Abstract
Background & Aims
Looping is a common problem during colonoscopy that prolongs procedure time. We aimed to determine the efficacy and safety of ColoWrap, an external abdominal compression device, with respect to insertion time and other procedural outcomes.
Methods
We performed a prospective study of outpatients undergoing elective colonoscopy (40–80 years old; mean age, 60.5 years) at endoscopy facilities in the University of North Carolina Hospitals, from April 2013 through March 2014. Subjects were randomly assigned to groups that received either ColoWrap (n=175) or a sham device (control, n=175) during colonoscopy. Colonoscopists and staff were blinded to the application. The primary outcome was cecal intubation time (CIT). Secondary outcomes included use of manual pressure and position change.
Results
The mean CIT was similar for the control and ColoWrap groups (6.69 min vs 6.67 min; P=.98). There were no statistical differences between groups in the frequency of manual pressure (45% for controls vs 37% for ColoWrap group, P=.13) or position changes (4% for controls vs 2% for ColoWrap group, P=.36) between groups. Among patients with body mass index of 30–40 (n=78), CIT was significantly lower for patients who underwent ColoWrap (4.69 min) than controls (6.10 min) (P=.03). Adverse events were similar between groups.
Conclusions
In patients undergoing elective colonoscopy, application of an external abdominal compression device did not improve CIT or affect the frequency of ancillary maneuvers. It appears to benefit patients with body mass indices of 30–40, but further studies are needed. ColoWrap appears to be safe and could reduce looping in a subgroup of patients undergoing colonoscopy. ClinicalTrials.gov no: NCT02025504
Keywords: BMI, abdominal binder, nursing, difficult
INTRODUCTION
Colonoscopy is the gold standard test for colorectal cancer screening, and is also an important diagnostic and therapeutic procedure.1, 2 An estimated 14 million colonoscopies are performed annually in the US,3 and utilization of colonoscopy is on the rise worldwide.4–6 Therefore, the demand for well-performed colonoscopy is substantial.
High quality colonoscopy requires unique technical skills, including advancement of the colonoscope proximal to the ileocecal valve enabling full visualization of the cecum.7 Despite many technological advances in the field, achieving cecal intubation with colonoscopy can be challenging due to looping of the colon during insertion, particularly in the sigmoid region. Looping occurs in up to 90% of all colonoscopies, is a primary cause of patient pain and increased procedure time, and increases the risk of bowel perforation and splenic injury.8–12 Ancillary maneuvers such as abdominal pressure and patient position change are often employed to correct looping, yet these maneuvers are applied variably, require extra personnel, are incompletely successful, and can increase procedure time and cost.10, 13 Manual application of abdominal pressure (generally by a technician or nurse) is the most frequently used ancillary maneuver during colonoscopies, yet this is largely an unscientific and unregimented practice.14 There remains an unmet clinical need for a uniform, practical method to prevent looping, and a more standardized method of applying abdominal pressure may effectively address this need.
ColoWrap® is a recently developed, non-invasive abdominal compression device designed to prevent and reduce looping during the insertion phase of colonoscopy. We hypothesized that use of the ColoWrap device would be associated with reduced insertion time and use of ancillary maneuvers. Herein we report the results of a randomized trial of this device on colonoscopy performance and safety outcomes.
METHODS
Study design and population
This was a randomized, blinded, sham-controlled clinical trial. At a single center with multiple endoscopy facilities (University of North Carolina Hospitals), outpatients undergoing elective colonoscopy between the ages of 40–80 were recruited for participation from April 2013 to March 2014. Inclusion criteria included healthy subjects (American Society of Anesthesiologists Class I–III) who completed the entire purgative preparation prior to their procedure and described adequate cleansing, and could understand and read English. Exclusion criteria included known history of incomplete colonoscopy, problems with sedation or anesthesia, pregnancy, unsedated procedures, multiple planned procedures (e.g. bidirectional endoscopy), previous colon resection, known or suspected inflammatory bowel disease, history of colorectal cancer or other intra-abdominal malignancy, patients with recent wounds or skin rash on the anterior abdominal wall, history of cirrhosis or ascites and known ventral hernia. Patients with body mass index (BMI) >40 kg/m2 or waist circumference >45 inches were also excluded due to the manufacturer-recommended size limits of the device used in this study. This research was approved by the University of North Carolina Institutional Review Board, and all participants provided written informed consent. The study was registered prior to initiation (ClinicalTrials.gov number NCT02025504). This manuscript is reported in accordance with the CONSORT guidelines.15, 16 All authors had access to the study data and reviewed and approved the final manuscript.
Study procedures and device description
Age, sex, race, height, weight, and waist circumference were recorded in all subjects prior to their procedure. Body mass index (BMI) was calculated using the standard formula. All participants completed a study questionnaire regarding medical history, reason for colonoscopy, and concomitant gastrointestinal disorders. Once enrolled, participants were randomized to either the intervention or sham arm. Randomization was stratified by gender and was performed using a web-based portal linked to a previously computer-generated randomization table that was concealed to study coordinators, investigators, all clinical staff and participants.
The study coordinators obtained the randomization assignment and then fitted the intervention or sham device in a private bay alone with the patient. No other study or procedural personnel were present during application of the device (or sham) to preserve blinding. For participants randomized to intervention, an abdominal compression device (ColoWrap, LLC, Durham, NC) was applied around the circumference of the lower abdomen, just below the umbilicus. Designed specifically for compression during colonoscopy, the ColoWrap consists of a neoprene-composite adjustable primary wrap that provides general lower abdominal compression and a secondary support strap that provides focused compression to the area overlying the sigmoid colon. The device is equipped with a Velcro-closure to allow for individualized fitting. Although the manufacturer offers the device in several sizes, the regular size (100cm) ColoWrap was used on all participants in the study for uniformity. Once applied, participants were asked to assert that the wrap was fastened tightly but not uncomfortably. In participants randomized to the sham arm, a sham device, similar in appearance to the intervention (same color, logo, and material) was placed loosely around the lower abdomen such that no pressure was applied. All devices (intervention or sham) were applied by one of 2 coordinators (HC and RK) to ensure standardized application.
After application of either the study device or sham, an opaque sheet was placed transversely over the participants and was kept in place for the duration of the procedure. All patients were then placed in the left lateral decubitus position and underwent anesthesiologist-administered sedation and colonoscopy per standard procedure. Propofol was used in the vast majority of cases, and conscious sedation with fentanyl and midazolam was used in 2 cases. Patients and study coordinators were unblinded to the intervention, but endoscopists, nurses, technicians, and anesthesiologists were blinded to treatment assignment. Manual pressure and patient position change were performed typically by an endoscopy nurse or technician at the discretion of the endoscopist. Endoscopists or anesthesiologists were allowed to remove the study device at any point during the procedure if they determined it was necessary for clinical or safety reasons; this was classified as a protocol deviation.
Outcomes
The primary outcome for this study was cecal intubation time (CIT), which was measured in typical fashion from scope insertion to intubation of the cecal tip and visualization of the base. Time was subtracted for polyp removal during insertion, if this occurred. Main secondary outcomes included whether or not manual pressure or patient position change (e.g. from lateral decubitus to supine) was required during the case. Other secondary outcomes included frequency of prolonged cases (CIT >10 min and >20 min), colonoscopy completion rate, operator-assessed patient discomfort during the procedure as measured by the Gloucester score,17 dosage of anesthetic agents, and patient-reported pain, bloating, and satisfaction at discharge from the endoscopy unit. Pain and bloating were measured using 10 point visual analog scales, and global satisfaction was measured according to a 5 point scale. Secondary outcomes also included provider assessments of procedural difficulty and severity of endoscope looping. Any adverse events were also recorded. Patients with incomplete colonoscopies (e.g. due to poor prep) were excluded from primary outcome analysis (CIT), but were included for secondary outcome analyses. Planned a priori subgroup analyses included stratification by age, gender, and BMI. A per-protocol analysis was also performed excluding participants in the intervention arm in whom the ColoWrap device was removed or became loosened at some point between application of the device and cecal intubation.
Statistical analysis and sample size
Summary statistics and histogram of continuous variables were examined to ensure a normal distribution. Mean CIT for intervention and control groups were compared using Student’s t-tests. Categorical secondary outcomes (e.g. use of manual pressure and patient position change) were compared using chi-square or Fisher’s exact tests. Differences were considered statistically significant at an alpha level of < 0.05. The study biostatistician (JAG) was blinded to study group assignment.
Sample size was determined based on pilot data estimates of CIT and its SD in female patients. We calculated that we would need 166 women (83 per group) to detect a 90 second difference in CIT (SD 207 sec), with 80% power and a two-sided alpha of 0.05. Incorporating an anticipated dropout or exclusion rate of 5%, we planned to enroll 350 patients: 175 men and 175 women. Also based on pilot data and literature estimates of the frequency of ancillary maneuvers,14, 18–20 we determined that a sample size of 332 would be associated with 97.7% power to detect a 20% reduction in use of manual pressure. All statistical analyses were performed using SAS 9.4 (Cary, NC) and Stata 10.1 (College Station, TX).
RESULTS
Participant and procedure characteristics
350 patients were enrolled, 175 in each study arm (Figure 1) between April 2014 and March 2015. Study groups were similar with respect to age, sex, race, body mass index (BMI), waist circumference, and medical history (Table 1). The mean age of participants was 60.5 years (SD 8.4), and 216 (62%) were women. Colonoscopy was incomplete in 3 patients in the sham group vs 1 patient in the ColoWrap group (p=0.32). Reasons for aborted procedures were poor bowel prep (n=2, both in sham arm), looping (n=1 in sham arm), and medical instability (n=1 in ColoWrap arm). There were no device failures. Study colonoscopies were performed by 23 different endoscopists, including 238 cases by senior faculty (≥10 years in practice), 63 cases by junior faculty (<10 years in practice), and 49 cases where gastroenterology fellows worked in tandem with supervising attending physicians. The highest volume endoscopist performed 119 study colonoscopies.
Figure 1.
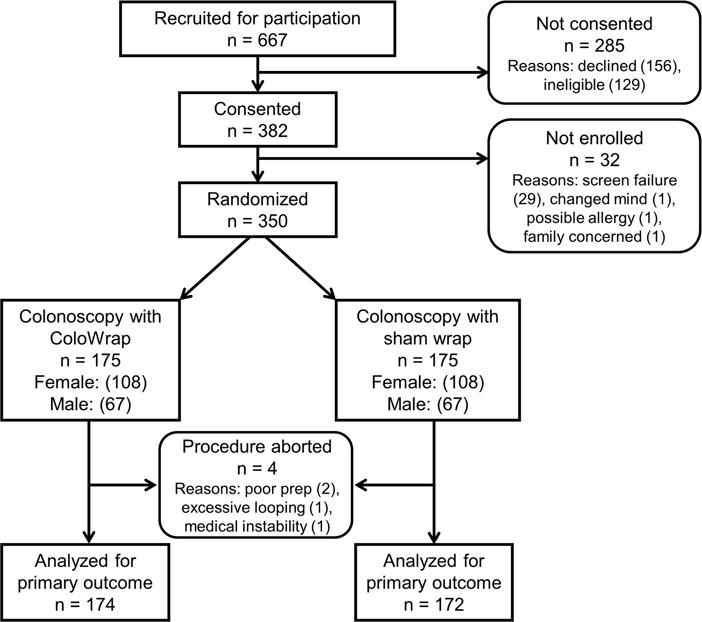
Study flow chart showing flow of participants through trial.
Table 1.
Characteristics of study population and procedures
| Characteristics | Sham n = 175 | ColoWrap n = 175 | pa |
|---|---|---|---|
|
| |||
| Sex | 1.00 | ||
| Male, n (%) | 67 (38) | 67 (38) | |
| Female, n (%) | 108 (62) | 108 (62) | |
|
| |||
| Age | |||
| mean ± SD (years) | 61.1 ± 8.1 | 59.9 ± 8.7 | 0.20 |
| >60 years, n (%) | 97 (55) | 81 (46) | 0.09 |
|
| |||
| Race | 0.20 | ||
| Black, n (%) | 21 (12) | 32 (18) | |
| White, n (%) | 152 (87) | 138 (79) | |
| Asian, n (%) | 2 (1) | 4 (2) | |
| Other, n (%) | 0 (0) | 1 (0.6) | |
|
| |||
| BMI | |||
| mean ± SD | 26.6 ± 4.2 | 26.6 ± 4.3 | 0.97 |
| ≥30 (obese), n (%) | 40 (23) | 38 (22) | 0.80 |
|
| |||
| Waist circumference | 0.58 | ||
| mean ± SD | 35.8 ± 4.2 | 35.6 ± 4.3 | |
|
| |||
| Medical history | |||
| Hysterectomyb, n (%) | 27 (25) | 32 (30) | 0.45 |
| Endometriosisb, n (%) | 10 (9) | 19 (18) | 0.07 |
| Irritable bowel syndrome, n (%) | 10 (6) | 15 (9) | 0.30 |
| Constipation, n (%) | 19 (11) | 25 (14) | 0.33 |
| Diverticulosis, n (%) | 20 (11) | 27 (15) | 0.27 |
| Narcotic use, n (%) | 12 (7) | 12 (7) | 1.00 |
| Any of above, n (%) | 65 (37) | 77 (44) | 0.19 |
|
| |||
| Colonoscopy indication | 0.87 | ||
| Screening, n (%) | 81 (46) | 85 (49) | |
| Surveillance, n (%) | 78 (45) | 72 (41) | |
| Diagnostic, n (%) | 15 (9) | 16 (9) | |
|
| |||
| Endoscopistc | 0.78 | ||
| Senior faculty, n (%) | 116 (66) | 122 (70) | |
| Junior faculty, n (%) | 33 (19) | 30 (17) | |
| Fellow case, n (%) | 26 (15) | 23 (13) | |
|
| |||
| Type of colonoscope | 1.00 | ||
| Pediatric, n (%) | 151 (86) | 151 (86) | |
| Adult, n (%) | 23 (13) | 23 (13) | |
|
| |||
| Prep | 0.77 | ||
| Poor, n (%) | 4 (2) | 3 (2) | |
| Fair, n (%) | 17 (10) | 23 (13) | |
| Good, n (%) | 71 (41) | 70 (40) | |
| Excellent, n (%) | 83 (47) | 79 (45) | |
|
| |||
| Findings | |||
| Polyps | 120 (69) | 111 (63) | 0.31 |
| Diverticulosis, n (%) | 97 (55) | 88 (50) | 0.34 |
| Hemorrhoids, n (%) | 96 (55) | 89 (51) | 0.45 |
| IBD or colitis, n (%) | 4 (2) | 1 (1) | 0.18 |
| Otherd, n (%) | 44 (25) | 32 (18) | 0.12 |
: p values obtained via t-tests or Chi-squared tests comparing the ColoWrap group to the sham group.
: % calculated for women only
: Senior faculty included endoscopists with ≥10 years of endoscopy experience. Junior faculty had <10 years of experience. Fellow cases were those where gastroenterology fellows performed colonoscopy procedures with faculty supervision.
: examples of other findings include inflammation, bleeding, abnormal mucosa, lipoma, and hypertrophied anal papillae
BMI: Body mass index; SD: Standard deviation; IBD: inflammatory bowel disease
Main outcomes
In the intention-to-treat analysis, there was no significant reduction in the primary outcome; the mean CIT was similar for those in the sham and ColoWrap groups (6.69 min vs 6.67 min, p=0.98) (Table 2) (Figure 2A). Manual pressure and position change occurred more frequently in the sham arm compared to the ColoWrap arm, but this was not statistically significant (45% vs 37%, p=0.13, and 4% vs 2%, p=0.36, respectively).
Table 2.
Effect of ColoWrap on cecal intubation time, procedure completion, manual pressure, and patient repositioning
| Outcomes | Sham n = 175 | ColoWrap n = 175 | pa |
|---|---|---|---|
|
| |||
| Cecal intubation time (min) | |||
| Mean ± SD | 6.69 ± 4.38 | 6.67 ± 3.98 | 0.98 |
| Median (IQR) | 5.57 (4.03, 7.96) | 5.62 (4.02, 7.73) | |
| Range | 1.65 – 28.05 | 1.65 – 23.75 | |
| CIT ≥10 min | 28 (16.0) | 27 (15.4) | 0.88 |
| CIT ≥20 min | 7 (4.0) | 4 (2.3) | 0.36 |
|
| |||
| Incomplete colonoscopy, n (%) | 3 (1.7) | 1 (0.6) | 0.32 |
|
| |||
| Manual pressure, n (%) | 79 (45.1) | 65 (37.1) | 0.13 |
|
| |||
| Change in position, n (%) | 7 (4.0) | 4 (2.3) | 0.36 |
SD: Standard deviation; CIT: cecal intubation time; IQR: interquartile range; ITT: intention to treat analysis
: p value comparing ColoWrap ITT to sham
: p value comparing ColoWrap per-protocol to sham
Figure 2.
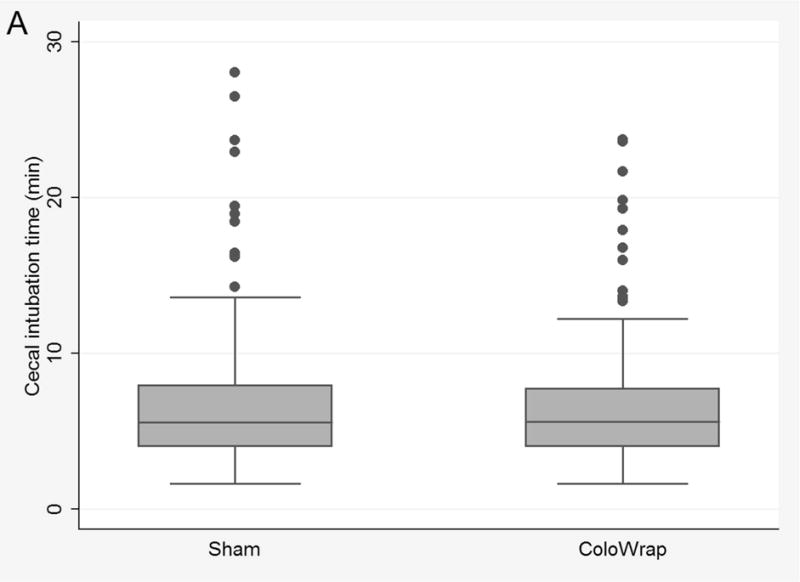
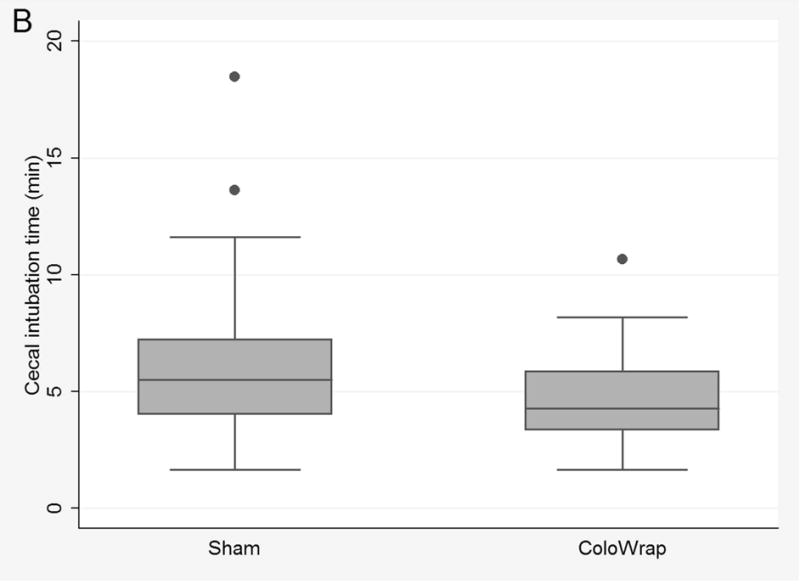
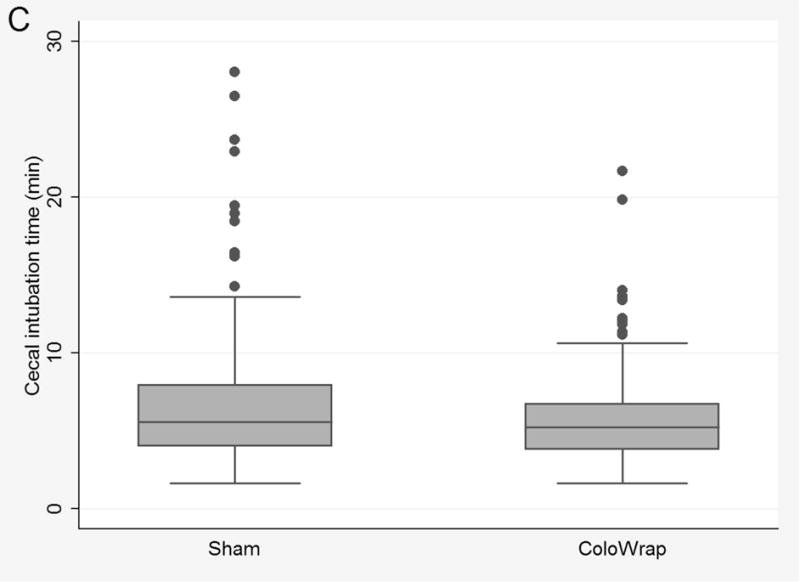
Box and whisker plots of cecal intubation time for ColoWrap and sham arms overall for intention to treat analysis (A), for participants with class I–II obesity (B), and per-protocol analysis (C).
Subgroup analyses
In intention-to-treat subgroup analyses, results were similar after stratification by age and gender. After stratifying by BMI, use of ColoWrap was associated with significantly lower mean CIT in participants with mild to moderate obesity (BMI ≥ 30 and ≤ 40) compared to the sham arm (4.69 min vs. 6.08 min, p=0.03) (Table 3) (Figure 2B). There was also a trend towards reduction in manual pressure and change of position in patients with mild to moderate obesity. When stratified by endoscopist experience, results were similar to overall results (Supplementary Table 1).
Table 3.
Effect of ColoWrap on cecal intubation time and ancillary maneuvers for subgroups of sex, age, and BMI.
| Outcomes | Sham | ColoWrap | pa |
|---|---|---|---|
|
| |||
| Sex stratification | |||
|
| |||
| Female | n=108 | n=108 | |
| Cecal intubation time (min) | |||
| mean ± SD | 6.99 ± 4.13 | 7.11 ± 4.11 | 0.83 |
| CIT ≥ 10 min | 20 (18.5) | 18 (16.7) | 0.72 |
| CIT ≥ 20 min | 5 (4.6) | 4 (3.7) | 1.00 |
| Manual pressure, n (%) | 52 (48.2) | 40 (37.0) | 0.10 |
| Patient repositioned, n (%) | 3 (2.8) | 2 (1.9) | 1.00 |
|
| |||
| Male | n=67 | n=67 | |
| Cecal intubation time (min) | |||
| mean ± SD | 6.22 ± 4.75 | 5.98 ± 3.69 | 0.74 |
| CIT ≥ 10 min | 8 (11.9) | 9 (13.4) | 0.80 |
| CIT ≥ 20 min | 2 (3.0) | 0 (0.0) | 0.50 |
| Manual pressure, n (%) | 27 (40.3) | 25 (37.3) | 0.72 |
| Patient repositioned, n (%) | 4 (6.0) | 2 (3.0) | 0.68 |
|
| |||
| Age stratification | |||
|
| |||
| Age ≤60 years | n=78 | n=94 | |
| Cecal intubation time (min) | |||
| mean ± SD | 6.33 ± 4.32 | 6.18 ± 3.39 | 0.81 |
| CIT ≥ 10 min | 10 (12.8) | 10 (10.6) | 0.66 |
| CIT ≥ 20 min | 3 (3.9) | 0 (0.0) | 0.09 |
| Manual pressure, n (%) | 35 (44.9) | 30 (31.9) | 0.08 |
| Patient repositioned, n (%) | 4 (5.1) | 1 (1.1) | 0.18 |
|
| |||
| Age >60 years | n=97 | n=81 | |
| Cecal intubation time (min) | |||
| mean ± SD | 6.98 ± 4.43 | 7.25 ± 4.54 | 0.69 |
| CIT ≥ 10 min | 18 (18.6) | 17 (21.0) | 0.68 |
| CIT ≥ 20 min | 4 (4.1) | 4 (4.9) | 1.00 |
| Manual pressure, n (%) | 44 (45.4) | 35 (43.2) | 0.77 |
| Patient repositioned, n (%) | 3 (3.1) | 3 (3.7) | 1.00 |
|
| |||
| BMI stratificationb | |||
|
| |||
| BMI <30 kg/m2 | n=135 | n=137 | |
| Cecal intubation time (min) | |||
| mean ± SD | 6.87 ± 4.64 | 7.23 ± 4.24 | 0.51 |
| CIT ≥ 10 min | 23 (17.0) | 26 (19.0) | 0.68 |
| CIT ≥ 20 min | 7 (5.2) | 4 (2.9) | 0.38 |
| Manual pressure, n (%) | 59 (43.7) | 53 (38.7) | 0.40 |
| Patient repositioned, n (%) | 5 (3.7) | 4 (2.9) | 0.75 |
|
| |||
| BMI ≥ 30 kg/m2 | n=40 | n=38 | |
| Cecal intubation time (min) | |||
| mean ± SD | 6.08 ± 3.37 | 4.69 ± 1.89 | 0.03 |
| CIT ≥ 10 min | 5 (12.5) | 1 (2.6) | 0.20 |
| CIT ≥ 20 min | 0 (0.0) | 0 (0.0) | 1.00 |
| Manual pressure, n (%) | 20 (50.0) | 12 (31.6) | 0.10 |
| Patient repositioned, n (%) | 2 (5.0) | 0 (0.0) | 0.49 |
p values obtained via Student’s t-tests or Chi-squared or Fisher’s exact tests comparing the ColoWrap and sham groups
patients with BMI >40 were excluded from the trial
BMI: Body mass index; CIT: cecal intubation time; SD: Standard deviation
Secondary outcomes
Results of secondary outcome analyses are presented in Table 4. There were no statistical differences in Gloucester comfort scale, propofol dose, provider assessments of difficulty or looping, or patient-assessed post-procedure pain, bloating, or global satisfaction. Among patients with mild to moderate obesity, we observed trends toward reduced provider difficulty and looping, but these were not statistically significant.
Table 4.
Effect of ColoWrap on procedural outcomes and post-procedure patient pain, bloating, and satisfaction.
| Secondary outcomes | Sham n = 175 | ColoWrap n = 175 | pa |
|---|---|---|---|
|
| |||
| Gloucester score (intraprocedural comfort) | |||
| Mean ± SD | 1.36 ± 0.72 | 1.49 ± 0.78 | 0.11 |
| Moderate or severe discomfort | 6 (3.5) | 5 (2.9) | 0.75 |
|
| |||
| Propofol (mg)c | |||
| Mean ± SD | 240 ± 92 | 241 ± 92 | 0.89 |
|
| |||
| Operator difficulty | |||
| Difficult or extremely difficult, n (%) | 17 (9.7) | 14 (8.0) | 0.57 |
|
| |||
| Operator assessed looping | |||
| Moderate to excessive looping, n (%) | 20 (11.9) | 19 (10.9) | 0.87 |
|
| |||
| Patient reported pain (0–10 scale) | |||
| Mean ± SD | 0.29 ± 1.03 | 0.25 ± 0.96 | 0.75 |
| Any pain (≥1/10), n (%) | 17 (9.7) | 16 (9.1) | 0.86 |
|
| |||
| Patient reported bloating (0–10 scale) | |||
| Mean ± SD | 1.43 ± 2.10 | 1.33 ± 2.02 | 0.64 |
| Any bloating (≥1/10), n (%) | 78 (44.6) | 74 (42.3) | 0.67 |
|
| |||
| Patient reported global satisfaction | |||
| Satisfied or very satisfied, n (%) | 172 (99.4) | 170 (100.0) | 0.32 |
: p values obtained via Student’s t-tests or Chi-squared or Fisher’s exact tests comparing the ColoWrap ITT and sham groups
: p values comparing ColoWrap per-protocol to sham
: excludes 2 participants who underwent conscious sedation with fentanyl and midazolam
Per-protocol analysis
In the per-protocol analysis, 31 participants who had the ColoWrap device loosened or unfastened during colonoscope insertion were excluded (Supplementary Table 2). In this analysis, ColoWrap was associated with significantly reduced mean CIT, CIT over 10 minutes, and those requiring ancillary maneuvers (Supplementary Table 3) (Figure 2C). Provider difficulty and looping scores were also lower in ColoWrap arm in the per-protocol analysis. Specifically, the proportion with moderate or excessive looping was 12% in sham arm vs 4% in the ColoWrap arm (p=0.009).
Adverse events
A total of 14 participants (7 in each group) reported adverse events during the study (Supplementary Table 4). All adverse events were judged by investigators to be likely attributable to the colonoscopy procedure itself or procedural sedation. There was 1 serious adverse event, a perforation, in the intervention group. This event was attributed to a polypectomy performed during the case and was felt to be unlikely related to use of the study device. The number of adverse events did not differ statistically between groups (p=1.00).
DISCUSSION
In this randomized, blinded, sham-controlled study, use of an external abdominal compression device did not lower overall cecal intubation times in all patients compared with sham. In the intention-to-treat analysis, there were also no differences in difficulty of procedure or degree of looping, patient pain or bloating following the procedure, patient global satisfaction, or adverse events. We did find that use of this device resulted in lower CIT for participants with mild to moderate obesity, however. The device was well-tolerated by participants.
One would expect that if ColoWrap use makes colonoscopy insertion easier for some patients, its use could improve the overall rate of cecal intubation, which is an established provider-level quality metric.21 We did not find that the colonoscopy completion percentage was statistically different in ColoWrap and control arms (99.4% vs. 98.2%), but incomplete procedures were rare overall. The possible reduction in need for manual pressure and patient positioning has implications not only for patient comfort, but for endoscopy unit staffing and procedural costs, given that extra personnel are often required to perform these maneuvers. These maneuvers are also felt to be a primary driver of endoscopy staff injury.22
Two previously published studies have evaluated the effect of abdominal binders on colonoscopy performance.23, 24 In a randomized, unblinded Japanese study of 212 patients, the use of an “abdominal bandage” was found to reduce patient-reported pain at a statistically significant level (p=0.03).24 There was, however, no significant difference in cecal intubation rate between groups. In a more recent Turkish study, 216 patients undergoing colonoscopy were randomized to receive either an “abdominal corset” or a conventional procedure.23 The investigators reported reduced CIT in the intervention arm (7.9 minutes vs. 11.4 minutes, p=0.001) as well as decreased frequency of ancillary maneuvers. Additionally, patient pain scores were significantly reduced in the corset arm. Results from these studies are also suggestive of a potential benefit of abdominal compression devices during colonoscopy, but prior to the current study, the benefit of this technique in the US population, where colonoscopy practice differs, was uncertain. Furthermore, the current study is the first to evaluate the benefit of an abdominal compression device designed specifically for use during colonoscopy in a sham-controlled trial. In another related study, Uddin et al. demonstrated that prone positioning of obese patients during colonoscopy reduced cecal intubation times and decreased need for patient repositioning.25 Their findings also support the potential benefit of abdominal splinting in obese patients undergoing colonoscopy.
Strengths of this study include its randomized design, use of sham controls, and blinding of providers to treatment group. These factors serve to minimize bias and confounding. Our study setting included a high volume academic practice site and multiple endoscopists including fellows. While this led to variability in colonoscopy performance and skill among endoscopists and may have limited our ability to identify statistically-significant differences in the main outcomes, it did maximize the generalizability of our results to other similar multi-physician settings. Results did not seem to differ substantially by endoscopist experience and somewhat surprisingly, a reduction in CIT in patients with mild to moderate obesity was seen even among senior endoscopists, a group we expected to be most adept at avoiding looping. Propofol anesthesia was used for the vast majority of cases in this study. As such, generalizability to settings where conscious sedation is performed may be limited, and the device may have different effects in patients under lighter or no sedation. The use of propofol sedation also likely limited the use of position changes, which occurred infrequently in this study. For this reason, the trial was largely underpowered to detect clinically-meaningful differences in position change maneuvers.
Though randomization and concealed allocation was performed, there were slight (but not statistically-significant) imbalances among groups with respect to age and medical history in particular, which could have led to a small amount of residual confounding. Another potential limitation is that patients were unblinded for this study. However, we did not feel that blinding of patients was possible, safe, or methodologically sound given the manufacturer-recommended application method, which requires patient input to determine comfort of fitting. Furthermore, we felt it was highly unlikely that participant knowledge of ColoWrap placement could have influenced the procedurally-oriented primary outcome (CIT) or use of ancillary maneuvers as all participants were sedated. Coordinators, who were unblinded, could have introduced bias as they recorded study data used to determine outcomes, although we feel this is unlikely since CIT is generally determined by patient and provider factors and is recorded as a standard measure in our colonoscopy units. It is also possible that endoscopy unit staff became unblinded during some cases, particularly if manual pressure was applied, which could have introduced bias.
Results of the per-protocol analysis should be interpreted with caution as some patients were excluded, which could introduce confounding. This analysis excluded 31 participants (17.7%) in the intervention group. We did not have accurate accounting of reasons for removal of the device, so cannot exclude the possibility that the ColoWrap was removed more commonly in “difficult” cases, which could lead to a biased estimate. Nevertheless, in participants who had the ColoWrap fastened at the appropriate tightness during the entire period of colonoscope insertion, CIT was significantly reduced and colonoscopies were judged to be easier, with less looping. Results of subgroup analyses should also be interpreted cautiously, especially since obese subjects represented a minority of study participants. However, the fact that patients with mild to moderate obesity also experienced trends in reduced ancillary maneuvers, procedural difficulty and looping suggests there is a true benefit of use of the device in this population. Patients with BMI >40 and large waist circumferences were excluded in the trial due to concern for fitting of the device. In retrospect this is an unfortunate limitation given the apparent benefit in obese patients. Due to this limitation and those of subgroup analyses in general, further studies specifically in obese subjects will be important to confirm a benefit in this population.
We did not find a reduction in CIT in women, non-obese or elderly participants, groups we were particularly interested in studying given literature reports of difficult or prolonged colonoscopy in these populations.26 Reasons for lack of demonstrated efficacy in these groups is uncertain, but could be related to the fact that they received less pressure from the device than obese patients due to smaller pelvic circumference, or that more focal abdominal pressure was needed to address looping in these patients. Some authors have postulated that there are two main causes of difficult colonoscope insertions: redundant colons (in obese, tall, or chronically-constipated persons), and sigmoid angulation (in thin patients, and those with diverticulosis or a history of pelvic surgery).27, 28 Our results would suggest that abdominal pressure (particularly the type of pressure generated by the ColoWrap device) may be more effective for looping associated with longer, redundant colons.
In sum, this randomized trial did not show benefit of the ColoWrap device in all patients undergoing colonoscopy with respect to CIT and ancillary maneuvers. We did demonstrate a possible benefit of this device in patients with mild to moderate obesity specifically, but these findings should be interpreted cautiously given small numbers in this subgroup and lack of extremely obese (BMI>40) participants in the trial. There was no decrement in safety or patient-reported outcomes with use of this device. Further study is needed to confirm a beneficial effect of ColoWrap in obese patients and in other settings and patient groups. Nevertheless, these data suggest that this simple device may improve the performance of colonoscopy in some patients.
Supplementary Material
Acknowledgments
Grant support: ColoWrap LLC funded this study. Drs. Crockett and Dellon designed the study, had full access to all study data, performed data analyses and interpretation independently, and drafted manuscript without assistance from industry sponsor. Dr. Crockett’s effort was also supported in part by grants from the NIH (KL2TR001109) and the American College of Gastroenterology (ACG-JR-000-2012). Dr. Galanko’s and Mr. Martin’s effort are supported in part by a grant from the NIH (P30 DK 034987).
Abbreviations
- CIT
cecal intubation time
- BMI
body mass index
- SD
standard deviation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
|
| ||
| Author | Contributions | Disclosures |
|
| ||
| Crockett | Designed the study, obtained funding, oversaw study performance, performed statistical analyses and interpreted the data, and drafted and revised the manuscript. | Has received research funding from ColoWrap (this study only), Boston Scientific, and Exact Sciences. |
|
| ||
| Cirri | Recruited and enrolled patients, collected data, interfaced with Sponsor, and maintained study database. | None to declare |
|
| ||
| Kelapure | Recruited and enrolled patients, collected data, and maintained study database. | None to declare |
|
| ||
| Galanko | Performed data analysis and interpreted the data, and critically revised the manuscript for publication. | None to declare |
|
| ||
| Martin | Assisted with study design, data analysis and interpretation, and critically revised the manuscript for publication. | None to declare |
|
| ||
| Dellon | Assisted with study design, data analysis and interpretation, and critically revised the manuscript for publication. | Has received research funding from Miraca Life Sciences, Meritage, Receptos, and Regeneron, has received an educational grant from Diagnovus. Has been a consultant for Aptalis, Novartis, Receptos, Regeneron, and Roche. |
|
| ||
References
- 1.Davila RE, Rajan E, Baron TH, et al. ASGE guideline: colorectal cancer screening and surveillance. Gastrointest Endosc. 2006;63:546–57. doi: 10.1016/j.gie.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 2.Dominic OG, McGarrity T, Dignan M, et al. American College of Gastroenterology Guidelines for Colorectal Cancer Screening 2008. Am J Gastroenterol. 2009;104:2626–7. doi: 10.1038/ajg.2009.419. author reply 2628–9. [DOI] [PubMed] [Google Scholar]
- 3.Seeff LC, Richards TB, Shapiro JA, et al. How many endoscopies are performed for colorectal cancer screening? Results from CDC’s survey of endoscopic capacity. Gastroenterology. 2004;127:1670–7. doi: 10.1053/j.gastro.2004.09.051. [DOI] [PubMed] [Google Scholar]
- 4.Peery AF, Dellon ES, Lund J, et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology. 2012;143:1179–87. e1–3. doi: 10.1053/j.gastro.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Turenhout ST, Terhaar sive Droste JS, Meijer GA, et al. Anticipating implementation of colorectal cancer screening in The Netherlands: a nation wide survey on endoscopic supply and demand. BMC Cancer. 2012;12:46. doi: 10.1186/1471-2407-12-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stock C, Haug U, Brenner H. Population-based prevalence estimates of history of colonoscopy or sigmoidoscopy: review and analysis of recent trends. Gastrointest Endosc. 2010;71:366–381. e2. doi: 10.1016/j.gie.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 7.Rex DK. Still photography versus videotaping for documentation of cecal intubation: a prospective study. Gastrointest Endosc. 2000;51:451–9. doi: 10.1016/s0016-5107(00)70447-0. [DOI] [PubMed] [Google Scholar]
- 8.Shah SG, Brooker JC, Thapar C, et al. Patient pain during colonoscopy: an analysis using real-time magnetic endoscope imaging. Endoscopy. 2002;34:435–40. doi: 10.1055/s-2002-31995. [DOI] [PubMed] [Google Scholar]
- 9.Rex DK, Goodwine BW. Method of colonoscopy in 42 consecutive patients presenting after prior incomplete colonoscopy. Am J Gastroenterol. 2002;97:1148–51. doi: 10.1111/j.1572-0241.2002.05681.x. [DOI] [PubMed] [Google Scholar]
- 10.Shah SG, Saunders BP, Brooker JC, et al. Magnetic imaging of colonoscopy: an audit of looping, accuracy and ancillary maneuvers. Gastrointest Endosc. 2000;52:1–8. doi: 10.1067/mge.2000.107296. [DOI] [PubMed] [Google Scholar]
- 11.Cheng WB, Moser MA, Kanagaratnam S, et al. Analysis of and mathematical model insight into loop formation in colonoscopy. Proc Inst Mech Eng H. 2012;226:858–67. doi: 10.1177/0954411912453263. [DOI] [PubMed] [Google Scholar]
- 12.Shankar S, Rowe S. Splenic injury after colonoscopy: case report and review of literature. Ochsner J. 2011;11:276–81. [PMC free article] [PubMed] [Google Scholar]
- 13.Hsieh YH, Tseng KC, Chou AL. Patient self-administered abdominal pressure to reduce loop formation during minimally sedated colonoscopy. Dig Dis Sci. 2010;55:1429–33. doi: 10.1007/s10620-009-0876-3. [DOI] [PubMed] [Google Scholar]
- 14.Hansel SL, Prechel JA, Horn B, et al. Observational study of the frequency of use and perceived usefulness of ancillary manoeuvres to facilitate colonoscopy completion. Dig Liver Dis. 2009;41:812–6. doi: 10.1016/j.dld.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 15.Schulz KF, Altman DG, Moher D, et al. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med. 2010;152:726–32. doi: 10.7326/0003-4819-152-11-201006010-00232. [DOI] [PubMed] [Google Scholar]
- 16.Boutron I, Moher D, Altman DG, et al. Extending the CONSORT statement to randomized trials of nonpharmacologic treatment: explanation and elaboration. Ann Intern Med. 2008;148:295–309. doi: 10.7326/0003-4819-148-4-200802190-00008. [DOI] [PubMed] [Google Scholar]
- 17.National Health Service-United Kingdom Cancer Screening Programme. Quality assurance guidelines for colonoscopy. publication no. 6. Available at: http://www.cancerscreening.nhs.uk/bowel/publications/nhsbcsp06.pdf.
- 18.Waye JD, Yessayan SA, Lewis BS, et al. The technique of abdominal pressure in total colonoscopy. Gastrointest Endosc. 1991;37:147–51. doi: 10.1016/s0016-5107(91)70673-1. [DOI] [PubMed] [Google Scholar]
- 19.Church JM. Ancillary colonoscope insertion techniques. An evaluation. Surg Endosc. 1993;7:191–3. doi: 10.1007/BF00594106. [DOI] [PubMed] [Google Scholar]
- 20.Eickhoff A, Pickhardt PJ, Hartmann D, et al. Colon anatomy based on CT colonography and fluoroscopy: impact on looping, straightening and ancillary manoeuvres in colonoscopy. Dig Liver Dis. 2010;42:291–6. doi: 10.1016/j.dld.2009.04.022. [DOI] [PubMed] [Google Scholar]
- 21.Rex DK, Bond JH, Winawer S, et al. Quality in the technical performance of colonoscopy and the continuous quality improvement process for colonoscopy: recommendations of the U.S. Multi-Society Task Force on Colorectal Cancer. Am J Gastroenterol. 2002;97:1296–308. doi: 10.1111/j.1572-0241.2002.05812.x. [DOI] [PubMed] [Google Scholar]
- 22.Drysdale SA. The incidence of upper extremity injuries in endoscopy nurses working in the United States. Gastroenterol Nurs. 2013;36:329–38. doi: 10.1097/SGA.0b013e3182a6e05d. [DOI] [PubMed] [Google Scholar]
- 23.Toros AB, Ersoz F, Ozcan O. Does a fitted abdominal corset makes colonoscopy more tolerable? Dig Endosc. 2012;24:164–7. doi: 10.1111/j.1443-1661.2011.01207.x. [DOI] [PubMed] [Google Scholar]
- 24.Tsutsumi S, Fukushima H, Kuwano H. Colonoscopy using an abdominal bandage. Hepatogastroenterology. 2007;54:1983–4. [PubMed] [Google Scholar]
- 25.Uddin FS, Iqbal R, Harford WV, et al. Prone positioning of obese patients for colonoscopy results in shortened cecal intubation times: a randomized trial. Dig Dis Sci. 2013;58:782–7. doi: 10.1007/s10620-012-2468-x. [DOI] [PubMed] [Google Scholar]
- 26.Anderson JC, Messina CR, Cohn W, et al. Factors predictive of difficult colonoscopy. Gastrointest Endosc. 2001;54:558–62. doi: 10.1067/mge.2001.118950. [DOI] [PubMed] [Google Scholar]
- 27.Rex DK, Chen SC, Overhiser AJ. Colonoscopy technique in consecutive patients referred for prior incomplete colonoscopy. Clin Gastroenterol Hepatol. 2007;5:879–83. doi: 10.1016/j.cgh.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 28.Waye JD. Completing colonoscopy. Am J Gastroenterol. 2000;95:2681–2. doi: 10.1111/j.1572-0241.2000.03172.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


