Abstract
There is a growing recognition that gliomas are complex tumors composed of neoplastic and non-neoplastic cells, which each individually contribute to cancer formation, progression and response to treatment. The majority of the non-neoplastic cells are tumor-associated macrophages (TAMs), either of peripheral origin or representing brain-intrinsic microglia, that create a supportive stroma for neoplastic cell expansion and invasion. TAMs are recruited to the glioma environment, have immune functions, and can release a wide array of growth factors and cytokines in response to those factors produced by cancer cells. In this manner, TAMs facilitate tumor proliferation, survival and migration. Through such iterative interactions, a unique tumor ecosystem is established, which offers new opportunities for therapeutic targeting.
Solid cancers develop in complex tissue environments that dramatically influence tumor growth, transformation and metastasis. In the microenvironment of most solid tumors are various non-neoplastic cell types, including fibroblasts, immune system cells and endothelial cells. Each of these stromal cell types produce growth and survival factors, chemokines, extracellular matrix constituents, and angiogenic molecules with the capacity to change the local milieu in which neoplastic cells grow and infiltrate. In the case of the most common brain tumor (glioma or astrocytoma), monocytes (macrophages and microglia) represent rich sources of these stromal factors. Moreover, the fact that as many as 30–50% of the cells in gliomas are microglia or macrophages1–4 raises the intriguing possibility that targeting microglia and macrophages might emerge as an adjuvant therapy for these difficult to manage cancers. In this review, we discuss the current understanding of these critical stromal elements in glioma.
Origins of glioma associated microglia and macrophages
Microglia are the resident macrophages of the CNS. These mono-nuclear cells are distributed throughout the brain, where they function as key immune effector cells of the CNS. Originally discovered and characterized almost a century ago by Pio Del Rio Hortega5, the tissue origins of microglia and the mechanisms regulating their homeostasis in health and disease have been debated for many decades6. Contributing to the confusion was the use of particular experimental systems, including chimera mice generated by bone marrow (BM) transplantation of lethally irradiated recipients, and monocyte classification schemes reliant on the expression of specific cell surface antigens. Using bone-marrow transplantation, investigators concluded that, under homeostatic conditions, a considerable percentage of microglia are replaced by donor-derived monocytes7. Similar studies have also suggested that increases in microglia density in response to CNS damage involve both the expansion of endogenous resident microglia and the active recruitment of BM-derived microglial progenitors from the bloodstream8–12. Leveraging analogous methods, other reports demonstrated little or no contribution of circulating progenitors to the brain microglia pool. These studies argued that the expansion of microglia during microgliosis (microglial activation) results mainly from the local expansion of existing resident microglia13. These seemingly contradictory findings were finally resolved when chimeric animals generated by parabiosis were employed, which does not require either irradiation or transplantation. Using two models of acute and chronic microglia activation (axotomy and neurodegeneration), no microglial recruitment from the blood circulation was found13. In addition, acute peripheral recruitment of monocytes was observed in an experimental mouse model of autoimmune encephalitis (EAE); however, these infiltrating cells vanished following remission and did not contribute to the resident microglia pool14.
Notably, recent fate-mapping studies have identified immature yolk sac progenitors as the predominant source of brain microglia. Using sub-lethally irradiated C57BL/6 CD45.2+ newborns with hematopoietic cells isolated from CD45.1+ congenic mice, investigators found that, 3 months after transplantation, 95% of adult microglia remained of host origin. Second, they employed Cx3cr1GFP/+ knock-in mice to demonstrate that myeloid cells expressing CD45 and the adult macrophage markers CD11b, F4/80 and CX3CR1 were detectable in the developing brain beginning at embryonic day 9.5 (E9.5). Third, in Csf1r-deficient mice, colony stimulating factor receptor (CSF1R) deficiency markedly reduced the development of microglia, whereas the circulating monocytes were unimpaired. Fourth, leveraging the Rosa26R26R-eYFP/R26R-eYFP reporter strain intercrossed with mice in which the tamoxifen-inducible CreER recombinase gene was under the control of one of the endogenous runt-related transcription factor 1 (Runx1) locus promoters, the authors found that Runx1+ progenitors migrate from the yolk sac into the brain between E8.5 and E9.5, where they serve as the cells of origin for microglia15. Lastly, microglia derived from primitive c-kit+ erythromyeloid precursors subsequently develop into CD45+ c-kit+ CX3CR1neg immature cells (A1), which then mature into CD45+ c-kitneg CX3CR1+ (A2) cells following CD31 downregulation and upregulation of F4/80 and CSF1R16. Together, these studies reveal that mouse myeloid progenitors from the blood do not substantially contribute to the pool of adult microglia after birth, establishing that the majority of adult microglia are yolk sac–derived and maintain themselves by virtue of longevity and limited self-renewal13,15,17. In this regard, resident microglia represent a distinct population of myeloid cells (Fig. 1).
Figure 1.
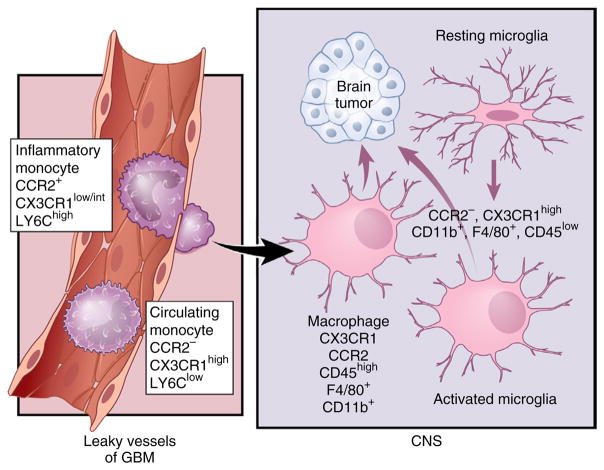
Microglia and monocytes have distinct cellular origins. Under steady-state conditions, these different mononuclear cell populations reside in separate locations. In adult life, monocytes are generated from HSCs that differentiate into granulocyte-macrophage progenitors (GMPs) and then into monocyte-dendritic cell progenitors (MDPs). Mature Ly6Chi CCR2+ CX3CR1low/int inflammatory monocytes are released into circulation20, where they can migrate to tissues in response to specific pathological conditions. These cells can also give a rise to circulating monocytes. Microglia originate from yolk sac progenitors in the neuroepithelium beginning around E8.5 in the mouse. In the adult brain, they express high levels of CX3CR1, CD11b and F4/80, but low levels of CD45 and no CCR2. Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 2015. All rights reserved.
Whereas the naive CNS is occupied by resident microglia only, the diseased CNS presents a different picture. In many neuropathological conditions, the blood brain barrier is impaired, resulting in an infiltration of monocytes from the periphery. Understanding the differences between macrophages and microglia is critically important, as it is well-documented that they can react differently to various types of CNS insults. Recent studies using a complex parabiosis model with highly efficient permanent labeling of blood monocytes showed that peripheral mononuclear cells invade the inflamed CNS during EAE pathogenesis and have a primary role in disease progression14.
Monocytes originate from hematopoietic stem cells (HSCs) via progeny called macrophage-DC precursors. These cells differentiate in the bone marrow into monocytes, which are subsequently released into the blood circulation to colonize peripheral organs under both normal and inflammatory conditions18. Mouse monocytes can be further subdivided into two main populations: Ly6C+ CX3CR1int CCR2+ inflammatory monocytes and Ly6C− CX3CR1hi CCR2− circulating monocytes (Fig. 1)19,20. In the future, a clear distinction should be made between the contributions of microglia and blood monocyte to disease pathogenesis, further underscoring the need to better understand the fate and origins of blood monocytes.
Approaches to distinguishing microglia from invading monocytes have traditionally relied on the use of CD45 antibodies to separate resident microglia (CD45low) from macrophages of hematopoietic origin (CD45high)21. Analysis of human glioma samples by fluorescence- activated cell sorting has revealed that the CD45high population is larger than the CD45low population, suggesting that gliomas contain more recruited monocytes than microglia22. This concept was recently challenged by a study using irradiation chimeras, which demonstrated that the majority of TAMs are intrinsic microglia and that these microglial cells upregulate their CD45 expression to constitute a significant proportion of the CD45high monocyte population in gliomas23. In this study, the investigators protected the head from radiation to avoid a massive infiltration of monocytes as a result of a disrupted blood-brain barrier23. Another study using only single staining with either antibodies for CX3CR1 or CCR2 concluded that the majority of TAMs are mainly monocyte-derived macrophages (CCR2+ CX3C1−) and, to a smaller extent, resident microglia (CCR2− CX3C1+)24. Although interesting, this study has several limitations, which include the lack of lineage tracing experiments to conclusively demonstrate that macrophages were derived from monocytes. Moreover, others have demonstrated that CX3CR1 is expressed by blood monocytes and that its expression is upregulated during monocyte differentiation into macrophages, implying that CX3CR1 does not represent a microglia-specific marker in either the naive brain19,20 or the context of glioma25 (Fig. 2). The discrepancies in the literature resulting from the use of bone-marrow chimeras and cell surface antibodies highlight the urgent need to re-evaluate these published conclusions and to perform lineage-tracing experiments using reporter mice that accurately distinguish microglia from monocytes and macrophages relative to their distinct roles in glioma formation, maintenance and progression (Fig. 2).
Figure 2.
Microglia and monocytes converge in high-grade glioma (HGG). HGG cells induce local inflammation that compromises the integrity of the blood-brain barrier (BBB) and results in Ly6Chi CCR2+ CX3CR1low/int monocytes infiltrating into the tumor14. Once in the CNS, these cells can differentiate into tumor-associated macrophages and become nearly indistinguishable from activated resident microglia. Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 2015. All rights reserved.
TAMs and low-grade glioma
Similar to their high-grade counterparts, the majority of World Health Organization (WHO) grade I and II astrocytomas contain microglia and macrophages26. Using CD68 and Iba1 antibodies, the percentage of monocytes in these low-grade tumors has been estimated at 15–30%, as compared with 10–15% in normal non-neoplastic brain specimens2. Depending on the region in which the tumor arises, the microglia fraction can be as high as 35–50%, as observed in WHO grade I pilocytic astrocytomas1. Notably, the percent of proliferating CD68+ cells may be higher in WHO grade I pilocytic astrocytomas (32%) relative to malignant WHO grade III–IV astrocytomas (8.6–13.4%)27. The importance of these immune system–like cells (macrophages and microglia) to glioma behavior is further underscored by two clinical observations: the number of CD68+ cells increases with increasing malignancy grade28 and the recurrence-free survival of patients with pilocytic astrocytoma is inversely related to the percentage of CD68+ cells in the tumor29.
To gain insights into the contributions of microglia to low-grade glioma biology, we previously leveraged a murine model of neurofibromatosis type 1 (NF1) optic glioma. 15–20% of children with the NF1 inherited cancer predisposition syndrome develop pilocytic astrocytomas involving the optic pathway30. These children are born with a germline NF1 gene mutation (NF1+/−) and develop brain tumors following somatic NF1 gene inactivation in cells of the astroglial lineage1. Similarly, Nf1+/− mice with somatic Nf1 gene inactivation in neuroglial progenitors develop low-grade glial neoplasms involving the optic nerve and chiasm31. As observed in their human counterparts, these murine low-grade tumors are infiltrated by TAMs32. The majority of the TAMs in Nf1 mouse optic gliomas are CD11bhigh CD45low, and therefore most likely microglia33, which are evident early during tumorigenesis34. The role of these stromal cells in mediating glioma growth has been revealed by preclinical studies in which pharmacologic (minocycline, c-Jun-NH(2)-kinase inhibition) or genetic (gangciclovir treatment of CD11b− thymidine kinase–expressing mouse line) silencing of microglial function results in reduced tumor proliferation2,35,36. Moreover, Nf1 optic glioma mice with reduced expression of a chemokine receptor responsible for directional macrophage migration (CX3CR1) demonstrate delayed tumor formation33. Collectively, these data establish critical functions for microglia in murine low-grade glioma formation and maintenance. Notably, similar requirements for monocytes in another low-grade glial (Schwann) cell tumor have been reported. In these studies, mast cells and macrophages are the stromal cell types essential for neurofibroma development and continued growth37,38.
Although the mechanisms underlying microglia stimulation of low grade glioma growth have not been fully elucidated, Nf1+/− TAMs produce paracrine factors and chemokines capable of increasing Nf1-deficient astroglial cell proliferation35. One such chemokine, stroma-derived factor-1 (SDF-1 or CXCL12), is increased in Nf1+/− TAM relative to wild-type (normal) microglia39,40. SDF-1 operating through the CXCR4 receptor promotes optic glioma cell survival, such that CXCR4 inhibition reduces tumor growth in vivo. A more complete characterization of TAMs support of tumor maintenance has been performed using optimized RNA-sequencing methods41,42.
TAMs and high-grade (malignant) glioma
TAM activation
Macrophages and microglia are mononuclear cell types characterized by considerable diversity and plasticity. As such, different types of macrophage activation have been defined following in vitro stimulation. The pro-inflammatory M1 phenotype is typically acquired after stimulation with Toll-like receptor 4 (TLR4) ligands and IFN-γ, while the alternative M2 phenotype occurs after IL-4, IL-10 and IL-13 exposure43. Alternative macrophage activation can be further subdivided into M2a (Th2 responses, type II inflammation, killing of pathogens, allergy), M2b (Th2 activation, immunoregulation) and M2c (immunoregulation, matrix deposition, tissue remodeling) activation states43,44. These polarized subpopulations of macrophages differ with respect to receptor expression, effector function, and cytokine and chemokine production45. Given that the definitions of these mutually exclusive activation states are based on in vitro conditions, they do not translate well to the in vivo setting.
Several studies have analyzed the expression of polarization marker genes in TAMs either in vitro or in vivo46–48. Similar to solid tumors arising in other organs, TAMs exhibit alternative macrophage activation, including increased production of anti-inflammatory molecules (for example, transforming growth factor β (TGF-β) ARG1 and IL-10) as well as those that support tissue remodeling and angiogenesis (for example, VEGF, MMP2, MMP9 and MT1-MMP). In addition, TAMs also produce pro-inflammatory molecules (for example, TNF-α, IL1-β and CXCL10)46,48–52.
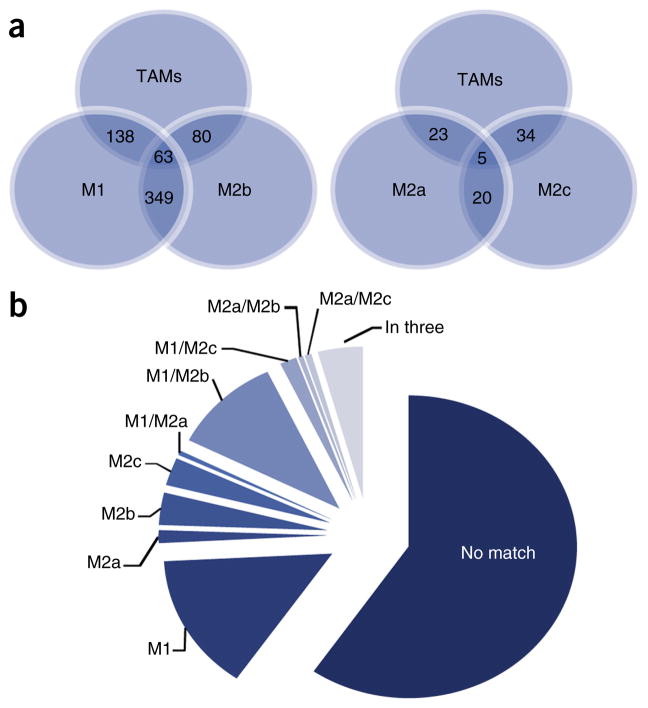
Using RNA microarray analyses, the expression profiles of glioma-associated microglia and macrophages, and control microglia were compared with those from control animals obtained by CD11b antibody–mediated magnetic-activated cell sorting. Approximately 1,000 transcripts were differentially expressed by twofold or more in glioma-associated microglia and macrophages relative to control microglial cells. This expression pattern had only partial overlap with reported gene signatures for M1-, M2a-, M2b- and M2c-polarized macrophages (Fig. 3)53.
Figure 3.
M1/M2 profile of TMAs. Comparison of TAMs with M1- and M2a-, M2b- and M2c-stimulated macrophage data sets53 (http://www.ebi.ac.uk/arrayexpress/experiments/E-GEOD-32690/) containing macrophages stimulated for 24 h in vitro into different polarization states (M0 (unstimulated), M1 (IFNγ + LPS), M2a (IL4), M2b (IFNγ + complexed Ig) and M2c (dexamethasone)), which were compared with TAMs. (a) A graphical representation of the overlap of upregulated genes in TAMs and the four macrophage data sets. The TAMs gene expression profile shows the greatest overlap with M1- and M2b-polarized macrophages. The number of overlapping genes is indicated. (b) Using Gene Set Enrichment Analysis reveals that only a minority of genes that were upregulated in TAMs were also induced in the M1 to M2c phenotype; 59.5% of the genes upregulated in TAMs were not regulated in any of the four macrophage phenotypes.
Similarly, other investigators have performed correlative analyses to determine whether the survival of patients with high-grade gliomas is associated with the expression of either M1 or M2 polarization- specific markers. One such M1 polarization marker, CD74, was found to be expressed by human TAMs and was positively correlated with increased patient survival54. In another study, F11R was established as a monocyte prognostic marker for glioblastoma, where it negatively correlates with patient survival and may be critical for defining a subpopulation of stromal cells for future potential therapeutic intervention55.
Based on the current literature, it is clear that the current M1 and M2 classification schemes are not absolute, but constitute relative definitions when studying TAMs in vivo. In this regard, TAMs express markers that are characteristic of either the M1 or M2 phenotype. As such, glioma-derived M-CSF induces a shift of microglia and macrophages toward the M2 phenotype, which increases tumor growth56. Similarly, mTOR57 or CSF-1 (ref. 56) inhibition shifts to the M1 phenotype. Similar anti-tumor effects have been shown by dopamine or targeting miR-142-3p, which affects the M2-polarization of TAMs58,59. On the basis of these studies, the identification of targeting approaches that convert M2 macrophages to M1 macrophages has been suggested as a potential therapeutic strategy to reduce glioma growth. However, other studies have suggested that M1 specific markers or associated pathways positively correlate with glioma growth. For example, IL1-β was shown to promote glioma growth25. Considering the plasticity and the fact that the M1 and M2 phenotype is a classification scheme defined in cultured macrophages, the phenotype of TAMs in vivo is more complex, and strategies should focus on targeting specific pathways or molecules that TAMs employ to interact with gliomas and promote their growth.
TAM recruitment
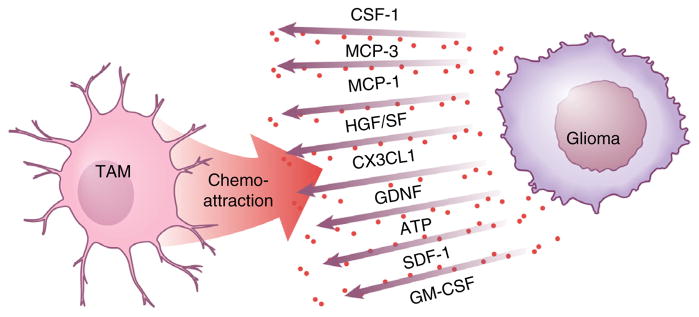
Microglial cells and macrophages accumulate in and around glioma tissue and acquire an amoeboid morphology. There are many factors that mediate microglia chemoattraction, including chemokines, ligands of complement receptors, neuro-transmitters and ATP. It is presently unclear whether there exist distinct factors that recruit intrinsic (resident) microglia or peripheral macrophages to the tumor. The first chemoattractant factor identified was monocyte chemoattractant protein-1 (MCP-1), also known as CCL2. Ectopic expression of CCL2 in rat glioma cells resulted in a tenfold higher density of Ox42-positive cells in vivo and the tumors generated with CCL2-expressing glioma cells were more than threefold larger in size, resulting in reduced rat survival60. The importance of MCP-1 to human glioma biology has recently been challenged, with a stronger correlation being observed between MCP-3, rather than MCP-1, expression and the density of infiltrating microglia and macrophages61.
Hepatocyte growth factor and scatter factor released by glioma cells similarly function as chemoattractants for microglia, but this has only been shown using a microglial cell line62. CXCL12 (SDF-1) is another potent microglia and macrophage recruiting molecule, especially for attracting TAMs to hypoxic areas63. In the normal brain, the receptor for the cytokine CX3CL1 (fractalkine), CX3CR1, is mostly expressed by microglial cells, where it has been established as a reliable marker for in vivo microglia imaging. The CX3CL1 and CX3CR1 signaling cascade is important for neuron-microglia communication, such that deletion of CX3CR1 impairs synapse plasticity during development64. However, conflicting data exist regarding the importance of CX3CL1 in tumor-directed TAM migration25,65,66.
The growth factor glial cell–derived neurotrophic factor (GDNF) was initially identified as a released factor from the glial cell line B49, and was found to promote the survival and differentiation of dopaminergic neurons. Mouse and human gliomas also secrete GDNF, which serves as a strong chemoattractant for microglia. When glioma cells were encapsulated in hollow fibers to allow for the passage of molecules, but not cells, microglia accumulated around these fibers following brain implantation. GDNF mediated this attraction, as revealed by GDNF knockdown in the encapsulated glioma or by over-expression of GDNF in an encapsulated fibroblast cell line. Notably, the upregulation of GFAP in astrocytes around hollow fibers was not affected by GDNF knockdown, indicating that GDNF largely acts on microglia67.
Lastly, CSF-1 is released by glioma cells, where it can also function as a microglia chemoattractant. Treatment of mice with a blood-brain barrier–permeable CSF-1R antagonist reduces the density of TAMs and attenuated glioblastoma invasion in vivo68. In addition, granulocyte- macrophage colony-stimulating factor (GM-CSF) can serve as a chemoattractant for microglia, as GM-CSF knockdown reduces microglia-dependent invasion in organotypic brain slices as well as attenuated the growth of intracranial gliomas in vivo69. In conclusion, there are many factors that can attract TAMs to the glioma (Fig. 4).
Figure 4.
Glioma cells release several factors, which attract TAMs to the tumor tissue. Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 2015. All rights reserved.
TAM regulation of glioma growth and migration
The accumulation of TAMs in and around glioma has raised the question as to whether these mononuclear cells are bystanders or whether they actively influence glioma growth and invasion. Accumulating evidence indicates that TAMs promote glioma growth and invasion. One study noted that, in the presence of microglial cells, the motility of the murine glioma cells was increased threefold in vitro70. In contrast, oligodendrocytes and endothelial cells only weakly promote glioma motility70. In situ, glioma growth can be monitored using organotypic brain slices. These slices can be depleted of microglia using liposomes filled with the toxin clodronate, resulting in reduced glioma invasion and growth71. A complementary in vivo approach entails the use of transgenic mice expressing the herpes simplex virus thymidine kinase gene under the control of the Cd11b promoter. In the CNS, CD11b is specifically expressed by microglia. When ganciclovir is infused into the brain, there is a marked reduction in microglia number, which concomitantly results in attenuated glioma growth in vivo50.
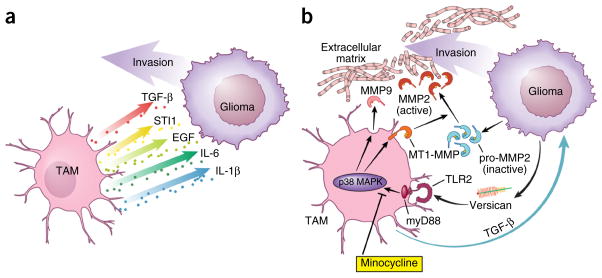
Several factors released from microglia have been reported to promote glioma proliferation and/or migration. Microglia synthesize and release stress-inducible protein 1 (STI1), a cellular prion protein ligand that increases the proliferation and migration of glioblastomas in vitro and in vivo72. In addition, microglia release epidermal growth factor (EGF), which also stimulates glioblastoma cell invasion68. This glioma-promoting activity by microglia is triggered by CSF-1, which is constitutively released by the tumor cells. As described above, CSF-1 is a chemoattractant for microglia and, at the same time, converts microglia into a pro-tumorigenic phenotype56. CCL2 is another factor released from human glioma cell lines and acts on the CCL2 receptor (CCR2) expressed on microglia73. CCL2 can trigger the release of IL-6 from microglia, which in turn, promotes the invasiveness of glioma cells74. It should be noted that there may be species differences, as it was recently described that mouse microglia do not express CCR2 (ref. 75).
Transforming growth factor-β (TGF-β) also increases the migration of glioma cells through processes that likely involve increased integrin expression and function76. TGF-β is predominantly released from microglia when studied in co-culture systems, such that blocking TGF-β signaling impairs glioma growth77. In addition, TGF-β2 induces the expression of matrix metalloprotease-2 (MMP2) and suppresses the expression of tissue inhibitor of metalloproteinases (TIMP)-2, which degrades the extracellular matrix to promote glioma invasion76. Although antagonizing TGF-β function was initially considered as a potential anti-tumor therapy, it has severe side effects, as systemic inhibition or lack of TGF-β signaling results in acute inflammation and disruption of immune system homeostasis77.
MMP2 enzyme is released in a pro-form that needs to be cleaved to become active. The prominent enzyme for pro-MMP2 cleavage is the membrane-bound metalloprotease MT1-MMP. Under normal conditions, microglia do not elaborate MT1-MMP (membrane type 1–matrix metalloproteinase), but, when exposed to glioma cells, they do upregulate MT1-MMP expression. Microglial MT1-MMP expression then increases glioma growth in organotypic slices. In this regard, slices obtained from MT1-MMP–deficient mice harbor substantially smaller tumors. Moreover, when microglia are depleted from MT1-MMP– deficient organotypic slices, glioma growth is further reduced, indicating that MT1-MMP is not the only glioma-promoting factor expressed by microglia. In human glioma samples, MT1-MMP expression positively correlates with the increasing glioma malignancy grade50.
The involvement of the TLR signaling cascade in glioma-microglia interactions was initially inferred by the observation that deletion of MyD88, an adaptor protein of most Toll-like receptors, inhibits MT1-MMP induction in microglia. Toll-like receptors are prominent detectors of DNA fragments or bacterial cell wall components, and are important for mediating immunologic responses to pathogens78. In microglia, TLR2 was identified as the major TLR involved in triggering MT1-MMP upregulation. In this manner, gliomas implanted into Tlr2-deficient mice are substantially smaller, and the survival of these mice is prolonged. TLR2 forms heterodimers with TLR1 and TLR6, which is important for modulating MT1-MMP expression; deletion of both TLR1 and TLR6 results in reduced MT1-MMP expression. In addition, treatment with TLR2-neutralizing antibodies reduces glioma-induced microglial MT1-MMP expression and attenuates glioma growth79.
In a screen for endogenous ligands released from glioma cells, versican was identified as a candidate molecule for triggering TLR2 signaling80. Versican exists as different splice variants, V0, V1 and V2. The V0 and V1 isoforms are highly expressed in mouse and human gliomas, and reduced glioma versican expression is associated with decreased microglial MT1-MMP expression in vitro and in vivo. Furthermore, inoculation of versican-silenced gliomas results in smaller tumors and longer survival rates relative to controls. Notably, the effect of versican signaling on glioma growth depends on the presence of microglia. The ability of glioma-produced versican to trigger increased TLR2 expression converts microglia into a pro-tumorigenic phenotype characterized by the upregulation of MT1-MMP and MMP9 expression. This feed-forward loop establishes an interdependent circuit of cellular interactions that increases glioma growth and invasion81.
TAMs not only target glioma cells, but also affect angiogenesis to indirectly affect tumor growth. Signaling through the receptor for advanced glycation end product (RAGE) is important for the process. RAGE ablation abrogates angiogenesis, which can be reconstituted with wild-type microglia or macrophages. This TAM activity correlates with the expression of VEGF, an important pro-angiogenic factor82 (Fig. 5).
Figure 5.
TAM glioma crosstalk. (a) TAMs release several factors that promote glioma cell invasion. (b) Microglia release TGF-β, which triggers the release of pro-MMP2 from glioma cells. Pro-MMP2 is then cleaved into active MMP2 by microglia-expressed MT1-MMP. Microglial MT1-MMP expression is stimulated by versican, which is released from glioma cells. Versican activates TLR2 and p38- MAP-kinase signaling in microglial cells, which leads to MT1-MMP upregulation. TLR2 signaling in microglia also triggers MMP9 release. Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 2015. All rights reserved.
The effects of microglia and macrophages on glioma stem cells
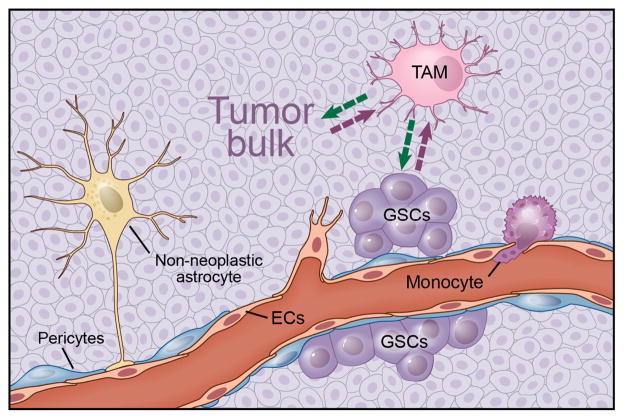
Glioblastomas contain a subpopulation of cells with stem cell–like properties (self-renewal, multi-lineage differentiation) capable of reconstituting the native tumor following implantation into naive hosts. These glioma stem cells (GSCs) reside in the perivascular niche, where they can be highly resistant to radiation and chemotherapy83–85 (Fig. 6). The importance of GSCs to microglia attraction is also underscored by a positive correlation between the density of GSCs and TAMs, indicating that GSCs may recruit TAMs more efficiently than their more differentiated neoplastic counterparts86. A recent study showed that GSCs release periostin, which accumulates in the perivascular niche. Periostin acts as a chemoattractant for TAMs, which is mediated by signaling through the integrin receptor αvβ3 (ref. 24). TAMs also influence the properties of GSCs, in that TGF-β released from TAMs induce MMP-9 expression and increase GSC invasiveness52. In addition, naive microglia can reduce the sphere-forming ability of human stem cells to suppress glioma growth, whereas microglia or monocytes cultured from glioma patients lack this anti-tumorigenic potential87. Supernatants from glioma stem cells likewise inhibit the phagocytosis activity of TAMs and induce the secretion of interleukin-10 and TGF-β88.
Figure 6.
Illustration of the complexity and cellular composition of glioma. Gliomas consist of neoplastic tumor cells and non-neoplastic cells from microenvironment, including endothelial cells, pericytes, infiltrating monocytes, activated astrocytes and TAMs. TAMs are recruited to the tumor by tumor bulk and GSCs. These recruited and reprogrammed TAMs secrete soluble factors that both expand the tumor bulk and GSCs as well. Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 2015. All rights reserved.
Microglia and macrophages as targets for glioma therapy
For several decades, our understanding of glioma biology has largely been driven by studies focused on the genetic and molecular changes that occur in the cancer cells and their contributions to deregulated cell growth. Over the past several years, work from numerous laboratories, including our own groups, has revealed that glioma growth is dependent on growth regulatory signals that emanate from the tumor microenvironment. In this regard, it is important to recognize that brain tumors are complex microcosms in which the communication between neoplastic and non-neoplastic cells will influence not only gliomagenesis89, but may also modify glioma responses to standard therapy. The identification of these glioma microenvironment-derived signals represents an initial step toward developing stroma-directed glioma therapies, with the ultimate goal of combining these therapies with anti-neoplastic cell–targeted therapies.
In this regard, the induction of HIF-1 following glioma radiation results in the recruitment of bone marrow–derived myeloid cells, partially due to the activation of stromal cell–derived factor-1 (SDF-1) and its receptor, CXCR4. As such, activation of SDF-1 and CXCR4 promotes vasculogenesis and tumor recurrence. These findings support the notion that better outcomes for glioblastoma might be achieved using a combination of radiotherapy and the clinically approved small molecule inhibitor of CXCR4 signaling, AMD3100 (ref. 90). These observations are further supported by a different glioma model, which showed that tumor-secreted SDF-1 is one important factor in radiotherapy-induced tumor invasiveness, where it exerts its primary effect through macrophage mobilization and tumor revascularization91. Similar observations were made when human recurrent glioblastomas were treated with anti-angiogenic therapy. The increased TAM number correlated with poor survival, suggesting that TAMs may participate in the escape from anti-angiogenic therapy, and therefore represent a potential biomarker of resistance as well as a logical therapeutic target for recurrent glioblastoma treatment92. In support of these human correlative data, murine glioma studies revealed that glioblastoma resistance to anti-VEGF therapy is associated with increased myeloid cell infiltration93.
Interfering with CSF-1 signaling is another potential approach to targeting TAM regulation of glioma growth. One study used an inhibitor of the CSF-1 receptor in a mouse proneural glioblastoma model to increase survival and shrink established tumors56. Periostin has also emerged as an interesting target for attenuating the tumor-supportive phenotype of TAMs by interrupting integrin αvβ3 signaling24. Interfering with this pathway with a blocking peptide impairs TAM recruitment. Moreover, it may be possible to exploit the interaction of TAMs with glioma initiating cells. Using this strategy, in a drug screen, Amphotericin B was identified as a molecule that enhanced the microglial effect on brain tumor initiating cell (BTIC) cycle growth arrest and differentiation87, whereas Stat3 inhibition has been shown to activate TAMs and inhibit glioma growth in mice94.
Minocycline, an antibiotic, interferes with the process of microglia activation. A rat model of glioma revealed synergistic activity when systemic BCNU (chemotherapy) treatment was combined with the local delivery of minocycline to impair microglia activation95. Currently, investigators at the University of Utah are recruiting patients for a phase I clinical trial using minocycline as adjuvant therapy (clinicaltrials.gov, #NCT02272270, https://clinicaltrials.gov/ct2/show/NCT02272270?term=NCT02272270&rank=1). In addition, immunotherapy using activated natural killer (NK) cells combined with the antibody mAb9.2.27 directed against the proteoglycan Neuroglial-2 (NG2) has shown beneficial effects, which are partly a result of a reversal of the tumor-promoting effects of TAMs96. Collectively, these studies suggest that TAMs modify the glioma response to standard and anti-angiogenic therapy.
Conclusions
It is now evident that TAMs home to the evolving glioma and interact in a complex fashion with the tumor environment to promote glioma growth in mouse models and in human patients (Fig. 6). However, there are still many unanswered questions. It is not clear what factors are truly responsible for mediating the interaction between glioma cells and microglia and macrophages. In this respect, we do not know how microglia and macrophages interact in the tumor, and whether they acquire distinct properties and execute distinct functions. It remains also an open question as to whether TAMs acquire different functional phenotypes depending on individual glioma types (low-grade versus high-grade, glioblastoma molecular subtypes). Similarly, even in a given tumor, TAMs might interact differently with different neoplastic cell types (GSCs, differentiated astrocytoma cells). Nonetheless, after decades of applying treatments directed against the tumor cells directly, TAMs have emerged as exciting targets for therapeutic intervention. Further investigation into the mechanisms and interactions between TAM populations and the variety of neoplastic and non-neoplastic cells in these tumors may one day yield new glioma treatment strategies.
Acknowledgments
The authors would like to thank D. Schumick for his great work with illustrations. This work was supported by the Deutsche Forschungsgemeinschaft (TR 43, KE 329/30-1; H.K.) and Neurocure (H.K.) as well as funding from the Department of Defense (W81XWH-13-1-0094, D.H.G.) and James S. McDonnell Foundation (D.H.G.) and a collaborative U01 grant from the National Cancer Institute (U01-CA160882; D.H., D.H.G. and H.K.).
Footnotes
AUTHOR CONTRIBUTIONS
D.H., D.H.G. and H.K. wrote and edited the manuscript.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
References
- 1.Gutmann DH, et al. Somatic neurofibromatosis type 1 (NF1) inactivation characterizes NF1-associated pilocytic astrocytoma. Genome Res. 2013;23:431–439. doi: 10.1101/gr.142604.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simmons GW, et al. Neurofibromatosis-1 heterozygosity increases microglia in a spatially and temporally restricted pattern relevant to mouse optic glioma formation and growth. J Neuropathol Exp Neurol. 2011;70:51–62. doi: 10.1097/NEN.0b013e3182032d37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morantz RA, Wood GW, Foster M, Clark M, Gollahon K. Macrophages in experimental and human brain tumors. Part 2: studies of the macrophage content of human brain tumors. J Neurosurg. 1979;50:305–311. doi: 10.3171/jns.1979.50.3.0305. [DOI] [PubMed] [Google Scholar]
- 4.Rossi ML, Hughes JT, Esiri MM, Coakham HB, Brownell DB. Immunohistological study of mononuclear cell infiltrate in malignant gliomas. Acta Neuropathol. 1987;74:269–277. doi: 10.1007/BF00688191. [DOI] [PubMed] [Google Scholar]
- 5.Hortega PDR. El tercer elemento de los centros nerviosos. Bol Soc Esp d Biol. 1919;9:69–120. [Google Scholar]
- 6.Ling EA, Wong WC. The origin and nature of ramified and amoeboid microglia: a historical review and current concepts. Glia. 1993;7:9–18. doi: 10.1002/glia.440070105. [DOI] [PubMed] [Google Scholar]
- 7.Biffi A, et al. Correction of metachromatic leukodystrophy in the mouse model by transplantation of genetically modified hematopoietic stem cells. J Clin Invest. 2004;113:1118–1129. doi: 10.1172/JCI19205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simard AR, Rivest S. Bone marrow stem cells have the ability to populate the entire central nervous system into fully differentiated parenchymal microglia. FASEB J. 2004;18:998–1000. doi: 10.1096/fj.04-1517fje. [DOI] [PubMed] [Google Scholar]
- 9.Priller J, et al. Targeting gene-modified hematopoietic cells to the central nervous system: use of green fluorescent protein uncovers microglial engraftment. Nat Med. 2001;7:1356–1361. doi: 10.1038/nm1201-1356. [DOI] [PubMed] [Google Scholar]
- 10.Flügel A, Bradl M, Kreutzberg GW, Graeber MB. Transformation of donor-derived bone marrow precursors into host microglia during autoimmune CNS inflammation and during the retrograde response to axotomy. J Neurosci Res. 2001;66:74–82. doi: 10.1002/jnr.1198. [DOI] [PubMed] [Google Scholar]
- 11.Hickey WF, Kimura H. Perivascular microglial cells of the CNS are bone marrow–derived and present antigen in vivo. Science. 1988;239:290–292. doi: 10.1126/science.3276004. [DOI] [PubMed] [Google Scholar]
- 12.Massengale M, Wagers AJ, Vogel H, Weissman IL. Hematopoietic cells maintain hematopoietic fates upon entering the brain. J Exp Med. 2005;201:1579–1589. doi: 10.1084/jem.20050030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ajami B, Bennett JL, Krieger C, Tetzlaff W, Rossi FM. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat Neurosci. 2007;10:1538–1543. doi: 10.1038/nn2014. [DOI] [PubMed] [Google Scholar]
- 14.Ajami B, Bennett JL, Krieger C, McNagny KM, Rossi FM. Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nat Neurosci. 2011;14:1142–1149. doi: 10.1038/nn.2887. [DOI] [PubMed] [Google Scholar]
- 15.Ginhoux F, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kierdorf K, et al. Microglia emerge from erythromyeloid precursors via Pu.1- and Irf8-dependent pathways. Nat Neurosci. 2013;16:273–280. doi: 10.1038/nn.3318. [DOI] [PubMed] [Google Scholar]
- 17.Elmore MR, et al. Colony-stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain. Neuron. 2014;82:380–397. doi: 10.1016/j.neuron.2014.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol. 2011;11:762–774. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 20.Yona S, et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013;38:79–91. doi: 10.1016/j.immuni.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Badie B, Schartner JM. Flow cytometric characterization of tumor-associated macrophages in experimental gliomas. Neurosurgery. 2000;46:957–961. doi: 10.1097/00006123-200004000-00035. discussion 961–962. [DOI] [PubMed] [Google Scholar]
- 22.Parney IF, Waldron JS, Parsa AT. Flow cytometry and in vitro analysis of human glioma-associated macrophages. Laboratory investigation. J Neurosurg. 2009;110:572–582. doi: 10.3171/2008.7.JNS08475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muller A, Brandenburg S, Turkowski K, Muller S, Vajkoczy P. Resident microglia, and not peripheral macrophages, are the main source of brain tumor mononuclear cells. Int J Cancer. 2014;137:278–288. doi: 10.1002/ijc.29379. [DOI] [PubMed] [Google Scholar]
- 24.Zhou W, et al. Periostin secreted by glioblastoma stem cells recruits M2 tumour-associated macrophages and promotes malignant growth. Nat Cell Biol. 2015;17:170–182. doi: 10.1038/ncb3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feng X, et al. Loss of CX3CR1 increases accumulation of inflammatory monocytes and promotes gliomagenesis. Oncotarget. 2015;6:15077–15094. doi: 10.18632/oncotarget.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang I, Han SJ, Sughrue ME, Tihan T, Parsa AT. Immune cell infiltrate differences in pilocytic astrocytoma and glioblastoma: evidence of distinct immunological microenvironments that reflect tumor biology. J Neurosurg. 2011;115:505–511. doi: 10.3171/2011.4.JNS101172. [DOI] [PubMed] [Google Scholar]
- 27.Klein R, Roggendorf W. Increased microglia proliferation separates pilocytic astrocytomas from diffuse astrocytomas: a double labeling study. Acta Neuropathol. 2001;101:245–248. doi: 10.1007/s004010000286. [DOI] [PubMed] [Google Scholar]
- 28.Komohara Y, Ohnishi K, Kuratsu J, Takeya M. Possible involvement of the M2 anti-inflammatory macrophage phenotype in growth of human gliomas. J Pathol. 2008;216:15–24. doi: 10.1002/path.2370. [DOI] [PubMed] [Google Scholar]
- 29.Dorward IG, et al. Postoperative imaging surveillance in pediatric pilocytic astrocytomas. J Neurosurg Pediatr. 2010;6:346–352. doi: 10.3171/2010.7.PEDS10129. [DOI] [PubMed] [Google Scholar]
- 30.Bajenaru ML, et al. Optic nerve glioma in mice requires astrocyte Nf1 gene inactivation and Nf1 brain heterozygosity. Cancer Res. 2003;63:8573–8577. [PubMed] [Google Scholar]
- 31.Listernick R, Charrow J, Greenwald MJ, Esterly NB. Optic gliomas in children with neurofibromatosis type 1. J Pediatr. 1989;114:788–792. doi: 10.1016/s0022-3476(89)80137-4. [DOI] [PubMed] [Google Scholar]
- 32.Kim KY, Ju WK, Hegedus B, Gutmann DH, Ellisman MH. Ultrastructural characterization of the optic pathway in a mouse model of neurofibromatosis-1 optic glioma. Neuroscience. 2010;170:178–188. doi: 10.1016/j.neuroscience.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pong WW, Higer SB, Gianino SM, Emnett RJ, Gutmann DH. Reduced microglial CX3CR1 expression delays neurofibromatosis-1 glioma formation. Ann Neurol. 2013;73:303–308. doi: 10.1002/ana.23813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bajenaru ML, Garbow JR, Perry A, Hernandez MR, Gutmann DH. Natural history of neurofibromatosis 1-associated optic nerve glioma in mice. Ann Neurol. 2005;57:119–127. doi: 10.1002/ana.20337. [DOI] [PubMed] [Google Scholar]
- 35.Daginakatte GC, Gutmann DH. Neurofibromatosis-1 (Nf1) heterozygous brain microglia elaborate paracrine factors that promote Nf1-deficient astrocyte and glioma growth. Hum Mol Genet. 2007;16:1098–1112. doi: 10.1093/hmg/ddm059. [DOI] [PubMed] [Google Scholar]
- 36.Daginakatte GC, Gianino SM, Zhao NW, Parsadanian AS, Gutmann DH. Increased c-Jun-NH2-kinase signaling in neurofibromatosis-1 heterozygous microglia drives microglia activation and promotes optic glioma proliferation. Cancer Res. 2008;68:10358–10366. doi: 10.1158/0008-5472.CAN-08-2506. [DOI] [PubMed] [Google Scholar]
- 37.Prada CE, et al. Neurofibroma-associated macrophages play roles in tumor growth and response to pharmacological inhibition. Acta Neuropathol. 2013;125:159–168. doi: 10.1007/s00401-012-1056-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang FC, et al. Nf1-dependent tumors require a microenvironment containing Nf1+/– and c-kit–dependent bone marrow. Cell. 2008;135:437–448. doi: 10.1016/j.cell.2008.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Warrington NM, et al. Spatiotemporal differences in CXCL12 expression and cyclic AMP underlie the unique pattern of optic glioma growth in neurofibromatosis type 1. Cancer Res. 2007;67:8588–8595. doi: 10.1158/0008-5472.CAN-06-2220. [DOI] [PubMed] [Google Scholar]
- 40.Warrington NM, et al. Cyclic AMP suppression is sufficient to induce gliomagenesis in a mouse model of neurofibromatosis-1. Cancer Res. 2010;70:5717–5727. doi: 10.1158/0008-5472.CAN-09-3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Solga AC, et al. RNA-sequencing reveals oligodendrocyte and neuronal transcripts in microglia relevant to central nervous system disease. Glia. 2014;63:531–548. doi: 10.1002/glia.22754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Solga AC, et al. RNA-sequencing of tumor-associated microglia reveals Ccl5 as a stromal chemokine critical for neurofibromatosis-1 glioma growth. Neoplasia. 2015;17:777–788. doi: 10.1016/j.neo.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 44.Mantovani A, et al. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 45.Kennedy BC, et al. Tumor-associated macrophages in glioma: friend or foe? J Oncol. 2013;2013:486912. doi: 10.1155/2013/486912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gabrusiewicz K, et al. Characteristics of the alternative phenotype of microglia/macrophages and its modulation in experimental gliomas. PLoS One. 2011;6:e23902. doi: 10.1371/journal.pone.0023902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kees T, et al. Microglia isolated from patients with glioma gain antitumor activities on poly (I:C) stimulation. Neurooncol. 2012;14:64–78. doi: 10.1093/neuonc/nor182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Umemura N, et al. Tumor-infiltrating myeloid-derived suppressor cells are pleiotropic-inflamed monocytes/macrophages that bear M1- and M2-type characteristics. J Leukoc Biol. 2008;83:1136–1144. doi: 10.1189/jlb.0907611. [DOI] [PubMed] [Google Scholar]
- 49.Li W, Graeber MB. The molecular profile of microglia under the influence of glioma. Neurooncol. 2012;14:958–978. doi: 10.1093/neuonc/nos116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Markovic DS, et al. Gliomas induce and exploit microglial MT1-MMP expression for tumor expansion. Proc Natl Acad Sci USA. 2009;106:12530–12535. doi: 10.1073/pnas.0804273106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sica A, Schioppa T, Mantovani A, Allavena P. Tumour-associated macrophages are a distinct M2 polarized population promoting tumour progression: potential targets of anti-cancer therapy. Eur J Cancer. 2006;42:717–727. doi: 10.1016/j.ejca.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 52.Ye XZ, et al. Tumor-associated microglia/macrophages enhance the invasion of glioma stem-like cells via TGF-β1 signaling pathway. J Immunol. 2012;189:444–453. doi: 10.4049/jimmunol.1103248. [DOI] [PubMed] [Google Scholar]
- 53.Szulzewsky F, et al. Glioma-associated microglia/macrophages display an expression profile different from M1 and M2 polarization and highly express Gpnmb and Spp1. PLoS One. 2015;10:e0116644. doi: 10.1371/journal.pone.0116644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zeiner PS, et al. MIF receptor CD74 is restricted to microglia/macrophages, associated with a M1-polarized immune milieu and prolonged patient survival in gliomas. Brain Pathol. 2014;25:491–504. doi: 10.1111/bpa.12194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pong WW, et al. F11R is a novel monocyte prognostic biomarker for malignant glioma. PLoS One. 2013;8:e77571. doi: 10.1371/journal.pone.0077571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pyonteck SM, et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat Med. 2013;19:1264–1272. doi: 10.1038/nm.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lisi L, Laudati E, Navarra P, Dello Russo C. The mTOR kinase inhibitors polarize glioma-activated microglia to express a M1 phenotype. J Neuroinflammation. 2014;11:125. doi: 10.1186/1742-2094-11-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Qin T, et al. Dopamine induces growth inhibition and vascular normalization through reprogramming M2-polarized macrophages in rat C6 glioma. Toxicol Appl Pharmacol. 2015;286:112–123. doi: 10.1016/j.taap.2015.03.021. [DOI] [PubMed] [Google Scholar]
- 59.Xu S, et al. Effect of miR-142-3p on the M2 macrophage and therapeutic efficacy against murine glioblastoma. J Natl Cancer Inst. 2014;106:dju162. doi: 10.1093/jnci/dju162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Platten M, et al. Monocyte chemoattractant protein-1 increases microglial infiltration and aggressiveness of gliomas. Ann Neurol. 2003;54:388–392. doi: 10.1002/ana.10679. [DOI] [PubMed] [Google Scholar]
- 61.Okada M, et al. Tumor-associated macrophage/microglia infiltration in human gliomas is correlated with MCP-3, but not MCP-1. Int J Oncol. 2009;34:1621–1627. doi: 10.3892/ijo_00000292. [DOI] [PubMed] [Google Scholar]
- 62.Badie B, Schartner J, Klaver J, Vorpahl J. In vitro modulation of microglia motility by glioma cells is mediated by hepatocyte growth factor/scatter factor. Neurosurgery. 1999;44:1077–1082. doi: 10.1097/00006123-199905000-00075. discussion 1082–1083. [DOI] [PubMed] [Google Scholar]
- 63.Wang SC, Hong JH, Hsueh C, Chiang CS. Tumor-secreted SDF-1 promotes glioma invasiveness and TAM tropism toward hypoxia in a murine astrocytoma model. Lab Invest. 2012;92:151–162. doi: 10.1038/labinvest.2011.128. [DOI] [PubMed] [Google Scholar]
- 64.Paolicelli RC, Bisht K, Tremblay ME. Fractalkine regulation of microglial physiology and consequences on the brain and behavior. Front Cell Neurosci. 2014;8:129. doi: 10.3389/fncel.2014.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu C, Luo D, Streit WJ, Harrison JK. CX3CL1 and CX3CR1 in the GL261 murine model of glioma: CX3CR1 deficiency does not impact tumor growth or infiltration of microglia and lymphocytes. J Neuroimmunol. 2008;198:98–105. doi: 10.1016/j.jneuroim.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rodero M, et al. Polymorphism in the microglial cell-mobilizing CX3CR1 gene is associated with survival in patients with glioblastoma. J Clin Oncol. 2008;26:5957–5964. doi: 10.1200/JCO.2008.17.2833. [DOI] [PubMed] [Google Scholar]
- 67.Ku MC, et al. GDNF mediates glioblastoma-induced microglia attraction but not astrogliosis. Acta Neuropathol. 2013;125:609–620. doi: 10.1007/s00401-013-1079-8. [DOI] [PubMed] [Google Scholar]
- 68.Coniglio SJ, et al. Microglial stimulation of glioblastoma invasion involves epidermal growth factor receptor (EGFR) and colony stimulating factor 1 receptor (CSF-1R) signaling. Mol Med. 2012;18:519–527. doi: 10.2119/molmed.2011.00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sielska M, et al. Distinct roles of CSF family cytokines in macrophage infiltration and activation in glioma progression and injury response. J Pathol. 2013;230:310–321. doi: 10.1002/path.4192. [DOI] [PubMed] [Google Scholar]
- 70.Bettinger I, Thanos S, Paulus W. Microglia promote glioma migration. Acta Neuropathol. 2002;103:351–355. doi: 10.1007/s00401-001-0472-x. [DOI] [PubMed] [Google Scholar]
- 71.Markovic DS, Glass R, Synowitz M, Rooijen Nv, Kettenmann H. Microglia stimulate the invasiveness of glioma cells by increasing the activity of metalloprotease-2. J Neuropathol Exp Neurol. 2005;64:754–762. doi: 10.1097/01.jnen.0000178445.33972.a9. [DOI] [PubMed] [Google Scholar]
- 72.Carvalho da Fonseca AC, et al. Increased expression of stress inducible protein 1 in glioma-associated microglia/macrophages. J Neuroimmunol. 2014;274:71–77. doi: 10.1016/j.jneuroim.2014.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang J, et al. A dialog between glioma and microglia that promotes tumor invasiveness through the CCL2/CCR2/interleukin-6 axis. Carcinogenesis. 2012;33:312–319. doi: 10.1093/carcin/bgr289. [DOI] [PubMed] [Google Scholar]
- 74.Saederup N, et al. Selective chemokine receptor usage by central nervous system myeloid cells in CCR2-red fluorescent protein knock-in mice. PLoS One. 2010;5:e13693. doi: 10.1371/journal.pone.0013693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mizutani M, et al. The fractalkine receptor but not CCR2 is present on microglia from embryonic development throughout adulthood. J Immunol. 2012;188:29–36. doi: 10.4049/jimmunol.1100421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wick W, Platten M, Weller M. Glioma cell invasion: regulation of metalloproteinase activity by TGF-beta. J Neurooncol. 2001;53:177–185. doi: 10.1023/a:1012209518843. [DOI] [PubMed] [Google Scholar]
- 77.Wesolowska A, et al. Microglia-derived TGF-beta as an important regulator of glioblastoma invasion—an inhibition of TGF-beta-dependent effects by shRNA against human TGF-beta type II receptor. Oncogene. 2008;27:918–930. doi: 10.1038/sj.onc.1210683. [DOI] [PubMed] [Google Scholar]
- 78.Lehnardt S. Innate immunity and neuroinflammation in the CNS: the role of microglia in Toll-like receptor–mediated neuronal injury. Glia. 2010;58:253–263. doi: 10.1002/glia.20928. [DOI] [PubMed] [Google Scholar]
- 79.Vinnakota K, et al. Toll-like receptor 2 mediates microglia/brain macrophage MT1-MMP expression and glioma expansion. Neurooncol. 2013;15:1457–1468. doi: 10.1093/neuonc/not115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hu F, et al. Glioma-derived versican promotes tumor expansion via glioma-associated microglial/macrophages Toll-like receptor 2 signaling. Neurooncol. 2015;17:200–210. doi: 10.1093/neuonc/nou324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hu F, et al. Glioma-associated microglial MMP9 expression is upregulated by TLR2 signaling and sensitive to minocycline. Int J Cancer. 2014;135:2569–2578. doi: 10.1002/ijc.28908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen X, et al. RAGE expression in tumor-associated macrophages promotes angiogenesis in glioma. Cancer Res. 2014;74:7285–7297. doi: 10.1158/0008-5472.CAN-14-1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bao S, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 84.Hambardzumyan D, Squatrito M, Holland EC. Radiation resistance and stem-like cells in brain tumors. Cancer Cell. 2006;10:454–456. doi: 10.1016/j.ccr.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 85.Bleau AM, et al. PTEN/PI3K/Akt pathway regulates the side population phenotype and ABCG2 activity in glioma tumor stem-like cells. Cell Stem Cell. 2009;4:226–235. doi: 10.1016/j.stem.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yi L, et al. Glioma-initiating cells: a predominant role in microglia/macrophages tropism to glioma. J Neuroimmunol. 2011;232:75–82. doi: 10.1016/j.jneuroim.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 87.Sarkar S, et al. Therapeutic activation of macrophages and microglia to suppress brain tumor-initiating cells. Nat Neurosci. 2014;17:46–55. doi: 10.1038/nn.3597. [DOI] [PubMed] [Google Scholar]
- 88.Wu A, et al. Glioma cancer stem cells induce immunosuppressive macrophages/microglia. Neurooncol. 2010;12:1113–1125. doi: 10.1093/neuonc/noq082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hoelzinger DB, Demuth T, Berens ME. Autocrine factors that sustain glioma invasion and paracrine biology in the brain microenvironment. J Natl Cancer Inst. 2007;99:1583–1593. doi: 10.1093/jnci/djm187. [DOI] [PubMed] [Google Scholar]
- 90.Kioi M, et al. Inhibition of vasculogenesis, but not angiogenesis, prevents the recurrence of glioblastoma after irradiation in mice. J Clin Invest. 2010;120:694–705. doi: 10.1172/JCI40283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang SC, Yu CF, Hong JH, Tsai CS, Chiang CS. Radiation therapy-induced tumor invasiveness is associated with SDF-1-regulated macrophage mobilization and vasculogenesis. PLoS One. 2013;8:e69182. doi: 10.1371/journal.pone.0069182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lu-Emerson C, et al. Increase in tumor-associated macrophages after antiangiogenic therapy is associated with poor survival among patients with recurrent glioblastoma. Neurooncol. 2013;15:1079–1087. doi: 10.1093/neuonc/not082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Piao Y, et al. Glioblastoma resistance to anti-VEGF therapy is associated with myeloid cell infiltration, stem cell accumulation, and a mesenchymal phenotype. Neurooncol. 2012;14:1379–1392. doi: 10.1093/neuonc/nos158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang L, et al. Stat3 inhibition activates tumor macrophages and abrogates glioma growth in mice. Glia. 2009;57:1458–1467. doi: 10.1002/glia.20863. [DOI] [PubMed] [Google Scholar]
- 95.Frazier JL, et al. Local delivery of minocycline and systemic BCNU have synergistic activity in the treatment of intracranial glioma. J Neurooncol. 2003;64:203–209. doi: 10.1023/a:1025695423097. [DOI] [PubMed] [Google Scholar]
- 96.Poli A, et al. Targeting glioblastoma with NK cells and mAb against NG2/CSPG4 prolongs animal survival. Oncotarget. 2013;4:1527–1546. doi: 10.18632/oncotarget.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]