Abstract
Retinoblastoma (RB) is an ocular malignancy of early childhood. Although mutations in the Rb1 gene and expression of stem cell markers have been identified in RB, additional information on RB-specific alterations in signaling pathways and protein expression would be useful for the design of targeted RB therapies. Here we have evaluated the expression of HER2 (ERBB2) in RB. HER2 is a member of the epidermal growth factor family, which is overexpressed in breast, ovarian, gastric, colorectal, pancreatic, and endometrial cancers in a stratified manner. Overexpression and gene amplification of HER2 is associated with aggressive malignancies, accompanied by chemoresistance and poor outcomes. In this study, we present the first evidence of HER2 immunoreactivity in retinoblastoma, as shown by immunocytochemistry, flow cytometry, and western immunoblot, with validation by reverse transcription PCR (RT-PCR) in both RB cell lines and clinical RB tumors. Our results suggest that the HER2 protein expressed in RB is a truncated version that spares the trastuzumab binding site, while HER2 is not detected in normal ocular tissues. Our discovery of HER2 expression in RB may lead to innovative and targeted drug treatment options designed to spare the eye and preserve vision in RB patients.
Keywords: Retinoblastoma, HER2, ERBB2, Target discovery, Drug target, Targeted therapy
Background
Human epidermal growth factor receptor 2 (HER2) is a trans-membrane protein encoded by the ERBB2 gene on chromosome 17q21–22 [1]. The overexpression of HER2, detected in 15–30 % of breast cancers, is typically associated with poor clinical outcomes [2]. HER2 expression has been exploited as a therapeutic drug target in the form of trastuzumab [3, 4], a humanized monoclonal antibody targeted to the extracellular domain of HER2 [review, see 5]. In addition to breast cancer, HER2 is also associated with other malignancies including ovarian cancer [6], gastric cancer [7] (including premalignant lesions [8]), colon cancer [9], pancreatic cancer [10], and bladder cancer [11]. The identification of HER2 expression in other malignancies has led to HER2-directed therapeutic strategies for additional neoplastic diseases. As such, HER2 has become an important drug target for malignancies other than breast cancer [12].
The purpose of the present study was to determine the expression of the HER2 target in retinoblastoma (RB), a childhood tumor of the retina that can spread and metastasize via the optic nerve to the brain [13]. In previous studies, our group has reported on expression of stem cell markers, such as ABCG2, Oct4, and Nanog, in RB [14, 15]. ABCG2, in particular, is a stem cell marker that can confer resistance to a number of common chemotherapeutic agents [16]. The specter of chemoresistance underscores the need to identify new markers for targeted RB therapies. Here we investigate the expression of the cell surface marker HER2/ERBB2, since there has been very little information on the expression of HER2 in RB. To date, there has been no HER2-directed therapy tested on RB patients, mainly because early indications suggested that the HER2 target would not be expressed in RB [17]. However, that study relied upon only one HER2 antibody and one method, i.e., immunohistochemistry. Thus, if we can thoroughly evaluate and establish the expression of HER2 in RB, the RB patients may significantly benefit from existing treatment approaches originally designed for other malignancies expressing HER2.
In this manuscript, we have undertaken a more comprehensive examination of HER2 expression in RB, using multiple methods including immunohistochemistry, immunocytochemistry, reverse transcription PCR (RT-PCR), flow cytometry, and western immunoblot. Here, for the first time, we present HER2 immunoreactivity in retinoblastoma, a finding that may lead to novel prognostic indicators and treatment options for RB, including the potential for utilizing FDA-approved anti-HER2 agents like trastuzumab.
Results
Immunoreactivity of HER2 in RB tissues in situ
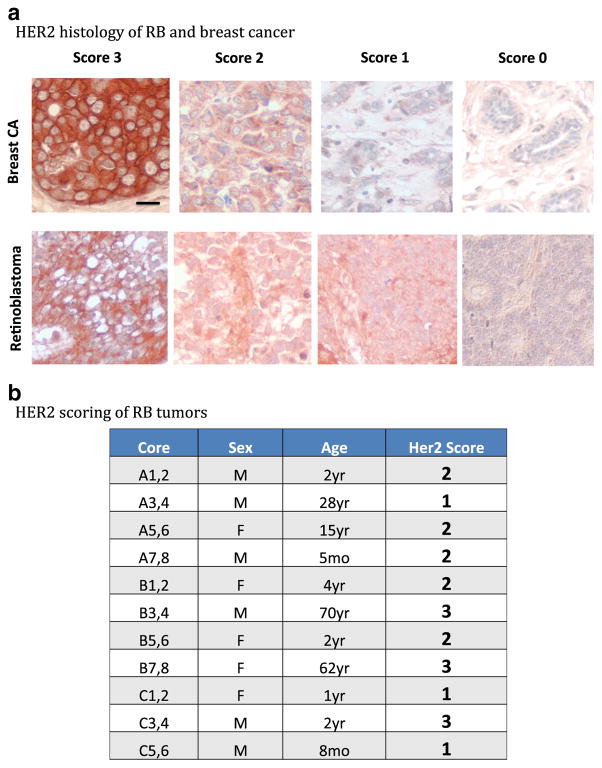
HER2 immunoreactivity was compared between a breast cancer tumor array and a retinoblastoma tumor array comprised of patient tissue samples. A breast cancer array with a variety of HER2-positive cores was chosen, as HER2 is most commonly associated with expression in breast cancer and could serve as a good positive control for HER2 immunoreactivity. In Fig. 1a, HER2 immunoreactivity was assessed based on accepted clinical standards (scale 0–3) for both tumor arrays. The retinoblastoma array contained cores derived from RB patients that covered a range of HER2 clinical scores, and representative fields are shown side-by-side with breast cancer fields of corresponding scores. Our results showed that just as with breast cancer, RB tumors from different patients, as well as fields within the same core, had varying degrees of HER2 immunoreactivity. Figure 1b shows a summary of HER2 clinical scores for the RB tumor array, with a range from 1 to 3.
Fig. 1.
Immunoreactivity of HER2 in retinoblastoma tissues in situ. a An RB tumor array and a human breast cancer tumor array were immunostained for HER2 as described in “Methods.” Representative fields of HER2 clinical scores are shown, ranging from 0 to 3, with corresponding breast cancer fields placed above RB fields for comparison. Scale bar=10 microns. b HER2 immunoreactivity was scored for the retinoblastoma tumor array on a scale of 0–3
Immunoreactivity of HER2 in RB cell lines
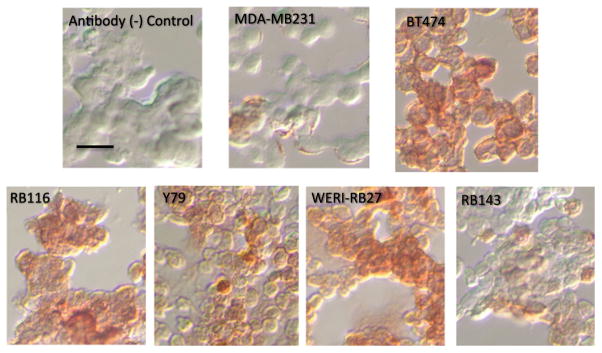
Next, we examined HER2 immunoreactivity in four RB cell lines, all derived from different patients—Y79, WERI-RB27, RB116, and RB143. In Fig. 2, we compared HER2 immunoreactivity in these four RB cell lines with two breast cancer cell lines—BT474 (HER2-high) and MDA-MB231 (HER2-low). As evident in the photomicrographs, all RB cell lines displayed some HER2 immunoreactivity. WERI-RB27 cells appeared to exhibit the strongest staining. For better quantitation, we then measured HER2 immunoreactivity in RB cells by flow cytometry.
Fig. 2.
Immunoreactivity of HER2 in RB cell lines. Four RB cell lines (Y79, WERI-RB27, RB116, and RB143) were compared with BT474 (HER2-high) and MDA-MB231 (HER2-low) human breast cancer cells. The negative control panel received isotype control antibody instead of primary antibody. All RB cells tested displayed HER2 immunoreactivity greater than MDA-MB-231, but less than BT474. Scale bar=10 microns
Quantitation of HER2 in RB cell lines
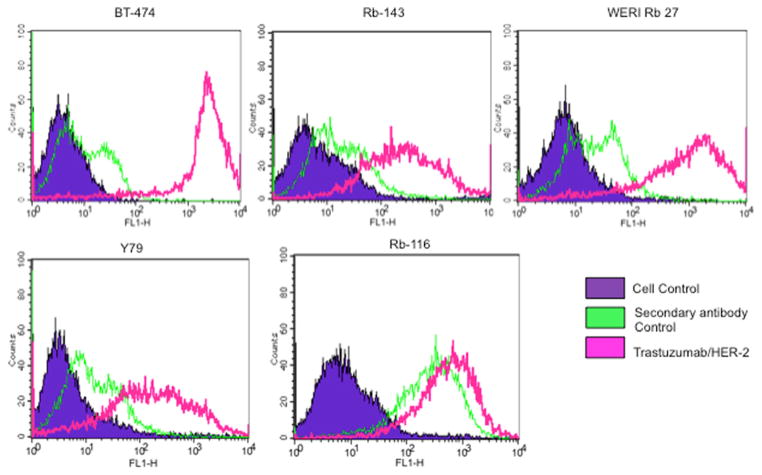
Flow cytometry allowed us to measure HER2 immunoreactivity in a large population of RB cells and compare them with the HER2-high breast cancer cell line BT474. For the flow cytometry experiments, we utilized trastuzumab as the primary antibody for added validation and specificity. As seen in Fig. 3, all RB cell lines contained populations of HER2-positive cells that were able to bind to trastuzumab. Here as well, WERI-RB27 cells appeared to exhibit the strongest HER2 immunoreactivity among RB cell lines.
Fig. 3.
Quantitation of HER2 immunoreactivity in retinoblastoma cell lines. RB cells (Y79, WERI-RB27, RB116, and RB143) were compared with HER2-positive BT-474 cells for the expression of HER2 using trastuzumab as primary antibody. Peak corresponding with the violet color shows the unstained control cells, green corresponds to cells stained with fluorochrome-conjugated secondary antibody alone, and the pink corresponds to the HER2-positive population of cells
HER2 mRNA expression in RB tumors
We carried out RT-PCR as an additional validation of HER2 expression at the messenger RNA (mRNA) level in both RB tumors and cell lines using HER2-specific primers. As shown in Fig. 4, RT-PCR on mRNA isolated from primary tumors derived from three RB patients (different tumor samples than those used for immunohistochemistry), as well as mRNA isolated from RB cell lines (SJ, RBL355, and Y79), displayed a band of the predicted size (130 bp), which is consistent with HER2 transcript expression in these samples. There was no amplification of HER2 in tumor or cell line samples in which RT was omitted (data not shown), indicating that the HER2 expression was from transcribed complementary DNA (cDNA) and not from contaminating genomic DNA.
Fig. 4.
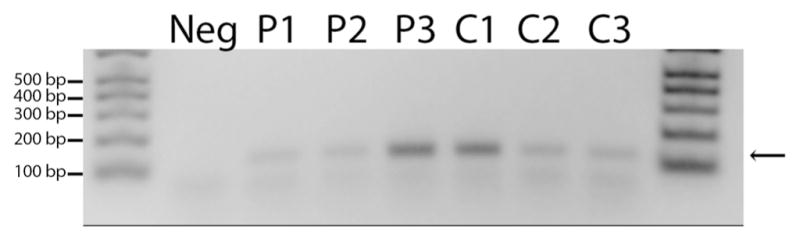
HER2 mRNA in RB tumors. Amplification of an expected 130-bp band (arrow) is shown in three RB patient samples (P1, P2, P3) and three RB cell lines (C1 [SJ], C2 [RBL355], and C3 [Y79]). The “no template”lane (Neg) is a negative control for the PCR reaction that omitted the Taq polymerase, while molecular weight markers are shown at each end
Evidence for possible truncation of HER2 in RB
Western blot analysis was used to visualize HER2 immunoreactive band(s) in RB cell lysates. The four RB cell lines (Y79, WERI-RB27, RB116, and RB143) were analyzed alongside the BT474-positive control, and the results are shown in Fig. 5a–c. The Cell Signaling anti-HER2 antibody detected the appropriate HER2 band only in BT474- and N-87-positive control lanes, with no visible bands for the RB lanes. For the Genetex and Sigma-Aldrich anti-HER2 antibodies, we saw bands of lower molecular weight than the expected 185 kDa, a potential sign of HER2 truncation or splice variation. Figure 5d shows positive immunostaining for BT474, but lack of immunoreactivity in the RB cell lines with the Cell Signaling anti-HER2 antibody, the same antibody that was used in the western blot of Fig. 5a that lacked HER2 bands for RB samples. Taken together, these results suggest a truncation or splice variation of HER2 in RB.
Fig. 5.
Evidence of possible truncation for HER2 in RB. a–c Western blot analysis with three anti-HER2 antibodies. a Cell Signaling Technology antibody showed high expression of HER2 in BT474 and N-87 cells with band sized 185 kDa; however, all the RB cell lines were negative for HER2 expression. b Antibody from Sigma-Aldrich showed high expression of HER2 in BT474 cells with the band size 185 kDa, but it was not observed in RB cells, instead all the RB cells showed three different bands corresponding to 75, 48, and ~40 kDa. c The Genetex antibody also positively detected a 185-kDa band in BT474 cells that was again missing in RB cells. The RB cells showed bands corresponding to 120, 48, and ~40 kDa with the Genetex antibody. d Immunocytochemistry of HER2 using the Cell Signaling Technologies antibody with a binding site at the amino terminal end of HER2. Note the absence of immunoreactivity for all RB cell lines, with the positive control (BT474) still immunoreactive. Scale bar=10 microns
Herstatin immunoreactivity in RB
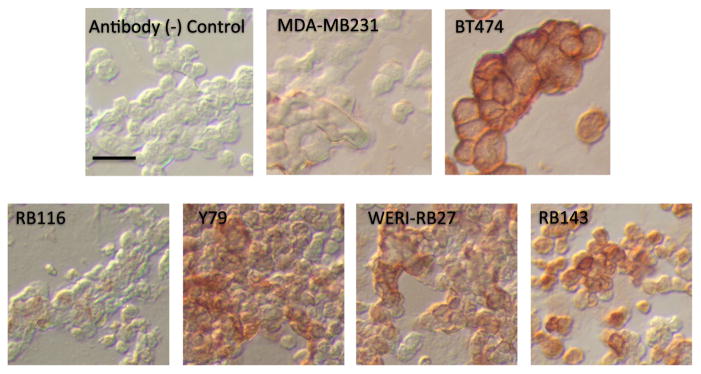
A splice variant of HER2, Herstatin (HST), is expressed in a number of malignancies and may regulate HER2 activity [18]. In Fig. 6, Herstatin immunoreactivity was most intense in BT474 breast cancer cells, with immunoreactivity evident in Y79, WERI-RB27, and RB143 cells. RB116 cells showed slight immunoreactivity, which was comparable to MDA-MB231 breast cancer cells.
Fig. 6.
Herstatin immunoreactivity in RB cell lines. RB cell lines (Y79, WERI-RB27, RB143, and RB116) and breast cancer cells (MDA-MB231 and BT474) were immunostained for Herstatin as described in “Methods.”BT474 was used as the positive control for the most intense staining, with MDA-MB231 cells as a Herstatin-low control. Scale bar=10 microns
Immunoreactivity of HER2 in normal human eye
Considering HER2 as a potential ocular drug target, it would be important to know the extent to which HER2 is expressed in normal human eyes to avoid toxic cross-reactivity during intra-ocular treatment. To this end, we carried out studies to determine the degree of HER2 immunoreactivity in normal human eyes, free of retinoblastoma. In Fig. 7, we examined a variety of ocular structures, including the retina, cornea, optic nerve, and lens. In all cases, there was no detectable HER2 immunoreactivity in normal ocular tissues.
Fig. 7.
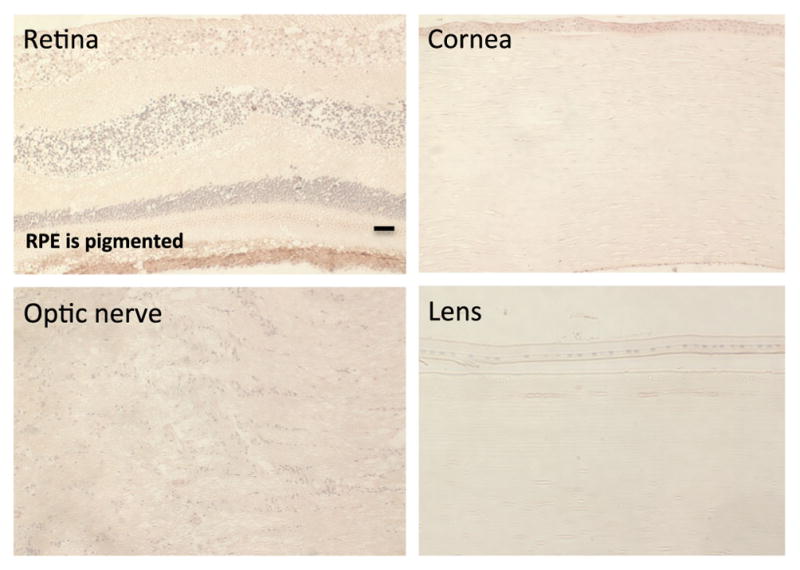
HER2 immunoreactivity in normal human eyes. a Normal human eyes were stained with anti-HER2 antibody. Images of several ocular structures, including the optic nerve, cornea, lens, and retina, show no specific staining for HER2. The retinal pigmented epithelial region has naturally occurring pigment that does not constitute HER2 immunoreactivity. Scale bar=30 microns
Discussion
Immunoreactivity of HER2 in RB
To our knowledge, this is the first report of HER2 expression in retinoblastoma, both in RB cell lines and RB patient samples. A previous study examined HER2, along with other markers in RB, but did not find HER2 immunoreactivity under those experimental conditions [17]. Negative HER2 results in that study may have been due to (a) differences in sample preparation, (b) the use of only one anti-HER2 antibody (that may have been confounded by the truncation in RB), and (c) examination by immunohistochemistry alone. In our study, we have shown HER2 in RB by immunocytochemistry of RB cell lines and immunohistochemistry of RB tumors with more than one anti-HER2 antibody, trastuzumab binding by flow cytometry, mRNA expression by RT-PCR, as well as immunoreactive bands by western blot that suggest splice variants or truncation of the HER2 protein in RB. Interestingly, HER2 immunoreactivity in RB tumors, as scored in Fig. 1b, appears to be patient specific, just as it is for other malignancies, including breast cancer [19]. This finding suggests the possibility of using a personalized medicine approach for the treatment of HER2+ RB tumors.
Truncation of HER2 in RB
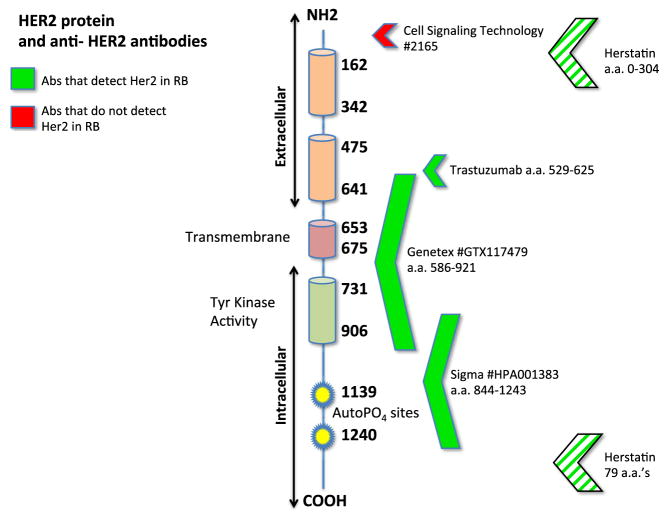
Splice variants for HER2 have been reported previously in breast cancer [20]. Some HER2 splice variations may delete the trastuzumab binding site, rendering the tumor chemo resistant [21] . Our western blot and immunocytochemistry data suggest a truncation of the HER2 protein expressed by RB cells and immunoreactivity for the Herstatin splice variant, yet the trastuzumab binding site appears to be intact based on binding of the antibody to RB cell lines in Fig. 3. With the binding site present, it may be feasible to test trastuzumab as a potential therapeutic agent on RB xenografts in vivo. A summary of the information we have on HER2 protein expressed by RB (as determined by the antibodies used in this study) is shown in Fig. 8. Additional sequencing studies of HER2 will pinpoint the extent of protein truncation in HER2 expressed by RB cells. Based on our trastuzumab immunoreactivity data, we predict that this truncation would not affect the efficacy of trastuzumab in vivo. Preclinical studies, using animal models of human RB, will help determine the feasibility of using anti-HER2 therapy for human RB.
Fig. 8.
HER2 antibodies and the HER2 protein. Antibodies used for this study and their binding sites along the HER2 protein are shown. Green arrows indicate positive detection of HER2, while red arrows show negative results. Based on these results, it appears that the HER2 truncation is at the amino terminal end. Note that the Herstatin antibody recognizes a splice variant that includes both amino and carboxy-terminal ends (striped arrows)
HER2 in normal human eye
HER2 immunoreactivity was not seen in normal human ocular structures (cornea, lens, retina, optic nerve), using both the Genetex and Sigma-Aldrich anti-HER2 antibodies. Thus, the treatment of HER2-expressing ocular tumors with trastuzumab would more likely spare non-tumor cells that lack HER2 on the cell surface, resulting in more efficient and targeted killing of the tumor with fewer ocular side effects. In vitro and in vivo studies of anti-HER2 therapies with attention to non-tumor cells of ocular origin will provide additional information regarding the degree of off-target effects and ocular safety of HER2 targeted therapies. Death of RB tumor cells, coupled with retention of healthy surrounding ocular tissue, would be the most promising result for anti-HER2 experimental therapies.
Conclusions
In summary, we have detected HER2 in retinoblastoma by immunocytochemistry, immunohistochemistry, flow cytometry, and RT-PCR, while normal ocular tissues were immunonegative for HER2. Western blot analysis, coupled with immunocytochemistry, suggests a possible truncation or splice variation of HER2 in RB that spares the trastuzumab binding site. Further studies will pinpoint the truncation and determine the feasibility of repurposing trastuzumab as a potential targeted therapeutic agent for retinoblastoma.
Methods
Cell culture
Human retinoblastoma cell lines (Y79, WERI-Rb27, RB116, RB143, SJ, and RBL355) were grown in suspension (37 °C with 95 % air, 5 % CO2) in RPMI-1640 medium with 10 % fetal calf serum. For RB116 cells, RPMI-1640 medium was supplemented with 1 % 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES; Life Technologies, Carlsbad, CA). Cytospins of cell lines for immunocytochemistry were prepared by 10-min fixation in cytospin solution (72 % isopropanol, 19 % acetone, 7.6 % glycerol) and centrifugation onto slides using a Shandon Cytospin II.
Immunohistochemistry
Staining of paraffin sections (RB tissue array and breast cancer tissue array—USBiomax Rockville, MD), archival human retinal specimens, and cytospins was carried out as described previously [14, 15]. Cytospins and deparaffinized tissues were stained using the CRF Anti-Polyvalent HRP Polymer kit (ScyTek, Inc. Logan, Utah). Anti-HER2 antibodies (GTX117479, Genetex, Irvine, CA; Sigma-Aldrich HPA001383, St. Louis, MO; Cell Signaling Technologies, Beverly, MA) or Herstatin antibody (Novus, Littleton, CO) were used as the primary antibody (5 μg/ml) and incubated for 1 h. Negative controls received non-specific rabbit antibody instead of primary antibody. HRP Polymer and diaminobenzidine (DAB) were used according to the CRF kit to develop brown reaction product. After DAB staining and a water rinse, paraffin sections were counterstained with hematoxylin for 1 min, rinsed in water, then 15 s in acid ethanol, a water rinse, followed by dehydration through a series of graded alcohols to xylene. Paraffin slides were mounted in Permount (Fisher Scientific, Pittsburgh, PA). Cell line cytospins were mounted with KPL aqueous mounting medium (KPL, Gaithersburg, MD). Digital images were captured with a SONY ICX 285AL SPOT camera (Diagnostic Instruments, Sterling Heights, MI).
Flow cytometry
RB cells and a positive control (BT474 breast cancer cells) were labeled for flow cytometry using trastuzumab as the primary antibody. Cells were suspended in phosphate-buffered saline (PBS) containing 0.5 % BSA and incubated with (10 μg/ml) trastuzumab (Genentech, South San Francisco, CA) for 1 h on ice. After incubation, they were washed three times with PBS, resuspended in PBS containing 0.5 % bovine serum albumin (BSA) and incubated with anti-Human AlexaFluor 488-conjugated secondary antibody (Life Technologies, Grand Island, NY) for 30 min in the dark. Cells were washed again three times with PBS and resuspended in PBS containing 0.5 % BSA. Labeled cells were analyzed using a BD FACSCalibur with CellQuest Pro software.
Western immunoblot
Cellular proteins (50 μg per sample) obtained by lysis of RB cells along with positive control were separated in an 8 % SDS-PAGE under reduced conditions. Protein was transferred onto a PVDF membrane and the membrane was blocked with 5 % milk for an hour. The membrane was incubated overnight with anti-HER2 antibody at 4 °C, washed, and then incubated with horseradish peroxidase-conjugated secondary antibody. Finally, the blot was probed and imaged with enhanced chemiluminescent reagents using BioRad ChemiDoc MP imaging system.
RT-PCR
RT-PCR was performed as described in our previous publication [15]. One microgram of total RNA from human RB tissues or cell lines was reversed transcribed into cDNA using ThermoScript (Invitrogen) according to manufacturer’s directions, followed by incubation with RNase H for 30 min at 37 °C. The HER2 gene was amplified from cDNA samples using PCR with 45 s denaturation at 94 °C, 45 s annealing at 61 °C, and 60 s extension at 72 °C. A negative control cDNA that did not have reverse transcriptase was used to distinguish amplification from contaminating genomic DNA. A control PCR sample without template was included for each reaction. The PCR primers [22] were designed to span an intron and include all possible transcript variants of HER2, with the sequences as follows : Erbb2 - 2686F : ATCTGCCTGACATCCACG and Erbb2-2814R: GCAATCTGCATACACCAGTTC.
Acknowledgments
The authors thank Dr. Bruce Ksander for providing the RB116 and RB143 cells. We also thank Dr. John Ludlow for providing the WERI-RB27 cells. GMS is supported by the Cornell Center on the Microenvironment and Metastasis through Award Number U54CA143876 from the National Cancer Institute, as well as a grant from the SUNY Brain Network of Excellence. ASH is supported by the Karl Kirchgessner Foundation. Institutional support to Bascom Palmer Eye Institute was provided by a Research to Prevent Blindness Unrestricted Grant and an NEI Center Core Grant P30 EY014801. SS is supported by a Postdoctoral Fellowship grant from Roche Inc. to DKS. This work was in part supported by NIH grant GM114179 to DKS, and funding from the Center for Protein Therapeutics at the State University of New York at Buffalo.
Footnotes
Compliance with ethical standards
Conflicts of interest
None
Authors’ contributions All authors (GMS, SS, ASH, and DKS) participated in experimental design, analysis, and manuscript preparation. GMS carried out immunohistochemical and immunocytochemical experiments and analysis. SS performed western blot and flow cytometry with analysis. ASH provided RT-PCR results and analysis. All authors have read and approved of the final manuscript.
Contributor Information
G. M. Seigel, Email: gseigel@frontiernet.net.
S. Sharma, Email: sharadsh@buffalo.edu.
A. S. Hackam, Email: Ahackam@med.miami.edu.
Dhaval K. Shah, Email: dshah4@buffalo.edu.
References
- 1.Hynes NE, Stern DF. The biology of erB-2/neu/HER-2 and its role in cancer. Biochim Biophys Acta. 1994;1198:165–84. doi: 10.1016/0304-419x(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 2.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–82. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 3.Balselga J, Norton L, Albanell J, et al. Recombinant humanized anti-HER2 antibody enhances the antitumor activity of paclitaxel and doxorubicin against HER2/neu overexpression human breast cancer xenografts. Cancer Res. 1998;58:2825–31. [PubMed] [Google Scholar]
- 4.Arteaga CL, Engelman JA. ERBB receptors: from oncogene discovery to basic science to mechanism-based cancer therapeutics. Cancer Cell. 2014;25(3):282–303. doi: 10.1016/j.ccr.2014.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kulhari H, Pooja D, Rompicharla SV, Sistla R, Adams DJ. Biomedical applications of trastuzumab: as a therapeutic agent and a targeting ligand. Med Res Rev. 2015;35(4):849–76. doi: 10.1002/med.21345. [DOI] [PubMed] [Google Scholar]
- 6.Färkkilä A, Andersson N, Bützow R, Leminen A, Heikinheimo M, Anttonen M, et al. HER2 and GATA4 are new prognostic factors for early-stage ovarian granulosa cell tumor—a long-term follow-up study. Cancer Med. 2014;3(3):526–36. doi: 10.1002/cam4.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gravalos C, Jimeno A. HER2 in gastric cancer: a new prognostic factor and a novel therapeutic target. Ann Oncol. 2008;19(9):1523–9. doi: 10.1093/annonc/mdn169. [DOI] [PubMed] [Google Scholar]
- 8.Ieni A, Barresi V, Rigoli L, Caruso RA, Tuccari G. HER2 status in premalignant, early, and advanced neoplastic lesions of the stomach. Dis Markers. 2015;2015:234851. doi: 10.1155/2015/234851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arnaout AH, Dawson PM, Soomro S, Taylor P, Theodorou NA, Feldmann M, et al. HER2 (c-erbB-2) oncoprotein expression in colorectal adenocarcinoma: an immunohistological study using three different antibodies. J Clin Pathol. 1992;45(8):726–7. doi: 10.1136/jcp.45.8.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hansel DE, Ashfaq R, Rahman A, Wanzer D, Yeo CJ, Wilentz RE, et al. A subset of pancreatic adenocarcinomas demonstrates coamplification of topoisomerase II alpha and HER2/neu: use of immunolabeling and multicolor FISH for potential patient screening and treatment. Am J Clin Pathol. 2005;123(1):28–35. [PubMed] [Google Scholar]
- 11.Carneiro BA, Meeks JJ, Kuzel TM, Scaranti M, Abdulkadir SA, Giles FJ. Emerging therapeutic targets in bladder cancer. Cancer Treat Rev. 2015;41(2):170–8. doi: 10.1016/j.ctrv.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 12.English DP, Roque DM, Santin AD. HER2 expression beyond breast cancer: therapeutic implications for gynecologic malignancies. Mol Diagn Ther. 2013;17(2):85–99. doi: 10.1007/s40291-013-0024-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.MacKay CJ, Abramson DH, Ellsworth RM. Metastatic patterns of retinoblastoma. Arch Ophthalmol. 1984;102(3):391–6. doi: 10.1001/archopht.1984.01040030309025. [DOI] [PubMed] [Google Scholar]
- 14.Seigel GM, Campbell LM, Narayan M, Gonzalez-Fernandez F. Cancer stem cell characteristics in retinoblastoma. Mol Vis. 2005;11:729–37. [PubMed] [Google Scholar]
- 15.Seigel GM, Hackam AS, Ganguly A, Mandell LM, Gonzalez-Fernandez F. Human embryonic and neuronal stem cell markers in retinoblastoma. Mol Vis. 2007;13:823–32. [PMC free article] [PubMed] [Google Scholar]
- 16.Sharom FJ. ABC multidrug transporters: structure, function and role in chemoresistance. Pharmacogenomics. 2008;9(1):105–27. doi: 10.2217/14622416.9.1.105. [DOI] [PubMed] [Google Scholar]
- 17.Bösch D, Pache M, Simon R, Schraml P, Glatz K, Mirlacher M, et al. Expression and amplification of therapeutic genes in retino-blastoma. Graefes Arch Clin Exp Ophthalmol. 2005;243(2):156–62. doi: 10.1007/s00417-004-1036-2. [DOI] [PubMed] [Google Scholar]
- 18.Koletsa T, Kostopoulos I, Charalambous E, Christoforidou B, Nenopoulou E, Kotoula V. A splice variant of HER2 corresponding to Herstatin is expressed in the noncancerous breast and in breast carcinomas. Neoplasia. 2008;10(7):687–96. doi: 10.1593/neo.08314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoff ER, Tubbs RR, Myles JL, Procop GW. HER2/neu amplification in breast cancer: stratification by tumor type and grade. Am J Clin Pathol. 2002;117(6):916–21. doi: 10.1309/4NTU-N6K4-F8JF-EWRX. [DOI] [PubMed] [Google Scholar]
- 20.Omenn GS, Guan Y, Menon R. A new class of protein cancer biomarker candidates: differentially expressed splice variants of ERBB2 (HER2/neu) and ERBB1 (EGFR) in breast cancer cell lines. J Proteomics. 2014;107:103–12. doi: 10.1016/j.jprot.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jackson C, Browell D, Gautrey H, Tyson-Capper A. Clinical significance of HER-2 splice variants in breast cancer progression and drug resistance. Int J Cell Biol. 2013;2013:97358. doi: 10.1155/2013/973584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hillig T, Thode J, Breinholt MF, Franzmann MB, Pedersen C, Lund F, et al. Assessing HER2 amplification by IHC, FISH, and real-time polymerase chain reaction analysis (real-time PCR) following LCM in formalin-fixed paraffin embedded tissue from 40 women with ovarian cancer. APMIS. 2012;120(12):1000–7. doi: 10.1111/j.1600-0463.2012.02929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]