Abstract
Environmental lead (Pb) exposure and prenatal stress (PS) are co-occurring risk factors for impaired cognition and other disorders/diseases in adulthood and target common biological substrates in the brain. Sex-dependent differences characterize the neurochemical and behavioral responses of the brain to Pb and PS and sexually dimorphic histone modifications have been reported to occur in at-risk brain regions (cortex and hippocampus) during development. The present study sought to examine levels and developmental timing of sexually dimorphic histone modifications (i.e., H3K9/14Ac and H3K9Me3) and the extent to which they may be altered by Pb ± PS. Female C57/Bl6 mice were randomly assigned to receive distilled deionized drinking water containing 0 or 100 ppm Pb acetate for 2 months prior to breeding and throughout lactation. Half of the dams in each group were exposed to restraint stress (PS, three restraint sessions in plastic cylindrical devices 3×/day at for 30 min/day (1000, 1300, and 1600h)) from gestational day 11–19 or no stress (NS). At delivery (PND0) and postnatal day 6 (PND6), pups were euthanized and frontal cortex and hippocampus were removed, homogenized, and assayed for levels of H3K9/14Ac and H3K9Me3. Sex-dependent differences in both levels of histone modifications as well as the developmental trajectory of changes in these levels were observed in both structures and these parameters were differentially affected by Pb ± PS in a sex and brain-region-dependent manner. Disruptions of these epigenetic processes by developmental Pb ± PS may underlie some of the sex-dependent neurobehavioral differences previously observed in these animals.
Keywords: H3K9/14Ac, H3K9Me3, hippocampus, frontal cortex, lead, prenatal stress
1. INTRODUCTION
Many aspects of both normal and pathological brain functioning exhibit important yet poorly understood sex differences (Wizemann, 2001; McCarthy et al., 2012). Sex differences have been described for various cognitive functions, as well as for anxiety and stress responses, outcomes from traumatic brain injury, risk and outcomes from various neurodegenerative diseases, and the risk for developing various emotional and affective disorders (see McCarthy et al., 2012 for review). Sex-dependent differences also characterize the response of the brain to the neurotoxicant lead (Pb) and to prenatal stress (PS). Developmental Pb exposure and PS are important co-occurring risk factors for a variety of adverse health outcomes in adulthood as well as for various cognitive/behavioral problems during childhood, with effects expressed differently in males and females (Virgolini et al., 2008a; Virgolini et al., 2008b; Cory-Slechta et al., 2010; Cory-Slechta et al., 2012; Giesbrecht et al., 2015; Wainstock et al., 2015; Zohar et al., 2015).
The precise molecular mechanisms underlying the effects of developmental Pb exposure and of PS on the brain are not yet completely known, nor are the molecular underpinnings for the sex-related differences in the responses of the brain to such risk factors. However, it seems likely that epigenetic mechanisms are involved (Auger and Auger, 2011). Epigenetic outcomes resulting from environmental perturbations are frequently found to be sex-dependent, particularly in relation to neuroendocrine-acting systems. These sex differences likely reflect reported sex differences in such aspects of epigenetic programming as levels of the DNA methyltransferases (DNMTs) (McCarthy et al., 2009; Kolodkin and Auger, 2011) or DNA methylation patterns, and expression of methyl-binding proteins and co-repressor proteins (McCarthy et al., 2009). The existence of such sex differences early in development has been suggested to be critical to brain differentiation (Auger and Auger, 2011). These differences also establish a different biological substrate for each sex on which environmental stressors, including chemical exposures, then act.
In the mammalian brain, chromatin-level modifications play pivotal roles in coordinating developmental programs. Gene regulation during development is regulated by the binding of a variety of regulatory proteins to gene promoter regions and through epigenetic modification of chromatin by post-translational histone modifications and DNA methylation. The role of epigenetics in determining sex differences in the brain (McCarthy et al., 2009) can also be significantly influenced by the period of development during which these environmental factors act. For example, higher levels of DNMT3a mRNA expression in female amygdala were reported at postnatal day 1, but had disappeared by postnatal day 10, with these changes related to sex differences in steroid exposure levels (Kolodkin and Auger, 2011). These dynamics in the developmental trajectory could influence the extent to which environmental risk factors affect the epigenome in a sex-dependent manner.
Epigenetic alterations may occur in the germline as well as in somatic cells (Skinner et al., 2008), both of which are modifiable by environmental stimuli such as steroid hormones and endocrine-disrupting chemicals (McCarthy et al., 2009; Gore, 2008) and potentially, by environmental factors such as developmental Pb exposure and PS. It is possible that an interaction between genes and sex hormones during development may make a variety of brain regions, including the frontal cortex and hippocampus, which are particularly sensitive to the effects of Pb and PS (Cory-Slechta et al., 1998; Cory-Slechta et al., 1999; Berger et al., 2002; Barros et al., 2004; Virgolini et al., 2008a; Martinez-Tellez et al., 2009; Rossi-George et al., 2011; Neal et al., 2012; Stansfield et al., 2012; Baranowska-Bosiacka et al., 2013; Hu et al., 2014; Li et al., 2014; Li et al., 2015), and differentially susceptible to epigenetic alterations, such as post-translational histone modifications, that are associated with modulation of transcriptional regulation.
As sexually dimorphic histone modifications have been reported to occur in cortex and hippocampus during development (Tsai et al., 2009), the present study examined the extent to which these histone modifications may be influenced by Pb and/or PS and thus contribute to the sex-dependent differences in the patterns of effects observed in response to these environmental risk factors alone and in combination (Cory-Slechta et al., 2004b; Virgolini et al., 2006; Cory-Slechta et al., 2008; Virgolini et al., 2008a; Virgolini et al., 2008b; Cory-Slechta et al., 2010; Rossi-George et al., 2011). The goal of the present study was to examine levels and developmental timing of sexually dimorphic histone modifications, H3K9/14Ac (associated with gene activation) and H3K9Me3 (associated with gene silencing) (Tsai et al., 2009) and the extent to which they may be altered by Pb and/or PS (Pb ± PS). Modifications of these histone marks by Pb ± PS could have wide-ranging effects on gene transcription as well as later behavioral/cognitive functions.
2. MATERIALS AND METHODS
2.1 Animals and Lead Exposure and Prenatal Stress
The use of animals was in compliance with NIH Guidelines for the Care and Use of Laboratory Animals and the study was approved by the institutional animal care and use committee at the University of Rochester School of Medicine. Two-week-old female C57/Bl6 mice (Jackson Laboratories, Bar Harbor ME) were randomly assigned to receive distilled deionized water drinking containing 0 or 100 ppm Pb acetate for 2 months prior to breeding and throughout lactation. Standard rat chow diet (Lab Diet, Laboratory Rodent Diet) was provided ad libitum. Female mice were mated with males (1:1) for 4 to 5 days to cover the duration of an estrous cycle. Gestational day 1 (GD1) was designated as the second day after pairing.
Pregnant females in the 0 and 100 ppm Pb-treated groups were weighed and further randomly subdivided to a non-stress (NS) or prenatal stress (PS) condition. Half of the dams in each Pb group were exposed to restraint stress (PS) 3×/day at for 30 min/day (1000, 1300, and 1600h) from gestational day 11–19 or no stress (NS). The stress procedure consisted of three 30-min restraint sessions in plastic cylindrical devices, a protocol previously verified to elevate corticosterone levels (Cory-Slechta et al., 2004a). Non-stressed dams were left undisturbed in their home cages. This yielded 4 treatment groups/sex: 0-NS, 0-PS, 100-NS and 100-PS.
2.2 Blood Collection For Lead Determinations
Blood Pb measurements were determined from trunk blood of PND5-6 pups and from dams (collected at weaning so as not to produce maternal stress) by anodic stripping voltammetry using the Lead Care II system with a detection limit of 3.3 µg/dl.
2.3 Measurement of Post-Translational Histone Modifications
At delivery (PND0) and postnatal day 6 (PND6), pups were euthanized and frontal cortex and hippocampus were removed. Genomic DNA was extracted from tail snips and the sex of the pups was confirmed by Jarid 1C (X-chromosome-specific gene)/Jarid 1D (Y-chromosome-specific gene) PCR (Clapcote and Roder, 2005). To measure H3K9/14Ac and H3K9Me3, hippocampus and prefrontal cortex from each animal was homogenized in hypotonic lysis buffer (10mM HEPES, 1.5mM MgCl2 and 10mM KCl, pH 7.9) with 1× HALT protease inhibitor followed by addition of 1M HCl, Samples were incubated at 4°C for 30 min and then vortexed, centrifuged for 10 min at 15000 rpm, and supernatant containing acid soluble proteins was transferred to fresh tubes at 4°C and protein was quantified using the BCA reaction (Pierce Inc.). Samples (10 µg) were separated on 4–12% Bis-Tris gels (Invitrogen Inc.) run for 1hr at 200V and then transferred to 0.2 µm nitrocellulose membranes using semi-dry transfer (Bio-rad Inc.) for 15 min/membrane at 15V. Membranes were washed in tris-buffered saline (TBS) containing tween-20 (TBS-T) and blocked in 5% milk for 1hr at room temperature. Primary antibody, rabbit anti-acetyl H3K9/14 at 1:10,000 (Millipore), anti-acetyl H3K9 Me3 at 1:5,000 (Millipore) or rabbit anti-β-actin at 1:2,000 (Imgenex), was added for 1 hour at room temperature. Following multiple washes in TBS-T, secondary antibody (horseradish peroxidase conjugated goat anti rabbit at 1:20,000; Thermo Fisher Scientific) was added for 1hour. Membranes were washed repeatedly in TBS-T and exposed to chemiluminescent developing reagent (Pierce Pico or Dura, Thermo Fisher Scientific). Densitometry was used to quantify the amount of H3K9/14Ac and H3K9Me3 relative to β-actin for each sample (MCID™ Basic V 7.2, Interfocus Imaging LTD). Relative optical density was normalized to β-actin.
2.4 Statistical Analyses
Statistical analyses were carried out using between factor ANOVAs. Specifically, initial overall analyses included sex, Pb treatment, prenatal stress and day (PND0 and PND6) for each region and histone marker separately (hippocampus and frontal cortex). Because of the extensive effects of sex and interactions of sex with other variables, subsequent analyses were undertaken using Pb, stress and day separately for each histone marker in each brain region for each sex. This allowed the assessment of impact of developmental period of measurement of histone changes and made characterization of the effects of Pb and/or PS clearer for each time point for each sex. Analyses were undertaken using JMP Pro11. Subsequent post-hoc tests (Fisher’s least protected difference tests) were carried out as appropriate depending upon outcomes of main effects or interaction effects. Data are presented as mean ± S.E. While not continuous outcomes, data across treatment groups are presented in a linear rather than bar fashion as it provides easier visualization of outcomes.
3. RESULTS
3.1 Dam and Offspring Weights and Litter Size
Body weights of dams were collected at 3 different time points (gestational days 4, 10 and 17) over the course of gestation. No significant differences in body weight were observed until day 17, when a main effect of stress was found (F=4.53, p<0.0001). This reflected a small decrement in body weight of stressed dams (approximately 3 gm), with mean ± SE values of 32.73 ± 0.62, 29.75 ± 0.69, 33 ± 0.61 and 30.19 ± 0.64 grams for the 0-NS, 0-PS, 100-NS and 100-PS groups, respectively.
Litter weight at birth did not differ significantly in response to treatment conditions (mean ± SE values of 7.43 ± 0.44, 8.11 ± 0.53, 8.63 ±0.43 and 8.5 ± 0.45 grams for the 0-NS, 0-PS, 100-NS and 100-PS groups, respectively. When normalized to litter size, a small decrement in response to PS was found in statistical analyses (F=2.16, p=0.034), where group mean ± SE values of weight/pup were 1.36 ± 0.039, 1.25 ± 0.046, 1.36 ± 0.038 and 1.29 ± 0.04 for the 0-NS, 0-PS, 100-NS and 100-PS groups, respectively. Total litter sizes did not differ significantly by any of the treatments, with group mean ± SE values of 5.6 ± 0.4, 6.35 ± 0.47, 6.63 ± 0.39 and 6.77 ± 0.41 for the 0-NS, 0-PS, 100-NS and 100-PS groups, respectively.
3.2 Blood Lead Concentrations
The blood Pb levels of the dams in the 0-NS and 0-PS groups were comparable (0.22 ± 0.07 µg/dl and 0.23 ± 0.84 µg/dl, respectively), as were the blood Pb levels of the dams in the 100-NS and 100-PS groups (12.61 ± 1.11 µg/dl and 13.76 ± 1.40 µg/dl, respectively). Pups with water only mothers had blood lead levels averaging 0.37 ug/dl (±0.3 S.D.; N = 7), while pups with dams drinking 100 ppm lead water had blood lead levels of 10.2 ug/dl (±1.3 S.D.; N + 9). Blood lead levels were not differentially influenced by prenatal stress.
3.3 Hippocampal Post-Translational Histone Modifications
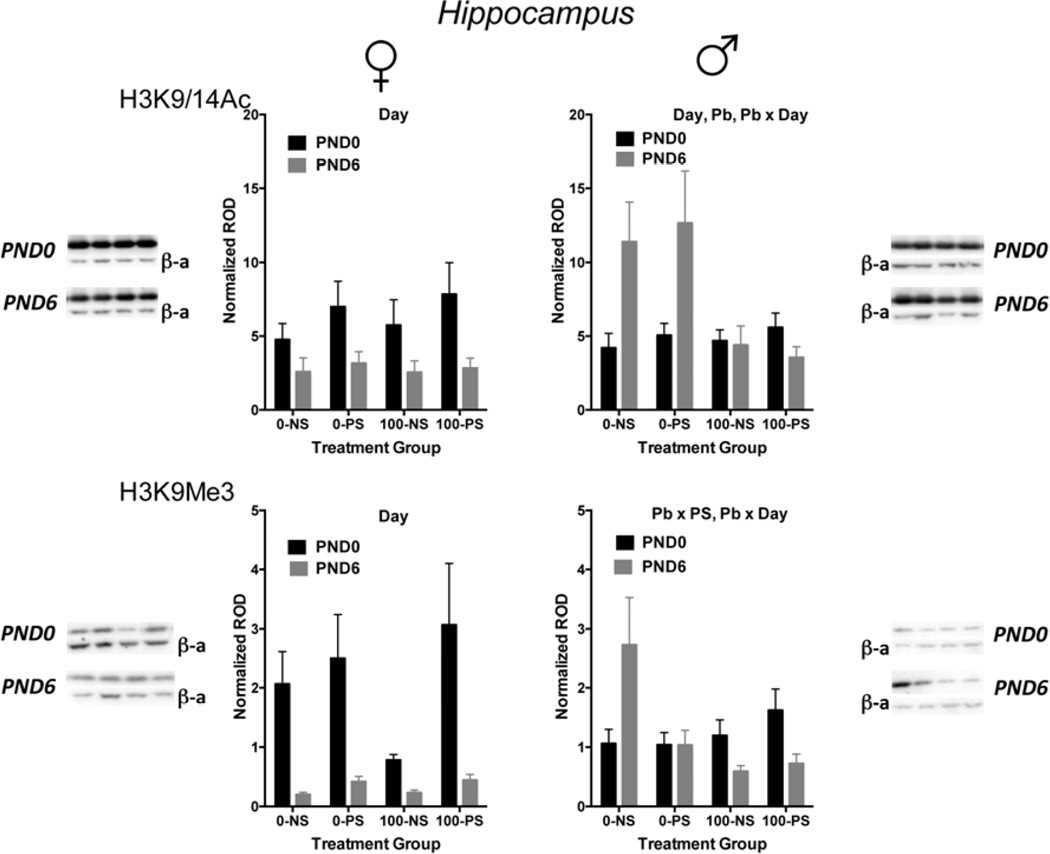
Figure 1 depicts the changes in H3K9/14Ac (top row) and H3K9Me3 (bottom row) in relation to treatment conditions for females (left column) and males (right column) at PND0 and PND6. In females, levels of both histones were reduced between PND0 and PND6 by approximately 50% in the case of H3K9/14Ac (main effect of day: F(1,68=13.20, p=0.0005), and by almost 10-fold in the case of H3K9Me3 (main effect of day: F(1,62)=17.71, p<0.0001). However, there were no effects of Pb or of PS or any associated interaction despite an apparent reduction in the 100-NS group for H3K9Me3 levels at PND0.
FIGURE 1.
Mean ± S.E. normalized relative optical density (ROD) of H3K9/14Ac (top row) and H3K9Me3 (bottom row) in females (left column) and males (right column) at PND0 (closed circles) and at PND6 (open circles) in hippocampus for each of the indicated treatment groups (0-NS = no Pb, non-stress; 0-PS = no Pb + prenatal stress; 100-NS = 100 ppm Pb, non-stress; 100-PS = 100 ppm Pb + prenatal stress). Sample sizes range from 7–13. Results of statistical analysis: Day=significant main effect of Day, Pb=significant main effect of Pb; PS=significant main effect of PS; Pb × Day=significant interaction of Pb by Day; Pb × PS=significant interaction of Pb by PS. For representative gel images associated with each graph, PND0 is the top set, PND6 is the bottom set. Within each set of gel images, H3K9/14Ac or H3K9Me3 is the top row and the beta actin (β-a) is the bottom row. The lanes are in the same order as the treatment groups on the graph.
In contrast to females, levels of these post-translational histone modifications increased between PND0 and PND6 in males (cf. PND0 vs. PND6 for the 0-NS group) and, further, were modified by Pb and/or stress. In the case of H3K9/14Ac, Pb exposure effects significantly differed by developmental time point, with no effects at PND0, but greater than 50% reductions in response to Pb at PND6 (main effect of Pb: F(1,74)=6.72, p=0.0115; Pb by day: F(1,74)=8.65, p=0.004). In the case of H3K9Me3 post-translational histone modifications, there were no significant changes in response to either Pb or PS at PND0. At PND6, however, there was a significant reduction in response to Pb (main effect of Pb: F(1,33)=7.123, p=0.0117) and a nearly significant interaction of Pb by PS (F(1,33)=3.96, p=0.055). Indeed, comparisons of treatment groups to control at PND6 revealed significantly lower values of each relative to 0-NS control (post hoc p values: 0-PS=0.0121, 100-NS =0.0027, 100-PS=0.0028).
3.4 Frontal Cortex Post-Translational Histone Modifications
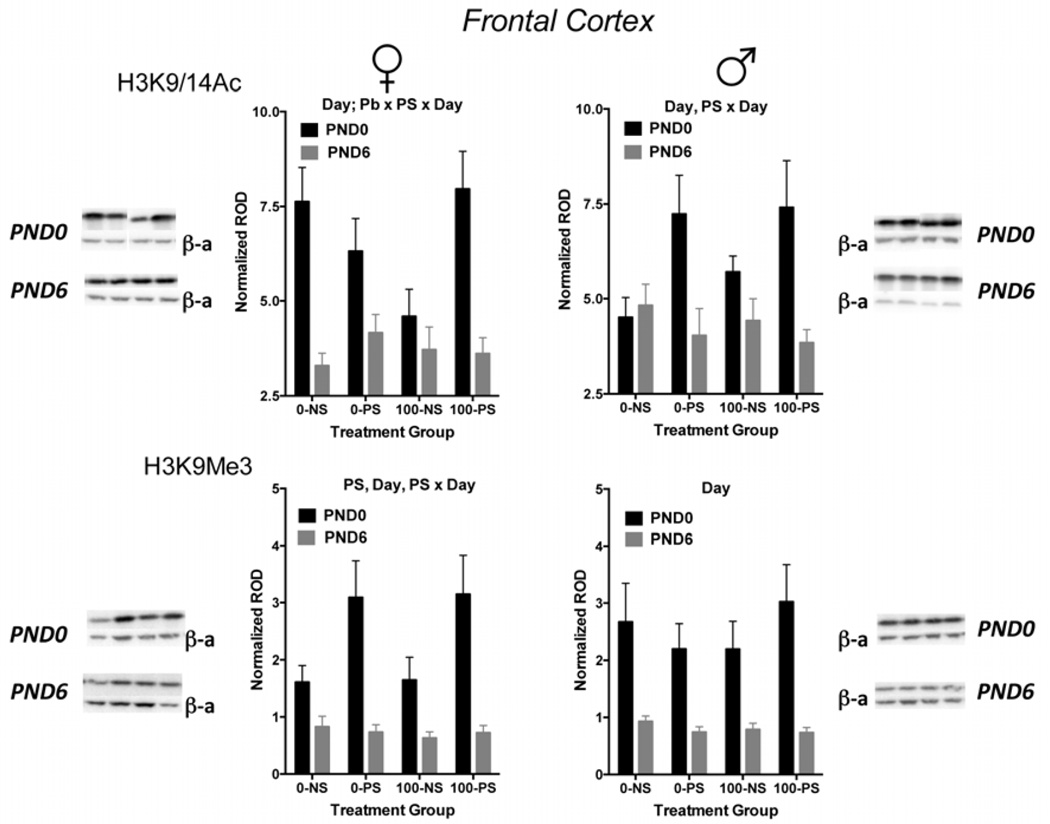
In contrast to the sex-related differences in the developmental trajectory of post-translational histone modifications in hippocampus, reductions in levels of both H3K9/14Ac (females only) and H3K9Me3 were seen in frontal cortex between PND0 and PND6 in both sexes (Figure 2). In females, H3K9/14Ac modifications levels were reduced by 57% in the 0-NS group (main effect of day: F(1,61,=32.95, p<0.0001) and for H3K9Me3 by approximately 49% (F(1,64)=31.58, p<0.0001). While no differences were seen between PND0 and PND6 in levels of H3K9/14Ac when examined in 0-NS groups in males, reductions of approximately 65% were found between PND0 and PND6 in levels of H3K9Me3 (main effect of day: F(1,69)=37.12, p<0.0001) (Figure 2).
FIGURE 2.
Mean ± S.E. normalized relative optical density (ROD) of H3K9/14Ac (top row) and H3K9Me3 (bottom row) in females (left column) and males (right column) at PND0 (closed circles) and at PND6 (open circles) in frontal cortex for each of the indicated treatment groups (0-NS = no Pb, non-stress; 0-PS = no Pb + prenatal stress; 100-NS = 100 ppm Pb, non-stress; 100-PS = 100 ppm Pb + prenatal stress). Sample sizes range from 7–10. Results of statistical analysis: Day=significant main effect of day, Pb=significant main effect of Pb; PS=significant main effect of PS; PS×Day=significant interaction of PS by Day; Pb×PS×Day=significant 3-way interaction of Pb by PS by Day. For representative gel images associated with each graph, PND0 is the top set, PND6 is the bottom set. Within each set of gel images, H3K9/14Ac or H3K9Me3 is the top row and the beta actin (β-a) is the bottom row. The lanes are in the same order as the treatment groups on the graph.
Histone levels in frontal cortex were also influenced by Pb and/or prenatal stress (Figure 2), and these effects were more evident at PND0 than PND6 in both sexes in contrast to the PND6 effects of Pb ± PS in males in the hippocampus. In the case of frontal cortex H3K9/14Ac in females, an interaction of Pb by PS by day was confirmed in the statistical analysis (F(1,61)=7.653, p=0.0075). Subsequent post-hoc testing revealed that while there were no significant effects of Pb or of PS on PND6, there was a significant approximately 50% reduction in response to Pb alone (0-NS vs. 100-NS, p=0.039). Despite the reduction in the 100-NS group, and a trend towards reduction in response to PS alone (0-PS), the impact of combined Pb and PS (100-PS) precluded any changes that would have occurred to either factor alone. In males, PS increased levels of H3K9/14Ac by approximately 60% at PND0, but, again these effects were no longer evident at PND6 (prenatal stress by day: F(1,63)=7.536, p=0.008).
In contrast to Pb-induced reductions at PND0 in females in H3K9/14Ac, levels of H3K9Me3 were nearly doubled in response to PS at PND0 (stress by day: F(1,64)=6.5, p=0.0132), but these effects had disappeared by PND6. Levels of H3K9Me3 were not influenced by either Pb or PS or the combination at either PND0 or PND6 in males (Figure 2).
4. DISCUSSION
Epigenetic changes in response to a stressor can persist long after the stressor has ended and can underlie persisting functional changes in the brain. In this study, we examined the effects of Pb and PS on H3K9/14Ac and H3K9Me3 levels in two brain regions known to be sensitive to the effects of Pb and PS, frontal cortex and hippocampus, in male and female offspring at PND0 and PND6, two time points previously suggested to show different sexually dimorphic effects on these two histone marks (Tsai et al., 2009). Results showed examples of sex-dependent differences in both levels of histone modifications and the developmental trajectory of changes in these levels. For example, in the hippocampus, there were no sexually dimorphic effects at either histone mark at PND0, but at PND6, expression of both H3K9/14Ac and H3K9Me3 were greater in males than in females, as levels had decreased in females but increased in males between PND0 and PND6. In frontal cortex, sexually dimorphic expression of H3K9/14Ac (associated with gene activation) was observed at PND0, with significantly higher expression in females compared to males. In contrast, at PND6, expression of H3K9/14Ac was higher in males compared to females, consistent with reductions in levels between PND0 and PND6 in females only. Expression of H3K9Me3 (associated with gene silencing and heterochromatin formation) in the frontal cortex was not different in males and females at either PND0 or PND6.
These data generally support the hypothesis that histone modifications are sexually dimorphic in the developing mouse brain (Tsai et al., 2009), but are not in complete agreement with previous findings. Tsai et al. (Tsai et al., 2009) reported higher levels of H3K9/14Ac in males at E18 and PND0 and this difference no longer existed at PND6. Higher levels of H3K9Me3 were also found in males at both PND0 and PND6, but no sex differences were observed earlier at E18 (Tsai et al., 2009). Differences in results between our study and the prior one could be related to methodological differences: we examined frontal cortex and hippocampus tissues separately, whereas Tsai et al. ((Tsai et al., 2009)) analyzed combined samples of cortex (region not specified) and hippocampus. As histone modifications appear to be brain region specific (Tsai et al., 2009; Stadler et al., 2005; Graff et al., 2012), results could differ based on the precise brain regions examined. Additionally, in the prior study, western blots were normalized to total H4 (which did not differ between males and females at any time point) whereas our data were normalized to beta actin. It is unlikely that changes in H3K9/14ac and H3K9me3 levels observed presently were related to changes in total H3 as H3K9/14ac and H3K9me3 levels did not always change in unison within a treatment group, as would be expected if the data reflected changes in total H3 and not changes in these specific marks. Thus, although we did not measure total H3 (or H4) levels, we do not believe that our experimental manipulations altered total H3 levels within a sex and an experimental group. Other potential differences that could impact the comparability of data across studies are the source of the animals and their diets. Although it is not possible from our data to draw firm conclusions regarding timing of any developmental shifts in sex differences in acetylation or methylation, at least for the histone modifications studied, sexual dimorphism in H3K9Me3 and H3K9/14Ac expression in the hippocampus appears to begin later, whereas sexual dimorphism in H3K9/14Ac in frontal cortex appears earlier but shifts over time. These changes in histone acetylation and trimethylation would be expected to modify the regulation of gene expression involved in various aspects of development in these brain regions.
With respect to the impact of developmental Pb and/or PS on these histone marks, it was notable that only males showed sensitivity to such changes in hippocampus, with reductions at PND0 in response to Pb alone in levels of H3K9/14Ac, and in response to both Pb alone and PS alone in levels of H3K9Me3. Further, these were seen only at PND6, and were not present at PND0. While behavioral functions associated with these histone changes are not yet fully understood, it is notable that levels of H3K9/14Ac were found to be increased in the dorsal raphe nucleus of male rats that exhibited less resilience in response to a chronic social defeat stress experience (Kenworthy et al., 2014). Recently, a role of this histone modification was reported in epigenetic regulation of presenilin 1 and 2 in mouse cerebral cortex (Kumar and Thakur, 2015), proteases that are crucial for brain development (Kumar and Thakur, 2014). Further, hippocampal H3 histone methylation was found to be influenced by acute and chronic stress in adult male rats, including increases in levels of H3K9Me3 in dentate gyrus and CA1 in response to acute stress, increased levels following 7 days of restraint stress, and reduced levels in the dentate gyrus in response to a 21 day chronic restraint stress (Hunter et al., 2009). Thus our findings are consistent with prior reports of both marks being associated with stress, while this is the first report of changes in these marks associated with developmental Pb exposure and assessed in the developing brain. Previously, Luo et al. (2014) reported that extended exposure to Pb (gestation through postnatal day 60) resulted in elevated acetylate histone H3 levels in the adult hippocampus. However, these animals had also been tested in free and forced open field exploration which may have influenced the expression of this epigenetic mark.
In the frontal cortex, significant changes in expression of H3K9Me3 and H3K9/14Ac due to our experimental manipulations were restricted to the PND0 time point. There was an interaction between Pb and prenatal stress on H3K9/14Ac expression in females whereas in males, only prenatal stress influenced (increased) H3K9/14Ac expression. H3K9Me3 levels were significantly increased by prenatal stress in females while H3K9Me3 levels in males were unaffected by the experimental manipulations. In contrast, in the hippocampus, significant changes in expression of H3K9Me3 and H3K9/14Ac due to our experimental manipulations were restricted to the PND6 time point. These findings suggest that developmental processes occurring in the frontal cortex at PND0 (and potentially earlier) may be particularly susceptible to effects of PS and Pb, resulting in aberrant modulation of gene expression through the affected epigenetic mechanisms. Even though modified expression of H3K9/14ac and H3K9Me3 was not detected in the frontal cortex at PND6, it is possible that the modulation of gene expression through control of histone acetylation and methylation that occurred earlier may have significantly changed gene expression profiles of genes, leading to altered gene function at later stages of development, consistent with the concept of a fetal basis of adult disease.
The frontal cortex and hippocampus play important roles in controlling a variety of cognitive functions and social behaviors many of which are sexually dimorphic. In the hippocampus, the influences of Pb and PS were observed primarily at PND6 and were greater in males than in females. Sex differences in hippocampal morphology have been reported in rats and suggested to be related to a greater degree of neurogenesis in males during the first postnatal week, although in mice, this effect may differ by strain (Tabibnia et al., 1999; Zhang et al., 2008). Thus it is possible that PS and Pb may exert greater effects on the hippocampus at a time when sexually dimorphic processes are maturing and this effect may be greater in males than in females.
5. CONCLUSIONS
In conclusion, sex differences in histone modifications that manifest early in development may be involved in the expression of sex-dependent differences in cognitive functions observed later in life. Disruptions of these processes by developmental Pb exposure, PS, and the combination of these factors may underlie some of the sex-dependent neurobehavioral differences observed in these animals in adulthood (Virgolini et al., 2008a; Cory-Slechta et al., 2010; Virgolini et al., 2006; Virgolini et al., 2008b). The studies described here are a necessary first step in exploring the role of histone modifications in the response of specific brain regions to Pb exposure and prenatal stress. Additional work is now needed to extend these findings and examine effects of Pb exposure and prenatal stress on the dynamic modulation of histone modifications on specific genes of interest or, using ChIP-seq, to identify genome-wide profiles of histone modifications resulting from these environmental perturbations.
Highlights.
H3K9/14Ac and H3K9Me3 are suggested to be sexually dimorphic histone modifications.
H3K9/14Ac and H3K9Me3 were examined for modification by lead and prenatal stress
Sex-dependent differences were seen in both histone modifications
Effects of lead and stress were sex and brain-region-dependent.
Acknowledgments
This work was supported by NIH grant R01 ES021534-01 (Cory-Slechta, D.A.; Schneider, J.S. multi-PIs).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicts of interest to declare.
REFERENCES
- Auger AP, Auger CJ. Epigenetic turn ons and turn offs: chromatin reorganization and brain differentiation. Endocrinology. 2011;152:349–353. doi: 10.1210/en.2010-0793. [DOI] [PubMed] [Google Scholar]
- Baranowska-Bosiacka I, Struzynska L, Gutowska I, Machalinska A, Kolasa A, Klos P, Czapski GA, Kurzawski M, Prokopowicz A, Marchlewicz M, Safranow K, Machalinski B, Wiszniewska B, Chlubek D. Perinatal exposure to lead induces morphological, ultrastructural and molecular alterations in the hippocampus. Toxicology. 2013;303:187–200. doi: 10.1016/j.tox.2012.10.027. [DOI] [PubMed] [Google Scholar]
- Barros VG, Berger MA, Martijena ID, Sarchi MI, Perez AA, Molina VA, Tarazi FI, Antonelli MC. Early adoption modifies the effects of prenatal stress on dopamine and glutamate receptors in adult rat brain. J Neurosci Res. 2004;76:488–496. doi: 10.1002/jnr.20119. [DOI] [PubMed] [Google Scholar]
- Berger MA, Barros VG, Sarchi MI, Tarazi FI, Antonelli MC. Long-term effects of prenatal stress on dopamine and glutamate receptors in adult rat brain. Neurochemical research. 2002;27:1525–1533. doi: 10.1023/a:1021656607278. [DOI] [PubMed] [Google Scholar]
- Clapcote SJ, Roder JC. Simplex PCR assay for sex determination in mice. Biotechniques. 2005;38 doi: 10.2144/05385BM05. 702, 704, 706. [DOI] [PubMed] [Google Scholar]
- Cory-Slechta DA, O'Mara DJ, Brockel BJ. Nucleus accumbens dopaminergic mediation of fixed interval schedule-controlled behavior and its modulation by low-level lead exposure. Journal of Pharmacology and Experimental Therapeutics. 1998;286:794–805. [PubMed] [Google Scholar]
- Cory-Slechta DA, O'Mara DJ, Brockel BJ. Learning versus performance impairments following regional administration of MK-801 into nucleus accumbens and dorsomedial striatum. Behav Brain Res. 1999;102:181–194. doi: 10.1016/s0166-4328(99)00015-7. [DOI] [PubMed] [Google Scholar]
- Cory-Slechta DA, Stern S, Weston D, Allen JL, Liu S. Enhanced learning deficits in female rats following lifetime Pb exposure combined with prenatal stress. Toxicological Sciences. 2010;117:427–438. doi: 10.1093/toxsci/kfq221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cory-Slechta DA, Virgolini MB, Liu S, Weston D. Enhanced stimulus sequence-dependent repeated learning in male offspring after prenatal stress alone or in conjunction with lead exposure. Neurotoxicology. 2012;33:1188–1202. doi: 10.1016/j.neuro.2012.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cory-Slechta DA, Virgolini MB, Rossi-George A, Thiruchelvam M, Lisek R, Weston D. Lifetime consequences of combined maternal lead and stress. Basic & clinical pharmacology & toxicology. 2008;102:218–227. doi: 10.1111/j.1742-7843.2007.00189.x. [DOI] [PubMed] [Google Scholar]
- Cory-Slechta DA, Virgolini MB, Thiruchelvam M, Weston DD, Bauter MR. Maternal stress modulates the effects of developmental lead exposure. Environ Health Perspect. 2004a;112:717–730. doi: 10.1289/ehp.6481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cory-Slechta DA, Virgolini MB, Thiruchelvam M, Weston DD, Bauter MR. Maternal stress modulates the effects of developmental lead exposure. Environ Health Perspect. 2004b;112:717–730. doi: 10.1289/ehp.6481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giesbrecht GF, Campbell T, Letourneau N. Sexually dimorphic adaptations in basal maternal stress physiology during pregnancy and implications for fetal development. Psychoneuroendocrinology. 2015;56:168–178. doi: 10.1016/j.psyneuen.2015.03.013. [DOI] [PubMed] [Google Scholar]
- Gore AC. Developmental programming and endocrine disruptor effects on reproductive neuroendocrine systems. Front Neuroendocrinol. 2008;29:358–374. doi: 10.1016/j.yfrne.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff J, Woldemichael BT, Berchtold D, Dewarrat G, Mansuy IM. Dynamic histone marks in the hippocampus and cortex facilitate memory consolidation. Nat Commun. 2012;3:991. doi: 10.1038/ncomms1997. [DOI] [PubMed] [Google Scholar]
- Hu F, Xu L, Liu ZH, Ge MM, Ruan DY, Wang HL. Developmental lead exposure alters synaptogenesis through inhibiting canonical Wnt pathway in vivo and in vitro. PLoS One. 2014;9:e101894. doi: 10.1371/journal.pone.0101894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter RG, McCarthy KJ, Milne TA, Pfaff DW, McEwen BS. Regulation of hippocampal H3 histone methylation by acute and chronic stress. Proc Natl Acad Sci U S A. 2009;106:20912–20917. doi: 10.1073/pnas.0911143106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenworthy CA, Sengupta A, Luz SM, Ver Hoeve ES, Meda K, Bhatnagar S, Abel T. Social defeat induces changes in histone acetylation and expression of histone modifying enzymes in the ventral hippocampus, prefrontal cortex, and dorsal raphe nucleus. Neuroscience. 2014;264:88–98. doi: 10.1016/j.neuroscience.2013.01.024. [DOI] [PubMed] [Google Scholar]
- Kolodkin MH, Auger AP. Sex difference in the expression of DNA methyltransferase 3a in the rat amygdala during development. J Neuroendocrinol. 2011;23:577–583. doi: 10.1111/j.1365-2826.2011.02147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Thakur MK. Analysis of Presenilin 1 and 2 interacting proteins in mouse cerebral cortex during development. International Journal of Developmental Neuroscience. 2014;38:138–146. doi: 10.1016/j.ijdevneu.2014.08.010. [DOI] [PubMed] [Google Scholar]
- Kumar A, Thakur MK. Epigenetic regulation of presenilin 1 and 2 in the cerebral cortex of mice during development. Developmental Neurobiology. 2015;75:1165–1173. doi: 10.1002/dneu.22274. [DOI] [PubMed] [Google Scholar]
- Li N, Zhang P, Qiao M, Shao J, Li H, Xie W. The effects of early life lead exposure on the expression of P2X7 receptor and synaptophysin in the hippocampus of mouse pups. Journal of Trace Elements in Medicine and Biology. 2015;30:124–128. doi: 10.1016/j.jtemb.2014.12.001. [DOI] [PubMed] [Google Scholar]
- Li N, Zhao G, Qiao M, Shao J, Liu X, Li H, Li X, Yu Z. The effects of early life lead exposure on the expression of insulin-like growth factor 1 and 2 (IGF1, IGF2) in the hippocampus of mouse pups. Food and Chemical Toxicology. 2014;63:48–52. doi: 10.1016/j.fct.2013.10.037. [DOI] [PubMed] [Google Scholar]
- Luo M, Yi X, Cai R, Tang Y, Ge M-M, Liu Z-H, Xu L, Hu F, Ruan D-Y, Wang H-L. Epigenetic histone modification regulates developmental lead exposure induced hyperactivity in rats. Toxicology Letters. 2014;225:78–85. doi: 10.1016/j.toxlet.2013.11.025. [DOI] [PubMed] [Google Scholar]
- Martinez-Tellez RI, Hernandez-Torres E, Gamboa C, Flores G. Prenatal stress alters spine density and dendritic length of nucleus accumbens and hippocampus neurons in rat offspring. Synapse. 2009;63:794–804. doi: 10.1002/syn.20664. [DOI] [PubMed] [Google Scholar]
- McCarthy MM, Arnold AP, Ball GF, Blaustein JD, De Vries GJ. Sex differences in the brain: the not so inconvenient truth. J Neurosci. 2012;32:2241–2247. doi: 10.1523/JNEUROSCI.5372-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, Auger AP, Bale TL, De Vries GJ, Dunn GA, Forger NG, Murray EK, Nugent BM, Schwarz JM, Wilson ME. The epigenetics of sex differences in the brain. J Neurosci. 2009;29:12815–12823. doi: 10.1523/JNEUROSCI.3331-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal AP, Stansfield KH, Guilarte TR. Enhanced nitric oxide production during lead (Pb(2)(+)) exposure recovers protein expression but not presynaptic localization of synaptic proteins in developing hippocampal neurons. Brain Res. 2012;1439:88–95. doi: 10.1016/j.brainres.2011.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi-George A, Virgolini MB, Weston D, Thiruchelvam M, Cory-Slechta DA. Interactions of Lifetime Lead Exposure and Stress: Behavioral; Neurochemical and HPA Axis Effects. Neurotoxicology. 2011:83–99. doi: 10.1016/j.neuro.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner MK, Anway MD, Savenkova MI, Gore AC, Crews D. Transgenerational epigenetic programming of the brain transcriptome and anxiety behavior. PLoS One. 2008;3:e3745. doi: 10.1371/journal.pone.0003745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler F, Kolb G, Rubusch L, Baker SP, Jones EG, Akbarian S. Histone methylation at gene promoters is associated with developmental regulation and regionspecific expression of ionotropic and metabotropic glutamate receptors in human brain. J Neurochem. 2005;94:324–336. doi: 10.1111/j.1471-4159.2005.03190.x. [DOI] [PubMed] [Google Scholar]
- Stansfield KH, Pilsner JR, Lu Q, Wright RO, Guilarte TR. Dysregulation of BDNF-TrkB signaling in developing hippocampal neurons by Pb(2+): implications for an environmental basis of neurodevelopmental disorders. Toxicological Sciences. 2012;127:277–295. doi: 10.1093/toxsci/kfs090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabibnia G, Cooke BM, Breedlove SM. Sex difference and laterality in the volume of mouse dentate gyrus granule cell layer. Brain Res. 1999;827:41–45. doi: 10.1016/s0006-8993(99)01262-7. [DOI] [PubMed] [Google Scholar]
- Tsai HW, Grant PA, Rissman EF. Sex differences in histone modifications in the neonatal mouse brain. Epigenetics. 2009;4:47–53. doi: 10.4161/epi.4.1.7288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virgolini MB, Bauter MR, Weston DD, Cory-Slechta DA. Permanent alterations in stress responsivity in female offspring subjected to combined maternal lead exposure and/or stress. Neurotoxicology. 2006;27:11–21. doi: 10.1016/j.neuro.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Virgolini MB, Rossi-George A, Lisek R, Weston DD, Thiruchelvam M, Cory-Slechta DA. CNS effects of developmental Pb exposure are enhanced by combined maternal and offspring stress. Neurotoxicology. 2008a;29:812–827. doi: 10.1016/j.neuro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virgolini MB, Rossi-George A, Weston D, Cory-Slechta DA. Influence of low level maternal Pb exposure and prenatal stress on offspring stress challenge responsivity. Neurotoxicology. 2008b;29:928–939. doi: 10.1016/j.neuro.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainstock T, Shoham-Vardi I, Glasser S, Anteby E, Lerner-Geva L. Fetal sex modifies effects of prenatal stress exposure and adverse birth outcomes. Stress. 2015;18:49–56. doi: 10.3109/10253890.2014.974153. [DOI] [PubMed] [Google Scholar]
- Wizemann TMaPM. Exploring the biological contributions to human health: Does sex matter? Washington, D.C: National Academies Press; 2001. [PubMed] [Google Scholar]
- Zhang JM, Konkle AT, Zup SL, McCarthy MM. Impact of sex and hormones on new cells in the developing rat hippocampus: a novel source of sex dimorphism? Eur J Neurosci. 2008;27:791–800. doi: 10.1111/j.1460-9568.2008.06073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zohar I, Dosoretz-Abittan L, Shoham S, Weinstock M. Sex dependent reduction by prenatal stress of the expression of 5HT1A receptors in the prefrontal cortex and CRF type 2 receptors in the raphe nucleus in rats: reversal by citalopram. Psychopharmacology (Berl) 2015;232:1643–1653. doi: 10.1007/s00213-014-3803-z. [DOI] [PubMed] [Google Scholar]