Abstract
Background
Prior studies have shown that switching patients from inducing antiepileptic drugs (AEDs) to lamotrigine, levetiracetam, or topiramate reduces serum lipids and C-reactive protein (CRP). These studies were all of short duration, and some drugs, such as zonisamide, have not been investigated.
Methods
We recruited 41 patients taking phenytoin or carbamazepine who were being switched to zonisamide, lamotrigine, or levetiracetam. We measured serum lipids and CRP before the switch, >6 weeks after, and >6 months after. An untreated control group (n=14) underwent similar measurement. We combined these data with those of our previous investigation (n=34 patients and 16 controls) of a very similar design.
Results
There were no differences in outcome measures between the two inducing AEDs, nor among the three non-inducing AEDs. Total cholesterol (TC), atherogenic lipids, and CRP were higher under inducer treatment than in controls. All measures were elevated under inducer treatment relative to non-inducer treatment, including TC (24 mg/dL higher, 95% CI: 17.5–29.9, p<0.001) and CRP (72% higher, 95% CI: 41–111%, p<0.001). The difference between drug treatments was clinically meaningful for atherogenic lipids (16%, 95% CI: 11–20%, p<0.001) but small for high-density-lipoprotein cholesterol (5%, 95% CI: 1–9%, p<0.05). All measures were stable between 6 weeks and 6 months after drug switch.
Conclusions
We demontrate that switching from inducing to non-inducing AEDs produces an enduring reduction in serum lipids and CRP. These results provide further evidence that inducing AEDs may be associated with elevated vascular disease risk. These are the first vascular risk marker data in patients taking zonisamide, which shows a profile similar to that of other non-inducing AEDs.
Search terms: antiepileptic drugs, vascular disease, lipids, C-reactive protein
Introduction
Many studies have demonstrated that use of antiepileptic drugs (AEDs) which induce the cytochrome P450 (CYP450) system is associated with higher serum lipid levels and elevated C-reactive protein (CRP). This has been shown in cross-sectional studies1,2 and in repeated-measures analyses3. Most compelling are studies directly demonstrating that switching patients from carbamazepine (CBZ) or phenytoin (PHT) to non-inducing AEDs results in significant reductions in these vascular risk markers. To date, reduction in total cholesterol has been documented upon switch from these inducing agents to lamotrigine (LTG), levetiracetam (LEV), oxcarbazepine, or topiramate4–6. However, all of these studies have been short in duration, with lipids assessed 6–8 weeks after the change in medication. Without longer-term studies, there is no way to ascertain whether this is a transient change which will be undone by homeostatic mechanisms, or a permanent change from withdrawal of the inducing AED. In fact, there are reports that some AED-induced lipid changes are transient in nature7,8.
Thus, our goal was to determine whether improvements in lipids and CRP due to changes in AED therapy are enduring. We utilized a repeated-measures design, so that each patient could effectively serve as his or her own control. In addition, we extend our findings here to include the non-inducing AED zonisamide (ZNS), an agent for which there are no data with respect to lipids to date, to our knowledge.
Methods
Our study population consisted of a group of adult patients with focal epilepsy (age 18 years or older) who were being treated in monotherapy with either PHT or CBZ, and who were being crossed over to monotherapy with one of three newer-generation non-inducing AEDs: ZNS, LTG, or LEV. The large majority of these patients had focal epilepsy. Patients taking a lipid-lowering agent were excluded from the study to avoid having drug interactions confound the results. A group of normal subjects, also age 18 years or older, without epilepsy and not taking any AED or any lipid-lowering agent, served as controls; this was a convenience sample of people available to the investigators and all were newly-recruited for the present investigation.
The decision to switch AEDs was made on clinical grounds by the managing physician at the Jefferson Comprehensive Epilepsy Center and was not, itself, part of the study; patients may have been switched due to side effects from their existing AED, continued seizures, pregnancy planning, or concern about potential long-term AED effects, but in all cases this plan was undertaken by the managing physician for the patient’s benefit. Only once that therapeutic plan was made was the patient was asked to consent to participate in the study so that serologic data could be provided while the drug switch was being pursued. The rate of initiation of therapy with the new agent and taper of the old agent was at the discretion of the treating physician and was individualized for each patient. The minimum target dose for the new AED was 200 mg daily for LTG or ZNS, and 1000 mg daily for LEV.
The design of the study entailed each drug-treated patient having blood drawn after a minimum of 10 hours fasting on 3 occasions: first at baseline, while still taking the old AED; a second time early after switching, at least 6 weeks after the last dose of the older AED was taken; and a third time long after switching, at least 4.5 months after the second draw (constituting at least 6 months after the medication crossover was completed). Likewise, control subjects were to have fasting blood samples drawn at baseline, 10 weeks later (to simulate the time that would be required for cross-titration of medication), and 6 months after baseline. Samples were analyzed for serum lipids, including lipid fractions, and CRP.
The design and population for this study were very similar to those of our previous investigation, in which patients taking CBZ or PHT were crossed over to either LTG or LEV; however, in that study, patients had blood drawn only twice: before the switch, while taking the EIAED, and a second time at least 6 weeks after switch to the newer drug. In 11 of those patients, we were able to obtain a third blood sample, at least 4.5 months after the second one, to mirror the design of the present study; this represents additional data which was not available at the time of the original report. Because of the similarities in design and population, data from the remainder of the prior cohort, who had had only two blood draws, were included in the present analysis in order to maximize the amount of data on each individual AED for the statistical model. A few patients from the newly-recruited cohort were lost to follow-up and also provided only 2 blood samples; their data was included as well, for the same reason. Analogously, a cohort of controls who provided 3 serologic samples was recruited for the present study, and combined with the control group from the prior study, who provided 2 blood samples. Two participant patients, one taking LTG and one taking LEV, had their AED changed owing to significant side effects after providing the second blood sample; both were switched to ZNS at the discretion of the treating physician, and they remained in the study, providing a third blood sample that was analyzed as having been obtained under ZNS treatment. (We considered this “per protocol” analysis, rather than an intention-to-treat analysis, to be most appropriate because the study outcome measures are objective and thus unbiased.) Serology was performed by a specialty lipid laboratory (Liposcience, Raleigh NC) according to the specifications documented in our previous report5.
For statistical analysis, the repeated measures Total Cholesterol (TC) and log transformed measures of CRP, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), triglycerides (TRIG), and non-HDL cholesterol (TC minus HDL-C) were analyzed in separate linear mixed effects models adjusting for correlation among repeated draws per patient. After log transformations, and excluding one LDL observation as potential outlier, the normal distribution assumption was valid as determined by examination of the residuals and estimates of the patient random effects. The initial models included the following covariates (predictors): drug group (OLD=(CBZ or PHT), NEW=(LEV, LTG, ZNS) or NONE), draw (1, 2, or 3) and gender. The difference between draws was not significant in any of the models and was not included in the final models. Gender was retained in the final models if it was a significant predictor. Data were analyzed in SAS 9.1 (SAS Institute Inc., Cary, NC, USA) and S-Plus (Insightful Corporation, Seattle, Washington). This study was approved by the Institutional Review Board of Thomas Jefferson University.
Results
Our analysis included a total of 75 drug-treated epilepsy patients (33 female), with a mean age of 40 (±14) years. This included 41 newly-recruited for this study, all but 3 of whom provided all 3 serologic samples. An additional 34 patients from the prior study were also included in the analysis, of whom 11 provided a third blood sample. Thus, there were 49 patients who provided all 3 samples, and 26 who provided just the first 2 (pre-switch, and >6 weeks after switch). The control group consisted of 30 subjects (18 female), with a mean age of 35 (±13) years, of whom 14 provided 3 blood samples, and 16 provided the first two. The mean time between the first two serologic tests was 113 (± 51) days in the patients and 80 (± 33) days in the controls; this accounts for both the time taken to taper the original AED and the waiting period of at least 6 weeks after it was stopped (along with any delays in getting the patient back to the clinic for the follow-up visit). The mean time between the second and third samples was 250 (± 71) days in the drug-treated patients and 257 (± 31) days in the controls, again accounting for both the AED taper and the waiting period of at least 6 months after it was stopped. Neither age nor gender differed significantly between the patient and control groups, and age did not impact upon any of the outcome measures. Gender did have an impact on some of the lipid measures, however, and this was accounted for in the statistical models when appropriate.
Analysis demonstrated that there was no difference in any outcome measure between the individual inducing AEDs (PHT and CBZ), nor between the individual non-inducing AEDs (LEV, LTG, and ZNS). Furthermore, the sequence of blood draws (1, 2, or 3) had no association with the outcome measures once the drug was taken into account. Thus, the final statistical model combined all samples taken during inducing AED treatment in one common group, all samples taken during non-inducing AED treatment as a second common group, and all samples taken from the untreated controls in a third common group. These data are presented in the Table. All lipid measures and CRP were significantly higher during inducing AED treatment than during non-inducing AED treatment. Specifically, TC was higher on average by 24 mg/dL (95% CI: 18–30; p<0.001), non-HDL-C was higher on average by 16% (95% CI: 11%–20%; p<0.001), HDL-C was higher on average by 5% (95% CI: 1%–9%; p=0.017), TRIG were higher on average by 36% (95% CI: 23%–50%; p<0.001), LDL-C was higher on average by 7% (95% CI: 2%–12%; p=0.010), and CRP was higher on average by 72% (95% CI: 41%–111%; p<0.001). These differences are reported in percentage terms (except for TC) because the data underwent log transformation for statistical analysis. TC was also elevated in patients during inducing AED treatment relative to untreated controls (mean difference=18mg/dL, 95% CI: 4–33; p=0.015), as were non-HDL-C (mean difference=13%, 95% CI: 1%–25%; p=0.030) and CRP (mean difference=141%, 95% CI: 39%–319%; p=0.002). There were no differences between the values obtained in patients during non-inducing AED treatment and those seen in controls except for TRIG, which was lower in the former (by an average of 23%, 95% CI: 3%–38%, p<0.05).
Table.
Mean values (with 95% confidence intervals) of lipid measures and C-reactive protein (CRP) in patients during treatment with an inducing AED, in the same patients during treatment with a non-inducing AED, and in controls treated with no AED. For inducing AED-treated patients, values from samples 2 and 3 averaged together. For controls, values from all samples averaged together. All measures in mg/dL, except CRP in mg/L. HDL = high-density lipoprotein. LDL = low-density lipoprotein.
|
Group → Measure↓ |
Inducing AED | Non-inducing AED | No AED | p-values |
|---|---|---|---|---|
| Total cholesterol |
213.0 (204.6 – 221.5) |
189.3 (181.3 – 197.3) |
194.7 (182.7 – 206.8) |
<0.001* <0.05† |
| non-HDL cholesterol |
146.3 (137.8 – 155.3) |
126.4 (119.3 – 133.8) |
130.0 (119.1 – 141.9) |
<0.001* <0.05† |
| LDL cholesterol |
116.4 (108.9 – 124.4) |
109.3 (102.6 – 116.4) |
113.5 (103.2 – 124.8) |
<0.02* |
| HDL cholesterol |
59.2 (55.9 – 62.6) |
56.5 (53.5 – 59.6) |
57.7 (53.2 – 62.7) |
<0.02* |
| Triglycerides |
100.3 (87.4 – 115.1) |
73.7 (65.1 – 83.4) |
95.3 (79.1 – 114.9) |
<0.001* <0.05§ |
| CRP |
3.1 (2.2 – 4.2) |
1.8 (1.3 – 2.4) |
1.3 (0.8 – 2.0) |
<0.001* <0.01† |
For difference between inducing and non-inducing AED
For difference between inducing AED and no AED
p<0.01 for difference between inducing AED and no AED
For difference between non-inducing AED and no AED
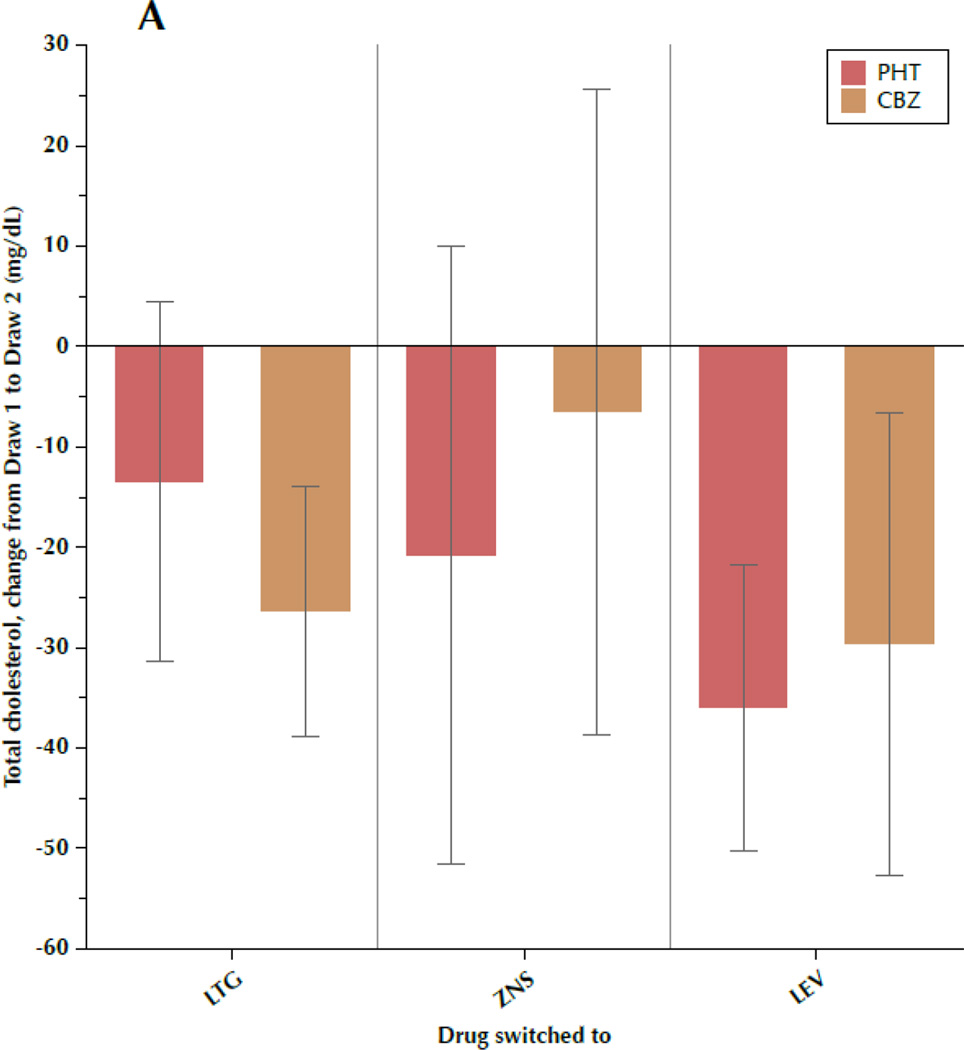
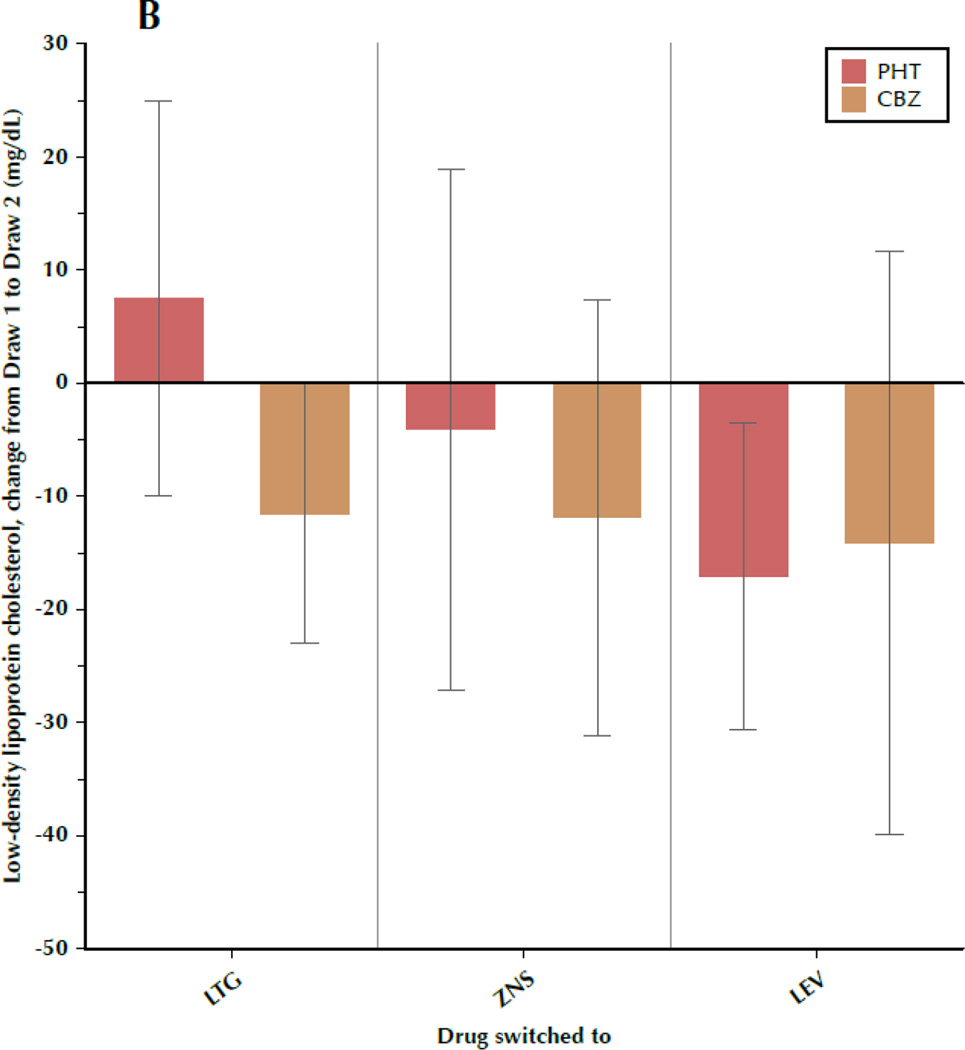
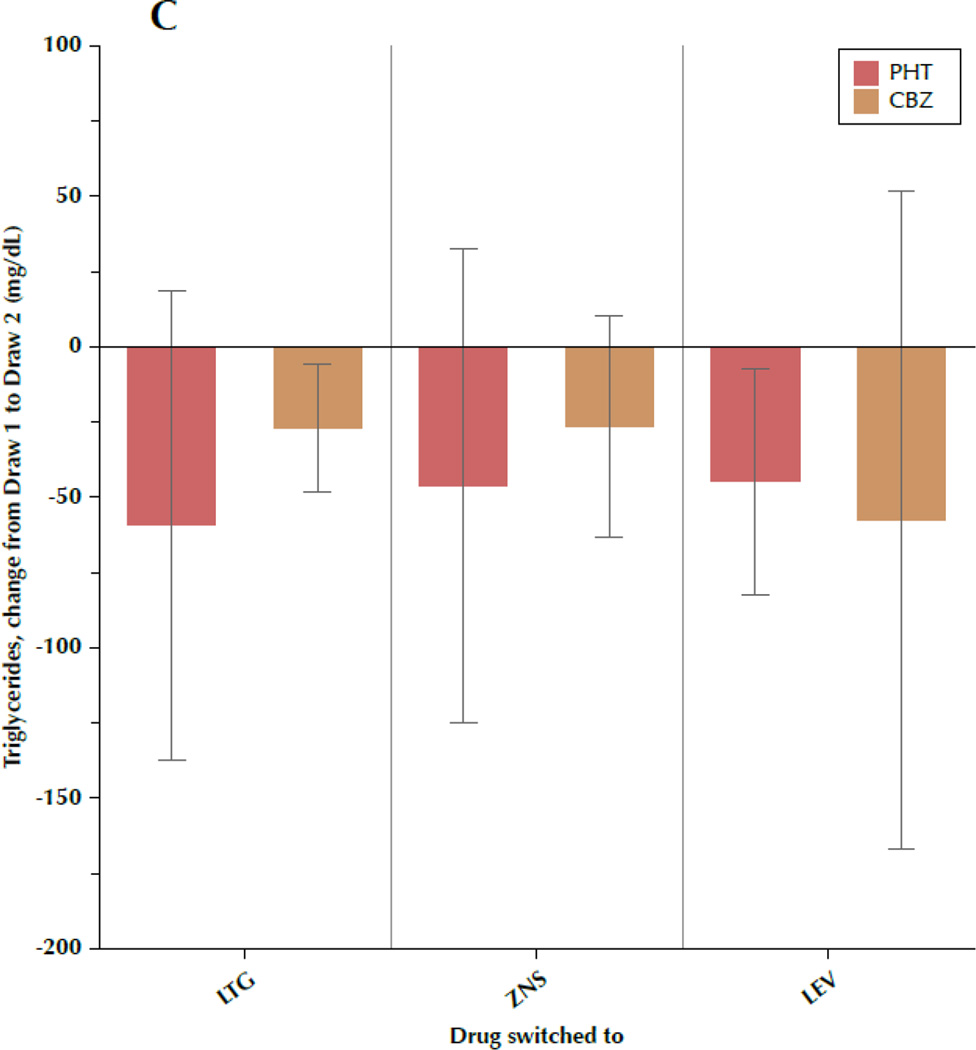
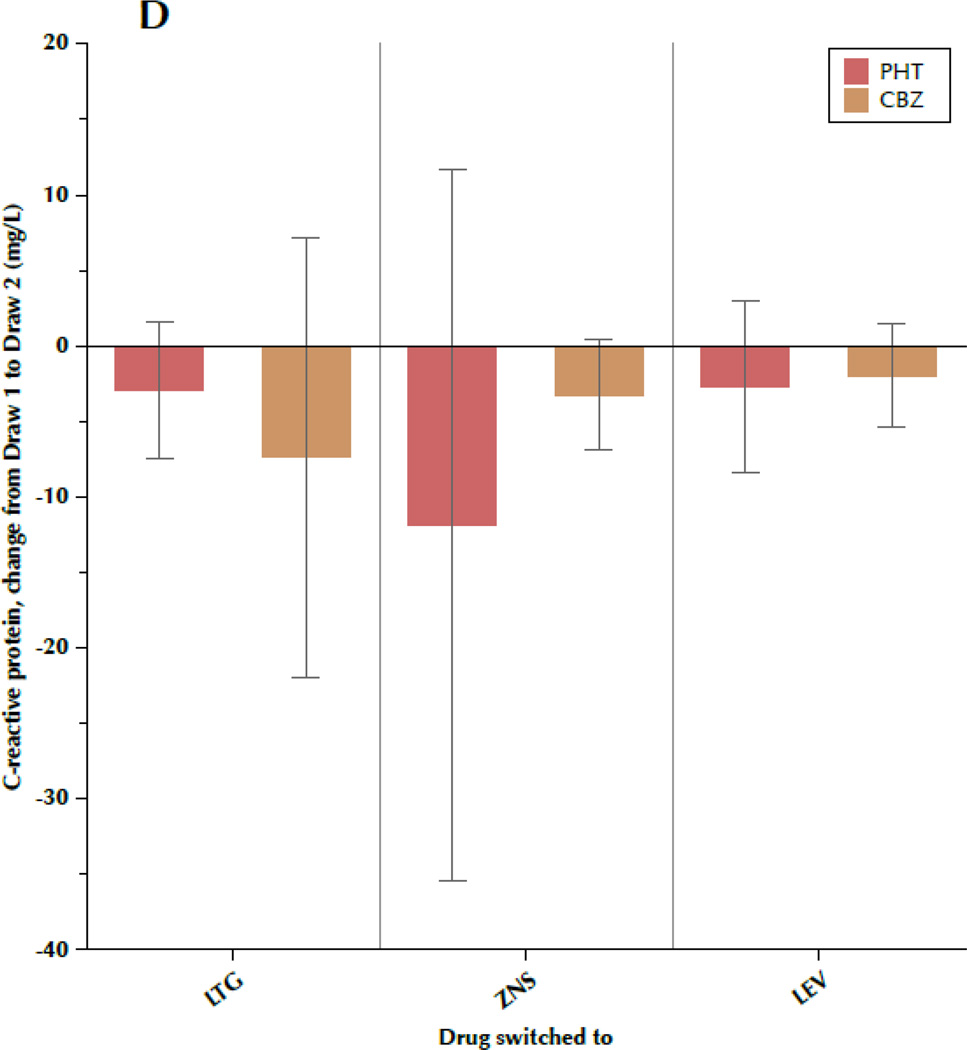
Figure 1 shows the change in TC, LDL-C, TRIG, and CRP between draw 1 and draw 2, subdivided by both the drug the patient was initially taking and the drug to which the patient was switched. Most results were fairly consistent, regardless of specific AED, with some variation between the individual drugs that is not significant. Particular uniformity was seen regarding changes in TRIG and CRP, while TC and LDL-C exhibited more intra-group variability, as evidenced by the large standard deviations.
Figure 1.
A–D. Change in outcome measures between the first and second blood samples, subdivided by the drug the patient was originally taking (phenytoin[PHT] or carbamazepine[CBZ]) and the drug to which the patient was switched (lamotrigine[LTG], levetiracetam[LEV], or zonisamide[ZNS]). Data shown are means and 95% confidence intervals for total cholesterol (A), low-density lipoprotein cholesterol (B), triglycerides (C), and C-reactive protein (D). For LEV, n=8 patients originally taking CBZ and n=20 originally taking PHT. For LTG, n=19 switched from CBZ and n=11 switched from PHT. For ZNS, n=9 patients switched from CBZ and n=8 switched from PHT.
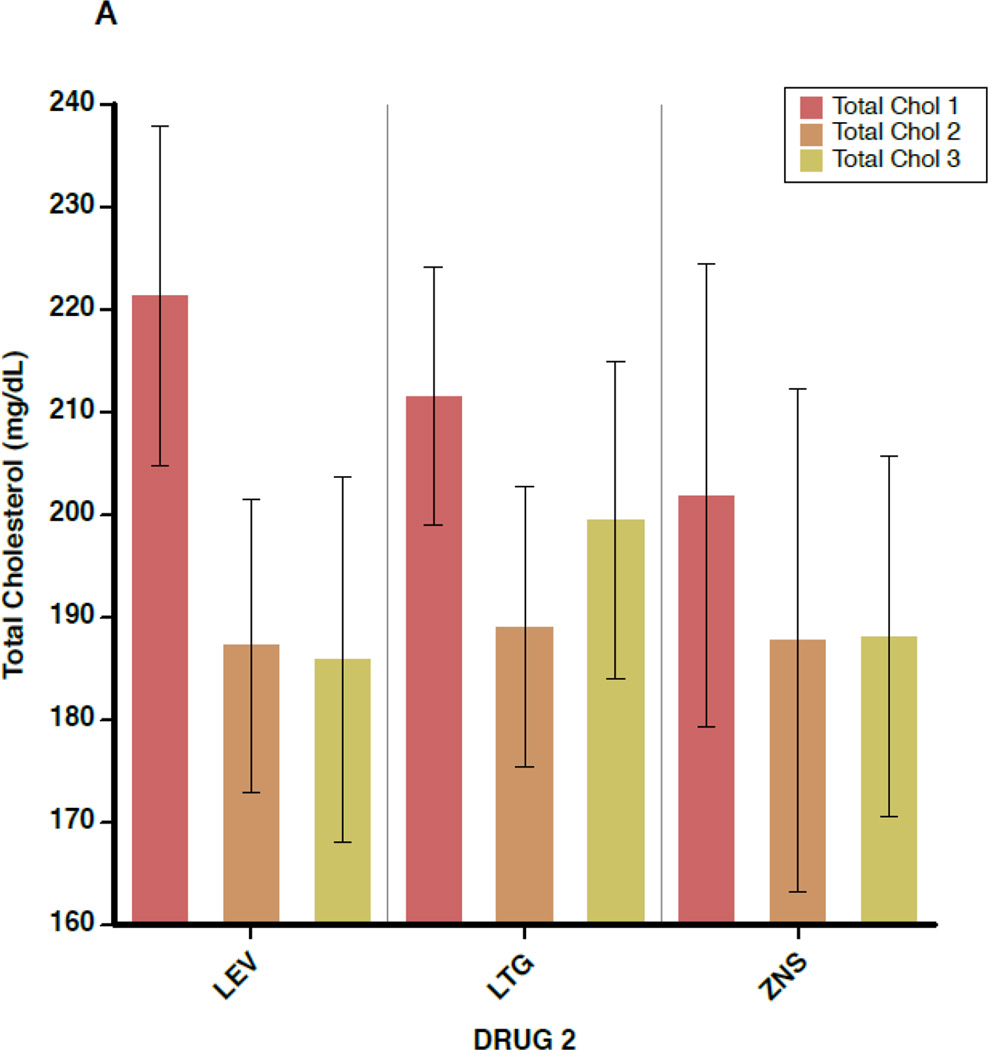
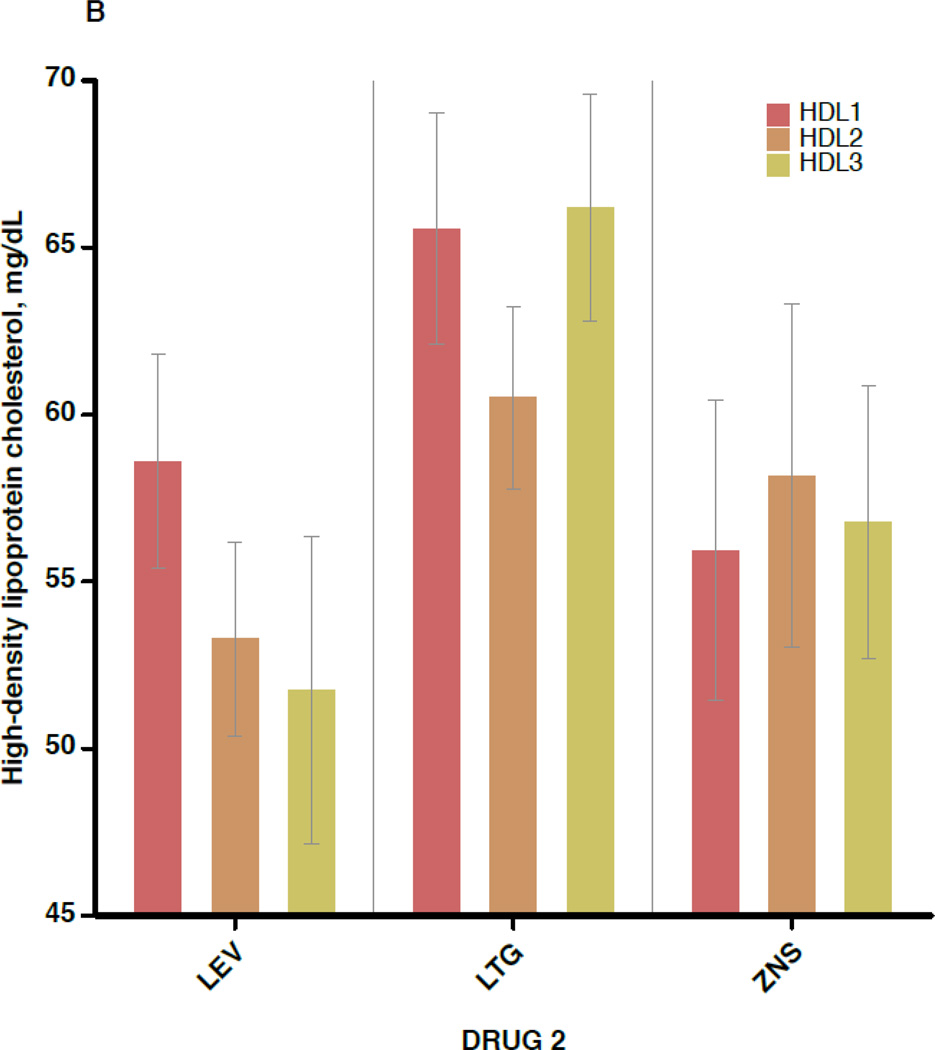
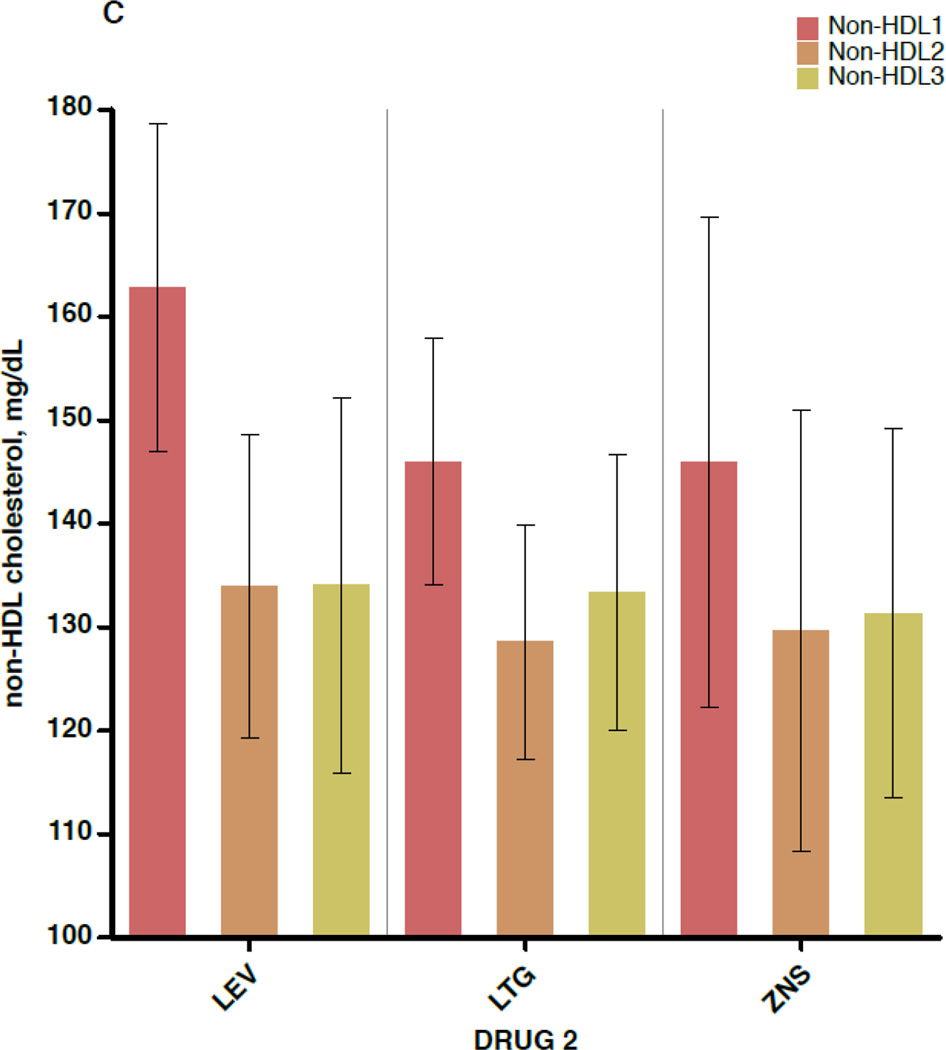
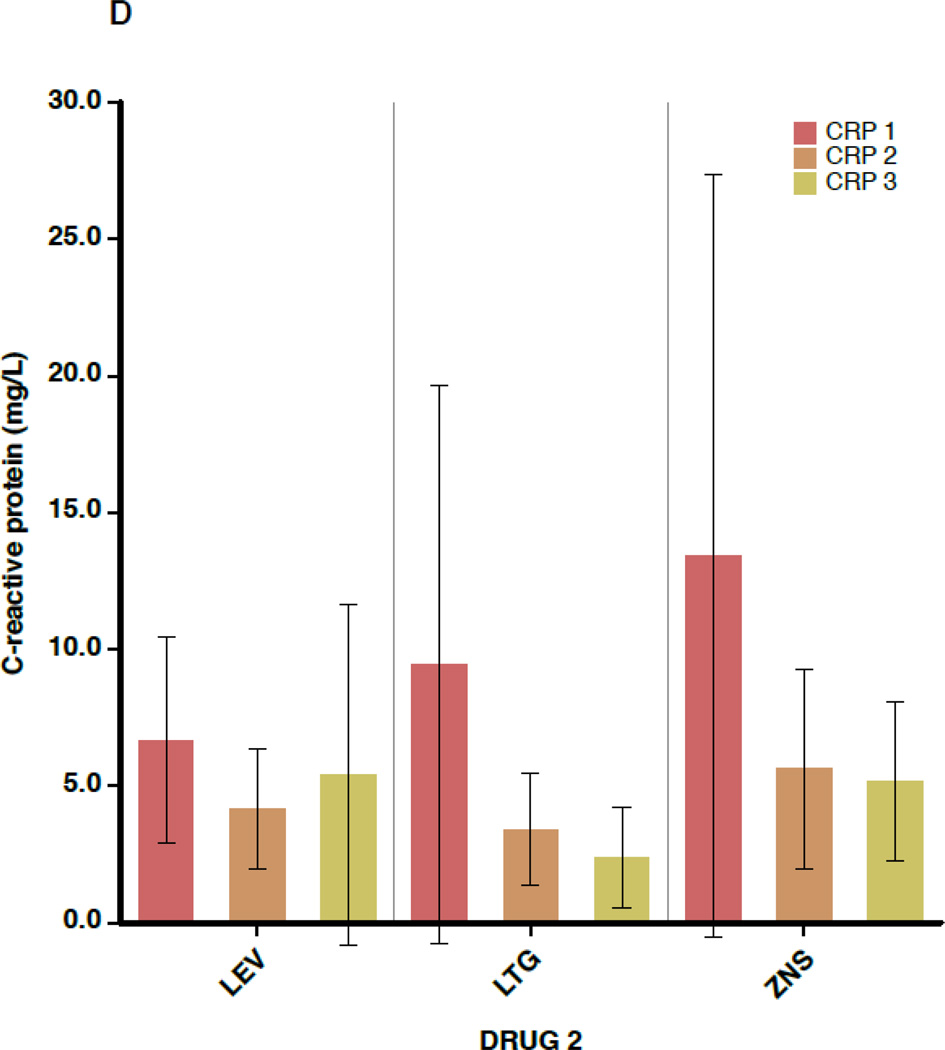
Figure 2 shows the mean values for TC, non-HDL-C, HDL-C, and CRP separately for each of the 3 blood draws (before switch, ≥6 weeks after switch, and ≥6 months after switch), subdivided by the non-inducing AED to which the patient was switched. Again, outcome values were consistent across the 3 AEDs. In addition, little change is seen in mean values between draw 2 and draw 3, indicating that the effects of AED switch appear to have taken full effect by 6 weeks after completion of the crossover.
Figure 2.
A–D. Outcome measures for each of the three serologic samples, subdivided by the drug to which the patient was switched (lamotrigine[LTG], levetiracetam[LEV], or zonisamide[ZNS]). Data shown are means and 95% confidence intervals for total cholesterol (A), high-density lipoprotein cholesterol (B), non-high-density lipoprotein cholesterol (C), and C-reactive protein (D). For the LEV patients, n=28 for the first and second sample times and n=11 for the third. For LTG patients, n=30 for the first two samples and n=19 for the third. For ZNS patients, n=17 for the first two and n=16 for the third.
Discussion
We show here, for the first time, that switching patients from the enzyme-inducing agents PHT or CBZ to non-inducing AEDs produces a reduction in serum cholesterol and CRP that is enduring, rather than the product of a temporary homeostatic reaction. While one group has reported changes in lipids with various AEDs have resolved after a year of therapy or more 8,9, this stands in contrast to many cross-sectional studies in adults and children (reviewed in LoPinto-Khoury and Mintzer10) in which patients under long-term treatment with CBZ have elevated lipid values relative to controls. Some of our data comes from continued observation of patients enrolled in our prior study, but more than half (41 of 75) were newly-recruited patients, and this, along with the size of the total cohort, are among the strengths of the present report.
This study is also, to our knowledge, the first to measure lipid levels in patients taking ZNS, a broad-spectrum AED which has been used in Asia and the United States for many years, and was more recently approved in Europe following a large randomized trial in newly-diagnosed focal epilepsy patients which demonstrated very similar efficacy to CBZ11. Data now exist to verify that lipids and CRP will decline if patients taking inducing AEDs are switched to ZNS, LTG, LEV, TPM, or OXC4–6.
Our study provides further strong evidence that inducing AEDs elevate serum lipids and CRP. The particular value of the “drug switch” paradigm is that it demonstrates that changes in lipids and CRP are due to the change in drug treatment, rather than due to differences in patient population, as could be the case in a cross-sectional study. In theory, this could also be due to some lipid-lowering effect of LEV, LTG, and ZNS. But all outcome measures (except TRIG) were similar between the newer AED group and the controls, with the older AED group higher, suggesting that it is the inducing AEDs that cause the derangement. The cross-sectional studies mentioned above also point to the former hypothesis. Furthermore, there is clinical and pharmacologic reasoning to apply. The second hypothesis implies that LEV, LTG, ZNS, topiramate, and oxcarbazepine, 5 drugs which are wholly dissimilar in their chemical structure and pharmacologic action, have in common some property which leads to a comparable reduction in lipids. Thus, whatever property is responsible for changes in lipids after AED switch must differ between PHT and CBZ on the one hand, and LTG, LEV, ZNS, oxcarbazepine, and topiramate on the other. Induction of the CYP450 system is by far the most likely culprit, particularly in view of the involvement of CYP450 enzymes in the cholesterol synthetic pathway12. Oxcarbazepine and topiramate are only limited (and possibly dose-dependent) inducers of CYP450 enzymes13–15, and LTG, LEV, and ZNS have no effect upon the P450 system, whereas PHT and CBZ are broad and powerful inducers. Furthermore, there is evidence in laboratory animals that CYP450 inhibition leads to reduced cholesterol production12 and that patients exposed to valproate, a CYP450 inhibitor, have lower cholesterol levels2,16. Thus, the sum total of evidence strongly implies that PHT and CBZ elevate lipids and CRP because of their enzyme-inducing capacity, and that the changes are enduring, clinically significant, and reversible.
In this study, differences between the groups in LDL-C were modest. This is the case despite the fact that both TC and non-HDL-C (the total of atherogenic lipid components, calculated as TC minus HDL-C), are significantly elevated by the use of inducing AEDs. Non-HDL-C is mainly comprised of VLDL-C and LDL-C. It appears that enzyme induction from AEDs results in variable increases in subfractions of atherogenic lipids, with more accruing in the VLDL-C fraction than the LDL-C fraction. VLDL are the predominant carriers of TRIG, so the large difference seen in TRIG values between inducing and non-inducing AED use corroborates this. One odd finding of our study is that TRIG values in controls were similar to those seen with inducing AED treatment, and lower than those seen with non-inducing AED treatment. This is incongruous with the rest of the data, and there is no obvious explanation; it may be a statistical fluke, but this should be examined in future studies. Our study also confirmed that inducing AEDs, while they increase atherogenic lipids, also increase the beneficial HDL-C, though apparently too modestly (2–3 mg/dL) to have much clinical impact on cardiovascular disease incidence.
Our study has verified that CRP declines prominently and in an enduring fashion when patients are switched from inducing to non-inducing AEDs. Since drugs which lower cholesterol also lower CRP, it is possible that this is an epiphenomenon. Alternatively, it is possible that inducing AEDs also produce alterations in the inflammatory pathways, of which CRP is the most commonly-measured marker. Further studies should examine whether other markers of inflammation are likewise affected by AED treatment.
A limitation of the study is that we did not account for other factors that could influence the primary outcome markers, such as body weight, diet, exercise, or smoking. However, since each patient serves as his or her own control, inter-subject differences would be very unlikely to have an impact on the results; there would have to be changes in these factors within patients over the course of the study, and it is highly doubtful that any such changes would account for our findings. Another limitation is that we did not measure other markers associated with vascular disease, such as apolipoprotein levels or homocysteine; whether or not these markers contribute meaningfully to vascular risk stratification beyond the more established markers is the subject of much debate within the cardiovascular community.
Studies in the literature demonstrate that the relative risk of a major vascular event changes by roughly 1% for each change in TC of 1 mg/dL (see supplementary material in Mintzer et al 5). Thus, based simply on the change in TC, one of the oldest and most established surrogate markers in medicine, we would expect that exposure to an inducing AED should raise the risk of vascular disease by approximately 25%. Thus is mitigiated only very slightly -- around 5% -- by the small concomitant elevation in HDL-C. Once the change in CRP -- an independent risk factor -- is factored in, we would expect the increase in relative vascular risk with inducing AED treatment to be around 33%, which is clinically meaningful by anyone’s standards, particularly given the enormous number of patients worldwide who are treated with these agents both for neurological and psychiatric purposes.
Both CBZ and PHT remain commonly-used treatment options throughout the world, but mounting evidence demonstrates that newer drugs are equally effective for focal epilepsy 11,17–20. We now have good evidence to demonstrate that each of these non-inducing drugs avoids the elevation of vascular risk markers — and possible promotion of atherosclerosis1 — which occurs with the inducing AEDs. CBZ and PHT are also responsible for a plethora of other metabolic derangements, including alteration of vitamin levels5,21 bone metabolism22,23, male reproductive function24, and many drug interactions25. In view of these findings, there is increasing reason to believe that the use of enzyme-inducing AEDs is problematic. This is particularly true in early-stage disease, because if patients do well, but are subsequently found to have metabolic side effects, there is a modest but measurable risk of recurrence from switch to a different AED26. Thus, choosing the right drug in the first place may avoid problems later.
In summary, switching patients from inducing to non-inducing AEDs produces long-term improvement in serologic markers of vascular risk. Since inducing AEDs appear to be the culprits, patients taking PHT and CBZ should be screened for vascular risk (via lipid and CRP studies, and possibly stress tests or other measures). Patients taking phenobarbital or primidone, both of which are also potent inducers of the CYP450 system, may be at risk too and should likely be screened for hyperlipidemia and other metabolic derangements10. If non-HDL-C or CRP are elevated they may be treated, but many lipid- lowering agents are themselves induced by the CYP450 system, so higher doses may be needed. Alternatively, the patient may be switched to any of several non-inducing AEDs to reverse the problem for the long-term. We have here demonstrated the benefit of doing so, though if the patient is seizure-free this must be weighed against the modest but meaningful chance of recurrence with AED switch26.
Highlights.
Changing from inducing to non-inducing AED reduces serum lipids and CRP within 6 weeks.
These changes persist for ≥6 months rather than being a transient phenomenon.
The change is similar regardless of which inducer or non-inducer the patient was taking.
This suggests that CYP450 induction increases lipids and CRP, and de-induction reverses this.
Acknowledgments
Study funded by NIH (K23NS058669, Mintzer PI)
Dr. Mintzer has engaged in consulting for UCB, Upsher-Smith, Lundbeck, and Acorda, and has been engaged as a speaker by UCB and Glaxo SmithKline.
Dr. Nei reports research support from Upsher-Smith.
Dr. Skidmore reports being a site principal investigator for a NeuroPace trial.
Dr. Sperling has engaged in consulting for UCB and has served as a site principal investigator for trials from SK Life Sciences, UCB, Sunovion, Eisai, Marinus, Lundbeck, Acorda, Upsher-Smith, Glaxo SmithKline, Brain Sentinel, Medtronic, and Visualase. He also reports personal income from serving previously as associate editor of Epilepsia, and research support from NIH and DARPA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Ms. Miller and Dr. Chervoneva have no disclosures relevant to the manuscript.
REFERENCES
- 1.Chuang YC, Chuang HY, Lin TK, et al. Effects of long-term antiepileptic drug monotherapy on vascular risk factors and atherosclerosis. Epilepsia. 2012;53:120–128. doi: 10.1111/j.1528-1167.2011.03316.x. [DOI] [PubMed] [Google Scholar]
- 2.Nikolaos T, Stylianos G, Chryssoula N, et al. The effect of long-term antiepileptic treatment on serum cholesterol (TC, HDL, LDL) and triglyceride levels in adult epileptic patients on monotherapy. Medical Science Monitor. 2004;10:MT50–MT52. [PubMed] [Google Scholar]
- 3.Bramswig S, Sudhop T, Luers C, et al. Lipoprotein(a) concentration increases during treatment with carbamazepine. Epilepsia. 2003;44:457–460. doi: 10.1046/j.1528-1157.2003.44802.x. [DOI] [PubMed] [Google Scholar]
- 4.Isojarvi JI, Pakarinen AJ, Rautio A, et al. Liver enzyme induction and serum lipid levels after replacement of carbamazepine with oxcarbazepine. Epilepsia. 1994;35:1217–1220. doi: 10.1111/j.1528-1157.1994.tb01792.x. [DOI] [PubMed] [Google Scholar]
- 5.Mintzer S, Skidmore CT, Abidin CJ, et al. Effects of antiepileptic drugs on lipids, homocysteine, and C-reactive protein. Ann Neurol. 2009;65:448–456. doi: 10.1002/ana.21615. [DOI] [PubMed] [Google Scholar]
- 6.Mintzer S, Skidmore CT, Rankin SJ, et al. Conversion from enzyme-inducing antiepileptic drugs to topiramate: Effects on lipids and c-reactive protein. Epilepsy Res. 2012;98:88–93. doi: 10.1016/j.eplepsyres.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 7.Isojarvi JI, Pakarinen AJ, Myllyla VV. Serum lipid levels during carbamazepine medication. A prospective study. Archives of Neurology. 1993;50:590–593. doi: 10.1001/archneur.1993.00540060030012. [DOI] [PubMed] [Google Scholar]
- 8.Verrotti A, Basciani F, Domizio S, et al. Serum lipids and lipoproteins in patients treated with antiepileptic drugs. Pediatric Neurology. 1998;19:364–367. doi: 10.1016/s0887-8994(98)00084-8. [DOI] [PubMed] [Google Scholar]
- 9.Verrotti A, Domizio S, Angelozzi B, et al. Changes in serum lipids and lipoproteins in epileptic children treated with anticonvulsants. Journal of Paediatrics & Child Health. 1997;33:242–245. doi: 10.1111/j.1440-1754.1997.tb01588.x. [DOI] [PubMed] [Google Scholar]
- 10.Lopinto-Khoury C, Mintzer S. Antiepileptic drugs and markers of vascular risk. Curr Treat Options Neurol. 2010;12:300–308. doi: 10.1007/s11940-010-0080-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baulac M, Brodie MJ, Patten A, et al. Efficacy and tolerability of zonisamide versus controlled-release carbamazepine for newly diagnosed partial epilepsy: a phase 3, randomised, double-blind, non-inferiority trial. Lancet Neurol. 2012;11:579–588. doi: 10.1016/S1474-4422(12)70105-9. [DOI] [PubMed] [Google Scholar]
- 12.Gibbons GF. The Role of Cytochrome P450 in the Regulation of Cholesterol Biosynthesis. Lipids. 2002;37:1163–1170. doi: 10.1007/s11745-002-1016-x. [DOI] [PubMed] [Google Scholar]
- 13.Patsalos PN, Zakrzewska JM, Elyas AA. Dose dependent enzyme induction by oxcarbazepine? Eur J Clin Pharmacol. 1990;39:187–188. doi: 10.1007/BF00280057. [DOI] [PubMed] [Google Scholar]
- 14.Doose DR, Wang SS, Padmanabhan M, et al. Effect of topiramate or carbamazepine on the pharmacokinetics of an oral contraceptive containing norethindrone and ethinyl estradiol in healthy obese and nonobese female subjects. Epilepsia. 2003;44:540–549. doi: 10.1046/j.1528-1157.2003.55602.x. [DOI] [PubMed] [Google Scholar]
- 15.Rosenfeld WE, Doose DR, Walker SA, et al. Effect of topiramate on the pharmacokinetics of an oral contraceptive containing norethindrone and ethinyl estradiol in patients with epilepsy. Epilepsia. 1997;38:317–323. doi: 10.1111/j.1528-1157.1997.tb01123.x. [DOI] [PubMed] [Google Scholar]
- 16.Eiris JM, Lojo S, Del Rio MC, et al. Effects of long-term treatment with antiepileptic drugs on serum lipid levels in children with epilepsy. Neurology. 1995;45:1155–1157. doi: 10.1212/wnl.45.6.1155. [DOI] [PubMed] [Google Scholar]
- 17.Marson AG, Al-Kharusi AM, Alwaidh M, et al. The SANAD study of effectiveness of carbamazepine, gabapentin, lamotrigine, oxcarbazepine, or topiramate for treatment of partial epilepsy: an unblinded randomised controlled trial. Lancet. 2007;369:1000–1015. doi: 10.1016/S0140-6736(07)60460-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brodie MJ, Richens A, Yuen AW. Double-blind comparison of lamotrigine and carbamazepine in newly diagnosed epilepsy. UK Lamotrigine/Carbamazepine Monotherapy Trial Group. Lancet. 1995;345:476–479. doi: 10.1016/s0140-6736(95)90581-2. [DOI] [PubMed] [Google Scholar]
- 19.Brodie MJ, Perucca E, Ryvlin P, et al. Comparison of levetiracetam and controlled-release carbamazepine in newly diagnosed epilepsy. Neurology. 2007;68:402–408. doi: 10.1212/01.wnl.0000252941.50833.4a. [DOI] [PubMed] [Google Scholar]
- 20.Privitera MD, Brodie MJ, Mattson RH, et al. Topiramate, carbamazepine and valproate monotherapy: double-blind comparison in newly diagnosed epilepsy. Acta Neurol Scand. 2003;107:165–175. doi: 10.1034/j.1600-0404.2003.00093.x. [DOI] [PubMed] [Google Scholar]
- 21.Linnebank M, Moskau S, Semmler A, et al. Antiepileptic drugs interact with folate and vitamin B12 serum levels. Ann Neurol. 2011;69:352–359. doi: 10.1002/ana.22229. [DOI] [PubMed] [Google Scholar]
- 22.Pack AM, Morrell MJ, Randall A, et al. Bone health in young women with epilepsy after one year of antiepileptic drug monotherapy. Neurology. 2008;70:1586–1593. doi: 10.1212/01.wnl.0000310981.44676.de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mintzer S, Boppana P, Toguri J, et al. Vitamin D levels and bone turnover in epilepsy patients taking carbamazepine or oxcarbazepine. Epilepsia. 2006;47:510–515. doi: 10.1111/j.1528-1167.2006.00460.x. [DOI] [PubMed] [Google Scholar]
- 24.Sivaraaman K, Mintzer S. Hormonal consequences of epilepsy and its treatment in men. Curr Opin Endocrinol Diabetes Obes. 2011;18:204–209. doi: 10.1097/MED.0b013e328345e533. [DOI] [PubMed] [Google Scholar]
- 25.Brodie MJ, Mintzer S, Pack AM, et al. Enzyme induction with antiepileptic drugs: cause for concern? Epilepsia. 2013;54:11–27. doi: 10.1111/j.1528-1167.2012.03671.x. [DOI] [PubMed] [Google Scholar]
- 26.Wang SP, Mintzer S, Skidmore CT, et al. Seizure recurrence and remission after switching antiepileptic drugs. Epilepsia. 2013;54:187–193. doi: 10.1111/j.1528-1167.2012.03652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]