Abstract
Mycobacterium tuberculosis (Mtb), the causative agent of tuberculosis, is responsible for 1.5 million deaths annually. We previously showed that Mtb infection in mice induces expression of the carbon monoxide (CO) producing enzyme heme oxygenase (HO1) and that CO is sensed by Mtb to initiate a dormancy program. Further, mice deficient in HO1 succumb to Mtb infection more readily than wild type mice. While mouse macrophages control intracellular Mtb infection through several mechanisms such as nitric oxide synthase, the respiratory burst, acidification and autophagy, how human macrophages control Mtb infection remains less well understood. Here we show that Mtb induces and colocalizes with HO1 in both mouse and human tuberculosis lesions in vivo, and that Mtb induces and colocalizes with HO1 during primary human macrophage infection in vitro. Surprisingly, we find that chemical inhibition of HO1 both reduces inflammatory cytokine production by human macrophages and restricts intracellular growth of mycobacteria. Thus, induction of HO1 by Mtb infection may be a mycobacterial virulence mechanism to enhance inflammation and bacterial growth.
Introduction
One third of the world’s population is latently infected with Mycobacterium tuberculosis (Mtb), leading to 9 million new cases of tuberculosis every year and another 1–2 million deaths (1). Mtb is spread from person to person through aerosolized droplets (2). Following infection, the bacteria are taken up by alveolar macrophages found in the terminal alveoli of the lungs (3, 4). Cytokines produced by these macrophages recruit a large number of immune cells to the lung, including dendritic cells, B cells, T cells and additional monocytes. In the majority of cases, bacterial replication is restricted, leading to latent infection (5, 6). However, in about 5% of early cases, bacterial replication is not controlled, which leads to active disease. Importantly, how the human immune system responds to Mtb infection remains poorly understood.
We (7) and others (8) have shown that Mtb infection in mice induces heme oxygenase (HO1), and Mtb senses CO, a downstream product of HO1, to alter its transcriptional program (7, 9). HO1 catalyzes the breakdown of heme into carbon monoxide, biliverdin, and iron (7, 10). We found that Mtb infection of mouse macrophages in vitro results in production of HO1, and HO1 accumulation is observed within macrophages in Mtb infected mice (7). Additionally, HO1 knockout mice show increased susceptibility to Mycobacterium bovis BCG and Mycobacterium avium infection (11, 12), likely due to the cytotoxic affects of free heme accumulation (12). Although HO1 is important for controlling murine Mtb infection, whether HO1 plays a similar role in human infection is not known. Based on previous work, we hypothesized that HO1 would be produced by human macrophages in response to Mtb infection and that HO1 expression would impact tuberculosis pathogenesis.
Here we report that HO1 was robustly expressed both in human tuberculosis lesions and in human macrophages in response to Mtb infection, and HO1 directly colocalized with Mtb within the phagosomes of infected human macrophages. Moreover, HO1 regulated inflammatory cytokine production such that significant reductions in pro-inflammatory cytokines were observed when HO1 was inhibited in both macrophage cell lines and primary human macrophages. Surprisingly, when HO1 was inhibited in primary human macrophages infected with Mtb, intracellular growth of Mtb was diminished coincident with reduced autocrine exposure to pro-inflammatory cytokines. Together our results suggest a vital role for HO1 during human tuberculosis by mediating inflammatory cytokine production and facilitating intracellular growth.
Materials and Methods
Strains and media
The Erdman wild type strain of Mtb was grown in Middlebrook 7H9 medium or on Middlebrook 7H11 agar plates supplemented with 10% oleic acid-albumin-dextrose-catalase (Thermo-Fisher). Tween-80 (Fisher) was added to liquid medium to a final concentration of 0.05%. Mtb Erdman expressing GFP or mCherry was used for immunofluorescence experiments and was grown in the same 7H9 broth medium as above.
Cell maintenance and macrophage differentiation
THP1 and U937 cells were grown in suspension in RPMI media supplemented with 10% FBS, 2.5% D-Glucose, 2 mM L-Glutamine, +/− penicillin/streptomycin (100 U/mL and 100 µg/mL, respectively), 10 mM HEPES, and 1 mM sodium pyruvate. To differentiate THP1 and U937 cells into macrophages, cells were treated with Phorbol-12-Myristate-13-Acetate (PMA, 100 ng/mL, 16561-29-8 Fisher) and plated at a density of 5 × 105 cells per well of a 24 welled-plate. Cells were kept at 37°C, 5% CO2 for 2 days at which time the media was changed to antibiotic-free media. Cells were kept at 37°C, 5% CO2 for an additional 2 days prior to Mtb infection.
Primary human macrophages were collected from 30 mL of citrate-treated blood samples and prepared according to standard techniques (13). Briefly, blood was separated on a Ficoll-Paque Plus gradient by centrifugation at 750 × g for 20 minutes without braking. Autologous serum was collected and frozen for Mtb infection. The lymphocyte/monocyte layer was transferred to 50 mL centrifuge tube, diluted to 50 mL with phosphate buffered saline (PBS), and centrifuged at 350 × g for 20 minutes at 4°C without braking. Monocytes were washed three more times in PBS at 4°C. Finally, monocytes were resuspended in 5 mL RPMI + 10% autologous human serum. To differentiate into macrophages, monocytes were diluted with RPMI + 10% human serum + 100 ng/mL Macrophage Colony Stimulating Factor (M-CSF, 216-MC-025, R&D Systems) and plated in 24 well Primaria Plates (353847, Corning). Cells were allowed to attach at 37°C 5% CO2 for 4 hours before being rinsed with PBS and adding fresh RPMI + 10% human serum + M-CSF. Cells were differentiated for 7 days and media was changed every other day before infection.
Western Blotting
THP-1 cells were infected at an MOI of 10. At 24 hours after infection, cells were lysed in 0.5% Triton X-100 (Sigma) plus protease inhibitors (Roche) and samples were filtered twice with a 0.22 µm microcentrifuge filter (Millipore). Blotting was performed with rabbit anti-HO1 polyclonal antibody (1:1000, ADI-SPA-896-F, Enzo Life Sciences) or mouse anti-β actin antibody (1:1000, Santa Cruz, sc-47778) and donkey anti-rabbit-HRP conjugate secondary antibody (1:5000, sc-2305, Santa Cruz) or goat anti-mouse-HRP conjugate (1:5000, sc-2005, Santa Cruz), respectively. Supersignal West Femto Chemiluminescent Substrate (Pierce) was used for signal detection.
HO1 ELISA
The HO1 ELISA was performed according to manufacturer’s instructions (HO1 ELISA Kit, ADI-EKS-800, Enzo). Clinical samples of serum from individuals with active tuberculosis were obtained from the Houston Tuberculosis Initiative (14–17). Briefly, primary human macrophages were lysed in extraction buffer at various time points after infection or hemin treatment. Primary human macrophage lysates were diluted to 20 ng/mL total protein with kit provided sample diluent and human Mtb patient serum was diluted 1:10 in sample diluent. 100 uL of each diluted sample and standard was added in duplicate to a 96-welled plate and plate incubated at room temperature for 30 minutes. Excess sample was flicked off the plate and wells were washed 6× with 200 µl of kit provided wash buffer. Each well was treated with 100 µl of kit provided rabbit anti-human HO1 antibody for 1 hour at room temperature. After washing and drying, 100 µl of kit provided anti-rabbit IgG:HRP conjugate was added to each well for 30 minutes at room temperature. The plate was washed again and 100 µl of kit provided TMB Substrate was added to each well for 15 minutes at room temperature followed directly by 100 µl of kit provided Stop Solution. The plate was read immediately on a Synergy HT plate reader (BioTek Instruments) at 450 nm.
Quantitative PCR
THP-1 cells were infected at an MOI of 10. At 24 hours, infected cells were lysed using Triazol and the RNA purified. cDNA was prepared using the iScript cDNA synthesis kit (Bio-Rad). For qPCR analysis, Fast Sybr Green (Life Technologies) was used. Primers were from Sigma. qPCR was performed on an Applied Biosystems Vii7 using the following primers: HO-1 forward (5’-CAACAAAGTGCAAGATTCTG-3’), HO-1 reverse (5’-TGCATTCACATGGCATAAAG-3’), IFN-β forward (5’CGCTGCGTTCCTGCTGTGCTT-3’), IFN-β reverse (5’-AGGTGAGGTTGATCTTTCCATTCA-3’), Beta-Actin forward (5’-GGTGTGATGGTGGGAATGG-3’), Beta-Actin reverse (5’-GCCTCGTCACCCACATAGGA-3’). All fold change calculations for gene expression were made using the ΔΔCt method (18).
Mouse infections
BALBc mice (Jackson Laboratories) were infected using a Glas-Col aerosol-exposure chamber to deliver ~200 bacilli per mouse as previously described (19, 20). Prior to aerosolization, bacteria were washed repeatedly and sonicated to generate a single-cell suspension. At day zero, we plated total organ homogenates from both lungs (5 mice per group) to determine the initial inoculum. At subsequent time points, lungs from infected animals were fixed in 10% neutral buffered formalin for 24 hours and paraffin embedded. Animal experiments were reviewed and approved by the Institutional Animal Care and Use Committee of the University of Texas (UT) Southwestern.
Immunohistochemistry and immunofluorescence of tissue specimens
Sections from paraffin-embedded mouse and human lung were deparaffinized in xylene, subjected to heat-mediated antigen-retrieval in 1 mM EDTA with 0.05% Tween 20 (pH 8.0), endogenous peroxide activity was quenched in 2.5% hydrogen peroxide solution in methanol, slides were permeabilized in 0.1% Triton-100 (Sigma) and blocked in SuperBlock Blocking Buffer (Thermo Scientific). For immunohistochemistry, sections were stained with rabbit anti-HO1 (1:100, ADI-SPA-896-F, Enzo Life Sciences), rabbit anti-HA (1:50, sc-805 Santa Cruz), or mouse anti-CD68 (1:100, ab955 Abcam). HRP-conjugated secondary antibodies were obtained from Jackson Immunochemicals and used at 1:250. Staining was amplified with AB reagent (Vectastain) and detected using DAB reagent (Thermo Scientific). Images were acquired using a Zeiss Axioplan 2 microscope. For immunofluorescence, HO1 was identified using rabbit anti-HO1 (1:100) and a donkey anti-rabbit-HRP conjugate secondary antibody (1:500, Santa Cruz) followed by amplification with Cyanine 3 tyramide (1:100, Perkin Elmer). Mtb was identified using guinea pig anti-Mtb (1:25, NR-13818, NR-13823 BEI) and an Alexa 488 conjugated donkey anti-guinea pig secondary antibody (1:100, 706-545-148 Jackson Immunochemicals). All commercial antibodies were prepared without Freund’s adjuvant. Images were acquired using a Leica TCS SP5 confocal microscope.
Quantification of immunohistochemistry in mouse lungs
DAB-stained sections were imaged as above and analyzed using Image J using the color deconvolution plug-in (http://wiki.imagej.net/Colour_Deconvolution) as described (21). Briefly, images opened in Image J and then processed using the color deconvolution tool to separate brown and purple images. The area of brown staining was then quantified, and divided by the total (purple) area to yield a percent staining area. Three mice were analyzed per time point.
Mycobacterial preparation for macrophage infections
Bacteria for infection were prepared as described previously (7, 22). Briefly, Mtb were grown to late-log phase, and washed repeatedly with PBS. After the final wash, bacterial clumps were removed by slow-speed centrifugation (300×g), and the resulting supernatant was then sonicated to break up any remaining clumps and generate a single-cell suspension. After sonication, the bacterial OD600 was recorded and the bacteria were diluted into macrophage-infection media (RPMI plus 10% horse serum [THP1 and U937 cells] or 10% autologous human serum [primary cells]).
Immunofluorescence of macrophages infected in vitro
U937 or THP-1 cells were differentiated as described above. Cells were infected at an MOI of 5 and 24 hours after infection, cells were washed with PBS and fixed in 4% paraformaldehyde (Alfa Aesar). Cells were permeabilized with 0.1% Triton X-100 and blocked with SuperBlock Blocking Buffer (Thermo Scientific). HO1 was identified using rabbit anti-HO1 (1:100) and an HRP-conjugated donkey anti-rabbit secondary (1:500, Santa Cruz) followed by amplification with Cyanine 3 tyramide (1:100, Perkin Elmer). Mtb expressing GFP were used for infections in THP1 and U937 cells. Images were acquired as z-stacks using a Zeiss Axioplan 2 microscope and were deconvoluted using Imaris and Autoquant softwares. Additional antibodies used to identify the location of HO1 positive bacteria were mouse anti-LAMP1 (lysosome, 1:100, sc-20011, Santa Cruz), mouse anti-Rab7 (late endosome, 1:100, ab50533, Abcam), mouse anti-SQSTM1 (for p62/sequestosome, 1:2000, H00008878-MO1, AbNova), and mouse anti-Sec22B (ER-Golgi intermediate compartment, 1:50, sc-101267, Santa Cruz).
Immunofluorescence of mycobacteria
Mtb expressing mCherry was grown in 7H9 media to log phase and then fixed in 4% paraformaldehyde for 30 minutes. Fixed Mtb was then incubated with primary antibodies against Mtb (rabbit anti-Mtb), HO1 (rabbit anti-HO1; Enzo Life Sciences) or rabbit IgG all at a 1:100 dilution. Then, an Alexa 488 conjugated donkey anti-rabbit secondary (1:500, A21206, Life Technologies) was added. After incubation, Mtb were immobilized on glass slides and imaged using a Zeiss Axioplan 2 fluorescence microscope.
Magpix cytokine analysis
U937 cells were infected at an MOI of 10 for 2 hours. Cells were washed with PBS and fresh medium +/− 0.1 M NaOH (vehicle control) or tin protoporphorin (SnPP; 50 µM final concentration) was added. SnPP blocks the active site of HO1 leading to competitive inhibition with a nanomolar Ki (23). At 24 hours, conditioned medium was collected and filtered twice through a 0.22 µm filter. Cytokine levels were measured using a 29-plex Magpix cytokine assay (HCYTMAG-60K-PX29, Millipore).
Primary human cells were infected at an MOI of 10 for 2 hours. Cells were washed with PBS and fresh medium +/− SnPP (50 µM) or cobalt protoporphyrin (CoPP; 10 µM final concentration) was added (24). At 24 hours, conditioned medium was collected and filtered twice through a 0.22 µm filter. IL-1β, IL-8, TNF-α, IL-6, and GM-CSF levels were measured using Human Inflammatory Magnetic 5-Plex Panel Assay (LHC0003M, ThermoFisher). SnPP and CoPP were both from Fisher Scientific. Both were dissolved in 0.1 M NaOH and sterile filtered prior to use.
Survival in macrophages
Primary human macrophages were infected at an MOI of 0.1 for 2 hours with a single-cell suspension of bacteria prepared as described above using autologous serum for each donor during the initial infection period. Macrophages were washed and macrophage medium without antibiotics (+/− 50 µM SnPP or 10 µM CoPP) was then replaced. To avoid the need for antibiotics, cells were gently washed with PBS daily and fresh medium +/− SnPP or CoPP added. On days 0, 3 and 6, cells were lysed using 0.5% Triton X-100 (Sigma) and serial dilutions plated to 7H11 agar without antibiotics to determine CFU values.
Human studies
The Institutional Review Boards of the University of Texas Southwestern and Methodist Hospital approved all research using human specimens.
Statistical analysis
Quantitative PCR was analyzed by unpaired Student’s t-test. Cytokine data from conditioned media was first analyzed by a two way ANOVA followed by head-to-head comparisons with Tukey’s correction for multiple comparisons. Colocalization of Mtb with markers was analyzed by unpaired Student’s t-test. HO1 cytokine data in serum was analyzed by unpaired Student’s t-test. All analyses were performed using Prism 6 (GraphPad Software, Inc.).
Results
HO1 colocalizes with Mtb during chronic aerosol infection
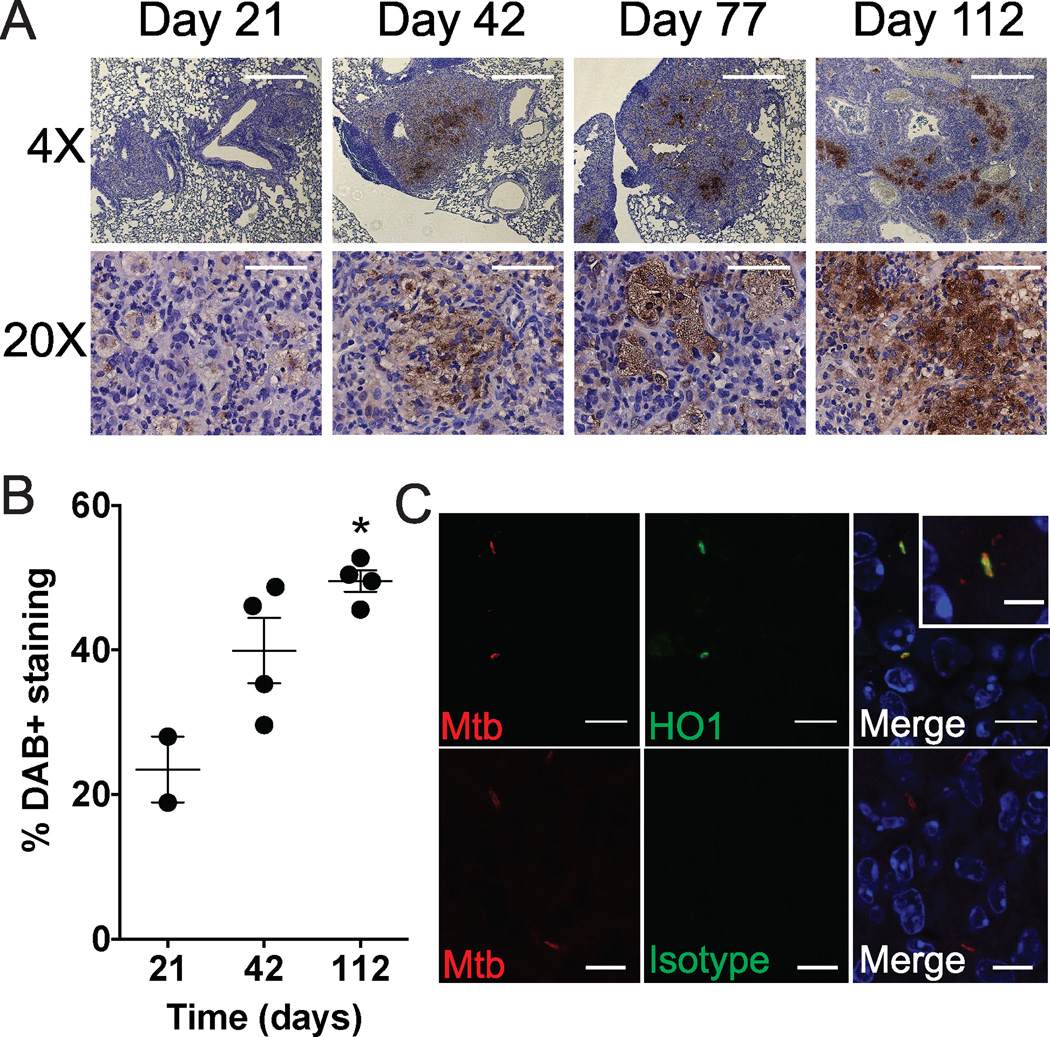
Previously we showed that HO1 is induced 10 days after intravenous infection of mice with Mtb (7). To determine if HO1 expression is induced during a more physiologically relevant Mtb infection, we used a mouse aerosol infection model. We performed an aerosol infection with a low dose of Mtb Erdman (200 CFU/mouse), harvested lungs from BALB/c mice 21, 42, 77 and 112 days after infection and assessed HO1 and Mtb expression by immunohistochemistry and immunofluorescence microscopy. By immunohistochemistry we found that HO1 was modestly expressed at 21 days, and then gradually increased over time so that at 112 days it was robustly expressed within characteristic foamy macrophages (Fig. 1A,B). To show that HO1 and Mtb exist within the same cellular organelles, we used confocal microscopy and found that within infected cells, HO1 and Mtb colocalized, with HO1 expression often surrounding individual bacteria (Fig. 1C). Thus, HO1 expression appeared to increase over the course of infection, and HO1 and Mtb colocalized to the same cells and organelles.
Fig. 1. HO1 colocalizes to Mtb infected macrophages in an aerosol model of infection.
(A) BALB/c mice were infected with Mtb via a low dose aerosol infection and lungs harvested at days 21, 42, 77, and 112. Shown is immunohistochemistry of paraffin sections with anti-HO1 (brown) and counterstained with hematoxylin (purple). Scale bars are 500 µm for 4× images and 100 µm for 20× images. (B) The extent of DAB positivity was quantified using Image J and graphed over time as percent positive area (3 mice/time point). (C) Immunofluorescence of day 112 paraffin sections with rabbit anti HO-1 and guinea pig anti-Mtb. Scale bars are 10 µm.
HO1 is expressed in CD68+ cells within tissue from Mtb infected individuals
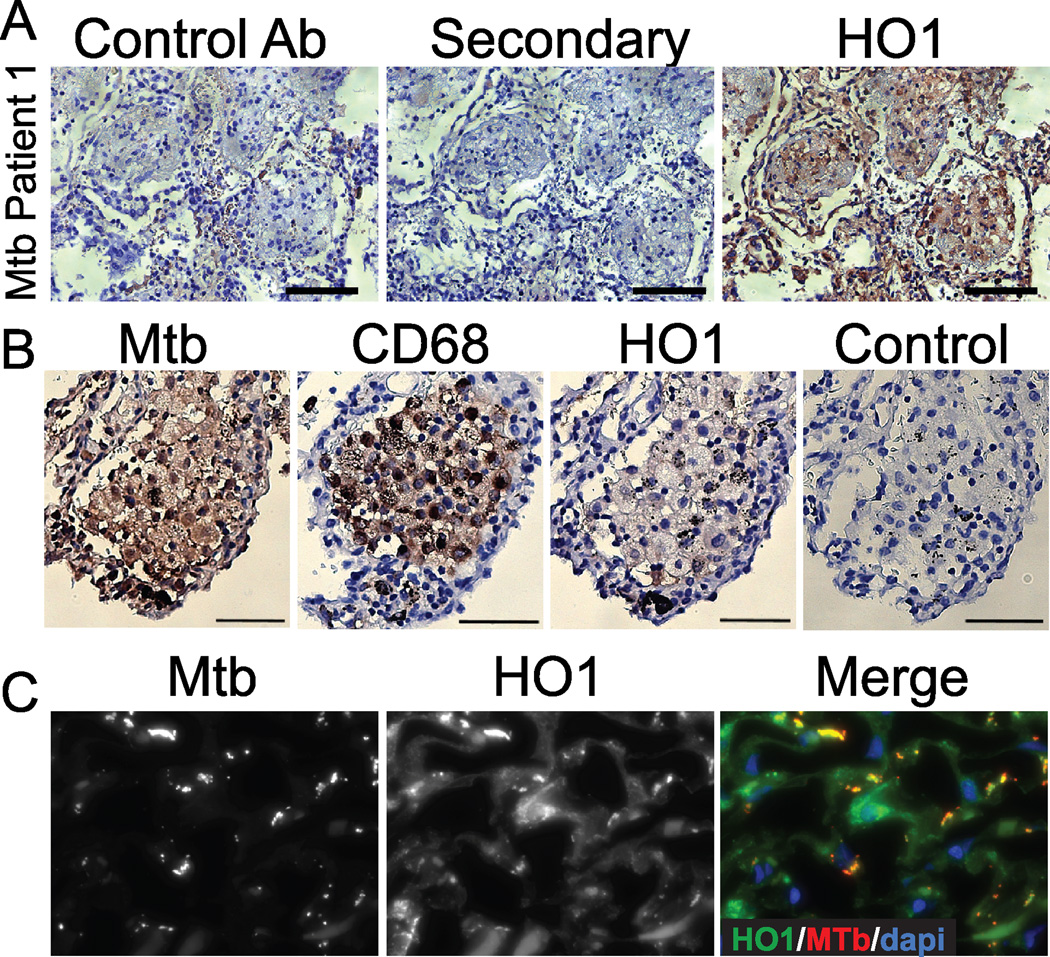
To determine the role of HO1 in human tuberculosis, we first examined HO1 expression by immunohistochemistry and immunofluorescence microscopy in lung biopsies from ten Mtb infected individuals. These individuals and their specimens were randomly selected from a pool of individuals identified by the pathology department at Parkland Hospital (Dallas, TX) as being positive for Mtb by AFB staining and culture. A representative patient sample demonstrated an accumulation of HO1 in infected tissues, compared to staining with secondary antibody alone or with an isotype antibody control, both of which showed minimal DAB staining (Fig. 2A). HO1 expression was found in 10/10 tested individuals to various degrees, while HO1 expression was only rarely observed in 4/4 autopsy specimens from uninfected individuals (Fig. 2A, Supplemental Fig. 1, and 5 other Mtb-infected individuals not shown). Occasionally, HO1 staining was found in areas outside of traditional granuloma, including in epithelial cells and cells with a monocytic appearance, although the majority of HO1 was found within granuloma. To exclude that the HO1 antibody was detecting Mtb antigens, we stained fluorescent Mtb with a rabbit anti-Mtb antibody, rabbit polyclonal immunoglobulin or rabbit anti-HO1 antibody and found that the HO1 antibody did not cross-react with Mtb alone (Supplemental Fig. 2). To determine if HO1 expression was increased in Mtb-infected monocytes and macrophages, we stained serial sections for HO1, Mtb or the macrophage/monocyte marker CD68. We found that HO1 and Mtb positive cells were also CD68 positive (Fig 2B). By deconvolution of z-stacked immunofluorescence microscopy images we found that, similar to our observations of Mtb-infected mouse tissue (Fig. 1), HO1 colocalized to Mtb infected cells in lungs from individuals with active tuberculosis (Fig. 2C). Furthermore, HO1 appeared to envelope discrete bacteria (Fig. 2C) in the same staining pattern as Mtb-infected mouse lungs (Fig. 1). Thus HO1 was expressed in human lung specimens from Mtb infected individuals within monocytes and macrophages.
Fig. 2. HO1 is expressed in human tuberculosis specimens.
(A) Immunohistochemistry was performed on paraffin embedded lung sections from Mtb infected individuals using anti-HO1 antibody and DAB detection (brown). Sections were counterstained with hematoxylin (purple). Control specimens were treated with either rabbit polyclonal serum (control Ab) or secondary antibody alone. Scale bars are 100 µm. (B) Serial sections were stained with either anti-HO1, anti-CD68, or anti-Mtb as in A. (C) Immunofluorescence was performed on paraffin sections with anti HO-1 (green) and anti Mtb (red). Scale bars are 10 µm.
Mtb infection is sufficient to induce HO1 expression in human macrophages
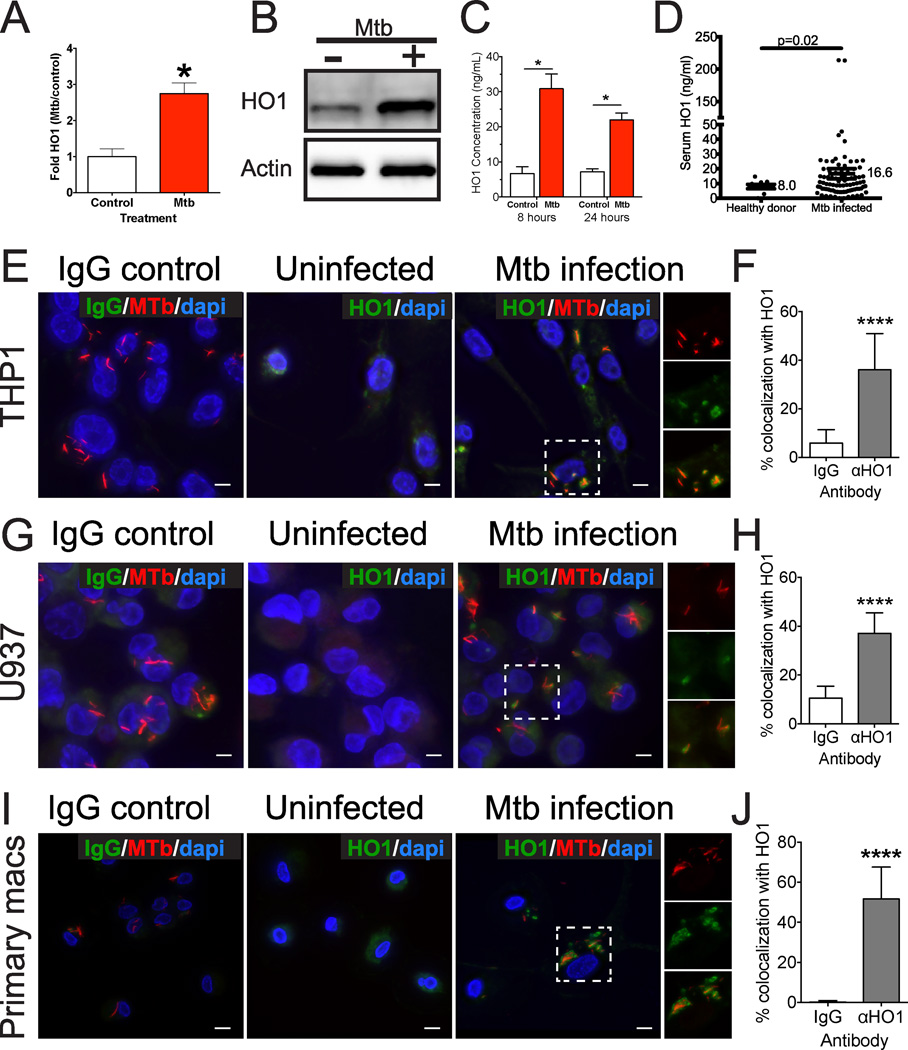
To ask if Mtb infection alone is sufficient to induce HO1 in human macrophages, we measured the regulation of HO1 by Mtb infection and the association of HO1 with Mtb in the human cell lines U937 and THP1, which when stimulated with phorbol myristate acetate (PMA) differentiate into macrophage-like cells, and in primary human macrophages differentiated from healthy donor monocytes. In THP1 cells we observed that Mtb infection was sufficient to induce HO1 transcription (Fig. 3A) and accumulation of HO1 protein by Western blotting (Fig. 3B). We also quantified HO1 using a specific HO1 ELISA in primary human macrophages and noted a 3-fold increase in HO1 after 8 hours of infection (Fig. 3C). Furthermore, we confirmed a prior report (25) that individuals with active tuberculosis have increased serum HO1 by comparing serum HO1 from healthy donors to those from individuals with active tuberculosis (Fig. 3D). We next infected macrophages with fluorescent Mtb, and found by deconvolution of z-stacked microscopy images that Mtb-infected THP1, U937, and primary human macrophages induced HO1 expression in a very focal distribution compared to uninfected cells (Fig. 3 E,G,I, Supplemental Fig. 3 and Supplemental Movie 1). Whereas uninfected cells demonstrated faint and diffuse HO1 staining, during Mtb infection HO1 frequently co-localized with Mtb (Fig. 3E,G,I). In particular, HO1 staining surrounded individual bacteria in infected macrophages (Fig. 3 E,G,I and insets, Supplemental Fig. 3, Supplemental Movie 1), with about 40–50% of bacteria colocalizing with HO1 (Fig. 3F,H,J).
Fig. 3. Mtb infection is sufficient to induce HO1 expression in human macrophages.
(A) qPCR analysis of HO1 gene expression in THP1 cells following Mtb infection (MOI 10) for 24 h. *p = < 0.05 by Student’s t-test (B) Western blot of lysates from THP1 cells mock-infected or infected with Mtb (MOI 10) for 24 hours and probed with anti-HO1 or anti-actin antibody. (C) HO1 ELISA from macrophages derived from a healthy donor and then infected with Mtb for 8 or 24 h. *p = < 0.05 by Student’s t-test (D) HO1 concentration in the serum of Mtb infected versus healthy control individuals was determined by ELISA. (E–J) Confocal microscopy and quantification showing HO1 localization in THP1 (E,F), U937 (G,H) or primary human macrophages (I,J) following Mtb infection at an MOI of 5 and stained 24 hours after infection. (E,G,I) HO1 (green) was detected using anti-HO1 antibody and Mtb (shown in red) was detected by its GFP fluorescence and false-colored to red in ImageJ. Scale bars are 10 µm. (F, H, J) Quantification of HO1 and Mtb colocalization in macrophages. 100 bacteria were counted in triplicate slides, and colocalization assessed by deconvolution of z-stacked images. Percent colocalization reflects the number of HO1-colocalizing bacteria per 100 total bacteria counted. Data are representative of at least two similar experiments. **** p<0.0001 by Student's t-test.
To further determine the Mtb containing organelle to which HO1 was recruited, we stained Mtb infected THP1 macrophages with markers for the ER-Golgi intermediate compartment also known as ERGIC (Sec22B), late endosome (Rab7), autophagosome (p62) and lysosome (LAMP1) (Fig. 4 and Supplementary Fig. 3, 4). In these experiments, we consistently observed 40% of Mtb colocalizing with HO1. Of the HO1 positive, Mtb-containing organelles, about 40% also colocalized with LAMP1 (Fig. 4A–D, Supplemental Fig. 3, Supplemental Movie 2). Thus, a total of ~20% of total intracellular Mtb were HO1+/Lamp1+. Additionally, we observed that although Mtb-containing organelles also colocalized with Rab7 (~20% colocalization, Fig. 4E–H) and with p62 (~20% colocalization, Supplemental Fig. 4), these Mtb-containing organelles were not HO1 positive. Neither HO1 nor Mtb colocalized with the ERGIC marker Sec22B (Supplemental Fig. 4). Thus, Mtb infection of human macrophages in vitro upregulated HO1 expression, and HO1 was targeted to Mtb-containing organelles, some of which appeared to be lysosomes. Additionally, some bacteria that were not HO1 positive also localized to either late endosomes or autophagosomes.
Fig. 4. HO1 colocalizes with Mtb in LAMP1 positive organelles in human macrophages.
Confocal microscopy and quantification showing HO1 colocalization with LAMP1 (A–D) or Rab7 (E–H) in THP1 cells following Mtb infection at an MOI of 5 and stained 24 hours after infection. HO1 (red) was detected using anti-HO1 antibody, LAMP1 or Rab7 (green) were detected with specific antibodies, and Mtb (blue) was detected by its GFP fluorescence and false-colored to blue in ImageJ. Nuclei (cyan) were detected using DAPI. Scale bars are 10 µm. For quantification of HO1, Mtb or LAMP1/Rab7 colocalization in macrophages, 100 bacteria were counted on triplicate slides, and colocalization assessed by deconvolution. Percent colocalization reflects the number of bacteria colocalizing with HO1 per 100 total bacteria counted (B,F), LAMP1 or Rab7 with Mtb (C,G) or HO1+ Mtb with LAMP1/Rab7 (D,H). Data are representative of two similar experiments. * p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001 by Student's t-test.
HO1 inhibition prevents inflammatory cytokine production by Mtb infected macrophages
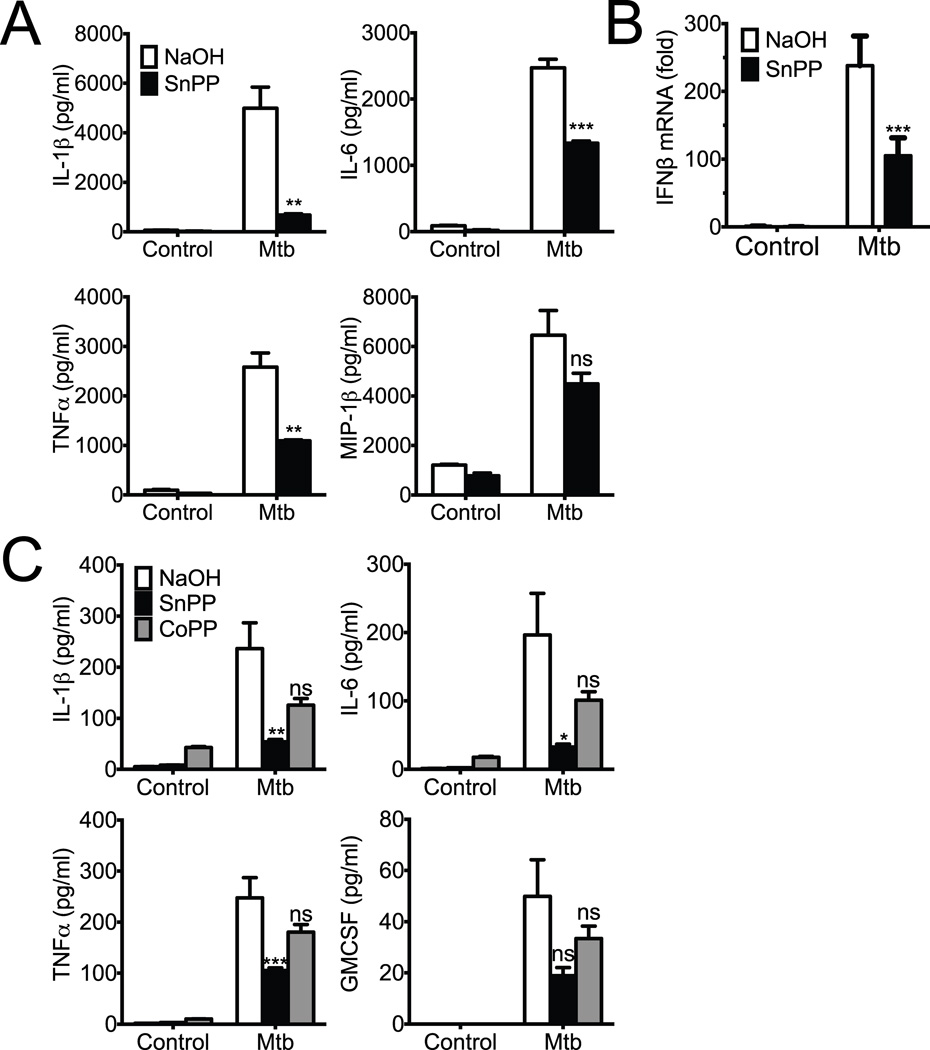
To explore the functional significance of HO1 activity during Mtb infection, we infected U937 macrophages in the presence or absence of the HO1 inhibitor SnPP and determined the effect on inflammatory cytokine production. As has been previously observed, Mtb infection of human macrophages induced a number of cytokines including IL-1β, TNFα, and IL-6 (Fig. 5A). Surprisingly, inhibition of HO1 activity significantly decreased the accumulation of IL-1β, TNFα, and IL-6 in the conditioned media (Fig 5A). Inhibition of HO1 did not uniformly affect all cytokines, as another Mtb-induced cytokine MIP1β was unaffected by treatment with SnPP (Fig. 5A). We also measured the induction of IFNβ by qPCR and found that HO1 inhibition significantly reduced its transcription in Mtb infected macrophages (Fig. 5B). To confirm these results, we tested the role of HO1 in cytokine induction in primary human macrophages derived from healthy human donors. Similar to the results with the U937 cell line, primary human macrophages also induced IL-1β, TNFα, IL-6, and GM-CSF when infected with Mtb, and this Mtb-dependent induction was partially blunted by SnPP treatment (Fig. 5C). In contrast, treatment with the HO1 inducer cobalt protoporphyrin (CoPP) (24) did not have a statistically significant effect on cytokine production. Thus, we conclude that HO1 activity is important for the production of some, but not all inflammatory cytokines following Mtb infection.
Fig. 5. HO1 inhibition reduces inflammatory cytokine production during Mtb infection.
(A,B) U937 cells were infected with Mtb or treated with PBS in the presence of tin protoporphyrin (SnPP) or NaOH control for 24 hours. (A) Supernatants were collected and assayed for a IL-1β, IL-6, TNFα, and MIP1β using Magpix multiplexing technology. Data are mean ± S.D. of samples in triplicate and are representative of two similar experiments. * p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001 by Student's t-test. (B) U937 cells were infected as described above with and without the presence of SnPP for 24 hours. Macrophage RNA was collected and qPCR was performed using IFNβ specific primers with actin as the internal control. ***p = < 0.001 by Student’s t-test (C) Primary human macrophages were infected with Mtb or treated with PBS in the presence of SnPP, CoPP, or control for 24 hours. Supernatants were collected and assayed for a IL-1β, IL-6, TNFα, and GMCSF using Magpix multiplexing technology. Data are mean ± S.D. of samples in triplicate and are representative of two similar experiments. * p<0.05, ** p<0.01, *** p<0.001 by Student's t-test.
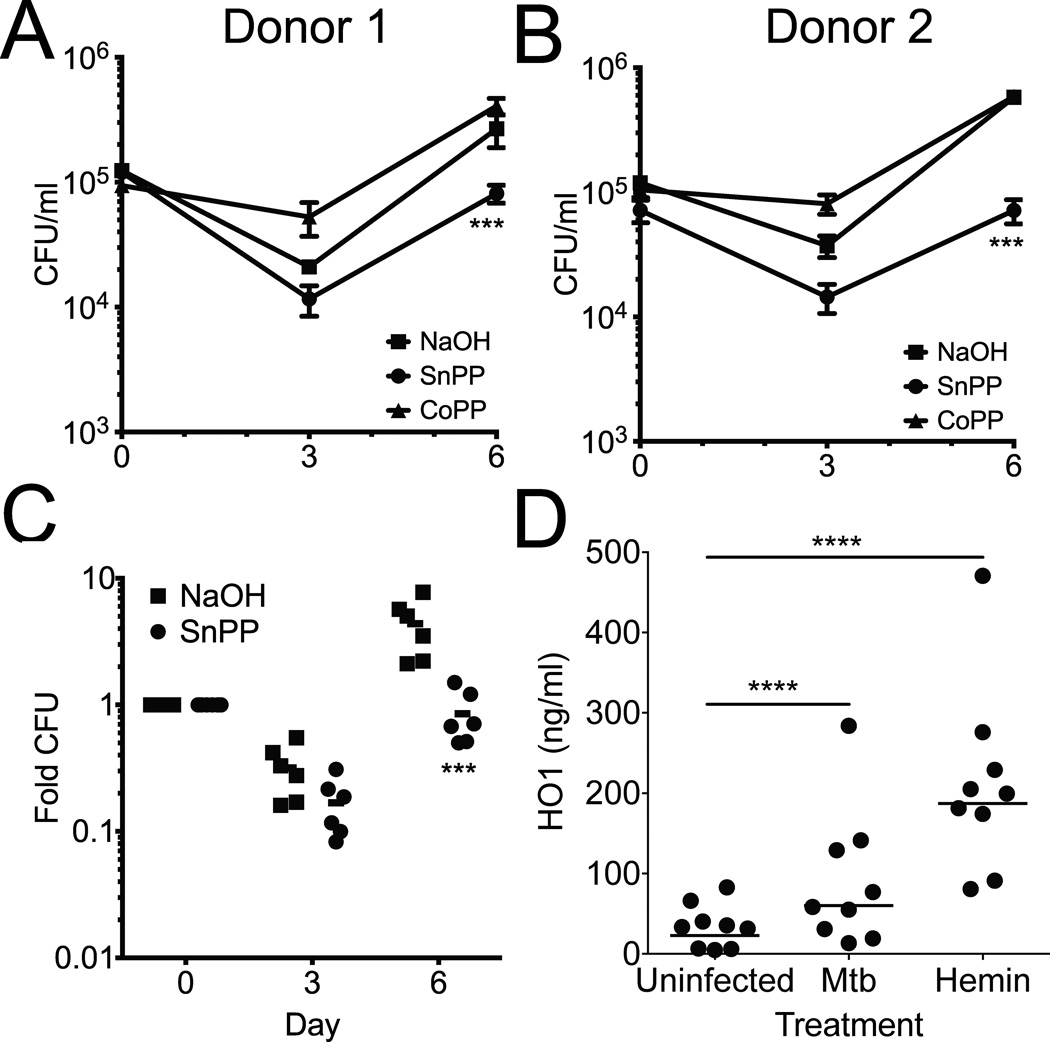
HO1 activity permissive for Mtb growth within primary human macrophages
Since HO1 deficient mice are hypersusceptible to mycobacterial infection (11, 12), we hypothesized that HO1 induction in human macrophages might have antimicrobial activity. We first confirmed that SnPP or CoPP alone had no direct effect on Mtb by growing the bacteria with and without SnPP added to the media and performing CFU assays over the course of 6 days. No significant growth difference was seen between bacteria treated with SnPP, CoPP and untreated controls (not shown). We next infected primary human macrophages with Mtb in the presence or absence of SnPP or CoPP and measured CFU from infected macrophages 3 and 6 days post-infection. Control, SnPP and CoPP treated macrophages all demonstrated an initial inhibition of Mtb growth (Fig. 6A–C). However, by 6 days after infection, Mtb growth was restricted in SnPP treated macrophages compared to CoPP treated and control macrophages (Fig. 6A,B). The effect of SnPP treatment was consistent across 6 independent human donors, where SnPP treated macrophages had on average a 4–5 fold lower Mtb CFU 6 days after infection (Fig. 6C). All of the donors induced HO1 in response to both Mtb and hemin (Fig. 6D). Thus, we conclude that HO1 activity facilitates growth of Mtb within primary human macrophages.
Fig. 6. HO1 inhibition reduces bacterial growth in primary human macrophages.
(A,B) Primary human macrophages from two independent donors were infected in quadruplicate with Mtb in the presence of NaOH, SnPP or CoPP and CFU enumerated at 0, 3 and 6 days after infection. Data are mean ± S.D.. *** p<0.001 by ANOVA. (C) Primary human macrophages from six donors were infected in quadruplicate with Mtb in the presence of NaOH or SnPP and CFU enumerated at 0, 3 and 6 days after infection. Fold CFU was calculated by dividing the mean CFU at each time point by the day zero CFU for each donor. Data include donor 1 and 2 from panels A,B. *** p<0.001 by ANOVA. (D) HO1 ELISA was performed on lysates from donor macrophages infected with Mtb or treated with hemin. **** p<0.001 by Student’s t-test.
Discussion
We and others have shown previously that HO1 is induced shortly after Mtb infection in mice (7, 8). HO1 deficient mice are less able to control mycobacterial infection compared to wild type mice as indicated by increased bacterial numbers and mortality following infection (11, 12). Here we demonstrate that HO1 accumulated throughout the course of a chronic low dose mouse infection. Modest HO1 expression was observed at day 21 and increased over time so that by day 112, robust levels of HO1 were observed. Additionally, HO1 colocalized with Mtb within mouse macrophages, appearing to surround the bacteria. Taken together this data further supports an important role for HO1 in murine Mtb infection.
Human tuberculosis and experimental murine tuberculosis infection differ in many ways, including how infected macrophages kill Mtb (26, 27), what cell types are found in experimental infections (28), how Mtb antigens are presented to the adaptive immune system (29, 30) and the architecture of infected tissue (31). The experimental models used in mice such as intravenous infection could potentially explain some of these differences. However, even the aerosol mouse model, while more physiological, still differs significantly from human infection (32, 33). Thus, it is important to test if the functions of host genes identified in mouse models are shared in humans.
To determine the role of HO1 in human tuberculosis, we examined tissue samples from individuals infected with Mtb and identified that HO1 is expressed in human tuberculosis lesions. Similar to its localization in the lungs of mice infected with tuberculosis, HO1 colocalized with Mtb in human granulomas both at the cellular and subcellular level. The observation that HO1 is expressed within infected human lungs is consistent with the recent finding that plasma HO1 levels are elevated during active tuberculosis in humans, and that HO1 levels return to baseline following successful treatment (25). Because HO1 is known to be upregulated by a variety of stimuli, we wanted to ask if Mtb infection alone is sufficient to induce HO1. We found that in human macrophage-like cell lines and in primary human macrophages derived from healthy donors, HO1 was increased following infection and colocalized with Mtb, commonly colocalizing with a lysosomal marker. However, about 50% of the HO1 and Mtb colocalization is in organelles not marked by p62, Sec22B, Rab7 or Lamp1, and some of the HO1 colocalization may be with intracytoplasmic Mtb (34).
Previously, HO1 was found to regulate expression of multiple cytokines (10). In purely inflammatory disease models, HO1 is anti-inflammatory (35, 36). Splenocytes from HO1 knockout mice secrete increased levels of pro-inflammatory cytokines following mitogenic stimulation with LPS or anti CD3/anti CD28 antibodies (37). Additionally, HO1 transgenic mice that constitutively express HO1 in the lung show a significant reduction in the production of proinflammatory cytokines and chemokines in response to hypoxia (38). However, in microbial infectious disease models, recent studies suggest HO1 may be involved in the induction of inflammatory cytokines. In mice, conditional deletion of HO1 from myeloid cells results in impaired IFNβ production as well as decreased production of IRF3 dependent target genes such as RANTES, IP-10, and MCP-1 in peritoneal macrophages in the setting of both viral and bacterial infection (39). Additionally, HIV infection of LPS activated primary human monocyte-derived macrophages leads to increased HO1 expression and increased levels the inflammatory cytokines MIP1α and MIP1β which is attenuated following treatment with the HO1 inhibitor SnPP (40). Finally, it was recently demonstrated that CO derived from HO1 causes bacteria to release ATP, thus triggering P2×7 receptors to activate the NALP3 inflammasome and release the pro-inflammatory cytokine IL-1β (41). In our study we found that inhibition of HO1 concurrent with Mtb infection in both macrophage cell lines and primary human macrophages suppressed the accumulation of multiple inflammatory cytokines, including IL-1β. Taking a human host-centric view, HO1 might therefore act as an important early component of the innate immune response leading to the production of pro inflammatory cytokines such as TNFα and IL-1β. Conversely, taking a mycobacteria-centric view, induction of HO1 may be a virulence mechanism. Recent studies in both zebrafish and mice indicate that granuloma formation in the context of M. marinum infection requires an appropriate level of TNFα (42, 43), which can be induced by bacterial factors (44). Indeed, it was recently shown that HO1 could be induced in macrophages via the region of difference 1 (RD1) locus and its effector ESAT-6 (45).
The cytokines produced by Mtb-infected macrophages are IFN-β, IL-1β, IL-6, and TNF-α (46). A number of genetic determinants for Mtb susceptibility have been described (47, 48) including polymorphisms in the genes for TNFα (49, 50), IL-1β (51), and IL-6 (52). Furthermore, TNFα blockade is an established risk factor for developing active tuberculosis (53). While we have demonstrated that secretion of these cytokines was significantly reduced following HO1 inhibition, what remains unclear is the mechanism behind this inhibition. One possibility is that reduced intracellular mycobacterial growth broadly diminishes the signals for cytokine production. Another possibility is that HO1 dependent production of pro-inflammatory cytokines is mediated through one of the products of HO1, such as iron, biliverdin or CO, as has been reported for Enterococcus faecalis infected macrophages (41). Alternatively, cytokine induction may be due to direct interaction of HO1 with other host molecules. For example, Mtb can directly activate the cytoplasmic DNA surveillance pathway through cGAS, resulting in IRF-3 phosphorylation and IFN-β production by infected macrophages (19, 54, 55). Interestingly, HO1 directly interacts with IRF3, and HO1 deficient macrophages show a reduction in the expression of IFNβ and other IRF3 target genes (39), and we also observed diminished IFN-β transcription in infected macrophages. Whether inhibition of HO1 activity would also alter binding to and nuclear translocation of IRF-3 is unknown.
HO1 is important to control a variety of infections in mice, including M. avium (12), Listeria monocytogenes (56), Plasmodium falciparum (57) and Toxoplasma gondii (58). In human cells, HO1 can inhibit the replication of viruses such as HIV (40), Ebola (59) and HCV (60). However, a direct role for HO1 in human macrophages infected with Mtb infection has not previously been established. We found that, in contrast to HO1−/− mice infected with mycobacteria, inhibition of HO1 in primary human macrophages was more restrictive to Mtb growth. This unexpected result is consistent with a recent report demonstrating reduced Mycobacterium abscessus growth in THP-1 cells when HO-1 was inhibited by SnPP treatment or siRNA-mediated silencing of HO1 (24). For M. abscessus infected macrophages, the reduced growth was attributed to enhanced phagolysosomal fusion and increased reactive oxygen intermediate production (24). Another explanation for the enhanced growth of Mtb in human cells in an HO1-dependent manner is that HO1 may increase the availability of intracellular and intraphagosomal iron through heme catabolism. Since iron acquisition is essential for Mtb survival in the host (61, 62), increasing the free iron concentration could increase the ability of Mtb to replicate (63, 64). Indeed, for a number of intracellular pathogens including Salmonella typhimurium, Chlamydia species and Legionella pneumophila, increased intracellular iron enhances replication (65, 66). Thus, the cell-autonomous affect of HO1 in the context of Mtb infection of macrophages could be beneficial for Mtb growth.
In conclusion, we show that HO1 has an important dual role in human tuberculosis infection. HO1 was induced by aerosol Mtb infection in mice and in human macrophages where it colocalized within infected cells. HO1 also mediated pro-inflammatory cytokine production in a human macrophage cell line and primary human macrophages, while at the same time created a permissive environment for intracellular bacterial replication. Future studies will be necessary to determine if HO1 is beneficial or detrimental during human tuberculosis infection.
Supplementary Material
Acknowledgments
We thank D. Cavuoti for help with pathology specimens, B. Greenberg, D. Greenberg and S. Hughes for help with human blood donors and members of the Shiloh lab for helpful discussions.
Funding:This study was supported by NIH RO1 AI099439, R21 AI111023 and U19 AI109725 (M.U.S.), T32 AI005284 (C.R.S.), T32 AI007520 (C.E.S.), R01 DK099478 (D.K.M) and R01 DA09238 (E.A.G.) Clinical specimens used for this project were partially supported by the NIH (NIAID) under contract N01-AO02738. M.U.S. acknowledges support from the Disease Oriented Clinical Scholars Program at UT Southwestern.
Footnotes
Author contributions: C.R.S., A.C.C. and M.U.S. conceived and designed the study; C.R.S., C.E.S., A.C.C., and V.R.N. performed all of the experiments; C.R.S., A.C.C., V.R.N. and D.K.M. obtained microscopy images; E.A.G. provided human serum samples from the Houston Tuberculosis Initiative; C.R.S., A.C.C. and M.U.S. drafted the manuscript; all authors edited and approved the final manuscript.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: The data and materials for this study are available from the authors upon request.
Bibliography
- 1.WHO. WHO Press, World Health Organization; 2011. Global Tuberculosis Control. [Google Scholar]
- 2.Fennelly KP, Martyny JW, Fulton KE, Orme IM, Cave DM, Heifets LB. Cough-generated aerosols of Mycobacterium tuberculosis: a new method to study infectiousness. Am J Respir Crit Care Med. 2004;169:604–609. doi: 10.1164/rccm.200308-1101OC. [DOI] [PubMed] [Google Scholar]
- 3.Smith DW, Wiegeshaus E, Navalkar R, Grover AA. Host-parasite relationships in experimental airborne tuberculosis. I. Preliminary studies in BCG-vaccinated and nonvaccinated animals. J Bacteriol. 1966;91:718–724. doi: 10.1128/jb.91.2.718-724.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dannenberg AM., Jr Pathogenesis of pulmonary tuberculosis. Am Rev Respir Dis. 1982;125:25–29. doi: 10.1164/arrd.1982.125.3P2.25. [DOI] [PubMed] [Google Scholar]
- 5.Silva Miranda M, Breiman A, Allain S, Deknuydt F, Altare F. The tuberculous granuloma: an unsuccessful host defence mechanism providing a safety shelter for the bacteria? Clin Dev Immunol. 2012:139127. doi: 10.1155/2012/139127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Modlin RL, Bloom BR. TB or Not TB: That Is No Longer the Question. Sci Transl Med. 5:213sr216. doi: 10.1126/scitranslmed.3007402. [DOI] [PubMed] [Google Scholar]
- 7.Shiloh MU, Manzanillo P, Cox JS. Mycobacterium tuberculosis senses host-derived carbon monoxide during macrophage infection. Cell host & microbe. 2008;3:323–330. doi: 10.1016/j.chom.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar A, Deshane JS, Crossman DK, Bolisetty S, Yan BS, Kramnik I, Agarwal A, Steyn AJ. Heme oxygenase-1-derived carbon monoxide induces the Mycobacterium tuberculosis dormancy regulon. J Biol Chem. 2008;283:18032–18039. doi: 10.1074/jbc.M802274200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zacharia VM, Shiloh MU. Effect of carbon monoxide on Mycobacterium tuberculosis pathogenesis. Medical gas research. 2012;2:30. doi: 10.1186/2045-9912-2-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grochot-Przeczek A, Dulak J, Jozkowicz A. Haem oxygenase-1: non-canonical roles in physiology and pathology. Clin Sci (Lond) 2012;122:93–103. doi: 10.1042/CS20110147. [DOI] [PubMed] [Google Scholar]
- 11.Regev D, Surolia R, Karki S, Zolak J, Montes-Worboys A, Oliva O, Guroji P, Saini V, Steyn AJ, Agarwal A, Antony VB. Heme oxygenase-1 promotes granuloma development and protects against dissemination of mycobacteria. Laboratory investigation; a journal of technical methods and pathology. 2012;92:1541–1552. doi: 10.1038/labinvest.2012.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silva-Gomes S, Appelberg R, Larsen R, Soares MP, Gomes MS. Heme Catabolism by Heme Oxygenase-1 Confers Host Resistance to Mycobacterium Infection. Infect Immun. 2013 doi: 10.1128/IAI.00251-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Erbel C, Rupp G, Helmes CM, Tyka M, Linden F, Doesch AO, Katus HA, Gleissner CA. An in vitro model to study heterogeneity of human macrophage differentiation and polarization. Journal of visualized experiments : JoVE. 2013:e50332. doi: 10.3791/50332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma X, Dou S, Wright JA, Reich RA, Teeter LD, El Sahly HM, Awe RJ, Musser JM, Graviss EA. 5' dinucleotide repeat polymorphism of NRAMP1 and susceptibility to tuberculosis among Caucasian patients in Houston, Texas. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 2002;6:818–823. [PubMed] [Google Scholar]
- 15.Feske ML, Teeter LD, Musser JM, Graviss EA. Giving TB wheels: Public transportation as a risk factor for tuberculosis transmission. Tuberculosis (Edinburgh, Scotland) 2011;91(Suppl 1):S16–S23. doi: 10.1016/j.tube.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Marquez L, Feske ML, Teeter LD, Musser JM, Graviss EA. Pediatric tuberculosis: the litmus test for tuberculosis control. The Pediatric infectious disease journal. 2012;31:1144–1147. doi: 10.1097/INF.0b013e318266b6c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teeter LD, Ha NP, Ma X, Wenger J, Cronin WA, Musser JM, Graviss EA. Evaluation of large genotypic Mycobacterium tuberculosis clusters: contributions from remote and recent transmission. Tuberculosis (Edinburgh, Scotland) 2013;93(Suppl):S38–S46. doi: 10.1016/S1472-9792(13)70009-X. [DOI] [PubMed] [Google Scholar]
- 18.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 19.Collins AC, Cai H, Li T, Franco LH, Li XD, Nair VR, Scharn CR, Stamm CE, Levine B, Chen ZJ, Shiloh MU. Cyclic GMP-AMP Synthase Is an Innate Immune DNA Sensor for Mycobacterium tuberculosis. Cell host & microbe. 2015;17:820–828. doi: 10.1016/j.chom.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zacharia VM, Manzanillo PS, Nair VR, Marciano DK, Kinch LN, Grishin NV, Cox JS, Shiloh MU. cor, a novel carbon monoxide resistance gene, is essential for Mycobacterium tuberculosis pathogenesis. MBio. 2013;4:e00721-e00713. doi: 10.1128/mBio.00721-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruifrok AC, Johnston DA. Quantification of histochemical staining by color deconvolution. Analytical and quantitative cytology and histology / the International Academy of Cytology [and] American Society of Cytology. 2001;23:291–299. [PubMed] [Google Scholar]
- 22.Stanley SA, Raghavan S, Hwang WW, Cox JS. Acute infection and macrophage subversion by Mycobacterium tuberculosis require a specialized secretion system. Proc Natl Acad Sci U S A. 2003;100:13001–13006. doi: 10.1073/pnas.2235593100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drummond GS, Kappas A. Prevention of neonatal hyperbilirubinemia by tin protoporphyrin IX, a potent competitive inhibitor of heme oxidation. Proc Natl Acad Sci U S A. 1981;78:6466–6470. doi: 10.1073/pnas.78.10.6466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abdalla MY, Ahmad IM, Switzer B, Britigan BE. Induction of heme oxygenase-1 contributes to survival of Mycobacterium abscessus in human macrophages-like THP-1 cells. Redox Biol. 2015;4:328–339. doi: 10.1016/j.redox.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andrade BB, Pavan Kumar N, Mayer-Barber KD, Barber DL, Sridhar R, Rekha VV, Jawahar MS, Nutman TB, Sher A, Babu S. Plasma heme oxygenase-1 levels distinguish latent or successfully treated human tuberculosis from active disease. PLoS One. 2013;8:e62618. doi: 10.1371/journal.pone.0062618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Philips JA, Ernst JD. Tuberculosis pathogenesis and immunity. Annual review of pathology. 2012;7:353–384. doi: 10.1146/annurev-pathol-011811-132458. [DOI] [PubMed] [Google Scholar]
- 27.Stanley SA, Cox JS. Host-pathogen interactions during Mycobacterium tuberculosis infections. Current topics in microbiology and immunology. 2013;374:211–241. doi: 10.1007/82_2013_332. [DOI] [PubMed] [Google Scholar]
- 28.Huynh KK, Joshi SA, Brown EJ. A delicate dance: host response to mycobacteria. Curr Opin Immunol. 2011;23:464–472. doi: 10.1016/j.coi.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 29.Abebe F, Bjune G. The protective role of antibody responses during Mycobacterium tuberculosis infection. Clin Exp Immunol. 2009;157:235–243. doi: 10.1111/j.1365-2249.2009.03967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Behar SM. Antigen-specific CD8(+) T cells and protective immunity to tuberculosis. Adv Exp Med Biol. 2013;783:141–163. doi: 10.1007/978-1-4614-6111-1_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsai MC, Chakravarty S, Zhu G, Xu J, Tanaka K, Koch C, Tufariello J, Flynn J, Chan J. Characterization of the tuberculous granuloma in murine and human lungs: cellular composition and relative tissue oxygen tension. Cell Microbiol. 2006;8:218–232. doi: 10.1111/j.1462-5822.2005.00612.x. [DOI] [PubMed] [Google Scholar]
- 32.Via LE, Lin PL, Ray SM, Carrillo J, Allen SS, Eum SY, Taylor K, Klein E, Manjunatha U, Gonzales J, Lee EG, Park SK, Raleigh JA, Cho SN, McMurray DN, Flynn JL, Barry CE., 3rd Tuberculous granulomas are hypoxic in guinea pigs, rabbits, and nonhuman primates. Infect Immun. 2008;76:2333–2340. doi: 10.1128/IAI.01515-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nuermberger E. Using animal models to develop new treatments for tuberculosis. Seminars in respiratory and critical care medicine. 2008;29:542–551. doi: 10.1055/s-0028-1085705. [DOI] [PubMed] [Google Scholar]
- 34.van der Wel N, Hava D, Houben D, Fluitsma D, van Zon M, Pierson J, Brenner M, Peters PJ. M. tuberculosis and M. leprae translocate from the phagolysosome to the cytosol in myeloid cells. Cell. 2007;129:1287–1298. doi: 10.1016/j.cell.2007.05.059. [DOI] [PubMed] [Google Scholar]
- 35.Willis D, Moore AR, Frederick R, Willoughby DA. Heme oxygenase: a novel target for the modulation of the inflammatory response. Nat Med. 1996;2:87–90. doi: 10.1038/nm0196-87. [DOI] [PubMed] [Google Scholar]
- 36.Paine A, Eiz-Vesper B, Blasczyk R, Immenschuh S. Signaling to heme oxygenase-1 and its anti-inflammatory therapeutic potential. Biochem Pharmacol. 80:1895–1903. doi: 10.1016/j.bcp.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 37.Kapturczak MH, Wasserfall C, Brusko T, Campbell-Thompson M, Ellis TM, Atkinson MA, Agarwal A. Heme oxygenase-1 modulates early inflammatory responses: evidence from the heme oxygenase-1-deficient mouse. Am J Pathol. 2004;165:1045–1053. doi: 10.1016/S0002-9440(10)63365-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Minamino T, Christou H, Hsieh CM, Liu Y, Dhawan V, Abraham NG, Perrella MA, Mitsialis SA, Kourembanas S. Targeted expression of heme oxygenase-1 prevents the pulmonary inflammatory and vascular responses to hypoxia. Proc Natl Acad Sci U S A. 2001;98:8798–8803. doi: 10.1073/pnas.161272598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tzima S, Victoratos P, Kranidioti K, Alexiou M, Kollias G. Myeloid heme oxygenase-1 regulates innate immunity and autoimmunity by modulating IFN-beta production. J Exp Med. 2009;206:1167–1179. doi: 10.1084/jem.20081582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou ZH, Kumari N, Nekhai S, Clouse KA, Wahl LM, Yamada KM, Dhawan S. Heme oxygenase-1 induction alters chemokine regulation and ameliorates human immunodeficiency virus-type-1 infection in lipopolysaccharide-stimulated macrophages. Biochem Biophys Res Commun. 435:373–377. doi: 10.1016/j.bbrc.2013.04.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wegiel B, Larsen R, Gallo D, Chin BY, Harris C, Mannam P, Kaczmarek E, Lee PJ, Zuckerbraun BS, Flavell R, Soares MP, Otterbein LE. Macrophages sense and kill bacteria through carbon monoxide-dependent inflammasome activation. The Journal of clinical investigation. 2014;124:4926–4940. doi: 10.1172/JCI72853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tobin DM, Vary JC, Jr, Ray JP, Walsh GS, Dunstan SJ, Bang ND, Hagge DA, Khadge S, King MC, Hawn TR, Moens CB, Ramakrishnan L. The lta4h locus modulates susceptibility to mycobacterial infection in zebrafish and humans. Cell. 2010;140:717–730. doi: 10.1016/j.cell.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tobin DM, Roca FJ, Oh SF, McFarland R, Vickery TW, Ray JP, Ko DC, Zou Y, Bang ND, Chau TT, Vary JC, Hawn TR, Dunstan SJ, Farrar JJ, Thwaites GE, King MC, Serhan CN, Ramakrishnan L. Host genotype-specific therapies can optimize the inflammatory response to mycobacterial infections. Cell. 2012;148:434–446. doi: 10.1016/j.cell.2011.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Watkins BY, Joshi SA, Ball DA, Leggett H, Park S, Kim J, Austin CD, Paler-Martinez A, Xu M, Downing KH, Brown EJ. Mycobacterium marinum SecA2 promotes stable granulomas and induces tumor necrosis factor alpha in vivo. Infect Immun. 2012;80:3512–3520. doi: 10.1128/IAI.00686-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Andrade BB, Pavan Kumar N, Amaral EP, Riteau N, Mayer-Barber KD, Tosh KW, Maier N, Conceicao EL, Kubler A, Sridhar R, Banurekha VV, Jawahar MS, Barbosa T, Manganiello VC, Moss J, Fontana JR, Marciano BE, Sampaio EP, Olivier KN, Holland SM, Jackson SH, Moayeri M, Leppla S, Sereti I, Barber DL, Nutman TB, Babu S, Sher A. Heme Oxygenase-1 Regulation of Matrix Metalloproteinase-1 Expression Underlies Distinct Disease Profiles in Tuberculosis. J Immunol. 2015;195:2763–2773. doi: 10.4049/jimmunol.1500942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Torrado E, Cooper AM. Cytokines in the balance of protection and pathology during mycobacterial infections. Adv Exp Med Biol. 2013;783:121–140. doi: 10.1007/978-1-4614-6111-1_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Azad AK, Sadee W, Schlesinger LS. Innate immune gene polymorphisms in tuberculosis. Infect Immun. 80:3343–3359. doi: 10.1128/IAI.00443-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sivangala R, Ponnana M, Thada S, Joshi L, Ansari S, Hussain H, Valluri V, Gaddam S. Association of cytokine gene polymorphisms in Tuberculosis patients and their House hold contacts. Scand J Immunol. doi: 10.1111/sji.12136. [DOI] [PubMed] [Google Scholar]
- 49.Correa PA, Gomez LM, Cadena J, Anaya JM. Autoimmunity and tuberculosis. Opposite association with TNF polymorphism. J Rheumatol. 2005;32:219–224. [PubMed] [Google Scholar]
- 50.Merza M, Farnia P, Anoosheh S, Varahram M, Kazampour M, Pajand O, Saeif S, Mirsaeidi M, Masjedi MR, Velayati AA, Hoffner S. The NRAMPI, VDR and TNF-alpha gene polymorphisms in Iranian tuberculosis patients: the study on host susceptibility. Braz J Infect Dis. 2009;13:252–256. doi: 10.1590/s1413-86702009000400002. [DOI] [PubMed] [Google Scholar]
- 51.Awomoyi AA, Charurat M, Marchant A, Miller EN, Blackwell JM, McAdam KP, Newport MJ. Polymorphism in IL1B: IL1B-511 association with tuberculosis and decreased lipopolysaccharide-induced IL-1beta in IFN-gamma primed ex-vivo whole blood assay. J Endotoxin Res. 2005;11:281–286. doi: 10.1179/096805105X58706. [DOI] [PubMed] [Google Scholar]
- 52.Amirzargar AA, Rezaei N, Jabbari H, Danesh AA, Khosravi F, Hajabdolbaghi M, Yalda A, Nikbin B. Cytokine single nucleotide polymorphisms in Iranian patients with pulmonary tuberculosis. Eur Cytokine Netw. 2006;17:84–89. [PubMed] [Google Scholar]
- 53.O'Garra A, Redford PS, McNab FW, Bloom CI, Wilkinson RJ, Berry MP. The immune response in tuberculosis. Annual review of immunology. 2013;31:475–527. doi: 10.1146/annurev-immunol-032712-095939. [DOI] [PubMed] [Google Scholar]
- 54.Manzanillo PS, Shiloh MU, Portnoy DA, Cox JS. Mycobacterium tuberculosis activates the DNA-dependent cytosolic surveillance pathway within macrophages. Cell host & microbe. 2012;11:469–480. doi: 10.1016/j.chom.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Watson RO, Bell SL, MacDuff DA, Kimmey JM, Diner EJ, Olivas J, Vance RE, Stallings CL, Virgin HW, Cox JS. The Cytosolic Sensor cGAS Detects Mycobacterium tuberculosis DNA to Induce Type I Interferons and Activate Autophagy. Cell host & microbe. 2015;17:811–819. doi: 10.1016/j.chom.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tachibana M, Hashino M, Nishida T, Shimizu T, Watarai M. Protective role of heme oxygenase-1 in Listeria monocytogenes-induced abortion. PLoS One. 6:e25046. doi: 10.1371/journal.pone.0025046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Seixas E, Gozzelino R, Chora A, Ferreira A, Silva G, Larsen R, Rebelo S, Penido C, Smith NR, Coutinho A, Soares MP. Heme oxygenase-1 affords protection against noncerebral forms of severe malaria. Proc Natl Acad Sci U S A. 2009;106:15837–15842. doi: 10.1073/pnas.0903419106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Araujo EC, Barbosa BF, Coutinho LB, Barenco PV, Sousa LA, Milanezi CM, Bonfa G, Pavanelli WR, Silva JS, Ferro EA, Silva DA, Cunha-Junior JP, Silva NM. Heme oxygenase-1 activity is involved in the control of Toxoplasma gondii infection in the lung of BALB/c and C57BL/6 and in the small intestine of C57BL/6 mice. Vet Res. 44:89. doi: 10.1186/1297-9716-44-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hill-Batorski L, Halfmann P, Neumann G, Kawaoka Y. The cytoprotective enzyme heme oxygenase-1 suppresses ebola virus replication. J Virol. 87:13795–13802. doi: 10.1128/JVI.02422-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen MH, Lee MY, Chuang JJ, Li YZ, Ning ST, Chen JC, Liu YW. Curcumin inhibits HCV replication by induction of heme oxygenase-1 and suppression of AKT. Int J Mol Med. 30:1021–1028. doi: 10.3892/ijmm.2012.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rodriguez GM. Control of iron metabolism in Mycobacterium tuberculosis. Trends Microbiol. 2006;14:320–327. doi: 10.1016/j.tim.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 62.Rodriguez GM, Smith I. Identification of an ABC transporter required for iron acquisition and virulence in Mycobacterium tuberculosis. J Bacteriol. 2006;188:424–430. doi: 10.1128/JB.188.2.424-430.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ganz T. Iron in innate immunity: starve the invaders. Curr Opin Immunol. 2009;21:63–67. doi: 10.1016/j.coi.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Soares MP, Weiss G. The Iron age of host-microbe interactions. EMBO Rep. 2015;16:1482–1500. doi: 10.15252/embr.201540558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nairz M, Theurl I, Ludwiczek S, Theurl M, Mair SM, Fritsche G, Weiss G. The co-ordinated regulation of iron homeostasis in murine macrophages limits the availability of iron for intracellular Salmonella typhimurium. Cell Microbiol. 2007;9:2126–2140. doi: 10.1111/j.1462-5822.2007.00942.x. [DOI] [PubMed] [Google Scholar]
- 66.Paradkar PN, De Domenico I, Durchfort N, Zohn I, Kaplan J, Ward DM. Iron depletion limits intracellular bacterial growth in macrophages. Blood. 2008;112:866–874. doi: 10.1182/blood-2007-12-126854. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.