Abstract
Background
For patients with non-small cell lung cancer (NSCLC) metastatic to hilar lymph nodes (N1), guidelines recommend surgery and adjuvant chemotherapy in operable patients and chemoradiation (CRT) for those deemed inoperable. However, it is unclear how these recommendations are applied nationally.
Methods
The National Cancer Database (NCDB) was queried to identify patients with tumors < 7cm (T1/T2) with clinically positive N1 nodes. Patients undergoing CRT (chemotherapy and radiation >45Gy) or surgical resection were considered adequately treated. Remaining patients were classified as receiving inadequate or no treatment.
Results
Of 20,366 patients that met study criteria, 63% underwent adequate treatment [48% surgical resection, 15% CRT]. The remainder received inadequate (23%) or no treatment (14%). In univariate analysis, patients undergoing inadequate or no treatment were older, non-Caucasian, had lower income, and a higher comorbidity score. Patients undergoing adequate treatment had improved overall survival (OS) compared with those receiving inadequate or no treatment (median OS=34.0 vs. 11.7 months, p<0.001). Of those receiving adequate treatment, logistic regression identified several variables associated with surgical resection including treatment at an academic facility, Caucasian race, and annual income >$35,000. Increasing age and T2 stage were associated with non-operative management. Following propensity score matching of 2,308 patient-pairs undergoing surgery or CRT, resection was associated with longer median OS (34.1 vs. 22.0 months, p<0.001).
Conclusions
Despite established guidelines, many patients with T1-2N1 NSCLC do not receive adequate treatment. Surgery is associated with prolonged survival in selected patients. Surgical input in the multidisciplinary evaluation of these patients should be mandatory.
INTRODUCTION
Node-positive non-small cell lung cancer (NSCLC) is an aggressive disease with high mortality.1 However, patients with disease limited to pulmonary and hilar lymph nodes (N1) may experience long-term survival with aggressive, multi-modality therapy.2 In patients with acceptable operative risk, surgical resection with adjuvant chemotherapy forms the cornerstone of treatment for hilar node-positive (N1) disease.3 Although studies directly evaluating treatment of medically inoperable patients with N1 disease are lacking, extrapolation of data from stage III patients suggests that chemoradiation is generally the preferred standard of care.4–7 Despite established guidelines outlining these treatment paradigms, adherence to these recommendations at a national level is unclear.
The National Cancer Database (NCDB) is a joint program developed in 1989 by the Commission on Cancer, the American College of Surgeons, and the American Cancer Society.8 Data is submitted by more than 1,500 accredited cancers centers across the United States and Puerto Rico, and it captures approximately 70% of all new cancer cases diagnosed in the U.S. annually. In order to better characterize the treatment of N1 disease nationwide, we queried the NCDB to examine patterns of care regarding N1 (T1 or T2) NSCLC in the United States. We hypothesized that despite established guidelines, physician practice and surgical referral for this disease would vary considerably.
METHODS
We queried the NCDB to identify patients treated for clinical N1 node-positive NSCLC (hilar, interlobar, lobar, or segmental nodes) between 1998 and 2010.9 All information was de-identified so IRB approval for the study was waived at Washington University. Analysis was limited to patients with T1 or T2 disease (generally representing stage II NSCLC according to the 7th edition AJCC staging manual).10 Those patients with clinical T3 or T4 tumors, or those with clinically positive mediastinal lymph nodes (N2 disease) were specifically excluded. Patients undergoing either surgical resection or chemoradiotherapy (CRT) with >45 Gy of radiation were considered adequately treated. Chemotherapy and radiation could be given in any order. Patients not meeting these treatment criteria were classified as receiving inadequate (some chemotherapy and/or radiation but not meeting the previously defined threshold for adequate therapy) or no treatment.
Information regarding patient- and tumor-related variables, treatment details, and short- and long-term outcomes was extracted. Using information on race, income, and population size of the area from which a patient presented, we created dichotomized groups in which a patient was either Caucasian or not Caucasian, had an annual income less than or greater than $35,000, and presented from a rural location (regional population less than 250,000) or an urban location, respectively. The Charlson/Deyo score was used as a measure of comorbidity. It was categorized as 0, 1, or ≥ to 2. The NCDB combines those with scores of 2 or greater into a single group, as very few patients have scores greater than two. Treatment facilities were classified as community cancer programs, comprehensive community cancer programs, and academic/research centers. For the analysis, community cancer programs and comprehensive community cancer programs were categorized as non-academic centers.
Last known vital status and the time between diagnosis and the follow-up date were used to determine survival. According to the NCDB, date of diagnosis refers to the date of histologic confirmation of NSCLC in cases where that information is available. In cases where the diagnosis was made based on imaging and patients proceeded directly to resection without biopsy, date of diagnosis refers to the date of radiologic imaging identifying the lesion.
All analyses were performed using SPSS 21.0 (SPSS 21.0 for Windows, SPSS Inc, Chicago, IL). Descriptive statistics were expressed as means +/− standard deviation unless otherwise specified. Independent samples t tests and one-way ANOVA were used to compare continuous variables. Chi-square tests were used to compare categorical data. Overall survival was estimated by the Kaplan-Meier method. Multivariate logistic regression models were fitted to evaluate variables associated with surgical resection. Factors accounted for in the multivariate analysis include: age, tumor size (mm), gender, race, facility type (academic vs. non-academic), income, urban location, Charlson score, and clinical T stage. Propensity score matching was performed to identify two equivalent cohorts of patients undergoing either surgery or adequate CRT. The propensity score was the probability of receiving chemoradiation during the study period, estimated using a logistic regression model including age, race, gender, income, facility type (academic vs. non-academic), Charlson score, urban location, clinical T stage and tumor size. These variables were selected from an initial univariate analysis comparing the surgery and chemotherapy groups and variables significantly different between the two groups were chosen for propensity matching. Patients for whom the propensity scores matched to the fourth decimal place were matched in 1:1 fashion. Automated matching was performed using the Fuzzy extension command in SPSS 21.0. For all analyses, p-values less than 0.05 were considered statistically significant.
RESULTS
A total of 20,366 patients that met study criteria were identified in the NCDB from 1998 to 2010. These patients were all noted to have NSCLC with clinically positive N1 lymph nodes. Of these, 12,857 (63%) underwent adequate treatment as defined in the Methods section [surgical resection, n=9,719 (48%); definitive chemoradiation, n= 3,138 (15%)]. The remaining 7,509 patients (37%) received either inadequate CRT or no treatment [inadequate CRT, n=4,640 (23%); no treatment, n=2,869 (14%)].
Demographic, tumor and treatment-related information for patients receiving adequate vs. inadequate therapy is shown in Table 1. In univariate analysis, patients undergoing inadequate or no treatment were older, more likely non-Caucasian, had lower annual income, and a higher Charlson comorbidity score than those undergoing adequate treatment. These patients were also more likely to have slightly larger tumors, reflected by increased T stage and a higher incidence of Stage IIB vs. IIA disease. Of patients that received treatment but failed to meet the standard for adequate therapy, 72% received at least some radiation while only 48% received any chemotherapy (34% received a multi-agent regimen). Neoadjuvant therapy was infrequent, with 6% of patients receiving induction chemotherapy and 4% receiving pre-operative radiation. Use of adjuvant radiation was more common in those with a positive pathologic margin (42% vs. 15% in those with a negative margin, p<0.001).
Table 1.
Demographics and clinical characteristics of patients with clinical N1-positive NSCLC undergoing adequate vs. inadequate therapy – Continuous variables are displayed as mean +/− standard deviation. Categorical variables are displayed as number (% total).
| Patient Characteristics |
Inadequate or no treatment n= 7,509 |
Surgery or adequate CRT n= 12,857 |
p-Value | |
|---|---|---|---|---|
| Age at diagnosis (years) | 72.7 +/− 10.1 | 66.9 +/− 10.0 | <0.001 | |
| Male Gender | 4,059 (54%) | 7,099 (55%) | 0.11 | |
| Caucasian | 6,458 (86%) | 11,328 (88%) | <0.001 | |
| Academic Center | 1,728 (23%) | 3,928 (31%) | <0.001 | |
| Annual Income > $35,000 | 4,353 (61%) | 7,880 (65%) | <0.001 | |
| Urban Population Area | 4,735 (63%) | 7,955 (62%) | 0.09 | |
| Charlson/Deyo Score | 0 | 4,401 (59%) | 7,275 (57%) | <0.001 |
| 1 | 2,073 (28%) | 4,121 (32%) | ||
| 2 | 1,035 (14%) | 1,461 (11%) | ||
| Tumor Size (mm) | 41.1 +/− 31.9 | 39.8 +/− 35.1 | 0.01 | |
| AJCC Clinical T Stage | 1 | 2,296 (31%) | 4,629 (36%) | <0.001 |
| 2 | 5,213 (69%) | 8,228 (64%) | ||
| AJCC Clinical Stage Group | 2A | 2,517 (33%) | 5,322 (41%) | <0.001 |
| 2B | 4,658 (62%) | 7,143 (56%) | ||
| 2 NOS | 334 (4%) | 392 (3%) | ||
| Any radiotherapy | 3,342 (44%) | 4,910 (38%) | <0.001 | |
| Mean total radiation dose (cGy) | 5,334 +/− 3,264 | 6,284 +/− 3427 | <0.001 | |
| Any chemotherapy | 2,241 (30%) | 8,244 (64%) | <0.001 | |
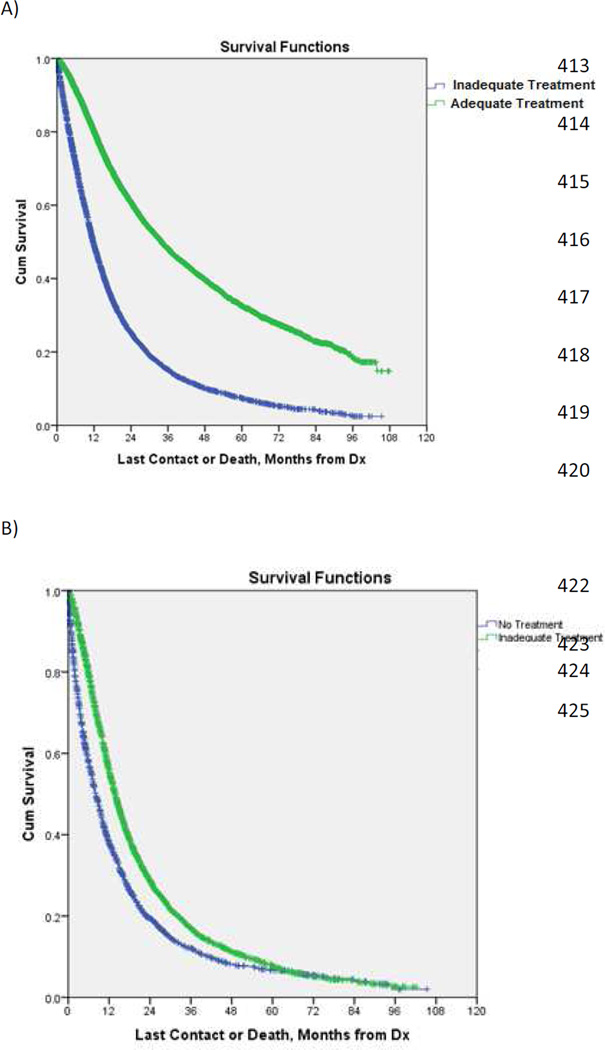
Results from Kaplan-Meier analysis of overall survival (OS) in patients undergoing various treatments are shown in Figure 1. Patients undergoing either surgical resection or adequate CRT had prolonged OS compared with those receiving inadequate or no treatment [median OS = 34.0 months (95% CI = 32.8–35.1) vs. 11.7 months (95% CI = 11.4–12.1), p<0.001] (Figure 1A). In addition, inadequate treatment was associated with longer OS compared with no therapy [median OS = 13.7 months (95% CI = 13.2–14.2) vs. 8.1 months (95% CI = 7.6–8.6), p<0.001] (Figure 1B).
Figure 1.
Kaplan-Meier curves for overall survival in patients with clinical N1-positive NSCLC undergoing adequate vs. inadequate or no treatment (A) and inadequate vs. no treatment (B) (both p<0.001). (See online supplement for 95% confidence intervals and number at risk.)
To investigate the variation in these treatment approaches over time, we divided the patient sample into 2 cohorts based on treatment date (2003–2006 and 2007–2010). Patients treated during the more recent time period (2007–2010) were significantly more likely to receive adequate treatment (70% vs. 54%).
Demographic and clinical characteristics of patients undergoing surgery vs. definitive CRT are shown in Table 2. On univariate analysis, patients undergoing surgical resection were younger, more likely to be female, live in an urban area, have an income > $35,000, and were more likely to undergo treatment at an academic facility. The majority of surgical patients underwent anatomic resection with either lobectomy (79%) or pneumonectomy (15%). Final pathologic analysis showed pN0 status in 18%, pN1 in 63%, and pN2/N3 in 11%. Mean length of stay for surgical patients was 7.7 days (standard deviation +/− 8.7, median = 6.0 days) and 30-day mortality in the surgical cohort was 3.5%.
Table 2.
Demographic and clinical information for patients with N1-positive NSCLC undergoing surgical resection vs. adequate definitive chemoradiation - Continuous variables are displayed as mean +/− standard deviation. Categorical variables are displayed as number (% total).
| Patient Characteristics |
Definitive chemoradiation n= 3,138 |
Surgical resection n= 9,719 |
p-Value | |
|---|---|---|---|---|
| Age at diagnosis (years) | 69.4 +/− 9.5 | 66.0 +/− 10.1 | <0.001 | |
| Male Gender | 1,793 (57%) | 5,306 (55%) | 0.01 | |
| Caucasian | 2,741 (87%) | 8,587 (88%) | 0.13 | |
| Academic Center | 645 (21%) | 3,283 (34%) | <0.001 | |
| Annual Income > $35,000 | 1,811 (60%) | 6,069 (66%) | <0.001 | |
| Urban Population Area | 1,844 (59%) | 6,111 (63%) | <0.001 | |
| Charlson/Deyo Score | 0 | 1,864 (59%) | 5,411 (56%) | <0.001 |
| 1 | 895 (28%) | 3,226 (33%) | ||
| 2 | 379 (12%) | 1,082 (11%) | ||
| Tumor Size (mm) | 39.9 +/− 28.1 | 39.8 +/− 36.8 | 0.84 | |
| AJCC Clinical T Stage | 1 | 937 (30%) | 3,692 (38%) | <0.001 |
| 2 | 2,201 (70%) | 6,027 (62%) | ||
| AJCC Clinical Stage Group | 2A | 1,090 (35%) | 4,232 (43%) | <0.001 |
| 2B | 1,963(63%) | 5,180 (53%) | ||
| 2 NOS | 85 (3%) | 307 (3%) | ||
| Type of Resection | None | 3,138 (100%) | 0 (n/a) | <0.001 |
| Wedge | 0 (n/a) | 612 (6%) | ||
| Lobectomy | 0 (n/a) | 7,657 (79%) | ||
| Pneumonectomy | 0 (n/a) | 1,450 (15%) | ||
| AJCC Pathologic T Stage | 0/IS | n/a | 47 (<1%) | n/a |
| 1 | n/a | 2,922 (30%) | ||
| 2 | n/a | 5,279 (54%) | ||
| 3 | n/a | 398 (4%) | ||
| 4 | n/a | 298 (3%) | ||
| X | n/a | 775 (8%) | ||
| AJCC Pathologic N Stage | 0 | n/a | 1,758 (18%) | n/a |
| 1 | n/a | 6,127 (63%) | ||
| 2 | n/a | 1,006 (10%) | ||
| 3 | n/a | 17 (<1%) | ||
| X | n/a | 811 (8%) | ||
| Any radiotherapy | 3,138 (100%) | 1,772 (18%) | <0.001 | |
| Mean total radiation dose (cGy) | 6,669 +/− 3,695 | 5,423 +/− 2533 | <0.001 | |
| Chemotherapy | None | n/a | 4,613 (47%) | <0.001 |
| Single Agent | 320 (10%) | 239 (2%) | ||
| Multi-agent | 2,509 (80%) | 4,328 (45%) | ||
| Chemo NOS | 309 (10%) | 539 (6%) | ||
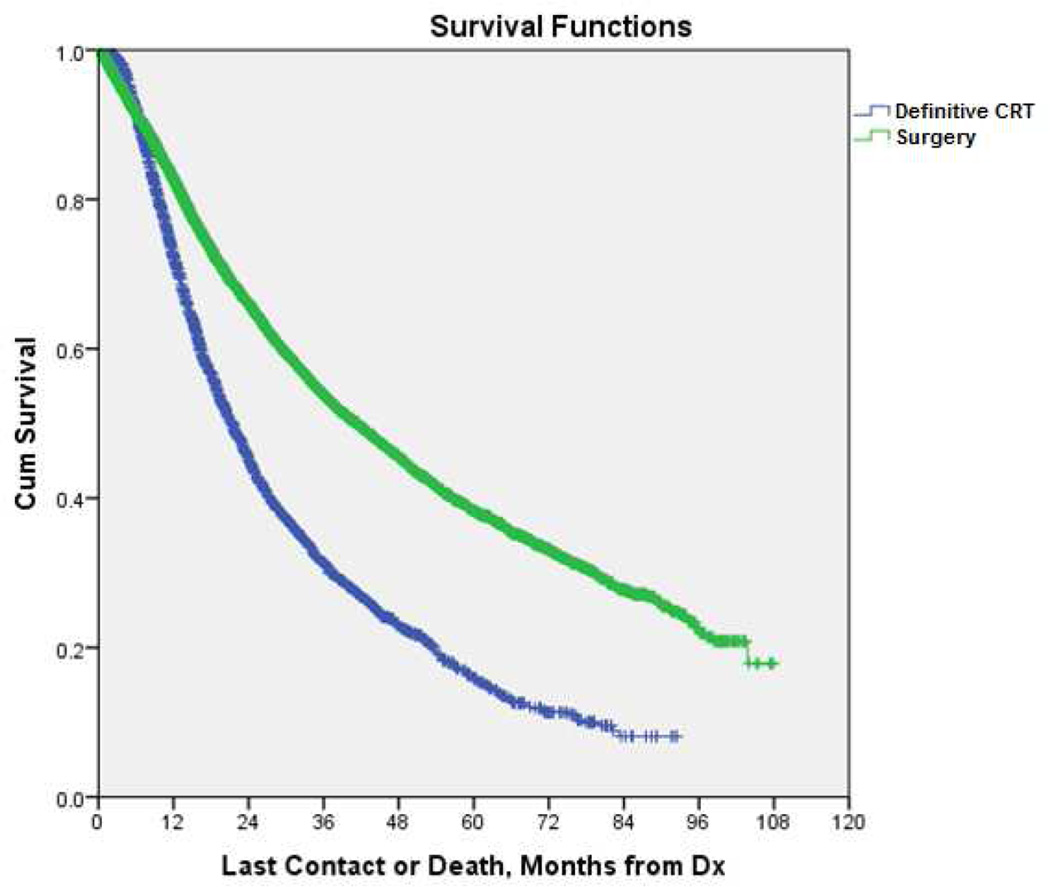
Multivariate logistic regression modeling identified several variables associated with surgical resection including treatment at an academic facility, Caucasian race, and a higher annual income (Table 3). Increasing age and T2 status were associated with non-operative management. Unadjusted median OS was greater in those undergoing surgery than those receiving CRT [41.5 months (95% CI= 39.8–43.2) vs. 21.4 months (95% CI= 20.5–22.4), p<0.001] (Figure 2).
Table 3.
Multivariable logistic regression analysis identifying variables associated with surgical resection in clinical N1 node-positive NSCLC.
| Patient and Treatment Variables |
Odds ratio (OR) with 95% Confidence Interval (CI) |
p-Value |
|---|---|---|
| Age | 0.96 (0.96–0.97) | <0.001 |
| Academic facility | 1.91 (1.71–2.12) | <0.001 |
| Caucasian race | 1.34 (1.17–1.54) | <0.001 |
| Annual income > $35,000 | 1.22 (1.10–1.35) | <0.001 |
| Urban area | 1.10 (0.99–1.22) | 0.05 |
| Charlson Score = 1 | 1.27 (1.15–1.40) | <0.001 |
| Charlson Score = 2 | 1.06 (0.92–1.22) | 0.42 |
| Clinical T2 status | 0.72 (0.65–0.80) | <0.001 |
Figure 2.
Kaplan-Meier curve for overall survival in patients with clinical N1-positive NSCLC undergoing definitive CRT vs. surgical resection (p<0.001). (See online supplement for 95% confidence intervals and number at risk.)
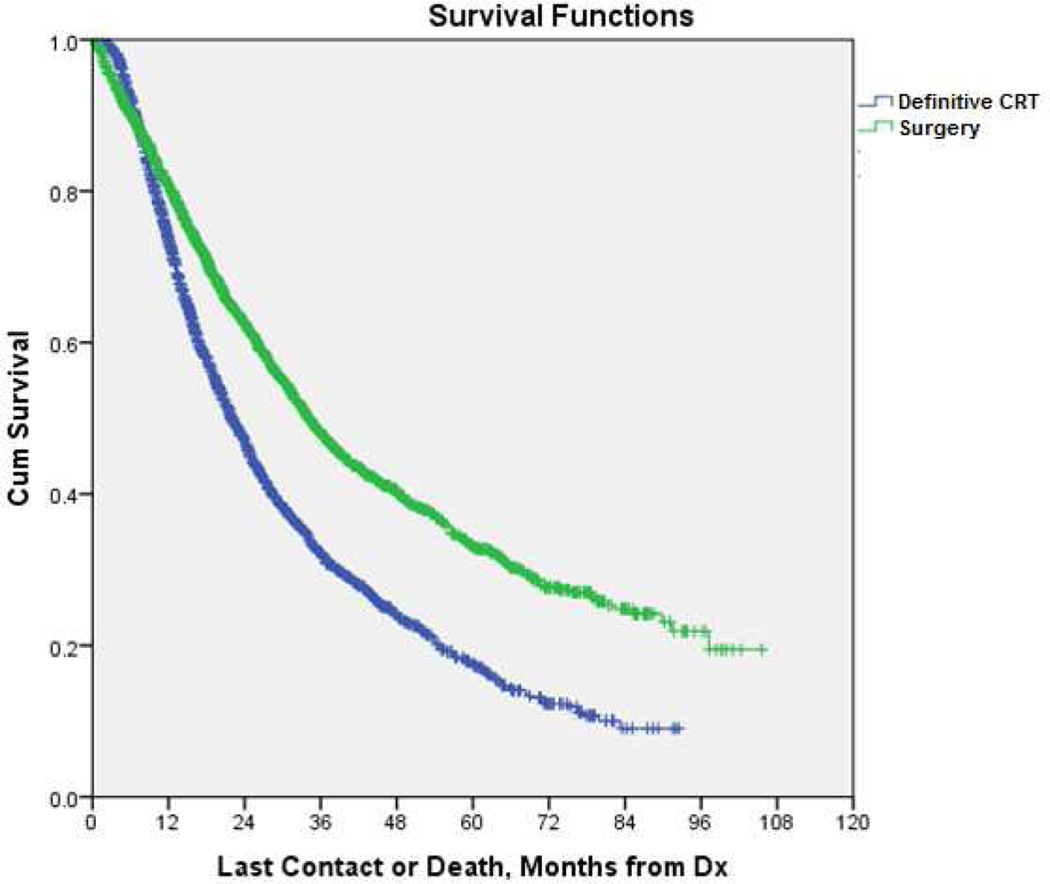
Adjusted survival analysis was performed using propensity matching based on criteria previously described in the Methods section. Propensity score matching identified 2,308 patient-pairs undergoing either surgical resection or definitive chemoradiation. Results of propensity score matching are shown in Table 4. In this matched pair analysis, surgical resection was associated with longer median OS compared with definitive CRT [median OS = 34.1 months (95% CI= 31.8–36.3) vs. 22.0 months (95% CI= 20.8–23.2), p<0.001] (Figure 3).
Table 4.
Results of propensity score matching of 2,308 patient-pairs undergoing either surgical resection or definitive CRT. Patients were matched for variables including: age, race, gender, income, type of treatment facility, comorbidity score, and tumor size. Continuous variables are displayed as mean +/− standard deviation. Categorical variables are displayed as number (% total).
| Patient Characteristics |
Definitive chemoradiation n= 2,308 |
Surgical resection n= 2,308 |
p-Value | |
|---|---|---|---|---|
| Age at diagnosis (years) | 68.9 +/− 9.0 | 68.9 +/− 8.9 | 0.81 | |
| Male Gender | 1,279 (55%) | 1,292 (56%) | 0.72 | |
| Caucasian | 2,030 (88%) | 2,024 (88%) | 0.82 | |
| Academic Center | 508 (22%) | 511 (22%) | 0.94 | |
| Annual Income > $35,000 | 1,450 (63%) | 1,415 (61%) | 0.30 | |
| Charlson/Deyo Score (CCI) | 0 | 1,317 (57%) | 1,304 (56%) | 0.91 |
| 1 | 710 (31%) | 715 (31%) | ||
| 2 | 281 (12%) | 289 (13%) | ||
| Tumor Size (mm) | 39.8 +/− 28.6 | 39.7 +/− 34.2 | 0.90 | |
| Radiotherapy | 2.308 (100%) | 397 (17%) | <0.001 | |
| Mean total radiation dose (cGy) | 6,619 +/− 3,409 | 5,293 +/− 1,580 | <0.001 | |
| Chemotherapy | None | 0 (0%) | 1,172 (51%) | <0.001 |
| Single Agent | 230 (10%) | 50 (2%) | ||
| Multi-agent | 1,862 (81%) | 954 (41%) | ||
| Chemo NOS | 216 (9%) | 132 (6%) | ||
Figure 3.
Kaplan-Meier curve for overall survival in 2,308 propensity score matched patient-pairs undergoing either surgical resection or definitive CRT for the treatment of N1 node-positive NSCLC. (p<0.001). (See online supplement for 95% confidence intervals and number at risk.)
To assess the impact of adjuvant chemotherapy, we performed an additional survival analysis in surgically resected patients that were found to be pathologic N1 or higher. Of resected, pathologic node-positive patients, 53% received adjuvant chemotherapy. Adjuvant treatment in these patients was associated with significantly longer OS [median OS = 48.2 months (95% CI=45.0–51.4) vs. 31.7 months (95% CI= 29.1–34.3), p<0.001].
In a parallel analysis, we studied NCDB patients who had undergone complete resection for pathologic T1-2N1 disease (n=7,317) and received adjuvant treatment. Of these, 6,438 (88%) received adjuvant chemotherapy while 879 (12%) received adjuvant chemoradiation. The only factor associated with the administration of radiation therapy in addition to chemotherapy in the adjuvant setting was pathologic T2 status (OR= 1.55, 95% CI=1.29–1.85), while those associated with chemotherapy alone were treatment at an academic center (OR= 0.50, 95% CI=0.42–0.61) and lobectomy/pneumonectomy (OR= 0.39, 95% CI=0.29–0.52 and 0.24, 95% CI=0.16–0.36, respectively compared with sublobar resection). In a multivariable cox PH model, lobectomy and pneumonectomy were associated with prolonged survival in this group (HR= 0.65, 95% CI=0.54–0.79 and 0.74, 95% CI=0.59–0.93 compared with sublobar resection), while those factors associated with shorter overall survival include age (HR= 1.02, 95% CI=1.01–1.02), male gender (HR= 1.29, 95% CI=1.18–1.42), Caucasian race (HR= 1.24, 95% CI=1.06–1.45), pT2 status (HR=1.15, 95% CI=1.03–1.28), and treatment with CRT versus chemo alone (HR= 1.56, 95% CI=1.38–1.77).
DISCUSSION
This analysis of data from the NCDB brings to light several interesting points regarding the treatment of clinical N1 node-positive NSCLC in the United States. First, despite evidence showing significant long-term survival with surgical resection and clear guidelines recommending its use in this population, less than half of patients (48%) presenting with clinical T1-2/N1 disease underwent surgical resection as part of their treatment.2–4 Our propensity matched analysis confirms that patients selected for surgical resection demonstrated longer OS than patients treated with adequate definitive chemoradiation (median OS = 34.1 vs. 22.0 months, p<0.001). Although the discrepancy between guidelines and nationwide practice is likely multifactorial, our regression analysis identifies several variables that are significantly associated with resection including younger age and smaller tumors. In addition, this multivariate analysis reveals that demographic and socioeconomic factors such as race and income also play a role.
Interestingly, undergoing treatment at an academic center was associated with a higher likelihood of surgical resection. Although there is some evidence that academic institutions may be associated with improved outcomes following surgery for lung cancer, the use of surgical resection itself has not previously been used as a quality measure.11 As academic centers tend to be associated with higher volume and surgeon experience, potential explanations for this relationship between academic institutions and the use of surgical resection include more strict adherence to published guidelines, greater willingness to attempt resection in cases where it may not be technically feasible, or a higher threshold for declaring patients medically inoperable.
Regardless, even in light of these factors the relatively low incidence of surgical resection in this population is surprising. Perhaps the most straightforward assumption would be that patients are not offered resection due to medical or technical in-operability. However, it is interesting to note that of adequately treated patients, there was a higher percentage with Charlson Comorbidity Scores of 1 or ≥2 in the surgical resection group than in the CRT cohort (44% vs. 41%, p<0.001). Even patients undergoing inadequate or no treatment had lower rates of Charlson Score ≥ 1 (41% and 42%, respectively) than those undergoing surgery. Thus there was no clear evidence from the NCDB data that these patients were denied surgery based on medical comorbidity. However, it is important to consider that the true extent of a patient’s operability extends beyond the measure of Charlson score. Unfortunately the NCDB does not capture more detailed information regarding a patient’s pulmonary function such as pre-operative forced expiratory volume in 1 second (FEV1) or diffusion capacity (DLCO).
Assuming that all patients failing to undergo resection are truly medically inoperable, non-operative treatment of this population falls short of established guidelines. Although it must be noted that randomized trials specifically evaluating medically inoperable patients with N1-node positive NSCLC are lacking, extrapolation of data from patients with Stage IIIA and IIIB disease has supported combined chemoradiation as the standard of care.4–7 In light of that, our data reveal that of 10,647 patients with T1-2N1 NSCLC not undergoing resection, only 29% received what we had defined as adequate chemoradiotherapy (chemotherapy in conjunction with >4500 cGy of radiation in any order). In patients undergoing some form of therapy but failing to meet the standard of adequate treatment, the major barrier appears to be chemotherapy. Of inadequately treated patients, the majority (72%) received external beam radiation (mean radiation dose = 5334 cGy) while over half (52%) failed to receive any chemotherapy and another 6% received only single agent treatment. Although several large trials have shown adjuvant chemotherapy to be beneficial in patients with completely resected stage II NSCLC, our data suggest that chemotherapy also appears to be difficult to administer in the adjuvant setting.12–14 According to this data from the NCDB, only 53% of pathologic N1 node-positive patients undergoing resection received peri-operative chemotherapy. Once again, this suggests a lack of adherence to accepted guidelines which state that surgical resection alone is not a recommended therapy for this patient population.3
Perhaps one of the most encouraging aspects of this analysis is that the vast majority of patients undergoing surgery received an anatomic resection. According to the NCDB, of those with clinical T1-2N1 NSCLC, 79% underwent lobectomy, 15% received pneumonectomy, and only 6% were treated with wedge resection. Thus, once patients proceeded to surgery, their treatment appeared to be in line with current standards of care regarding operative intervention.15–16
The use of adjuvant radiation following complete resection of NSCLC is controversial. Several studies suggest lack of benefit and even diminished survival in early stage patients.17–19 However, some centers still include adjuvant radiotherapy in treatment protocols for this patient population. Our data from the NCDB support the notion that adjuvant radiation for completely resected patients with T1-2N1 NSCLC is associated with shorter overall survival than those treated with adjuvant chemotherapy alone. Unless additional studies provide strong evidence for benefit in this population, routine treatment with radiotherapy should be avoided in these patients.
One additional factor that warrants discussion is the discrepancy between clinical and pathologic staging in a substantial proportion of cases. Several previous studies have documented poor correlation between clinical and pathologic staging in patients with NSCLC.20–22 In 2011, Darling and colleagues evaluated 149 patients undergoing both positron emission tomography / computed tomography (PET-CT) and invasive mediastinal staging (either mediastinoscopy, thoracotomy, or both) to evaluate the accuracy of the pre-operative staging workup.22 Overall they found fairly poor correlation between the two methods (64% positive predictive value, 95% negative predictive value). Of 13 patients with clinical N1 disease on PET-CT, 1 (8%) was found to be pathologic node negative and 4 (31%) were found to have N2 disease on final pathology. Our study confirms these findings in a much larger cohort of patients. According to the NDCB, of patients with clinical N1-node positive NSCLC, 18% were found to have no evidence of nodal disease on final pathologic evaluation. It is possible that node- positivity on clinical staging evaluation biases providers against referral for surgical resection. If so, these data suggest that false-positive clinical staging evaluations may prevent resection in a substantial proportion of patients with true pathologic stage I disease. Conversely, although the study excluded patients with clinical evidence of mediastinal lymph node involvement, the incidence of pathologic N2 or N3 disease was over 10% in resected patients. These data once again highlight the relative inadequacy of current clinical staging paradigms.
The discrepancy between published standards of care and the reality of actual physician practice highlighted in this study underscores the need for additional measures to improve the treatment of patients with N1 node-positive NSCLC across the country. Demographic and clinical factors such as patient age and tumor size are fairly immutable. However, the impact of socioeconomic variables suggests that efforts to improve access to healthcare in disadvantaged populations could potentially yield benefits by making surgery available to a greater proportion of operable patients. It is possible that popularization of healthcare-based internet portals and mobile device applications may allow for improved delivery of care to these underserved populations.
Although a certain subset of patients will undoubtedly be inoperable due to medical comorbidity, it is hard to believe that this would constitute over half of patients identified in the database. This finding prompts speculation that these patients are not referred for surgical resection due to reluctance on behalf of either primary physicians or the patients themselves. Multi-disciplinary evaluation of these patients should be mandatory and improved communication between surgeons and referring physicians may eliminate preconceived misconceptions regarding the safety and efficacy of surgery in this population. Similarly, enhanced dialogue between surgeons and their patients can help to alleviate additional barriers to operative intervention such as anxiety regarding postoperative pain and lack of perceived benefit.
There are some limitations to the current study. Although the NCDB contains a wealth of patient data, the information is still retrospectively reviewed and treatment groups are not randomized. Despite techniques such as multivariate analysis and propensity score matching, it is possible that more subtle difference in patient populations exist and therefore bias results. Similarly, there are several relevant patient variables that are not captured by the database. As a result, factors such as staging modalities and pre-operative FEV1 or other measures of operative fitness cannot be compared in the current analysis. Although the majority of patients likely underwent clinical staging with CT and or PET, the use of these modalities is not mandated, nor recorded by the NCDB. In addition, this analysis only includes patients treated before 2010. It is possible that practice patterns have since changed, as the prevalence of minimally invasive surgical modalities has increased and data supporting adjuvant therapy has grown. Lastly, although the American College of Surgeons Commission on Cancer requires at least a 90% patient follow-up rate as part of its accreditation program, our de-identified patient information cannot be used to further validate the accuracy of survival information submitted to the NCDB.
Our review of the NCDB reveals that treatment of patients with N1-node positive NSCLC falls short of established guidelines. Although data from the NCDB confirm a longer overall survival associated with surgery, a large number of patients fail to undergo resection. In addition, medical therapy of patients not selected for surgery is suboptimal in a large percentage of cases. More research is needed to elucidate the reasons behind these delinquencies and improve patient care in the future. The impact of factors such as socioeconomic status and treatment at an academic center highlight the need for improved access to care in certain patient populations. In addition, these data highlight the importance of multi-disciplinary evaluation in this patient population. Perhaps better communication between surgeons and referring physicians, as well as better dialogue between surgeons and their patients may alleviate barriers to surgical resection in operable patients.
Supplementary Material
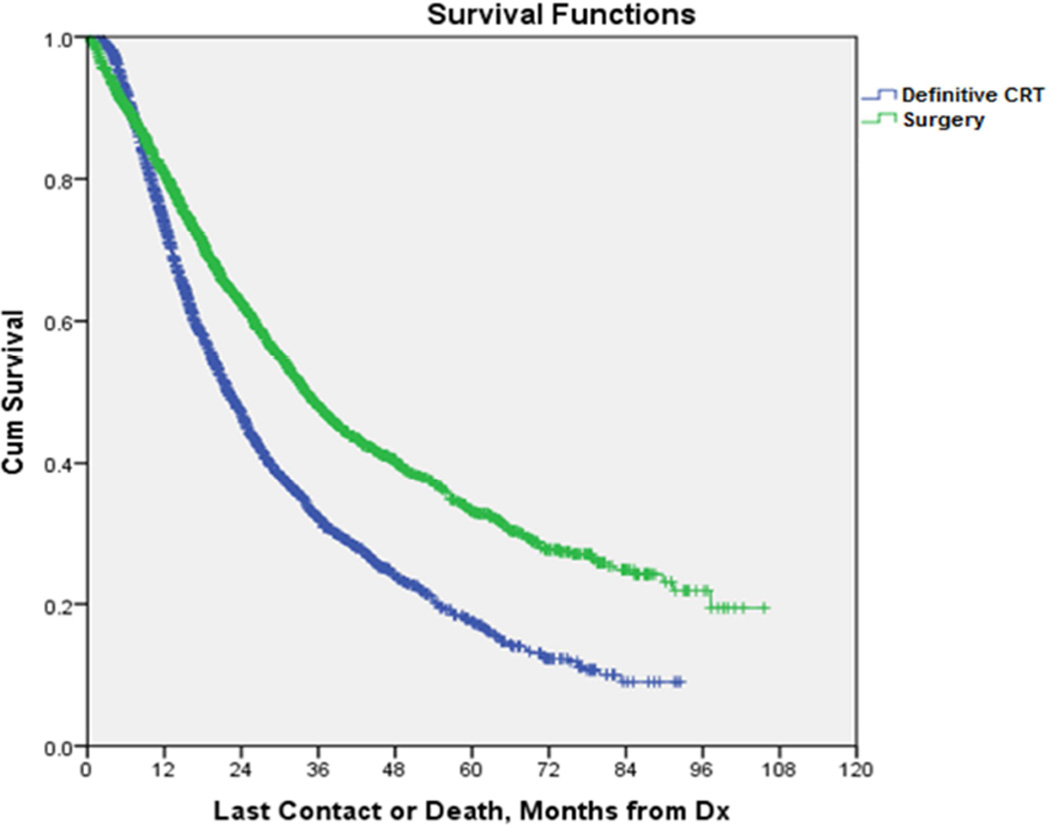
Central Picture.
Propensity-matched survival for patients undergoing surgery vs CRT for N1-positive NSCLC
Perspective Statement
Published guidelines recommend surgery as the primary treatment modality for operable patients with hilar node-positive (N1) NSCLC and chemoradiation (CRT) for those deemed inoperable. Our analysis of a large national database shows that adherence to these guidelines is poor. We show that less than 50% of T1-2N1 patients undergo resection and many selected for CRT receive inadequate therapy.
Central Message
Despite guidelines, many patients with T1-2N1 NSCLC do not receive adequate treatment. Surgical evaluation should be mandatory.
Acknowledgments
Grant Support
Varun Puri - NIH K07CA178120, K12CA167540-02 (Paul Calabresi Award)
Abbreviations and Acronyms
- AJCC
American Joint Committee on Cancer
- ANOVA
Analysis of Variance
- CI
Confidence Interval
- CRT
Chemoradiation
- CT
Computed tomography
- DLCO
Diffusion Capacity
- FEV1
Forced expiratory volume in 1 second
- NCDB
National Cancer Database
- NSCLC
Non-small cell lung cancer
- OR
Odds Ratio
- OS
Overall survival
- PET
Positron emission tomography
Biography

Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented in oral format at the 41st Annual Meeting of the Western Thoracic Surgical Association, Whistler, British Columbia, June 25, 2015.
The authors report no relevant disclosures and financial conflict of interest.
REFERENCES
- 1.Detterbeck FC, Gibson CJ. Turning Gray: The Natural History of Lung Cancer Over Time. J Thorac Oncol. 2008;3:781–792. doi: 10.1097/JTO.0b013e31817c9230. [DOI] [PubMed] [Google Scholar]
- 2.Chansky K, Sculier JP, Crowley JJ, Giroux D, Van Meerbeeck J, Goldstraw P. The International Association for the Study of Lung Cancer Staging Project – Prognostic Factors and Pathologic TNM Stage in Surgically Managed Non-small Cell Lung Cancer. J Thorac Oncol. 2009;4:792–801. doi: 10.1097/JTO.0b013e3181a7716e. [DOI] [PubMed] [Google Scholar]
- 3.Howington JA, Blum MG, Chang AC, Balekian AA, Murthy SC. Treatment of Stage I and II Non-small Cell Lung Cancer: Diagnosis and Management of Lung Cacner, 3rd ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. CHEST. 2013;143(5 supp):e278s–e313S. doi: 10.1378/chest.12-2359. [DOI] [PubMed] [Google Scholar]
- 4.National Comprehensive Cancer Network. NCCN Guidelines Version 5.2015 - Non-Small Cell Lung Cancer. http://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf. [Google Scholar]
- 5.Auperin A, Le Pechoux C, Rolland E, Curran WJ, Furuse K, Fournel P, et al. Meta-Analysis of Concomitant Versus Sequential Radiochemotherapy in Locally Advanced Non-Small-Cell Lung Cancer. J Clin Oncol. 2010;28:2181–2190. doi: 10.1200/JCO.2009.26.2543. [DOI] [PubMed] [Google Scholar]
- 6.O’Rourke N, I Figuls MR, Bernado NF, Macbeth F. Concurrent chemoradiotherapy in non-small cell lung cancer. Cochrane Database Systematic Reviews. 2010;(6) doi: 10.1002/14651858.CD002140.pub3. [DOI] [PubMed] [Google Scholar]
- 7.Curran WJ, Paulus R, Langer CJ, Komaki R, Lee JS, Hauser S, et al. Sequential vs Concurrent Chemoradiation for Stage III Non-Small Cell Lung Cancer: Randomized Phase III Trial RTOG 9410. J Natl Cancer Inst. 2011;103:1452–1460. doi: 10.1093/jnci/djr325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bilimoria KY, Stewart AK, Winchester D, Ko CY. The National Cancer Data Base: A Powerful Initiative to Improve Cancer Care in the United States. Ann Surg Onc. 2008;15(3):683–690. doi: 10.1245/s10434-007-9747-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rusch VW, Asamura H, Watanabe H, Giroux DJ, Rami-Porta R, Goldstraw P. The IASLC Lung Cancer Staging Project: A Proposal for a New International Lymph Node Map in the Forthcoming Seventh Edition of the TNM Classification of Lung Cancer. J Thorac Oncol. 2009;4:568–577. doi: 10.1097/JTO.0b013e3181a0d82e. [DOI] [PubMed] [Google Scholar]
- 10.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC cancer staging manual. 7th. New York, NY: Springer; 2010. [Google Scholar]
- 11.Meguid RA, Brooke BS, Chang DC, Sherwood JT, Brock MV, Yang SC. Are Surgical Outcomes for Lung Cancer Resections Improved at Teaching Hospitals? Ann Thorac Surg. 2008;85:1015–1025. doi: 10.1016/j.athoracsur.2007.09.046. [DOI] [PubMed] [Google Scholar]
- 12.Winton T, Livingston R, Johnson D, Riga J, Johnston M, Butts C, et al. Vinorelbine plus Cisplatin vs. Observation in Resected Non-Small-Cell Lung Cancer. NEJM. 2005;352:2589–2597. doi: 10.1056/NEJMoa043623. [DOI] [PubMed] [Google Scholar]
- 13.Douillard J, Rosell F, De Lena M, Carpagnano F, Ramlau R, Gonzales-Larriba JL, et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer: a randomized controlled trial. Lancet Oncol. 2006;7:719–727. doi: 10.1016/S1470-2045(06)70804-X. [DOI] [PubMed] [Google Scholar]
- 14.Pignon JP, Tribodet H, Scagliotti G, Douillard JY, Shepherd FA, Stephens RJ, et al. Lung Adjuvant Cisplatin Evaluation: A Poole Analysis by the LACE Collaborative Group. J Clin Oncol. 26:3552–3559. doi: 10.1200/JCO.2007.13.9030. [DOI] [PubMed] [Google Scholar]
- 15.Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1N0 non-small cell lung cancer. Ann Thorac Surg. 1995;60:615–622. doi: 10.1016/0003-4975(95)00537-u. [DOI] [PubMed] [Google Scholar]
- 16.Miller DL, Rowland CM, Deschamps C, Allen MS, Trastek VF, Pairolero PC. Surgical treatment of non-small cell lung cancer 1cm or less in diameter. Ann Thorac Surg. 2002;73:1545–1550. doi: 10.1016/s0003-4975(02)03525-7. [DOI] [PubMed] [Google Scholar]
- 17.Burdett S, Rydzewska L, Tierney JF, Fisher DJ. A closer look at the effects of postoperative radiotherapy by stage and nodal status: Updated results of an individual participant data meta-analysis in non-small-cell lung cancer. Lung Cancer. 2013;80:350–352. doi: 10.1016/j.lungcan.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 18.Feng QF, Wang M, Wang LJ, Yang ZY, Zhang YG, Zhang DW, et al. A study of postoperative radiotherapy in patients with non-small-cell lung cancer: a randomized trial. Int J Radiat Oncol Biol Phys. 2000;47:925–929. doi: 10.1016/s0360-3016(00)00509-5. [DOI] [PubMed] [Google Scholar]
- 19.Dautzenberg B, Arriagada R, Chammard AB, Jarema A, Mezzetti M, Mattson K, et al. A controlled study of postoperative radiotherapy for patients with completely resected nonsmall cell lung carcinoma. Cancer. 1999;86:265–273. doi: 10.1002/(sici)1097-0142(19990715)86:2<265::aid-cncr10>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 20.D’Cunha J, Corfits AL, Herndon JE, Kern JA, Kohman LJ, Patterson GA, et al. Molecular staging of lung cancer: real-time polymerase chain reaction estimation of lymph node micrometastatic tumor cell burden in stage I non-small cell lung cancer – preliminary results of Cancer and Leukemia Group B Trial 9761. J Thorac Cardiovasc Surg. 2002;123(3):484–491. doi: 10.1067/mtc.2002.119883. [DOI] [PubMed] [Google Scholar]
- 21.Lopez-Encuentra A, Garcia-Lujan R, Rivas JJ, Rodriguez-Rodriguez J, Torres-Lanza J, Varela-Simo G. Comparison Between Clinical and Pathologic Staging in 2,994 Cases of Lung Cancer. Ann Thorac Surg. 2005;79:974–979. doi: 10.1016/j.athoracsur.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 22.Darling GE, Maziak DE, Inculet RI, Gulenchyn KY, Driedger AA, Ung YC, et al. Positron Emission Tomography-Computed Tomography Compared with Invasive Mediastinal Staging in Non-small Cell Lung Cancer – Results of the Mediastinal Staging in the Early Lung Positron Emission Tomography Trial. J Thorac Oncol. 2011;6:1367–1372. doi: 10.1097/JTO.0b013e318220c912. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.