Abstract
Alumina is an inorganic material, which is widely used in ceramics, catalysts, catalyst supports, ion exchange and other fields. The micromorphology of alumina determines its application in high tech and value-added industry and its development prospects. This paper gives an overview of the liquid phase synthetic method of alumina preparation, combined with the mechanism of its action. The present work focuses on the effects of various factors such as concentration, temperature, pH, additives, reaction system and methods of calcination on the morphology of alumina during its preparation.
Keyword: Alumina, Particle morphology, Reaction mechanism, Calcination method
Review
Introduction
Different materials are essential for the social development. Generally, certain material structure and morphology are required for their applications in a specific field. Inorganic materials are an important branch of materials, which promote development of science and technology. Alumina is an inexpensive and widely used inorganic material. It has a complex structure and many crystalline polymorphic phases such as α-Al2O3, β-Al2O3, γ-Al2O3, δ-Al2O3, θ-Al2O3, η-Al2O3, κ-Al2O3, χ-Al2O3 and ρ-Al2O3. The phase transition temperatures are different for different precursors during their calcination as shown in Fig. 1 [1].
Fig. 1.
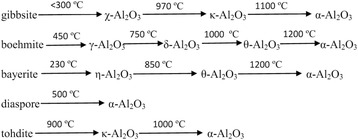
Phase transformation of alumina
Among the numerous crystal forms of alumina, α-Al2O3 and γ-Al2O3 are the two most common kinds. α-Al2O3 has some excellent physical and chemical properties such as good acid, alkali and heat resistances and high hardness and strength. It is widely used in different fields such as ceramics, surface protective layer materials, refractory materials, catalysts and catalyst supports and optical materials [2–5]. γ-Al2O3, which is also called activated alumina, has a large surface area, strong adsorption capacity, good catalytic activity and wear resistance. It is also widely applied in various fields such as adsorbents [6], ceramics [7], catalysts and catalyst supports [8].
The application performance of alumina depends on not only the size of ultra-fine particles but also the particle shape [9–11]. Alumina has a variety of shapes such as rod [12], fibrous structure [13], flake [14] and sphere [15]. Different shapes of alumina have different physical and chemical properties and applications. For example, the fibrous nano-alumina has a very strong anti-sintering property [16], which is often used as an additive for epoxy resin to improve its tensile strength and rigidity. The flake-like alumina is generally used as a seed crystal added to ceramics, which significantly enhances the toughness of ceramics [17].
Alumina is a common catalyst support, whose pore structure is closely related to the activity, selectivity and lifetime of the catalyst. Alumina is divided into different categories such as microporous alumina, mesoporous alumina and macroporous alumina according to its pore size. The pore size of mesoporous alumina is between 2 and 50 nm. It is a rigid porous material with a mutually interconnected or isolated network structure. It has not only the characteristics of a crystalline phase of alumina but also the characteristics of a porous material. Mesoporous alumina is widely used in the catalysis [18], adsorption [19] and other fields due to its adjustable pore structure, relatively large internal and external surface area and pore volume.
The morphology, purity, surface acidity and hydrothermal stability, the pore structure and other properties restrict the application of alumina. The research is ongoing on the pore structure, surface acidity and hydrothermal stability [20]. Morphology, as one of the important parameters of particle characterization, has a substantial effect on the properties and applications of the products. The morphology of particles is influenced and controlled by its crystallization habit during the preparation using liquid-phase method [21, 22], which is restricted by the environment and the growth conditions. This article reviews the research carried out on the preparation of alumina starting from the liquid-phase method for its synthesis including its mechanism and discusses the effect of different factors such as reactant concentration, temperature, pH, additives, system environment and calcination methods on the micromorphology of particles.
Liquid-Phase Method for Synthesis of Alumina
There are some common liquid-phase methods for synthesis of alumina, such as sol-gel method, hydrothermal method, template method, precipitation method, emulsion method or microemulsion method and electrolysis method. Alumina with different morphologies can be obtained by using different synthesis methods and optimizing the reaction conditions.
Hydrothermal Method
Hydrothermal method is an approach where the mixed solution is poured into a sealed reactor. Utilization of the relatively high temperature in the reactor and the high-pressure growth environment promotes the dissolution and recrystallization of poorly soluble or insoluble material. Hydrothermal methods include hydrothermal synthesis, hydrothermal treatment and hydrothermal reactions. During the hydrothermal process, the crystal grows to its largest possible size under the non-restricted conditions and its characteristics (various shapes, high degree of crystallinity, small size, uniform distribution, lighter particle agglomeration, etc.) form [23, 24]. The development of crystal face and the morphology of the crystal formed by hydrothermal synthesis are closely related to the hydrothermal conditions such as water temperature, pressure and the permittivity and viscosity and diffusion coefficient of the solution. The same type of crystal can be produced with different morphology under different hydrothermal conditions [25].
Li et al. [26] let ammonium aluminum sulfate, dispersant PEG2000 and urea disperse in deionized (DI) water and stirred them vigorously to form a solution. Then, the mixed solution was poured into a stainless steel pressure reactor with a teflon-lining. By changing the temperature of the water, mesoporous alumina with different morphologies was obtained. In the course of the reaction, the following reactions take place:
| 1 |
| 2 |
| 3 |
As Fig. 2a shows, when the temperature is 90 °C, the particles obtained are different size spheres. As it is shown in Fig. 2b, at the temperature of 120 °C, the particles are superfine fiber-shaped. As Fig. 2c shows, massive fiber-shaped particles are obtained at the temperature of 150 °C. The crystal orientation is dependent on the temperature which affects the growth rate of the crystal face; consequently, the morphology can be controlled by regulating the temperature. These results indicate that the morphology of the particles substantially changes with the increasing water temperature [27].
Fig. 2.
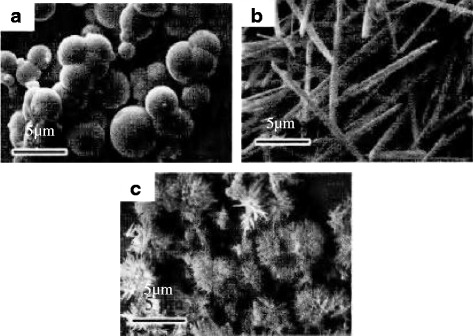
TEM image of alumina prepared under different synthetic temperatures. a 90 °C. b 120 °C. c 150 °C
Zhao et al. [28] prepared flat hexagonal-shaped nano-alumina by hydrothermal synthesis, using aluminum nitrate as aluminum source and sodium nitrate as additive. During the reaction, the Na+ of sodium nitrate was adsorbed onto the surface, which hindered the accumulation of Al3+ and OH− ions. This affected the appearance of the particles. By changing the amount of sodium nitrate additive to control the growth of certain crystal face of alumina, hexagon-shaped alumina with different parameters was obtained. When the amount of sodium nitrate was 0.2 mol, the width of the particle was reduced and its length and thickness remained unchanged. When sodium nitrite was 0.4 and 0.6 mol, the thickness increased and the length and width remained unchanged. The hexagon-shaped particles were gradually transformed into thicker particles as the sodium nitrate concentration increased as shown in Fig. 3.
Fig. 3.
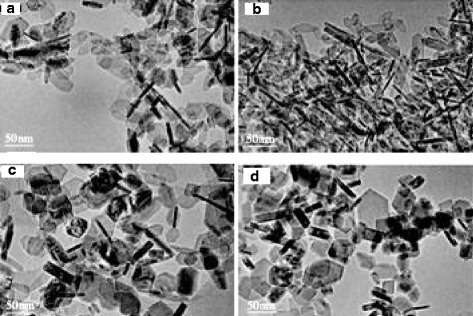
TEM image of nano-alumina with sodium nitrate concentration of a 0 mol, b 0.2 mol, c 0.4 mol and d 0.6 mol
Depending on different reaction systems, particles have accordingly different crystal habits. Pramod K. Sharma’s group and Shi’s group [29, 30] synthesized needle-like and plate-like α-Al2O3, respectively, in water and alcohol-water reaction systems by hydrothermal treatment method using Al(OH)3 colloid as precursor, as shown in Fig. 4.
Fig. 4.
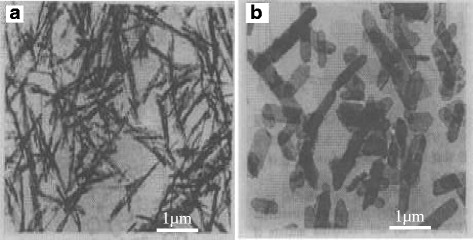
TEM image of α-Al2O3. a Needle-like α-Al2O3. b Plate-like α-Al2O3
Mikhailov et al. [31] prepared hexagonal flake-shaped γ-Al2O3 by hydrothermal method with Al2(SO4)3·18H2O and ammonia as raw materials. This study has shown that pH of solution has a significant impact on the morphology of precursor. Under acidic conditions, the H+ in solution will bind with the hydroxyl, which is on the surface of the γ-AlOOH layered structure, thereby destroying this structure, eventually forming a rod-like nanostructure by rolling growth mechanism [32]. On the contrary, under alkaline conditions, it retains its layered structure, forming plate-shaped nanostructure. Figure 5 shows that when pH is 5, the product is rod-like; when pH is 7, the product is transformed from rod-like to plate-shaped nanostructure; when pH is 9, the product has hexagonal shape. Boehmite converts into a γ-Al2O3 in the firing process, but its shape and size do not change [33, 34]. Calcining the plate-like precursor at 600 °C for 4 h resulted in the original hexagonal γ-Al2O3 with basically same size.
Fig. 5.
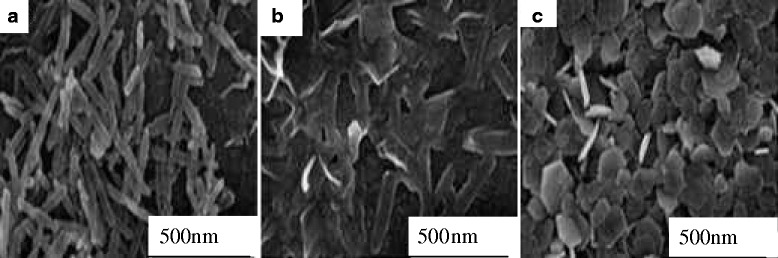
TEM image of γ-AlOOH. a pH = 5. b pH = 7. c pH = 9
Sol-Gel Method
The sol-gel method refers to inorganic or organic alkoxide dispersed in solution. Using the transparent sol formed by hydrolysis and condensation of the precursor, a gel with certain structure is formed during the aging process by the aggregation between the gel particles. During the sol-gel process, the microstructure of the material is controlled and cut at the mesoscopic level by means of low-temperature chemical method, which changes the morphology and structure of the particles [35, 36].
Ning et al. [37], using AcOH as additive and adopting two-step alkoxide hydrolysis sol-gel method, synthesized spherical and fibrillar Al2O3 nano-powder in organic phase. The results showed that the amount of AcOH has a decisive effect on the morphology of the particles. As the amount of AcOH increased, the shape of the particles gradually shifted from the fibrillar to the spherical shape, as shown in Fig. 6. During the reaction, AcOH and other organic molecules containing functional groups N, O and S (ethylacetoacetate, polyamide carboxylic acid salt) as additives coordinate with inorganic ion or are adsorbed onto the surface of crystal nucleus, which changes the growth rate of crystal face. This leads to the change in the morphology of particles.
Fig. 6.
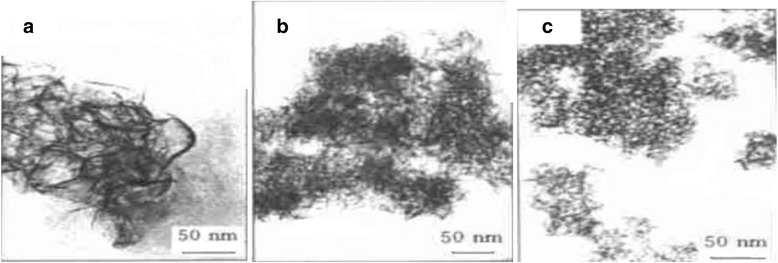
TEM image of alumina nanoparticles with different amount of AcOH. a No AcOH. b m(AcOH)/m[Al(Opri)3] < 0.05. c m(AcOH)/m[Al(Opri)3]≈0.1
Masouleh’s group and Ji’ group [38, 39], using aluminum isopropoxide as aluminum source, [Bmim] PF6 as ionic liquid and adopting sol-gel method to change the molar ratio of [Bmim] PF6 and aluminum isopropoxide, successfully synthesized uniform rod-like mesoporous γ-Al2O3. This study has shown that ionic liquid plays a very important role in the morphology of products. As it is shown in Fig. 7, with the molar ratio of [Bmim]PF6, aluminum isopropoxide increases from 0 to 0.18; the morphology of the products shows a highly homogeneous rod shape. When the molar ratio of [Bmim]PF6 and aluminum isopropoxide is 0.18, the morphology of the products with rod shape has the best homogeneity. If this ratio exceeds 0.18, it is not conducive enough to form the rod shape.
Fig. 7.
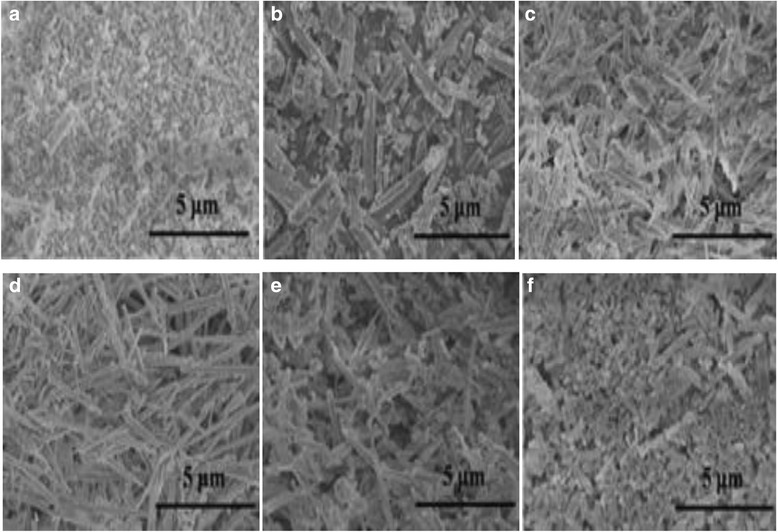
SEM image of alumina particles with different molar ratio of [Bmin]PF6 and aluminum isopropoxide. a Al2O3-0. b Al2O3-0.03. c Al2O3-0.12. d Al2O3-0.18. e Al2O3-0.24. f Al2O3-0.30
Template Method
Template method is a cutting-edge technology developed in the 1990s. It is widely applied in recent years, and it is an effective synthetic method for controlling the structure, particle size and morphology of materials through a utilization of template. Depending on the differences in template structure, template method can be divided into two groups called hard and soft template methods.
Hard Template
In hard template method, precursor is uniformly dispersed in the pore of the hard template or absorbed onto its surface, thereby converting it into a complex product. Then, by choosing an appropriate method (dissolution, sintering, etching, etc.), the target product can be obtained. The special structure of hard template restricts the crystallization or polymerization of the precursor during the process of synthesis, which leads to formation of a mesoscopic phase with an opposite-phase structure of the template.
The hard template is often used as a microreactor during the synthesis. The type of hard template and the reaction conditions such as concentration of reactants, time of immersion, temperature of immersion and the temperature of heat treatment affect the structure and morphology of the product. Especially, the temperature of heat treatment has a great impact on product. The excessively high temperature causes microscopic particles to gather together which in turn affects the order of the micromorphology and its structure [40].
Pang et al. [41] successfully prepared alumina bubble with tunable pore size using colloidal carbon spheres as template and aluminum nitrate as the aluminum source as shown in Fig. 8a. This study has shown that the concentration of aluminum nitrate has no significant effect on the morphology and pore size of alumina. Also, the adsorption time does not affect the morphology; however, the pore diameter increases gradually with increasing time. The adsorption temperature as well has an effect on the morphology. The surface of the particles becomes smooth, and the wall thickness increases with increasing temperature as shown in Fig. 8b, c.
Fig. 8.
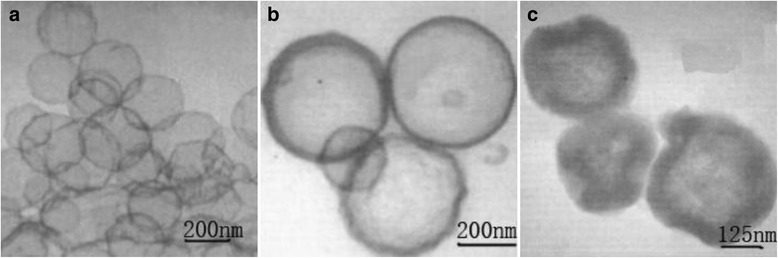
TEM image of alumina bubble with different synthetic temperatures. a 25 °C b 45 °C and c 55 °C
Soft Template Method
Soft template utilizes the intermolecular or intramolecular interaction forces, such as hydrogen bonds and bond and static electricity, to form aggregates with certain structural characteristics (liquid crystal, vesicles, micelle, microemulsion, self-assembled film, etc.) during the reaction. The reactants use these aggregates as template to generate a particle with certain morphology and structural features.
In the synthesis by soft template method, it is usually thought that the interaction between liquid crystalline phase and organic/inorganic interface plays a decisive role in the morphology of mesoporous materials [42, 43]. The liquid crystalline phase formed by the surfactant in solution has a rich structure such as lamellar phase, cubic phase and hexagonal phase and is easy to construct and adjust [44]. The interaction of the organic/inorganic interface is a weak hydrogen bond force in the strong acid environment while it is a strong electrostatic attraction force in the strong alkaline environment [45].
Gu et al. [46] successfully synthesized plate-like and rod-like mesoporous alumina, using F127 as soft template and aluminum isopropoxide as alumina source, and changing the mole ratio of aluminum isopropoxide and F127, as shown in Fig. 9. This study showed that the molar ratio of aluminum isopropoxide and F127 has an obvious effect on the morphology of the product. It is gradually transformed from square to plate and rod, and eventually, all become rod-like as the molar ratio increases. The result of crystalline phase analysis showed the diffraction peaks that are indexed at (311), (222), (400) and (440) associated with γ-alumina which become wider from curve a to f (Fig. 10). This XRD results suggest that the crystallite size can be smaller by increasing of F127 amount. Soft template F127 gives a good performance to weaken the crystallization process.
Fig. 9.
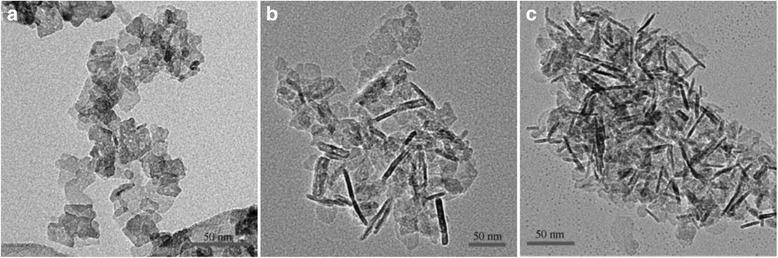
TEM image of alumina with different morphology. a Al2O3-∞. b Al2O3-60. c Al2O3-30
Fig. 10.
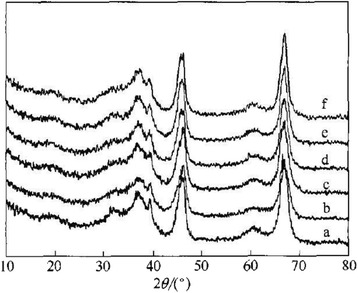
Wide-angle XRD patterns of alumina synthesized with different F127 molar ratio. a Al2O3-∞, b Al2O3-1500, c Al2O3-1000, d Al2O3-500, e Al2O3-60, f Al2O3-30
Groenewolt et al. [47] synthesized the ordered mesoporous γ-Al2O3 by using the soft template method. They have systematically studied the effects of various factors such as the type of aluminum source, the type of surfactant, the type of the acidity regulator and the reaction temperature on the structure and morphology of the products.
Precipitation Method
Precipitation method produces the target products by adding the precipitant agent to the metal solution and heat treating the precipitate. The particles with different morphology can be obtained by adjusting the reaction temperature, the concentration of the reaction, pH, etc.
Zhou et al. [48] synthesized fibrillar nano-Al2O3, using Al2(SO4)3·18H2O and NaOH as raw materials with direct precipitation method. They discussed, respectively, the effect of reaction temperature and the concentration of Al2(SO4)3 on the morphology (Fig. 11). The results showed that the fibrillar nano-Al2O3 with good dispersion was obtained at 65 °C.
Fig. 11.
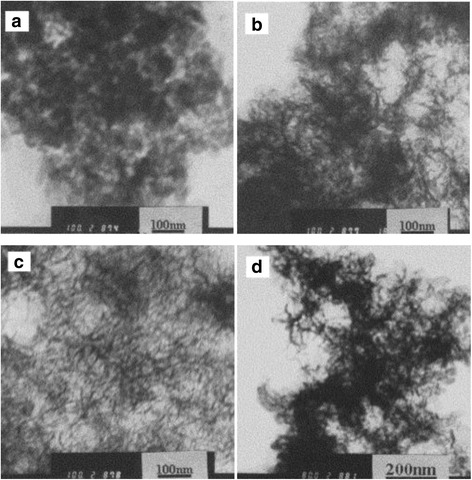
TEM image of alumina with different synthetic temperatures. a 40 °C. b 55 °C. c 65 °C. d 80 °C
The concentration of reactants is also one of the important factors which control the morphology and dispersion of the product. It has an effect on the formation and the growth rates of the crystal grain, and the effect on the formation rate of the grain is greater than that on the growth rate. As shown in Fig. 12, when the concentration of Al3+ is 1 mol L−1, the granular product can be obtained. When the concentration of Al3+ is 0.8 mol L−1, the fibrillar product of poor dispersion is formed. When the concentration of Al3+ is 0.3 and 0.5 mol L−1, the reticular and fibrillar products of good dispersion are formed, respectively.
Fig. 12.

TEM image of alumina of different concentration. a 1.0 mol L−1. b 0.8 mol L−1. c 0.5 mol L−1. d 0.3 mol L−1
The Effect of Calcination System on the Morphology of Alumina
The alumina calcination system is very important for obtaining nanoparticle powder with monodispersity and uniform morphology. Nano-Al2O3 powder, which is composed of widely used α-Al2O3, γ-Al2O3 and amorphous Al2O3, is generally obtained by alumina precursor calcined at different temperatures. Therefore, the compaction among alumina particles of high activity is inevitable at high temperature, which results in severe particle agglomeration and resintering of individual particles with surrounding ones after melting with a formation of dendritic structure called “neckformation” of particle [49]. The result of the experiments showed that the calcination temperature, holding time and heating rate have a significant effect on the morphology of alumina. While the temperature is less than 800 °C, alumina particles can continue to maintain their original morphology. If the temperature becomes higher than 800 °C, the activity of alumina particles is enhanced, and agglomeration begins to occur [50]. Ceresa et al. [51] first presented the relationship between temperature and phase transformation of alumina during the calcination process.
It can be seen from Fig. 13 that the calcination temperature and time have a significant influence on the transformation of alumina (crystal type). When alumina particles calcined at the desired temperature in order to obtain certain crystal types, the calcination time depends on the size of the precursor. The smaller the particle size of precursor is, the shorter the time required for the calcination is, and the higher the temperature of the heat treatment is, the shorter the time required for the calcination is. The method of controlling the temperature and time during the calcination of Al2O3 is well-known. This method ensures that while the Al2O3 particles go through a complete phase change, their morphology remains unaffected and the dispersion of particles is reduced [52].
Fig. 13.
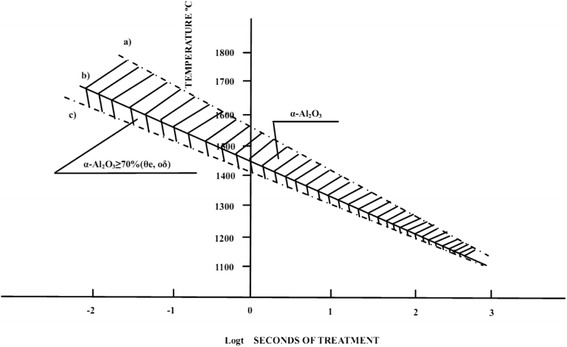
The relationship between temperature and phase transformation of alumina
A significant amount of research is carried out in this area, and effective methods are proposed to control the morphology of alumina particles such as using DI water, alcohol and organic solvent mixtures to wash precursor before calcination in order to prevent agglomeration, enhance the dispersion, and increase the specific surface area of alumina [53]. In addition, the sintering properties of the powder can be improved with ultrasonic pretreatment, so that the neckformation created by agglomeration of the particles will not occur until 1400 °C [54]. The phase transformation temperature of γ-Al2O3 to α-Al2O3 can be decreased if sintering is carried out under the CO2 or ethanol atmosphere; consequently, the well-crystalline spherical α-Al2O3 is eventually obtained [55].
Dispersants and surfactants also play an effective role in dispersion of particles and control of agglomeration. For example, using poly(methacrylic acid), organic acid, glucose, sucrose, inorganic salts, trimethylsilane and other additives [56], which results in a strong electrostatic repulsion among particles, eventually change the polarity of the particle surface from hydrophilic to hydrophobic (water-repellent). Polyacrylamide, silica gel and lignin and other polymer dispersants can form a protective layer with certain strength and thickness on the particle surface and prevents the agglomeration of the particles [57]. Surfactants can form a coating layer of several nanometers on the surface of the particles, which can reduce the surface energy and effectively hinder the interactions among the particles [58].
Besides, by adding 5 wt.% α-Al2O3 seed and 44 % NH4NO3 during the calcination process, the phase transition temperature can be decreased from 1200 to 900 °C [59]. Table 1 summarizes the effects of different calcination temperatures on the grain size of Al2O3 in the presence of various additives mentioned above.
Table 1.
Effect of calcination temperature on the size of alumina in the presence of additives
| Calcination temperature (°C) | 600 | 800 | 1050 | 1200 |
|---|---|---|---|---|
| Al2O3 crystal type | Amorphous Al2O3 | γ-Al2O3 | α-Al2O3 | α-Al2O3 |
| Grain size (nm) | – | 5~50 | 10~100 | ≥100 |
| Color of Al2O3 | Light yellow | White | White | White |
As shown in Table 1, the amorphous Al2O3 particles obtained at 600 °C are light yellow while the additive is still present on the surface of the particles. This coating gradually disappears while 800 °C is reached. In addition, some additives can decrease the phase transition temperature of α-Al2O3 to 1000 °C. As the temperature increases, the grain size of Al2O3 will inevitably increase, meanwhile the agglomeration will start to occur. This is due to the fact that when Al2O3 completely transformed to α phase, the spatial arrangement of the O2 in α-Al2O3 occurs, which is the reconstruction of phase transition from face-centered cubic to hexagonal close-packed lattice [60].
Conclusions
The morphology of Al2O3 can be influenced by various factors such as raw materials, concentrations, different synthesis methods, additives and heat treatment system. During the preparation of Al2O3, the morphology of the precursors and the protection of the particles during heat treatment play a decisive role in the final morphology of alumina. The morphology will not change during the low-temperature heat treatment. However, when high temperature is reached, the diffusion of the powder particles accelerates. Thus, the particles diffuse from the inside to the surface of the crystal lattice and spread to the surrounding resulting in the neckformation as well as the agglomeration of surrounded particles. Accordingly, the morphology of the particles changes. The use of various additives effectively reduces the calcination temperature; consequently, the problem of particle agglomeration can be solved. The utilization of template is a new research hotspot with the objective of improving the dispersibility of Al2O3 powder and controlling the shape of the sample particles.
Prospects
In the research field of the morphology of Al2O3 and its application performance, more work is needed to obtain nano-Al2O3 powder with different shapes, single morphology and good dispersion. There is also a need to expand the application range of this type of nano-Al2O3 powder and improve its application prospect in high tech and value-added product development fields.
Acknowledgements
This work was supported by the Natural Sciences and Engineering Research Council of Canada (NSERC). Technical support of the University Research Centre on Aluminum (CURAL) of the University of Quebec at Chicoutimi, Canada, and Civil, Architectural & Environmental Engineering (CAEE) of Drexel University, Philadelphia, PA, the United States of America.
Footnotes
Competing Interests
The authors declare that they have no competing interests.
Authors’ Contributions
YDX collected and reviewed the data and drafted the manuscript under the instruction of DK. DK and YK helped in modifying the draft. JC and WL suggested many helpful issues for improving the review paper. All authors read and approved the final manuscript.
Contributor Information
Duygu Kocaefe, Email: duygu_kocaefe@uqac.ca.
Wei Liu, Email: cjlw@gznu.edu.cn.
References
- 1.Digne M, Sautet P, Raybaud P, Toulhoat H, Artacho E. Structure and stability of aluminum hydroxides: a theoretical study. J Phys Chem B. 2002;106(20):5155–5162. doi: 10.1021/jp014182a. [DOI] [Google Scholar]
- 2.Xu PK, Dong YB. Corundum refractories. Beijing: Metallurgical Industry Press; 1999. pp. 56–61. [Google Scholar]
- 3.Wang LS. Special ceramics. Changsha: Central South University Press; 1994. pp. 133–138. [Google Scholar]
- 4.Lu XJ, Cui XQ, Song MN. Study on the alteration of chemical composition and structure parameters of modified montmorillonite. Miner Eng. 2003;16:1303–1306. doi: 10.1016/j.mineng.2003.05.003. [DOI] [Google Scholar]
- 5.Khatib K, Pons CH, Bottero JY, François M, Baudin I. Study of the structure of dimet hyldioctadecylammonium-montmorillonite by small angle X-ray scattering. J Colloid Interface Sci. 1995;172(2):317–323. doi: 10.1006/jcis.1995.1258. [DOI] [Google Scholar]
- 6.Zhang HY, Shan GB, Xing JM, Zhang HY. Preparation of (Ni/W)-γ-A12O3 microspheres and their application in adsorption desulfurization for model gasoline. Chem Eng Commun. 2007;194(7):938–945. doi: 10.1080/00986440701232387. [DOI] [Google Scholar]
- 7.Bartsch M, Saruhan B, Schmuecker M, Schneider H. Novel low-temperature processing route of dense mullite ceramics by reaction sintering of amorphous SiO2-coated γ-Al2O3 particle nanocomposites. J Am Ceram Soc. 2004;82(6):1388–1392. doi: 10.1111/j.1151-2916.1999.tb01928.x. [DOI] [Google Scholar]
- 8.Lietti L, Forzatti P, Nova I, Tronconi E. NOx storage reduction over Pt-Ba/γ-Al2O3 catalyst. J Catal. 2001;204:175–191. doi: 10.1006/jcat.2001.3370. [DOI] [Google Scholar]
- 9.Meijere AD, Meyer LU. Shape control of CdSe nanocrystals. Nature. 2000;404(6773):59–61. doi: 10.1038/35003535. [DOI] [PubMed] [Google Scholar]
- 10.Kolosnjaj J, Szwarc H, Moussa F. Toxicity studies of fullerenes and derivatives. Bio-applications of nanoparticles. New York: Springer; 2007. pp. 168–180. [DOI] [PubMed] [Google Scholar]
- 11.Ahmadi TS, Wang ZL, Green TC, Henglein A, El-Sayed MA. Shape-controlled synthesis of colloidal platinum nanoparticles. Science. 1996;272(5270):1924–1925. doi: 10.1126/science.272.5270.1924. [DOI] [PubMed] [Google Scholar]
- 12.Kim SM, Lee YJ, Bae JW, Potdar HS, Jun KW. Synthesis and characterization of a highly active alumina catalyst for methanol dehydration to dimethyl ether. Appl Catal A Gen. 2008;348(1):113–120. doi: 10.1016/j.apcata.2008.06.032. [DOI] [Google Scholar]
- 13.Glemza R. High pore volume and pore diameter aluminum phosphate: US, US RE34911 E[P]. 1995.
- 14.Seri O, Kamazawa R. Preparation of flake alumina by corrosion of aluminum in methanol. J Jpn Soc Matellurgy. 2012;59(6):307–310. [Google Scholar]
- 15.Kim KT, Dao TD, Han MJ, Anjanapura RV, Aminabhavi TM. Graphene coated with alumina and its utilization as a thermal conductivity enhancer for alumina sphere/thermoplastic polyurethane composite. Mater Chem Phys. 2015;153:291–300. doi: 10.1016/j.matchemphys.2015.01.016. [DOI] [Google Scholar]
- 16.Zhu HY, Riches JD, Barry JC. γ-alumina nanofibers prepared from aluminum hydrate with poly(ethylene oxide) surfactant. Chem Mater. 2002;14(5):2086–2093. doi: 10.1021/cm010736a. [DOI] [Google Scholar]
- 17.Yu JW, Liao QL. Effect of plate-like alumina seed on the fracture toughness of alumina ceramics. J Funct Mater. 2011;42(10):1833–1835. [Google Scholar]
- 18.Kim P, Kim Y, Kim H, Song IK, Yi JH. Synthesis and characterization of mesoporous alumina for use as a catalyst support in the hydrodechlorination of 1,2-dichloropropane: effect of preparation condition of mesoporous alumina. J Mol Catal A Chem. 2004;219(1):87–95. doi: 10.1016/j.molcata.2004.04.038. [DOI] [Google Scholar]
- 19.Thomas F, Schouller E, Bottero JY. Adsorption of salicylate and polyacrylate on mesoporous aluminas. Colloids Surf A Physicochem Eng Asp. 1995;95(2):271–279. doi: 10.1016/0927-7757(94)03022-R. [DOI] [Google Scholar]
- 20.Tang GQ, Zhang CF, Sun CS, Yan B, Yang GX, Dai W, et al. Research progress of γ-Al2O3 support. Chem Ind Eng Prog. 2011;8:1756–1765. [Google Scholar]
- 21.Li WJ, Shi EW, Yin ZW. Growth habit of crystal and the shape of coordination polyhedron. J Synth Cryst. 1999;28(4):368–372. [Google Scholar]
- 22.Zheng YQ, Shi EW, Li WJ, Wang BG, Hu XF. Research and development of the theories of crystal growth. J Inorg Mater. 1998;14(3):321–332. [Google Scholar]
- 23.Zhang Y, Wang YF, Yan YH. Development and application of hydrothermal method in growing low-dimensional artificial crystal. Bull Chin Ceram Soc. 2002;21(3):22–26. [Google Scholar]
- 24.Tian MY, Shi EW, Zhong WZ, Pang WQ, Guo JK. Nano ceramics and nano ceramic powder. J Inorg Mater. 1998;2:129–137. [Google Scholar]
- 25.Hu C C, Wu Y T, Chang K H. Low-Temperature Hydrothermal Synthesis of Mn3O4 and MnOOH Single Crystals: Determinant Influence of Oxidants[J]. Chemistry of Materials. 2008; 20(9):2890-894.
- 26.Li YH, Peng C, Zhao W, Bai MM, Rao PG. Morphology evolution in hydrothermal synthesis of mesoporous alumina. J Inorg Mater. 2014;29(10):1115–1120. doi: 10.15541/jim20140104. [DOI] [Google Scholar]
- 27.Ray JC, You KS, Ahn JW, Ahn WS. Synthesis of mesoporous alumina using anionic, nonionic and cationic surfactants. Stud Surf Sci Catal. 2007;165(07):275–278. doi: 10.1016/S0167-2991(07)80316-8. [DOI] [Google Scholar]
- 28.Zhang ZX, Shen ZQ, Ling FX, Xia CH. Impacts of sodium nitrate additive on alumina morphology. Pet Process Petrochem. 2013;44(9):47–50. [Google Scholar]
- 29.Pramod KS, Jilavi MH, Burgard D, Nass R. Hydrothermal synthesis of nanosize alpha-al2o3 from seeded aluminum hydroxide. J Am Ceram Soc. 2005;81(10):2732–2734. doi: 10.1111/j.1151-2916.1998.tb02687.x. [DOI] [Google Scholar]
- 30.Shi EW, Xia CT, Wang BG, Zhong WZ. Application and development of hydrothermal method. J Inorg Mater. 1996;2:193–206. [Google Scholar]
- 31.Mikhailov VI, Maslennikova TP, Krivoshapkin PV. Materials based on aluminum and iron oxides obtained by the hydrothermal method. Glass Phys Chem. 2014;40(6):650–656. doi: 10.1134/S1087659614060078. [DOI] [Google Scholar]
- 32.Hou HW, Xie Y, Yang Q, Guo QX, Tan CR. Preparation and characterization of γ-AlOOH nanotubes and nanorods. Nanotechnology. 2005;16(6):741. doi: 10.1088/0957-4484/16/6/019. [DOI] [Google Scholar]
- 33.Buining PA, Pathmamanoharan C, Jansen JBH, Lekkerkerker HNW. Preparation of colloidal boehmite needles by hydrothermal treatment of aluminum alkoxide precursors. J Am Ceram Soc. 1991;74(6):1303–1307. doi: 10.1111/j.1151-2916.1991.tb04102.x. [DOI] [Google Scholar]
- 34.Wang J, Zhang B, Xu XL. Influence of hydrothermal temperature on structural and microstructural properties of boehmite. Nonferrous Metals Extractive Metallurgy. 2007;5:23–26. [Google Scholar]
- 35.Varma HK, Mani TV, Damodaran AD, Warrier KGK, Balachandran U. Sol–spray preparation, particulate characteristics, and sintering of alumina powders. Advanced materials I. 1994. pp. 11–14. [Google Scholar]
- 36.Li J, Pan YB, Xiang CS, Ge QM, Guo JK. Low temperature synthesis of ultrafine α-Al2O3 powder by a simple aqueous sol–gel process. Ceram Int. 2006;32(5):587–591. doi: 10.1016/j.ceramint.2005.04.015. [DOI] [Google Scholar]
- 37.Ning GL, Chang YF. Shape-controlled synthesis of alumina nanoparticles by carboxy-containing organic molecules. Chem Res Chin Univ. 2004;23(3):345–348. [Google Scholar]
- 38.Masouleh NSG, Taghizadeh M, Yaripour F. Optimization of effective sol-gel parameters for the synthesis of mesoporous γ-Al2O3, using experimental design. Chem Eng Technol. 2014;37(9):1475–1482. doi: 10.1002/ceat.201300747. [DOI] [Google Scholar]
- 39.Ji XW, Tang SK, Gu L, Liu TC, Zhang XW. Synthesis of rod-like mesoporous γ-Al2O3 by an ionic liquid-assisted sol–gel method. Mater Lett. 2015;151:20–23. doi: 10.1016/j.matlet.2015.03.022. [DOI] [Google Scholar]
- 40.Roggenbuck J, Koch G, Tiemann M. Synthesis of mesoporous magnesium oxide by CMK-3 carbon structure replication. Chem Mater. 2006;18(17):4151–4156. doi: 10.1021/cm060740s. [DOI] [Google Scholar]
- 41.Pang LP, Zhao RH, Guo F, Chen JF, Cui WG. Preparation and characterization of novel alumina hollow spheres. Acta Phys -Chim Sin. 2008;24(6):1115–1119. [Google Scholar]
- 42.Zhao DY, Yang PD, Huo QS, Chmelka BF, Stucky GD. Topological construction of mesoporous materials. Curr Opin Solid State Mater Sci. 1998;3(1):111–121. doi: 10.1016/S1359-0286(98)80073-9. [DOI] [Google Scholar]
- 43.Brinker CJ, Dunphy DR. Morphological control of surfactant-templated metal oxide films. Curr Opin Colloid Interface Sci. 2006;11(2–3):126–132. doi: 10.1016/j.cocis.2005.10.006. [DOI] [Google Scholar]
- 44.Anderson MT, Martin JE, Odinek JG, Newcomer PP. Surfactant-templated silica mesophases formed in water: cosolvent mixtures. Chem Mater. 1998;10(1):311–321. doi: 10.1021/cm9704600. [DOI] [Google Scholar]
- 45.Materna KL, Grant SM, Jaroniec M. Poly(ethylene oxide)-poly(butylene oxide)-poly(ethylene oxide)-templated synthesis of mesoporous alumina: effect of triblock copolymer and acid concentration. ACS Appl Mater Interfaces. 2012;4(7):3738–3744. doi: 10.1021/am3008642. [DOI] [PubMed] [Google Scholar]
- 46.Gu L, Cui XL, Tang SK, Zhang XW. Morphology-controlled synthesis of mesoporous alumina dependent on triblock copolymer. J Chem Ind Eng. 2015;66(9):3782–3787. [Google Scholar]
- 47.Groenewolt M, Brezesinski T, Schlaad H, Antonietti M, Groh P, Iván B. Polyisobutylene-block-poly(ethylene oxide) for robust templating of highly ordered mesoporous materials. Adv Mater. 2005;17(17):1158–1162. doi: 10.1002/adma.200401549. [DOI] [Google Scholar]
- 48.Zhou KG, Li YP, Li JC, He WW, Yang XJ. Preparation of fibrous nano-alumina by direct precipitation. J Hunan Univ. 2009;36(8):59–63. [Google Scholar]
- 49.Kao HC, Wei WC. Kinetics and microstructural evolution of heterogeneous transformation of θ-alumina to α-alumina. J Am Ceram Soc. 2000;83(2):362–368. doi: 10.1111/j.1151-2916.2000.tb01198.x. [DOI] [Google Scholar]
- 50.Sarikaya Y, Sevinç İ, Akinç M. The effect of calcinations temperature on some of the adsorptive properties of fine alumina powders obtained by emulsion evaporation technique. Powder Technol. 2001;116(1):109–114. doi: 10.1016/S0032-5910(00)00365-X. [DOI] [Google Scholar]
- 51.Ceresa EM, Gennaro A, Cortesi P (1987) α-alumina in the form of spherical non-aggregation particles having a narrow size distribution and size below 2 μm and process for preparing same. US Pat: 4818515
- 52.Kass MD, Cecala DM. Enhanced sinter ability of alumina particles by pretreating in liquid ammonia. Mater Lett. 1997;32(s2-3):55–58. doi: 10.1016/S0167-577X(97)00010-4. [DOI] [Google Scholar]
- 53.Bousquet C, Elissalde C, Aymonier C, Maglione M, Cansell F, Heintz JM. Tuning Al2O3 crystallinity under supercritical fluid conditions: effect on sintering. J Eur Ceram Soc. 2008;28(1):223–228. doi: 10.1016/j.jeurceramsoc.2007.06.005. [DOI] [Google Scholar]
- 54.Shek CH, Lai JKL, Gu TS, Lin GM. Transformation evolution and infrared absorption spectra of amorphous and crystalline nano-Al2O3 powders. Nanostruct Mater. 1997;8(5):605–610. doi: 10.1016/S0965-9773(97)00201-8. [DOI] [Google Scholar]
- 55.Xie ZP, Lu JW, Gao LC, Li WC, Xu LH, Wang XD. Influence of different seeds on transformation of aluminum hydroxides and morphology of alumina grains by hot pressing. Mater Des. 2003;24(3):209–214. doi: 10.1016/S0261-3069(02)00147-4. [DOI] [Google Scholar]
- 56.Li JG, Sun X. Synthesis and sintering behavior of a nanocrystalline α-Al2O3 powder. Acta Mater. 2000;48:3103–3112. doi: 10.1016/S1359-6454(00)00115-4. [DOI] [Google Scholar]
- 57.Karagedov GR, Lyakhov NZ. Preparation and sintering of nano-sized α-Al2O3 powder. Nanostruct Mater. 1999;11(99):559–572. doi: 10.1016/S0965-9773(99)00331-1. [DOI] [Google Scholar]
- 58.Strekopytov S, Exley C. Thermal analyses of aluminum hydroxide and hydroxyaluminosilieates. Polyhedron. 2006;25(8):1707–1713. doi: 10.1016/j.poly.2005.11.011. [DOI] [Google Scholar]
- 59.Liu HY, Ning GL, Gan ZH, Lin Y. Emulsion-based synthesis of unaggregated, spherical α-alumina. Mater Lett. 2008;62(10):1685–1688. doi: 10.1016/j.matlet.2007.09.059. [DOI] [Google Scholar]
- 60.Bagwell RB, Messing GL, Howell PR. The formation of α-Al2O3 from θ-Al2O3: the relevance of a “critical size” and: diffusional nucleation of “synchro-shear”. J Mater Sci. 2001;36(7):1833–1841. doi: 10.1023/A:1017545213590. [DOI] [Google Scholar]


