Summary
Objective
Recombinant human leptin (metreleptin) improves glycemia and hypertriglyceridemia in patients with generalized lipodystrophy; antibody development with in vitro neutralizing activity has been reported. We aimed to characterize anti-metreleptin antibody development, including in vitro neutralizing activity.
Design
Two randomized controlled studies in patients with obesity (twice-daily metreleptin ± pramlintide for 20–52 weeks; 2006–2009); two long-term, open-label studies in patients with lipodystrophy (once-daily or twice-daily metreleptin for 2 months to 12.3 years; 2000–2014).
Patients
579 metreleptin-treated patients with obesity, 134 metreleptin-treated patients with lipodystrophy (antibody/neutralizing activity data: n=105).
Measurements
Anti-metreleptin antibodies, in vitro neutralizing activity.
Results
Anti-metreleptin antibodies developed in most patients (obese: 96–100%; lipodystrophy: 86–92%). Peak antibody titers (~1:125 to 1:3125) generally occurred within 4–6 months and decreased with continued therapy (lipodystrophy). Antibody development did not adversely impact efficacy or safety (patients with obesity), except for inflammatory injection site reactions, but was associated with elevated leptin concentrations. Three patients with obesity developed in vitro neutralizing activity coincident with weight gain. Weight later returned to baseline in one patient despite persistent neutralizing activity. Four patients with generalized lipodystrophy developed in vitro neutralizing activity concurrent with worsened metabolic control; two with confounding comorbidities had sepsis. One patient with lipodystrophy had resolution of neutralizing activity on metreleptin.
Conclusions
Development of in vitro neutralizing activity could be associated with loss of efficacy but has not been consistently associated with adverse clinical consequences. Whether neutralization of endogenous leptin with clinical consequences occurs remains unclear.
Keywords: Metreleptin, Immunogenicity, Antibody, Lipodystrophy, Antibody
Introduction
Leptin, the adipocyte-secreted protein product of the obese (OB) gene,1 plays a central role in regulation of energy homeostasis and fat and glucose metabolism.2 Metreleptin, a recombinant analog of human leptin differing from leptin by an additional methionine, is administered by subcutaneous injection once daily (QD) or twice daily (BID). It was initially studied as an obesity treatment but did not achieve clinically meaningful weight loss.3 The combination of metreleptin with pramlintide demonstrated greater weight loss for obesity than either therapy alone,4,5 but this program was discontinued.
Lipodystrophy is an extremely rare disorder characterized by loss of subcutaneous adipose tissue, leptin deficiency, ectopic lipid deposition, and metabolic abnormalities including diabetes mellitus and/or hypertriglyceridemia that can be extremely severe and often inadequately treated with conventional therapies.6–8 Clinical studies of metreleptin in patients with lipodystrophy have demonstrated substantially improved insulin resistance, diabetes, and/or hypertriglyceridemia.9–12
Metreleptin was recently approved as an adjunct to diet as replacement therapy to treat complications of leptin deficiency in patients with generalized lipodystrophy. Anti-metreleptin antibodies with in vitro neutralizing activity (NAc) have occurred in metreleptin-treated patients.13 Antibody development against therapeutic proteins is common14,15 and often without clinical relevance. However, anti-drug antibodies can sometimes lead to loss of efficacy by affecting pharmacokinetics, neutralizing drug activity, or adverse effects if neutralizing antibodies cross-react with an endogenous counterpart with key physiological functions. An example is pure red cell aplasia from anti-erythropoietin antibodies following recombinant human erythropoietin administration.16
For metreleptin-treated patients with lipodystrophy, it is important to understand the frequency of anti-metreleptin antibody development and characteristics including titer, onset, time course, and potential impact on efficacy and safety. Data from metreleptin studies in patients with obesity or lipodystrophy are presented herein.
Materials and methods
Clinical studies
Two randomized, double-blinded controlled studies (DFA101 [NCT00392925] and DFA102 [NCT00673387]) and one extension study (DFA102E [NCT00819234]) investigated metreleptin alone or in combination with pramlintide in patients with obesity (Table 1). A safety follow-up study (DFA106 [ClinicalTrials.gov identifier unavailable]) was conducted in patients who participated in DFA101 or DFA102/E to assess antibody status after discontinuing study drug. Two open-label studies (National Institutes of Health [NIH; NCT00025883] and FHA101 [NCT00677313]) investigated the safety and efficacy of metreleptin in patients with acquired or inherited lipodystrophy (excluding human immunodeficiency virus-associated lipodystrophy) (Table 1). Studies were conducted in accordance with the Declaration of Helsinki with protocols approved by each institutional review board. All patients provided written informed consent prior to study entry.
Table 1.
Summary of presented studies
| Clinical study and design | Inclusion criteria | Study therapy | Patient enrollment and demographics | Patients receiving metreleptin | Antibody and leptin levela assessments |
|---|---|---|---|---|---|
| Obesity | |||||
|
DFA101 24-wk, randomized, double-blind, active-drug-controlled study, starting in October 2006 |
Pts with obesity and BMI of 27–35 kg/m2 | 4-wk pramlintide (180–360 mcg BID) lead-in, then 20 wks treatment (3 arms):
|
177 enrolled:
139 randomized |
83 metreleptin-treated (56 combo, 27 mono) 94 (67.6%) completed randomized treatment |
Baseline and every 4 wks during study |
|
DFA102 28-wk, randomized, double-blind, placebo-controlled, dose-ranging study, starting in April 2008 |
Pts with obesity and BMI of 27–45 kg/m2 | 28-wks treatment (8 arms):
|
636 enrolled 608 randomized:
|
456 metreleptizn-treated (384 combo, 72 mono) 370 (60.9%) completed randomized treatment |
Baseline and every 4 wks during study |
|
DFA102E 24-wk extension of DFA102, starting in November 2008 |
Completed DFA102 | Placebo → placebo Monotherapy and low-dose pramlintide combination (180/2.5, 180/5.0) → high-dose pramlintide combination (360/1.25, 360/2.5, 360/5.0) |
274 enrolled | 199 completed study 44 not previously treated with metreleptin in DFA102 received metreleptin in DFA102E |
Baseline and every 4 wks during study |
|
DFA106 Safety follow-up study (single visit) |
Received ≥1 dose of study drug in DFA101, 102/E (N=784b eligible) | No treatment | 419 (53.4%) enrolled:
|
In prior study, 326c metreleptin-treated (mean exposure, 31.3 wks [0.1– 54.7], mean time since last dose, 3.0 yrs [2.1–4.8]), 51 pramlintide-treated, 42 placebo-treated | At study visit |
| Lipodystrophy | |||||
|
NIH Open-label, open-ended, investigator-sponsored, starting in August 2000 |
Acquired or inherited LD (excluding HIV-LD), age ≥6 mos, ≥1 metabolic abnormality (DM, fasting TG >200 mg/dL, or fasting insulin >30 μIU/mL), fasting leptin level <12 (females) or <8 (males) ng/mL | Metreleptin QD or BID, weight-based dosing depending on sex and age, with subsequent adjustment based on individual response | 107d enrolled | Metreleptin exposure at time of last NAc assessment: mean 3.9 yrs, median 2.6 yrs (range, 2 mos–12.3 yrs) | N=81 with NAc data on metreleptin, a subset (N=43) of whom had anti-metreleptin Ab data (2–4 time points per patient due to sample volume limitations) |
|
FHA101 Open-label, open-ended, industry-sponsored expanded access protocol, starting in March 2009 |
Acquired or inherited LD (excluding HIV-LD), age ≥5 yrs, DM and/or fasting TG >200 mg/dL | Metreleptin QD or BID, weight-based dosing depending on sex and age, with subsequent adjustment based on individual response | 27e enrolled at University of Michigan site where protocol specified Ab and leptin level assessment | Metreleptin exposure at time of last NAc assessment: mean and median 2.6 yrs (range, 0.6–4.8 yrs) | Baseline and every 3 mos during study N=24 with anti-metreleptin Ab and NAc data on metreleptin |
Leptin levels for NIH study measured using RIA;
1 patient who enrolled in DFA101 did not receive any study medication;
324 of 326 patients had binding Ab data at both the end of the prior treatment study and the safety follow-up;
N=2 discontinued metreleptin before any follow-up assessments could be performed, N=2 with only baseline NAc assessment, N=22 with no NAc or antibody assessment;
N=3 discontinued metreleptin before any follow-up assessments could be performed.
Ab, antibody; BID, twice daily; BMI, body mass index; DM, diabetes mellitus; HIV-LD, human immunodeficiency virus lipodystrophy; LD, lipodystrophy; mos, months; NAc, neutralizing activity; NIH, National Institutes of Health; pts, patients; QD, once daily; TG, triglycerides; wks, week; yrs, years.
Bioanalytical methods
Plasma leptin concentration was determined using a commercially available (Millipore, Bedford, MA, USA) and validated, solid-phase sandwich enzyme-linked immunosorbent assay (ELISA) using a polyclonal capture antibody (obesity studies, FHA101) or radioimmunoassay (RIA; NIH study). The signal is directly proportional to total leptin (ie, metreleptin and endogenous human leptin) in the sample. ELISA results were quantified by comparison to a standard curve of defined metreleptin concentrations. RIA results were quantified using kit standards. The lower limit of quantification for both methods is 0.7 ng/mL. Both metreleptin and endogenous human leptin are detected with equivalent efficiency. Agreement between methods is high (ρ>0.9). Anti-metreleptin antibodies present in ELISA-tested samples did not interfere with quantification until titers >1:3125. The ELISA used in this study was found to have good quantitative correlation with a previously published method for leptin determination using liquid chromatography-tandem mass spectrometry (LC-MS/MS).17
Anti-metreleptin antibody titer was determined from serum samples tested by bridging ELISA. The solution phase method incorporates two modified metreleptin conjugates combined with a sample to allow subsequent capture and detection of antibody-metreleptin complexes (Supplemental Fig. 1). The method allows detection of all antibody isotypes and does not distinguish between isotypes. A competition step is included to confirm specificity of an antibody result. Treatment-emergent antibody-positive was defined as titer ≥1:5 following a negative or missing baseline titer or titer increasing ≥2 dilutions from a detectable baseline titer.
In vitro NAc to metreleptin was determined by measuring the inhibition of a murine, interleukin (IL)-3- dependent lymphoblastoid cell line18 modified to express a recombinant, chimeric receptor comprising the extracellular domain of the leptin receptor and intracellular domain of the erythropoietin receptor, when patient plasma was added to the culture (Supplemental Fig. 2). If the sample inhibited IL-3-dependent metabolism, the result was reported as non-specific. Evidence of in vitro NAc is based on potency and specificity of response. If the result was less than the assay cut point upon initial testing, retesting, or ten-fold dilution, the sample was considered to have no evidence of NAc. If the result was greater than the assay cut point after ten-fold dilution and inhibitory activity was specific for metreleptin, the sample had evidence of high potency, reproducible in vitro NAc to metreleptin. In vitro NAc is reported relative to a reference sample (eg, patient’s pre-metreleptin sample or a pool of human serum from metreleptin-naïve donors).
Results
Characterization of anti-drug antibodies
Obesity studies
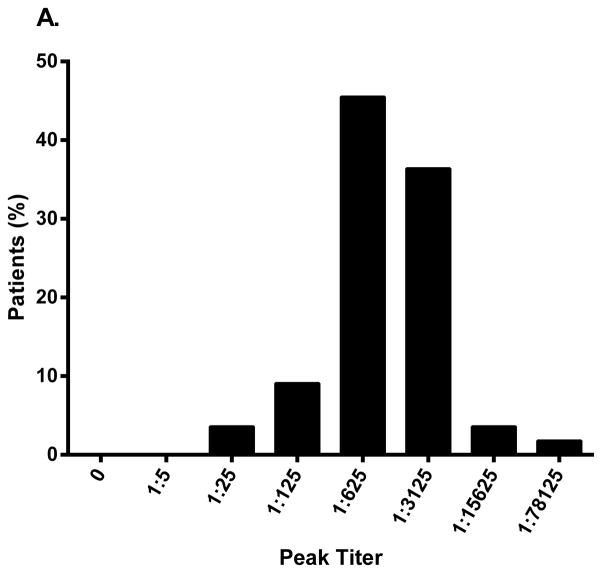
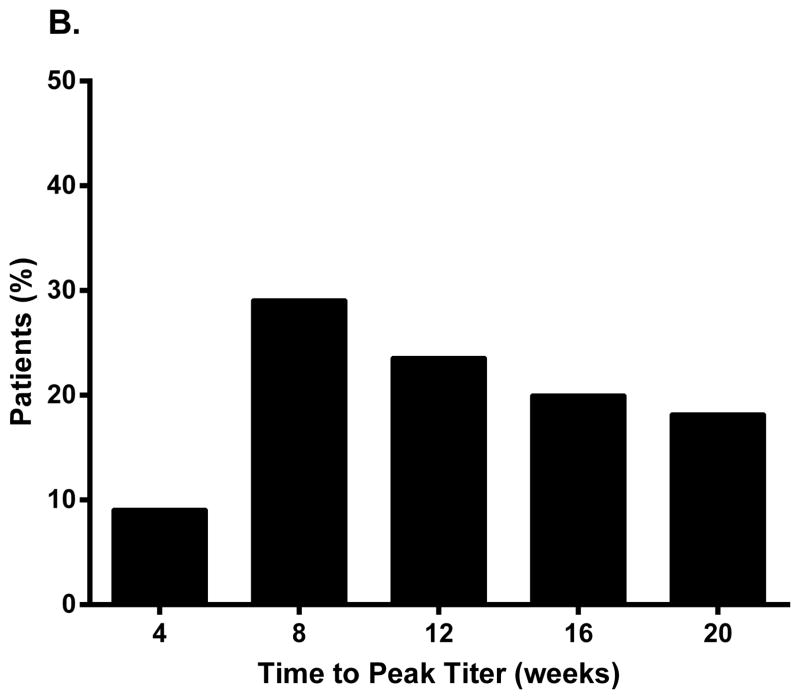
In Study DFA101, all patients randomized to metreleptin (±pramlintide) were antibody-positive at Week 8, and all except one patient receiving pramlintide+metreleptin) were still antibody-positive at Week 20 (study termination). The majority had a peak titer of 1:625 or 1:3125 (Fig. 1A), and ~80% reached peak titer by Week 16 (Fig. 1B). The mean (standard deviation) percent weight loss at Week 20 in patients randomized to pramlintide+metreleptin was similar across peak antibody titer groups: −11.0 (0.4)% with titer 1:25 [n=2], −11.2 (9.1)% for 1:125 (n=3), −10.9 (4.5)% for 1:625 (n=14), −11.5 (6.2)% for 1:3125 (n=15), and −18.7 (2.9)% for 1:15625 (n=2).
Fig. 1.
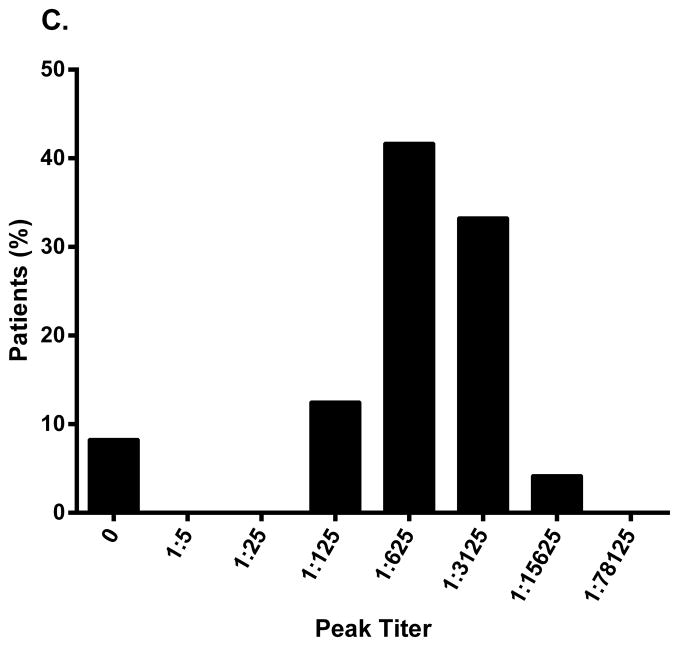
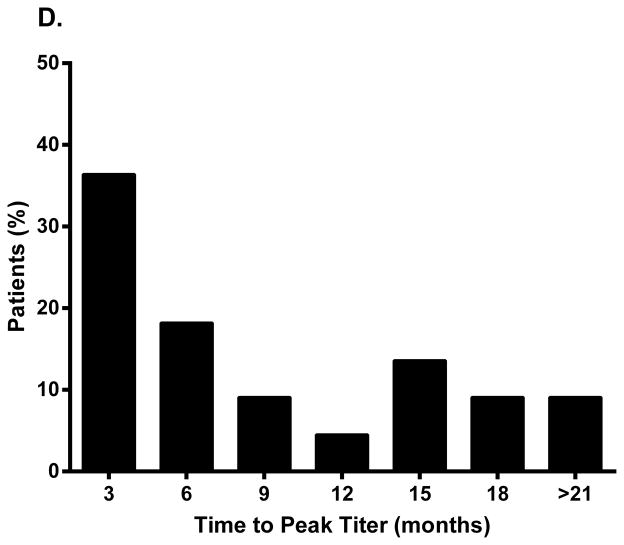
Peak binding antibody titer and time to peak titer in (A, B) patients with obesity (Study DFA101) and (C, D) patients with lipodystrophy (Study FHA101). Study DFA101: black bar, metreleptin 5 mg BID and pramlintide 360 mcg BID + metreleptin 5 mg BID combined (N=55). Study FHA101: black bar, metreleptin treatment (N=24). BID, twice daily.
In Study DFA102, antibody formation was minimal at Week 4 in metreleptin-treated patients (±pramlintide), but ≥96% were antibody-positive by Week 28 (study termination). Peak antibody titer occurred at Week 16 for the 2.5- and 5-mg dose groups, with average titers around 1:125 to 1:625. For the 1.25-mg dose group, antibody titer peaked around Week 8 and plateaued. The 2.5- and 5-mg dose groups were generally associated with higher antibody titer earlier in treatment. The mean percent weight loss at Week 28 in the pramlintide-metreleptin group tended to be lower in the 1:3125 (vs 1:125 and 1:625) peak titer groups across different dose combinations, although there were fewer patients with peak titer 1:3125.
The incidence of potentially immune-related adverse events (AEs) in DFA102 (mostly inflammatory injection site AEs) increased with increasing antibody titer, whereas the incidence of all other AEs was similar across titer groups. The incidence of inflammatory injection site AEs was 42.7% (n=96/225) in those with peak titer ≤1:125, 67.2% (n=117/174) for 1:625, and 85.1% (n=40/47) for ≥1:3125.
The majority of metreleptin-exposed patients with antibody data (80.9% [n=262/324]) from DFA106 were antibody-negative at safety follow-up, while 19.1% (n=62/324) were antibody-positive an average of 3 years after the last dose of metreleptin in the prior study. Of 62 antibody-positive patients at safety follow-up, the majority had low titer of 1:5 (11.3% [n=7]), 1:25 (50.0% [n=31]), or 1:125 (33.9% [n=21]), while 3.2% (n=2) and 1.6% (n=1) had titers of 1:625 and 1:78125, respectively. All patients who remained antibody-positive at the safety follow-up had a lower titer compared with the last value from the previous treatment study except for three patients (one shifted from 1:5 to 1:25, one from negative to 1:625, and one from 1:625 to 1:78125).
The proportions of patients in various categories of weight change from the previous treatment study baseline to the safety follow-up (weight loss, weight increase of >0% to ≤10%, or weight increase of >10% to ≤50%) were similar between those receiving placebo, pramlintide, or metreleptin±pramlintide in the previous study, and were also similar in metreleptin-treated patients who were antibody-positive vs antibody-negative at safety follow-up (Supplemental Table 1). Two patients had notable increases in body weight (>50% vs previous study baseline) at the safety follow-up: one who received placebo (weight gain of 77.3% vs prior study baseline; 34.7% from prior study end), and one who received pramlintide-metreleptin (see In Vitro NAc).
Similarly, the proportion of patients reporting AEs of interest including diabetes or infection leading to hospitalization occurring between the end of the previous study and the safety follow-up was similar between those receiving placebo, pramlintide, or metreleptin (diabetes: 4.8% [n=2/42], 5.9% [n=3/51], and 5.5% [n=18/326], respectively; infection: 4.8% [n=2/42], 2.0% [n=1/51], and 1.5% [n=5/326], respectively). All metreleptin-treated patients who experienced infection leading to hospitalization were antibody-negative.
Lipodystrophy studies
Of 43 patients with lipodystrophy and anti-metreleptin antibody data in the NIH study, 86% (n=37) developed antibodies during metreleptin treatment with titers ranging from 1:5 to 1:78125. The duration of treatment at the time of antibody assessment ranged from 4–138 months.
Similarly, of 24 patients with lipodystrophy and antibody data in the FHA101 study, 92% (n=22) developed antibodies during metreleptin treatment. The majority had a peak titer of 1:625 or 1:3125 (Fig. 1C), and time to peak titer ranged from 3 to >21 months (Fig. 1D). Most patients remained antibody-positive on treatment, but titer generally decreased over time, despite continued metreleptin exposure.
Impact of antibodies on leptin concentrations: obesity and lipodystrophy studies
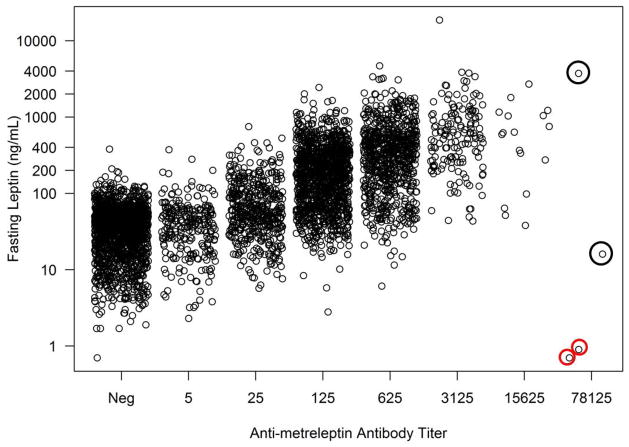
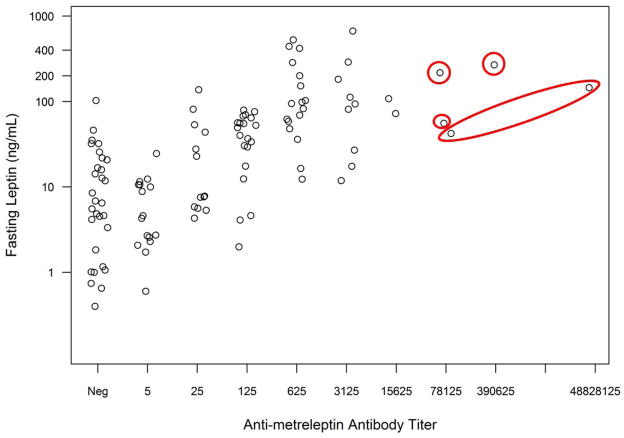
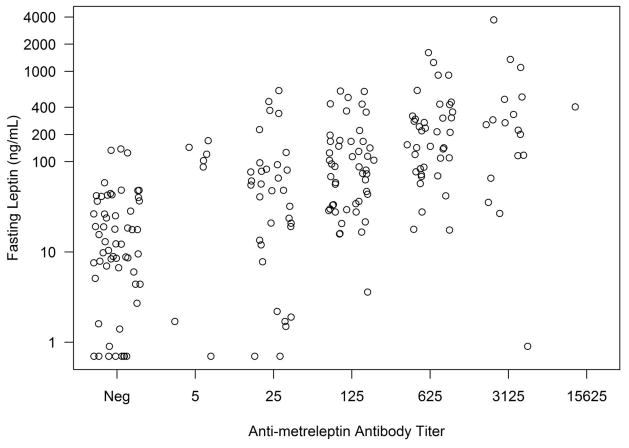
Antibody development in patients with obesity or lipodystrophy was associated with higher leptin concentrations, and higher antibody titers were associated with higher leptin concentrations (Figs. 2A–C). Very high antibody titer (≥1:78125) was observed infrequently. One DFA101 patient had a 1:78125 titer with leptin concentration of 616 ng/mL (4 weeks prior), which decreased to 1:25 (with leptin concentration of 3.2 ng/mL) at the safety follow-up 4.6 years after discontinuing metreleptin. Two DFA102 patients had a 1:78125 titer (see In Vitro NAc). One DFA102E patient had a 1:78125 titer at Week 40 that decreased to 1:15625 at Week 52 (study termination) and decreased further to 1:125 (with leptin concentration of 45.2 ng/mL) at the safety follow-up 2.6 years after discontinuing metreleptin.
Fig. 2.
Fasting leptin concentrations vs antibody titer at baseline and during metreleptin treatment in (A) patients with obesity (DFA101 and DFA102/E studies) and patients with lipodystrophy in the (B) NIH study and (C) FHA101 study. Red circles around individual plots denote patients who developed in vitro NAc. Black circles around individual plots denote patients with very high antibody titer who did not develop in vitro NAc. NAc, neutralizing activity; Neg, negative.
In vitro NAc: obesity and lipodystrophy studies
Seven patients (obese, n=3; lipodystrophy, n=4) were identified with in vitro NAc from the DFA and NIH clinical studies; no patients with lipodystrophy with in vitro NAc have been identified from FHA101. Table 2 provides relevant medical history, baseline characteristics, labs, timing of NAc, and clinical course.
Table 2.
Patients identified with in vitro neutralizing activity (NAc) from the DFA and NIH clinical studies of metreleptin
| Demographics | Relevant medical history | Baseline characteristics and labs | Metreleptin treatment | Initial identification of NAc and subsequent course | Clinical course |
|---|---|---|---|---|---|
| 40-yr-old female with obesity | None | BMI: 42.5 kg/m2 Weight: 129.9 kg FPG: 93–101 mg/dL |
Metreleptin 5 mg BID x 28 wks | At 20 wks of metreleptin to 8 mos after discontinuing metreleptin – no further follow-up | Initial weight loss followed by weight regain 4.5 kg above baseline at ST, 26 kg above baseline 8 mos after discontinuing metreleptin |
| 48-yr-old male with obesity | None | BMI: 38.2 kg/m2 Weight: 127.9 kg FPG: 116 mg/dL |
Metreleptin 2.5 mg – Pramlintide 180 mcg BID x 28 wks | At 24 wks of metreleptin to 6 yrs after discontinuing metreleptin – continuing follow-up | Initial weight loss followed by weight regain 3.3 kg above baseline at ST, 12.6 kg above baseline 12 mos after discontinuing metreleptin. Diagnosis of DM 4 mos after ST (treated with metformin and glimepiride). Over next 4 yrs, progressive weight loss back to baseline, with discontinuation of DM meds and A1C 6.4%. Slight increase in body weight at last available follow-up |
| 39-yr-old female with obesity | None | BMI: 33 kg/m2 Weight: 85.6 kg Normal FPG |
Metreleptin 2.5 mg – Pramlintide 360 mcg BID x 28 wks | At ~3 yrs after discontinuing metreleptin to 3 yrs later – continuing follow-up | Weight gain 64.4 kg above baseline (to 150 kg) at ~3 yrs after discontinuing metreleptin. Diagnosed with DM (A1C 7.3%) 6 mos after the DFA106 safety follow-up (treated with glipizide and insulin). Weight decrease by 15 kg 3 yrs after DFA106 safety follow-up |
| 16-yr-olda female with congenital generalized LD | DM, high TG, cirrhosis, portal hypertension, hepatic encephalopathy, esophageal varices, proteinuria, cardiomyopathy, poor dental hygiene | BMI: 21.6 kg/m2 A1C: 9.8% TG: 600 mg/dL |
Metreleptin 2.25 – 2.5 mg BID x 4.5 yrs | At ~3.5 yrs of metreleptin until withdrawn from treatment 1 yr later | Good initial metabolic response to metreleptin while reducing insulin from 300–100 units/day. After 18 mos, gradual worsening of metabolic status back to baseline at 4 yrs. Multiple episodes of sepsis (Gemella, Streptococcus, Stenotrophomonas, Acinetobacter species) from 2 mos prior to identification of NAc to several mos after. Died 7 mos after withdrawn from metreleptin from gastrointestinal bleed from pre-existing varices |
| 13-yr-olda female with congenital generalized LD | DM, high TG, cirrhosis, portal hypertension, hepatopulmonary syndrome, chronic hypoxia, poor dental hygiene | BMI: 16.4 kg/m2 A1C: 8.6% TG: 49 mg/dL |
Metreleptin 3 – 5 mg per day x 5.5 yrs – lost to follow-up | At ~5.5 yrs of metreleptin – no further follow-up | Good metabolic response to metreleptin during first 2 yrs (A1C reduced to 5.8%) but worsened glycemic control (A1C 13.1%) at 3 yrs. Reported episode of sepsis at 4 yrs treatment (fever, abdominal swelling), no organism identified; treated with antibiotics, recovered, no further episodes |
| 20-yr-olda female with congenital generalized LD | DM, high TG, pancreatitis, fatty liver, proteinuria, polycystic ovary disease, diabetic neuropathy | BMI: 27.4 kg/m2 A1C: 9.7% TG: 1510 mg/dL |
Metreleptin 7.5 – 10 mg BID – ongoing | At baseline in NIH study (prior metreleptin treatment in another study x 2.5 yrs) and after 4 yrs in NIH study. Resolved after 4.5 yrs in NIH study | Fluctuating metabolic control in NIH study but generally suboptimal (A1C often >9%, TG 1000– 5000 mg/dL), but poor compliance with metreleptin and DM/lipid meds. A1C and TG improved and insulin treatment discontinued at 4.5 yrs in NIH study when NAc resolved |
| 11-yr-olda female with acquired generalized LD | Severe insulin resistance (no DM), high TG, steatohepatitis | BMI: 16.6 kg/m2 A1C: 5.3% TG: 368 mg/dL |
Metreleptin 2.65 – 2.8 mg QD – ongoing | >3 yrs of metreleptin – continuing follow-up | Stable A1C and TG during first 3 yrs of treatment. Diagnosed with localized anaplastic large cell lymphoma after 2 yrs of metreleptin (excised, treated with chemo without recurrence). When NAc identified, A1C 8.3% |
Age at study enrollment.
A1C, glycated hemoglobin; Ab, antibody; BID, twice daily; BMI, body mass index; DM, diabetes mellitus; FPG, fasting plasma glucose; kg, kilogram; LD, lipodystrophy; mos, months; NAc, neutralizing activity; NIH, National Institutes of Health; QD, once daily; ST, study termination; TG, triglycerides; wks, weeks; yrs, years.
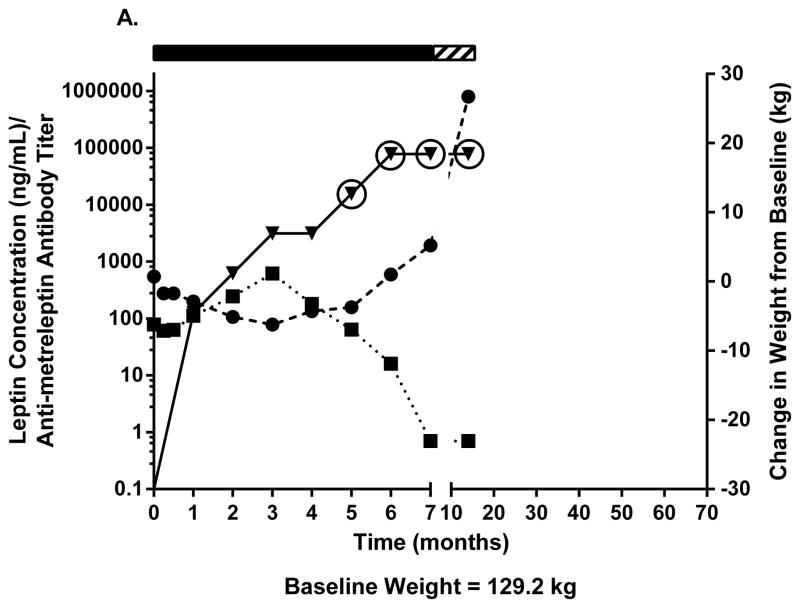
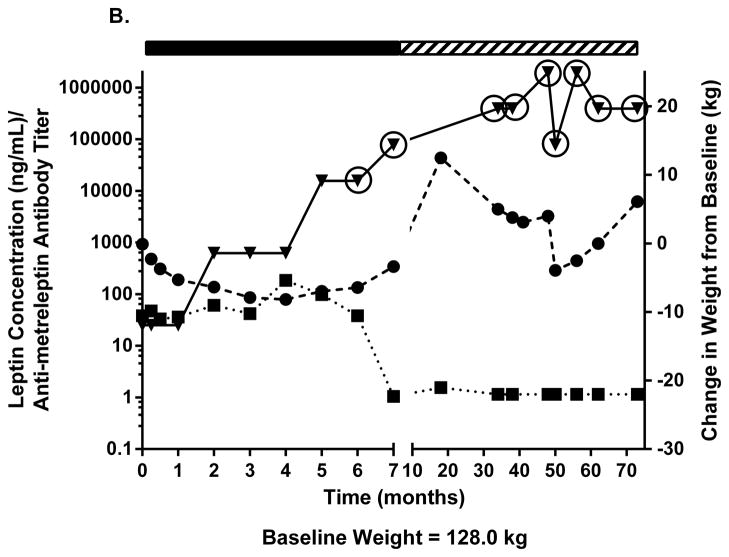
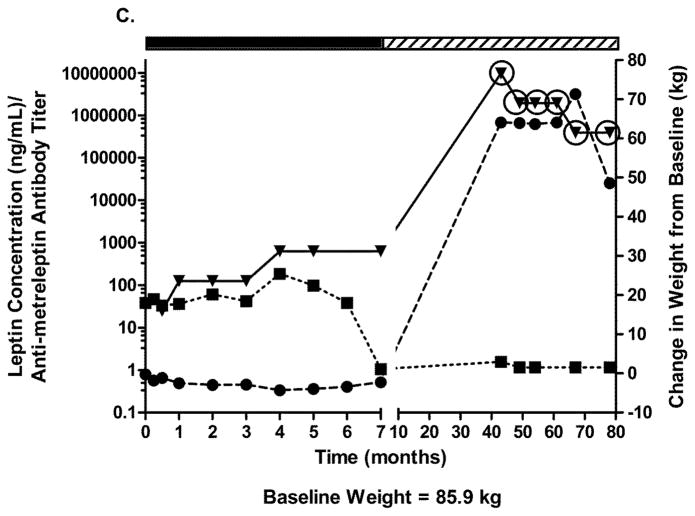
Two patients with obesity had NAc after 20–24 weeks of metreleptin treatment in DFA102. Figs. 3A–C show body weight, plasma leptin concentration, antibody titer, and NAc during and after discontinuing metreleptin. Initial weight loss was followed by weight regain occurring in parallel with decreasing leptin concentration and increasing antibody titers with NAc identified at 20–24 weeks. One patient had weight gain 8 months later but no further follow-up (Fig. 3A). The other patient entered a safety follow-up program and had progressive weight loss over several years back to his pre-metreleptin baseline (slight increase at last follow-up) despite persistent NAc (Fig. 3B). A third patient with obesity had no NAc at DFA102 termination, but NAc was identified at the DFA106 safety follow-up ~3 years after DFA102. She had substantial weight gain but had decreased weight 3 years later (Fig. 3C).
Fig. 3.
Changes in body weight, fasting leptin concentration, and antibody titer over time in (A–C) three patients with obesity with evidence of in vitro NAc. (■), fasting leptin concentration; (▼), antibody titer; (●), body weight. Solid rectangle above the plot represents time on metreleptin treatment. Hashed rectangle represents time off metreleptin treatment. Circles around individual plots denote the detection of in vitro NAc. NAc, neutralizing activity.
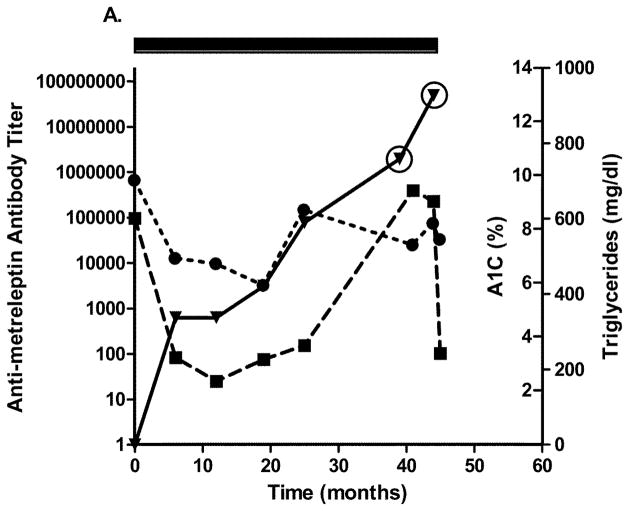
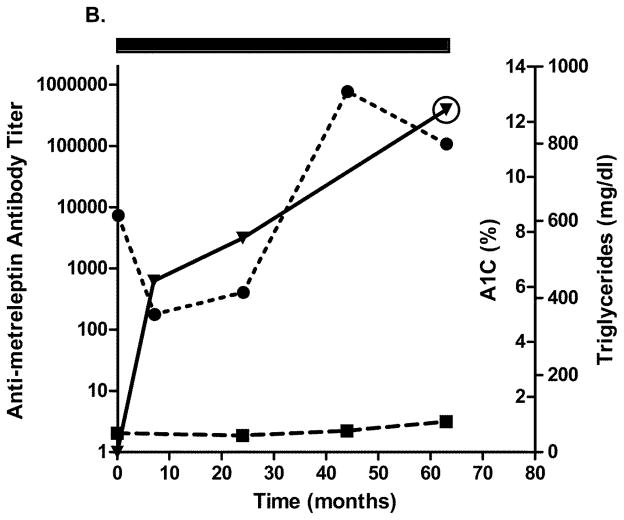
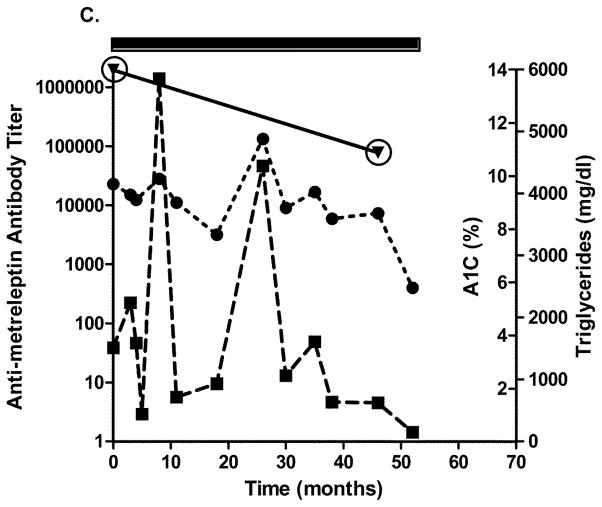
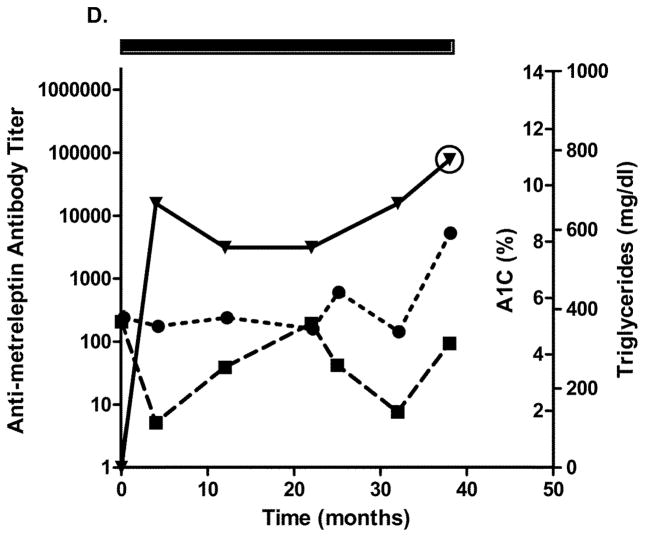
Four patients with generalized lipodystrophy in the NIH study had in vitro NAc detected after 2.5 years of metreleptin treatment. Relatively infrequent sampling limited the ability to precisely determine when NAc developed. Figs. 4A–D show glycated hemoglobin, triglycerides, antibody titer, and NAc during the study. One patient with lipodystrophy received metreleptin for ~2.5 years in another investigator’s study (NCT00896298) and had NAc at the NIH study baseline. Development of in vitro NAc in all four patients was concurrent with poor or worsened metabolic control. Two patients with lipodystrophy (both with advanced liver disease and poor dentition) had episodes of sepsis: One had multiple episodes with different organisms a few months prior to, and for several months after, NAc detection, and the other had a single episode ~1.5 years before NAc identification (NAc status at the time of sepsis unknown). Sources of infection for the first patient were unclear, but risk factors included severe liver disease, poor dentition, and peripherally inserted central catheters for broad-spectrum antibiotics. Notably, one patient with lipodystrophy (with NAc upon transfer into the NIH study) had resolution of NAc 4 years later (while continuing metreleptin) that was associated with substantially improved metabolic abnormalities (Fig. 4C).
Fig. 4.
Changes in A1C, triglycerides, and antibody titer over time in (A–D) four patients with lipodystrophy with evidence of in vitro NAc. (●), A1C; (■), triglycerides; (▼), antibody titer. Solid rectangle above the plot represents time on metreleptin treatment. Circles around individual plots denote the detection of in vitro NAc. A1C, glycated hemoglobin; NAc, neutralizing activity.
One FHA101 patient with familial partial lipodystrophy and prior history of recurrent urinary tract infections and lactic acidosis had multiple episodes of urinary tract and bladder infections over the first 3 years of metreleptin treatment but showed no evidence of NAc.
Discussion
Biologic drugs, being immunologically foreign peptides or proteins, commonly induce anti-drug immune responses,14,15 and metreleptin is no exception. Anti-metreleptin antibodies have been detected in >85–90% of patients with obesity or lipodystrophy receiving metreleptin in clinical studies. This high incidence of antibody development may relate to physicochemical properties of metreleptin and non-physiologic administration (subcutaneous injection vs secretion into circulation). However, the exact mechanism by which metreleptin elicits this high rate of antibodies is not definitively established.
In most patients, anti-metreleptin antibodies occurred at a moderate titer detected within 4–8 weeks following treatment initiation, reached peak titer or plateau within 4–6 months, and tended to decrease over time with long-term treatment but generally did not disappear completely despite chronic antigen presentation during continued therapy. Antibodies were still detectable in ~20% of patients with obesity approximately 3 years after discontinuing metreleptin, although titers decreased to a low level in almost all patients.
Anti-metreleptin antibody development was not associated with an adverse impact on efficacy or safety in patients with obesity, except for increased frequency of inflammatory injection site AEs. In patients with obesity or lipodystrophy, anti-metreleptin antibodies resulted in increased leptin concentrations to supraphysiologic ranges (the leptin assay measures both metreleptin and endogenous leptin), likely due to delayed clearance of antibody-bound metreleptin. As previously described in another method using LC-MS/MS,17 the increase in leptin levels observed during metreleptin treatment results from accumulation of metreleptin as endogenous leptin concentrations remain stable. Higher antibody titers were associated with higher leptin concentrations although there was considerable patient variability. This effect confounds accurate determination of leptin concentrations during metreleptin treatment and precludes their use for correlating drug levels with response to therapy.
In some patients, in vitro NAc and very high antibody titer (≥1:78125) were identified. Although this very high titer was observed in all cases in which NAc was identified, very high antibody titer was also observed in the absence of NAc and therefore is not specific for NAc. In the three patients with obesity and NAc, leptin concentration was below the assay limit according to the primary ELISA method used (leptin concentrations using ELISA were unavailable for the NIH study). However, interference occurs with antibody titer >1:3125 using this ELISA. Thus, the finding of undetectable leptin in these patients should not be construed as indicating loss of all endogenous leptin action since leptin measurement with very high antibody titer is unreliable. Leptin measurement in these samples using an immunoassay with a different detection antibody, or mass spectrometry,17 confirmed that leptin could be detected in all samples subjected to these alternative methods (data not shown). Development of a more sensitive assay that will help further characterize the clinical impact of in vitro NAc is underway.
Clinical consequences coincident with in vitro NAc could include interference with activity of metreleptin, resulting in loss of efficacy (although non-compliance with metreleptin should be considered first). This may not be surprising in the context of “replacement therapy” in leptin-deficient patients. NAc development could also potentially affect normal physiological processes regulated by leptin (including immune function).19 This is difficult to definitively establish through statistical comparisons given the small number of cases identified to date, especially because the cases associated with severe infections were confounded by complex coexisting medical conditions (eg, severe chronic liver disease, poor dentition).
In individuals with obesity, the concern with NAc is the potential for associated development of a phenotype resembling congenital leptin deficiency with severe hyperphagia, substantial weight gain, and other obesity-related metabolic abnormalities (eg, diabetes, dyslipidemia).20, 21 While the development of in vitro NAc in the three patients with obesity was concurrent with weight gain/regain, long-term follow-up in one patient demonstrated weight loss and improved diabetes despite persistent NAc, indicating that in vitro NAc is not always associated with adverse clinical consequences. Additionally, weight gain commonly occurs in patients with obesity, and one DFA106 patient who received placebo in the prior treatment study had a similar amount of weight gain as the DFA106 metreleptin-treated patient with in vitro NAc. None of the patients with obesity had any remarkable medical history or clinical characteristics that might indicate why they developed this laboratory finding.
Two of the patients with obesity developed in vitro NAc during metreleptin treatment, but NAc developed after metreleptin discontinuation in the third. Individuals with obesity have high endogenous leptin concentrations,22 which may serve as a persistent immune stimulus. This may be less relevant for patients with generalized lipodystrophy and very low endogenous leptin concentrations,23 although the minute antigen levels needed for immune stimulus may make this distinction irrelevant. Further studies evaluating metreleptin immunogenicity in the post-marketing setting, including long-term follow-up during treatment and after discontinuation, may provide more information on the frequency of in vitro NAc after discontinuing treatment and any associated clinical consequences.
In three of four patients with lipodystrophy and in vitro NAc, there appeared to be an association of in vitro NAc with loss of efficacy (mainly loss of glycemic control and increased triglycerides in one), though the number of patients was too small to draw statistically significant conclusions. In the patient with lipodystrophy previously treated with metreleptin who had NAc at the NIH study baseline, metabolic control was generally poor during the first 3 years in the NIH study, although she was also was noncompliant with medications. However, she had markedly improved metabolic parameters coincident with a sample demonstrating resolution of NAc. Thus, in vitro NAc can resolve during metreleptin treatment, allowing these patients to potentially continue deriving benefit from treatment.
In patients with lipodystrophy who are leptin deficient at baseline (especially generalized lipodystrophy), the consequences of neutralizing endogenous leptin are less clear. Sepsis was reported in two patients with lipodystrophy with in vitro NAc, but both also had end-stage liver disease and poor dentition that could have predisposed them to infection. Notably, no sepsis events were reported in any other patient (obesity or lipodystrophy) with NAc who did not have advanced liver disease. In addition, another metreleptin-treated patient with lipodystrophy and multiple urinary and bladder infections had no evidence of NAc. While the possibility that NAc could increase risk of infection in susceptible individuals cannot be excluded, there is no evidence to date that in vitro NAc would cause severe infection in a patient not otherwise susceptible.
While the concern for potential clinical consequences of NAc may theoretically be greater for patients with partial lipodystrophy who often have higher endogenous leptin concentrations,23 all four lipodystrophy NAc cases to date occurred in patients with generalized lipodystrophy. Two-thirds of NIH study patients had generalized lipodystrophy, so the finding of NAc only in patients with generalized lipodystrophy could be due to the greater number in this subgroup. Although leptin concentrations are usually very low in patients with generalized lipodystrophy, they can still make leptin and thus are distinct from patients with congenital leptin deficiency due to OB gene mutations who cannot synthesize leptin and for whom leptin (and metreleptin) is a foreign protein. Transient NAc in association with loss of efficacy was reported in two patients with congenital leptin deficiency treated with metreleptin20 but resolved with continuing treatment.
In eight metreleptin-treated children with congenital generalized lipodystrophy from a separate French study, some patients who had beneficial metabolic effects with metreleptin in an earlier study24 developed metreleptin resistance in a follow-up study, although adherence to therapy was not reported.25 NAc measurement using an in-house primary cell culture in two of three patients with a “negative” clinical response suggested an immunological origin for their metreleptin resistance.25 In vitro NAc was not assessed in the other patients (one other “negative” responder, two “partial” responders, and three “complete” responders). Samples from these eight patients were tested using the NAc assay described herein, and in vitro NAc was confirmed in one of the two patients reported with immunological resistance, but not the rest (data on file). This patient demonstrated a lack of response to metreleptin from treatment initiation rather than initial response followed by loss of efficacy, and testing several years after discontinuing metreleptin showed resolution of in vitro NAc (data on file).
When deciding on metreleptin treatment for a patient with generalized lipodystrophy, consideration should be given to potential benefits of improved metabolic control vs potential risks of developing anti-metreleptin antibodies with NAc, along with other potential benefits and risks. Management of metabolic abnormalities in patients with lipodystrophy can be challenging with standard therapies because of the underlying pathophysiology (ectopic lipid deposition) and severity of metabolic abnormalities. While caution is warranted in using metreleptin, especially in patients who may be at high risk for severe infections, metreleptin has also been shown to improve metabolic abnormalities (diabetes and/or hypertriglyceridemia) in patients with generalized lipodystrophy, resulting in substantial improvement in some cases. Based on currently available data, it is expected that the benefit-risk profile of metreleptin treatment would be favorable for the majority of patients with generalized lipodystrophy and complications of leptin deficiency.
Development of in vitro NAc in patients with lipodystrophy may be associated with loss of efficacy, although the number of cases is too small to be definitive. In patients with obesity, in vitro NAc has not been consistently associated with adverse clinical consequences. Whether neutralization of endogenous leptin with clinical consequences occurs remains unclear.
Supplementary Material
Acknowledgments
Funding: The National Institutes of Health (NIH) study was supported by AstraZeneca (AZ), Bristol-Myers Squibb (BMS), and Amylin Pharmaceuticals (a member of the AstraZeneca group of companies) with provision of metreleptin and by the NIH for all other aspects of the study. FHA101 was an industry-sponsored (Amylin Pharmaceuticals Inc., BMS, AZ) expanded access protocol and used infrastructure at the University of Michigan supported by NIH Clinical and Translational Science Award (CTSA) grant UL1TR000433 and Nutrition Obesity Research Center (NORC) grant P30 DK089503. The DFA101, DFA102/E, and DFA106 studies were industry-sponsored clinical trials conducted by Amylin Pharmaceuticals, Inc. Development of the manuscript was supported by AstraZeneca.
Robert Schupp, PharmD, CMPP, of inScience Communications, Springer Healthcare, provided medical writing support funded by Aegerion Pharmaceuticals and AstraZeneca.
Non-Author Contributions: We gratefully thank the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Core Laboratory for assistance with sample processing and management and Michelle Ashmus for coordination of samples for antibody testing for the NIH study. Adam H. Neidert, MS, helped in the coordination, and Drs. Nevin Aljuni and Suma Amarnath participated in the execution of the FHA101 study. We appreciate the efforts of Bradly Boone, Manju Saxena, and Edwin Golez in refining and improving the immunogenicity test methods and ensuring timely and accurate testing of samples.
Footnotes
Clinical Trial Registration Numbers: NCT00392925, NCT00673387, NCT00819234, NCT00025883, NCT00677313
Disclosure Statement: JLC and JK were employees of Bristol-Myers Squibb, and JSH was an employee of AstraZeneca, at the time of manuscript preparation. EKC, PG, and RJB have no conflicts of interest to disclose. EAO was supported in part by NIH grant R01DK088114 and has received grant support and served as a consultant for Amylin Pharmaceuticals Inc., Bristol-Myers Squibb, and AstraZeneca.
References
- 1.Zhang Y, Proenca R, Maffei M, et al. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 2.Friedman J. 20 years of leptin: Leptin at 20: an overview. J Endocrinol. 2014;223:T1–T8. doi: 10.1530/JOE-14-0405. [DOI] [PubMed] [Google Scholar]
- 3.Heymsfield SB, Greenberg AS, Fujioka K, et al. Recombinant leptin for weight loss in obese and lean adults: a randomized, controlled, dose-escalation trial. JAMA. 1999;282:1568–1575. doi: 10.1001/jama.282.16.1568. [DOI] [PubMed] [Google Scholar]
- 4.Ravussin E, Smith SR, Mitchell JA, et al. Enhanced weight loss with pramlintide/metreleptin: an integrated neurohormonal approach to obesity pharmacotherapy. Obesity (Silver Spring) 2009;17:1736–1743. doi: 10.1038/oby.2009.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roth JD, Roland BL, Cole RL, et al. Leptin responsiveness restored by amylin agonism in diet-induced obesity: evidence from nonclinical and clinical studies. Proc Natl Acad Sci U S A. 2008;105:7257–7262. doi: 10.1073/pnas.0706473105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan JL, Oral EA. Clinical classification and treatment of congenital and acquired lipodystrophy. Endocr Pract. 2010;16:310–323. doi: 10.4158/EP09154.RA. [DOI] [PubMed] [Google Scholar]
- 7.Garg A. Acquired and inherited lipodystrophies. N Engl J Med. 2004;350:1220–1234. doi: 10.1056/NEJMra025261. [DOI] [PubMed] [Google Scholar]
- 8.Garg A. Clinical review#: Lipodystrophies: genetic and acquired body fat disorders. J Clin Endocrinol Metab. 2011;96:3313–3325. doi: 10.1210/jc.2011-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan JL, Lutz K, Cochran E, et al. Clinical effects of long-term metreleptin treatment in patients with lipodystrophy. Endocr Pract. 2011;17:922–932. doi: 10.4158/EP11229.OR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chong AY, Lupsa BC, Cochran EK, Gorden P. Efficacy of leptin therapy in the different forms of human lipodystrophy. Diabetologia. 2010;53:27–35. doi: 10.1007/s00125-009-1502-9. [DOI] [PubMed] [Google Scholar]
- 11.Javor ED, Cochran EK, Musso C, et al. Long-term efficacy of leptin replacement in patients with generalized lipodystrophy. Diabetes. 2005;54:1994–2002. doi: 10.2337/diabetes.54.7.1994. [DOI] [PubMed] [Google Scholar]
- 12.Oral EA, Simha V, Ruiz E, et al. Leptin-replacement therapy for lipodystrophy. N Engl J Med. 2002;346:570–578. doi: 10.1056/NEJMoa012437. [DOI] [PubMed] [Google Scholar]
- 13.AstraZeneca. MYALEPT (metreleptin) prescribing informaiton. 2014. [Google Scholar]
- 14.Baker MP, Reynolds HM, Lumicisi B, Bryson CJ. Immunogenicity of protein therapeutics: The key causes, consequences and challenges. Self Nonself. 2010;1:314–322. doi: 10.4161/self.1.4.13904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schnabel CA, Fineberg SE, Kim DD. Immunogenicity of xenopeptide hormone therapies. Peptides. 2006;27:1902–1910. doi: 10.1016/j.peptides.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 16.Weeraratne DK, Kuck AJ, Chirmule N, Mytych DT. Measurement of anti-erythropoiesis-stimulating agent IgG4 antibody as an indicator of antibody-mediated pure red cell aplasia. Clin Vaccine Immunol. 2013;20:46–51. doi: 10.1128/CVI.00435-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y, Heilig JS. Differentiation and quantification of endogenous and recombinant-methionyl human leptin in clinical plasma samples by immunocapture/mass spectrometry. J Pharm Biomed Anal. 2012;70:440–446. doi: 10.1016/j.jpba.2012.06.018. [DOI] [PubMed] [Google Scholar]
- 18.Crouse JA, Elliott GE, Burgess TL, et al. Altered cell surface expression and signaling of leptin receptors containing the fatty mutation. J Biol Chem. 1998;273:18365–18373. doi: 10.1074/jbc.273.29.18365. [DOI] [PubMed] [Google Scholar]
- 19.Procaccini C, Jirillo E, Matarese G. Leptin as an immunomodulator. Mol Aspects Med. 2012;33:35–45. doi: 10.1016/j.mam.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 20.Farooqi IS, Matarese G, Lord GM, et al. Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J Clin Invest. 2002;110:1093–1103. doi: 10.1172/JCI15693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Licinio J, Caglayan S, Ozata M, et al. Phenotypic effects of leptin replacement on morbid obesity, diabetes mellitus, hypogonadism, and behavior in leptin-deficient adults. Proc Natl Acad Sci U S A. 2004;101:4531–4536. doi: 10.1073/pnas.0308767101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Considine RV, Sinha MK, Heiman ML, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334:292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 23.Haque WA, Shimomura I, Matsuzawa Y, Garg A. Serum adiponectin and leptin levels in patients with lipodystrophies. J Clin Endocrinol Metab. 2002;87:2395. doi: 10.1210/jcem.87.5.8624. [DOI] [PubMed] [Google Scholar]
- 24.Beltrand J, Beregszaszi M, Chevenne D, et al. Metabolic correction induced by leptin replacement treatment in young children with Berardinelli-Seip congenital lipoatrophy. Pediatrics. 2007;120:e291–296. doi: 10.1542/peds.2006-3165. [DOI] [PubMed] [Google Scholar]
- 25.Beltrand J, Lahlou N, Le Charpentier T, et al. Resistance to leptin-replacement therapy in Berardinelli-Seip congenital lipodystrophy: an immunological origin. Eur J Endocrinol. 2010;162:1083–1091. doi: 10.1530/EJE-09-1027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.