Abstract
Interleukin 10 (IL-10) is an important regulatory cytokine that modulates a wide range of immune cells. While it is best known for its ability to suppress immune responses, IL-10 has been found to be pathogenic in several human and animal studies of immune-mediated diseases. There is a considerable gap in our understanding of the molecular mechanisms behind the stimulatory effects of IL-10 during allergic inflammation. IL-10 treatment has been shown to suppress mast cell TNF production. In this study we report that while TNF secretion was reduced, IL-10 surprisingly enhanced IgE-mediated protease and cytokine production both in vitro and in vivo. This stimulatory effect was consistent in mouse and human skin mast cells. IL-10 enhanced activation of the key FcεRI signaling proteins Stat5, JNK, and ERK. We demonstrate that IL-10 effects are dependent on Stat3 activation, eliciting miR-155 expression, with a resulting loss of SOCS-1. The importance of miR-155 was demonstrated by the inability of IL-10 to enhance anaphylaxis in miR-155-deficient mice. Taken together, our results reveal an IL-10-induced, Stat3-miR-155 signaling pathway that can promote mast cell responses.
Keywords: mast cell, miR-155, IL-10, Stat3, SOCS1, immunoglobulin E, allergy, signal transduction, anaphylaxis, Inflammation
Introduction
Interleukin 10 (IL-10) is a pleiotropic immunoregulatory cytokine that is secreted by macrophages, T helper 1 (Th1) and Th2 cells, regulatory T and B cells, cytotoxic T cells and mast cells (1, 2). Traditionally IL-10 is known to inhibit production of pro-inflammatory cytokines such as tumor necrosis factor (TNF), by altering antigen presentation in monocytes and macrophages (2, 3).
The IL-10 receptor (IL-10R) is a member of the class II cytokine receptor family, employing JAK1 and Tyk2 kinases and signaling through several pathways, most notably the latent transcription factor, Stat3 (2, 3). The majority of pre-clinical data from patients and mouse models with defects in IL-10 or IL-10R suggest IL-10 is a major immunosuppressive cytokine, particularly in the context of intestinal mucosal homeostasis (4) (5). Thus these studies suggested IL-10 to be a favorable candidate for cytokine-based immunosuppressive therapies. However, animal studies and clinical administration of IL-10 disclosed undesirable pro-inflammatory properties. Several studies of IL-10 administration in experimental endotoxemia (6) as well as studies of patients with Crohn’s disease (7), systemic lupus erythematosus (SLE) (8), and psoriasis (9) have shown immunostimulatory effects of IL-10 that correlated with disease severity.
Mast cells are master effector cells in allergic responses, present in skin and mucosal tissues. Along with producing IL-10, mast cells are also responsive to IL-10. It is well established that the suppressive effects of IL-10 are largely Stat3-dependent (3). In mast cells, we and others have shown that IL-10 suppresses FcεRI-mediated TNF production in a Stat3-dependent manner (10, 11), an effect noted after prolonged (3–4 day) treatment with IL-10 (10–12). In this study we find that while IL-10 does reduce FcεRI-mediated TNF secretion, production of several inflammatory cytokines and chemokines are enhanced in a Stat3-dependent manner. Additional experiments suggest that Stat3 exerts its activity through the induction of miR-155, which suppresses SOCS1. These stimulatory effects were consistent among mouse and human mast cells in vitro and in an in vivo mouse assay of systemic anaphylaxis. Taken together, these data show that IL-10 can promote IgE-mediated mast cell activation.
Materials and Methods
Reagents
Recombinant mouse IL-3, SCF and IL-10 were purchased from Biolegend (San Diego, CA). Purified mouse IgE (clone C38-2, κ isotype) was purchased from BD Biosciences (Pharmingen division, San Diego, CA). Dinitrophenyl-coupled human serum albumin (DNP-HSA) and LPS (Cat# L-6529) were purchased from Sigma-Aldrich (St. Louis, MO). Histamine was purchased from Enzo Life Sciences (Farmingdale, NY). All western blotting antibodies were purchased from Cell Signaling Technology (Danvers, MA). Dr. Daniel Conrad (VCU) generously provided purified mouse anti-DNP IgE for in vivo experiments. Antibodies for c-Kit (PE anti-mouse CD117) and FcεRI (APC anti-mouse FcεRIα) were purchased from Biolegend (San Diego, CA) and used at a concentration 1:100. LPS levels were tested using Toxin Sensor™ Chromogenic LAL Endotoxin Assay Kit from GenScript (Piscataway, NJ). IL-10 resuspended in PBS and PBS alone used in in vivo studies had LPS content of <0.1 EU/mol. Media and IL-10 resuspended in media for in vitro studies had LPS content of ~1.0 EU/mol. There was no significant difference between IL-10 and the respective vehicle control used when comparing LPS levels using unpaired Student’s t-test.
Animals
C57BL/6J and C57BL/6J-background miR-155−/− (B6.Cg-Mir155 tm1.1 Risky/J) mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and used at a minimum of 8 weeks old, with approval from the Virginia Commonwealth University institutional Animal Care and Use Committee.
Mouse mast cell cultures
Mouse bone marrow-derived mast cells (BMMC) were cultured as described (13). Briefly, BMMC were derived by harvesting bone marrow from femurs of mice and culturing the bone marrow extract in complete RPMI (crimp) 1640 medium (Nitrogen Life Technologies, Carlsbad, CA) containing 10% FBS, 2 mom L-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, 1 mom sodium private, and 10 mom HEPES (Befouled, Rockville, MD). crimp was supplemented with IL-3 containing supernatant from WEHI-3 cells and SCF-containing supernatant from BHK-MKL cells for 21 d. The final concentration of IL-3 and SCF was adjusted to 1.5 and 15 nag/ml, respectively, as measured by ELISA. Mast cell purity was determined to be 99%. Peritoneal cells were extracted in PBS, then expanded in culture for 12 days as described for BMMCs. Peritoneal mast cell purity was 83% as determined via flow cytometry for c-Kit- and FcεRI-double positive cells.
Human mast cell culture
Protocols involving human tissues were approved by the human studies Internal Review Board at the University of South Carolina. Surgical skin samples were obtained from the Cooperative Human Tissue Network of the National Cancer Institute. Skin MCs were cultured as described previously (14) and were used after 8–16 weeks. The purity of mast cells was 100% as determined by olivine blue staining.
IgE-mediated activation and cytokine, chamomile, protease measurement by ELISA
Human MC, BMMC, and peritoneal derived MC were sensitized overnight with DNP-specific mouse IgE (1.0 &ML/ml for human MC; 0.5 &ML/ml for BMMC and peritoneal mast cells). Cells were then washed and resuspended at 1×106 cells/ml in complete media with cytokines. Cells were stimulated for 16 hours with DNP-HSA (30ng/ml for human MC and 50ng/ml for mouse BMMC) for assessment of cytokines in supernatant. Marine IL-13 ELISA kit was purchased from bioscience (San Diego, CA). IL-6, MCP-1 (CCL2) and TNF ELISA kits were purchased from Bilged (San Diego, CA). MIP-1α (CCL-3) ELISA kit was purchased from Prepotency (Rocky Hill, NJ). Human MCP-1 and TNF Elisa’s were purchased from R&D Systems (Minneapolis, MN). Elisa’s were performed using culture supernatants according to the manufacturer’s protocol, and developed using BD Optima reagents from BD Biosciences (San Diego, CA). Cyst levels were measured using Certainly Leukotriene ELISA kit from Cayman Chemicals (Ann Arbor, Michigan) which had the following cross reactivity: LTC4 100%, LTD4 100%, LTE4 79%, 5,6-DiHETE 3.7%, LTB4 1.3%, 5(S)-HETE 0.04%, Arachidonic Acid <0.01%. For in vitro deregulation assay, 1×106 cells were treated and activated as described above. Cells were polluted and lyses in 1 mol of PBS + 1% Ideal ™ CA-630 purchased from USB Corporation (Cleveland, OH). Supernatant and lists were analyzed with mMCPT-1 ELISA purchased bioscience (San Diego, CA). Percent mMCPT-1 released was calculated by dividing the amount of mMCPT-1 in the culture supernatant by the sum of mMCPT-1 detected in the supernatant and cell pellet.
Cytokine mina and miR-155 RT-per
BMMC were cultured with or without 50ng/ml of IL-10 for the indicated time points. Cells were harvested and total RNA was extracted with Trio reagent (Life Technologies, Grand Island, NY). RNA was quantified using the Thermo Scientific NanoDrop™ 1000 UV–vies Spectrophotometer (Thermo Scientific, Waltham, MA) according to the manufacturer’s recommended protocol. For cytokine mina detection, cDNA was synthesized using the qScript™ cDNA Synthesis from Quanta Biosciences (Gaithersburg, MD). For miR-155 detection, cDNA was synthesized using qScript™ microRNA Quantification System using mouse primers for miR-155-5p, miR-155-3p, and SNORD47 from Quanta Biosciences (Gaithersburg, MD) according to the manufacturer’s instructions. BioRad CFX96 Touch™ Real-Time PCR Detection System (Hercules, CA) was used to amplify message using PerfeCTa SYBR Green SuperMix (Quantabio, Gaithersburg, MD). Primers for IL-6 (forward: 5′TCCAGTTGCCTTCTTGGGAC3′, reverse: TCCAGTTGCCTTCTTGGGAC3′), TNF (forward: 5′AGCACAGAAAGCATCATCCGC3′, Reverse: 5′TGCCACAAGCAGGAATGAGAAG3′), β-actin (forward: 5′GATGACGATATCGCTGCGC3′, Reverse: 5′CTCGTCACCCACATAGGAGTC3′), GAPDH (forward: 5′GATGACATCAAGAAGGTGGTG3′, Reverse: 5′GCTGTAGCCAAATTCGTTGTC3′), SOCS1 (forward 5′CAGGTGGCAGCCGACAATGCGATC3′, Reverse: 5′CGTAGTGCTCCAGCAGCTCGAAAA3′), and SHIP-1 (forward: 5′GGTGGTACGGTTTGGAGAGA3′, Reverse: 5′ATGCTGAGCCTCTGTGGTCT3′) were purchased from Eurofins MWG Operon (Huntsville, AL). Primers for mmu-miR-155-5p, 5′-UUAAUGCUAAUUGUGAUAGGGGU-3′, mmu-miR-155-3p, 5′-CUCCUACCUGUUAGCAUUAAC-3′, and SNORD47, 5′-GUGAUGAUUCUGCCAAAUGAUACAAAGUGAUAUCACCUUUAAACCGUUCAUUUUAUUUCUGAGG-3′ were purchased from Quanta Biosciences (Gaithersburg, MD). Amplification conditions for microRNA detection were set to heat-activation at 95°C for 2 minutes followed by 40 cycles of denaturation at 95°C for 5 seconds and annealing at 60°C for 30 seconds. Amplification conditions for all other reactions consisted of a heat-activation step at 95°C for 2 minutes followed by 40 cycles of 95°C for 15 seconds, 55°C for 30 seconds and 60°C for 1 minute. All melting curve analysis was performed between 50°C and 95°C. Results were normalized to housekeeping genes using relative Livak Method.
Passive Systemic Anaphylaxis (PSA)
Age-matched groups of mice (8–16 weeks old) received 3 intra-peritoneal (IP) injections of 4μg recombinant IL-10 in 200μl of PBS 24 hours prior to receiving DNP-HSA. Anti-DNP IgE (50 μg) was IP injected 11.5 hours before IP injection of DNP-HSA (100 mg). For histamine-induced PSA, mice were given IL-10 injections as stated above and 8mg of histamine was injected IP 24 hours after the first IL-10 injection. The core body temperature of each mouse was measured using a rectal microprobe (Physitemp Instruments). Mice were euthanized with CO2 asphyxiation, and blood was collected via cardiac puncture 120 or 180 minutes after DNP-HSA injection. ELISA was used to analyze plasma cytokine levels.
Western blot analysis
Western blotting was performed using 40 mg total cellular protein as described previously (13) using the following antibodies from Biolegend (San Diego, CA): Phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204) #9101, p44/42 MAPK (Erk1/2) #9102, Phospho-SAPK/JNK (Thr183/Tyr185) (G9) Mouse mAb #9255, SAPK/JNK Antibody #9252, Phospho-Stat5 (Tyr694) (C71E5) Rabbit mAb #9314, Stat5 Antibody #9363, Fyn Antibody #4023, Lyn Antibody #2732, Syk Antibody #2712, GAPDH (14C10) Rabbit mAb #2118, Phospho-Stat3 (Tyr705) Antibody #9131 and Stat3 Antibody #9132. All primary antibodies were used at a 1:1000 dilution in Blocker™ Casein (Thermofisher Scientific, Rockford, IL) in TBS with 0.1% TWEEN® 20 Sigma-Aldrich) and incubated at 4°C overnight (72 hours for pStat5 and Stat5) with gentle rocking. All secondary HRP-linked anti-IgG (goat anti-rabbit DyLight800 or goat anti-mouse DyLight680) from Cell Signaling (Danvers, MA) were used at a 1:5,000 dilution and in Blocker™ Casein in TBS with 0.1% TWEEN® 20 and incubated at room temperature for 1 hour with gentle rocking. Blots were visualized and quantified using a LiCor Odyssey CLx Infrared imaging system (Lincoln, NE). After background subtraction, fluorescence intensity for the protein of interest was normalized to the signal intensity for the relevant loading control, using Image Studio 4.0 (Li-Cor, Lincoln, Nebraska).
Cell transfection for siRNA knockdown and miR-mimic
BMMC were transfected with 100 nM STAT3-, or SOCS1-specific or scrambled FlexiTube siRNAs (a pool of 4 targeting sequences) from Qiagen (Valencia, CA). miR-155-5p and miR-155-3p mimics were purchased from Exiqon (Woburn, MA) and used at 50nM concentration. All transfection experiments were done using Amaxa Nucleofector from Lonza (Allendale, NJ) with program T-5 in Dulbecco’s modified Eagle’s medium with 20% FBS and 50 mom Hepes (pH 7.5) (10). Cells were used 48 hours after being transfected.
Flow cytometric analysis
For surface c-Kit and FcεRI expression, cells were treated with IL-10 (50ng/ml) for 24 hours and with washed in PBS. Cell pellets were incubated in 10μl rat anti-mouse FcgRII/III clone 2.4G2 (10mg/ml) + staining or isotype control antibodies for 20 minutes at 4°C, washed with PBS and resuspended in FACS buffer (PBS, 3% FBS, 0.1% Sodium azide), and analyzed by flow cytometry using a BD FACSCaliber.
Statistical analysis
Data presented are the mean ± SEM of at least 3 independent experiments (unless otherwise stated). Paired or unpaired Student’s t-test, one-way analysis of variance with Tukey post hoc tests or area under curve (AUC) were used when appropriate, using GraphPad Prism software. Statistical significance was set at p< .05. In all figures, *p<.05; **p<.01; ***p<.001; ****p<.0001.
Results
IL-10 enhances FcεRI-mediated cytokine production in BMMC
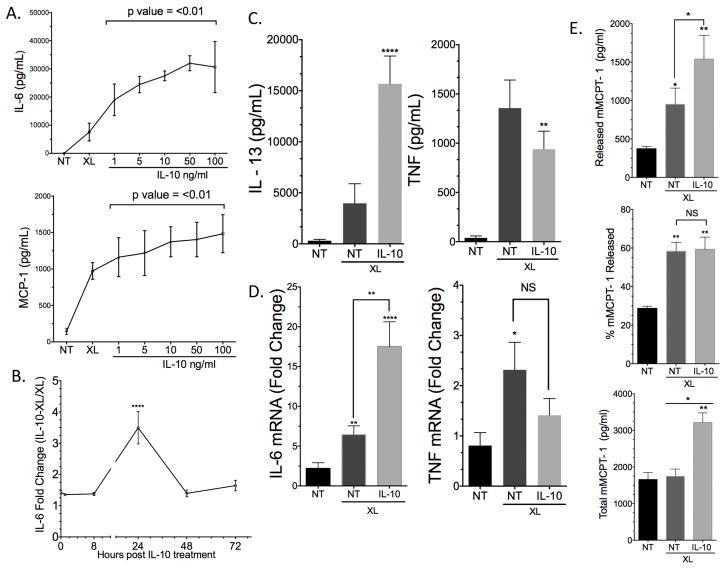
We previously reported IL-10 suppressed FcεRI-mediated TNF production in BMMC in vitro and ameliorated PSA (11). In a follow-up study examining a broader array of mast cell mediators, we were surprised to find that IL-10 enhanced IgE-mediated IL-6 and MCP-1 production, with the greatest effects using 50ng/ml IL-10 24 hours prior to antigen exposure (Figure 1A–B). IL-13 production was also enhanced, while TNF secretion was suppressed, as we previously reported (11) (Figure 1C). RT-per data showed that IL-6 but not TNF mina was upregulated by IL-10 treatment (Figure 1D). IL-10 treatment for 24 hours prior to activation also increased mast cell deregulation as indicated by increased mMCPT-1 release (Figure 1E). Interestingly, the percent mMCPT-1 release was unchanged by IL-10 addition, which was explained by an increase in total mMCPT-1 content induced by IL-10 (Figure 1E).
Figure 1.
24 hour IL-10 treatment enhances FcεRI-mediated cytokine production and deregulation in mouse BMMC. (A) BMMC were incubated with IgE and the indicated IL-10 concentrations for 24 hours prior to antigen-induced IgE crosslinking (XL) for 16 hours. Cytokines were measured using ELISA. (B) BMMC were treated as in (A) using 50ng/ml IL-10 for the indicated times. (C) BMMC were treated as in (A) with 50ng/ml IL-10 prior to IgE XL for 16 hours to measure IL-13 and TNF release. (D) BMMC were treated with IL-10 for 4hrs and mina levels were measured by RT-per relative to β-actin. (E) BMMC were treated with 50 ML/ml IL-10 or 24 hours prior to IgE XL for 15 minutes to measure mMCPT-1 levels by ELISA. Cytokines were measured by ELISA. Data are means ± SEM of four (A,C) and three (B, D, E) independent experiments done in triplicate. Post hoc comparisons using Tukey HSD test indicated that the mean score for 24-hour treatment was significantly different than all other treatment times.
It has been reported that mast cells expanded in IL-3 alone have a mucosal mast cell phenotype while cells grown in IL-3+SCF resemble a connective tissue phenotype (15, 16). IL-10 has also been shown to enhance the growth factor activity of SCF in rat MC (17). To test if our results were due to enhanced SCF signaling, we generated BMMC in WEHI/IL-3 for 21 days with no SCF. These cells were then treated with IL-10 for 24 hours. The stimulatory effects of IL-10 were preserved and comparable to SCF-treated cells (Figure S1A). Further, we noted that 24-hour treatment with IL-10 enhanced IL-6 production at 5 or 16 hours after antigen-induced IgE crosslinking, while TNF suppression was only apparent 16 hours after antigen addition (Figure S1B). We also tested BMMC from various mouse strains (129/SvImJ, CBA) bearing known IL-10R polymorphisms, and found that the stimulatory effects of IL-10 were consistent with our C57BL/6J results (data not shown). Together these data indicate that 24 hours of IL-10 treatment has pro-inflammatory effects on IgE responses in mouse mast cells, effects that do not require SCF.
IL-10 enhances FcεRI-mediated cytokine production in peritoneal-derived mouse mast cells and human skin mast cells
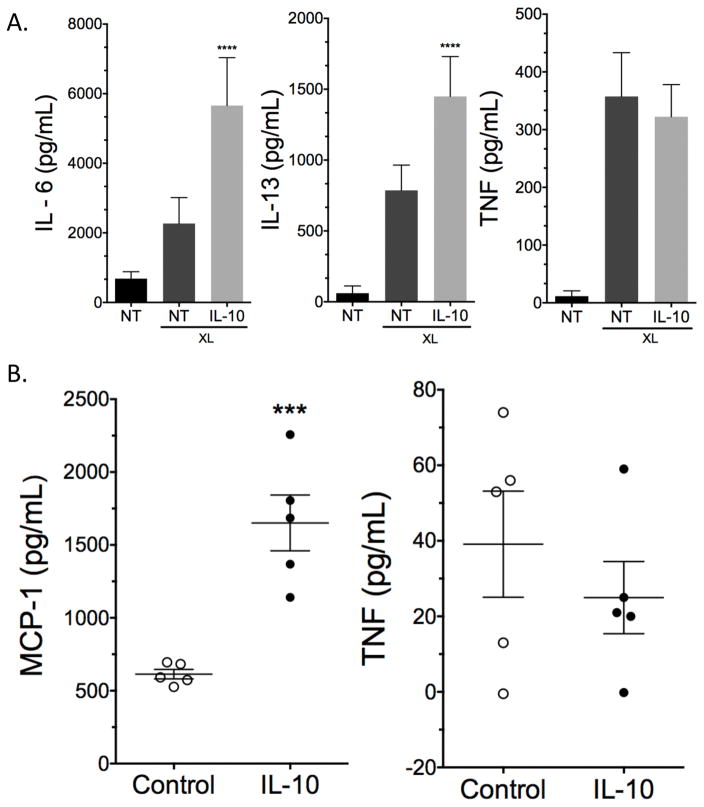
We next expanded peritoneal-derived mast cells that matured in vivo and treated them with IL-10 for 24 hours prior to IgE-induced activation. Peritoneal-derived mast cells also showed enhanced inflammatory cytokines with IL-10 treatment (Figure 2A), suggesting these effects were not due to in vitro mast cell differentiation. Finally, human skin mast cells from 5 different donors treated with IL-10 24 hours prior to Ag crosslinking showed significantly enhanced MCP-1 secretion, while TNF production was unchanged (Figure 2B). These data suggest that the pro-inflammatory effects of IL-10 are not limited to BMMC and can be observed in primary mast cells from mouse or human sources.
Figure 2.
24-hour IL-10 treatment enhances FcεRI-mediated cytokine production in peritoneal derived mouse or human skin mast cells. (A) Mouse peritoneal derived mast cells and (B) human skin mast cells were incubated with IgE and 50ng/ml of IL-10 for 24 hours prior to antigen-induced IgE crosslinking (XL) for 16 hours. Data are means ± SEM of (A) three independent experiments done in triplicates or (B) cells from 5 donors tested 6 times. Cytokines were measured using ELISA.
Administration of IL-10 for 24 hours before Ag exacerbates IgE-induced anaphylaxis
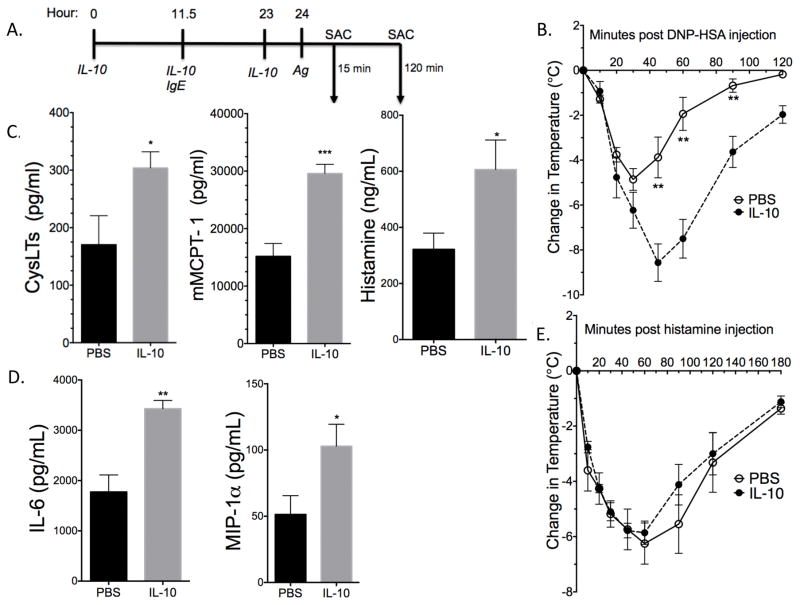
To test the functional relevance of IL-10 treatment under our conditions, we administered three IL-10 injections (4μg each) prior to inducing IgE-mediated PSA, as described in Figure 3A. Core body temperature drop in IL-10-treated mice was almost double compared to controls (Figure 3B). The drop in body temperature was significant using both ANOVA and AUC analysis with p value ≤ 0.05. IL-10-treated mice also showed significantly enhanced plasma mMCPT-1, Cyst and histamine levels after 15 minutes of antigen exposure (Figure 3C) and elevated plasma IL-6 and MIP-1α after 120 minutes (Figure 3D). The more severe drop in body temperature could reflect either greater mast cell activation in the presence of IL-10, or a stronger vascular response to mast cell mediators, particularly histamine. To assess the effects of IL-10 on the vasculature, we administered three IL-10 injections 24 hours prior to injecting 8mg of histamine. No difference was seen between IL-10-treated and control mice (Figure 3E), indicating no change in vascular responsiveness to histamine. Under the same conditions, IL-10 administration did not alter plasma IgE levels (data not shown). These data indicate that the pro-inflammatory effects of IL-10 are functionally significant in vivo, and that this is likely due to exacerbated mast cell activation.
Figure 3.
IL-10 treatment exacerbates PSA. (A) Schematic of PSA assay. SAC = sacrifice, Ag = Antigen. C57BL/6 mice received IL-10 injections and were subjected to PSA as described in (A) and Materials and Methods. (B) Depicts change in core body temperature. (C) Plasma CysLTs, mMCPT-1, and Histamine levels from mice sacrificed 15 minutes post Ag administration. (D) Plasma cytokine and chamomile levels 120 minutes post Ag administration. Levels in (C) and (D) were determined by ELISA. (E) Mice were injected with IL-10 as described in (A). Histamine was injected at hour 24 in place of antigen and mice were sacrificed 180 minutes post histamine injection. (B, C) n = 5, representative of three independent experiments. (D, E) n = 5. Data are means ± SEM. In (B) and (E) "*" show significant value determined via ANOVA. AUC was also used to determine significance between IL-10 and PBS treatment in (B) (p value ≤ 0.05) and in (E) (p value ≥ 0.05).
IL-10 enhances FcεRI signaling
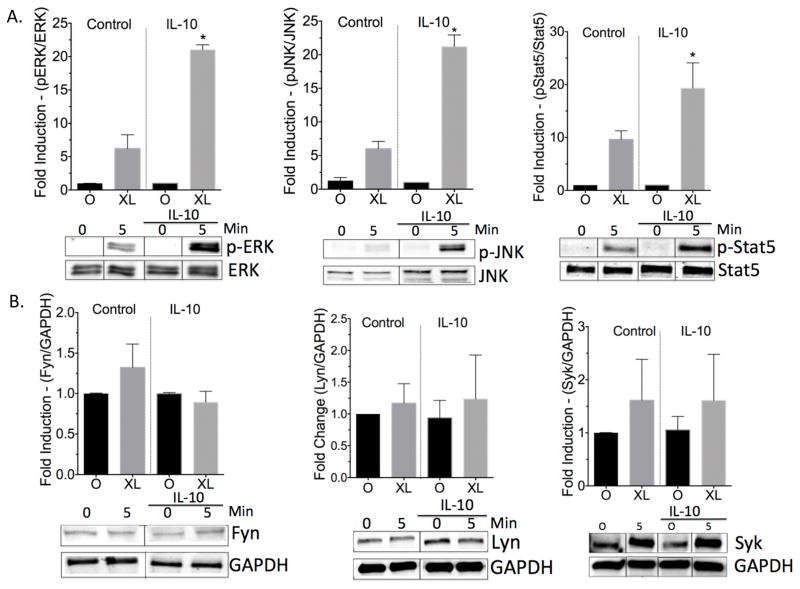
We previously reported that 3-day culture with IL-10 suppressed FcεRI and c-Kit expression (11, 12), suggesting that perhaps 24-hour IL-10 exposure might have an opposite effect. However, IL-10 treatment for 24 hours did not alter c-Kit or FcεRI surface expression, as assessed by flow cytometry (Figures S2). This indicated that the effects of IL-10 are likely to be due to changes in FcεRI signal transduction. In fact, IL-10 selectively enhanced activation of key signaling proteins downstream of the IgE receptor. Western blot data showed that IL-10 significantly increased IgE-mediated phosphorylation of JNK, ERK, and Stat5 (Figure 4A). Although IL-10 has been reported to suppress total Fyn levels in mast cells (10), we observed no significant changes in total Fyn, Lyn and Syk levels with IL-10 treatment for 24 hours (Figure 4B).
Figure 4.
24-hour IL-10 treatment enhances FcεRI signaling. BMMC were pre-sensitized with IgE and treated +/− 50ng/ml of IL-10 for 24 hours, then left unactivated (0) or activated by IgE XL for 5 minutes. Lists were collected and subjected to western blotting using parameters described in Materials and Methods. Data are means ± SEM of three independent experiments done in triplicate. Representative blots are shown for each protein; bands show quantified fluorescence emission and were cropped to show one time point.
Stimulatory effects of IL-10 require Stat3
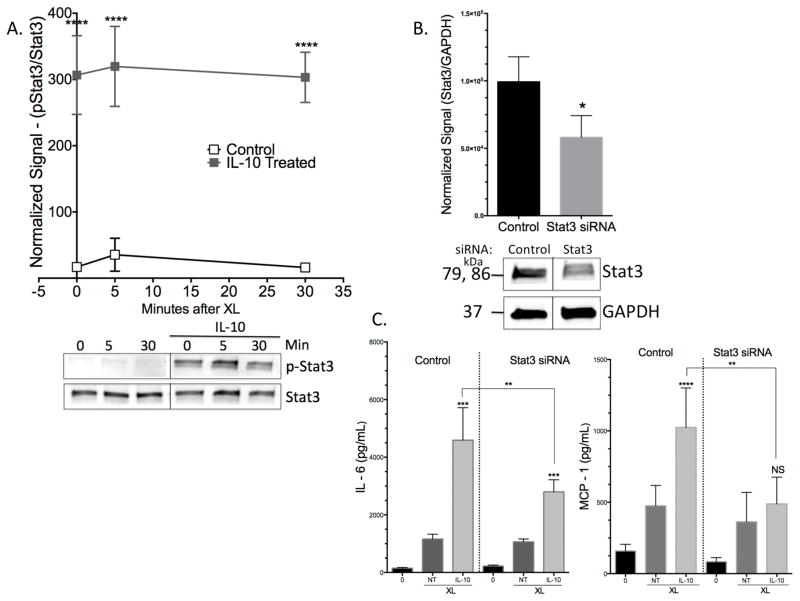
To examine the mechanism by which IL-10 enhances IgE effects, we first investigated Stat3 phosphorylation. As the main transcription factor associated with IL-10 signaling, Stat3 is central to IL-10 effects (18). Twenty four-hour IL-10 treatment enhanced Stat3 Tyr705 phosphorylation, as expected (Figure 5A). We suppressed Stat3 expression via siRNA transfection (Figure 5B), and found that the ability of IL-10 to enhance FcεRI-mediated IL-6 or MCP-1 was significantly reduced (Figure 5C). Thus Stat3 is critical for IL-10 to increase IgE-mediated cytokine production.
Figure 5.
IL-10-mediated effects are Stat3-dependent. (A) BMMC were pre-sensitized with IgE and treated +/− 50ng/ml IL-10 for 24 hours prior to antigen-induced IgE crosslinking (XL) for indicated times (bands were cropped to show indicated time points). Lists were collected and subjected to western blotting. Graph shows normalized data from n= 3 samples. (B) BMMC were transfected with control or Stat3-targeting siRNA and lists were collected 48 hours later for western blot analysis. Representative blot is shown for Stat3 suppression (bands were cropped to show 1 population). (C) Transfected cells were treated as described in (A), with IgE XL for 16 hours. Supernatants were collected for ELISA analysis. Data are means ± SD of three (A) and two (B) independent experiments done in triplicate.
IL-10 induces mir-155
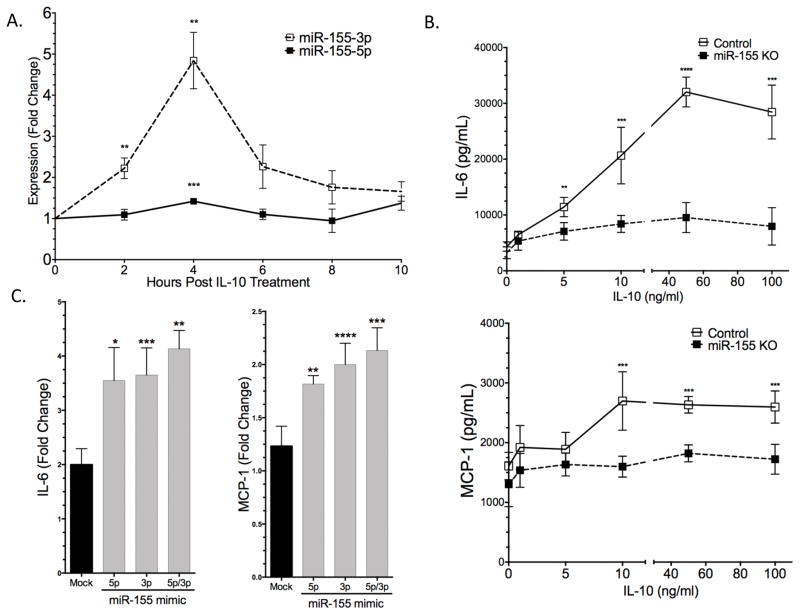
miR-155 is known to be a major contributor to inflammatory diseases and has recently been shown to control mast cell activation by FcεRI (19, 20). To determine the expression profile of miR-155 in IL-10-treated BMMC, we treated cells with IL-10 and measured 155-5p and 155-3p induction via per. IL-10 enhanced both miR-155-5p and miR-155-3p, with peak effects after 4 hours of treatment (Figure 6A). It is noteworthy that miR-155-3p enhancement was several-fold higher than miR-155-5p (Figure 6A). There is previous evidence that in bone marrow-derived macrophages, IL-10 suppresses LPS-induced miR-155 expression (21). To test the importance of lineage and stimulus, BMMC were treated with 1mg/ml LPS +/− 50ng/ml IL-10. Under these conditions, IL-10 enhanced miR-155-5p and did not change miR-155-3p induction by LPS (Figure S3A), suggesting that cell lineage alters IL-10 effects on miR-155. To determine the functional significance of miR-155, we treated control and miR-155 KO BMMC with IL-10 for 24 hours prior to FcεRI activation. The stimulatory effects of IL-10 seen in control cells were significantly reduced in miR-155 KO cells, as measured by MCP-1 and IL-6 production (Figure 6B).
Figure 6.
IL-10 induces miR-155 expression. (A) BMMC were treated with 50ng/ml IL-10 for indicated times. per was used to measure miR-155-5p and miR-155-3p expression relative to Snord47. Fold change relative to untreated cells is shown. (B) C57BL/6 and miR-155KO BMMC were pre-sensitized with anti-DNP IgE and treated +/− IL-10 at the indicated concentrations for 24 hours prior to IgE XL for 16 hours. Cytokines were measured via ELISA. (C) miR-155KO BMMC were mock transfected or received 50nM miR-155-5p, miR-155-3p or miR-155-5p/3p mimic 48 hours prior to treatment +/- 50ng/ml of IL-10. Cells were activated as described in (B) and cytokines were measured via ELISA. Fold change is relative to cells not receiving IL-10. Data are means ± SEM of five (A), three (B) or two (C) independent experiments done in triplicate.
Because miR-155-5p and -3p have complementary sequences, they likely have distinct target sets. To determine the importance of each strand, miR-155 KO BMMC were transfected with control ("mock"), miR-155-5p and/or miR-155-3p mimics. Transfection efficiency was measured via per (Figure S4). We compared IgE-induced cytokine production in the presence or absence of IL-10. The IL-10-mediated fold enhancement of IL-6 and MCP-1 secretion, shown in Figure 6C, showed that transfecting either miR-155-5p or -3p increased the IL-10 response. These results indicate that miR-155 plays a critical role in the ability of IL-10 to enhance FcεRI signaling, with partially redundant effects of each miR strand.
IL-10 enhances miR-155 in a Stat3-dependent manner
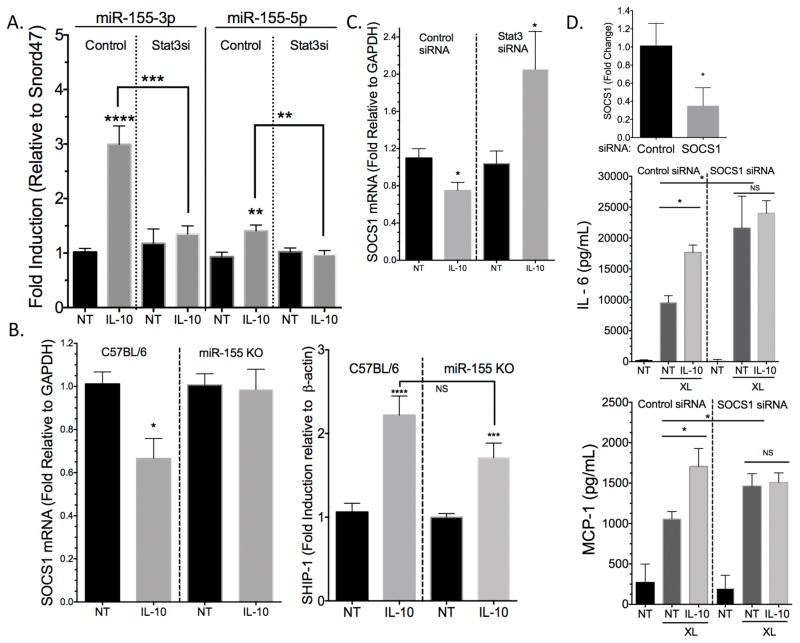
There is evidence that Stat3 directly binds the miR-155 promoter, inducing its expression in Th17 cells (22). To determine if Stat3 is required for IL-10-induced miR-155 expression in mast cells, Stat3 was suppressed by siRNA, and miR-155 expression was measured 4 hours after IL-10 treatment. Stat3 suppression diminished IL-10’s ability to induce miR-155-5p and -3p (Figure 7A). As a measure of Stat3-miR-155 functional relevance, we next examined IL-10-mediated changes in expression of the negative regulators SOCS1 and SHIP-1, known miR-155 targets (23–25). IL-10 significantly suppressed SOCS1 mina, an effect that was absent in miR-155 KO cells (Figure 7B). To our surprise, IL-10 enhanced SHIP-1 mina levels, and this was conserved in miR-155 KO cells (Figure 7B). Furthermore, IL-10-mediated SOCS1 suppression was reversed when Stat3 was knocked down (Figure 7C). To confirm that the enhancement of cytokines with IL-10 treatment was due SOCS1 suppression, we transfected C57BL/6 derived BMMC with SOCS1 siRNA. We saw that in cells with low SOCS1 levels, baseline FcεRI-mediated cytokine production was enhanced as expected, but IL-10 treatment gave no further increase (Figure 7D). These data support the hypothesis that IL-10 uses Stat3 to induce miR-155, which suppresses SOCS1, resulting in enhanced cytokine production.
Figure 7.
IL-10 induction of miR-155 is Stat3-dependent. (A) C57BL/6 BMMC were transfected with control or Stat3 siRNA and treated +/− 50ng/ml IL-10 for 4 hours before mina was collected. per was used to measure miR-155-5p and miR-155-3p levels relative to Snord47. (B) miR-155 KO and Control BMMC were treated with 50ng/ml IL-10 for 4 hours. SOCS1 and SHIP-1 mina were measured via RT-per. (C) C57BL/6J BMMC were transfected with control or Stat3 siRNA as described in (A) and SOCS1 mina levels were measured using RT-per. (D) C57BL/6J BMMC were transfected with control or SOCS1 siRNA, and SOCS1 mina suppression was measured via RT-per. Cells were treated +/− 50ng/ml IL-10 for 24 hours prior to IgE XL for 16 hours. Cytokines were measured by ELISA. Data are means ± SEM of three (A–B) or two (C–D) independent experiments done in triplicate. NT = not treated (no IL-10).
miR-155 KO mice are resistant to IL-10-mediated enhancement of anaphylaxis
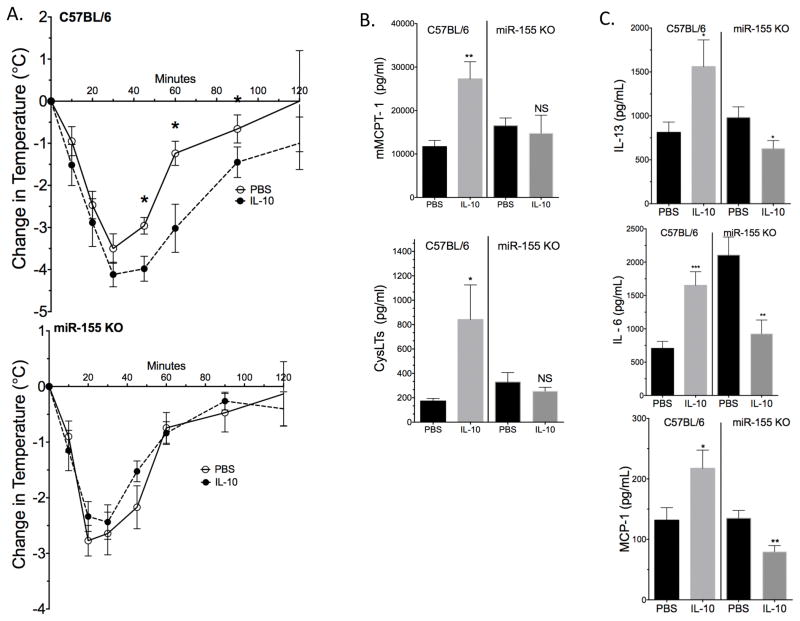
To test the functional relevance of IL-10-induced miR-155 expression in vivo, we repeated our PSA model using miR-155 KO mice. Control C57BL/6 and miR-155 KO mice were given three IL-10 injections over the course of 24 hours before inducing IgE-mediated PSA, as described in Figure 3A. Similar to the results in Figure 3, C57BL/6 mice treated with IL-10 experienced a more severe drop in core body temperature than PBS-treated mice. In contrast, there was no difference between PBS- and IL-10-injected miR-155 KO mice (Figure 8A). Drop in body temperature was analyzed using both ANOVA and AUC analysis. Consistent with temperature change, plasma mMCPT-1 and CysLTs were significantly elevated in IL-10-treated C57BL/6, but not miR-155 KO mice (Figure 8B). Similarly, plasma IL-13, MCP-1, and IL-6 were significantly elevated by IL-10 treatment only among C57BL/6 mice (Figure 8C). Baseline IL-13 and MCP-1 levels between C57BL/6 and miR-155 KO mice were comparable (Figure S3B). Baseline IL-6 levels were undetectable (assay limit of detection was approximately 60pg/ml). These results indicate that miR-155 is required for the stimulatory effects of IL-10, and that loss of miR-155 can even result in IL-10-mediated inhibitory effects.
Figure 8.
IL-10 treatment does not affect PSA in miR-155 KO mice. (A) C57BL/6J and miR-155KO mice were subjected to IL-10 or PBS IP injections and PSA as described in Figure 3A/B. (B) Plasma mMCPT-1/CysLTs (15 minutes post Ag injection) and cytokines (120 minutes post Ag injection) were measured by ELISA. Data are means ± SEM of eight mice for temperature change and cytokine measurements and five mice for MCPT-1 and Cyst. In (A) "*" shows significant value determined via ANOVA. AUC was also used to determine significance between IL-10 and PBS treatment in C57BL/6 mice (p value ≤ 0.05) and miR-155 KO mice (p value ≥ 0.05).
Discussion
IL-10 was originally termed cytokine synthesis inhibitory factor (CSIF) in the context of Th1/Th2 cross-regulation (1). However, support for IL-10-mediated anti-inflammatory effects is not absolute. This study shows for the first time that IL-10 can enhance mast cell activation and exacerbate anaphylaxis, through a Stat3- and miR-155-dependent process. Previous studies of IL-10 effects on mast cells have focused mostly on TNF production. We previously showed that 4 day treatment with IL-10 suppresses TNF production in BMMC (11). There are also data from rat peritoneal mast cells that show TNF mina and protein suppression with 18 hours and 24 hours of IL-10 treatment, respectively (17, 26). Interestingly, the same study also showed increased histamine production with 24 hour IL-10 (50ng/ml) treatment (17). Under our current conditions, IL-10 did diminish TNF release, confirming that this inhibitory response is consistent. In contrast, IL-10 significantly enhanced IgE-induced production of key inflammatory cytokines and chemokines, as well as deregulation. These data call into question the role of IL-10 in mast cell biology, and how IL-10 may participate in allergic and inflammatory diseases involving mast cell activation.
Similar to our earlier work addressing IL-10-mediated anti-inflammatory effects (11), we found that Stat3 expression is required for IL-10’s ability to enhance mast cell activation. The mechanisms by which Stat3 contributes to both positive and negative IL-10 effects are enigmatic, but clues are apparent in the literature. A recent study employing dendritic cells (DC) addressed Stat3 effects during IL-6 and IL-10 treatment, which cause pro-inflammatory and anti-inflammatory effects, respectively (18, 27). Braun et al. found that the duration of Stat3 activation determines the cytokine response. They found that at the early phase of Stat3 tyrosine phosphorylation, both IL-6 and IL-10 treatment in DC led to the same genome-wide pro-inflammatory transcriptional responses. However, at later time points, the transcriptional responses were the opposite, with IL-10-activated Stat3 upregulating anti-inflammatory genes (27). This is an attractive theory to explain the current data, as we have previously found that the suppressive effects of IL-10 on mast cells require 3–4 days to manifest (11). One possibility is that the duration of Stat3 activation alters its pairing with other transcriptional regulators, allowing for differential gene expression.
MicroRNAs are small non-coding RNAs that are involved in many developmental and pathological processes. miR-155 expression is present in both myeloid and lymphoid cells at varying levels of expression. miR-155 has been shown to play a critical role in a variety of cancers, viral infections and other inflammatory diseases (2). Like most miRs, miR-155 can bind the 3′UTR of its target with perfect complementarity to degrade mina. It can also cause a modest change in mina levels by binding seed regions with mismatches. Most studies of miR-155 have assessed miR-155-5p, although there is evidence that miR-155-3p is functionally active (28). According to in silico target site predictors (DianaMicroT and miRBase), the 3p and 5p forms of miR-155 have the potential to regulate different sets of genes (20). miR-155 has several confirmed targets that lead to both pro- and anti-inflammatory effects. Of note are targets that are negative regulators, yielding stimulatory phenotypes when suppressed. Examples of these include SOCS1 and SHIP-1 (29, 30). There is also evidence that miR-155 can be anti-inflammatory. One study of mast cells revealed that miR-155 targets PI3Kγ (19), suppressing mast cell activation. Paradoxically, miR-155 can be both pro and anti-inflammatory in the same cell system. For example, it is well known that in LPS-activated macrophages, Toll Like Receptor (TLR)-induced miR-155 inhibits TLR signaling by targeting IL-1R-associated kinase (IRAK)1 and TNF receptor-associated factor (TRAF) in a negative feedback loop to suppress macrophage activation (31). However, miR-155 overexpression was shown to enhance LPS-induced TNF production both in vitro and in vivo (32).
Our data show that Stat3-dependent miR-155 induction is required for IL-10 to enhance mast cell activation and exacerbate PSA. Recent data in miR-155 KO BMMC confirm that miR-155 ablation does not alter mast cell numbers or FcεRIα and c-Kit expression (19). Other than the previously published PI3Kγ (19), no other target of miR-155 has been identified in mast cells. We found that IL-10 can suppress SOCS1 mina, a confirmed miR-155 target (23–25), in a Stat3-dependent manner, and that SOCS1 suppression is lost in miR-155 KO mast cells. Because SOCS1 is an important negative regulator of mast cell signaling (33), reducing SOCS1 levels is a logical contributor to the inflammatory effects of IL-10. Our data confirmed this hypothesis, since IL-10 was unable to enhance IgE-mediated cytokine production after SOCS-1 depletion.
To our surprise, IL-10 treatment enhanced SHIP-1 mina, an effect that persisted in miR-155 KO BMMC. This result might be explained by recent studies of IL-10 signaling in macrophages showing that IL-10 can induce SHIP-1 in a Stat3-independent pathway. The same authors also show that IL-10 uses SHIP-1 to suppress TNF translation without affecting its mina level (34). This could explain why we did not observe suppression of TNF mina when TNF protein is reduced. It is plausible that Stat3-independent anti-inflammatory effects, such as the use of SHIP-1, are not affected at the 24 hour IL-10 treatment time.
IL-10 is upregulated in many human inflammatory diseases and in animal models such as endotoxemia (6). High serum IL-10 has been suggested as an indicator of poor prognosis in cancers (35) and sepsis (36). However, these data are mostly correlative. Therefore it is still not understood if enhanced IL-10 is a feedback mechanism to suppress inflammation or part of disease etiology. There is however some evidence, including data from the current study in regards to mast cells, that designate IL-10 as a possible inducer of inflammation.
One noteworthy example is IL-10’s role in allergic asthma. Several studies have found that IL-10 is needed to resolve the late phase of eosinophilic inflammation. Surprisingly, these studies also show that in the early phase of asthma IL-10 is required for airway hyperresponsiveness (AHR). These results were confirmed both in IL-10-deficient mice and with intratracheal administration of recombinant IL-10 (37–39). In animal models one mechanism of AHR development is through mast cell activation and Th2 cytokine release (40). Because allergic sensitization is required for IL-10 to induce AHR and IL-10 does not directly trigger smooth muscle contraction (39), we speculate that IL-10 might act partly by enhancing IgE induced signaling in mast cells to upregulate Th2 cytokine production. To our knowledge, there are no studies addressing the effect of IL-10 on mast cells during AHR development. In addition, a recent study in miR-155 KO mice revealed that miR-155 is required for allergen-induced eosinophilic airway inflammation (41). Clinical studies show detectable IL-10 in the bronchoalveolar lavage fluid of asthmatic patients. These data also indicate a positive correlation between IL-10 gene polymorphisms and the development of asthma (37–39). There is however no substantial data on the role of recombinant IL-10 therapy in asthmatic patients. It would be of interest to study the role of IL-10-induced miR-155 expression in a mast cell-dependent asthma model.
Our data show IL-10 stimulatory effects on human mast cells, suggesting that IL-10 could exacerbate disease states where mast cells are active. There is support for this. For instance, human skin mast cells have been found to be major producers of the IL-10 homolog IL-22 in psoriasis patients, leading to increased psoriatic plaques (42). One clinical study found that IL-10 therapy in psoriasis patients exacerbated inflammation and increased Th2 cytokines (9). While miR-155 expression was found to be lower among benign skin disorders (including psoriasis) than in cutaneous T cell lymphoma (43), no comparison of miR-155 expression in healthy and psoriatic skin has been published. Our data suggest that it may be productive to study miR-155 expression and function among psoriatic lesions.
This study sheds light on a novel IL-10-induced, Stat3- and miR-155-dependent signaling pathway in mast cells. While the exact mechanism requires further study, our data indicate that the loss of miR-155 might be beneficial in curbing unwanted inflammation in conditions where IL-10 production and mast cell activation coincide.
Supplementary Material
Acknowledgments
The authors would like to thank Dr. Daniel Conrad’s lab, in particular Andrea Elkovich, for measuring serum IgE levels.
This work was supported by NIH grants 1R01AI101153 and 2R01AI059638 to JJR, and 1R01 AI095494 to CAO.
Abbreviations
- BMMC
bone marrow-derived mast cell
- DNP-HSA
dinitophenyl-coupled human serum albumin
- Stat3
Signal transducer and activator of transcription 3
- PSA
passive systemic anaphylaxis
References
- 1.Mosmann TR, Moore KW. The role of IL-10 in crossregulation of TH1 and TH2 responses. Immunol Today. 1991;12:A49–53. doi: 10.1016/S0167-5699(05)80015-5. [DOI] [PubMed] [Google Scholar]
- 2.Trifunović J, Miller L, Debeljak Ž, Horvat V. Pathologic patterns of interleukin 10 expression--a review. Biochem Med (Zagreb) 2015;25:36–48. doi: 10.11613/BM.2015.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murray PJ. The primary mechanism of the IL-10-regulated antiinflammatory response is to selectively inhibit transcription. Proc Natl Acad Sci USA. 2005;102:8686–8691. doi: 10.1073/pnas.0500419102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kühn R, Löhler J, Rennick D, Rajewsky K, Müller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 5.Moran CJ, Walters TD, Guo CH, Kugathasan S, Klein C, Turner D, Wolters VM, Bandsma RH, Mouzaki M, Zachos M, Langer JC, Cutz E, Benseler SM, Roifman CM, Silverberg MS, Griffiths AM, Snapper SB, Muise AM. IL-10R polymorphisms are associated with very-early-onset ulcerative colitis. Inflamm Bowel Dis. 2013;19:115–123. doi: 10.1002/ibd.22974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lauw FN, Pajkrt D, Hack CE, Kurimoto M, van Deventer SJ, van der Poll T. Proinflammatory effects of IL-10 during human endotoxemia. J Immunol. 2000;165:2783–2789. doi: 10.4049/jimmunol.165.5.2783. [DOI] [PubMed] [Google Scholar]
- 7.Tilg H, van Montfrans C, van den Ende A, Kaser A, van Deventer SJH, Schreiber S, Gregor M, Ludwiczek O, Rutgeerts P, Gasche C, Koningsberger JC, Abreu L, Kuhn I, Cohard M, LeBeaut A, Grint P, Weiss G. Treatment of Crohn’s disease with recombinant human interleukin 10 induces the proinflammatory cytokine interferon gamma. Gut. 2002;50:191–195. doi: 10.1136/gut.50.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peng H, Wang W, Zhou M, Li R, Pan HF, Ye DQ. Role of interleukin-10 and interleukin-10 receptor in systemic lupus erythematosus. Clin Rheumatol. 2013;32:1255–1266. doi: 10.1007/s10067-013-2294-3. [DOI] [PubMed] [Google Scholar]
- 9.Döcke WD, Asadullah K, Belbe G, Ebeling M, Höflich C, Friedrich M, Sterry W, Volk HD. Comprehensive biomarker monitoring in cytokine therapy: heterogeneous, time-dependent, and persisting immune effects of interleukin-10 application in psoriasis. J Leukoc Biol. 2009;85:582–593. doi: 10.1189/jlb.0408249. [DOI] [PubMed] [Google Scholar]
- 10.Kim HS, Kim AR, Kim DK, Kim HW, Park YH, Jang GH, Kim B, Park YM, You JS, Kim HS, Beaven MA, Kim YM, Choi WS. Interleukin-10-producing CD5+ B cells inhibit mast cells during immunoglobulin E-mediated allergic responses. Sci Signal. 2015;8:ra28–ra28. doi: 10.1126/scisignal.2005861. [DOI] [PubMed] [Google Scholar]
- 11.Kennedy Norton S, Barnstein B, Brenzovich J, Bailey DP, Kashyap M, Speiran K, Ford J, Conrad D, Watowich S, Moralle MR, Kepley CL, Murray PJ, Ryan JJ. IL-10 suppresses mast cell IgE receptor expression and signaling in vitro and in vivo. J Immunol. 2008;180:2848–2854. doi: 10.4049/jimmunol.180.5.2848. [DOI] [PubMed] [Google Scholar]
- 12.Gillespie SR, DeMartino RR, Zhu J, Chong HJ, Ramirez C, Shelburne CP, Bouton LA, Bailey DP, Gharse A, Mirmonsef P, Odom S, Gomez G, Rivera J, Fischer-Stenger K, Ryan JJ. IL-10 inhibits Fc epsilon RI expression in mouse mast cells. J Immunol. 2004;172:3181–3188. doi: 10.4049/jimmunol.172.5.3181. [DOI] [PubMed] [Google Scholar]
- 13.Fernando J, Faber TW, Pullen NA, Falanga YT, Kolawole EM, Oskeritzian CA, Barnstein BO, Bandara G, Li G, Schwartz LB, Spiegel S, Straus DB, Conrad DH, Bunting KD, Ryan JJ. Genotype-dependent effects of TGF-β1 on mast cell function: targeting the Stat5 pathway. J Immunol. 2013;191:4505–4513. doi: 10.4049/jimmunol.1202723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kambe N, Kambe M, Kochan JP, Schwartz LB. Human skin-derived mast cells can proliferate while retaining their characteristic functional and protease phenotypes. Blood. 2001;97:2045–2052. doi: 10.1182/blood.v97.7.2045. [DOI] [PubMed] [Google Scholar]
- 15.Hundley TR, Gilfillan AM, Tkaczyk C, Andrade MV, Metcalfe DD, Beaven MA. Kit and FcepsilonRI mediate unique and convergent signals for release of inflammatory mediators from human mast cells. Blood. 2004;104:2410–2417. doi: 10.1182/blood-2004-02-0631. [DOI] [PubMed] [Google Scholar]
- 16.Ito T, Smrž D, Jung M-Y, Bandara G, Desai A, Smržová Š, Kuehn HS, Beaven MA, Metcalfe DD, Gilfillan AM. Stem cell factor programs the mast cell activation phenotype. J Immunol. 2012;188:5428–5437. doi: 10.4049/jimmunol.1103366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin TJ, Befus AD. Differential regulation of mast cell function by IL-10 and stem cell factor. J Immunol. 1997;159:4015–4023. [PubMed] [Google Scholar]
- 18.Niemand C, Nimmesgern A, Haan S, Fischer P, Schaper F, Rossaint R, Heinrich PC, Müller-Newen G. Activation of STAT3 by IL-6 and IL-10 in primary human macrophages is differentially modulated by suppressor of cytokine signaling 3. J Immunol. 2003;170:3263–3272. doi: 10.4049/jimmunol.170.6.3263. [DOI] [PubMed] [Google Scholar]
- 19.Biethahn K, Orinska Z, Vigorito E, Goyeneche-Patino DA, Mirghomizadeh F, Föger N, Bulfone-Paus S. miRNA-155 controls mast cell activation by regulating the PI3Kγ pathway and anaphylaxis in a mouse model. Allergy. 2014;69:752–762. doi: 10.1111/all.12407. [DOI] [PubMed] [Google Scholar]
- 20.O’Connell RM, Chaudhuri AA, Rao DS, Baltimore D. Inositol phosphatase SHIP1 is a primary target of miR-155. Proc Natl Acad Sci USA. 2009;106:7113–7118. doi: 10.1073/pnas.0902636106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCoy CE, Sheedy FJ, Qualls JE, Doyle SL, Quinn SR, Murray PJ, O’Neill LAJ. IL-10 inhibits miR-155 induction by toll-like receptors. J Biol Chem. 2010;285:20492–20498. doi: 10.1074/jbc.M110.102111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Escobar T, Yu CR, Muljo SA, Egwuagu CE. STAT3 activates miR-155 in Th17 cells and acts in concert to promote experimental autoimmune uveitis. Invest Ophthalmol Vies Sci. 2013;54:4017–4025. doi: 10.1167/iovs.13-11937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rao R, Rieder SA, Nagarkatti P, Nagarkatti M. Staphylococcal enterotoxin B-induced microRNA-155 targets SOCS1 to promote acute inflammatory lung injury. Infect Immun. 2014;82:2971–2979. doi: 10.1128/IAI.01666-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kohanbash G, Okada H. MicroRNAs and STAT interplay. Semin Cancer Biol. 2012;22:70–75. doi: 10.1016/j.semcancer.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu LF, Thai TH, Calado DP, Chaudhry A, Kubo M, Tanaka K, Loeb GB, Lee H, Yoshimura A, Rajewsky K, Rudensky AY. Foxp3-dependent microRNA155 confers competitive fitness to regulatory T cells by targeting SOCS1 protein. Immunity. 2009;30:80–91. doi: 10.1016/j.immuni.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marshall JS, Leal-Berumen I, Nielsen L, Glibetic M, Jordana M. Interleukin (IL)-10 inhibits long-term IL-6 production but not preformed mediator release from rat peritoneal mast cells. J Clin Invest. 1996;97:1122–1128. doi: 10.1172/JCI118506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Braun DA, Fribourg M, Sealfon SC. Cytokine response is determined by duration of receptor and signal transducers and activators of transcription 3 (STAT3) activation. J Biol Chem. 2013;288:2986–2993. doi: 10.1074/jbc.M112.386573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vigorito E, Kohlhaas S, Lu D, Leyland R. miR-155: an ancient regulator of the immune system. Immunol Rev. 2013;253:146–157. doi: 10.1111/imr.12057. [DOI] [PubMed] [Google Scholar]
- 29.Kurowska-Stolarska M, Alivernini S, Ballantine LE, Asquith DL, Millar NL, Gilchrist DS, Reilly J, Ierna M, Fraser AR, Stolarski B, McSharry C, Hueber AJ, Baxter D, Hunter J, Gay S, Liew FY, McInnes IB. MicroRNA-155 as a proinflammatory regulator in clinical and experimental arthritis. Proc Natl Acad Sci USA. 2011;108:11193–11198. doi: 10.1073/pnas.1019536108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li X, Tian F, Wang F. Rheumatoid arthritis-associated microRNA-155 targets SOCS1 and upregulates TNF-α and IL-1β in PBMCs. Int J Mol Sci. 2013;14:23910–23921. doi: 10.3390/ijms141223910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun Y, Cai J, Ma F, Lü P, Huang H, Zhou J. miR-155 mediates suppressive effect of progesterone on TLR3, TLR4-triggered immune response. Immunol Lett. 2012;146:25–30. doi: 10.1016/j.imlet.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 32.Tili E, Michaille JJ, Cimino A, Costinean S, Dumitru CD, Adair B, Fabbri M, Alder H, Liu CG, Calin GA, Croce CM. Modulation of miR-155 and miR-125b levels following lipopolysaccharide/TNF-alpha stimulation and their possible roles in regulating the response to endotoxin shock. J Immunol. 2007;179:5082–5089. doi: 10.4049/jimmunol.179.8.5082. [DOI] [PubMed] [Google Scholar]
- 33.De Sepulveda P, Okkenhaug K, Rose JL, Hawley RG, Dubreuil P, Rottapel R. Socs1 binds to multiple signalling proteins and suppresses steel factor-dependent proliferation. EMBO J. 1999;18:904–915. doi: 10.1093/emboj/18.4.904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chan CS, Ming-Lum A, Golds GB, Lee SJ, Anderson RJ, Mui ALF. Interleukin-10 inhibits lipopolysaccharide-induced tumor necrosis factor-α translation through a SHIP1-dependent pathway. J Biol Chem. 2012;287:38020–38027. doi: 10.1074/jbc.M112.348599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lippitz BE. Cytokine patterns in patients with cancer: a systematic review. Lancet Oncol. 2013;14:e218–28. doi: 10.1016/S1470-2045(12)70582-X. [DOI] [PubMed] [Google Scholar]
- 36.Heper Y, Akalin EH, Mistik R, Akgöz S, Töre O, Göral G, Oral B, Budak F, Helvaci S. Evaluation of serum C-reactive protein, procalcitonin, tumor necrosis factor alpha, and interleukin-10 levels as diagnostic and prognostic parameters in patients with community-acquired sepsis, severe sepsis, and septic shock. Eur J Clin Microbiol Infect Dis. 2006;25:481–491. doi: 10.1007/s10096-006-0168-1. [DOI] [PubMed] [Google Scholar]
- 37.Matsumoto K, Inoue H, Tsuda M, Honda Y, Kibe A, Machida K, Yoshiura Y, Nakanishi Y. Different roles of interleukin-10 in onset and resolution of asthmatic responses in allergen-challenged mice. Respirology. 2005;10:18–26. doi: 10.1111/j.1440-1843.2005.00647.x. [DOI] [PubMed] [Google Scholar]
- 38.Mäkelä MJ, Kanehiro A, Dakhama A, Borish L, Joetham A, Tripp R, Anderson L, Gelfand EW. The failure of interleukin-10-deficient mice to develop airway hyperresponsiveness is overcome by respiratory syncytial virus infection in allergen-sensitized/challenged mice. Am J Respir Crit Care Med. 2002;165:824–831. doi: 10.1164/ajrccm.165.6.2105062. [DOI] [PubMed] [Google Scholar]
- 39.Justice JP, Shibata Y, Sur S, Mustafa J, Fan M, Van Scott MR. IL-10 gene knockout attenuates allergen-induced airway hyperresponsiveness in C57BL/6 mice. Am J Physiol Lung Cell Mol Physiol. 2001;280:L363–8. doi: 10.1152/ajplung.2001.280.2.L363. [DOI] [PubMed] [Google Scholar]
- 40.Kobayashi T, Miura T, Haba T, Sato M, Serizawa I, Nagai H, Ishizaka K. An essential role of mast cells in the development of airway hyperresponsiveness in a murine asthma model. J Immunol. 2000;164:3855–3861. doi: 10.4049/jimmunol.164.7.3855. [DOI] [PubMed] [Google Scholar]
- 41.Malmhäll C, Alawieh S, Lu Y, Sjöstrand M, Bossios A, Eldh M, Rådinger M. MicroRNA-155 is essential for T(H)2-mediated allergen-induced eosinophilic inflammation in the lung. J Allergy Clin Immunol. 2014;133:1429–38. 1438.e1–7. doi: 10.1016/j.jaci.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 42.Mashiko S, Bouguermouh S, Rubio M, Baba N, Bissonnette R, Sarfati M. Human mast cells are major IL-22 producers in patients with psoriasis and atopic dermatitis. J Allergy Clin Immunol. 2015;136:351–359.e1. doi: 10.1016/j.jaci.2015.01.033. [DOI] [PubMed] [Google Scholar]
- 43.Ralfkiaer U, Hagedorn PH, Bangsgaard N, Løvendorf MB, Ahler CB, Svensson L, Kopp KL, Vennegaard MT, Lauenborg B, Zibert JR, Krejsgaard T, Bonefeld CM, Søkilde R, Gjerdrum LM, Labuda T, Mathiesen AM, Grønbæk K, Wasik MA, Sokolowska-Wojdylo M, Queille-Roussel C, Gniadecki R, Ralfkiaer E, Geisler C, Litman T, Woetmann A, Glue C, Røpke MA, Skov L, Odum N. Diagnostic microRNA profiling in cutaneous T-cell lymphoma (CTCL) Blood. 2011;118:5891–5900. doi: 10.1182/blood-2011-06-358382. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.