Summary
G-protein signaling is known to be required for cell-cell contacts during the development of the Drosophila dorsal vessel. However, the identity of the G protein-coupled receptor (GPCR) that regulates this signaling pathway activity is unknown. Here we describe the identification of a novel cardiac specific GPCR, called Gia, for “GPCR in aorta”. Gia is the only heart-specific GPCR identified in Drosophila to date and it is specifically expressed in cardioblasts that fuse at the dorsal midline to become the aorta. Gia is the only Drosophila gene so far identified for which expression is entirely restricted to cells of the aorta. Deletion of Gia led to a broken-hearted phenotype, characterized by pericardial cells dissociated from cardioblasts and abnormal distribution of cell junction proteins. Both phenotypes were similar to those observed in mutants of the heterotrimeric cardiac G proteins. Lack of Gia also led to defects in the alignment and fusion of cardioblasts in the aorta. Gia forms a protein complex with G-αo47A, the alpha subunit of the heterotrimeric cardiac G proteins and interacts genetically with G-αo47A during cardiac morphogenesis. Our study identified Gia as an essential aorta-specific GPCR that functions upstream of cardiac heterotrimeric G proteins and is required for morphological integrity of the aorta during heart tube formation. These studies lead to a redefinition of the bro phenotype, to encompass morphological integrity of the heart tube as well as cardioblast-pericardial cell spatial interactions.
Keywords: Gia, Methuselah-like, Drosophila, heart development, G protein coupled receptor
Graphical Abstract-Highlights
We show that Gia, a novel Methuselah-like G protein coupled receptor (GPCR), is required for heart development in Drosophila. Gia signals through G-αo47A to activate the heterotrimeric G protein signaling pathway which is required for septate junction protein membrane localization during cardiac morphogenesis.
Introduction
The Drosophila heart is a linear tube composed of two rows of contractile myocardial cells called cardioblasts, flanked by non-contractile pericardial cells. Although relatively simple in structure, Drosophila heart development resembles early phases of mammalian heart formation. Genetic pathways evolutionarily conserved from Drosophila to humans control heart development, thus the fly heart is a valuable experimental model system for understanding mammalian heart development and human cardiac disease (Bodmer and Venkatesh, 1998; Olson, 2006; Yi et al., 2006).
A genetic screen for genes regulating heart tube morphogenesis identified a unique cardiac phenotype called broken hearted in which pericardial cells were dissociated from cardioblasts in the dorsal vessel at the end of embryogenesis (Yi et al., 2006). This phenotype is caused by mutations in the gene encoding HMG-CoA reductase (HMGCR) as well as downstream enzymes involved in the mevalonate pathway, or proteins modified by this pathway (Yi et al., 2006). The mevalonate pathway mediates geranylgeranylation of the G protein gamma subunit Gγ1, which is required to maintain cardiac integrity (Yi et al., 2006). Since G proteins usually function as a heterotrimeric complex, we subsequently examined loss-of-function mutations in all alpha and beta subunits of G proteins in the Drosophila genome and identified G-αo47A and Gβ13F as the alpha and beta subunits that function together with Gγ1 during heart development (Yi et al., 2008). Over-expression of an activated form of G-αo47A also induced a bro phenotype, suggesting that both over-activation and loss of G-αo47A activity can lead to cardiac defects (Yi et al., 2008). These results suggested tight regulation of G-αo47A activity, presumably through an upstream G protein-coupled receptor (GPCR). Disruption of cardiac heterotrimeric G protein signaling also leads to abnormal distribution of septate junction proteins in cardioblasts and pericardial cells (Yi et al., 2008). The distribution of septate junction proteins in the Drosophila blood brain barrier (BBB) is also regulated by heterotrimeric G protein signaling, activated by the Moody GPCR (Bainton et al., 2005) (Schwabe et al., 2005). Moody is not expressed in the developing heart, however, and mutation of Moody does not affect heart development (data not shown).
Engagement of a GPCR with its ligand triggers a conformational change in the receptor, which then acts as a guanine nucleotide exchange factor (GEF) to replace GDP with GTP on the α subunit of an associated αβγ heterotrimeric G protein complex. The GTP bound α subunit dissociates from βγ to initiate intracellular signaling cascades (Malbon, 2005; Neves et al., 2002). While ubiquitous in metazoan development, a role for GPCRs in Drosophila heart development remains undefined.
A recent study examining the Drosophila embryonic expression profile of the Methuselah (Mth) and Methuselah-like (Mthl) family identified Mthl5 (CG6965) as a GPCR specifically expressed in the heart (Patel et al., 2012). The Mth/Mthl5 receptors form a subfamily (B3) of secretin-like receptors present in a variety of insects, potentially related to adhesion GPCRs (Harmar 2001). Adhesion GPCRs are known to be important in a diverse array of developmental processes important for development including cell movement and establishment of cell polarity (Langenhan et al., 2013).
Here we show that Mthl5 is a GPCR that is expressed in a highly specific pattern in the aorta during embryonic heart tube formation and cardiac morphogenesis. Deletion of Mthl5 produces the bro phenotype observed in mutants of the cardiac heterotrimeric G proteins, as well as adhesion and position defects between aorta cardioblasts. Mthl5 directly binds to G-αo47A in Drosophila S2 cells. Mthl5 and G-αo47A mutants genetically interact. Distribution of cardioblast junction proteins is disrupted in Mthl5 mutants. These observations suggest that Mthl5 is an aorta-specific GPCR that functions through G-αo47A to regulate cardioblast junction protein distribution, required for adhesion and position of both cardioblasts and pericardial cells during heart development. To highlight its distinct function and aorta-specific expression pattern we refer to Mthl5 as Gia, “GPCR in aorta”, in the following work.
Results
Generation of a Gia mutant
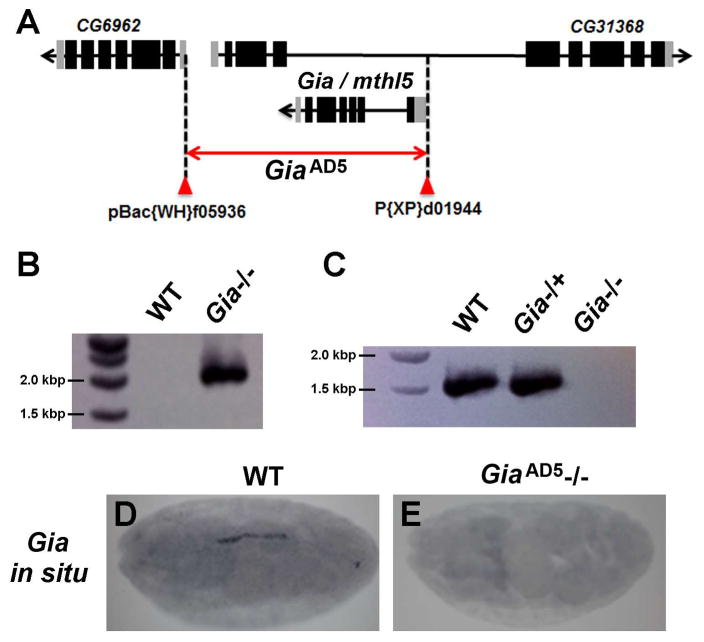
A Gia deletion allele was generated by FRT-mediated recombination between piggyBac and P-element insertions flanking the Gia (Mthl5) locus (Parks et al., 2004) (Fig. 1A). FRT-mediated recombination deleted the entire coding region of Gia, together with much of CG31368 and a portion of the 5′ UTR of CG6962 (Fig. 1A). Neither CG31368 nor CG6962 are expressed in cardiac cells or in the part of the mesoderm that differentiates into cardiac tissues, and they are therefore unlikely to contribute to heart development. A candidate mutant, GiaAD5, was homozygous lethal and PCR analysis showed an amplification product of predicted size for the expected deletion (Fig. 1B). RT-PCR analysis of homozygous and heterozygous GiaAD5 embryonic RNA (Fig. 1C) confirmed that GiaAD5 is a null allele. The absence of Gia mRNA in GiaAD5 homozygous mutant embryos was further confirmed by in situ hybridization (Fig. 1D–E), an experiment that highlighted normal heart specific Gia expression.
Figure 1. Generation and verification of the Gia mutant.
(A) A Gia null mutant was generated by FRT-mediated recombination between transposable element-based insertions flanking the Gia/Mthl5 locus. (B) Genomic PCR performed on DNA extracted from wild type (WT) and GiaAD5 homozygous mutant (Gia−/−) embryos produced the anticipated amplification product to confirm the chromosomal deletion. (C) RT-PCR analysis confirmed the absence of Gia mRNA in GiaAD5 homozygous mutant embryos, compared to wild type and GiaAD5 heterozygous mutant (Gia−/+) embryos. (D, E) In situ hybridization detected Gia mRNA in wild type but not in GiaAD5 homozygous mutant embryos.
Cardiac defects of GiaAD5 mutants
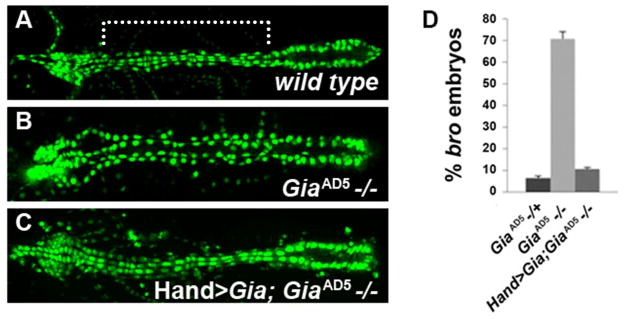
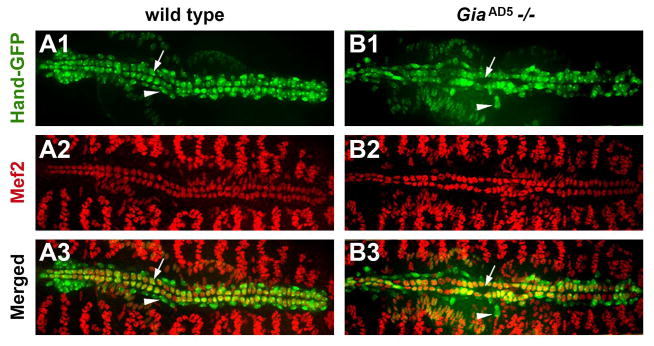
A lethal phase analysis showed that all homozygous GiaAD5 mutant embryos reached stage 17 and showed movement of visceral and body wall muscles. However, 100% failed to hatch into first instar larvae. Moreover, artificially hatched embryos incubated at 25°C in halocarbon oil died within two hours. Since Gia is specifically expressed in the embryonic heart, we examined GiaAD5 mutants in a Hand-GFP (Zhang et al., 2006) background to detect cardiac defects. The Hand-GFP reporter labels all heart cells, facilitating phenotypic analyses of mutations that alter the arrangement of cardioblasts and pericardial cells (Yi et al., 2006; Yi et al., 2008). GiaAD5 mutant embryos displayed significant heart defects, in particular the loss of cell-to-cell adhesion between cardioblasts and pericardial cells (Fig. 2A and B, comparing wild type and homozygous mutant embryonic dorsal vessels, respectively). The GiaAD5 heart phenotype was rescued by expression of a wild type Gia transgene driven by a Hand promoter (Fig. 2C, D), demonstrating that the GiaAD5 mutant phenotype is due specifically to deletion of Gia/Mthl5, and not to disruption of the CG31368 or CG6962 loci (Fig. 1A). The broken hearted phenotype was previously characterized in embryos harboring mutations in the three genes encoding the cardiac heterotrimeric G proteins (Yi et al., 2006; Yi et al., 2008). At stage 17 (after the onset of beating heart activity), approximately 70% of GiaAD5 homozygote mutant embryos displayed significant bro defects (Fig. 2D). Dissociation of pericardial cells was also observed in heterozygous GiaAD5/+ stage 17 embryos at a low frequency (6.5% of stage 17 heterozygous embryos, compared to 70% of homozygous embryos, P<0.001) (Fig. 2D). As previously seen for cardiac G protein mutants that also display the bro phenotype, dissociated pericardial cells are primarily observed in the aorta (mid-anterior region of the dorsal vessel) in contrast to the heart (posterior region) (Fig. 2B).
Figure 2. Gia mutants display broken hearted (Patel et al.) phenotype and cardioblast alignment defects.
(A–C) Hand-GFP expression in the dorsal vessel of stage 17 embryos. Anterior is to the left. Compared to wild type (A), homozygous GiaAD5−/− embryos (B) exhibited bro defects. The bro mutant phenotype was rescued by using Hand-Gal4 to drive a UAS-Gia transgene throughout the dorsal vessel (C). (D) Penetrance of the bro phenotype observed among Gia−/+, Gia−/− and Hand>Gia;Gia−/− embryos.
Gia activates G protein dependent pathways in the heart
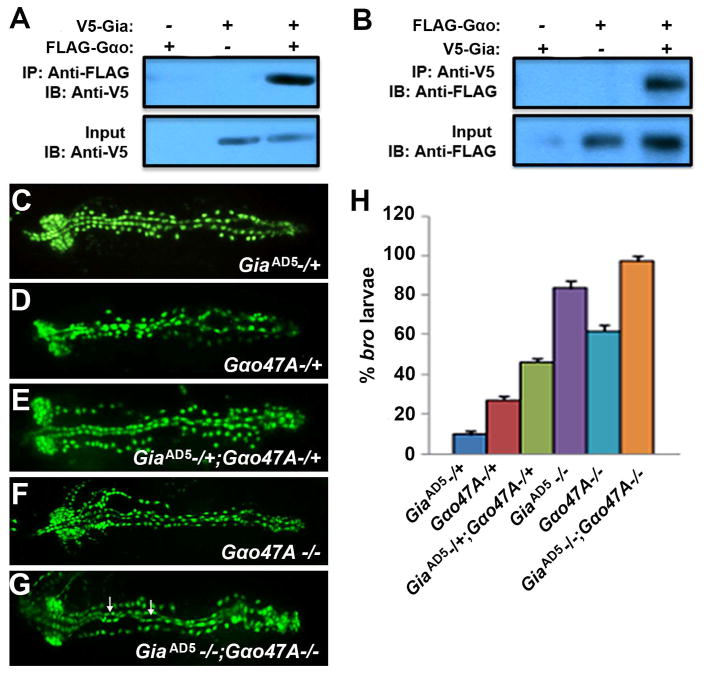
To test whether Gia interacts with G-αo47A, we performed coimmunoprecipitation assays targeting Gia and G-αo47A from Drosophila S2 cells transfected with V5-tagged Gia (V5-Gia) and/or Flag-tagged G-αo47A (Flag-Gαo). Immunoprecipitation of Flag-Gαo co-precipitated V5-Gia (Fig. 3A) and immunoprecipitation of V5-Gia coprecipitated Flag-Gαo (Fig. 3B), showing that Gia and G-αo47A associate in a protein complex. We also showed that Gia and G-αo47A interact genetically. The bro phenotype can be detected in a low percentage of heterozygous GiaAD5 (9.6%) or G-αo47A (27.6%) mutant embryos (Fig. 3C,D,H). For double heterozygote GiaAD5/+ ; G-αo47A/+ stage 17 embryos the percentage showing bro defects increased to 46% (Fig. 3E,H; P<0.001), and the severity of the pericardial dissociation phenotype was markedly increased (Fig. 3, compare C and D to E). Homozygous GiaAD5−/− and G-αo47A −/− embryos exhibited more penetrant bro defects (82% and 60%, respectively; P<0.001) (Fig. 3H). 100% (P<0.001) of GiaAD5−/−; G-αo47A−/− double homozygous mutant embryos exhibited the bro phenotype (Fig. 3G, H). The frequency of heart tube lumen abnormalities (Fig. 3G, arrows) was also increased in the aorta region in GiaAD5−/−; Goα47A−/− embryos. These results strongly suggest that Gia is the GPCR that initiates G protein signaling activity that is required for cardiac integrity.
Figure 3. Gia interacts with G-αo47A.
(A, B) Coimmunoprecipitation of Gia and G-αo47A from transfected Drosophila S2 cells. V5-tagged Gia was detected by immunoblotting (Kiyono et al.) after immunoprecipitation (Wang et al.) of Flag-tagged G-αo47A (A), and IB detected Flag-tagged G-αo47A after IP of V5-tagged Gia (B). (C–G) bro phenotypes visualized using Hand-GFP in GiaAD5 −/+ (C), G-αo47A−/+ (D), GiaAD5 −/+; G-αo47A−/+ (E), G-αo47A−/− (F), GiaAD5 −/− ; G-αo47A−/− (G) stage 17 embryos. Arrows in (G) point to heart tube lumen abnormalities. (H) Graphical representation of bro penetrance (P<0.001). A small percentage (9.6%) of GiaAD5 −/+ heterozygotes showed mild bro defects (C,H), whereas a larger percentage (27.6%) of G-αo47A−/+ heterozygotes showed mild bro defects (D,H). In GiaAD5 −/+; G-αo47A−/+ double heterozygotes the bro defects are much more severe (E) with higher (46%) penetrance (H). GiaAD5 −/−; G-αo47A−/− double homozygotes exhibited severe bro defects and abnormal lumen morphology in the aorta (G, arrows), with 100% bro penetrance (H).
Gia is an aorta-specific GPCR
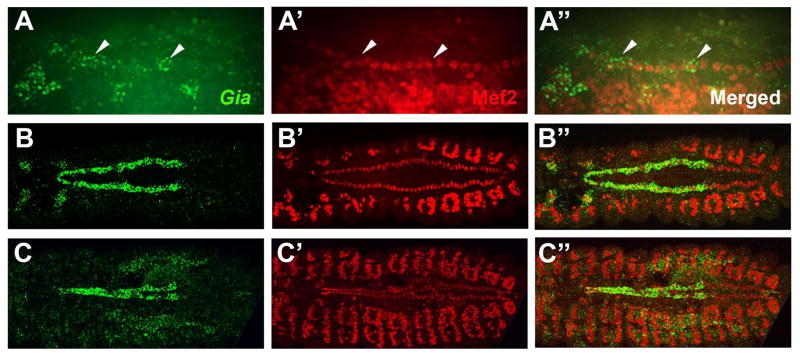
A previous study (Patel et al., 2012) showed that Gia mRNA was expressed in the late stage embryonic dorsal vessel, but did not identify the Gia expressing cell types. We studied Gia mRNA expression by in situ hybridization combined with immunofluorescence detection of Mef2, a transcription factor expressed specifically in cardioblasts, somatic muscles and visceral muscles. Expression of Gia mRNA in the dorsal vessel was first detected at stage 13 in the rows of cardioblasts on either side of the embryo (Fig. 4A–A″, lateral prospect). Gia was expressed most conspicuously in anterior cardioblasts and very weakly in posterior cardioblasts at stage 13 (Fig. 4A–A″, arrowheads). Gia mRNA expression increased in anterior segments and decreased in posterior segments from stage 13 to stage 15, becoming strongly expressed in cardioblasts of the anterior segments of the dorsal vessel that give rise to the aorta (Fig. 4B–B″, dorsal prospect) and disappearing from posterior segment cardioblasts. By stage 16, when the bilateral cardioblasts had fused at the dorsal midline to form the linear heart tube, Gia was expressed exclusively in aorta cardioblasts (Fig. 4C–C″).
Figure 4. Gia is an aorta specific GPCR.
(A–A″) Lateral view of a stage 13 embryo labeled with a Gia in situ hybridization probe (green) and Mef2 antibody (red). Gia expression appears in the cardioblasts from stage 13 (A–A″, arrowheads). Gia expression becomes stronger in the aorta cardioblasts and weaker in the heart cardioblasts from stage 13 to stage 15. (B–B″) Dorsal view of stage 15 embryo labeled with Gia in situ hybridization probe (green) and Mef2 antibody (red). Gia expression became restricted to aorta cardioblasts from stage 15 and remained highly specific to aorta cardioblasts at stage 16 (C–C″). The Mef2 antibody labels all dorsal vessel cardioblasts and somatic muscles.
Over-expression of Gia in the heart does not lead to cardiac defects
Because Gia expression was observed only in the anterior, aorta region during heart tube morphogenesis we tested whether ectopic expression of Gia throughout the entire heart could induce cardiac defects. We generated a transgenic UAS-Gia fly line and combined this with Hand-Gal4 to drive ectopic Gia expression in all cardioblasts and pericardial cells. We did not observe any morphological defects, and the heart was observed to beat properly (data not shown). Furthermore, adult flies bearing the Gia transgene eclosed in normal numbers, indicating an absence of overt lethal effects of ectopic Gia expression in posterior heart cells.
Gia is required for normal heart tube lumen morphology
Approximately 23% of GiaAD5−/− mutant embryos exhibited misaligned cardioblast cells in one or more aorta segments, resulting in an abnormally shaped heart tube lumen. These defects were largely rescued by wild type Gia transgene expression in the heart. By contrast, only 2.3% of GiaAD5 heterozygous embryos showed cardioblast misalignment, with subtle lumen defects. To further examine cardiac defects in GiaAD5−/− mutants we combined Mef2 immunofluorescence with Hand-GFP on stage 17 embryos. Compared to wild type, GiaAD5−/− mutants exhibited abnormal heart tube lumen shape (Fig. 5A1–3, B1–3, arrows), and dissociated pericardial cells (Fig. 5 A1, 3; B1, 3; arrowheads).
Figure 5. Gia is required for cardioblast alignment and lumen morphology in the aorta.
Dorsal views of stage 17 Hand-GFP embryos labeled with Mef2 antibody (red). Compared to wild type (A1–A3), GiaAD5−/− mutant embryos exhibited dissociated pericardial cells (arrowheads), misaligned cardioblasts, and abnormal heart tube lumen in the aorta (arrows).
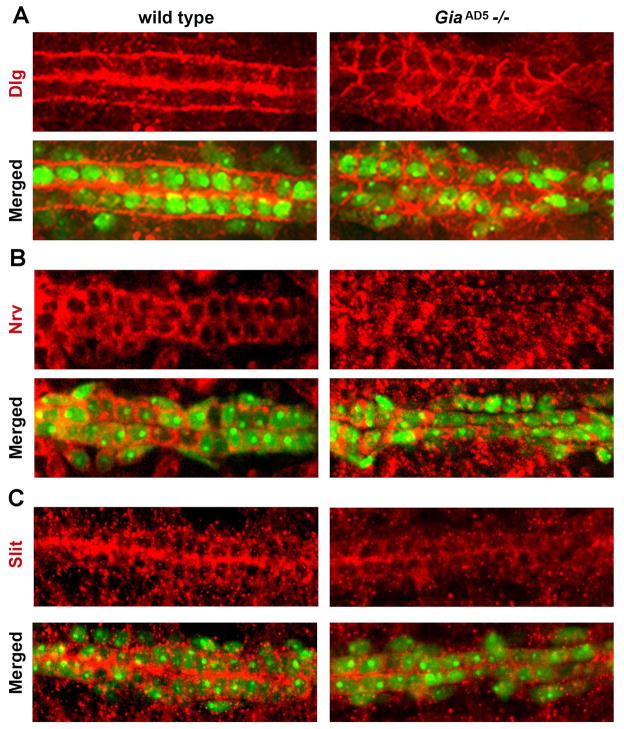
Discs-large (Dlg) is required for septate junctions and cell polarity in Drosophila epithelial cells (Bellaiche et al., 2001; Kiyono et al., 1997). In wild type stage 17 cardioblasts Dlg was normally expressed at the apical and basal membrane domains (Fig. 6 A1–4). Compared to wild type stage 17 embryos, GiaAD5 homozygous mutant embryos exhibited irregular cardioblast alignment and distention of the aorta heart tube lumen (Fig. 6 A1, B1; arrowheads), as well as dissociated pericardial cells (Fig. 6B1, arrow). In the mutant cardioblasts the distribution of Dlg was dramatically altered. Rather than being restricted to apical and basal domains as in wild type, in the GiaAD5−/− mutant the Dlg protein is found along the entire circumference of the cell (Fig. 6B2, compare to 6A2; arrows). Also, Dlg protein associated with the mutant apical (lumenal) domain appeared significantly reduced compared to wild type (Fig. 6B2–4, compare to 6A2–4). Loss of Gia thus resulted in severe disruption of polarized Dlg (and by inference, septate junction protein) localization at cardioblast apical and basal membrane domains in the aorta. We also examined Dystroglycan (Parks et al.) protein localization in aorta cardioblasts of wild type and mutant stage 17 embryos (data not shown). Dg is a receptor for multiple extracellular matrix (ECM) molecules including laminin, agin and perlecan, and plays an essential role in linking the ECM to the actin cytoskeleton (Deng et al., 2003). Dg was predominantly localized to the apical (lumenal) and basal membranes of cardioblasts in wild type embryos and this localization of Dg was also observed in GiaAD5−/− embryo cardioblasts (data not shown). These results suggested that loss of Gia did not abrogate cardioblast cell polarity per se, but more specifically disrupted localization of septate junction proteins produced by cardioblasts.
Figure 6. Gia is required for normal distribution of Dlg.
(A, B) Dorsal views of Hand-GFP stage 17 embryos labeled with Dlg antibody (red). Compared to wild type (A1–A4), GiaAD5 mutants exhibited dissociation of pericardial cells (B1, arrow), misaligned cardioblasts, and abnormal lumen in the aorta (arrowhead, compare to arrowhead in A1). In wild type embryos, Dlg was mainly distributed in the cardioblast apical (lumenal) and basal membrane domains (A2), whereas in the GiaAD5 mutants Dlg was distributed uniformly around the cardioblast cell membrane (B2, arrow). Higher magnification views (A4, B4) of merged images (A3, B3) show clearly the degree of Dlg mislocalization in the mutant aorta.
Discussion
Heterotrimeric G-protein signaling is known to be important for the development of the Drosophila heart. Here, we linked G-protein signaling in the heart to an upstream GPCR, Gia, which is differentially expressed in cardioblasts of the aorta and is important for cell adhesion and cell junction formation between cardioblasts and pericardial cells, as well as between opposing cardioblasts that join to form the heart tube.
The biochemical and genetic interaction assays between Gia and G-αo47A strongly suggest that Gia functions through the heterotrimeric G proteins previously identified as important for cardiac morphological integrity (Yi et al., 2006; Yi et al., 2008). In that earlier study we demonstrated that the distribution of septate junction proteins is altered in mutants of the cardiac heterotrimeric G proteins, (Yi et al., 2008). In this study, we showed that the apical-basal distribution of septate junction components (as represented by Dlg localization) was altered in Gia mutants.
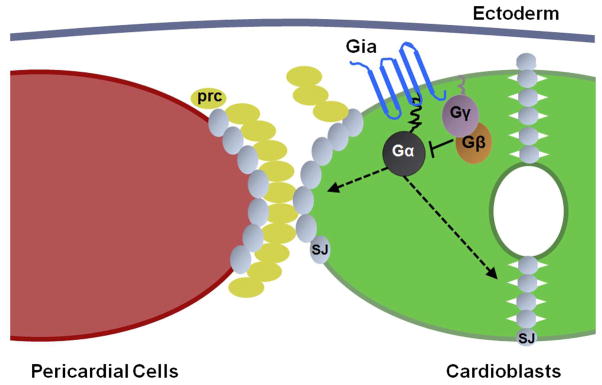
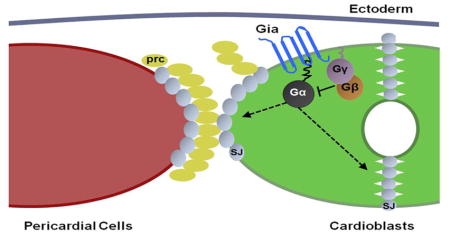
We propose a model to explain Gia’s role in aorta morphogenesis via downstream activation of heterotrimeric G proteins and effector proteins (Fig. 7). In this model, Gia is bound by an as yet unknown ligand, triggering a conformational change and activation of intrinsic GEF activity to exchange GTP for GDP on G-αo47A. Activated G-αo47A then dissociates from the β and γ subunits (Gβγ). Gβγ, modified by the mevalonate pathway and translocated to specific intracellular target regions, regulates the distribution of septate junction proteins. The formation of normal junctions between cardioblasts and pericardial cells and between cardioblasts is required for normal aorta morphology. In the Gia null mutant, the signaling pathway is not activated, leading to the bro defects described previously, as well as heart tube morphology defects. Figure 7 depicts the most severe phenotypic expression of the Gia null mutant phenotype in which pericardial cells are disassociated from cardioblasts, and cardioblasts themselves misposition and exhibit failure to fuse and abnormal aorta heart tube lumen.
Figure 7. A Model for Gia function in Drosophila dorsal vessel development.
(A) In normal (wild type) dorsal vessel the Gia transmembrane receptor is expressed by cardioblasts (CB) of the aorta. Engagement of Gia by extracellular ligand(s) triggers intrinsic GEF activity resulting in GTP (yellow circle) binding and activation of the Gia-associated Gα subunit of the heterotrimeric G protein. Gα dissociates from Gβγ subunits. Gβγ directs the apical and basal localization of septate junction proteins (represented by Dlg, red). Polarized septate junction protein localization is required for the normal close association of pericardial cells (Parks et al.) with CBs, and normal apposition and fusion of CBs with formation of the aorta heart tube lumen. (B) In the Gia mutant aorta lacking the Gia receptor the Gα subunit of the heterotrimeric G protein is in an inactive GDP (blue oval)-bound state and remains associated with the Gβγ subunits. Apical-basal localization of septate junction proteins is abrogated (represented by circumcellular Dlg protein, red). PCs are not positioned correctly, cardioblasts do not fuse, and aorta heart tube lumen morphology is abnormal.
The aorta specific expression of Gia suggests that another GPCR activates G protein signaling in the heart (the posterior region of the dorsal vessel). G-αo47A is expressed in all cardioblasts (both aorta and heart) and the heart region also undergoes similar heart tube fusion and cell-cell junction formation. The fact that we could rescue the bro phenotype and lethality of Gia mutants by expressing a wild type Gia transgene throughout the dorsal vessel (both aorta and heart) shows that ectopic Gia expression in cells of the heart does not lead to obvious morphological abnormalities or lethality. It is possible that the Gia ligand is also restricted to the aorta region.
Our analysis of Gia mutants suggests a role for Gia in cell-cell adhesion, as might be expected of an adhesion GPCR. Comparative sequence analysis indicates, however, that Gia differs from known adhesion GPCRs. Gia was originally named Mthl5 based on similarity to Mth and Mthl family members, but two independent analyses placed Mthl5 in a separate ancient clade of insect specific receptors (Patel et al., 2012; Li et al., 2013). In these analyses Gia is more closely related to adhesion GPCRs, consistent with a shared evolutionary history between adhesion and secretin receptors (Hamar, 2001). In a BLAST analysis against human and mouse sequences Gia shows greatest similarity to GPR133 and latrophilin receptors, especially within the transmembrane domains. Gia and latrophilin receptors also share similarity in the C-terminal domain, possibly reflecting the ability of both receptor types to activate Gαo (Lelianova et al., 1997). On the other hand, the N-terminal extracellular domain of Gia has adhesion-like domains but lacks the GAIN and GPS domains characteristic of most adhesion GPCRs. The GAIN domain is thought to promote self-cleavage at the GPS site (Arac et al., 2012; Promel et al., 2013), producing a “split-receptor” at the membrane with important physiological consequences (Langenhan et al., 2012; Promel et al., 2013). We consider Gia to possess “adhesion-like” GPCR functional attributes. The name change from Mthl5 to Gia (GPCR in aorta) is intended to highlight its unique sequence features while underscoring its function and aorta specific expression pattern.
In summary, we have identified Gia as the GPCR that activates G protein signaling in the dorsal vessel aorta, an important component of cellular processes underlying cell-cell adhesive interactions that, if blocked, result in a bro phenotype. The studies reported here lead us to a reassessment of the bro phenotype to include not only displacement of pericardial cells but also misalignment of cardioblasts and heart tube lumen morphological abnormalities.
Future studies of Gia addressing structure-function and identification of binding partners (ligands or other cell adhesion and/or receptor molecules) will further clarify Gia’s role in Drosophila dorsal vessel development and its functional relationship to conventional adhesion GPCRs. Ultimately, these studies have the potential to yield important insights into heart development in higher organisms.
Materials and Methods
Gia mutant generation
FLP-FRT deletion generation methods were performed essentially as described (Parks et al., 2004). Transposable elements pBac{WH}f05936 and P{XP}d01944 inserted on either side of the Gia/Mthl5 locus were utilized. Candidate deletion mutants were analyzed by genomic PCR using primer pairs WH5′ PLUS – GACGCATGATTATCTTTTACGTGAC, AND XP5′ MINUS – AATGATTCGCAGTGGAAGGCT.
RT PCR analysis
Mutant embryos were identified as heterozygous versus homozygous based on the presence or absence of a balancer chromosome YFP reporter. Total RNA was extracted and used as a template for RT PCR analysis using the following Mthl5 gene specific primers, 5′-ACAATAGCGGCCGCATATGCTCGTAAAAAC and 3′-GTATCTAGAGTAATCGTTGCCGTTCATATA.
In situ hybridization
In situ hybridization was carried out as previously described (Kosman et al., 2004).
Coimmunoprecipitation
GalphaO and Gia cDNA were cloned into the pAc5.1 vector (Invitrogene) with N-terminal Flag tag and V5 tag, respectively. Cell transfection and coimmunoprecipitation experiments in Drosophila S2 cells were carried out as described (Han et al., 2004; Wang et al., 2003).
Cloning
A full-length Gia cDNA clone in pUAST was generated from total embryonic cDNA using 5′-AATAGCGGCCGCATGCTCGTAAAAAC and 3′-CCGCTCTAGATCAGTAATCGTTGCC primers.
Drosophila strains
Expression of transgenes was accomplished using the Gal4-UAS system employing a Hand-Gal4 driver (Han and Olson, 2006). Oregon-R was used as wild type reference strain. The G-αo47A mutant strain that was used has been previously described (Fremion et al., 1999).
Immunohistochemistry and microscopy
Embryos were collected and stained with various antibodies as previously described (Han and Olson, 2006). The following primary antibodies were used: rabbit anti-Dmef2, 1:1000 (gift from B. Paterson); rabbit anti-Discs large, 1:200 (Developmental Studies Hybridoma Bank); rabbit anti-dystroglycan 1:100 (gift from W.M. Deng). Cy2, Cy3, Cy5 or Biotin-conjugated secondary antibodies (from Jackson Lab) were used. Images were obtained with a Zeiss LSM510-meta confocal microscope or an Olympus BX-51 disc-spin confocal microscope.
Acknowledgments
We thank the Bloomington Stock Center for providing the fly stocks and the Developmental Studies Hybridoma Bank for antibodies. Z.H. was supported by grants from the National Institute of Health (R01HL090801 and R01DK098410) and the American Heart Association (AHA-0630178N).
Footnotes
Author contribution:
Zhe Han overlooked the design of the research; Meghna Patel, Jun-yi Zhu, Zhiping Jiang performed the research; Meghna Patel, Adam Richman, Mark VanBerkum and Zhe Han analyzed the data and wrote the paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bainton RJ, Tsai LT, Schwabe T, DeSalvo M, Gaul U, Heberlein U. moody encodes two GPCRs that regulate cocaine behaviors and blood-brain barrier permeability in Drosophila. Cell. 2005;123:145–156. doi: 10.1016/j.cell.2005.07.029. [DOI] [PubMed] [Google Scholar]
- Bellaiche Y, Radovic A, Woods DF, Hough CD, Parmentier ML, O’Kane CJ, Bryant PJ, Schweisguth F. The Partner of Inscuteable/Discs-large complex is required to establish planar polarity during asymmetric cell division in Drosophila. Cell. 2001;106:355–366. doi: 10.1016/s0092-8674(01)00444-5. [DOI] [PubMed] [Google Scholar]
- Bodmer R, Venkatesh TV. Heart development in Drosophila and vertebrates: conservation of molecular mechanisms. Dev Genet. 1998;22:181–186. doi: 10.1002/(SICI)1520-6408(1998)22:3<181::AID-DVG1>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Deng WM, Schneider M, Frock R, Castillejo-Lopez C, Gaman EA, Baumgartner S, Ruohola-Baker H. Dystroglycan is required for polarizing the epithelial cells and the oocyte in Drosophila. Development. 2003;130:173–184. doi: 10.1242/dev.00199. [DOI] [PubMed] [Google Scholar]
- Han Z, Li X, Wu J, Olson EN. A myocardin-related transcription factor regulates activity of serum response factor in Drosophila. Proc Natl Acad Sci U S A. 2004;101:12567–12572. doi: 10.1073/pnas.0405085101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyono T, Hiraiwa A, Fujita M, Hayashi Y, Akiyama T, Ishibashi M. Binding of high-risk human papillomavirus E6 oncoproteins to the human homologue of the Drosophila discs large tumor suppressor protein. Proc Natl Acad Sci U S A. 1997;94:11612–11616. doi: 10.1073/pnas.94.21.11612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosman D, Mizutani CM, Lemons D, Cox WG, McGinnis W, Bier E. Multiplex detection of RNA expression in Drosophila embryos. Science. 2004;305:846. doi: 10.1126/science.1099247. [DOI] [PubMed] [Google Scholar]
- Lelianova VG, Davletov BA, Sterling A, Rahman MA, Grishin EV, Totty NF, Ushkaryov YA. Alpha-latrotoxin receptor, latrophilin, is a novel member of the secretin family of G protein-coupled receptors. J Biol Chem. 1997;272:21504–21508. doi: 10.1074/jbc.272.34.21504. [DOI] [PubMed] [Google Scholar]
- Malbon CC. G proteins in development. Nat Rev Mol Cell Biol. 2005;6:689–701. doi: 10.1038/nrm1716. [DOI] [PubMed] [Google Scholar]
- Neves SR, Ram PT, Iyengar R. G protein pathways. Science. 2002;296:1636–1639. doi: 10.1126/science.1071550. [DOI] [PubMed] [Google Scholar]
- Olson EN. Gene regulatory networks in the evolution and development of the heart. Science. 2006;313:1922–1927. doi: 10.1126/science.1132292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks AL, Cook KR, Belvin M, Dompe NA, Fawcett R, Huppert K, Tan LR, Winter CG, Bogart KP, Deal JE, Deal-Herr ME, Grant D, Marcinko M, Miyazaki WY, Robertson S, Shaw KJ, Tabios M, Vysotskaia V, Zhao L, Andrade RS, Edgar KA, Howie E, Killpack K, Milash B, Norton A, Thao D, Whittaker K, Winner MA, Friedman L, Margolis J, Singer MA, Kopczynski C, Curtis D, Kaufman TC, Plowman GD, Duyk G, Francis-Lang HL. Systematic generation of high-resolution deletion coverage of the Drosophila melanogaster genome. Nat Genet. 2004;36:288–292. doi: 10.1038/ng1312. [DOI] [PubMed] [Google Scholar]
- Patel MV, Hallal DA, Jones JW, Bronner DN, Zein R, Caravas J, Husain Z, Friedrich M, Vanberkum MF. Dramatic expansion and developmental expression diversification of the methuselah gene family during recent Drosophila evolution. J Exp Zool B Mol Dev Evol. 2012;318:368–387. doi: 10.1002/jez.b.22453. [DOI] [PubMed] [Google Scholar]
- Schwabe T, Bainton RJ, Fetter RD, Heberlein U, Gaul U. GPCR signaling is required for blood-brain barrier formation in drosophila. Cell. 2005;123:133–144. doi: 10.1016/j.cell.2005.08.037. [DOI] [PubMed] [Google Scholar]
- Wang Z, Wang DZ, Pipes GC, Olson EN. Myocardin is a master regulator of smooth muscle gene expression. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:7129–7134. doi: 10.1073/pnas.1232341100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi P, Han Z, Li X, Olson EN. The mevalonate pathway controls heart formation in Drosophila by isoprenylation of Ggamma1. Science. 2006;313:1301–1303. doi: 10.1126/science.1127704. [DOI] [PubMed] [Google Scholar]
- Yi P, Johnson AN, Han Z, Wu J, Olson EN. Heterotrimeric G proteins regulate a noncanonical function of septate junction proteins to maintain cardiac integrity in Drosophila. Developmental cell. 2008;15:704–713. doi: 10.1016/j.devcel.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Zhou W, Yin C, Chen W, Ozawa R, Ang LH, Anandan L, Aigaki T, Hing H. Misexpression screen for genes altering the olfactory map in Drosophila. Genesis. 2006;44:189–201. doi: 10.1002/dvg.20202. [DOI] [PubMed] [Google Scholar]