Abstract
Using neuroimaging techniques to explore the central mechanism of acupuncture gains increasing attention, but the quality control of acupuncture-neuroimaging study remains to be improved. We searched the PubMed Database during 1995 to 2014. The original English articles with neuroimaging scan performed on human beings were included. The data involved quality control including the author, sample size, characteristics of the participant, neuroimaging technology, and acupuncture intervention were extracted and analyzed. The rigorous inclusion and exclusion criteria are important guaranty for the participants' homogeneity. The standard operation process of acupuncture and the stricter requirement for acupuncturist play significant role in quality control. More attention should be paid to the quality control in future studies to improve the reproducibility and reliability of the acupuncture-neuroimaging studies.
1. Introduction
Acupuncture, a traditional therapy originated from China, has been gradually accepted as an alternative and complementary therapy by the Western medical community for its undeniable efficacy for pain and chronic diseases [1–5]. As acupuncture is widely used all over the world, its underlying mechanism attracts increasing attention. Since the 1970s, several studies of acupuncture on experimental animals have proven that the integration of central nervous system (CNS) plays an important role in acupuncture efficacy [6, 7]. With the development of neuroimaging techniques such as functional Magnetic Resonance Imaging (fMRI), Positron Emission Tomography (PET), Single-Photon Emission Computed Tomography (SPECT), electroencephalography (EEG), and magnetoencephalography (MEG), using these techniques to investigate the cerebral responses to acupuncture stimulations in vivo [8] has gradually become a spotlight in acupuncture mechanism research. Over the past two decades, there are around 200 original articles having been published in English, and a growing body of evidence demonstrates the involvement of CNS in acupuncture mechanism [9]. However, it was found that the results of acupuncture-neuroimaging studies were untenable. For example, some studies on acupoint specificity showed that the cerebral responses to acupoint stimulation significantly differed from acupoint to sham acupoint [10–13]. Some studies demonstrated that there was no significant difference in cerebral reaction between acupoint and sham acupoint [14–16]. Some investigators held that the significant difference in cerebral responses between acupoint and sham acupoint was only found during Deqi (needle sensation) state [17]. Methodology issues might contribute to the conflict results.
As we know, design and quality control are key points which may affect the result of a study, and strict quality control plays an essential role in the guarantee of a high repeatability, especially in acupuncture-neuroimaging study for relative smaller sample size, complexity of cerebral function, and integrity of acupuncture effect. So this study aims to investigate the current status of the quality control in acupuncture-neuroimaging studies from sample size, subjects screening, manipulation procedure, and scanning mode by analyzing the original articles published in English in the latest two decades, so as to provide ideas for the development of quality control criteria in future acupuncture-neuroimaging study.
2. Methods
2.1. Searching Strategy
We searched the original articles published during 1995 to 2014 on PubMed (http://www.ncbi.nlm.nih.gov/pubmed/) using the following MeSH terms and search strategies: ((“Acupuncture” [Mesh] OR “Acupuncture Therapy” [Mesh] OR “Acupuncture, Ear” [Mesh] OR “Acupuncture Points” [Mesh] OR “Acupuncture Analgesia” [Mesh])) AND ((“Neuroimaging” [Mesh] OR “Functional Neuroimaging” [Mesh] OR “Functional MRI” [Mesh] OR “PET” [Mesh] OR “EEG” [Mesh])).
We screened the bibliographies of identified trials and reviewed articles for further potentially relevant publication. Subsequently, we screened the full texts and assessed whether these articles met the inclusion criteria.
2.2. Inclusion and Exclusion Criteria
The articles would be included if they were (1) original articles; (2) acupuncture-neuroimaging studies on human beings; (3) published in English; and (4) published during 1995 to 2014.
The articles would be excluded if they were (1) reviews or editorials or trial protocols; (2) acupuncture-neuroimaging studies on animals; or (3) duplicate articles.
2.3. Data Extraction and Analysis
We extracted the data including the author (nationality, affiliation, and component), sample size, characteristics of the participant (patients or the health, age, gender, race, handedness, emotional state, acupuncture experience, and accompanying symptoms), neuroimaging technology, acupuncture intervention (method of intervention, manipulation procedure, Deqi/needle sensation, and acupuncturist), and ethical review. The data analysis was conducted after data extraction.
3. Results
168 [7, 10–176] original articles were included in this study.
Most of the studies were conducted in China (80 studies) [10, 11, 13, 14, 16, 18–24, 26, 27, 29–31, 33–36, 43–46, 48, 51, 53, 54, 56, 58–66, 68–70, 72–75, 77, 78, 83, 84, 86, 87, 89, 90, 93, 95, 97, 99–101, 104, 111, 113, 115, 123, 137, 138, 142, 144–147, 154, 159, 161–166] and in USA (40 studies) [15, 28, 37, 38, 42, 57, 70, 71, 76, 80, 88, 91, 94, 96, 105–107, 118–120, 124–127, 129–131, 133–136, 140, 141, 148, 151, 152, 155, 156, 158, 174]. The investigators in Korea (16 studies) [7, 47, 49, 52, 55, 82, 85, 98, 102, 109, 112, 114, 116, 117, 122, 153], Taiwan (6 studies) [79, 110, 128, 170, 171, 175], Austria (6 studies) [132, 139, 150, 160, 168, 176], Germany (5 studies) [32, 50, 157, 167, 169], Australia (4 studies) [40, 41, 92, 121], Japan (4 studies) [39, 108, 149, 173], UK (4 studies) [17, 67, 81, 103], Italy (2 studies) [25, 172], and Denmark (1 study) [143] also published articles on acupuncture-neuroimaging (Figure 1). 60 studies [16, 19, 23, 25, 26, 37, 38, 42, 45, 46, 55, 56, 60, 62, 65, 67, 70–72, 75, 78, 81, 87, 88, 90, 93, 97, 100, 101, 104, 106, 107, 110, 111, 113–115, 117–121, 123, 126–129, 132, 134, 137, 142, 145–147, 150, 157, 160, 162, 168, 175] were performed with the cooperation of more than two countries.
Figure 1.
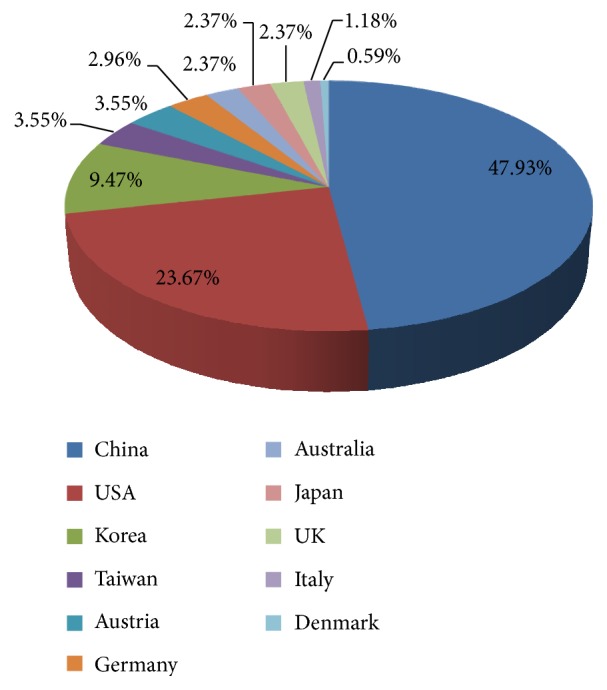
The nationality distribution of acupuncture-neuroimaging studies.
3.1. Sample Size
The average sample size of these studies was 15 participants per group. For the studies performed on patients, the average sample size was 16 participants per group, while the maximal and minimal sample sizes per group were 55 participants and 1 participant, respectively. For those performed on healthy subjects, the average sample size was 14 per group, and the maximal and minimal sample sizes per group were 48 participants and 1 participant, respectively.
3.2. The Status of Participants
3.2.1. Classification of Participants
122 studies [7, 11–13, 15–17, 24, 25, 30–33, 35, 36, 39, 41–45, 50, 53–57, 63, 64, 66–70, 72–76, 78–83, 85–100, 102–104, 106, 107, 109–111, 113–130, 132, 135, 136, 140, 143, 144, 147–176] were performed on healthy subjects. 25 studies were [10, 26–29, 34, 38, 49, 51, 58–60, 62, 65, 71, 84, 101, 105, 108, 112, 134, 137, 139, 142, 145] performed on patients. 21 studies [14, 18–23, 37, 40, 46–48, 52, 61, 77, 131, 133, 138, 141, 146, 165] recruited both healthy subjects and patients. 25 kinds of diseases were involved in these studies (Table 1). 19 studies [10, 14, 18, 19, 29, 34, 35, 40, 46, 51, 52, 59, 62, 65, 77, 84, 112, 134, 146] classified the subtypes of diseases.
Table 1.
The diseases involved in acupuncture-neuroimaging studies.
| Category | Disease | Number of studies |
|---|---|---|
| Neurology | Stroke | 12 studies |
| Alzheimer's disease | 2 studies | |
| Bell's palsy | 2 studies | |
| Mild cognitive impairment | 2 studies | |
| Parkinson's disease | 2 studies | |
| Vascular aphasia | 1 study | |
| Carpal tunnel syndrome | 5 studies | |
| Migraine | 2 studies | |
|
| ||
| Pain | Chronic low back pain | 1 study |
| Fibromyalgia | 1 study | |
| Chronic knee osteoarthritis pain | 1 study | |
| Musculoskeletal disease | 1 study | |
|
| ||
| Psychonosology | Depression | 2 studies |
| Heavy smoker | 1 study | |
| Heroin addicts | 1 study | |
|
| ||
| Gastroenterology | Functional diarrhea | 1 study |
| Irritable bowel syndrome—diarrhea | 2 studies | |
| Functional dyspepsia | 1 study | |
|
| ||
| Pediatrics | Childhood autism | 1 study |
| Children with visual disorder | 1 study | |
| Children with a severe type of cerebral palsy | 1 study | |
|
| ||
| Rheumatology | Rheumatoid arthritis | 1 study |
|
| ||
| Dermatology | Atopic dermatitis | 1 study |
|
| ||
| Myopia | Myopia | 1 study |
3.2.2. Age
100 studies [7, 10, 16–19, 23, 24, 26, 27, 30, 34, 35, 39–42, 44–48, 51–54, 57, 59, 60, 62, 63, 65, 69–74, 76, 77, 80–82, 84, 85, 87, 89, 92, 97, 98, 101–103, 105, 107–109, 112, 114, 116, 117, 120–122, 124, 125, 129, 131–135, 137, 139, 141–145, 148–153, 157, 158, 160, 162, 164, 166–168, 171–176] described the age range of participants. For the healthy subjects, the average age range was 18.3 years old, and the maximal age range was 62, while the minimal age range was 3. For the patients, the average age range was 29 years old, and the maximal age range was 57, while the minimal age range was 6. Taking studies on the stroke for instance, the maximal age range of the stroke patients was 52, while the minimal age range was 7. Furthermore, 66 studies [11, 13–15, 20–22, 25, 28, 29, 31–33, 36–38, 43, 49, 50, 55, 56, 58, 61, 64, 66–68, 75, 78, 79, 83, 86, 88, 90, 91, 93, 95, 96, 99, 100, 104, 106, 110, 111, 113, 115, 118, 119, 123, 126–128, 130, 136, 138, 140, 146, 147, 154–156, 159, 161, 163, 169, 176] described the average age of participants. Three studies [94, 163, 170] did not mention the age of participants.
3.2.3. Gender
159 studies [7, 10–26, 28–79, 81–93, 95–114, 116–123, 125–131, 133–151, 153–169, 172–176] described the gender of the participants (56.7% male and 43.3% female). Nine studies [27, 80, 94, 115, 124, 132, 152, 170, 171] did not mention the gender of the participants.
3.2.4. Race and Handedness
14 studies [66, 75, 87, 90, 99, 105, 115, 126, 135, 148, 151, 164, 167, 174] described and restricted the race of participants. 136 [10–24, 26, 28, 30–36, 39–56, 58, 59, 61–80, 83–87, 89–93, 95, 96, 98–100, 103–107, 109–111, 113–115, 117–131, 133, 135–138, 140–142, 144, 146–149, 151–157, 159, 162–164, 166, 168, 169, 171, 173–175] studies asked for the right-hand participants in inclusion criteria.
3.2.5. Emotional State
The psychological assessment on the participants was performed in 4 studies [21, 31, 46, 92, 93]. The self-rating depression scale (SDS) and the self-rating anxiety scale (SAS) were used in 2 studies [31, 46]. The Beck Depression Inventory (BDI) [92] and the State Trait Anxiety Inventory (STAI) [93] were used in 1 study, respectively. Nine studies [19–22, 24, 65, 76, 92, 146] have excluded the participants with claustrophobia.
3.2.6. Accompanying Symptoms
38 studies [11, 13, 15, 21, 31, 36, 42, 43, 46, 54, 57, 64, 65, 68, 73, 76, 78, 81, 87, 93, 100, 102, 104, 106, 110–121, 123, 128, 133, 135, 141, 148, 151, 153, 156, 158, 165, 174] excluded the participants with head trauma, and some studies [24, 46, 54, 73, 100, 121, 122] excluded the participants suffering from pain (including headache and dysmenorrhea).
3.2.7. Acupuncture Experience
81 articles [13–16, 18, 22, 24, 26, 28, 29, 31, 33–35, 37, 38, 42, 51, 54–59, 63, 64, 67, 68, 70, 71, 73, 75, 78, 83, 85–87, 89, 90, 92, 93, 95, 96, 99, 101, 103–106, 108, 111, 113–115, 117–121, 123, 127, 130, 135, 138, 140, 144, 147, 148, 151–153, 156–158, 162, 166–169, 174] described the acupuncture experience of participants. Among these articles, 73 articles [12–16, 18, 22, 28, 29, 31, 33–35, 37, 38, 42, 51, 55, 57–59, 63, 64, 67, 68, 70, 71, 73, 75, 78, 83, 85–87, 89, 90, 93, 95, 96, 99, 101, 103–106, 108, 111, 113–115, 117–121, 123, 127, 130, 135, 138, 140, 144, 147, 148, 151–153, 157, 158, 166–169] described the participants as acupuncture naive.
3.3. Neuroimaging Technology
137 studies [7, 11, 12, 14–26, 28, 29, 31–42, 44–50, 52, 53, 55–61, 63, 64, 66, 68–79, 84–87, 89–101, 103, 104, 106, 107, 109–115, 117–120, 122, 123, 125–129, 131–136, 138–141, 144, 146–159, 161, 162, 164, 166–168, 170, 171, 173–175] used fMRI (82.14%) to investigate the cerebral responses to acupuncture stimulation. Six studies [43, 80, 88, 116, 130, 160] used the combination of two imaging technologies. The application of the techniques in acupuncture-neuroimaging studies was shown in Figure 2.
Figure 2.
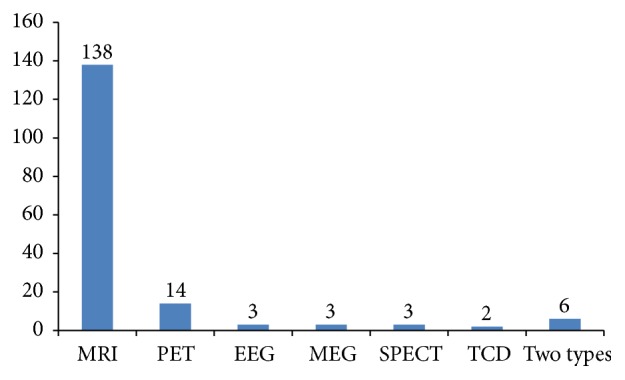
The techniques used in acupuncture-neuroimaging studies. MRI: Magnetic Resonance Imaging, PET: Positron Emission Tomography, EEG: electroencephalography, MEG: magnetoencephalography, SPECT: Single-Photon Emission Computed Tomography, and TCD: Transcranial Doppler.
3.4. Acupuncture Intervention
3.4.1. Method of Intervention
111 studies [7, 10, 12–14, 16–26, 28, 29, 31–34, 36, 39, 42, 43, 46–59, 61, 63, 64, 66–68, 75, 78, 82, 83, 85–88, 90, 91, 93–95, 97–100, 102–106, 108–114, 125–129, 132, 135–139, 142, 144, 145, 148, 149, 151, 153, 154, 156, 157, 163–165, 167, 172, 174–176] chose manual acupuncture as intervention method. 32 studies [27, 37, 38, 44, 60, 62, 65, 69, 70, 72, 76, 77, 80, 84, 89, 96, 101, 107, 115–117, 124, 140, 146, 152, 159, 162, 166, 169–171, 173] chose electroacupuncture as intervention method. Besides, the transcutaneous electric acupoint stimulation was performed in 6 studies [45, 73, 74, 81, 141, 143], the laser acupuncture in 6 studies [40, 79, 92, 150, 160, 168], heat stimulation on acupoints in 2 studies [147, 155], and the magnetic stimulation on acupoints in 1 study [30]. There were 10 studies [11, 15, 35, 41, 71, 131, 133, 134, 158, 161] using at least two types of acupuncture methods (Figure 3).
Figure 3.
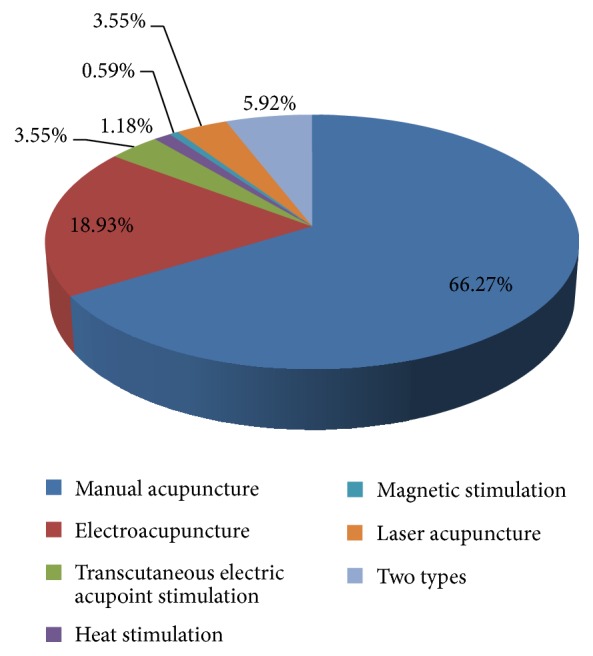
The acupuncture methods used in acupuncture-neuroimaging studies.
3.4.2. Manipulation Procedure
134 articles [10–20, 22, 24, 26, 27, 29, 30, 34–39, 41, 42, 46–52, 54–70, 72, 73, 75–130, 132, 133, 135–138, 142, 144–146, 148, 151–154, 156–158, 163, 167–170, 172, 174–176] have described the manipulation procedure of acupuncture.
3.4.3. Deqi (Needle Sensation)
82 studies [10, 15, 16, 22, 23, 26, 28, 29, 34, 35, 37, 38, 41–47, 50, 54, 56, 57, 64, 68, 69, 72, 73, 75–77, 80, 87, 89–91, 93–96, 98–101, 103, 105–111, 114, 115, 117, 118, 120–124, 127, 130, 133, 135–138, 140, 144, 146, 148, 153, 157, 158, 166–168, 172, 174–176] required Deqi (needle sensation) during acupuncture stimulation.
56 studies [10, 14–18, 28, 35, 38, 42, 43, 50, 53, 55, 57, 58, 61, 64, 66, 68, 69, 71, 73, 75–77, 87, 89–91, 93–96, 99–101, 103, 104, 106–111, 115, 117, 120, 124, 127–130, 140, 166, 169] have evaluated needle sensation after acupuncture stimulation. The 10-point Visual Analogue Scale (VAS), the Massachusetts General Hospital Acupuncture Sensation Scale (MASS), the Subject Acupuncture Sensation Scale (SASS), the 6-point Likert scale, the Park questionnaire, the Psychophysical Rating of Needling Sensation, and the Needle Sensation Questionnaire (NSQ) were used to evaluate the needle sensation (Figure 4).
Figure 4.
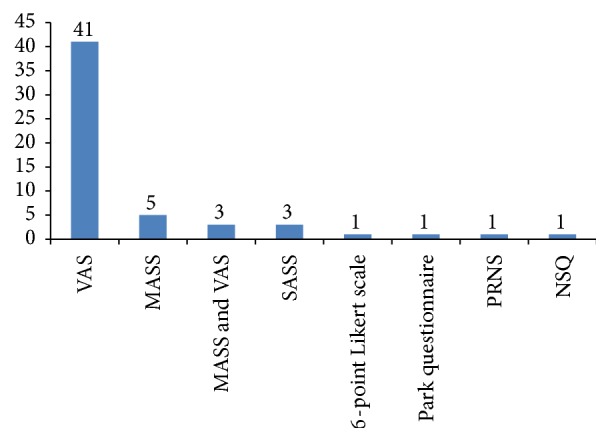
The scales/questionnaire used in needle sensation evaluation. VAS: 10-point Visual Analogue Scale, MASS: Massachusetts General Hospital Acupuncture Sensation Scale, SASS: Subject Acupuncture Sensation Scale, PRNS: Psychophysical Rating of Needling Sensation, and NSQ: Needle Sensation Questionnaire.
3.4.4. Qualification of Acupuncturists
99 articles [12, 14–19, 22–24, 26, 28, 29, 31–42, 44, 46–50, 52, 54–61, 63, 64, 66, 67, 69, 71, 72, 75, 78, 80, 82–84, 86, 87, 89–91, 93–98, 100–104, 106, 108–112, 114–117, 119–124, 126–129, 133, 135, 139, 140, 142, 144, 150, 158, 166, 174] have mentioned the qualification of acupuncturists.
3.5. The Ethical Review
139 studies [10–18, 20–29, 31–36, 38–45, 47–58, 60, 63–79, 81–86, 88, 90–93, 96–107, 109–115, 117–121, 123–125, 127, 128, 130–133, 135, 136, 138–148, 150–156, 158–162, 164, 166–168, 172, 174, 175] have mentioned the ethical review in the study.
4. Discussion
Owning to the complexity of the brain function and the diversity of acupuncture manipulations, the different or even reversed results occurring in similar acupuncture-neuroimaging studies become a common phenomenon. Seeking reasonable and practical methods is essential to improve the reproducibility and reliability of results in acupuncture-neuroimaging studies. Based on the open published literatures, this study analyzed the status of quality control in acupuncture-neuroimaging study for the first time and tried to provide some new ideas for future studies.
4.1. Sample Size
The appropriate sample size is important for designing an acupuncture-neuroimaging study. Bigger sample size increases statistical power because the standard error of the mean decreases by the square root of number (N). Due to the potential radioactivity (PET/SPECT) and the costs of imaging, the sample size in most of the neuroimaging studies was small. Some investigators suggested that 12 to 15 subjects per group could get statistical power in fMRI studies [177, 178]. Others held that the method should ensure large sample size to use rigorous corrections for multiple tests [179]. In this study, we found that the average sample size was 15 participants per group, and the average sample size for patients was slightly bigger than that for healthy subjects (16 versus 14 per group). Nowadays, most investigators agreed that, to achieve the stable statistical power, bigger sample size (at least 20 participants per group) was needed in the future acupuncture-neuroimaging study [180].
4.2. The Selection of Participants
The rigorous inclusion and exclusion criteria, as important guaranty for the homogeneity of the participants, are of great significance in the quality control of clinical trial.
4.2.1. Classification of Participants
This study indicated that the majority of neuroimaging studies (72.78%) were performed on healthy subjects. It might be a reason for the inconsistent results. Because the traditional Chinese acupuncture theory holds that acupuncture treatment focuses on strengthening the body resistance to removing pathogenic factors and restoring the balance of Yin and Yang, the efficacy of acupuncture treatment is specific to the pathological conditions (imbalance of Yin and Yang), not the physiological state (Yin and Yang in equilibrium). So during the pathological conditions, the actions of acupoints are disease-oriented, while in the physiological state, the acupoint keeps in silence and the actions of acupoints lack orientation. In this case, patient is the better choice for acupuncture-neuroimaging studies.
In this study, we found that there was a preponderance of nervous system disorders such as stroke among the diseases involved in acupuncture-neuroimaging studies. The result indicated that acupuncture stimulation promoted the action of neural rehabilitation and its mechanism is a focus of acupuncture study. Furthermore, we noticed that some studies were performed on functional disorders such as functional dyspepsia and irritable bowel syndrome [65, 101]. As we know, regulating functional disorder is the advantage of acupuncture, so functional disorder might be a new approach in future studies.
Moreover, the subtypes of a disease should be taken into consideration when you choose patients as the participants in neuroimaging study for the patients with different subtypes might have functional or/and structural differences in brain. For example, schizophrenic subjects with predominantly negative symptoms have greater metabolic abnormalities than subjects with predominantly positive symptoms [181]. So, in acupuncture-neuroimaging study, it is better to choose the same subtype of a disease to ensure the homogeneity of participants.
4.2.2. Demographic Characteristics of Participants
Some demographic characteristics of participants including age, gender, race, and handedness should be defined in the inclusion criteria.
The changes of cerebral function and structure come with age. Some studies indicated that the cerebral glucose metabolism decreased unevenly and brain tissues began aging after 40 years old [182, 183]. Older age directly correlated with reduced gray matter volume in bilateral rostral and right dorsal ACC [184]. So the age range of participants should not be ignored in neuroimaging studies. However we found that most of these studies (101 studies) described the age range of participants, that the average age range of healthy subjects was 18.3 years old, and that the average age range of patients was 29 years old. Even in some studies, the age range was more than 50 years old. It is better to keep the age range within 20 years to reduce the effect of outlier.
The functional and structural differences in human brain induced by handedness have long been investigated [185–188], although the mechanism remains unclear. So the majority of the current studies (137 studies) choose right-hand participants.
Furthermore, gender differences of the human brain are an important issue in neuroimaging studies. It has been identified that gender has significant influence on the regional neuronal activity [189, 190] and brain structure [191]. Race differences may lead to differences of brain function and structure. For example, it is reported that brain size varies by race [192]. In this study, we found that 14 studies (8.33%) described and restricted the race of the participants. Taking gender and race as covariates is needed when designing acupuncture-neuroimaging experiments.
4.2.3. Emotional State
The psychological factors have significant influence on the function and structure of human brain. For example, Drevets et al. have found an area of abnormally decreased activity in the prefrontal cortex ventral to the genu of the corpus callosum in both unipolar depressives and bipolar depressives [193]. Our study shows that only 4 articles described the psychological assessment performed on the participants; more attention should be paid to the emotional state of subjects during the inclusion and data analysis in future studies, except for the study which focuses on the mechanism of acupuncture treating for psychological disorders.
Furthermore, our study demonstrated that participants with claustrophobia have been excluded in 9 studies. Claustrophobia is a phobic disorder which will cause panic, fear, or anxiety in the confined space. Scanning cannot be accomplished when it is performed on a participant with claustrophobia. So the participants with claustrophobia should be excluded in acupuncture-neuroimaging studies.
4.2.4. Menstrual Period
In this study, we found that female participants were involved in 145 studies. Recently, some studies performed on healthy subjects indicated the cerebral functional and structural changes in menstrual period. For example, Veldhuijzen et al. [194] found that the pain-related cerebral activation varied significantly across the menstrual cycle. Hagemann et al. [195] found a significant gray matter volume peak and cerebral spinal fluid loss at the time of ovulation in females. So, for female participants, scanning should be performed during the same physiological period to avoid the possible changes in brain size and activity in menstrual cycles.
4.2.5. Accompanying Symptoms/Disorders
The accompanying symptoms/disorders such as head trauma, pain (including headache and dysmenorrhea), and insomnia should be excluded as possible as we could considering their influence on the neuroimaging data. Tu et al. [196, 197] found that abnormal gray matter volume changes are presented in primary dysmenorrhea patients even in the absence of pain. Furthermore, some investigators held that blood coagulation disorders should be excluded in acupuncture studies [6].
4.2.6. Acupuncture Experience
Some studies have reported the significant differences in cerebral response between the participant with acupuncture experience and the participant without acupuncture experience [198]. In our study, we found that participants in 44.04% of the studies were acupuncture naive. To ensure the consistency of the baseline of the participants and the comparability of consequence, the acupuncture experience of participants should be taken into consideration.
4.3. Image Technology
Among the neuroimaging technologies, fMRI (82.14%) was most commonly used in the acupuncture-neuroimaging studies. But we also found that multimodel imaging techniques became a new trend in acupuncture-neuroimaging studies for their significant advantages in improving spatial/temporal resolutions and lowing noise.
4.4. Acupuncture Intervention
4.4.1. Method of Intervention
We found that, during 1995 to 2014, 66.27% studies have used manual acupuncture as intervention method, and 18.93% studies have used the electroacupuncture as intervention method. Among those manual acupuncture studies, 78.57% studies have described the needle manipulation. During 2005–2014, the majority of acupuncture-neuroimaging studies (68.71%) still used manual acupuncture as intervention method, and 17.01% studies used the electroacupuncture as intervention method. Among the manual acupuncture studies, 79.21% studies have described the needle manipulation. The results indicated that (1) although the stimulation of manual acupuncture is hard to be quantified for the individual differences of manipulation induced by different practitioners, manual acupuncture, as the traditional acupuncture intervention, is easier to be accepted by investigators and (2) the majority of these studies with manual acupuncture treatment describe the acupuncture manipulation including sterilization, the angle and depth of needle insertion, and the duration of retaining needle to ensure consistency of acupuncture treatment. Considering the influence of different manipulation results, we should not allocate too many acupuncturists in one neuroimaging study. In order to ensure the accuracy of the results, it is better to perform acupuncture manipulation with one acupuncturist.
Meanwhile, transcutaneous electric acupoint stimulation, magnetic stimulation, heat stimulation, and laser acupuncture were used in some neuroimaging studies. The results indicated that not only manual acupuncture and electroacupuncture, but also other acupuncture-related interventions attract increasing interests of investigators. In the future, more attention should be paid to those acupuncture methods which have not or seldom been touched such as ear acupuncture, abdominal acupuncture, and wrist and ankle acupuncture.
4.4.2. Qualification of Acupuncturist and Operation Procedure
The qualification of acupuncturist and operation procedure is important in quality control of acupuncture trial. In our study, 59.5% of the studies have mentioned the qualification of acupuncturists and 79.88% of the studies have described the manipulation procedure of acupuncture. For the defined influence of the qualification of acupuncturist and manipulation procedure on clinical efficacy, the needling details including numbers of needle, depth of insertion, elicited response, and needle retention time and the background of practitioners including the duration of relevant training, length of clinical experience, and details of expertise in treating the specific condition being evaluated as well as any other experience that may be relevant to the trial should be reported according to the standards for reporting interventions in controlled trials of acupuncture (The STRICTA Recommendations) [199].
4.4.3. Deqi (Needle Sensation) and Evaluation of Sensation
Deqi (needle sensation) plays an important role in acupuncture efficacy. Clinical trials have demonstrated that acupuncture with needle sensation was superior to acupuncture without needle sensation for analgesia [200] and paralysis [201]. A neuroimaging study [54] also showed the significant differences of cerebral responses under the Deqi and non-Deqi condition. So it is important to record the Deqi sensation in acupuncture studies.
In this study, we found that the questionnaire-based forms such as 10-point VAS, MASS, SASS, Park questionnaire, Psychophysical Rating of Needling Sensation, and NSQ were used to assess the needle sensation in acupuncture-neuroimaging studies. Among them, 10-point VAS was the most commonly used (68.3%). However, the liabilities and validities of some specific or nonspecific questionnaires/scales for needle sensation need further investigation.
4.5. The Author Nationality and Ethical Review
In this study, we found that 63.7% of the studies were accomplished by the cooperation of more than two countries. The international cooperation improved the study level and quality control in some degree. To get more international recognition and perfecting research, the acupuncture-neuroimaging studies need more international cooperation. Furthermore, ethics has got growing attention by the researchers. The interests of the participants should be taken in the first place. The ethics was an essential component which should be considered during the whole acupuncture-neuroimaging studies.
In conclusion, to improve the reproducibility and reliability of the acupuncture-neuroimaging studies, more attention should be paid to the quality control including sample size, participants screening, and acupuncture manipulations in future studies. A practical and standard quality control criterion should be developed to improve the acupuncture-neuroimaging studies.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (no. 81473602), the State Key Program for Basic Research of China (973 Program, no. 2012CB518501), the Education Ministry's New Century Excellent Talents Supporting Plan, the Special Fund for the Authors Who Win the 100 Top Doctoral Dissertations Award of China (no. 2014084), and the Sichuan Youth Science & Technology Foundation (no. 15QNJJ0008).
Disclosure
Ke Qiu, Miaomiao Jing, and Ruirui Sun are co-first authors and contributed equally to this paper.
Competing Interests
The authors declare that they have no competing interests.
References
- 1.Diehl D. L., Kaplan G., Coulter I., Glik D., Hurwitz E. L. Use of acupuncture by American physicians. Journal of Alternative and Complementary Medicine. 1997;3(2):119–126. doi: 10.1089/acm.1997.3.119. [DOI] [PubMed] [Google Scholar]
- 2.Eisemberg D. M., Kessler R. C., Foster C., et al. Unconventional medicine in the United States. The New England Journal of Medicine. 1993;328:246–252. doi: 10.1056/NEJM199301283280406. [DOI] [PubMed] [Google Scholar]
- 3.Elsenberg D. M., Davis R. B., Ethmer S. C. Trend in alternative medicines use in the United States. The Journal of the American Medical Association. 1990;280:1569–1575. [Google Scholar]
- 4.Yu Z., Cao X., Xia Y., et al. Electroacupuncture stimulation at CV12 inhibits gastric motility via TRPV1 receptor. Evidence Based Complementary and Alternative Medicine. 2013;2013:6. doi: 10.1155/2013/294789.294789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kerr Grieve J., Flucker S., O'Riordan J. Acupuncture is an effective treatment for pain and other mssymptoms. Journal of Neurology, Neurosurgery & Psychiatry. 2013;84, article e2 [Google Scholar]
- 6.Han J.-S. Acupuncture: neuropeptide release produced by electrical stimulation of different frequencies. Trends in Neurosciences. 2003;26(1):17–22. doi: 10.1016/s0166-2236(02)00006-1. [DOI] [PubMed] [Google Scholar]
- 7.Cho Z. H., Chung S. C., Jones J. P., et al. New findings of the correlation between acupoints and corresponding brain cortices using functional MRI. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(5):2670–2673. doi: 10.1073/pnas.95.5.2670. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Zeng F., Liu X.-G., Tang Y., Liang F.-R. Application of PET-CT technique to the research on central mechanism of acupuncture effects. Zhen Ci Yan Jiu. 2008;33(4):284–286. [PubMed] [Google Scholar]
- 9.Cho Z.-H., Oleson T. D., Alimi D., Niemtzow R. C. Acupuncture: the search for biologic evidence with functional magnetic resonance imaging and positron emission tomography techniques. Journal of Alternative and Complementary Medicine. 2002;8(4):399–401. doi: 10.1089/107555302760253577. [DOI] [PubMed] [Google Scholar]
- 10.Yang J., Zeng F., Feng Y., et al. A PET-CT study on the specificity of acupoints through acupuncture treatment in migraine patients. BMC Complementary and Alternative Medicine. 2012;12, article 123 doi: 10.1186/1472-6882-12-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ren X.-J., Chen H.-Y., Wang B.-G., et al. Regional homogeneity analysis on acupoint specificity with resting-state functional magnetic resonance imaging. Chinese Medical Journal. 2012;125(9):1627–1632. doi: 10.3760/cma.j.issn.0366-6999.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 12.Dong M., Qin W., Sun J., et al. Tempo-spatial analysis of vision-related acupoint specificity in the occipital lobe using fMRI: an ICA study. Brain Research. 2012;1436:34–42. doi: 10.1016/j.brainres.2011.11.050. [DOI] [PubMed] [Google Scholar]
- 13.Feng Y., Bai L., Zhang W., et al. Investigation of acupoint specificity by whole brain functional connectivity analysis from fMRI data. Proceedings of the 33rd Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBS '11); August 2011; Boston, Mass, USA. pp. 2784–2787. [DOI] [PubMed] [Google Scholar]
- 14.Liu Z., Wei W., Bai L., et al. Exploring the patterns of acupuncture on mild cognitive impairment patients using regional homogeneity. PLoS ONE. 2014;9(6) doi: 10.1371/journal.pone.0099335.e99335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kong J., Kaptchuk T. J., Webb J. M., et al. Functional neuroanatomical investigation of vision-related acupuncture point specificity—a multisession fMRI study. Human Brain Mapping. 2009;30(1):38–46. doi: 10.1002/hbm.20481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fang J., Jin Z., Wang Y., et al. The salient characteristics of the central effects of acupuncture needling: limbic-paralimbic-neocortical network modulation. Human Brain Mapping. 2009;30(4):1196–1206. doi: 10.1002/hbm.20583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.MacPherson H., Green G., Nevado A., et al. Brain imaging of acupuncture: comparing superficial with deep needling. Neuroscience Letters. 2008;434(1):144–149. doi: 10.1016/j.neulet.2008.01.058. [DOI] [PubMed] [Google Scholar]
- 18.Xie Z., Cui F., Zou Y., Bai L. Acupuncture enhances effective connectivity between cerebellum and primary sensorimotor cortex in patients with stable recovery stroke. Evidence-Based Complementary and Alternative Medicine. 2014;2014:9. doi: 10.1155/2014/603909.603909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bai L., Tao Y., Wang D., et al. Acupuncture induces time-dependent remodelling brain network on the stable somatosensory first-ever stroke patients: combining diffusion tensor and functional MR imaging. Evidence-based Complementary and Alternative Medicine. 2014;2014:7. doi: 10.1155/2014/740480.740480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Z., Liang P., Zhao Z., et al. Acupuncture modulates resting state hippocampal functional connectivity in Alzheimer disease. PLoS ONE. 2014;9(3) doi: 10.1371/journal.pone.0091160.e91160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liang P., Wang Z., Qian T., Li K. Acupuncture stimulation of Taichong (Liv3) and Hegu (LI4) modulates the default mode network activity in Alzheimer's disease. American Journal of Alzheimer's Disease and Other Dementias. 2014;29(8):739–748. doi: 10.1177/1533317514536600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li J., Zhang J.-H., Yi T., Tang W.-J., Wang S.-W., Dong J.-C. Acupuncture treatment of chronic low back pain reverses an abnormal brain default mode network in correlation with clinical pain relief. Acupuncture in Medicine. 2014;32(2):102–108. doi: 10.1136/acupmed-2013-010423. [DOI] [PubMed] [Google Scholar]
- 23.He X., Zhu Y., Li C., et al. Acupuncture-induced changes in functional connectivity of the primary somatosensory cortex varied with pathological stages of Bell's palsy. NeuroReport. 2014;25(14):1162–1168. doi: 10.1097/WNR.0000000000000246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu C., Qu S., Zhang J., et al. Correlation between the effects of acupuncture at Taichong (LR3) and functional brain areas: a resting-state functional magnetic resonance imaging study using true versus sham acupuncture. Evidence-Based Complementary and Alternative Medicine. 2014;2014:7. doi: 10.1155/2014/729091.729091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Romoli M., Allais G., Airola G., et al. Ear acupuncture and fMRI: a pilot study for assessing the specificity of auricular points. Neurological Sciences. 2014;35(1):S189–S193. doi: 10.1007/s10072-014-1768-7. [DOI] [PubMed] [Google Scholar]
- 26.Zhao L., Liu J., Zhang F., et al. Effects of long-term acupuncture treatment on resting-state brain activity in migraine patients: a randomized controlled trial on active acupoints and inactive acupoints. PLoS ONE. 2014;9(6) doi: 10.1371/journal.pone.0099538.e99538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao Z.-Q., Jia S.-W., Hu S., Sun W. Evaluating the effectiveness of electro-acupuncture as a treatment for childhood autism using single photon emission computed tomography. Chinese Journal of Integrative Medicine. 2014;20(1):19–23. doi: 10.1007/s11655-014-1680-2. [DOI] [PubMed] [Google Scholar]
- 28.Hashmi J. A., Kong J., Spaeth R., Khan S., Kaptchuk T. J., Gollub R. L. Functional network architecture predicts psychologically mediated analgesia related to treatment in chronic knee pain patients. Journal of Neuroscience. 2014;34(11):3924–3936. doi: 10.1523/JNEUROSCI.3155-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen J., Wang J., Huang Y., et al. Modulatory effect of acupuncture at Waiguan (TE5) on the functional connectivity of the central nervous system of patients with ischemic stroke in the left basal ganglia. PLoS ONE. 2014;9(6) doi: 10.1371/journal.pone.0096777.e96777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo L., Wang Y., Yu H., Yin N., Li Y. Study of brain functional network based on sample entropy of EEG under magnetic stimulation at PC6 acupoint. Bio-Medical Materials and Engineering. 2014;24(1):1063–1069. doi: 10.3233/BME-130904. [DOI] [PubMed] [Google Scholar]
- 31.Zhao L., Qin W., Liu J. X., et al. Two sets of acupoint combination of similar functions engage shared neural representation: a functional magnetic resonance imaging study. Chinese Journal of Integrative Medicine. 2014;20(3):184–193. doi: 10.1007/s11655-014-1744-3. [DOI] [PubMed] [Google Scholar]
- 32.Usichenko T. I., Wesolowski T., Lotze M. Verum and sham acupuncture exert distinct cerebral activation in pain processing areas: a crossover fMRI investigation in healthy volunteers. Brain Imaging and Behavior. 2014;9(2):236–244. doi: 10.1007/s11682-014-9301-4. [DOI] [PubMed] [Google Scholar]
- 33.Cheng H., Yan H., Bai L.-J., Wang B.-G. Exploration of whole brain networks modulated by acupuncture at analgesia acupoint ST36 using scale-specific wavelet correlation analysis. Chinese Medical Journal. 2013;126(13):2459–2464. doi: 10.3760/cma.j.issn.0366-6999.20122681. [DOI] [PubMed] [Google Scholar]
- 34.Huang Y., Chen J.-Q., Lai X.-S., et al. Lateralisation of cerebral response to active acupuncture in patients with unilateral ischaemic stroke: an fMRI study. Acupuncture in Medicine. 2013;31(3):290–296. doi: 10.1136/acupmed-2012-010299. [DOI] [PubMed] [Google Scholar]
- 35.Jiang Y., Wang H., Liu Z., et al. Manipulation of and sustained effects on the human brain induced by different modalities of acupuncture: an fMRI study. PLoS ONE. 2013;8(6) doi: 10.1371/journal.pone.0066815.e66815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu J., Nan J., Xiong S., Li G., Qin W., Tian J. Additional evidence for the sustained effect of acupuncture at the vision-related acupuncture point, GB37. Acupuncture in Medicine. 2013;31(2):185–194. doi: 10.1136/acupmed-2012-010251. [DOI] [PubMed] [Google Scholar]
- 37.Maeda Y., Kettner N., Lee J., et al. Acupuncture evoked response in contralateral somatosensory cortex reflects peripheral nerve pathology of carpal tunnel syndrome. Medical Acupuncture. 2013;25(4):275–284. doi: 10.1089/acu.2013.0964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maeda Y., Kettner N., Lee J., et al. Acupuncture-evoked response in somatosensory and prefrontal cortices predicts immediate pain reduction in carpal tunnel syndrome. Evidence-Based Complementary and Alternative Medicine. 2013;2013:13. doi: 10.1155/2013/795906.795906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murase T., Umeda M., Fukunaga M., Tanaka C., Higuchi A. T. Deconvolution analyses with tent functions reveal delayed and long-sustained increases of BOLD signals with acupuncture stimulation. Magnetic Resonance in Medical Sciences. 2013;12(2):121–127. doi: 10.2463/mrms.2012-0070. [DOI] [PubMed] [Google Scholar]
- 40.Quah-Smith I., Suo C., Williams M. A., Sachdev P. S. The antidepressant effect of laser acupuncture: a comparison of the resting brain's default mode network in healthy and depressed subjects during functional magnetic resonance imaging. Medical Acupuncture. 2013;25(2):124–133. doi: 10.1089/acu.2012.0901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Quah-Smith I., Williams M. A., Lundeberg T., Suo C., Sachdev P. Differential brain effects of laser and needle acupuncture at LR8 using functional MRI. Acupuncture in Medicine. 2013;31(3):282–289. doi: 10.1136/acupmed-2012-010297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang X., Chan S.-T., Fang J., et al. Neural encoding of acupuncture needling sensations: evidence from a fMRI study. Evidence-Based Complementary and Alternative Medicine. 2013;2013:15. doi: 10.1155/2013/483105.483105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.You Y., Bai L., Dai R., et al. Altered hub configurations within default mode network following acupuncture at ST36: a multimodal investigation combining fMRI and MEG. PLoS ONE. 2013;8(5) doi: 10.1371/journal.pone.0064509.e64509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang G., Qu S., Zheng Y., et al. Key regions of the cerebral network are altered after electro acupuncture at the Baihui (GV20) and Yintang acupuncture points in healthy volunteers: an analysis based on resting fcMRI. Acupuncture in Medicine. 2013;31(4):383–388. doi: 10.1136/acupmed-2012-010301. [DOI] [PubMed] [Google Scholar]
- 45.Zhang Y., Jiang Y., Glielmi C. B., et al. Long-duration transcutaneous electric acupoint stimulation alters small-world brain functional networks. Magnetic Resonance Imaging. 2013;31(7):1105–1111. doi: 10.1016/j.mri.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 46.Zhou S., Zeng F., Liu J., et al. Influence of acupuncture stimulation on cerebral network in functional diarrhea. Evidence-Based Complementary and Alternative Medicine. 2013;2013:9. doi: 10.1155/2013/975769.975769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cho S.-Y., Kim M., Sun J. J., et al. A comparison of brain activity between healthy subjects and stroke patients on fMRI by acupuncture stimulation. Chinese Journal of Integrative Medicine. 2013;19(4):269–276. doi: 10.1007/s11655-013-1436-4. [DOI] [PubMed] [Google Scholar]
- 48.Li C., Yang J., Sun J., et al. Brain responses to acupuncture are probably dependent on the brain functional status. Evidence-Based Complementary and Alternative Medicine. 2013;2013:14. doi: 10.1155/2013/175278.175278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kang O.-S., Kim S.-Y., Jahng G.-H., et al. Neural substrates of acupuncture in the modulation of cravings induced by smoking-related visual cues: an FMRI study. Psychopharmacology. 2013;228(1):119–127. doi: 10.1007/s00213-013-3015-y. [DOI] [PubMed] [Google Scholar]
- 50.Beissner F., Deichmann R., Henke C., Bär K.-J. Acupuncture—deep pain with an autonomic dimension? NeuroImage. 2012;60(1):653–660. doi: 10.1016/j.neuroimage.2011.12.045. [DOI] [PubMed] [Google Scholar]
- 51.Huang Y., Tang C., Wang S., et al. Acupuncture regulates the glucose metabolism in cerebral functional regions in chronic stage ischemic stroke patients—a PET-CT cerebral functional imaging study. BMC Neuroscience. 2012;13(1, article 75) doi: 10.1186/1471-2202-13-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yeo S., Lim S., Choe I.-H., et al. Acupuncture stimulation on gb34 activates neural responses associated with parkinson's disease. CNS Neuroscience and Therapeutics. 2012;18(9):781–790. doi: 10.1111/j.1755-5949.2012.00363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu B., Chen J., Wang J., et al. Altered small-world efficiency of brain functional networks in acupuncture at ST36: a functional MRI study. PLoS ONE. 2012;7(6) doi: 10.1371/journal.pone.0039342.e39342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen J.-R., Li G.-L., Zhang G.-F., Huang Y., Wang S.-X., Lu N. Brain areas involved in acupuncture needling sensation of de qi: a single-photon emission computed tomography (SPECT) study. Acupuncture in Medicine. 2012;30(4):316–323. doi: 10.1136/acupmed-2012-010169. [DOI] [PubMed] [Google Scholar]
- 55.Napadow V., Lee J., Kim J., et al. Brain correlates of phasic autonomic response to acupuncture stimulation: an event-related fMRI study. Human Brain Mapping. 2013;34(10):2592–2606. doi: 10.1002/hbm.22091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang G., Yin H., Zhou Y.-L., et al. Capturing amplitude changes of low-frequency fluctuations in functional magnetic resonance imaging signal: a pilot acupuncture study on NeiGuan (PC6) Journal of Alternative and Complementary Medicine. 2012;18(4):387–393. doi: 10.1089/acm.2010.0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Claunch J. D., Chan S.-T., Nixon E. E., et al. Commonality and specificity of acupuncture action at three acupoints as evidenced by fMRI. American Journal of Chinese Medicine. 2012;40(4):695–712. doi: 10.1142/S0192415X12500528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu H., Xu J., Shan B., et al. Determining the precise cerebral response to acupuncture: an improved FMRI study. PLoS ONE. 2012;7(11) doi: 10.1371/journal.pone.0049154.e49154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shen Y., Li M., Wei R., Lou M. Effect of acupuncture therapy for postponing wallerian degeneration of cerebral infarction as shown by diffusion tensor imaging. The Journal of Alternative and Complementary Medicine. 2012;18(12):1154–1160. doi: 10.1089/acm.2011.0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chu W. C. W., Wu J. C. Y., Yew D. T. W., et al. Does acupuncture therapy alter activation of neural pathway for pain perception in irritable bowel syndrome?: a comparative study of true and sham acupuncture using functional magnetic resonance imaging. Journal of Neurogastroenterology and Motility. 2012;18(3):305–316. doi: 10.5056/jnm.2012.18.3.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Feng Y., Bai L., Ren Y., et al. FMRI connectivity analysis of acupuncture effects on the whole brain network in mild cognitive impairment patients. Magnetic Resonance Imaging. 2012;30(5):672–682. doi: 10.1016/j.mri.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 62.Fang Z., Ning J., Xiong C., Shulin Y. Effects of electroacupuncture at head points on the function of cerebral motor areas in stroke patients: a pet study. Evidence-Based Complementary and Alternative Medicine. 2012;2012:9. doi: 10.1155/2012/902413.902413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen S.-J., Meng L., Yan H., et al. Functional organization of complex brain networks modulated by acupuncture at different acupoints belonging to the same anatomic segment. Chinese Medical Journal. 2012;125(15):2694–2700. doi: 10.3760/cma.j.issn.0366-6999.2012.15.009. [DOI] [PubMed] [Google Scholar]
- 64.Sun J., Qin W., Jin L., et al. Impact of global normalization in fMRI acupuncture studies. Evidence-Based Complementary and Alternative Medicine. 2012;2012:22. doi: 10.1155/2012/467061.467061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zeng F., Qin W., Ma T., et al. Influence of acupuncture treatment on cerebral activity in functional dyspepsia patients and its relationship with efficacy. The American Journal of Gastroenterology. 2012;107(8):1236–1247. doi: 10.1038/ajg.2012.53. [DOI] [PubMed] [Google Scholar]
- 66.Zhong C., Bai L., Dai R., et al. Modulatory effects of acupuncture on resting-state networks: a functional MRI study combining independent component analysis and multivariate granger causality analysis. Journal of Magnetic Resonance Imaging. 2012;35(3):572–581. doi: 10.1002/jmri.22887. [DOI] [PubMed] [Google Scholar]
- 67.Asghar Aziz U. R., Johnson R. L., Woods W., Green G. G., Lewith G., Macpherson H. Oscillatory neuronal dynamics associated with manual acupuncture: a magnetoencephalography study using beam forming analysis. Frontiers in Human Neuroscience. 2012;6, article 303 doi: 10.3389/fnhum.2012.00303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sun J., Zhu Y., Jin L., et al. Partly separated activations in the spatial distribution between de-qi and sharp pain during acupuncture stimulation: an fMRI-based study. Evidence-Based Complementary and Alternative Medicine. 2012;2012:11. doi: 10.1155/2012/934085.934085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zheng Y., Qu S., Wang N. W., et al. Post-stimulation effect of electroacupuncture at Yintang (EX-HN3) and GV20 on cerebral functional regions in healthy volunteers: a resting functional MRI study. Acupuncture in Medicine. 2012;30(4):307–315. doi: 10.1136/acupmed-2011-010123. [DOI] [PubMed] [Google Scholar]
- 70.Zhang Y., Glielmi C. B., Jiang Y., et al. Simultaneous CBF and BOLD mapping of high frequency acupuncture induced brain activity. Neuroscience Letters. 2012;530(1):12–17. doi: 10.1016/j.neulet.2012.09.050. [DOI] [PubMed] [Google Scholar]
- 71.Napadow V., Li A., Loggia M. L., et al. The brain circuitry mediating antipruritic effects of acupuncture. Cerebral Cortex. 2014;24(4):873–882. doi: 10.1093/cercor/bhs363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fang J., Wang X., Liu H., et al. The limbic-prefrontal network modulated by electroacupuncture at CV4 and CV12. Evidence-Based Complementary and Alternative Medicine. 2012;2012:11. doi: 10.1155/2012/515893.515893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jiang Y., Hao Y., Zhang Y., et al. Thirty minute transcutaneous electric acupoint stimulation modulates resting state brain activities: a perfusion and BOLD fMRI study. Brain Research. 2012;1457:13–25. doi: 10.1016/j.brainres.2012.03.063. [DOI] [PubMed] [Google Scholar]
- 74.Bao R., Wei P., Li K., et al. Within-limb somatotopic organization in human SI and parietal operculum for the leg: an fMRI study. Brain Research. 2012;1445:30–39. doi: 10.1016/j.brainres.2012.01.029. [DOI] [PubMed] [Google Scholar]
- 75.Qin W., Bai L., Dai J., et al. The temporal-spatial encoding of acupuncture effects in the brain. Molecular Pain. 2011;7, article 19 doi: 10.1186/1744-8069-7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shukla S., Torossian A., Duann J.-R., Leung A. The analgesic effect of electroacupuncture on acute thermal pain perception-a central neural correlate study with fMRI. Molecular Pain. 2011;7, article 45 doi: 10.1186/1744-8069-7-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li G., Yang E. S. An fMRI study of acupuncture-induced brain activation of aphasia stroke patients. Complementary Therapies in Medicine. 2011;19(supplement 1):S49–S59. doi: 10.1016/j.ctim.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 78.Liu J., Qin W., Guo Q., et al. Divergent neural processes specific to the acute and sustained phases of verum and SHAM acupuncture. Journal of Magnetic Resonance Imaging. 2011;33(1):33–40. doi: 10.1002/jmri.22393. [DOI] [PubMed] [Google Scholar]
- 79.Hsieh C.-H., Hsieh C.-W., Wu J.-H., Wang Q.-F., Chen J.-H. Different brain network activations induced by modulation and nonmodulation laser acupuncture. Evidence-Based Complementary and Alternative Medicine. 2011;2011:8. doi: 10.1155/2011/951258.951258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Witzel T., Napadow V., Kettner N. W., Vangel M. G., Hämäläinen M. S., Dhond R. P. Differences in cortical response to acupressure and electroacupuncture stimuli. BMC Neuroscience. 2011;12, article 73 doi: 10.1186/1471-2202-12-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen M. J.-L., Thompson T., Kropotov J., Gruzelier J. H. Beneficial effects of electrostimulation contingencies on sustained attention and electrocortical activity. CNS Neuroscience & Therapeutics. 2011;17(5):311–326. doi: 10.1111/j.1755-5949.2010.00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jung W.-S., Park S.-U., Park J.-M., et al. Changes in SPECT cerebral blood flow following japanese style, superficial acupuncture at LI-4 and LI-11 in healthy volunteers. The Journal of Alternative and Complementary Medicine. 2011;17(4):357–362. doi: 10.1089/acm.2010.0331. [DOI] [PubMed] [Google Scholar]
- 83.Cheng H., Zhang X.-T., Yan H., et al. Differential temporal neural responses of pain-related regions by acupuncture at acupoint ST36: a magneto-encephalography study. Chinese Medical Journal. 2011;124(8):1229–1234. doi: 10.3760/cma.j.issn.0366-6999.2011.08.020. [DOI] [PubMed] [Google Scholar]
- 84.Duan D.-M., Tu Y., Jiao S., Qin W. The relevance between symptoms and magnetic resonance imaging analysis of the hippocampus of depressed patients given electro-acupuncture combined with fluoxetine intervention—a randomized, controlled trial. Chinese Journal of Integrative Medicine. 2011;17(3):190–199. doi: 10.1007/s11655-011-0666-6. [DOI] [PubMed] [Google Scholar]
- 85.Rheu K.-H., Jahng G.-H., Ryu C.-W., Lim S. Investigation of the delayed neuronal effects of acupuncture manipulations. The Journal of Alternative and Complementary Medicine. 2011;17(11):1021–1027. doi: 10.1089/acm.2010.0679. [DOI] [PubMed] [Google Scholar]
- 86.Feng Y., Bai L., Ren Y., et al. Investigation of the large-scale functional brain networks modulated by acupuncture. Magnetic Resonance Imaging. 2011;29(7):958–965. doi: 10.1016/j.mri.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 87.Xue T., Bai L., Chen S., et al. Neural specificity of acupuncture stimulation from support vector machine classification analysis. Magnetic Resonance Imaging. 2011;29(7):943–950. doi: 10.1016/j.mri.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 88.Li G., Liang J.-M., Li P.-W., et al. Physiology and cell biology of acupuncture observed in calcium signaling activated by acoustic shear wave. Pflügers Archiv—European Journal of Physiology. 2011;462(4):587–597. doi: 10.1007/s00424-011-0993-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu P., Zhou G., Yang X., et al. Power estimation predicts specific function action of acupuncture: an fMRI study. Magnetic Resonance Imaging. 2011;29(8):1059–1064. doi: 10.1016/j.mri.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 90.Liu P., Zhou G., Zhang Y., et al. The hybrid GLM-ICA investigation on the neural mechanism of acupoint ST36: an fMRI study. Neuroscience Letters. 2010;479(3):267–271. doi: 10.1016/j.neulet.2010.05.077. [DOI] [PubMed] [Google Scholar]
- 91.Qiu W. Q., Claunch J., Kong J., et al. The effects of acupuncture on the brain networks for emotion and cognition: an observation of gender differences. Brain Research. 2010;1362:56–67. doi: 10.1016/j.brainres.2010.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Quah-Smith I., Sachdev P. S., Wen W., Chen X., Williams M. A. The brain effects of laser acupuncture in healthy individuals: an FMRI investigation. PloS ONE. 2010;5(9) doi: 10.1371/journal.pone.0012619.e12619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bai L., Yan H., Li N., et al. Neural specificity of acupuncture stimulation at pericardium 6: evidence from an fMRI study. Journal of Magnetic Resonance Imaging. 2010;31(1):71–77. doi: 10.1002/jmri.22006. [DOI] [PubMed] [Google Scholar]
- 94.Hui K. K. S., Napadow V., Liu J., et al. Monitoring acupuncture effects on human brain by fMRI. Journal of Visualized Experiments. 2010;(38) doi: 10.3791/1190.e1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ren Y., Bai L., Feng Y., Tian J., Li K. Investigation of acupoint specificity by functional connectivity analysis based on graph theory. Neuroscience Letters. 2010;482(2):95–100. doi: 10.1016/j.neulet.2010.06.091. [DOI] [PubMed] [Google Scholar]
- 96.Zyloney C. E., Jensen K., Polich G., et al. Imaging the functional connectivity of the Periaqueductal Gray during genuine and sham electroacupuncture treatment. Molecular Pain. 2010;6, article 80 doi: 10.1186/1744-8069-6-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wu Y., Jin Z., Li K., et al. Functional magnetic resonance imaging activation of the brain in children: real acupoint versus sham acupoint. Journal of Child Neurology. 2010;25(7):849–855. doi: 10.1177/0883073809351314. [DOI] [PubMed] [Google Scholar]
- 98.Cho S.-Y., Jahng G.-H., Park S.-U., Jung W.-S., Moon S.-K., Park J.-M. FMRI study of effect on brain activity according to stimulation method at LI11, ST36: painful pressure and acupuncture stimulation of same acupoints. Journal of Alternative and Complementary Medicine. 2010;16(4):489–495. doi: 10.1089/acm.2009.0395. [DOI] [PubMed] [Google Scholar]
- 99.Li L., Qin W., Bai L., Tian J. Exploring vision-related acupuncture point specificity with multivoxel pattern analysis. Magnetic Resonance Imaging. 2010;28(3):380–387. doi: 10.1016/j.mri.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 100.Sun J., Qin W., Dong M., et al. Evaluation of group homogeneity during acupuncture stimulation in fMRI studies. Journal of Magnetic Resonance Imaging. 2010;32(2):298–305. doi: 10.1002/jmri.22238. [DOI] [PubMed] [Google Scholar]
- 101.Chu W. C. W., Wu J. C. Y., Yew D. T. W., et al. Does acupuncture therapy alter activation of neural pathway for pain perception in irritable bowel syndrome?: A comparative study of true and sham acupuncture using functional magnetic resonance imaging. Journal of Neurogastroenterology and Motility. 2012;18(3):305–316. doi: 10.5056/jnm.2012.18.3.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Park M.-S., Sunwoo Y.-Y., Jang K.-S., et al. Changes in brain FDG metabolism induced by acupuncture in healthy volunteers. Acta Radiologica. 2010;51(8):947–952. doi: 10.3109/02841851.2010.502541. [DOI] [PubMed] [Google Scholar]
- 103.Asghar A. U., Green G., Lythgoe M. F., Lewith G., MacPherson H. Acupuncture needling sensation: the neural correlates of deqi using fMRI. Brain Research. 2010;1315:111–118. doi: 10.1016/j.brainres.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 104.Bai L., Tian J., Zhong C., et al. Acupuncture modulates temporal neural responses in wide brain networks: evidence from fMRI study. Molecular Pain. 2010;6, article 73 doi: 10.1186/1744-8069-6-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Harris R. E., Zubieta J.-K., Scott D. J., Napadow V., Gracely R. H., Clauw D. J. Traditional Chinese acupuncture and placebo (sham) acupuncture are differentiated by their effects on μ-opioid receptors (MORs) NeuroImage. 2009;47(3):1077–1085. doi: 10.1016/j.neuroimage.2009.05.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bai L., Qin W., Tian J., et al. Time-varied characteristics of acupuncture effects in fMRI studies. Human Brain Mapping. 2009;30(11):3445–3460. doi: 10.1002/hbm.20769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Napadow V., Dhond R., Park K., et al. Time-variant fMRI activity in the brainstem and higher structures in response to acupuncture. NeuroImage. 2009;47(1):289–301. doi: 10.1016/j.neuroimage.2009.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sato M., Inubushi M., Shiga T., et al. Therapeutic effects of acupuncture in patients with rheumatoid arthritis: a prospective study using 18F-FDG-PET. Annals of Nuclear Medicine. 2009;23(3):311–316. doi: 10.1007/s12149-009-0238-4. [DOI] [PubMed] [Google Scholar]
- 109.Chae Y., Lee H., Kim H., Sohn H., Park J.-H., Park H.-J. The neural substrates of verum acupuncture compared to non-penetrating placebo needle: an fMRI study. Neuroscience Letters. 2009;450(2):80–84. doi: 10.1016/j.neulet.2008.11.048. [DOI] [PubMed] [Google Scholar]
- 110.Ho T.-J., Duann J.-R., Chen C.-M., et al. Temporally shifted hemodynamic response model helps to extract acupuncture-induced functional magnetic resonance imaging blood oxygenation-level dependent activities. Chinese Medical Journal. 2009;122(7):823–829. doi: 10.3760/cma.j.issn.0366-6999.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 111.Liu P., Zhang Y., Zhou G., et al. Partial correlation investigation on the default mode network involved in acupuncture: an fMRI study. Neuroscience Letters. 2009;462(3):183–187. doi: 10.1016/j.neulet.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 112.Chae Y., Lee H., Kim H., et al. Parsing brain activity associated with acupuncture treatment in Parkinson's diseases. Movement Disorders. 2009;24(12):1794–1802. doi: 10.1002/mds.22673. [DOI] [PubMed] [Google Scholar]
- 113.Zhang Y., Qin W., Liu P., et al. An fMRI study of acupuncture using independent component analysis. Neuroscience Letters. 2009;449(1):6–9. doi: 10.1016/j.neulet.2008.10.071. [DOI] [PubMed] [Google Scholar]
- 114.Yeo S., Choe I.-H., Van Den Noort M., Bosch P., Lim S. Consecutive acupuncture stimulations lead to significantly decreased neural responses. Journal of Alternative and Complementary Medicine. 2010;16(4):481–487. doi: 10.1089/acm.2009.0606. [DOI] [PubMed] [Google Scholar]
- 115.Liu P., Qin W., Zhang Y., et al. Combining spatial and temporal information to explore function-guide action of acupuncture using fMRI. Journal of Magnetic Resonance Imaging. 2009;30(1):41–46. doi: 10.1002/jmri.21805. [DOI] [PubMed] [Google Scholar]
- 116.An Y.-S., Moon S.-K., Min I.-K., Kim D.-Y. Changes in regional cerebral blood flow and glucose metabolism following electroacupuncture at LI 4 and LI 11 in normal volunteers. The Journal of Alternative and Complementary Medicine. 2009;15(10):1075–1081. doi: 10.1089/acm.2009.0257. [DOI] [PubMed] [Google Scholar]
- 117.Napadow V., Dhond R. P., Kim J., et al. Brain encoding of acupuncture sensation—coupling on-line rating with fMRI. NeuroImage. 2009;47(3):1055–1065. doi: 10.1016/j.neuroimage.2009.05.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kong J., Kaptchuk T. J., Polich G., et al. An fMRI study on the interaction and dissociation between expectation of pain relief and acupuncture treatment. NeuroImage. 2009;47(3):1066–1076. doi: 10.1016/j.neuroimage.2009.05.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bai L., Qin W., Tian J., et al. Acupuncture modulates spontaneous activities in the anticorrelated resting brain networks. Brain Research. 2009;1279:37–49. doi: 10.1016/j.brainres.2009.04.056. [DOI] [PubMed] [Google Scholar]
- 120.Hui K. K. S., Marina O., Claunch J. D., et al. Acupuncture mobilizes the brain's default mode and its anti-correlated network in healthy subjects. Brain Research. 2009;1287:84–103. doi: 10.1016/j.brainres.2009.06.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lai X., Zhang G., Huang Y., et al. A cerebral functional imaging study by positron emission tomography in healthy volunteers receiving true or sham acupuncture needling. Neuroscience Letters. 2009;452(2):194–199. doi: 10.1016/j.neulet.2009.01.052. [DOI] [PubMed] [Google Scholar]
- 122.Park S.-U., Shin A.-S., Jahng G.-H., Moon S.-K., Park J.-M. Effects of scalp acupuncture versus upper and lower limb acupuncture on signal activation of blood oxygen level dependent (BOLD) fMRI of the brain and somatosensory cortex. Journal of Alternative and Complementary Medicine. 2009;15(11):1193–1200. doi: 10.1089/acm.2008.0602. [DOI] [PubMed] [Google Scholar]
- 123.Li L., Liu H., Li Y.-Z., et al. The human brain response to acupuncture on same-meridian acupoints: evidence from an fMRI study. Journal of Alternative and Complementary Medicine. 2008;14(6):673–678. doi: 10.1089/acm.2008.0036. [DOI] [PubMed] [Google Scholar]
- 124.Dhond R. P., Witzel T., Hämäläinen M., Kettner N., Napadow V. Spatiotemporal mapping the neural correlates of acupuncture with MEG. The Journal of Alternative and Complementary Medicine. 2008;14(6):679–688. doi: 10.1089/acm.2007.0824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Deng G., Hou B. L., Holodny A. I., Cassileth B. R. Functional magnetic resonance imaging (fMRI) changes and saliva production associated with acupuncture at LI-2 acupuncture point: a randomized controlled study. BMC Complementary and Alternative Medicine. 2008;8, article 37 doi: 10.1186/1472-6882-8-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Qin W., Tian J., Bai L., et al. FMRI connectivity analysis of acupuncture effects on an amygdala-associated brain network. Molecular Pain. 2008;4, article 55 doi: 10.1186/1744-8069-4-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kong J., Kaptchuk T. J., Polich G., et al. Expectancy and treatment interactions: a dissociation between acupuncture analgesia and expectancy evoked placebo analgesia. NeuroImage. 2009;45(3):940–949. doi: 10.1016/j.neuroimage.2008.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ho T.-J., Duann J.-R., Chen C.-M., et al. Carryover effects alter fMRI statistical analysis in an acupuncture study. American Journal of Chinese Medicine. 2008;36(1):55–70. doi: 10.1142/S0192415X08005588. [DOI] [PubMed] [Google Scholar]
- 129.Dhond R. P., Yeh C., Park K., Kettner N., Napadow V. Acupuncture modulates resting state connectivity in default and sensorimotor brain networks. Pain. 2008;136(3):407–418. doi: 10.1016/j.pain.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dougherty D. D., Kong J., Webb M., Bonab A. A., Fischman A. J., Gollub R. L. A combined [11C]diprenorphine PET study and fMRI study of acupuncture analgesia. Behavioural Brain Research. 2008;193(1):63–68. doi: 10.1016/j.bbr.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Napadow V., Liu J., Li M., et al. Somatosensory cortical plasticity in carpal tunnel syndrome treated by acupuncture. Human Brain Mapping. 2007;28(3):159–171. doi: 10.1002/hbm.20261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Moncayo R., Rudisch A., Diemling M., Kremser C. In-vivo visualization of the anatomical structures related to the acupuncture points Dai mai and Shenmai by MRI: a single-case pilot study. BMC Medical Imaging. 2007;7, article 4 doi: 10.1186/1471-2342-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Napadow V., Kettner N., Liu J., et al. Hypothalamus and amygdala response to acupuncture stimuli in carpal tunnel syndrome. Pain. 2007;130(3):254–266. doi: 10.1016/j.pain.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Schaechter J. D., Connell B. D., Stason W. B., et al. Correlated change in upper limb function and motor cortex activation after verum and sham acupuncture in patients with chronic stroke. Journal of Alternative and Complementary Medicine. 2007;13(5):527–532. doi: 10.1089/acm.2007.6316. [DOI] [PubMed] [Google Scholar]
- 135.Hui K. K. S., Nixon E. E., Vangel M. G., et al. Characterization of the ‘deqi’ response in acupuncture. BMC Complementary and Alternative Medicine. 2007;7, article 33 doi: 10.1186/1472-6882-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wang S.-M., Constable R. T., Tokoglu F. S., Weiss D. A., Freyle D., Kain Z. N. Acupuncture-induced blood oxygenation level-dependent signals in awake and anesthetized volunteers: a pilot study. Anesthesia and Analgesia. 2007;105(2):499–506. doi: 10.1213/01.ane.0000270216.71234.f5. [DOI] [PubMed] [Google Scholar]
- 137.Huang Y., Chen J., Htut W. M., Lai X., Wik G. Acupuncture increases cerebral glucose metabolism in human vascular dementia. International Journal of Neuroscience. 2007;117(7):1029–1037. doi: 10.1080/00207450600936825. [DOI] [PubMed] [Google Scholar]
- 138.Liu S., Zhou W., Ruan X., et al. Activation of the hypothalamus characterizes the response to acupuncture stimulation in heroin addicts. Neuroscience Letters. 2007;421(3):203–208. doi: 10.1016/j.neulet.2007.04.078. [DOI] [PubMed] [Google Scholar]
- 139.Moncayo R., Rudisch A., Kremser C., Moncayo H. 3D-MRI rendering of the anatomical structures related to acupuncture points of the Dai mai, Yin qiao mai and Yang qiao mai meridians within the context of the WOMED concept of lateral tension: implications for musculoskeletal disease. BMC Musculoskeletal Disorders. 2007;8(1, article 33) doi: 10.1186/1471-2474-8-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kong J., Gollub R. L., Webb J. M., Kong J.-T., Vangel M. G., Kwong K. Test-retest study of fMRI signal change evoked by electroacupuncture stimulation. NeuroImage. 2007;34(3):1171–1181. doi: 10.1016/j.neuroimage.2006.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Napadow V., Kettner N., Ryan A., Kwong K. K., Audette J., Hui K. K. S. Somatosensory cortical plasticity in carpal tunnel syndrome-a cross-sectional fMRI evaluation. NeuroImage. 2006;31(2):520–530. doi: 10.1016/j.neuroimage.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 142.Wong V. C. N., Sun J.-G., Yeung D. W. C. Pilot study of efficacy of tongue and body acupuncture in children with visual impairment. Journal of Child Neurology. 2006;21(6):462–473. [PubMed] [Google Scholar]
- 143.Chen A. C. N., Liu F.-J., Wang L., Arendt-Nielsen L. Mode and site of acupuncture modulation in the human brain: 3D (124-ch) EEG power spectrum mapping and source imaging. NeuroImage. 2006;29(4):1080–1091. doi: 10.1016/j.neuroimage.2005.08.066. [DOI] [PubMed] [Google Scholar]
- 144.Li K., Shan B., Xu J., et al. Changes in fMRI in the human brain related to different durations of manual acupuncture needling. Journal of Alternative and Complementary Medicine. 2006;12(7):615–623. doi: 10.1089/acm.2006.12.615. [DOI] [PubMed] [Google Scholar]
- 145.Wong V. C. N., Sun J.-G., Yeung D. W. C. Pilot study of positron emission tomography (PET) brain glucose metabolism to assess the efficacy of tongue and body acupuncture in cerebral palsy. Journal of Child Neurology. 2006;21(6):455–462. doi: 10.2310/7010.2006.00101. [DOI] [PubMed] [Google Scholar]
- 146.Li G., Jack C. R., Jr., Yang E. S. An fMRI study of somatosensory-implicated acupuncture points in stable somatosensory stroke patients. Journal of Magnetic Resonance Imaging. 2006;24(5):1018–1024. doi: 10.1002/jmri.20702. [DOI] [PubMed] [Google Scholar]
- 147.Kong J., Gollub R. L., Rosman I. S., et al. Brain activity associated with expectancy-enhanced placebo analgesia as measured by functional magnetic resonance imaging. The Journal of Neuroscience. 2006;26(2):381–388. doi: 10.1523/jneurosci.3556-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hui K. K. S., Liu J., Marina O., et al. The integrated response of the human cerebro-cerebellar and limbic systems to acupuncture stimulation at ST 36 as evidenced by fMRI. NeuroImage. 2005;27(3):479–496. doi: 10.1016/j.neuroimage.2005.04.037. [DOI] [PubMed] [Google Scholar]
- 149.Ueda Y., Hayashi K., Kuriowa K. The application of fMRI to basic experiments in acupuncture. IEEE Engineering in Medicine and Biology Magazine. 2005;24(2):47–51. doi: 10.1109/MEMB.2005.1411348. [DOI] [PubMed] [Google Scholar]
- 150.Siedentopf C. M., Koppelstaetter F., Haala I. A., et al. Laser acupuncture induced specific cerebral cortical and subcortical activations in humans. Lasers in Medical Science. 2005;20(2):68–73. doi: 10.1007/s10103-005-0340-3. [DOI] [PubMed] [Google Scholar]
- 151.Napadow V., Makris N., Liu J., Kettner N. W., Kwong K. K., Hui K. K. S. Effects of electroacupuncture versus manual acupuncture on the human brain as measured by fMRI. Human Brain Mapping. 2005;24(3):193–205. doi: 10.1002/hbm.20081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Napadow V., Dhond R. P., Purdon P., et al. Correlating acupuncture fMRI in the human brainstem with heart rate variability. Proceedings of the IEEE 27th Annual Conference on Engineering in Medicine and Biology; September 2005; Shanghai, China. pp. 4496–4499. [DOI] [PubMed] [Google Scholar]
- 153.Jeun S.-S., Kim J.-S., Kim B.-S., et al. Acupuncture stimulation for motor cortex activities: a 3T fMRI Study. American Journal of Chinese Medicine. 2005;33(4):573–578. doi: 10.1142/s0192415x0500317x. [DOI] [PubMed] [Google Scholar]
- 154.Yan B., Li K., Xu J., et al. Acupoint-specific fMRI patterns in human brain. Neuroscience Letters. 2005;383(3):236–240. doi: 10.1016/j.neulet.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 155.Kong J., White N. S., Kwong K. K., et al. Using fMRI to dissociate sensory encoding from cognitive evaluation of heat pain intensity. Human Brain Mapping. 2006;27(9):715–721. doi: 10.1002/hbm.20213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yoo S.-S., Teh E.-K., Blinder R. A., Jolesz F. A. Modulation of cerebellar activities by acupuncture stimulation: evidence from fMRI study. NeuroImage. 2004;22(2):932–940. doi: 10.1016/j.neuroimage.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 157.Fang J. L., Krings T., Weidemann J., Meister I. G., Thron A. Functional MRI in healthy subjects during acupuncture: different effects of needle rotation in real and false acupoints. Neuroradiology. 2004;46(5):359–362. doi: 10.1007/s00234-003-1125-7. [DOI] [PubMed] [Google Scholar]
- 158.Liu W.-C., Feldman S. C., Cook D. B., et al. fMRI study of acupuncture-induced periaqueductal gray activity in humans. NeuroReport. 2004;15(12):1937–1940. doi: 10.1097/00001756-200408260-00021. [DOI] [PubMed] [Google Scholar]
- 159.Zhang W.-T., Jin Z., Luo F., Zhang L., Zeng Y.-W., Han J.-S. Evidence from brain imaging with fMRI supporting functional specificity of acupoints in humans. Neuroscience Letters. 2004;354(1):50–53. doi: 10.1016/j.neulet.2003.09.080. [DOI] [PubMed] [Google Scholar]
- 160.Litscher G., Rachbauer D., Ropele S., et al. Acupuncture using laser needles modulates brain function: first evidence from functional transcranial Doppler sonography and functional magnetic resonance imaging. Lasers in Medical Science. 2004;19(1):6–11. doi: 10.1007/s10103-004-0291-0. [DOI] [PubMed] [Google Scholar]
- 161.Li G., Cheung R. T. F., Ma Q.-Y., Yang E. S. Visual cortical activations on fMRI upon stimulation of the vision-implicated acupoints. NeuroReport. 2003;14(5):669–673. doi: 10.1097/00001756-200304150-00002. [DOI] [PubMed] [Google Scholar]
- 162.Zhang W.-T., Jin Z., Cui G.-H., et al. Relations between brain network activation and analgesic effect induced by low vs. high frequency electrical acupoint stimulation in different subjects: a functional magnetic resonance imaging study. Brain Research. 2003;982(2):168–178. doi: 10.1016/s0006-8993(03)02983-4. [DOI] [PubMed] [Google Scholar]
- 163.Yin L., Jin X., Qiao W., et al. PET imaging of brain function while puncturing the acupoint ST36. Chinese Medical Journal. 2003;116(12):1836–1839. [PubMed] [Google Scholar]
- 164.Li G., Liu H.-L., Cheung R. T. F., et al. An fMRI study comparing brain activation between word generation and electrical stimulation of language-implicated acupoints. Human Brain Mapping. 2003;18(3):233–238. doi: 10.1002/hbm.10098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Guan Y. H., Zuo C. T., Zhao J., Lin X. T. Regional cerebral metabolic changes after acupuncture by FDG PET: effects and methodology. Nuclear Science and Techniques. 2002;13(4):224–229. [Google Scholar]
- 166.Wu M.-T., Sheen J.-M., Chuang K.-H., et al. Neuronal specificity of acupuncture response: a fMRI study with electroacupuncture. NeuroImage. 2002;16(4):1028–1037. doi: 10.1006/nimg.2002.1145. [DOI] [PubMed] [Google Scholar]
- 167.Gareus I. K., Lacour M., Schulte A.-C., Hennig J. Is there a BOLD response of the visual cortex on stimulation of the vision-related acupoint GB 37? Journal of Magnetic Resonance Imaging. 2002;15(3):227–232. doi: 10.1002/jmri.10059. [DOI] [PubMed] [Google Scholar]
- 168.Siedentopf C. M., Golaszewski S. M., Mottaghy F. M., Ruff C. C., Felber S., Schlager A. Functional magnetic resonance imaging detects activation of the visual association cortex during laser acupuncture of the foot in humans. Neuroscience Letters. 2002;327(1):53–56. doi: 10.1016/S0304-3940(02)00383-X. [DOI] [PubMed] [Google Scholar]
- 169.Bäcker M., Hammes M. G., Valet M., et al. Different modes of manual acupuncture stimulation differentially modulate cerebral blood flow velocity, arterial blood pressure and heart rate in human subjects. Neuroscience Letters. 2002;333(3):203–206. doi: 10.1016/S0304-3940(02)01109-6. [DOI] [PubMed] [Google Scholar]
- 170.Wu M.-T., Sheen J.-M., Chin S.-L., et al. “Visual” and “auditroy” cortical activation by acupuncture: is there a specific acupoint-cortical correlation? A fMRI study using controlled electroacupuncture. NeuroImage. 2001;13(6):p. 1282. doi: 10.1016/S1053-8119(01)92596-0. [DOI] [Google Scholar]
- 171.Wu M.-T., Sheen J.-M., Chuang K.-H., et al. ‘Visual’ and “Auditroy” cortical activation by acupuncture has no specific cortical-acupoint correlation—a fMRI study of electroacupuncture with control stimulation. Proceednigs of the International Society for Magnetic Resonance in Medicine (ISMRM '01); 2001; p. p. 537. [Google Scholar]
- 172.Biella G., Sotgiu M. L., Pellegata G., Paulesu E., Castiglioni I., Fazio F. Acupuncture produces central activations in pain regions. NeuroImage. 2001;14(1):60–66. doi: 10.1006/nimg.2001.0798. [DOI] [PubMed] [Google Scholar]
- 173.Fukunaga M., Someya Y., Tanaka C., et al. Brain activation under electro-acupuncture stimulation using functional MRI. NeuroImage. 2000;11(5, supplement):p. s862. doi: 10.1016/s1053-8119(00)91790-7. [DOI] [Google Scholar]
- 174.Hui K. K. S., Liu J., Makris N., et al. Acupuncture modulates the limbic system and subcortical gray structures of the human brain: evidence from fMRI studies in normal subjects. Human Brain Mapping. 2000;9(1):13–25. doi: 10.1002/(sici)1097-0193(2000)9:1<13::aid-hbm2>3.0.co;2-f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wu M.-T., Hsieh J.-C., Xiong J., et al. Central nervous pathway for acupunture stimulation: localization of processing with functional MR imaging of the brain—preliminary experience. Radiology. 1999;212(1):133–141. doi: 10.1148/radiology.212.1.r99jl04133. [DOI] [PubMed] [Google Scholar]
- 176.Litscher G., Schwarz G., Sandner-Kiesling A., Hadolt I. Robotic transcranial Doppler sonography probes and acupuncture. International Journal of Neuroscience. 1998;95(1-2):1–15. doi: 10.3109/00207459809000645. [DOI] [PubMed] [Google Scholar]
- 177.Desmond J. E., Glover G. H. Estimating sample size in functional MRI (fMRI) neuroimaging studies: statistical power analyses. Journal of Neuroscience Methods. 2002;118(2):115–128. doi: 10.1016/s0165-0270(02)00121-8. [DOI] [PubMed] [Google Scholar]
- 178.Hayasaka S., Peiffer A. M., Hugenschmidt C. E., Laurienti P. J. Power and sample size calculation for neuroimaging studies by non-central random field theory. NeuroImage. 2007;37(3):721–730. doi: 10.1016/j.neuroimage.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Poldrack R. A. The future of fMRI in cognitive neuroscience. NeuroImage. 2012;62(2):1216–1220. doi: 10.1016/j.neuroimage.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Xu C. S., Li C. F., Yang J., et al. Effects of sample size on cerebral response to acupuncture with fMRI. Chinese Imaging Journal of Integrated Traditional and Western Medicine. 2011;9(4):289–308. [Google Scholar]
- 181.Potkin S. G., Alva G., Fleming K., et al. A PET study of the pathophysiology of negative symptoms in schizophrenia. American Journal of Psychiatry. 2002;159(2):227–237. doi: 10.1176/appi.ajp.159.2.227. [DOI] [PubMed] [Google Scholar]
- 182.Pardo J. V., Lee J. T., Sheikh S. A., et al. Where the brain grows old: decline in anterior cingulate and medial prefrontal function with normal aging. NeuroImage. 2007;35(3):1231–1237. doi: 10.1016/j.neuroimage.2006.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Chance S. A. Subtle changes in the ageing human brain. Nutrition and Health. 2006;18(3):217–224. doi: 10.1177/026010600601800303. [DOI] [PubMed] [Google Scholar]
- 184.Paradiso S., Vaidya J. G., McCormick L. M., Jones A., Robinson R. G. Aging and alexithymia: association with reduced right rostral cingulate volume. The American Journal of Geriatric Psychiatry. 2008;16(9):760–769. doi: 10.1097/jgp.0b013e31817e73b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Basic S., Hajnsek S., Poljakovic Z., Basic M., Culic V., Zadro I. Determination of cortical language dominance using functional transcranial Doppler sonography in left-handers. Clinical Neurophysiology. 2004;115(1):154–160. doi: 10.1016/S1388-2457(03)00281-5. [DOI] [PubMed] [Google Scholar]
- 186.Damore D., Rutledge J., Pan S., Knotek N., Ramundo M. Handedness effects on procedural training in pediatrics. Clinical Pediatrics. 2009;48(2):156–160. doi: 10.1177/0009922808323111. [DOI] [PubMed] [Google Scholar]
- 187.Siebner H. R., Limmer C., Peinemann A., et al. Long-term consequences of switching handedness: a positron emission tomography study on handwriting in ‘converted’ left-handers. The Journal of Neuroscience. 2002;22(7):2816–2825. doi: 10.1523/JNEUROSCI.22-07-02816.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.de La Fuente-Fernández R., Kishore A., Calne D. B., Ruth T. J., Stoessl A. J. Nigrostriatal dopamine system and motor lateralization. Behavioural Brain Research. 2000;112(1-2):63–68. doi: 10.1016/s0166-4328(00)00165-0. [DOI] [PubMed] [Google Scholar]
- 189.Hu Y., Xu Q., Li K., et al. Gender differences of brain glucose metabolic networks revealed by FDG-PET: evidence from a large cohort of 400 young adults. PLoS ONE. 2013;8(12) doi: 10.1371/journal.pone.0083821.e83821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Kong J., Tu P.-C., Zyloney C., Su T.-P. Intrinsic functional connectivity of the periaqueductal gray, a resting fMRI study. Behavioural Brain Research. 2010;211(2):215–219. doi: 10.1016/j.bbr.2010.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Lv B., Li J., He H., et al. Gender consistency and difference in healthy adults revealed by cortical thickness. NeuroImage. 2010;53(2):373–382. doi: 10.1016/j.neuroimage.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 192.Rushton J. P., Ankney C. D. Brain size and cognitive ability: correlations with age, sex, social class, and race. Psychonomic Bulletin & Review. 1996;3(1):21–36. doi: 10.3758/bf03210739. [DOI] [PubMed] [Google Scholar]
- 193.Drevets W. C., Price J. L., Simpson J. R., Jr., et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386(6627):824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- 194.Veldhuijzen D. S., Keaser M. L., Traub D. S., Zhuo J., Gullapalli R. P., Greenspan J. D. The role of circulating sex hormones in menstrual cycle-dependent modulation of pain-related brain activation. Pain. 2013;154(4):548–559. doi: 10.1016/j.pain.2012.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Hagemann G., Ugur T., Schleussner E., et al. Changes in brain size during the menstrual cycle. PLoS ONE. 2011;6(2) doi: 10.1371/journal.pone.0014655.e14655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Tu C.-H., Niddam D. M., Yeh T.-C., et al. Menstrual pain is associated with rapid structural alterations in the brain. Pain. 2013;154(9):1718–1724. doi: 10.1016/j.pain.2013.05.022. [DOI] [PubMed] [Google Scholar]
- 197.Tu C.-H., Niddam D. M., Chao H.-T., et al. Brain morphological changes associated with cyclic menstrual pain. Pain. 2010;150(3):462–468. doi: 10.1016/j.pain.2010.05.026. [DOI] [PubMed] [Google Scholar]
- 198.Naruse S., Mori K., Kurihara M., et al. Chorioretinal blood flow changes following acupuncture between thumb and forefinger. Nippon GankaGakkaiZasshi. 2000;104(10):717–723. [PubMed] [Google Scholar]
- 199.MacPherson H., White A., Cummings M., Jobst K. A., Rose K., Niemtzow R. C. Standards for reporting interventions in controlled trials of acupuncture: the STRICTA recommendations. Journal of Alternative & Complementary Medicine. 2002;8(1):85–89. doi: 10.1089/107555302753507212. [DOI] [PubMed] [Google Scholar]
- 200.Kong J., Gollub R., Huang T., et al. Acupuncture De Qi, from qualitative history to quantitative measurement. The Journal of Alternative and Complementary Medicine. 2007;13(10):1059–1070. doi: 10.1089/acm.2007.0524. [DOI] [PubMed] [Google Scholar]
- 201.Xu S.-B., Huang B., Zhang C.-Y., et al. Effectiveness of strengthened stimulation during acupuncture for the treatment of Bell palsy: a randomized controlled trial. Canadian Medical Association Journal. 2013;185(6):473–479. doi: 10.1503/cmaj.130319. [DOI] [PMC free article] [PubMed] [Google Scholar]


