Abstract
Despite the high medical burden experienced by patients with focal segmental glomerulosclerosis (FSGS), the etiology of the condition remains largely unknown. FSGS is highly heterogeneous in clinical and morphological manifestations. While this presents challenges for the development of new treatments, research investments over the last two decades have yielded a surfeit of potential avenues for therapeutic intervention. The development of many of those ideas and concepts into new therapies, however, has been very disappointing. Here, we describe some of the factors that have potentially contributed to the poor translational performance from this research investment, including the confidence we ascribe to a target, the conduct of experimental studies, and the availability of selective reagents to test hypotheses. We will discuss the significance of genetic and systems traits as well as other methods for reducing bias. We will analyze the limitations for a successful drug development. We will use specific examples hoping that these will guide a consensus for investment and drive greater translational quality. We hope that this substrate will serve to exemplify the tremendous opportunity for intervention as well as facilitate greater collaborative effort between industry, academia and private foundations in promoting appropriate validation of these targets. Only then will we have achieved our goal for curative therapies for this devastating disease.
Keywords: podocyte, proteinuria, focal segmental glomerulosclerosis
Epidemiology and key clinical considerations
FSGS is a common cause of primary nephrotic syndrome, with a peak age of onset between 10–45 years; with ~2000 individuals reaching end-stage kidney disease (ESKD) each year 1. As prevalence and incidence can not be precisely estimated due to lack of population based studies, the implementation of longitudinal cohorts of patients affected by nephrotic syndrome as well as the development of national and international registries will be key to understand disease pathogenesis, estimate disease impact, stratify patients, develop alternative clinically meaningful outcome measures and design feasible clinical trials. This is even more important if we consider that the clinical disease course of patients with biopsy proven FSGS is highly heterogeneous, as well as the recurrence rates after transplant, the response to treatment and the morphological pattern as reviewed elsewhere 2. Successful drug development in the field of FSGS has also been hampered by the fact that the etiology of FSGS remains largely unknown (with 80% of cases being idiopathic) and little consensus as well as confidence on key interventional nodes have been reached, as we will discuss.
While FSGS, and more broadly CKD, represents a silent killer, and affected vulnerable populations such as underrepresented minorities and children are often characterized by a worse clinical outcome, the investment in experimental studies has been somewhat limited in contrast to other disciplines. First, this clearly reflects the influence of the public opinion in the community, which overall is more sensitive to specific areas of research characterized by high morbidity and mortality, such as cancer and heart disease. Interestingly, however, the risk for dying from CKD is equal or superior to certain forms of cancer 3, yet, patients are surely more afraid to be informed they have cancer rather than CKD. While a cultural shift and education are needed to generate awareness about this, there is perhaps also a need to make the discipline more glamorous and attractive to researchers. Secondly, dialysis and transplantation remain a lucrative therapeutic alternative to preventive or curative strategies in kidney diseases, thus decreasing the attractiveness of drug development. Finally, the lack of alternative, regulatory acceptable outcome measures 4–6 as well as methods for better stratifying patients on their risk profile has discouraged industry to invest in drug development and requires further studies. Alternative biomarkers are being explored, for instance the measurement of podocyturia and urinary podocyte mRNA, which maybe especially relevant in FSGS where damage to the podocyte appears to be a key determinant in the disease pathogenesis. These alternatives may help stratify patients, predict disease progression or sensitivity to drug intervention 7 and certainly more investment as well as qualification is warranted.
The path moving forward
The limitations discussed in the prior paragraph are among the factors that have led to the limited success in the development of new effective drugs for kidney diseases. In fact Nephrology, among other subspecialties in Internal Medicine, has performed very poorly when it comes to conducting randomized clinical trials 8. Table 1 summarizes ongoing clinical trials in FSGS or treatment resistant nephrotic syndrome. There is a relative paucity of innovative and tailored approaches in development. This may be due to insufficient understanding of the disease pathophysiology, or the confidence in translation of experimental efficacy studies in animals to studies in humans or the cost of clinical development. With this in mind, the National Institute of Health established in 2012 a National Center for Advancing Translational Science (NCATS). Through two initiatives, the “Discovering New Therapeutic Uses for Existing Molecules (New Therapeutic Uses)” (http://www.ncats.nih.gov/ntu), and “Pfizer’s Centers for Therapeutic Innovation (CTI) for NIH Researchers” (http://www.ncats.nih.gov/cti) NCATS has facilitated the engagement of multi-disciplinary physician scientists capable of conducting clinical studies and Pharma to support the translation of new ideas. While the first initiative enables access to a diverse portfolio of clinically safe but developmentally curtailed agents and aims to re-purpose an asset for an alternative indication, to date no nephrology programs have been sponsored. This is somewhat surprising given the ingenuity and talent of the nephrology community for exploring therapeutic re-positioning of existing agents, as we will describe below. This contrasts the appetite of most Pharma and biotech companies, for de novo discovery and development of highly differentiated as well as novel approaches. As academicians often lack the expertise in drug discovery and Pharma has limited access to patient samples and clinical databases supporting their development plan, this makes approaches like CTI very attractive for both parties. Indeed, both initiatives place partnership between industry, academia, potentially private foundations and government funds at the center helping to fast track programs with high probability of success. It will also allow for the early implementation of precision medicine initiatives and for their translatability into the arena of clinical care. The role of private foundations such as NephCure Kidney International, engaging patients and families will be key for the success of any development, primarily when rare diseases such as FSGS are being targeted. Furthermore, foundations that are willing to serve as a partnering mediator between the academic investigators and the investors will undoubtedly facilitate the development of new therapeutic strategies. Access to patient registries, developed either by private foundations or supported by the NIH via consortia, that track a longitudinal cohort of affected individuals, as successfully implemented via national and international study networks such as NEPTUNE and CureGN, will be tremendously powerful.
Table 1.
A snapshot of ongoing clinical trials in FSGS captured from ClincalTrials.gov
| Identifier (NCT) |
Indication | Study design | Asset | Mechanism | Sponsor | Stage | Comments |
|---|---|---|---|---|---|---|---|
| 02382874 | FSGS | Single arm open label pilot safety study (n=5) |
Allogenic AD- MSC Transplant |
Not known | Royan Institute |
I | No substantial supporting pre-clinical data |
| 02235857 | FSGS | Open label POM study (n=35) Primary = change in UACR at 10 wks |
Liposorber LA-15 |
Not known | Kaneka Pharma America LLC |
Ib | No substantial supporting pre-clinical data |
| 02000440 | FSGS | Single arm open label POM study (n=24). Primary = 50% reduction in UACR at 24 wks |
Losmapimod | p38 kinase inhibition |
GSK | Ib | Pathway appears to be altered in FSGS. Some supporting pre- clinical data57,58 |
| 02585804 | FSGS | Single arm open label POM study (n=12) Primary =change in inulin GFR at 8 wks |
Dapagliflozin | SGLT2 inhibition |
AZ | Ib | Rationale is based on observed effects of SGLT2 inhibitors on renal hemodynamics in other diabetic populations (e.g. Renal hemodynamic effect of sodium- glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. 59 |
| 01164098 | FSGS (post- transplant) |
Randomized open label study (n=60) Primary = Prevention of proteinuria recurrence at 4 wks |
Rituximab | CD20 B cell depletion SMPDL3b |
Genentech/ University Miami |
II | Supported by clinical and pre-clinical observations 38 |
| 01573533 | FSGS (treatment resistant) |
Open label study in treatment Primary = remission & change in UACR at 12m |
Rituximab | CD20 B cell depletion SMPDL3b |
Genentech Mayo |
II | Supported by clinical observations |
| 02394106 | Nephrotic syndrome (treatment resistant) |
Double-blind, two- parallel-arm, placebo- controlled randomized study (n=50) Primary = remission and change in UACR at 6m |
Ofatumumab | CD20 B cell depletion |
Istituto Giannina Gaslini |
II | Supported by clinical observations |
| 01613118 | FSGS | Multi-dose randomized placebo control efficacy study (n=100) Primary = change in UACR at 8 wks |
RE-021/ sparsantan |
ETRA/ARB | Retrophin | II | ETRAs appear to improved UACR and fibrosis in pre-clinical models. As a class, though ETRAs have been associated with liver and CV AEs |
| 02257697 | Nephrotic syndrome |
Randomized controlled open label study (n=238) Primary = remission at 52 wks |
Mizoribine | IMPDH Podocyte target not known |
Asahi Kasei Pharma |
III | Pre-clinical supporting data 40 |
Legend. NCT: National Clinical Trial; POM: proof of mechanism; UACR: Urinary albumin to creatinine ratio; wks: weeks; AD-MSC: adipose tissue derived mesenchimal stem cells; IMPDH: Inosine-5′-monophosphate dehydrogenase; ETRA: Endothelin Receptor antagonists; CV AEs: cardiovascular adverse events
Strategies to identify clinically relevant targets
The glomerulus is a highly specialized structure that ensures the selective ultrafiltration of plasma so that essential proteins are retained in the blood 9. Podocytes, the primary cellular targets in FSGS, are specialized glomerular epithelial cells whose numerous foot processes support the formation of the slit diaphragm 10, 11. Podocytes contribute to the glomerular filtration barrier through a tight regulation of actin cytoskeleton remodeling 12, 13. A large variety of new experimental tools have allowed for the identification of specific drug targets that are predominantly expressed by podocytes in health and disease 14, 15. In fact, while nephrin was the only component of the glomerular filtration barrier when first discovered in 1998, a large body of new podocyte specific proteins and potential targets have now been identified 16, 17.
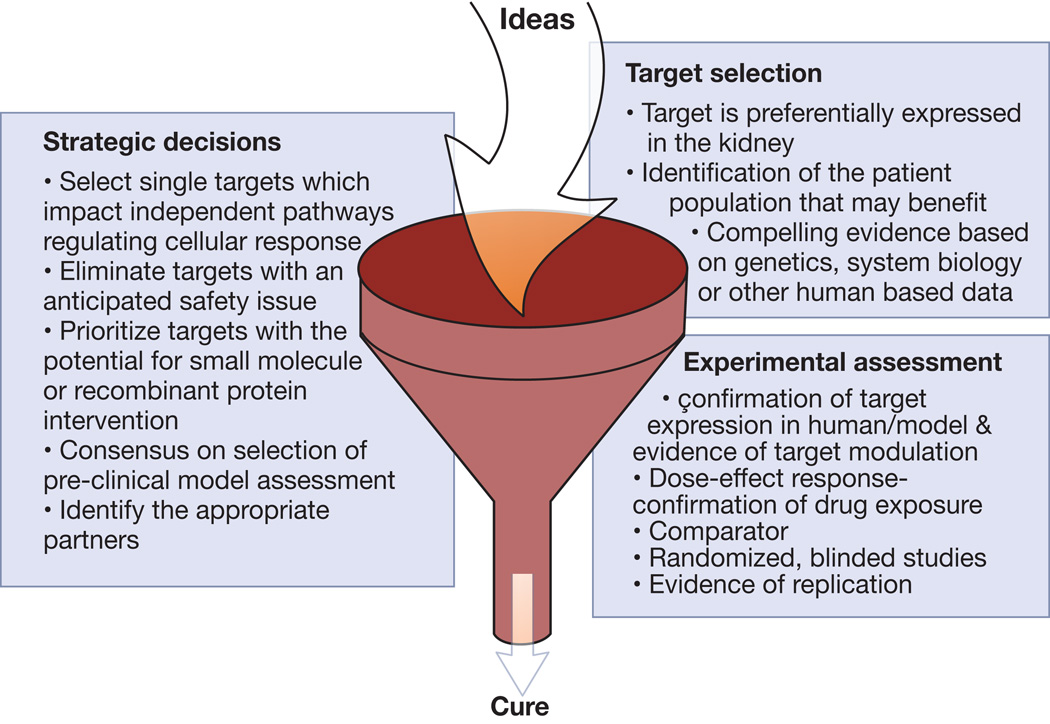
However, target validation has remained a problem and new strategies to increase the probability of success in drug development remains a matter of intense debate. Therefore, while we do not intend to discuss each of the new targets under experimental and clinical investigation for FSGS, we will provide some examples on how effective validation of new targets may lead to successful drug development. Some guidance criteria for strategic decisions, target selection and experimental assessment are summarized in Figure 1 and discussed below.
Figure 1. Considerations in intellectual and experimental triage of ideas to target validation.
Validation of clinical targets based on genetics
The discovery of Mendelian inherited genes, such as mutations in nephrin, podocin, etc, that pre-dispose individuals to FSGS signposted the significance of the podocyte in proteinuric disease. Their study has provided insight into potential mechanistic causes of proteinuric disease and opened avenues for patient stratification. Many of these genes have been studied in the context of transgenic and knockout systems, providing potentially important disease-relevant models for efficacy studies.
It has been increasingly recognized that the study of disease causing as well as protective alleles can yield to a rich source of important therapeutic targets. Recent industry analyses of clinical success and failure have indicated that portfolios that are rich in assets derived from genetic validation have a greater chance of success than those derived without 18–20. Many of the genes that have been shown to contribute to proteinuric disease are not easily or obviously targetable. Remarkably little investment has been made in developing approaches against known casual genes. If we take TRPC6 as an example, multiple gain-of function mutations have been mapped to FSGS21, 22. TRPC6 is a non-selective cation channel that sits at the slit diaphragm. It is a member of a class of channels that are believed to be chemically tractable drug targets. As hyperactivation of the channel is believed to promote podocyte motility, foot process effacement and apoptosis, antagonism of the channel might be desirable in a disease setting. Indeed, there is supporting evidence of increased expression of the channel in other glomerular diseases suggesting that this is a pathway that might have a key disease role23. However, for several potential reasons this target has not received much attention. Firstly, targeted knockdown or knockout mice alone do not appear to have a significant renal phenotype 24, 25, suggestive of potential compensation by other TRP channels or pathways 26. To this latter point, TRPC5 has also emerged as a potential podocyte target based on some pre-clinical observations 26 and we await evidence that TRPC5 activity is altered in human FSGS in order to underwrite the significance of these findings compared with what is known about TRPC6. As TRPC6 appears to be activated in an AngII-dependent fashion, if channel blockade is not superior to ACE inhibition or ARB treatment alone, then a TRPC6 antagonist might not have the additional clinical horsepower. TRPC6 is also expressed in many other cell types, and this raises potential safety concerns, especially cardiovascular safety. Finally, the quality and availability of selective TRPC6 tools has limited the validation of this approach; even ML204, which was used to assess the potential contribution of TRPC5 in podocytopathy, has significant polypharmacology at the concentrations tested that might limit interpretation of the pre-clinical findings 26,27. The barriers for initiating any de novo drug discovery program are considerable.
Increasingly researchers are trying to apply the knowledge of the underlying systems biology, transcriptional and omic networks with the genetic causes to re-purpose known drugs for therapeutic use 28, 29. This has been most elegantly demonstrated in patients with steroid resistant nephrotic syndrome and mutations in ADCK4 30. ADCK4 appears to localize to mitochondria, to interact with COQ6. Comparisons with related proteins and COQ mutant phenotypes suggested that ADCK4 promotes CoQ10 biosynthesis and Q10 deficiency might be driving the phenotype of individuals harboring ADCK4 mutations. Supporting in vitro studies and preliminary human data strongly suggest for this small group of individuals with ADCK4 mutations a restoration of renal function can be achieved with Q10 replacement. This early success suggests that more examples will emerge from the application of integrated functional genomics and the investigational pharmacopeia.
Validating clinically relevant targets through experimental studies
It is postulated that a hallmark of proteinuric disease is the sustained loss of podocytes from the glomerular tuft, either by injury or impaired function. As these cells have a poor capacity for self-renewal31, 32, the loss of functional podocyte mass on glomerular function is potentially irreversible, leads to progressive scarring and ultimately ESKD. Elegant experimental studies performed by the Wiggins group 33 have shown that selective depletion of 30–40% of glomerular podocyte is sufficient to induce an overt proteinuria and progressive glomerulosclerosis that is phenotypically reminiscent of the clinical situation. These observations, coupled with the high phenotypic penetrance of mutations in key proteins necessary for podocyte function in individuals that present with primary FSGS, have brought the podocyte to the center stage in the visualization and development of therapeutic approaches that seek to either preserve podocyte mass or restore function.
The goal of either an unbiased phenotypic screening approach or hypothesis-driven assessment of any potential target is to conduct studies that generate data that meaningfully signpost a pharmacological effect, offer an insight to differentiation compared with standard of care and a directionally compelling rationale for efficacy in the patient. There are several critical elements that are needed for the development and execution of a podocyte screening funnel; these include:
-
a)
disease relevant, human based in vitro assays and functional endpoints;
-
b)
orthologous reagents and in vivo systems that can be used to evaluate a compound(s) efficacy and concentration/dose response.
Of the >100 podocyte targets and pathways that have been identified from the phenotypic evaluation of transgenic animals, human genetics and systems biology approaches as potential causal contributors to nephrotic syndrome, the task to replicate the observation for each one and evaluate their therapeutic potential would be both time-consuming and cost prohibitive in the absence of sensitive, reproducible as well as predictive semi-high throughput assays. Podocytes are terminally differentiated cells and screening requires potentially large volumes of them. As primary cultures derived from sieved glomeruli are limited by tissue availability (human) or de-differentiate generating heterogenous populations in culture during expansion, many groups have therefore taken advantage of conditionally immortalized human and rodent lines 34, 35. However, these cells lack the distinctive morphology of the interdigitated foot process. Whether this is due to the clonal selection, the performance of these cells in the absence of an endothelium and glomerular basement matrix, the conventional 2D structure and lack of microfluidics or just simply that these cells do not express a full repertoire of podocyte proteins, especially those that form the structure of the slit diaphragm, is not clear. New cell lines and systems for deriving podocytes are being developed 36, 37 and may offer promise with respect to some of these deficiencies, but as we will discuss we are still far from a system that offers all of the desired features of bio-mimickry. Should we achieve the perfect conditions to culture podocytes, standardized protocols will need to be developed to assess the correlation between in vitro read outs of podocyte function and in vivo disease observation. Notwithstanding these limitations, some groups have been successful in deriving assays that might mimic some of the mechanistic processes associated with proteinuric disease 38, 39 and that can be used for drug screening purposes40. It will be interesting to determine if high throughput imaging system of podocyte function in vivo can be developed, and the scientific progress of this technique has been reviewed elsewhere 41.
The desire to have both rodent as well as human systems stems from two important observations. Firstly, one can not be confident of the translation of data generated in a rodent system to a patient in the absence of an experimental characterization in an equivalent human assay, for instance if there is a difference in receptor expression or pathway redundancy between systems. Much of the emphasis of the research community has focused on rodent derived podocytes as a primary screening assay, reflecting a desire to characterize a mechanism prior to assessment in a rodent in vivo efficacy model or that the human cells are more tricky to work with35. Secondly, not all compounds or reagents that one might use to interrogate a human target will have specificity and selectivity for the orthologous rodent target. There are many instances where the failure to understand the orthologous pharmacology of a reagent has generated misleading data.
Studies with cultured podocytes have principally focused on changes in actin cytoskeleton, for instance loss of F-actin stress fibers, which accompany a response to injury, usually induced with puromycin 40, 42, 43. This process is believed to underlie the increase in motility and loss of structural stiffness, which accompanies podocyte effacement and loss from the glomerular capillary into the urine. The development of sensitive high content screening (HCS) imaging has enabled the automation and quantitation of F-actin fibers in hundreds of cells/well by decoration with Alexa-labeled phalloidin. With the right reagents it would be possible to develop assays with alternative imaging and functional endpoints, for instance the localization of components of the slit diaphragm at the plasma membrane, the phosphorylation of VASP-1 44, activation of TRP channels 26, 45, accumulation of lipid droplets39 to complement conventional measures of cell morphology, survival, mitochondrial integrity, etc. The increasing availability of relatively affordable HCS technologies (e.g Perkin Elmer Opera) in academic settings are likely to transform the screening and triage of potential therapeutic targets for nephrotic syndrome, whether using podocytes or other cell types. However, these assays take considerable effort and care to develop to ensure robustness and specificity. Ideally the goal of such a system would be for the assay to report a Z’ of ≥0.6, that is an effect size that is greater than >10 standard deviations of assay variability; but in practice a Z’ >0.4 is usable. Despite the wide utilization of changes in actin cytoskeleton as a measure of effect, only one group have reportedly developed a murine podocyte HCS assay 40 that could be used to conduct a phenotypic screen. In these studies, puromycin was used to elicit a concentration-dependent decrease in cellular stress fibers and synaptopodin expression; an effect that could be attenuated by mizoribine as a positive control. Many companies and organizations have invested in developing libraries and ‘tool boxes’ of compounds with known pharmacology and specificity, for instance well-characterized development candidates and FDA approved drugs. In this study, the screen of a library of >2000 of chemically active compounds obtained from several commercial sources yielded a number of interesting hits. Whilst one might have expected certain classes of compounds to be identified, for instance ROCK inhibitors or other modifiers of the actin cytoskeleton, what was more compelling about the list of hits was that the vast majority did not have a known podocyte target pharmacology, for instance were antimicrobial drugs, or had a potency in the assay that exceeded that of its known pharmacology. Should we be more drawn to the targets that validate our own expectations and biases or observations that were unexpected? The paths to validation in both instances are somewhat similar. For the (apparently) known pharmacology there is always a need to independently validate the hit with a structurally distinct chemotype that has similar pharmacology at the receptor. For those examples without an obvious mammalian target, there is a desire to explore the structure-activity relationship of close-in analogues, which might reveal more potent adducts that could be used as bait in a chemical approach or other to identify the target, as has been recently achieved for thalidomide 46. Notwithstanding the value to the patient that could be realized by either opportunity, in the academic community there is likely to be less interest and fanfare of a target identified in an unbiased screen for which there was already published precedence. Similarly, if approved drugs already exist, the incentives and costs associated for repurposing these assets presents significant challenges, even before the consideration of the safety of those agents in the patient population. The critical niche then may be the lower hanging fruit of an unprecedented example where perhaps there are some potential good quality chemical or biological leads available with scope for innovation or that might fall under the NCATS initiative. With the potential utility of a PAN injury assay demonstrated, the opportunity afforded for RNAi or mAb library screening or access to more comprehensive chemical libraries through industry partnership should now be seen as a key driver in the expansion of commercially attractive targets.
We should also be cognizant that puromycin may not be the most appropriate disease adjuvant in this setting. It will be interesting to know from ongoing efforts if the phenotype of human podocytes exposed to the sera of affected patients can be utilized as a tool for drug library screening, as suggested by prior studies39. Still, the simplicity and reproducibility of a puromycin-induced cytoskeletal change, while attractive and has some clinical precedent, may not adequately mimick the genetic mechanisms of disease or the ‘circulating factor’ contribution. The target of puromycin in this setting is not known – whether ER stress, induction of autophagy or other – and this limits the confidence of this phenocopy to predict outcome in all nephrotic syndrome patients regardless of cause. To address this, there may be advantages in developing model systems incorporating known genetic mutations, for instance in podocin or TRPC6, which intrinsically drive a disease phenotype or subjecting cells to sera from FSGS patients to assess antagonism of the ‘circulating factor’ component 38, 44. Furthermore, as puromycin aminonucleoside nephrosis (PAN) is commonly used as a model of human minimal change nephropathy in rats induced by injection of puromycin, one should be wary of the value of approaches identified by screening for prevention of an in vitro puromycin phenotype and assessed in a PAN model. An in vivo assessment in an alternative disease-relevant model, such as LPS challenge, or one of the many genetic models of disease42 may provide more meaningful translational confidence from in vitro data. Alternatively, as circulating factors are involved in the pathogenesis of podocyte injury in FSGS, the use of appropriately controlled patient sera may set the stage for a clinically relevant phenotypic readouts that can be utilized for drug discovery and not solely as non invasive prognostic marker 38, 39.
Better still, if podocytes are challenging to maintain in culture, show diminished expression of key podocyte proteins, lack the distinctive morphology and functional characteristics, then perhaps these 2D systems should be evolved to more sophisticated microfluidics systems. Podocytes in vivo are subjected to considerable fluctuations in pressure, indeed both podocytes and isolated glomeruli show increased TRPC6 evoked currents and Ca2+ signaling in response to stretch 45, suggesting that flow is an important in podocyte function in normal physiology and perhaps in disease also. Podocytes are nourished by paracrine factors released by endothelial cells and sit on a unique glomerular basement membrane that is not mimicked in static 2D cultures. Recent studies 47, 48 have indicated that cells fabricated into a microfluidic environment more closely mimic the in vivo physiological situation. So far the attempts to develop a similar system mimicking the glomerulus that could be used to study the effects of AngII or other triggers of podocyte injury on filtration have not been successful. Earlier studies using sieved isolated whole glomeruli 49 had suggested that it might be possible to functionalize glomerular permeability screens, but development of these systems, even for calcium imaging 26, 45 has proven equally challenging or are limited by throughput. Despite the caveats and limitations of isolated immortalized podocyte cultures, there is still much innovation that can be made in supporting the identification and triage of novel therapeutic approaches using the guidance described above.
If in vitro studies are designed to identify potential targets and test robustly the pharmacology, then the next objective is often to qualify the target in an in vivo setting. Genetic models are particularly attractive as genocopy mimics of the known human mutations (e.g. nephrin, PKCε and CD2AP knockout) lead to persistent proteinuria and glomerulosclerosis 42. Similarly systems, such as those in the zebrafish, that can be used for screening suppressors or enhancers of a particular phenotype or in pharmacological screens 50 are also being increasingly used. However, because of availability, speed and cost, the PAN and LPS models appear to be the work horses of the community, although the models have a reversible phenotype and no efficacy/endpoint criteria that constitute a clinically meaningful effect have ever been formally recommended. In both instances, there is also a need for PAN/LPS titration, which has the potential to impact model performance and institution-to-institution replication. A number of disciplines have observed relatively poor translational performance of pre-clinical experimental studies 51–53, and the renal community are no less immune to the impact of data that are not reproducible54. The confidence of the community to seek to invest and develop new therapies is entirely dependent on the quality of the experimental research that underpins it. The absolute requirement of any study, therefore, is that the data can be replicated by others at different sites and independently by groups with different levels of expertise. Several features of inadequate design and execution have emerged that we shall briefly discuss here to raise awareness, namely:
-
1)
experimental bias: the lack of animal randomization (for instance a tendency to ‘dose cohorts’ rather than randomly assign animals to groups) and blinding in both in-life and data analysis phases can influence, both positively as well as negatively, the outcome of the study. This is particularly impactful for studies that are dependent on qualitative or semi-quantitative endpoints, for instance the selection of EM or histology images to support mechanism and efficacy endpoints.
-
2)
statistical rigor: it’s imperative to design the study with adequate power around a specific magnitude change in a primary endpoint, so that appropriate numbers of animals can be assigned to a given cohort. This assumes that a group has sufficient prior experience with a model, in particular the animal and endpoint variability. For models where there is high intrinsic variability or where the phenotype is acutely driven and subject to variable around the measurement day, this can result in study designs with large numbers of animals that might mean the study has to be randomized in phases. By convention studies are often conducted in a prophylactic dosing setting, rather than after the establishment of a disease phenotype, which might be preferred, and the sample sizes for a therapeutic dosing regimen may be very different from a prophylactic dosing regimen. A positive control, to set a reference benchmark on efficacy, is also desirable.
-
3)
evidence of pharmacology: the utilization of well characterized tools is essential as no compound is exquisitely selective at all concentrations 55. There is a strong desire for studies to test a dose-response and provide evidence of functional target engagement (proximal pharmacology) independently of the efficacy readout and confirm that the target is expressed in the desired compartment. This is important when one considers the potential difficulty of targeting of proteins expressed on the cell surface of the podocyte and how, for instance, a therapeutic antibody might access this cellular compartment from the vasculature or retain durable exposure at the site of action. Where many transgenic model systems which knockout or overexpress a gene/protein in the podocyte appear to have a renal phenotype, the absence of a correlative pharmacological intervention dilutes the significance of any phenotypic observation.
-
4)
replication: Whether knockout or transgenic animals, DNA, recombinant proteins, antibodies or small molecule compounds, a pre-requisite of publication should be that these reagents are made available to the community to facilitate replication and develop further evidence of significance of a given axis. Experimental protocols and methods should be sufficiently detailed and unambiguous that someone with sufficient expertise could repeat the studies. If we are to build a consensus on which approaches the community should invest in, a mechanism for replication should be actively encouraged. Where these criteria are not met, it is clear from the industrial experience of the last few decades 18, 52, 56 that the development of new medicines is stifled or leads to clinical failure.
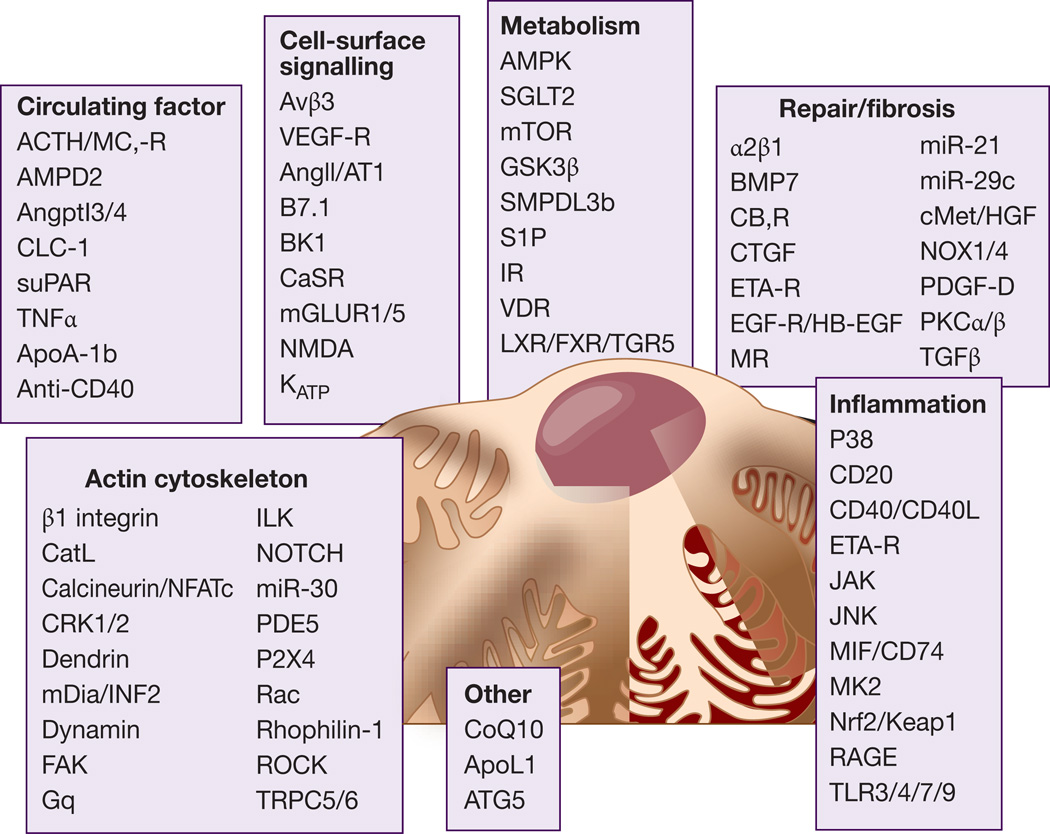
Our dependence on, sometimes multiple, rodent efficacy models is driving up research costs without significant translational impact. The many studies that have generated data which have never been replicated or were poorly conducted serves only to propagate uncertainty at a time when the community would benefit greatly from some notion of consensus and quality. Figure 2 highlights some of the potential targets for drug development in FSGS.
Figure 2. FSGS: clinical and potential experimental targets.
Some of the targets that are either in clinical development or that are supported by experimental studies are shown and subdivided into seven categories: 1) Cell-surface signaling, 2) Metabolism, 3) Actin cytoskeleton, 4) Circulating factors, 5) Inflammation, 6) Repair/fibrosis, 7) Others. A limited number of supporting citations about these targets is provided in supplemental materials.
CONCLUSIONS
FSGS and other glomerular diseases are an underestimated killer and there is a strong need for new therapeutic developments. Several new potential therapeutic targets have now been identified through human genetics or experimental studies. Yet, it will be only through a strong interaction across different disciplines on perhaps agreed mechanisms that will allow for data replication and success. A prioritization strategy to test clinically relevant hypothesis should be agreed by the scientific community. Strategies that are designed on compelling human biology to develop approaches focused on the modification of major disease nodes that are likely to benefit a large proportion of patients are also desirable. Finally, guidelines on experimental models, screening methods, biomarker research and clinical proof of mechanisms studies will be needed to fast track the development of successful drugs for FSGS and other glomerular diseases. 57, 58
Acknowledgments
A.F. is supported by the NIH grant numbers DK090316, DK104753, U24DK076169, U54DK083912, UM1DK100846, and 1UL1TR000460.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST. A.F. is inventor on pending patents aimed to diagnose or treat proteinuric renal diseases. She stands to gain royalties from their future commercialization. A.F. is consultant for Hoffman-La Roche, Genentech, Jannsen, Mesoblast, Abbvie, Boehringer Ingelheim, Alexion, Bristol Myers Squibb. N.P is an employee of Pfizer.
REFERENCES
- 1.USRDS. Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2011. [Google Scholar]
- 2.D’Agati VD, Kaskel FJ, Falk RJ. Focal segmental glomerulosclerosis. The New England journal of medicine. 2011;365:2398–2411. doi: 10.1056/NEJMra1106556. [DOI] [PubMed] [Google Scholar]
- 3.Murray CJ, Lopez AD. Measuring the global burden of disease. The New England journal of medicine. 2013;369:448–457. doi: 10.1056/NEJMra1201534. [DOI] [PubMed] [Google Scholar]
- 4.Coresh J, Turin TC, Matsushita K, et al. Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. JAMA. 2014;311:2518–2531. doi: 10.1001/jama.2014.6634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inker LA, Levey AS, Pandya K, et al. Early change in proteinuria as a surrogate end point for kidney disease progression: an individual patient meta-analysis. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2014;64:74–85. doi: 10.1053/j.ajkd.2014.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thompson A, Cattran DC, Blank M, et al. Complete and Partial Remission as Surrogate End Points in Membranous Nephropathy. J Am Soc Nephrol. 2015 doi: 10.1681/ASN.2015010091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wickman L, Afshinnia F, Wang SQ, et al. Urine podocyte mRNAs, proteinuria, and progression in human glomerular diseases. J Am Soc Nephrol. 2013;24:2081–2095. doi: 10.1681/ASN.2013020173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palmer SC, Sciancalepore M, Strippoli GF. Trial quality in nephrology: how are we measuring up? American journal of kidney diseases : the official journal of the National Kidney Foundation. 2011;58:335–337. doi: 10.1053/j.ajkd.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 9.Somlo S, Mundel P. Getting a foothold in nephrotic syndrome. Nature genetics. 2000;24:333–335. doi: 10.1038/74139. [DOI] [PubMed] [Google Scholar]
- 10.Ruotsalainen V, Ljungberg P, Wartiovaara J, et al. Nephrin is specifically located at the slit diaphragm of glomerular podocytes. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:7962–7967. doi: 10.1073/pnas.96.14.7962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tryggvason K. Unraveling the mechanisms of glomerular ultrafiltration: nephrin, a key component of the slit diaphragm. J Am Soc Nephrol. 1999;10:2440–2445. doi: 10.1681/ASN.V10112440. [DOI] [PubMed] [Google Scholar]
- 12.Kerjaschki D. Caught flat-footed: podocyte damage and the molecular bases of focal glomerulosclerosis. The Journal of clinical investigation. 2001;108:1583–1587. doi: 10.1172/JCI14629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Asanuma K, Mundel P. The role of podocytes in glomerular pathobiology. Clin Exp Nephrol. 2003;7:255–259. doi: 10.1007/s10157-003-0259-6. [DOI] [PubMed] [Google Scholar]
- 14.Boerries M, Grahammer F, Eiselein S, et al. Molecular fingerprinting of the podocyte reveals novel gene and protein regulatory networks. Kidney Int. 2013;83:1052–1064. doi: 10.1038/ki.2012.487. [DOI] [PubMed] [Google Scholar]
- 15.Grgic I, Hofmeister AF, Genovese G, et al. Discovery of new glomerular disease-relevant genes by translational profiling of podocytes in vivo. Kidney Int. 2014;86:1116–1129. doi: 10.1038/ki.2014.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mathieson PW. The podocyte as a target for therapies--new and old. Nature reviews Nephrology. 2012;8:52–56. doi: 10.1038/nrneph.2011.171. [DOI] [PubMed] [Google Scholar]
- 17.Arif EN, D Podocytes as a therapeutic target. Ann Clin Exp Hyperten. 2013;1:1003–1013. [Google Scholar]
- 18.Cook D, Brown D, Alexander R, et al. Lessons learned from the fate of AstraZeneca’s drug pipeline: a five-dimensional framework. Nature reviews Drug discovery. 2014;13:419–431. doi: 10.1038/nrd4309. [DOI] [PubMed] [Google Scholar]
- 19.Nelson MR, Tipney H, Painter JL, et al. The support of human genetic evidence for approved drug indications. Nature genetics. 2015;47:856–860. doi: 10.1038/ng.3314. [DOI] [PubMed] [Google Scholar]
- 20.Plenge RM, Scolnick EM, Altshuler D. Validating therapeutic targets through human genetics. Nature reviews Drug discovery. 2013;12:581–594. doi: 10.1038/nrd4051. [DOI] [PubMed] [Google Scholar]
- 21.Reiser J, Polu KR, Moller CC, et al. TRPC6 is a glomerular slit diaphragm-associated channel required for normal renal function. Nature genetics. 2005;37:739–744. doi: 10.1038/ng1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Winn MP, Conlon PJ, Lynn KL, et al. A mutation in the TRPC6 cation channel causes familial focal segmental glomerulosclerosis. Science. 2005;308:1801–1804. doi: 10.1126/science.1106215. [DOI] [PubMed] [Google Scholar]
- 23.Moller CC, Wei C, Altintas MM, et al. Induction of TRPC6 channel in acquired forms of proteinuric kidney disease. J Am Soc Nephrol. 2007;18:29–36. doi: 10.1681/ASN.2006091010. [DOI] [PubMed] [Google Scholar]
- 24.Hauser PV, Pippin JW, Kaiser C, et al. Novel siRNA delivery system to target podocytes in vivo. PloS one. 2010;5:e9463. doi: 10.1371/journal.pone.0009463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang L, Jirka G, Rosenberg PB, et al. Gq signaling causes glomerular injury by activating TRPC6. The Journal of clinical investigation. 2015;125:1913–1926. doi: 10.1172/JCI76767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schaldecker T, Kim S, Tarabanis C, et al. Inhibition of the TRPC5 ion channel protects the kidney filter. The Journal of clinical investigation. 2013;123:5298–5309. doi: 10.1172/JCI71165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller M, Shi J, Zhu Y, et al. Identification of ML204, a novel potent antagonist that selectively modulates native TRPC4/C5 ion channels. The Journal of biological chemistry. 2011;286:33436–33446. doi: 10.1074/jbc.M111.274167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martini S, Nair V, Keller BJ, et al. Integrative biology identifies shared transcriptional networks in CKD. J Am Soc Nephrol. 2014;25:2559–2572. doi: 10.1681/ASN.2013080906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kretzler M, Sedor JR. Introduction: Precision Medicine for Glomerular Disease: The Road Forward. Seminars in nephrology. 2015;35:209–211. doi: 10.1016/j.semnephrol.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ashraf S, Gee HY, Woerner S, et al. ADCK4 mutations promote steroid-resistant nephrotic syndrome through CoQ10 biosynthesis disruption. The Journal of clinical investigation. 2013;123:5179–5189. doi: 10.1172/JCI69000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hodgin JB, Bitzer M, Wickman L, et al. Glomerular Aging and Focal Global Glomerulosclerosis: A Podometric Perspective. J Am Soc Nephrol. 2015 doi: 10.1681/ASN.2014080752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peired A, Lazzeri E, Lasagni L, et al. Glomerular regeneration: when can the kidney regenerate from injury and what turns failure into success? Nephron Experimental nephrology. 2014;126:70. doi: 10.1159/000360669. [DOI] [PubMed] [Google Scholar]
- 33.Fukuda A, Wickman LT, Venkatareddy MP, et al. Angiotensin II-dependent persistent podocyte loss from destabilized glomeruli causes progression of end stage kidney disease. Kidney Int. 2012;81:40–55. doi: 10.1038/ki.2011.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saleem MA, O’Hare MJ, Reiser J, et al. A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J Am Soc Nephrol. 2002;13:630–638. doi: 10.1681/ASN.V133630. [DOI] [PubMed] [Google Scholar]
- 35.Ni L, Saleem M, Mathieson PW. Podocyte culture: tricks of the trade. Nephrology. 2012;17:525–531. doi: 10.1111/j.1440-1797.2012.01619.x. [DOI] [PubMed] [Google Scholar]
- 36.Da Sacco S, Lemley KV, Sedrakyan S, et al. A novel source of cultured podocytes. PloS one. 2013;8:e81812. doi: 10.1371/journal.pone.0081812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Song B, Smink AM, Jones CV, et al. The directed differentiation of human iPS cells into kidney podocytes. PloS one. 2012;7:e46453. doi: 10.1371/journal.pone.0046453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fornoni A, Sageshima J, Wei C, et al. Rituximab targets podocytes in recurrent focal segmental glomerulosclerosis. Science translational medicine. 2011;3:85ra46. doi: 10.1126/scitranslmed.3002231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Merscher-Gomez S, Guzman J, Pedigo CE, et al. Cyclodextrin protects podocytes in diabetic kidney disease. Diabetes. 2013 doi: 10.2337/db13-0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee HW, Khan SQ, Faridi MH, et al. A Podocyte-Based Automated Screening Assay Identifies Protective Small Molecules. J Am Soc Nephrol. 2015 doi: 10.1681/ASN.2014090859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peti-Peterdi J, Kidokoro K, Riquier-Brison A. Novel in vivo techniques to visualize kidney anatomy and function. Kidney Int. 2015;88:44–51. doi: 10.1038/ki.2015.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schiffer M, Teng B, Gu C, et al. Pharmacological targeting of actin-dependent dynamin oligomerization ameliorates chronic kidney disease in diverse animal models. Nat Med. 2015;21:601–609. doi: 10.1038/nm.3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yaddanapudi S, Altintas MM, Kistler AD, et al. CD2AP in mouse and human podocytes controls a proteolytic program that regulates cytoskeletal structure and cellular survival. The Journal of clinical investigation. 2011;121:3965–3980. doi: 10.1172/JCI58552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harris JJ, McCarthy HJ, Ni L, et al. Active proteases in nephrotic plasma lead to a podocin-dependent phosphorylation of VASP in podocytes via protease activated receptor-1. The Journal of pathology. 2013;229:660–671. doi: 10.1002/path.4149. [DOI] [PubMed] [Google Scholar]
- 45.Anderson M, Kim EY, Hagmann H, et al. Opposing effects of podocin on the gating of podocyte TRPC6 channels evoked by membrane stretch or diacylglycerol. American journal of physiology Cell physiology. 2013;305:C276–C289. doi: 10.1152/ajpcell.00095.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ito T, Ando H, Suzuki T, et al. Identification of a primary target of thalidomide teratogenicity. Science. 2010;327:1345–1350. doi: 10.1126/science.1177319. [DOI] [PubMed] [Google Scholar]
- 47.Jang KJ, Mehr AP, Hamilton GA, et al. Human kidney proximal tubule-on-a-chip for drug transport and nephrotoxicity assessment. Integrative biology : quantitative biosciences from nano to macro. 2013;5:1119–1129. doi: 10.1039/c3ib40049b. [DOI] [PubMed] [Google Scholar]
- 48.Huh D, Leslie DC, Matthews BD, et al. A human disease model of drug toxicity-induced pulmonary edema in a lung-on-a-chip microdevice. Science translational medicine. 2012;4:159ra147. doi: 10.1126/scitranslmed.3004249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Savin VJ, Sharma R, Sharma M, et al. Circulating factor associated with increased glomerular permeability to albumin in recurrent focal segmental glomerulosclerosis. The New England journal of medicine. 1996;334:878–883. doi: 10.1056/NEJM199604043341402. [DOI] [PubMed] [Google Scholar]
- 50.Sugano Y, Lindenmeyer MT, Auberger I, et al. The Rho-GTPase binding protein IQGAP2 is required for the glomerular filtration barrier. Kidney Int. 2015 doi: 10.1038/ki.2015.197. [DOI] [PubMed] [Google Scholar]
- 51.Pullen N, Birch CL, Douglas GJ, et al. The translational challenge in the development of new and effective therapies for endometriosis: a review of confidence from published preclinical efficacy studies. Human reproduction update. 2011;17:791–802. doi: 10.1093/humupd/dmr030. [DOI] [PubMed] [Google Scholar]
- 52.Prinz F, Schlange T, Asadullah K. Believe it or not: how much can we rely on published data on potential drug targets? Nature reviews Drug discovery. 2011;10:712. doi: 10.1038/nrd3439-c1. [DOI] [PubMed] [Google Scholar]
- 53.Begley CG, Ellis LM. Drug development: Raise standards for preclinical cancer research. Nature. 2012;483:531–533. doi: 10.1038/483531a. [DOI] [PubMed] [Google Scholar]
- 54.Spinale JM, Mariani LH, Kapoor S, et al. A reassessment of soluble urokinase-type plasminogen activator receptor in glomerular disease. Kidney Int. 2015;87:564–574. doi: 10.1038/ki.2014.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Leeson PD, Springthorpe B. The influence of drug-like concepts on decision-making in medicinal chemistry. Nature reviews Drug discovery. 2007;6:881–890. doi: 10.1038/nrd2445. [DOI] [PubMed] [Google Scholar]
- 56.Morgan P, Van Der Graaf PH, Arrowsmith J, et al. Can the flow of medicines be improved? Fundamental pharmacokinetic and pharmacological principles toward improving Phase II survival. Drug discovery today. 2012;17:419–424. doi: 10.1016/j.drudis.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 57.Koshikawa M, Mukoyama M, Mori K, et al. Role of p38 mitogen-activated protein kinase activation in podocyte injury and proteinuria in experimental nephrotic syndrome. J Am Soc Nephrol. 2005;16:2690–2701. doi: 10.1681/ASN.2004121084. [DOI] [PubMed] [Google Scholar]
- 58.Pengal R, Guess AJ, Agrawal S, et al. Inhibition of the protein kinase MK-2 protects podocytes from nephrotic syndrome-related injury. American journal of physiology Renal physiology. 2011;301:F509–F519. doi: 10.1152/ajprenal.00661.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cherney DZ, Perkins BA, Soleymanlou N, et al. Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation. 2014;129:587–597. doi: 10.1161/CIRCULATIONAHA.113.005081. [DOI] [PubMed] [Google Scholar]
References related to Figure 2.1–70
- 1.Barton M, Sorokin A. Endothelin and the glomerulus in chronic kidney disease. Seminars in nephrology. 2015;35:156–167. doi: 10.1016/j.semnephrol.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bu X, Zhou Y, Zhang H, et al. Systemic administration of naked plasmid encoding HGF attenuates puromycin aminonucleoside-induced damage of murine glomerular podocytes. American journal of physiology Renal physiology. 2011;301:F784–F792. doi: 10.1152/ajprenal.00210.2011. [DOI] [PubMed] [Google Scholar]
- 3.Buelli S, Rosano L, Gagliardini E, et al. beta-arrestin-1 drives endothelin-1-mediated podocyte activation and sustains renal injury. J Am Soc Nephrol. 2014;25:523–533. doi: 10.1681/ASN.2013040362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ju W, Nair V, Smith S, et al. Tissue transcriptome-driven identification of epidermal growth factor as a chronic kidney disease biomarker. Science translational medicine. 2015;7:316ra193. doi: 10.1126/scitranslmed.aac7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lai JY, Luo J, O’Connor C, et al. MicroRNA-21 in glomerular injury. J Am Soc Nephrol. 2015;26:805–816. doi: 10.1681/ASN.2013121274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin CL, Lee PH, Hsu YC, et al. MicroRNA-29a promotion of nephrin acetylation ameliorates hyperglycemia-induced podocyte dysfunction. J Am Soc Nephrol. 2014;25:1698–1709. doi: 10.1681/ASN.2013050527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takagi N, Tanizawa T, Kon V, et al. Mineralocorticoid Receptor Blocker Protects against Podocyte-Dependent Glomerulosclerosis. Nephron extra. 2012;2:17–26. doi: 10.1159/000334961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Helmering J, Juan T, Li CM, et al. A mutation in Ampd2 is associated with nephrotic syndrome and hypercholesterolemia in mice. Lipids in health and disease. 2014;13:167. doi: 10.1186/1476-511X-13-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wei C, Moller CC, Altintas MM, et al. Modification of kidney barrier function by the urokinase receptor. Nat Med. 2008;14:55–63. doi: 10.1038/nm1696. [DOI] [PubMed] [Google Scholar]
- 10.Yu CC, Fornoni A, Weins A, et al. Abatacept in B7-1-Positive Proteinuric Kidney Disease. The New England journal of medicine. 2013 doi: 10.1056/NEJMoa1304572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borza CM, Su Y, Chen X, et al. Inhibition of integrin alpha2beta1 ameliorates glomerular injury. J Am Soc Nephrol. 2012;23:1027–1038. doi: 10.1681/ASN.2011040367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pereira RL, Buscariollo BN, Correa-Costa M, et al. Bradykinin receptor 1 activation exacerbates experimental focal and segmental glomerulosclerosis. Kidney Int. 2011;79:1217–1227. doi: 10.1038/ki.2011.14. [DOI] [PubMed] [Google Scholar]
- 13.Lee HW, Khan SQ, Faridi MH, et al. A Podocyte-Based Automated Screening Assay Identifies Protective Small Molecules. J Am Soc Nephrol. 2015 doi: 10.1681/ASN.2014090859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oh J, Beckmann J, Bloch J, et al. Stimulation of the calcium-sensing receptor stabilizes the podocyte cytoskeleton, improves cell survival, and reduces toxin-induced glomerulosclerosis. Kidney Int. 2011;80:483–492. doi: 10.1038/ki.2011.105. [DOI] [PubMed] [Google Scholar]
- 15.George B, Verma R, Soofi AA, et al. Crk1/2-dependent signaling is necessary for podocyte foot process spreading in mouse models of glomerular disease. The Journal of clinical investigation. 2012;122:674–692. doi: 10.1172/JCI60070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gu L, Liang X, Wang L, et al. Functional metabotropic glutamate receptors 1 and 5 are expressed in murine podocytes. Kidney Int. 2012;81:458–468. doi: 10.1038/ki.2011.406. [DOI] [PubMed] [Google Scholar]
- 17.Giardino L, Armelloni S, Corbelli A, et al. Podocyte glutamatergic signaling contributes to the function of the glomerular filtration barrier. J Am Soc Nephrol. 2009;20:1929–1940. doi: 10.1681/ASN.2008121286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tamura Y, Tanabe K, Kitagawa W, et al. Nicorandil, a K(atp) channel opener, alleviates chronic renal injury by targeting podocytes and macrophages. American journal of physiology Renal physiology. 2012;303:F339–F349. doi: 10.1152/ajprenal.00158.2012. [DOI] [PubMed] [Google Scholar]
- 19.Yaddanapudi S, Altintas MM, Kistler AD, et al. CD2AP in mouse and human podocytes controls a proteolytic program that regulates cytoskeletal structure and cellular survival. The Journal of clinical investigation. 2011;121:3965–3980. doi: 10.1172/JCI58552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang L, Jirka G, Rosenberg PB, et al. Gq signaling causes glomerular injury by activating TRPC6. The Journal of clinical investigation. 2015;125:1913–1926. doi: 10.1172/JCI76767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu J, Zheng C, Wang X, et al. MicroRNA-30 family members regulate calcium/calcineurin signaling in podocytes. The Journal of clinical investigation. 2015;125:4091–4106. doi: 10.1172/JCI81061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Djudjaj S, Lue H, Rong S, et al. Macrophage Migration Inhibitory Factor Mediates Proliferative GN via CD74. J Am Soc Nephrol. 2015 doi: 10.1681/ASN.2015020149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hall G, Rowell J, Farinelli F, et al. Phosphodiesterase 5 inhibition ameliorates angiontensin II-induced podocyte dysmotility via the protein kinase G-mediated downregulation of TRPC6 activity. American journal of physiology Renal physiology. 2014;306:F1442–F1450. doi: 10.1152/ajprenal.00212.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ravani P, Ponticelli A, Siciliano C, et al. Rituximab is a safe and effective long-term treatment for children with steroid and calcineurin inhibitor-dependent idiopathic nephrotic syndrome. Kidney Int. 2013 doi: 10.1038/ki.2013.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nijenhuis T, Sloan AJ, Hoenderop JG, et al. Angiotensin II contributes to podocyte injury by increasing TRPC6 expression via an NFAT-mediated positive feedback signaling pathway. The American journal of pathology. 2011;179:1719–1732. doi: 10.1016/j.ajpath.2011.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heeringa SF, Chernin G, Chaki M, et al. COQ6 mutations in human patients produce nephrotic syndrome with sensorineural deafness. The Journal of clinical investigation. 2011;121:2013–2024. doi: 10.1172/JCI45693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gee HY, Saisawat P, Ashraf S, et al. ARHGDIA mutations cause nephrotic syndrome via defective RHO GTPase signaling. The Journal of clinical investigation. 2013;123:3243–3253. doi: 10.1172/JCI69134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Faul C, Donnelly M, Merscher-Gomez S, et al. The actin cytoskeleton of kidney podocytes is a direct target of the antiproteinuric effect of cyclosporine A. Nat Med. 2008;14:931–938. doi: 10.1038/nm.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clement LC, Mace C, Avila-Casado C, et al. Circulating angiopoietin-like 4 links proteinuria with hypertriglyceridemia in nephrotic syndrome. Nat Med. 2014;20:37–46. doi: 10.1038/nm.3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jo SK, Bajwa A, Awad AS, et al. Sphingosine-1-phosphate receptors: biology and therapeutic potential in kidney disease. Kidney Int. 2008;73:1220–1230. doi: 10.1038/ki.2008.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Janiak P, Poirier B, Bidouard JP, et al. Blockade of cannabinoid CB1 receptors improves renal function, metabolic profile, and increased survival of obese Zucker rats. Kidney Int. 2007;72:1345–1357. doi: 10.1038/sj.ki.5002540. [DOI] [PubMed] [Google Scholar]
- 32.Joy MS, Gipson DS, Powell L, et al. Phase 1 trial of adalimumab in Focal Segmental Glomerulosclerosis (FSGS): II Report of the FONT (Novel Therapies for Resistant FSGS) study group. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2010;55:50–60. doi: 10.1053/j.ajkd.2009.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCarthy ET, Sharma M, Savin VJ. Circulating permeability factors in idiopathic nephrotic syndrome and focal segmental glomerulosclerosis. Clin J Am Soc Nephrol. 2010;5:2115–2121. doi: 10.2215/CJN.03800609. [DOI] [PubMed] [Google Scholar]
- 34.Sampson MG, Robertson CC, Martini S, et al. Integrative Genomics Identifies Novel Associations with APOL1 Risk Genotypes in Black NEPTUNE Subjects. J Am Soc Nephrol. 2015 doi: 10.1681/ASN.2014111131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yokoi H, Mukoyama M, Mori K, et al. Overexpression of connective tissue growth factor in podocytes worsens diabetic nephropathy in mice. Kidney Int. 2008;73:446–455. doi: 10.1038/sj.ki.5002722. [DOI] [PubMed] [Google Scholar]
- 36.Ijaz A, Tejada T, Catanuto P, et al. Inhibition of C-jun N-terminal kinase improves insulin sensitivity but worsens albuminuria in experimental diabetes. Kidney Int. 2009;75:381–388. doi: 10.1038/ki.2008.559. [DOI] [PubMed] [Google Scholar]
- 37.Gorin Y, Cavaglieri RC, Khazim K, et al. Targeting NADPH oxidase with a novel dual Nox1/Nox4 inhibitor attenuates renal pathology in type 1 diabetes. American journal of physiology Renal physiology. 2015;308:F1276–F1287. doi: 10.1152/ajprenal.00396.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Menne J, Shushakova N, Bartels J, et al. Dual inhibition of classical protein kinase C-alpha and protein kinase C-beta isoforms protects against experimental murine diabetic nephropathy. Diabetes. 2013;62:1167–1174. doi: 10.2337/db12-0534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tossidou I, Teng B, Menne J, et al. Podocytic PKC-alpha is regulated in murine and human diabetes and mediates nephrin endocytosis. PloS one. 2010;5:e10185. doi: 10.1371/journal.pone.0010185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trachtman H, Fervenza FC, Gipson DS, et al. A phase 1, single-dose study of fresolimumab, an anti-TGF-beta antibody, in treatment-resistant primary focal segmental glomerulosclerosis. Kidney Int. 2011;79:1236–1243. doi: 10.1038/ki.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Roeyen CR, Eitner F, Boor P, et al. Induction of progressive glomerulonephritis by podocyte-specific overexpression of platelet-derived growth factor-D. Kidney Int. 2011;80:1292–1305. doi: 10.1038/ki.2011.278. [DOI] [PubMed] [Google Scholar]
- 42.Wang Y, Deb DK, Zhang Z, et al. Vitamin D receptor signaling in podocytes protects against diabetic nephropathy. J Am Soc Nephrol. 2012;23:1977–1986. doi: 10.1681/ASN.2012040383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pengal R, Guess AJ, Agrawal S, et al. Inhibition of the protein kinase MK-2 protects podocytes from nephrotic syndrome-related injury. American journal of physiology Renal physiology. 2011;301:F509–F519. doi: 10.1152/ajprenal.00661.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saurus P, Kuusela S, Lehtonen E, et al. Podocyte apoptosis is prevented by blocking the Toll-like receptor pathway. Cell death & disease. 2015;6:e1752. doi: 10.1038/cddis.2015.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stitt-Cavanagh EM, Faour WH, Takami K, et al. A maladaptive role for EP4 receptors in podocytes. J Am Soc Nephrol. 2010;21:1678–1690. doi: 10.1681/ASN.2009121234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamada S, Nakamura J, Asada M, et al. Twisted gastrulation, a BMP antagonist, exacerbates podocyte injury. PloS one. 2014;9:e89135. doi: 10.1371/journal.pone.0089135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yoshida S, Nagase M, Shibata S, et al. Podocyte injury induced by albumin overload in vivo and in vitro: involvement of TGF-beta and p38 MAPK. Nephron Experimental nephrology. 2008;108:e57–e68. doi: 10.1159/000124236. [DOI] [PubMed] [Google Scholar]
- 48.Agrawal S, Guess AJ, Chanley MA, et al. Albumin-induced podocyte injury and protection are associated with regulation of COX-2. Kidney Int. 2014;86:1150–1160. doi: 10.1038/ki.2014.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brosius FC, 3rd, He JC. JAK inhibition and progressive kidney disease. Curr Opin Nephrol Hypertens. 2015;24:88–95. doi: 10.1097/MNH.0000000000000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miyazaki Y, Shimizu A, Pastan I, et al. Keap1 inhibition attenuates glomerulosclerosis. Nephrol Dial Transplant. 2014;29:783–791. doi: 10.1093/ndt/gfu002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou LL, Cao W, Xie C, et al. The receptor of advanced glycation end products plays a central role in advanced oxidation protein products-induced podocyte apoptosis. Kidney Int. 2012;82:759–770. doi: 10.1038/ki.2012.184. [DOI] [PubMed] [Google Scholar]
- 52.Lal MA, Andersson AC, Katayama K, et al. Rhophilin-1 is a key regulator of the podocyte cytoskeleton and is essential for glomerular filtration. J Am Soc Nephrol. 2015;26:647–662. doi: 10.1681/ASN.2013111195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sun H, Schlondorff J, Higgs HN, et al. Inverted formin 2 regulates actin dynamics by antagonizing Rho/diaphanous-related formin signaling. J Am Soc Nephrol. 2013;24:917–929. doi: 10.1681/ASN.2012080834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ashraf S, Gee HY, Woerner S, et al. ADCK4 mutations promote steroid-resistant nephrotic syndrome through CoQ10 biosynthesis disruption. The Journal of clinical investigation. 2013;123:5179–5189. doi: 10.1172/JCI69000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Banas MC, Banas B, Hudkins KL, et al. TLR4 links podocytes with the innate immune system to mediate glomerular injury. J Am Soc Nephrol. 2008;19:704–713. doi: 10.1681/ASN.2007040395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Delville M, Sigdel TK, Wei C, et al. A circulating antibody panel for pretransplant prediction of FSGS recurrence after kidney transplantation. Science translational medicine. 2014;6:256ra136. doi: 10.1126/scitranslmed.3008538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fornoni A, Sageshima J, Wei C, et al. Rituximab targets podocytes in recurrent focal segmental glomerulosclerosis. Science translational medicine. 2011;3:85ra46. doi: 10.1126/scitranslmed.3002231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Forst AL, Olteanu VS, Mollet G, et al. Podocyte Purinergic P2×4 Channels Are Mechanotransducers That Mediate Cytoskeletal Disorganization. J Am Soc Nephrol. 2015 doi: 10.1681/ASN.2014111144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.George B, Vollenbroker B, Saleem MA, et al. GSK3beta inactivation in podocytes results in decreased phosphorylation of p70S6K accompanied by cytoskeletal rearrangements and inhibited motility. American journal of physiology Renal physiology. 2011;300:F1152–F1162. doi: 10.1152/ajprenal.00373.2010. [DOI] [PubMed] [Google Scholar]
- 60.Godel M, Hartleben B, Herbach N, et al. Role of mTOR in podocyte function and diabetic nephropathy in humans and mice. The Journal of clinical investigation. 2011;121:2197–2209. doi: 10.1172/JCI44774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kawakami T, Gomez IG, Ren S, et al. Deficient Autophagy Results in Mitochondrial Dysfunction and FSGS. J Am Soc Nephrol. 2015;26:1040–1052. doi: 10.1681/ASN.2013111202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kimura J, Ichii O, Miyazono K, et al. Overexpression of Toll-like receptor 8 correlates with the progression of podocyte injury in murine autoimmune glomerulonephritis. Scientific reports. 2014;4:7290. doi: 10.1038/srep07290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ma H, Togawa A, Soda K, et al. Inhibition of podocyte FAK protects against proteinuria and foot process effacement. J Am Soc Nephrol. 2010;21:1145–1156. doi: 10.1681/ASN.2009090991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Naves MA, Requiao-Moura LR, Soares MF, et al. Podocyte Wnt/ss-catenin pathway is activated by integrin-linked kinase in clinical and experimental focal segmental glomerulosclerosis. Journal of nephrology. 2012;25:401–409. doi: 10.5301/jn.5000017. [DOI] [PubMed] [Google Scholar]
- 65.Niranjan T, Bielesz B, Gruenwald A, et al. The Notch pathway in podocytes plays a role in the development of glomerular disease. Nat Med. 2008;14:290–298. doi: 10.1038/nm1731. [DOI] [PubMed] [Google Scholar]
- 66.Reiser J, Polu KR, Moller CC, et al. TRPC6 is a glomerular slit diaphragm-associated channel required for normal renal function. Nature genetics. 2005;37:739–744. doi: 10.1038/ng1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schaldecker T, Kim S, Tarabanis C, et al. Inhibition of the TRPC5 ion channel protects the kidney filter. The Journal of clinical investigation. 2013;123:5298–5309. doi: 10.1172/JCI71165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schiffer M, Teng B, Gu C, et al. Pharmacological targeting of actin-dependent dynamin oligomerization ameliorates chronic kidney disease in diverse animal models. Nat Med. 2015;21:601–609. doi: 10.1038/nm.3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang L, Ellis MJ, Fields TA, et al. Beneficial effects of the Rho kinase inhibitor Y27632 in murine puromycin aminonucleoside nephrosis. Kidney & blood pressure research. 2008;31:111–121. doi: 10.1159/000121531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lindskog A, Ebefors K, Johansson ME, et al. Melanocortin 1 receptor agonists reduce proteinuria. J Am Soc Nephrol. 2010;21:1290–1298. doi: 10.1681/ASN.2009101025. [DOI] [PMC free article] [PubMed] [Google Scholar]