Abstract
Women are more likely than men to suffer from post-traumatic stress disorder (PTSD) and major depression. In addition to their sex bias, these disorders share stress as an etiological factor and hyperarousal as a symptom. Thus, sex differences in brain arousal systems and their regulation by stress could help explain increased vulnerability to these disorders in women. Here we review preclinical studies that have identified sex differences in the locus coeruleus (LC)-norepinephrine (NE) arousal system. First, we detail how structural sex differences in the LC can bias females towards increased arousal in response to emotional events. Second, we highlight studies demonstrating that estrogen can increase NE in LC target regions by enhancing the capacity for NE synthesis, while reducing NE degradation, potentially increasing arousal in females. Third, we review data revealing how sex differences in the stress receptor, corticotropin releasing factor 1 (CRF1), can increase LC neuronal sensitivity to CRF in females compared to males. This effect could translate into hyperarousal in women under conditions of CRF hypersecretion that occur in PTSD and depression. The implications of these sex differences for the treatment of stress-related psychiatric disorders are discussed. Moreover, the value of using information regarding biological sex differences to aid in the development of novel pharmacotherapies to better treat men and women with PTSD and depression also is highlighted.
Keywords: corticotropin releasing hormone, sex differences, arousal, post-traumatic stress disorder, major depression, noradrenergic
1. Introduction
Women are roughly twice as likely as men to suffer from certain psychiatric disorders, such as posttraumatic stress disorder (PTSD) and major depression (Breslau, 2002; Freedman et al., 2002; Kessler, 2003; Kessler et al., 2012; Tolin and Foa, 2006). In addition to their shared sex bias, PTSD and depression are characterized by symptoms of hyperarousal that include: irritability, restlessness, agitation, sleep disturbance, and an inability to concentrate (American Psychiatric Association, 2013). There is some evidence that these hyperarousal symptoms are more pronounced in women. For example, women with these disorders suffer from insomnia more often than men (Hall et al., 2000; Kobayashi and Mellman, 2012). Additionally, ruminations, which are associated with high arousal states (Pedersen et al., 2011; Thomsen et al., 2003), are more common in depressed women than men (Mezo and Baker, 2012; Nolen-Hoeksema et al., 1999). One possible explanation for these data is that sex differences in brain arousal centers predispose women to disorders with hyperarousal as a core symptom. One candidate region for such sex differences is the locus coeruleus (LC), which regulates levels of arousal by releasing norepinephrine (NE) into forebrain regions (Aston-Jones and Cohen, 2005; Berridge and Waterhouse, 2003). A focus of this review will be to highlight preclinical literature revealing mechanisms by which estrogens can increase NE levels in LC target regions, perhaps contributing to hyperarousal in females.
This review also will detail the literature revealing sex differences in LC regulation by stress. Clinically, stress is of interest because it is linked to the etiology of PTSD and depression. In particular, the development of these disorders is attributed, at least in part, to the hypersecretion of the stress-related neuropeptide, corticotropin releasing factor (CRF; Hauger et al., 2009; Hauger et al., 2012; Nemeroff, 1996). Although CRF is primarily known for its role in initiating the hypothalamic pituitary adrenal axis in response to stress (Vale et al., 1981), it is the central effects of CRF that are thought to regulate behavioral stress responses and contribute to the symptoms of these “stress-related” psychiatric disorders (Bale and Vale, 2004; Bangasser and Kawasumi, 2015; Hauger et al., 2009; Owens and Nemeroff, 1991; Valentino and Van Bockstaele, 2002). There is evidence that the LC, in particular, is a target of CRF hypersecretion in depressed patients (Austin et al., 2003). Moreover, it has been proposed that subtypes of depression with symptoms of hyperarousal are attributable to a combined high CRF and high NE state (Gold and Chrousos, 1999; Gold and Chrousos, 2002; Koob, 1999). These studies, along with higher rates of stress-related psychiatric disorders in women, have prompted basic research revealing increased female LC neuronal sensitivity to CRF and the mechanisms underlying this effect (Bangasser et al., 2010; Bangasser et al., 2013; Curtis et al., 2006). These studies will be discussed, as will their implications for facilitating the treatment of stress-related psychiatric disorders in both men and women.
2. Sex differences in LC structure
2.1 Sex difference in LC neuronal number
The LC comprises a cluster of noradrenergic containing neurons located in the rostral rhombencephalic tegmental area within the pons (Aston-Jones, 2004). Despite its relatively small size (~2200 neurons in the adult rat), the LC has a wide efferent projection system that allows for regulation of brain regions throughout the neuroaxis (Aston-Jones, 2004; Guillamon et al., 1988; Swanson and Hartman, 1975). In fact, the LC is the major source of NE for the forebrain and the only NE source for the cortex and hippocampus (Abercrombie et al., 1988; Aston-Jones, 2004; Morrison et al., 1978).
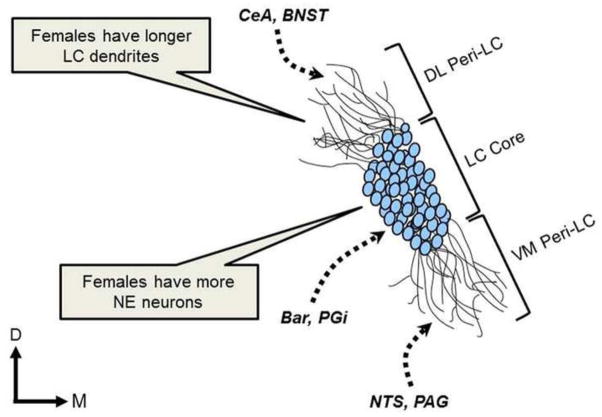
It has been reported that the LC of adult female rats is larger than that of adult males (Guillamon et al., 1988; Luque et al., 1992; Pinos et al., 2001), a difference that may affect the regulation of target regions (Fig. 1). This increase in size occurs because female rats have more NE containing neurons than male rats (Guillamon et al., 1988; Luque et al., 1992; Pinos et al., 2001). This sex difference emerges during development and, unlike sex differences in other structures (e.g., the sexually dimorphic nucleus of the preoptic area and the bed nucleus of the stria terminalis; (Chung et al., 2000; Davis et al., 1996), it is not attributable to a sex difference in apoptosis (Pinos et al., 2001). Instead, it is primarily attributed to LC neurogenesis that continues during puberty in females but not in males (Pinos et al., 2001). It is not fully understood how this sex difference is established or why the neurogenesis stops after puberty in females, but the presence of ovarian hormones throughout development is thought to play a role (Guillamon et al., 1988; Pinos et al., 2001). There is evidence for a different mechanism that masculinizes the male LC, involving prenatal testosterone (Pinos et al., 2001; Rodriguez-Zafra et al., 1993). Future studies are needed to further clarify the role of gonadal hormones in establishing sex differences in LC development.
Figure 1.
This image depicts the LC core and peri-LC regions, along with important LC afferents and structural sex differences in the rat LC. It has been reported that the female LC contains more noradrenergic neurons than the male LC. Additionally, the dendrites of female LC neurons are larger and more complex, extending further in the peri-LC region. This structure increases the probability that female LC dendrites will contact afferents that terminate in the peri-LC regions. Bar, Barrington’s nucleus; BNST, bed nucleus of the stria terminalis; CeA, central nucleus of the amygdala; DL Peri-LC; dorsolateral pericoerulear locus coeruleus; LC Core, locus coeruleus core; NTS, nucleus of the solitary tract; PAG, periaqueductal gray; PGi, nucleus paragigantoceullaris; VM Peri-LC, ventromedial pericoerulear locus coeruleus
The sex difference in rodent LC size and neuronal number is dependent on strain (Garcia-Falgueras et al., 2005; Garcia-Falgueras et al., 2006). Specifically, sex differences in LC size are observed in the ancestral Wistar strain, but not in the Long-Evans strain, an effect attributed to a loss of the sex difference as a result of inbreeding for other traits (Garcia-Falgueras et al., 2005; Garcia-Falgueras et al., 2006). Currently, behavioral data on the consequences of sex differences in LC size in Wistar, but not Long-Evans rats is lacking, but future studies addressing this issue could illuminate the consequences of a larger LC. Other studies suggest that the sex difference in LC size may be subregion specific, as the dorsal ascending projection zone of the LC is larger in male than female Sprague–Dawley rats (Babstock et al., 1997)
The implications of these nuanced sex differences in LC rodent size for human psychiatric diseases are unclear. Although, to our knowledge, no one has directly compared LC size in humans, two studies conducted separately by the same group reported the LC size as ~ 15,700 neurons in men and ~18,300 neurons in women, revealing a structural LC sex difference in humans (Busch et al., 1997; Ohm et al., 1997). These data suggest that the sex difference in LC size found in at least some rodent strains does, in fact, correspond to a similar phenomenon in humans. While the consequences of a larger LC in females is not entirely clear, it could increase female’s capacity for NE production and release.
2.2 Sex differences in LC dendritic morphology
In addition to sex differences in LC neuronal number, sex differences in the morphology of LC dendrites have also been identified. The majority of LC inputs innervate dendrites that extend far into the pericoerulear region (peri-LC; Shipley et al., 1996; Van Bockstaele et al., 2001). These dendrites, which expand into the ventromedial and dorsolateral peri-LC regions of female rats are denser than those of males (Bangasser et al., 2011). Further evidence for the complexity of female LC dendrites arises from morphological analysis of individual LC neurons, revealing that the LC dendritic trees of females are longer and more complex than those of males (Fig. 1; Bangasser et al., 2011). The more extensive dendrites in females appear to receive more synaptic input, as immunoreactivity for synaptophysin, a synaptic vesical protein, was greater in the LC of female rats than males, particularly in the dorsolateral peri-LC region (Bangasser et al., 2011). The sex difference in LC dendrites could bias the type of afferent information that the female LC receives. Although the information from the relatively few afferents that innervate the dendrites in the nuclear core of the LC (e.g., Barrington’s nucleus and the nucleus paragigantoceullaris; Aston-Jones et al., 1986; Valentino et al., 1996) is predicted to be similar in males and females, the longer and more complex dendritic trees found in females could increase contacts with afferents that synapse in the peri-LC region. This could translate into an increase in the amount of autonomic and nociceptive information coming into the ventromedial peri-LC from the nucleus of the solitary tract and the periaqueductal gray, respectively, that females receive (Van Bockstaele et al., 2001). Additionally, the sex difference in dendritic structure in the dorsolateral peri-LC could bias females to receive more CRF-containing afferents from limbic regions, including the central nucleus of the amygdala and the bed nucleus of the stria terminalis, which terminate in this region (Van Bockstaele et al., 1998; Van Bockstaele et al., 2001). This structure may be particularly relevant for the sex bias in stress-related psychiatric disorders, as it could provide a substrate for increased arousal in response to emotional events in females. In support of this idea, an imaging study found that noxious stimuli produced greater activation of the emotional-arousal circuit, which includes the amygdala and LC, in women when compared to men (Labus et al., 2008). Imaging studies examining the activation of this circuit following exposure to emotional stimuli in women and men could be designed to further test this hypothesis.
3. Regulation of NE synthesis, degradation, and receptors by estrogens
3.1 Estrogens increase NE in LC terminal regions
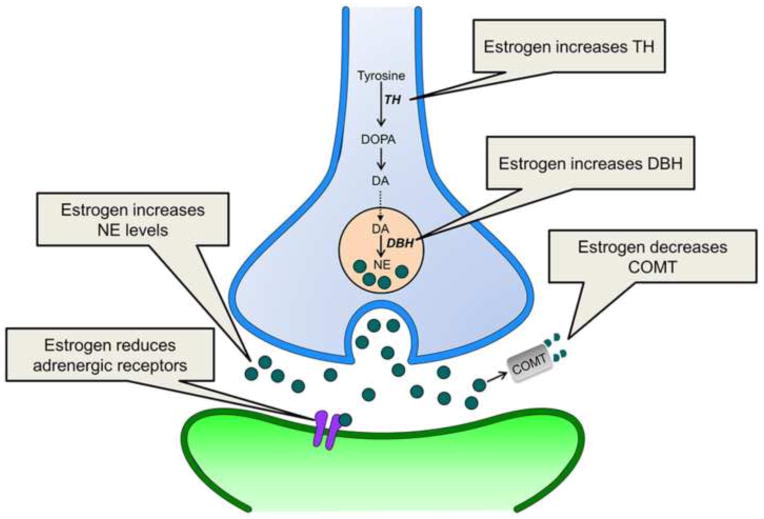
As noted, the LC has a wide efferent projection system through which it can release NE into the forebrain (Aston-Jones, 2004; Swanson and Hartman, 1975). It is through regulation of forebrain target regions that the LC alters states of arousal, attention, and vigilance (Chamberlain and Robbins, 2013; Sara, 2009; Szabadi, 2013). Thus, sex differences in LC-induced NE release could result in sex differences in these functions. There is evidence from microdialysis studies that ovarian hormones regulate NE levels in LC terminal regions (Fig. 2). For example, in the hypothalamus, NE levels change across the rat estrous cycle such that they are increased during the proestrus phase, when levels of ovarian hormones are higher (Selmanoff et al., 1976). This effect may be mediated by the LC as well as the A1 and A2 noradrenergic cell groups in the medulla that densely project to the hypothalamus (Aston-Jones, 2004). Estrogens appear to be key players in NE regulation because estradiol treatment increases norepinephrine levels in the ventral hippocampus, cortex, and hypothalamus of ovariectomized female rats (Alfinito et al., 2009; Lubbers et al., 2010). Effects in the hippocampus and cortex are mimicked by activation of either estrogen receptor subtype (ER), ERα and ERβ (Lubbers et al., 2010). Because both subtypes are expressed in the LC (Mitra et al., 2003; Shughrue et al., 1997), the increase of NE in target regions could result from direct estrogenic effects on this nucleus.
Figure 2.
This schematic illustrates how estrogen can regulate the NE system. Estrogen can increase NE levels by increasing NE synthesis within the LC neuron (blue) and reducing NE degradation. Estrogen also decreases adrenergic receptor (purple) levels on postsynaptic neurons (green), an effect that may be compensatory. DA, dopamine; DBH, dopamine β-hydroxylase; DOPA, L-dihydroxyphenylalanine; COMT, catechol-O-methyltransferase; NE, norepinephrine
3.2 Estrogens increase NE synthesis and decrease NE degradation
Ovarian hormones have been reported to increase NE levels in LC target regions via presynaptic modulation of NE release (Vathy and Etgen, 1988). This can occur through estrogen regulation of NE biosynthetic enzymes (Serova et al., 2002). For example, the expression of tyrosine hydroxylase (TH), which catalyzes the conversion of tyrosine to L-dihydroxyphenylalanine (DOPA), a precursor to NE, is increased by estradiol treatment of gonadectomized animals (Fig. 2; Maharjan et al., 2005; Pau et al., 2000; Serova et al., 2002; Serova et al., 2004). Given that TH is the rate limiting enzyme for catecholamine synthesis, estradiol-induced increases in TH could enhance the capacity for NE production (Serova et al., 2004). The effects of estrogens on TH appear to be limited to short-term estradiol treatments that mimic the preovulatory surge, as chronic estradiol treatment fails to alter TH expression in the LC (Serova et al., 2004; Tseng et al., 1997). This result then suggests that it is the cyclical nature of estrogen release that is critical, which is consistent with the finding that female rats have greater activation of TH neurons in the proestrus phase of their cycle when estrogens are higher (Maharjan et al., 2005). Although less studied, there also is evidence that estradiol treatment in ovariectomized female rats can increase the expression of dopamine β-hydroxylase (DBH), the enzyme that catalyzes the hydroxylation of dopamine into NE (Fig. 2; Serova et al., 2002). The mechanism by which estrogens regulate both TH and DBH is transcriptional (Maharjan et al., 2005; Serova et al., 2002; Serova et al., 2011). This regulation can occur through direct genomic effects of estrogens on estrogen responsive elements (EREs) found on the TH and DBH promoters (Maharjan et al., 2005; Serova et al., 2004). There is also evidence that estrogens act on plasma membrane ERs to initiate signaling cascades, which increase TH and DBH by regulating other elements on their promotor regions (Maharjan et al., 2005; Maharjan et al., 2010; Serova et al., 2011).
Another mechanism that could contribute to higher levels of NE in LC target regions involves gonadal hormone regulation of enzymes that degrade NE. Much of the research on this topic has focused on catechol-O-methyltransferase (COMT) because single nucleotide polymorphisms (SNPs) on the COMT gene have been associated with a range of psychiatric disorders that have sex differences in their prevalence and/or disease progression, including anxiety disorders (Domschke et al., 2007; Harrison and Tunbridge, 2008; Lewinsohn et al., 1998). Notably, there is a sex difference in COMT activity in human postmortem prefrontal cortex tissue such that men have higher COMT activity than women (Chen et al., 2004). Preclinical studies indicate that estrogen regulation could account for this sex difference (Fig. 2). In female rats, COMT levels are lowest in the proestrus cycle phase, indicating an inverse relationship between circulating estrogens and COMT (Parvez et al., 1978). In cell culture, estrogens decrease COMT activity and protein levels (Jiang et al., 2003), purportedly through the EREs on the COMT promotor (Xie et al., 1999). However, mRNA levels of COMT in human cortex are comparable between the sexes (Bray et al., 2003; Chen et al., 2004), suggesting that the sex difference in COMT activity in humans is not simply transcriptional, but may involve novel isoforms, cofactors, or interacting proteins (Harrison and Tunbridge, 2008). As noted, SNPs on the COMT gene are found in psychiatric disorders, and several studies have identified sex by genotype interactions (for review see, (Harrison and Tunbridge, 2008). The most common SNP implicated is the Val158Met polymorphism that alters COMT activity so that it is reduced in Met158 versus Val158 homozygous carriers (Chen et al., 2004). Several studies have found that the Met158 allele is associated with anxiety-related traits and disorders in women, particularly of Caucasian decent, but not men (Domschke et al., 2004; Domschke et al., 2007; Eley et al., 2003; Enoch et al., 2003; Olsson et al., 2005; Stein et al., 2005). As COMT degrades catecholamines in general, the specific neurotransmitter that contributes to anxiety in women with the Met158 allele is unclear. However, it is tempting to speculate that, at least in some women, the lower activity of COMT observed in females is exacerbated in carriers of the Met158 allele, and this potentiated reduction in activity increases NE in the prefrontal cortex to levels that contribute to greater hyperarousal symptoms.
3.3 Estrogens alter adrenergic receptors
In addition to estrogens’ regulation of NE synthesis and degradation, estrogens also can regulate adrenergic receptors. Most of the work on this topic has been conducted by Etgen and colleagues in the context of NE regulation of hypothalamic-mediated female reproductive behavior in rats (for review see, Etgen et al., 2001). In brief, estrogens shift the balance of NE receptor signaling away from β-adrenergic activation of the cAMP pathway, which inhibits luteinizing hormone (LH) and female reproductive behaviors, towards α1-adrenergic PLC-mediated signaling, which facilitates the LH surge and reproductive behaviors (Etgen et al., 1992; Etgen et al., 2001). Aside from its role in reproduction, estrogen regulation of adrenergic receptors in LC target regions implicated in arousal, attention, and vigilance, has received less attention. Yet, there is evidence that ovariectomized females treated with estrogen have reduced cortical postsynaptic α2-adrenoceptor binding and mRNA relative to their untreated counterparts (Ansonoff and Etgen, 2001; Karkanias et al., 1997, but see Shansky et al., 2009). Additionally, in cycling females, compared to ovariectomized females and intact males, β-adrenergic receptor binding is decreased in cortical membranes and there is less β-adrenergic receptor stimulated cAMP (Wagner and Davies, 1980). In the striatum—which primarily receives innervation from the A1 and A2 noradrenergic cell group (Aston-Jones, 2004)—conditions that reduce estrogens, such as menopause in women and ovariectomy in rats, increase β1-adrenergic receptor expression, while estrogen treatment of striatal neurons down-regulates β1-adrenergic receptor action (Meitzen et al., 2013). Taken together, these studies suggest that estrogens typically decrease adrenergic receptor expression and function in the cortex and striatum (Fig. 2). However, the consequences of this effect, and whether similar receptor regulation by estrogens occurs in other LC target regions, remain unclear.
Collectively, these studies reveal that estrogens can increase NE synthesis and decrease NE degradation, effects that can account for higher NE levels observed in LC target regions following estrogen treatment. However, in parallel with these effects, estrogens also reduce postsynaptic adrenergic receptors, decreasing the ability of NE to affect downstream structures. A similar pattern of high NE levels and decreased α2 adrenergic receptors is observed in the Flinders Sensitive Line, which is a genetic model of depressive-like behavior (Landau et al., 2015; Zangen et al., 1999). In this model, the decreased receptors are thought to reflect receptor downregulation following a persistently high NE tone (Landau et al., 2015). Estrogen-induced reductions in adrenergic receptors could similarly be compensatory in females, allowing them to maintain NE function at a level comparable to males under normal circumstances. Clearly, more studies are needed to test this hypothesis.
In stress-related psychiatric disorders, hyperarousal symptoms are likely caused by an imbalance between NE release and postsynaptic adrenergic receptors, resulting in too much NE tone. While this imbalance can occur in both males and females, perhaps the dynamic regulation of this system by estrogens in females increases the risk that this balance will be shifted into a high NE state. For example, a high NE state could occur if NE synthesis fails to decrease when estrogen levels are reduced, or if adrenergic receptors become insensitive to estrogen-induced downregulation. Such effects could increase the risk of hyperarousal symptoms in women, particularly during their reproducing years when estrogen levels fluctuate regularly. The data indicating that the rates of depression in women exceed that of men only following puberty and prior to menopause (Angold and Worthman, 1993; Kessler et al., 1993; Kessler, 2003), support the role of ovarian hormones in contributing to the pathophysiology of this disorder. However, more research assessing these effects throughout the female lifespan would help elucidate this issue.
4. Sex differences in CRF regulation of LC neurons and underlying mechanisms
4.1 CRF regulation of LC physiology
Changes in the electrophysiological responses of LC neurons shift states of arousal and attention. In awake animals, LC neurons discharge in a tonic fashion, and the rate of this tonic firing is positively correlated with electroencephalographic (EEG) and arousal (Aston-Jones and Bloom, 1981b; Berridge and Foote, 1991; Berridge et al., 1993). In addition to tonic firing, salient sensory stimuli can evoke a burst of synchronous discharge known as a phasic response (Aston-Jones and Bloom, 1981a; Foote et al., 1980). This phasic response precedes changes in behavior, implicating LC neurons in attentional processes (Aston-Jones and Bloom, 1981a; Foote et al., 1980). Further studies have demonstrated that phasic firing is associated with focused attention (Aston-Jones and Cohen, 2005; Clayton et al., 2004). In contrast, high tonic firing is associated with labile attention, behavioral flexibility, and losing focus (Aston-Jones and Cohen, 2005). Thus, the ability of LC neurons to switch between tonic and phasic firing can facilitate shifting between scanning the environment or focusing on a specific stimulus, allowing the animal to adapt to changing attentional demands (Aston-Jones and Cohen, 2005).
Stress shifts the mode of LC activity by increasing tonic firing, while decreasing sensory-evoked phasic responses (Curtis et al., 1997; Valentino and Wehby, 1988), and this effect is mediated by CRF. Specifically, local blockade of CRF1 receptors prevents stress-induced changes in LC activity (Curtis et al., 1994; Valentino and Wehby, 1988; Valentino et al., 1991). Moreover, administration of CRF directly into the LC mimics the effect of stress by increasing tonic firing, while attenuating phasic firing (Valentino and Foote, 1987; Valentino and Foote, 1988). One downstream consequence of CRF in the LC is an increase in cortical EEG, indicative of a higher arousal state (Curtis et al., 1997). Stress via activation of CRF receptors in the LC also increases cortical EEG, as well as NE release (Kawahara et al., 2000; Page et al., 1993; Smagin et al., 1997). When considered together, these studies reveal that stress via CRF activation of the LC regulates target regions to increase arousal and shift attention towards scanning the environment, responses that could help the organism identify and cope with threatening stimuli during a stressful event.
4.2 Sex differences in CRF regulation of LC physiology
The aforementioned studies that initially characterized the effects of stress and CRF on LC physiology were conducted exclusively in male rats. Curtis et al. (2006) extended these findings to females and found interesting sex differences. Although the physiology of LC neurons is similar in unstressed male and female rats, hypotensive stress—a physiological stressor elicited by a nitroprusside-induced drop in blood pressure— increased LC tonic firing more in females than males. This sex difference in physiology was mimicked by CRF. Specifically, the CRF dose-response curve for LC activation in females was shifted to the left, such that a low dose of CRF that failed to activate the LC in males increased firing in females (Curtis et al., 2006). The role of adult circulating gonadal hormones in this effect was investigated, and surprisingly, there was no effect of estrogens or any other circulating gonadal hormone on stress and CRF regulation of LC physiology (Curtis et al., 2006). These data suggest that this sex difference is attributable to hormonal surges during development or sex chromosome genes (Arnold, 2009; Schulz et al., 2009; Swerdloff et al., 1992).
Another physiological effect initially identified in males is that acute stressor exposure alters subsequent LC neuronal responses to CRF. Specifically, prior (24 h earlier) exposure to footshock, swim stress, and even CRF itself, desensitizes male LC neurons to high doses of CRF (Conti and Foote, 1995; Conti and Foote, 1996; Curtis et al., 1995; Curtis et al., 1999; Curtis et al., 2006). Exposure to these same stressors also increases LC neuronal sensitivity to low CRF doses in males when compared to their unstressed male counterparts (Curtis et al., 1995; Curtis et al., 1999; Curtis et al., 2006). In other words in males, a history of stressor exposure changes the CRF-dose response curve for LC activation so that it shifts to the left and has a lower plateau. Unlike males, a history of swim stress has no effect on the CRF-dose response curve for LC activation in females (Bangasser et al., 2010; Curtis et al., 2006). This is perhaps because female LC neurons are already very sensitive to low levels of CRF, even in the unstressed state, and they are less adaptable to high levels of CRF as will be detailed below.
4.3 Sex differences in CRF1 receptor signaling and coupling
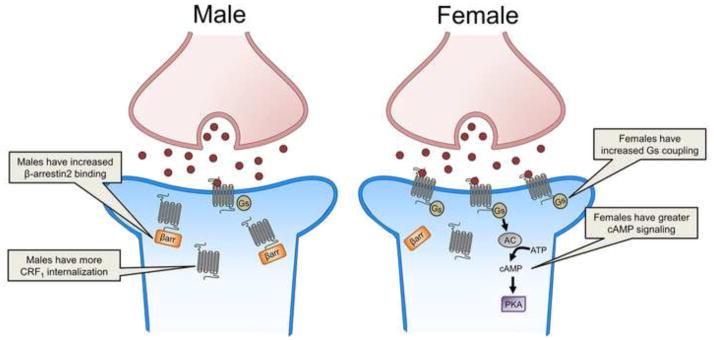
Given that sex differences in LC neuronal responses to CRF were observed following CRF administration, the underlying mechanism was predicted to be postsynaptic. Further investigation confirmed this by linking the physiological findings to sex differences in CRF1 receptor signaling. This receptor is a G-protein coupled receptor that preferentially couples to the Gs protein to activate the cAMP signaling pathway, although promiscuous coupling and initiation of additional signaling pathways is possible (Chen et al., 1986; Grammatopoulos et al., 2001; Hillhouse and Grammatopoulos, 2006). LC neuronal firing in response to CRF is thought to be primarily mediated by the cAMP signaling cascade, which when activated, purportedly increases neuronal firing via phosphorylation of a potassium channel (Jedema and Grace, 2004). To determine whether sex differences in neuronal responses to CRF were linked to sex differences in cAMP signaling, pretreatment with a cAMP antagonist was utilized. This manipulation revealed that LC neuronal responses to CRF in males were only partially cAMP mediated (Bangasser et al., 2010). In contrast, LC neuronal responses to CRF were completely cAMP mediated in females (Bangasser et al., 2010). Prior swim stressor exposure altered the signaling in males, such that their sensitized responses to CRF were completely cAMP mediated. This manipulation failed to sensitize the LC neuronal responses to CRF in females or alter the contribution of cAMP signaling (Bangasser et al., 2010). Together these studies suggest that in unstressed rats, CRF induces greater cAMP signaling in the LC of females than males (Fig. 3). In contrast, stressor exposure alters signaling in male, but not female rats. Specifically, 24 h after swim stressor exposure, male LC neuronal responses to CRF were completely cAMP mediated. Prior stressor exposure had no effect on the contribution of cAMP signaling to LC neuronal firing in females, which remained high. Given that cAMP signaling increases LC tonic firing (Jedema and Grace, 2004), these effects on cAMP signaling can account for the heightened sensitivity of LC neurons to CRF in male rats with a history of stress and females.
Figure 3.
This schematic depicts sex differences in the CRF1 receptor. The image on the left depicts increased β-arrestin2 binding to the CRF1 receptor (gray) and internalization in a male LC neuron (blue). The image on the right depicts CRF (red circle) binding to the CRF1 receptor (gray), which increases Gs coupling and signaling through the cAMP pathway in a female LC neuron (blue). AC, adenylyl cyclase; ATP, adenosine triphosphate; βarr., β-arrestin2; cAMP, cyclic adenosine monophosphate; PKA, protein kinase A
cAMP signaling is initiated by activation of the Gs protein, so sex differences in binding of the Gs protein to the CRF1 receptor could result in sex differences in the amount of cAMP signaling. Indeed, such sex differences are found in cortical tissue, where the CRF1 receptor of female rats is more highly coupled to Gs than in males (Bangasser et al., 2010). Prior stress increases coupling of Gs to the CRF1 receptor in males, but causes no further increase in females. Although technical limitations (i.e., small protein yield) prevented coupling from being assessed specifically in the LC, the sex difference in coupling in the cortex supports cAMP signaling results in the LC. Collectively, these findings support a model wherein increased CRF1 receptor-Gs coupling leads to greater cAMP signaling, thereby heightening tonic firing. Given that rapid tonic LC firing is correlated with high arousal, this sex differences in CRF1 receptor coupling and signaling could heighten arousal in females in response to stress. Perhaps there are situations where this is adaptive. However, during extremely stressful or prolonged stressful events, this physiology is predicted to predispose females towards shifting into the dysregulated state of hyperarousal that, as noted, characterizes certain psychiatric disorders.
4.4 Sex differences in CRF1 receptor trafficking
As noted, stress-related psychiatric disorders are characterized by CRF hypersecretion. (Hauger et al., 2009; Hauger et al., 2012; Nemeroff, 1996). Fortunately, there are some compensatory mechanisms that can protect neurons from excessive CRF signaling in an effort to maintain homeostasis. One such mechanism is internalization, where CRF1 receptors on the plasma membrane are trafficked into the cytosol in response to high concentrations of CRF. In vitro studies have revealed that the process of desensitization and subsequent internalization is typically initiated by GRK3 phosphorylation of serine and threonine residues on the carboxy terminus of the receptor, which promotes the recruitment and translocation of β-arrestin2 (Hauger et al., 2009; Oakley et al., 2007; Teli et al., 2005). Upon binding, β-arrestin2 uncouples the receptor from its cognate G protein and targets it for internalization (Hauger et al., 2009; Holmes et al., 2006; Oakley et al., 2007). These internalized receptors can either be recycled or degraded (Hauger et al., 2009; Reyes et al., 2006).
CRF1 receptor internalization has been demonstrated in the LC dendrites of male rats following the central administration of CRF and acute exposure to swim stress (Bangasser et al., 2010; Reyes et al., 2006; Reyes et al., 2008). Similarly, internalization is observed in male CRF overexpressing mice (Bangasser et al., 2013). CRF1 receptor internalization is apparent as soon as 5 minutes after receptor activation, but persists for at least 24 hours (later time points have not been tested; (Bangasser et al., 2010; Reyes et al., 2006; Reyes et al., 2008). Overtime, the proportion of internalized CRF1 receptors increases, as does their incorporation into multivesicular bodies, an effect indicative of receptor downregulation (Reyes et al., 2008). These studies indicate that, similar to the in vitro studies, the CRF1 receptor in male LC dendrites internalizes following high concentrations of ligand (Fig. 3). Remarkably, the same effect does not occur in females. Female rats exposed to acute swim stress and female CRF overexpressing mice have a larger proportion of CRF1 receptors on the plasma membrane of the LC dendrites than their unstressed counterparts (Bangasser et al., 2010; Bangasser et al., 2013). This result suggests that excessive ligand binding actually recruits CRF1 receptors to the plasma membrane in females. Physiologically, this sex difference in trafficking can account for the fact that, despite high levels of CRF in the LC, male CRF overexpressing mice maintain their tonic LC firing rate at wild type levels (Bangasser et al., 2013). In contrast, the LC neurons of CRF overexpressing female mice fire roughly three times faster than wild type mice. As LC neuronal firing translates into arousal (Aston-Jones and Bloom, 1981a; Berridge and Foote, 1991), a lack of CRF1 receptor internalization in females may predispose them to hyperarousal symptoms under conditions of CRF hypersecretion, as occur in stress-related psychiatric disorders (Hauger et al., 2009; Hauger et al., 2012; Nemeroff, 1996).
The mechanisms underlying the sex difference in internalization are thought to be linked to sex differences in β-arrestin2 recruitment to the CRF1 receptor, which, as previously mentioned, initiates receptor internalization. In cortical tissue, acute swim stress promotes β-arrestin2 binding to the CRF1 receptor in male rats (Bangasser et al., 2010). However, this increase in β-arrestin2 binding was not observed following the same stressor in females (Fig. 3). If these same mechanisms are occurring in the LC, it would explain the sex difference in CRF1 trafficking.
The sex difference in β-arrestin2 binding may have further implications for cellular function. In addition to its role in initiating internalization, β-arrestin2 can also activate its own suite of signaling cascades that are often distinct, and sometimes opposed to those activated by G proteins (Ahn et al., 2004; Lefkowitz and Shenoy, 2005; Violin and Lefkowitz, 2007). This has led to the proposal that the signaling of the CRF1 receptor is sex biased, such that it signals more through β-arrestin2 mediated pathways in males (Bangasser and Valentino, 2012; Valentino et al., 2012; Valentino et al., 2013a; Valentino et al., 2013b). As detailed above, the female CRF1 receptor signals more through the Gs-cAMP pathway (Bangasser et al., 2010). As the activation of different signaling cascades can lead to different cellular events, sex biased signaling may be an important way that sex differences in physiology, behavior, and even pathology are established (Bangasser and Valentino, 2012; Valentino et al., 2012; Valentino et al., 2013a; Valentino et al., 2013b).
5. Implications of sex differences in LC-NE system and its regulation by stress
Taken together, these studies reveal that the LC-NE system is regulated by estrogens and stress in a way that, under certain conditions, can increase susceptibility to states of high arousal in females relative to males. Despite this body of knowledge, the majority of studies have focused on understanding sex differences in the LC-NE system were conducted in adult rodents. It will be important to extend these findings to early development and aging to determine if, and how, sex differences in this important arousal system are established across the lifespan. Another goal of future studies should be to assess whether similar mechanisms occur in humans. Although some of the aforementioned approaches are currently restricted to preclinical models, neuroimaging data on sex differences in LC activation and further behavioral data on sex differences in stress-induced arousal would be valuable. Ultimately, if similar mechanisms are found to occur in humans, they could help explain the sex bias in psychiatric disorders that have hyperarousal as a key feature.
The identification of similar sex differences in the human LC-NE system would have implications for the treatment of certain stress-related psychiatric disorders. For example, if increased NE release is more common in women with these disorders, then they may respond better than men to a β-adrenergic receptor antagonist, such as propranolol, which has been used to reduce the consolidation (i.e. stabilization) and reconsolidation (i.e., the stabilization of a reactivated memory) of emotional memories (Cahill et al., 1994; Mueller and Cahill, 2010; Nader et al., 2000; Pitman et al., 2002). Corroborating this idea, a recent meta-analysis found that propranolol was more effective at reducing the reconsolidation of traumatic memories in women than men (Lonergan et al., 2013). Also, tricyclic antidepressants which increase the amount of NE in the synapse work better in men and post-menopausal women than in cycling women (Kornstein et al., 2000).
Sex differences in the CRF1 receptor also have implications for therapeutics, because CRF1 receptor antagonists are being developed to treat stress-related diseases (Holsboer and Ising, 2008; Ising et al., 2007; Nemeroff, 1996; Zobel et al., 2000). If increased sensitivity to CRF is driving hyperarousal symptoms, particularly in women, then they may better respond to treatments aimed at regulating CRF than men. Furthermore, the molecular studies suggest that there may be a conformational sex difference in the CRF1 receptor of females compared to males that biases it to bind Gs instead of βarrestin2. If true, this sex difference in conformation could extend to the extracellular domain where antagonist bind, thereby altering their efficacy. In support of this, sex differences in the efficacy of a CRF1 receptor antagonist in the dorsal raphe have been reported, such that the antagonist was effective at mitigating the effects of stress in male, but not female mice (Howerton et al., 2014). If this is a wider phenomenon, it might suggest this class of drug might have a reduced efficacy for women.
Perhaps even more importantly, the studies detailed here highlight that, by comparing males and females, new pharmacological targets can be identified. For example, biased ligands that shift the signaling of receptors away from G-protein mediated signaling and towards βarrestin2-mediated signaling have been developed for other receptors (Violin and Lefkowitz, 2007; Whalen et al., 2011). If such a compound could be developed for the CRF1 receptor, it may be particularly effective at treating hyperarousal symptoms in women (Bangasser and Valentino, 2012; Valentino et al., 2013a; Valentino et al., 2013b). As more researchers focus on investigating sex differences in the LC, it is expected that novel therapeutic targets will be identified that can lead to the development of better treatments for stress-related psychiatric disorders for both men and women.
Highlights.
Psychiatric disorders linked to stress and hyperarousal are more prevalent in women.
There are structural sex differences in the locus coeruleus (LC) arousal center.
Estrogens increase norepinephrine levels in LC target regions.
Female LC neurons are more sensitive to the stress neuropeptide CRF.
Collectively, these effects may contribute to sex biases in psychiatric disorders.
Acknowledgments
We would like to thank David Waxler for his helpful comments on the manuscript. This work was supported by MH092438 to D.A.B.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abercrombie ED, Keller RW, Zigmond MJ. Characterization of hippocampal norepinephrine release as measured by microdialysis perfusion: pharmacological and behavioral studies. Neuroscience. 1988;27:897–904. doi: 10.1016/0306-4522(88)90192-3. [DOI] [PubMed] [Google Scholar]
- Ahn S, Shenoy SK, Wei H, Lefkowitz RJ. Differential kinetic and spatial patterns of beta-arrestin and G protein-mediated ERK activation by the angiotensin II receptor. J Biol Chem. 2004;279:35518–25. doi: 10.1074/jbc.M405878200. [DOI] [PubMed] [Google Scholar]
- Alfinito PD, Chen X, Mastroeni R, Pawlyk AC, Deecher DC. Estradiol increases catecholamine levels in the hypothalamus of ovariectomized rats during the dark-phase. European journal of pharmacology. 2009;616:334–9. doi: 10.1016/j.ejphar.2009.06.045. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5. Vol. 5. American Psychiatric Publishing; Washington, D.C: 2013. [Google Scholar]
- Angold A, Worthman CW. Puberty onset of gender differences in rates of depression: a developmental, epidemiologic and neuroendocrine perspective. J Affect Disord. 1993;29:145–58. doi: 10.1016/0165-0327(93)90029-j. [DOI] [PubMed] [Google Scholar]
- Ansonoff MA, Etgen AM. Estrogen increases G protein coupled receptor kinase 2 in the cortex of female rats. Brain Res. 2001;898:186–9. doi: 10.1016/s0006-8993(01)02161-8. [DOI] [PubMed] [Google Scholar]
- Arnold AP. Mouse models for evaluating sex chromosome effects that cause sex differences in non-gonadal tissues. J Neuroendocrinol. 2009;21:377–86. doi: 10.1111/j.1365-2826.2009.01831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Bloom FE. Norepinephrine-containing locus coeruleus neurons in behaving rats exhibit pronounced responses to non-noxious environmental stimuli. J Neurosci. 1981a;1:887–900. doi: 10.1523/JNEUROSCI.01-08-00887.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Bloom FE. Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J Neurosci. 1981b;1:876–886. doi: 10.1523/JNEUROSCI.01-08-00876.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Ennis M, Pieribone VA, Nickell WT, Shipley MT. The brain nucleus locus coeruleus: restricted afferent control of a broad efferent network. Science. 1986;234:734–737. doi: 10.1126/science.3775363. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G. The locus coeruleus, A5 and A7 noradrenergic cell groups. In: Paxinos G, editor. The Rat Nervous System. Academic Press; 2004. pp. 259–287. [Google Scholar]
- Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci. 2005;28:403–50. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- Austin MC, Janosky JE, Murphy HA. Increased corticotropin-releasing hormone immunoreactivity in monoamine-containing pontine nuclei of depressed suicide men. Mol Psychiatry. 2003;8:324–32. doi: 10.1038/sj.mp.4001250. [DOI] [PubMed] [Google Scholar]
- Babstock D, Malsbury CW, Harley CW. The dorsal locus coeruleus is larger in male than in female Sprague-Dawley rats. Neurosci Lett. 1997;224:157–60. doi: 10.1016/S0304-3940(97)13462-0. [DOI] [PubMed] [Google Scholar]
- Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525–57. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- Bangasser DA, Curtis A, Reyes BA, Bethea TT, Parastatidis I, Ischiropoulos H, Van Bockstaele EJ, Valentino RJ. Sex differences in corticotropin-releasing factor receptor signaling and trafficking: potential role in female vulnerability to stress-related psychopathology. Mol Psychiatry. 2010;15:877, 896–904. doi: 10.1038/mp.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Zhang X, Garachh V, Hanhauser E, Valentino RJ. Sexual dimorphism in locus coeruleus dendritic morphology: a structural basis for sex differences in emotional arousal. Physiol Behav. 2011;103:342–51. doi: 10.1016/j.physbeh.2011.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Valentino RJ. Sex differences in molecular and cellular substrates of stress. Cell Mol Neurobiol. 2012;32:709–23. doi: 10.1007/s10571-012-9824-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Reyes BA, Piel D, Garachh V, Zhang XY, Plona ZM, Van Bockstaele EJ, Beck SG, Valentino RJ. Increased vulnerability of the brain norepinephrine system of females to corticotropin-releasing factor overexpression. Mol Psychiatry. 2013;18:166–73. doi: 10.1038/mp.2012.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Kawasumi Y. Cognitive disruptions in stress-related psychiatric disorders: A role for corticotropin releasing factor (CRF) Hormones and behavior. 2015 doi: 10.1016/j.yhbeh.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge CW, Foote SL. Effects of locus coeruleus activation on electroencephalographic activity in the neocortex and hippocampus. J Neurosci. 1991;11:3135–3145. doi: 10.1523/JNEUROSCI.11-10-03135.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge CW, Page ME, Valentino RJ, Foote SL. Effects of locus coeruleus inactivation on electroencephalographic activity in neocortex and hippocampus. Neuroscience. 1993;55:381–383. doi: 10.1016/0306-4522(93)90507-c. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Brain Res Rev. 2003;42:33–84. doi: 10.1016/s0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- Bray NJ, Buckland PR, Williams NM, Williams HJ, Norton N, Owen MJ, O’Donovan MC. A Haplotype Implicated in Schizophrenia Susceptibility Is Associated with Reduced COMT Expression in Human Brain. The American Journal of Human Genetics. 2003;73:152–161. doi: 10.1086/376578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslau N. Gender differences in trauma and posttraumatic stress disorder. J Gend Specif Med. 2002;5:34–40. [PubMed] [Google Scholar]
- Busch C, Bohl J, Ohm TG. Spatial, Temporal and Numeric Analysis of Alzheimer Changes in the Nucleus Coeruleus. Neurobiology of aging. 1997;18:401–406. doi: 10.1016/s0197-4580(97)00035-3. [DOI] [PubMed] [Google Scholar]
- Cahill L, Prins B, Weber M, McGaugh JL. Beta-adrenergic activation and memory for emotional events. Nature. 1994;371:702–4. doi: 10.1038/371702a0. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Robbins TW. Noradrenergic modulation of cognition: therapeutic implications. J Psychopharmacol. 2013;27:694–718. doi: 10.1177/0269881113480988. [DOI] [PubMed] [Google Scholar]
- Chen FM, Bilezikjian LM, Perrin MH, Rivier J, Vale W. Corticotropin releasing factor receptor-mediated stimulation of adenylate cyclase activity in the rat brain. Brain Res. 1986;381:49–57. doi: 10.1016/0006-8993(86)90688-8. [DOI] [PubMed] [Google Scholar]
- Chen J, Lipska BK, Halim N, Ma QD, Matsumoto M, Melhem S, Kolachana BS, Hyde TM, Herman MM, Apud J, Egan MF, Kleinman JE, Weinberger DR. Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): effects on mRNA, protein, and enzyme activity in postmortem human brain. Am J Hum Genet. 2004;75:807–21. doi: 10.1086/425589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung WC, Swaab DF, De Vries GJ. Apoptosis during sexual differentiation of the bed nucleus of the stria terminalis in the rat brain. J Neurobiol. 2000;43:234–43. [PubMed] [Google Scholar]
- Clayton EC, Rajkowski J, Cohen JD, Aston-Jones G. Phasic activation of monkey locus ceruleus neurons by simple decisions in a forced-choice task. J Neurosci. 2004;24:9914–20. doi: 10.1523/JNEUROSCI.2446-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti LH, Foote SL. Effects of pretreatment with corticotropin-releasing factor (CRF) on the electrophysiological responsivity of the locus coeruleus to subsequent CRF challenge. Neuroscience. 1995;69:209–219. doi: 10.1016/0306-4522(95)00222-5. [DOI] [PubMed] [Google Scholar]
- Conti LH, Foote SL. Reciprocal cross-desensitization of locus coeruleus electrophysiological responsivity to corticotropin-releasing factor and stress. Brain Res. 1996;722:19–29. doi: 10.1016/0006-8993(96)00175-8. [DOI] [PubMed] [Google Scholar]
- Curtis AL, Grigoriadis DE, Page ME, Rivier J, Valentino RJ. Pharmacological comparison of two corticotropin-releasing factor antagonists: in vivo and in vitro studies. J Pharmacol Exp Ther. 1994;268:359–65. [PubMed] [Google Scholar]
- Curtis AL, Pavcovich LA, Grigoriadis DE, Valentino RJ. Previous stress alters corticotropin-releasing factor neurotransmission in the locus coeruleus. Neuroscience. 1995;65:541–50. doi: 10.1016/0306-4522(94)00496-r. [DOI] [PubMed] [Google Scholar]
- Curtis AL, Lechner SM, Pavcovich LA, Valentino RJ. Activation of the locus coeruleus noradrenergic system by intracoerulear microinfusion of corticotropin-releasing factor: effects on discharge rate, cortical norepinephrine levels and cortical electroencephalographic activity. J Pharmacol Exp Ther. 1997;281:163–72. [PubMed] [Google Scholar]
- Curtis AL, Pavcovich LA, Valentino RJ. Long-term regulation of locus ceruleus sensitivity to corticotropin-releasing factor by swim stress. J Pharmacol Exp Ther. 1999;289:1211–9. [PubMed] [Google Scholar]
- Curtis AL, Bethea T, Valentino RJ. Sexually dimorphic responses of the brain norepinephrine system to stress and corticotropin-releasing factor. Neuropsychopharmacology. 2006;31:544–54. doi: 10.1038/sj.npp.1300875. [DOI] [PubMed] [Google Scholar]
- Davis EC, Popper P, Gorski RA. The role of apoptosis in sexual differentiation of the rat sexually dimorphic nucleus of the preoptic area. Brain Res. 1996;734:10–8. [PubMed] [Google Scholar]
- Domschke K, Freitag CM, Kuhlenbaumer G, Schirmacher A, Sand P, Nyhuis P, Jacob C, Fritze J, Franke P, Rietschel M, Garritsen HS, Fimmers R, Nothen MM, Lesch KP, Stogbauer F, Deckert J. Association of the functional V158M catechol-O-methyl-transferase polymorphism with panic disorder in women. Int J Neuropsychopharmacol. 2004;7:183–8. doi: 10.1017/S146114570400416X. [DOI] [PubMed] [Google Scholar]
- Domschke K, Deckert J, O’Donovan MC, Glatt SJ. Meta-analysis of COMT val158met in panic disorder: ethnic heterogeneity and gender specificity. American journal of medical genetics. Part B, Neuropsychiatric genetics : the official publication of the International Society of Psychiatric Genetics. 2007;144B:667–73. doi: 10.1002/ajmg.b.30494. [DOI] [PubMed] [Google Scholar]
- Eley TC, Tahir E, Angleitner A, Harriss K, McClay J, Plomin R, Riemann R, Spinath F, Craig I. Association analysis of MAOA and COMT with neuroticism assessed by peers. American journal of medical genetics. Part B, Neuropsychiatric genetics : the official publication of the International Society of Psychiatric Genetics. 2003;120B:90–6. doi: 10.1002/ajmg.b.20046. [DOI] [PubMed] [Google Scholar]
- Enoch MA, Xu K, Ferro E, Harris CR, Goldman D. Genetic origins of anxiety in women: a role for a functional catechol-O-methyltransferase polymorphism. Psychiatr Genet. 2003;13:33–41. doi: 10.1097/00041444-200303000-00006. [DOI] [PubMed] [Google Scholar]
- Etgen AM, Ungar S, Petitti N. Estradiol and progesterone modulation of norepinephrine neurotransmission: implications for the regulation of female reproductive behavior. J Neuroendocrinol. 1992;4:255–71. doi: 10.1111/j.1365-2826.1992.tb00167.x. [DOI] [PubMed] [Google Scholar]
- Etgen AM, Ansonoff MA, Quesada A. Mechanisms of ovarian steroid regulation of norepinephrine receptor-mediated signal transduction in the hypothalamus: implications for female reproductive physiology. Hormones and behavior. 2001;40:169–77. doi: 10.1006/hbeh.2001.1676. [DOI] [PubMed] [Google Scholar]
- Foote SL, Aston-Jones G, Bloom FE. Impulse activity of locus coeruleus neurons in awake rats and monkeys is a function of sensory stimulation and arousal. Proc Natl Acad Sci USA. 1980;77:3033–3037. doi: 10.1073/pnas.77.5.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman SA, Gluck N, Tuval-Mashiach R, Brandes D, Peri T, Shalev AY. Gender differences in responses to traumatic events: a prospective study. J Trauma Stress. 2002;15:407–13. doi: 10.1023/A:1020189425935. [DOI] [PubMed] [Google Scholar]
- Garcia-Falgueras A, Pinos H, Collado P, Pasaro E, Fernandez R, Segovia S, Guillamon A. The expression of brain sexual dimorphism in artificial selection of rat strains. Brain Res. 2005;1052:130–8. doi: 10.1016/j.brainres.2005.05.066. [DOI] [PubMed] [Google Scholar]
- Garcia-Falgueras A, Pinos H, Fernandez R, Collado P, Pasaro E, Segovia S, Guillamon A. Sexual dimorphism in hybrids rats. Brain Res. 2006;1123:42–50. doi: 10.1016/j.brainres.2006.09.053. [DOI] [PubMed] [Google Scholar]
- Gold PW, Chrousos GP. The endocrinology of melancholic and atypical depression: relation to neurocircuitry and somatic consequences. Proc Assoc Am Physicians. 1999;111:22–34. doi: 10.1046/j.1525-1381.1999.09423.x. [DOI] [PubMed] [Google Scholar]
- Gold PW, Chrousos GP. Organization of the stress system and its dysregulation in melancholic and atypical depression: high vs low CRH/NE states. Mol Psychiatry. 2002;7:254–75. doi: 10.1038/sj.mp.4001032. [DOI] [PubMed] [Google Scholar]
- Grammatopoulos DK, Randeva HS, Levine MA, Kanellopoulou KA, Hillhouse EW. Rat cerebral cortex corticotropin-releasing hormone receptors: evidence for receptor coupling to multiple G-proteins. J Neurochem. 2001;76:509–19. doi: 10.1046/j.1471-4159.2001.00067.x. [DOI] [PubMed] [Google Scholar]
- Guillamon A, de Blas MR, Segovia S. Effects of sex steroids on the development of the locus coeruleus in the rat. Brain Res. 1988;468:306–10. doi: 10.1016/0165-3806(88)90143-5. [DOI] [PubMed] [Google Scholar]
- Hall M, Buysse DJ, Nowell PD, Nofzinger EA, Houck P, Reynolds CF, 3rd, Kupfer DJ. Symptoms of stress and depression as correlates of sleep in primary insomnia. Psychosomatic medicine. 2000;62:227–30. doi: 10.1097/00006842-200003000-00014. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Tunbridge EM. Catechol-O-methyltransferase (COMT): a gene contributing to sex differences in brain function, and to sexual dimorphism in the predisposition to psychiatric disorders. Neuropsychopharmacology. 2008;33:3037–45. doi: 10.1038/sj.npp.1301543. [DOI] [PubMed] [Google Scholar]
- Hauger RL, Risbrough V, Oakley RH, Olivares-Reyes JA, Dautzenberg FM. Role of CRF receptor signaling in stress vulnerability, anxiety, and depression. Ann N Y Acad Sci. 2009;1179:120–43. doi: 10.1111/j.1749-6632.2009.05011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauger RL, Olivares-Reyes JA, Dautzenberg FM, Lohr JB, Braun S, Oakley RH. Molecular and cell signaling targets for PTSD pathophysiology and pharmacotherapy. Neuropharmacology. 2012;62:705–14. doi: 10.1016/j.neuropharm.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillhouse EW, Grammatopoulos DK. The molecular mechanisms underlying the regulation of the biological activity of corticotropin-releasing hormone receptors: implications for physiology and pathophysiology. Endocr Rev. 2006;27:260–86. doi: 10.1210/er.2005-0034. [DOI] [PubMed] [Google Scholar]
- Holmes KD, Babwah AV, Dale LB, Poulter MO, Ferguson SS. Differential regulation of corticotropin releasing factor 1alpha receptor endocytosis and trafficking by beta-arrestins and Rab GTPases. J Neurochem. 2006;96:934–49. doi: 10.1111/j.1471-4159.2005.03603.x. [DOI] [PubMed] [Google Scholar]
- Holsboer F, Ising M. Central CRH system in depression and anxiety--evidence from clinical studies with CRH1 receptor antagonists. European journal of pharmacology. 2008;583:350–7. doi: 10.1016/j.ejphar.2007.12.032. [DOI] [PubMed] [Google Scholar]
- Howerton AR, Roland AV, Fluharty JM, Marshall A, Chen A, Daniels D, Beck SG, Bale TL. Sex differences in corticotropin-releasing factor receptor-1 action within the dorsal raphe nucleus in stress responsivity. Biol Psychiatry. 2014;75:873–83. doi: 10.1016/j.biopsych.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ising M, Zimmermann US, Kunzel HE, Uhr M, Foster AC, Learned-Coughlin SM, Holsboer F, Grigoriadis DE. High-affinity CRF1 receptor antagonist NBI-34041: preclinical and clinical data suggest safety and efficacy in attenuating elevated stress response. Neuropsychopharmacology. 2007;32:1941–9. doi: 10.1038/sj.npp.1301328. [DOI] [PubMed] [Google Scholar]
- Jedema HP, Grace AA. Corticotropin-releasing hormone directly activates noradrenergic neurons of the locus ceruleus recorded in vitro. J Neurosci. 2004;24:9703–13. doi: 10.1523/JNEUROSCI.2830-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Xie T, Ramsden DB, Ho SL. Human catechol-O-methyltransferase down-regulation by estradiol. Neuropharmacology. 2003;45:1011–8. doi: 10.1016/s0028-3908(03)00286-7. [DOI] [PubMed] [Google Scholar]
- Karkanias GB, Li CS, Etgen AM. Estradiol reduction of alpha 2-adrenoceptor binding in female rat cortex is correlated with decreases in alpha 2A/D-adrenoceptor messenger RNA. Neuroscience. 1997;81:593–7. doi: 10.1016/s0306-4522(97)00359-x. [DOI] [PubMed] [Google Scholar]
- Kawahara H, Kawahara Y, Westerink BH. The role of afferents to the locus coeruleus in the handling stress-induced increase in the release of norepinephrine in the medial prefrontal cortex: a dual-probe microdialysis study in the rat brain. Eur J Pharmacol. 2000;387:279–286. doi: 10.1016/s0014-2999(99)00793-1. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Swartz M, Blazer DG, Nelson CB. Sex and depression in the National Comorbidity Survey. I: Lifetime prevalence, chronicity and recurrence. J Affect Disord. 1993;29:85–96. doi: 10.1016/0165-0327(93)90026-g. [DOI] [PubMed] [Google Scholar]
- Kessler RC. Epidemiology of women and depression. J Affect Disord. 2003;74:5–13. doi: 10.1016/s0165-0327(02)00426-3. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Petukhova M, Sampson NA, Zaslavsky AM, Wittchen HU. Twelvemonth and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int J Methods Psychiatr Res. 2012;21:169–84. doi: 10.1002/mpr.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi I, Mellman TA. Gender differences in sleep during the aftermath of trauma and the development of posttraumatic stress disorder. Behav Sleep Med. 2012;10:180–90. doi: 10.1080/15402002.2011.654296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Corticotropin-releasing factor, norepinephrine, and stress. Biol Psychiatry. 1999;46:1167–80. doi: 10.1016/s0006-3223(99)00164-x. [DOI] [PubMed] [Google Scholar]
- Kornstein SG, Schatzberg AF, Thase ME, Yonkers KA, McCullough JP, Keitner GI, Gelenberg AJ, Davis SM, Harrison WM, Keller MB. Gender differences in treatment response to sertraline versus imipramine in chronic depression. Am J Psychiatry. 2000;157:1445–52. doi: 10.1176/appi.ajp.157.9.1445. [DOI] [PubMed] [Google Scholar]
- Labus JS, Naliboff BN, Fallon J, Berman SM, Suyenobu B, Bueller JA, Mandelkern M, Mayer EA. Sex differences in brain activity during aversive visceral stimulation and its expectation in patients with chronic abdominal pain: a network analysis. Neuroimage. 2008;41:1032–43. doi: 10.1016/j.neuroimage.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau AM, Phan J-A, Iversen P, Lillethorup TP, Simonsen M, Wegener G, Jakobsen S, Doudet DJ. Decreased in vivo α2 adrenoceptor binding in the Flinders Sensitive Line rat model of depression. Neuropharmacology. 2015;91:97–102. doi: 10.1016/j.neuropharm.2014.12.025. [DOI] [PubMed] [Google Scholar]
- Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by beta-arrestins. Science. 2005;308:512–7. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- Lewinsohn PM, Gotlib IH, Lewinsohn M, Seeley JR, Allen NB. Gender differences in anxiety disorders and anxiety symptoms in adolescents. Journal of Abnormal Psychology. 1998;107:109. doi: 10.1037//0021-843x.107.1.109. [DOI] [PubMed] [Google Scholar]
- Lonergan MH, Olivera-Figueroa LA, Pitman RK, Brunet A. Propranolol’s effects on the consolidation and reconsolidation of long-term emotional memory in healthy participants: a meta-analysis. Journal of psychiatry & neuroscience : JPN. 2013;38:222–31. doi: 10.1503/jpn.120111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubbers LS, Zafian PT, Gautreaux C, Gordon M, Alves SE, Correa L, Lorrain DS, Hickey GJ, Luine V. Estrogen receptor (ER) subtype agonists alter monoamine levels in the female rat brain. The Journal of steroid biochemistry and molecular biology. 2010;122:310–7. doi: 10.1016/j.jsbmb.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Luque JM, de Blas MR, Segovia S, Guillamon A. Sexual dimorphism of the dopamine-beta-hydroxylase-immunoreactive neurons in the rat locus ceruleus. Brain Res Dev Brain Res. 1992;67:211–5. doi: 10.1016/0165-3806(92)90221-h. [DOI] [PubMed] [Google Scholar]
- Maharjan S, Serova L, Sabban EL. Transcriptional regulation of tyrosine hydroxylase by estrogen: opposite effects with estrogen receptors alpha and beta and interactions with cyclic AMP. J Neurochem. 2005;93:1502–14. doi: 10.1111/j.1471-4159.2005.03142.x. [DOI] [PubMed] [Google Scholar]
- Maharjan S, Serova LI, Sabban EL. Membrane-initiated estradiol signaling increases tyrosine hydroxylase promoter activity with ER alpha in PC12 cells. J Neurochem. 2010;112:42–55. doi: 10.1111/j.1471-4159.2009.06430.x. [DOI] [PubMed] [Google Scholar]
- Meitzen J, Perry AN, Westenbroek C, Hedges VL, Becker JB, Mermelstein PG. Enhanced striatal beta1-adrenergic receptor expression following hormone loss in adulthood is programmed by both early sexual differentiation and puberty: a study of humans and rats. Endocrinology. 2013;154:1820–31. doi: 10.1210/en.2012-2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezo PG, Baker RM. The moderating effects of stress and rumination on depressive symptoms in women and men. Stress Health. 2012;28:333–9. doi: 10.1002/smi.2417. [DOI] [PubMed] [Google Scholar]
- Mitra SW, Hoskin E, Yudkovitz J, Pear L, Wilkinson HA, Hayashi S, Pfaff DW, Ogawa S, Rohrer SP, Schaeffer JM, McEwen BS, Alves SE. Immunolocalization of Estrogen Receptor β in the Mouse Brain: Comparison with Estrogen Receptor α. Endocrinology. 2003;144:2055–2067. doi: 10.1210/en.2002-221069. [DOI] [PubMed] [Google Scholar]
- Morrison JH, Grzanna R, Molliver ME, Coyle JT. The distribution and orientation of noradrenergic fibers in neocortex of the rat: an immunofluorescence study. The Journal of comparative neurology. 1978;181:17–39. doi: 10.1002/cne.901810103. [DOI] [PubMed] [Google Scholar]
- Mueller D, Cahill SP. Noradrenergic modulation of extinction learning and exposure therapy. Behav Brain Res. 2010;208:1–11. doi: 10.1016/j.bbr.2009.11.025. [DOI] [PubMed] [Google Scholar]
- Nader K, Schafe GE, Le Doux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406:722–6. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB. The corticotropin-releasing factor (CRF) hypothesis of depression: new findings and new directions. Mol Psychiatry. 1996;1:336–42. [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Larson J, Grayson C. Explaining the gender difference in depressive symptoms. J Pers Soc Psychol. 1999;77:1061–72. doi: 10.1037//0022-3514.77.5.1061. [DOI] [PubMed] [Google Scholar]
- Oakley RH, Olivares-Reyes JA, Hudson CC, Flores-Vega F, Dautzenberg FM, Hauger RL. Carboxyl-terminal and intracellular loop sites for CRF1 receptor phosphorylation and beta-arrestin-2 recruitment: a mechanism regulating stress and anxiety responses. Am J Physiol Regul Integr Comp Physiol. 2007;293:R209–22. doi: 10.1152/ajpregu.00099.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohm TG, Busch C, Bohl J. Unbiased Estimation of Neuronal Numbers in the Human Nucleus Coeruleus during Aging. Neurobiology of aging. 1997;18:393–399. doi: 10.1016/s0197-4580(97)00034-1. [DOI] [PubMed] [Google Scholar]
- Olsson CA, Anney RJ, Lotfi-Miri M, Byrnes GB, Williamson R, Patton GC. Association between the COMT Val158Met polymorphism and propensity to anxiety in an Australian population-based longitudinal study of adolescent health. Psychiatr Genet. 2005;15:109–15. doi: 10.1097/00041444-200506000-00007. [DOI] [PubMed] [Google Scholar]
- Owens MJ, Nemeroff CB. Physiology and pharmacology of corticotropin-releasing factor. Pharmacol Rev. 1991;43:425–73. [PubMed] [Google Scholar]
- Page ME, Berridge CW, Foote SL, Valentino RJ. Corticotropin-releasing factor in the locus coeruleus mediates EEG activation associated with hypotensive stress. Neurosci Lett. 1993;164:81–84. doi: 10.1016/0304-3940(93)90862-f. [DOI] [PubMed] [Google Scholar]
- Parvez S, Ismahan G, Raza-Bukhari A, Youdim MB. Activity of catechol-o-methyltransferase in brain regions and adrenal gland during the oestrus cycle. J Neural Transm. 1978;42:305–12. doi: 10.1007/BF01673554. [DOI] [PubMed] [Google Scholar]
- Pau KY, Hess DL, Kohama S, Bao J, Pau CY, Spies HG. Oestrogen upregulates noradrenaline release in the mediobasal hypothalamus and tyrosine hydroxylase gene expression in the brainstem of ovariectomized rhesus macaques. J Neuroendocrinol. 2000;12:899–909. doi: 10.1046/j.1365-2826.2000.00549.x. [DOI] [PubMed] [Google Scholar]
- Pedersen WC, Denson TF, Goss RJ, Vasquez EA, Kelley NJ, Miller N. The impact of rumination on aggressive thoughts, feelings, arousal, and behaviour. Br J Soc Psychol. 2011;50:281–301. doi: 10.1348/014466610X515696. [DOI] [PubMed] [Google Scholar]
- Pinos H, Collado P, Rodriguez-Zafra M, Rodriguez C, Segovia S, Guillamon A. The development of sex differences in the locus coeruleus of the rat. Brain Res Bull. 2001;56:73–8. doi: 10.1016/s0361-9230(01)00540-8. [DOI] [PubMed] [Google Scholar]
- Pitman RK, Sanders KM, Zusman RM, Healy AR, Cheema F, Lasko NB, Cahill L, Orr SP. Pilot study of secondary prevention of posttraumatic stress disorder with propranolol. Biol Psychiatry. 2002;51:189–92. doi: 10.1016/s0006-3223(01)01279-3. [DOI] [PubMed] [Google Scholar]
- Reyes BA, Fox K, Valentino RJ, Van Bockstaele EJ. Agonist-induced internalization of corticotropin-releasing factor receptors in noradrenergic neurons of the rat locus coeruleus. Eur J Neurosci. 2006;23:2991–8. doi: 10.1111/j.1460-9568.2006.04820.x. [DOI] [PubMed] [Google Scholar]
- Reyes BA, Valentino RJ, Van Bockstaele EJ. Stress-induced intracellular trafficking of corticotropin-releasing factor receptors in rat locus coeruleus neurons. Endocrinology. 2008;149:122–30. doi: 10.1210/en.2007-0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Zafra M, De Blas MR, Perez-Laso C, Cales JM, Guillamon A, Segovia S. Effects of perinatal diazepam exposure on the sexually dimorphic rat locus coeruleus. Neurotoxicol Teratol. 1993;15:139–44. doi: 10.1016/0892-0362(93)90072-v. [DOI] [PubMed] [Google Scholar]
- Sara SJ. The locus coeruleus and noradrenergic modulation of cognition. Nature reviews Neuroscience. 2009;10:211–23. doi: 10.1038/nrn2573. [DOI] [PubMed] [Google Scholar]
- Schulz KM, Molenda-Figueira HA, Sisk CL. Back to the future: The organizational-activational hypothesis adapted to puberty and adolescence. Horm Behav. 2009;55:597–604. doi: 10.1016/j.yhbeh.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selmanoff MK, Pramik-Holdaway MJ, Weiner RI. Concentrations of dopamine and norepinephrine in discrete hypothalamic nuclei during the rat estrous cycle. Endocrinology. 1976;99:326–9. doi: 10.1210/endo-99-1-326. [DOI] [PubMed] [Google Scholar]
- Serova L, Rivkin M, Nakashima A, Sabban EL. Estradiol stimulates gene expression of norepinephrine biosynthetic enzymes in rat locus coeruleus. Neuroendocrinology. 2002;75:193–200. doi: 10.1159/000048237. [DOI] [PubMed] [Google Scholar]
- Serova LI, Maharjan S, Huang A, Sun D, Kaley G, Sabban EL. Response of tyrosine hydroxylase and GTP cyclohydrolase I gene expression to estrogen in brain catecholaminergic regions varies with mode of administration. Brain Res. 2004;1015:1–8. doi: 10.1016/j.brainres.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Serova LI, Nostramo R, Veerasirikul M, Cappell DB, Sabban EL. Varied mechanisms of oestradiol-mediated regulation of dopamine beta-hydroxylase transcription. J Neuroendocrinol. 2011;23:168–76. doi: 10.1111/j.1365-2826.2010.02086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shansky RM, Bender G, Arnsten AF. Estrogen prevents norepinephrine alpha-2a receptor reversal of stress-induced working memory impairment. Stress. 2009;12:457–63. doi: 10.1080/10253890802520988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipley MT, Fu L, Ennis M, Liu W, Aston-Jones G. Dendrites of locus coeruleus neurons extend preferentially into two pericoerulear zones. J Comp Neurol. 1996;365:56–68. doi: 10.1002/(SICI)1096-9861(19960129)365:1<56::AID-CNE5>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. The Journal of comparative neurology. 1997;388:507–25. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Smagin GN, Zhou J, Harris RB, Ryan DH. CRF receptor antagonist attenuates immobilization stress-induced norepinephrine release in the prefrontal cortex in rats. Brain Res Bull. 1997;42:431–4. doi: 10.1016/s0361-9230(96)00368-1. [DOI] [PubMed] [Google Scholar]
- Stein MB, Fallin MD, Schork NJ, Gelernter J. COMT polymorphisms and anxiety-related personality traits. Neuropsychopharmacology. 2005;30:2092–102. doi: 10.1038/sj.npp.1300787. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Hartman BK. The central adrenergic system. An immunofluorescence study of the location of cell bodies and their efferent connections in the rat utilizing dopamine-beta-hydroxylase as a marker. J Comp Neurol. 1975;163:467–505. doi: 10.1002/cne.901630406. [DOI] [PubMed] [Google Scholar]
- Swerdloff RS, Wang C, Hines M, Gorski R. Effect of androgens on the brain and other organs during development and aging. Psychoneuroendocrinology. 1992;17:375–83. doi: 10.1016/0306-4530(92)90042-6. [DOI] [PubMed] [Google Scholar]
- Szabadi E. Functional neuroanatomy of the central noradrenergic system. J Psychopharmacol. 2013;27:659–93. doi: 10.1177/0269881113490326. [DOI] [PubMed] [Google Scholar]
- Teli T, Markovic D, Levine MA, Hillhouse EW, Grammatopoulos DK. Regulation of corticotropin-releasing hormone receptor type 1alpha signaling: structural determinants for G protein-coupled receptor kinase-mediated phosphorylation and agonist-mediated desensitization. Mol Endocrinol. 2005;19:474–90. doi: 10.1210/me.2004-0275. [DOI] [PubMed] [Google Scholar]
- Thomsen DK, Yung Mehlsen M, Christensen S, Zachariae R. Rumination—relationship with negative mood and sleep quality. Personality and Individual Differences. 2003;34:1293–1301. [Google Scholar]
- Tolin DF, Foa EB. Sex differences in trauma and posttraumatic stress disorder: a quantitative review of 25 years of research. Psychol Bull. 2006;132:959–92. doi: 10.1037/0033-2909.132.6.959. [DOI] [PubMed] [Google Scholar]
- Tseng JY, Kolb PE, Raskind MA, Miller MA. Estrogen regulates galanin but not tyrosine hydroxylase gene expression in the rat locus ceruleus. Brain research Molecular brain research. 1997;50:100–6. doi: 10.1016/s0169-328x(97)00164-2. [DOI] [PubMed] [Google Scholar]
- Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–7. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Foote SL. Corticotropin-releasing factor disrupts sensory responses of brain noradrenergic neurons. Neuroendocrinology. 1987;45:28–36. doi: 10.1159/000124700. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Foote SL. Corticotropin-releasing hormone increases tonic but not sensory-evoked activity of noradrenergic locus coeruleus neurons in unanesthetized rats. J Neurosci. 1988;8:1016–25. doi: 10.1523/JNEUROSCI.08-03-01016.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentino RJ, Wehby RG. Corticotropin-releasing factor: evidence for a neurotransmitter role in the locus ceruleus during hemodynamic stress. Neuroendocrinology. 1988;48:674–7. doi: 10.1159/000125081. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Page ME, Curtis AL. Activation of noradrenergic locus coeruleus neurons by hemodynamic stress is due to local release of corticotropin-releasing factor. Brain Res. 1991;555:25–34. doi: 10.1016/0006-8993(91)90855-p. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Chen S, Zhu Y, Aston-Jones G. Evidence for divergent projections of corticotropin-releasing hormone neurons of Barrington’s nucleus to the locus coeruleus and spinal cord. Brain Res. 1996;732:1–15. doi: 10.1016/0006-8993(96)00482-9. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Van Bockstaele EJ. Corticotropin-releasing factor: putative neurotransmitter actions of a neurohormone. In: Pfaff AAD, Etgen A, Fahrbach S, Moss R, Rubin R, editors. Hormones, Brain and Behavior. Vol. 4. Academic Press; San Diego: 2002. pp. 81–102. [Google Scholar]
- Valentino RJ, Reyes B, Van Bockstaele E, Bangasser D. Molecular and cellular sex differences at the intersection of stress and arousal. Neuropharmacology. 2012;62:13–20. doi: 10.1016/j.neuropharm.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentino RJ, Bangasser D, Van Bockstaele EJ. Sex-biased stress signaling: the corticotropin-releasing factor receptor as a model. Molecular pharmacology. 2013a;83:737–45. doi: 10.1124/mol.112.083550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentino RJ, Van Bockstaele E, Bangasser D. Sex-specific cell signaling: the corticotropin-releasing factor receptor model. Trends Pharmacol Sci. 2013b;34:437–44. doi: 10.1016/j.tips.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Colago EE, Valentino RJ. Amygdaloid corticotropin-releasing factor targets locus coeruleus dendrites: substrate for the co-ordination of emotional and cognitive limbs of the stress response. J Neuroendocrinol. 1998;10:743–57. doi: 10.1046/j.1365-2826.1998.00254.x. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Bajic D, Proudfit H, Valentino RJ. Topographic architecture of stress-related pathways targeting the noradrenergic locus coeruleus. Physiol and Behav. 2001;73:273–283. doi: 10.1016/s0031-9384(01)00448-6. [DOI] [PubMed] [Google Scholar]
- Vathy I, Etgen AM. Ovarian steroids and hypothalamic norepinephrine release: studies using in vivo brain microdialysis. Life sciences. 1988;43:1493–9. doi: 10.1016/0024-3205(88)90396-7. [DOI] [PubMed] [Google Scholar]
- Violin JD, Lefkowitz RJ. Beta-arrestin-biased ligands at seven-transmembrane receptors. Trends Pharmacol Sci. 2007;28:416–22. doi: 10.1016/j.tips.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Wagner HR, Davies JN. Decreased β-adrenergic responses in the female rat brain are eliminated by ovariectomy: correlation of [3H]dihydroalprenolol binding and catecholamine stimulated cyclic AMP levels. Brain Research. 1980;201:235–239. doi: 10.1016/0006-8993(80)90792-1. [DOI] [PubMed] [Google Scholar]
- Whalen EJ, Rajagopal S, Lefkowitz RJ. Therapeutic potential of beta-arrestin- and G protein-biased agonists. Trends Mol Med. 2011;17:126–39. doi: 10.1016/j.molmed.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie T, Ho SL, Ramsden D. Characterization and implications of estrogenic down-regulation of human catechol-O-methyltransferase gene transcription. Molecular pharmacology. 1999;56:31–8. doi: 10.1124/mol.56.1.31. [DOI] [PubMed] [Google Scholar]
- Zangen A, Overstreet DH, Yadid G. Increased catecholamine levels in specific brain regions of a rat model of depression: normalization by chronic antidepressant treatment. Brain Research. 1999;824:243–250. doi: 10.1016/s0006-8993(99)01214-7. [DOI] [PubMed] [Google Scholar]
- Zobel AW, Nickel T, Kunzel HE, Ackl N, Sonntag A, Ising M, Holsboer F. Effects of the high-affinity corticotropin-releasing hormone receptor 1 antagonist R121919 in major depression: the first 20 patients treated. J Psychiatr Res. 2000;34:171–81. doi: 10.1016/s0022-3956(00)00016-9. [DOI] [PubMed] [Google Scholar]