopic calcification. Am. J. Pathol. 2002;161:2035–2046. doi: 10.1016/S0002-9440(10)64482-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Boskey AL, Spevak L, Paschalis E, Doty SB, McKee MD. Osteopontin deficiency increases mineral content and mineral crystallinity in mouse bone. Calcif. Tissue Int. 2002;71:145–154. doi: 10.1007/s00223-001-1121-z. [DOI] [PubMed] [Google Scholar]
- 159.Yuan Q, Jiang Y, Zhao X, Sato T, Densmore M, Schüler C, Erben RG, McKee MD, Lanske B. Increased osteopontin contributes to inhibition of bone mineralization in FGF23-deficient mice. J. Bone Miner. Res. 2014;29:693–704. doi: 10.1002/jbmr.2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Tagliabracci VS, Engel JL, Wen J, Wiley S, Worby CA, Kinch LN, Xiao J, Grishin NV, Dixon JE. Secreted kinase phosphorylates extracellular proteins that regulate mineralization. Science. 2012;336:1150–1153. doi: 10.1126/science.1217817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Tagliabracci VS, Wiley SE, Guo X, Kinch LN, Durrant E, Wen J, Xiao J, Cui J, Nguyen KB, Engel JL, Coon JJ, Grishin N, Pinna LA, Pagliarini DJ, Dixon JE. A Single Kinase Generates the Majority of the Secreted Phosphoproteome. Cell. 2015;161:1619–1632. doi: 10.1016/j.cell.2015.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Acevedo AC, Pouter JA, Alves PG, de Lima CL, Castro LC, Yamaguti PM, Paula LM, Parry DA, Logan CV, Smith CE, Johnson CA, Inglehearn CF, Mighell AJ. Variability of systemic and oro-dental phenotype in two families with non-lethal Raine syndrome with FAM20C mutations. BMC Med. Genet. 2015;16:8. doi: 10.1186/s12881-015-0154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wang SK, Samann AC, Hu JC, Simmer JP. FAM20C functions intracellularly within both ameloblasts and odontoblasts in vivo. J Bone Miner. Res. 2013;28:2508–2511. doi: 10.1002/jbmr.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]