Abstract
KLF4 and CD44 regulate cancer cell stemness, but their precise functions and roles in metastatic progression are not well understood. In this study, we used both inducible and genetic engineering approaches to assess whether the activities of these two factors intersect in pancreatic ductal adenocarcinoma (PDA). We found that genetic ablation of Klf4 in PDA cells isolated from Klf4flox/flox mice drastically increased CD44 expression and promoted the acquisition of stem-like properties, whereas tetracycline-inducible expression of KLF4 suppressed these properties in vitro and in vivo. Further mechanistic investigation revealed that KLF4 bound to the CD44 promoter to negatively regulate transcription and also the expression of the CD44 variant (CD44v). Moreover, in human PDA tissues, the expression patterns of KLF4 and CD44 were mutually exclusive, and this inverse relationship was particularly striking in human metastatic pancreatic tumors and in autochthonous mouse models of PDA. Taken together, our findings demonstrate that KLF4 acts as a tumor suppressor in PDA cells that restricts metastatic behaviors through direct negative regulation of CD44, providing support for the clinical investigation of therapeutic approaches focusing on targeted KLF4 activation in advanced tumors.
Keywords: KLF4, CD44, Cancer Stem Cell, Metastasis, Pancreatic Cancer
INTRODUCTION
The most devastating aspect of cancer is metastasis, which causes greater than 90% of cancer-associated mortality, particularly in patients with pancreatic cancer. PDA is the fourth leading cause of cancer-related death in the United State and has the worst prognosis among all malignant diseases due to its high propensity of early metastasis. Currently, metastasis is thought to originate from disseminated cancer stem cells (CSCs) (1,2), therapies that specifically target CSCs hold great promise for improving the survival and quality of life of cancer patients (3), especially those who suffer from metastatic pancreatic cancer. Advances in this field are dependent on our thorough understanding of the molecules and signaling pathways that regulate CSC properties.
CD44, the major hyaluronan (HA) receptor, is expressed as a wide variety of isoforms in many cells and has multifaceted functions in cell adhesion, migration, homing, proliferation and differentiation (4,5). Recently, CD44 was identified as an essential marker, individually or in combination with other marker(s), for enriching CSCs in various malignancies, including pancreatic cancer and it plays critical roles in the regulation of stemness and metastasis (5,6). Accordingly, a number of studies suggest that CD44 might be a promising marker for CSC-targeted therapy (2,7,8) however, progress has been impeded because little is known about the molecular mechanism by which CD44 expression is regulated in CSCs.
KLF4 transcription factor, a critical regulator of cell-fate decisions such as cell cycle progression, differentiation, and apoptosis, has important functions in cancer metastasis and CSCs(9,10). Mounting evidence from experimental and clinical studies indicates that KLF4 acts as a tumor suppressor (10,11), and targeted activation of KLF4 for therapeutic intervention of tumors has been approved for clinical trial (NCT01281592) (12). Acting as a critical negative regulator of epithelial-mesenchymal transition (EMT) (13,14), KLF4 has been shown to inhibit experimental metastasis (10,15). However, KLF4 had both inhibitory and promoting effects on breast cancer metastasis (16–18). As to KLF4’s functions in stem cells and CSCs, KLF4 was initially identified as one of the four Yamanaka factors (Oct3/4, Sox2, c-Myc, and KLF4) required for pluripotent stem cell reprogramming of somatic cells in both humans and mice (19,20), but later studies showed that KLF4 is dispensable for somatic stem cell reprogramming and the maintenance of self-renewal and pluripotency of ES cells (21–24). In both normal stem cells and CSCs, KLF4 was found to contribute to maintenance of telomerase activity through interaction with β-catenin to transactivate Tert expression (25,26), however, several other reports showed that KLF4 interacted with β-catenin and negatively regulated its function (27,28). More recently, a high level of KLF4 expression was reported to be essential for maintaining cancer stem cell populations in breast and DLD-1 colon cancer cells (18,29). In contrary to that report, restoration of KLF4 expression alone was sufficient to shut off tumor cell motility and eliminate the progenitor-like population of lung cancer cells (30,31). Although these findings are inconsistent, they all suggest an important role for KLF4 in cancer and highlight the need for further investigation of the causal role of KLF4 in CSCs and metastasis and the underlying molecular mechanisms. Additionally, although both KLF4 and CD44 are critical molecules involved in cancer stemness and metastasis, it is not known whether and, if so, how KLF4 and CD44 signaling pathways are integrated to regulate cancer stemness and metastasis, and ultimately, the clinical significance of these pathways.
In the present study, we used both inducible and genetically engineering approaches to modulate KFL4 expression and demonstrated for the first time that KLF4 is a negative regulator of CD44 and has stemness-suppressive functions in pancreatic cancer. Clinically, loss of KLF4 expression and increased CD44 expression predicted metastases in human pancreatic cancer. Our study establishes a strong rationale for developing therapeutic agents that target dysregulated KLF4-CD44 signaling to control CSCs and metastasis.
MATERIALS AND METHODS
Cell Lines and Culture Conditions
The human PDA cell lines BxPC-3, PANC-1, PANC-28, COLO357, and L3.7 and colon cancer cell line SW480 and SW620 cells, and 293T cells were directly purchased from American Type Culture Collection (ATCC, Manassas, VA) between years 2005–2015. The cell line characterization and authentication was performed using short tandem repeat profiling and the cells were passaged continuously for fewer than 6 months after receipt in our laboratory for relevant studies reported here.
For generation of paired Klf4 wild-type and knock-out mouse PDA cell lines, 723G and 924G cells, originally derived from pancreatic tumors induced by intra-pancreas injection of dimethylbenzanthracene into Klf4loxp/loxp mice(32), were infected with AdCre viruses, and the derived cells with Klf4 gene knockout were designated as 723K and 924K cells, respectively.
For generation of the CD44-knockdown stable cell line, L3.7 cells were infected with lenti-shCD44 or control viral particles (Santa Cruz Biotechnology, Dallas, Texas) and selected by puromycin treatment; the established cell lines were designated as L3.7shCD44 and L3.7sh-ctr, respectively.
For generation of the tetracycline-inducible KLF4 expression stable cell line, SW620 and L3.7 cells were infected with lenti-CMV-TetR viral particles (Gentarget, San Diego, CA) and selected by hygromycin treatment; next, the cells were infected with lenti-tet-O-hKLF4 viral particles (Gentarget) and selected by blasticidin treatment. The established stable cell lines were designated as RF4-SW620 and RF4-L3.7, respectively, and cultured in complete DMEM medium supplemented with 10% Tet System Approved fetal bovine serum (Clontech, Mountain View, CA). For induction of KLF4 expression, Dox was added to the cell culture medium at the concentration indicated.
Spheroid-Formation Assay
Human RF4-SW620 and RF4-L3.7 and mouse 723G/K and 924G/K cancer cells were cultured in tumorsphere culture medium of DMEM/Nutrient Mixture F-12 Ham medium containing 20ng/mL human recombinant epidermal growth factor, 10 ng/mL human recombinant basic fibroblast growth factor, and 2% N2 and 1% B27 supplements (Life Technologies) for 2~4 weeks (33). The typical primary spheres with >50 cells isolated from the culture spheres were digested in StemProAccutase (Life Technologies) to prepare a single-cell suspension for secondary spheroid colony formation assay in tumorsphere culture medium with or without addition of Dox. After 10 days to 2 weeks, each well was examined using a light microscope and total numbers of spheroid colonies were counted. In some experiments, the primary spheroid-forming cells in single-cell suspension were transfected with pCD44v plasmid DNA or CD44 siRNA oligos for secondary spheroid colony formation assay.
Genetically Engineered Mouse Models
The generation and use of mouse strains of Villin-Cre+;Klf4loxp/loxp and Villin-Cre+;Klf4+/+ were described previously (32). For genetically engineered mouse model of PDA progression, Pdx1-Cre mice (Stock Number: 014647, Jackson Laboratory) were across bred with LSL-KrasG12D/+ mice (Stock Number: 008180, Jackson Laboratory) to generate Pdx1-Cre;LSL-KrasG12D/+ mice (34). The mice were euthanized to harvest pancreas and liver tissues when they became old (>12 months) or very sick.
Statistical Analysis
All the in vitro and in vivo experiments, including Western blot and RT-PCR, were repeated 2 to 4 times or as indicated, and RT-PCR was routinely performed in triplicates. The data from independent experiments were shown as mean ± SEM or as indicated otherwise. Statistical significance was determined by Student’s t test (2-tailed), or analysis of variance (one-way or two-way) with Bonferroni posttest. For TMA analysis, associations between categorical variables were tested by the Pearson Chi-square test, Fisher’s exact tests, or Chi square for trend, when appropriate. All significance was defined at the p < 0.05 or p < 0.01 level.
SUPPLEMENTAL INFORMATION
Supplemental information for this article includes Supplemental Experimental Procedures, seven figures and four tables can be found with this article online.
RESULTS
Inducible Restoration of KLF4 Expression Suppressed the Stemness of Human Cancer Cells
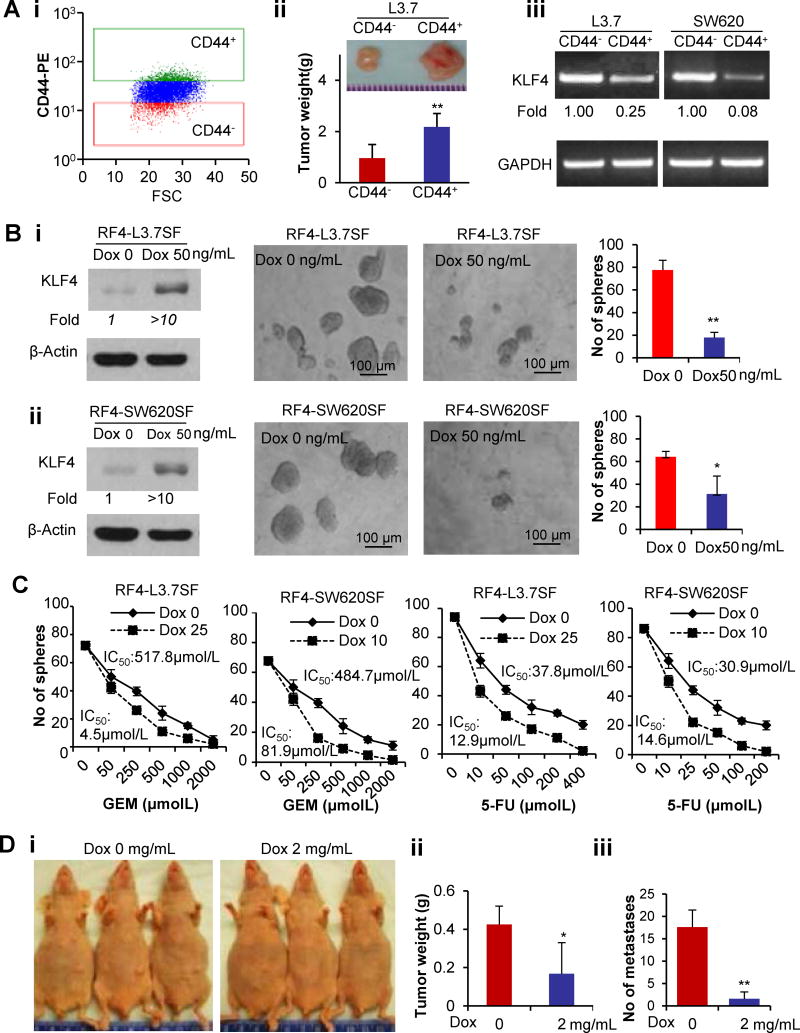
To investigate the expression of KLF4 in CSCs and its regulatory effect, we first isolated the 10% of cells with the highest (CD44+) or lowest (CD44−) CD44 expression, respectively, from L3.7, PANC-1, and PANC-28 pancreatic and SW620 colon cancer cells (Figure 1Ai). CD44+ L3.7 cells developed much larger tumors in nude mice than did CD44− L3.7 cells (Figure 1Aii). KLF4 expression was lower in CD44+ cells than in CD44− cancer cells (Figure 1Aiii; Figure S1A), suggesting that KLF4 may regulate the stemness of cancer cells.
Figure 1. Inducible KLF4 overexpression suppresses stem-like properties of human cancer cells.
(A) Top 10% of CD44-positive or -negative subpopulations of L3.7 PDA cells isolated by flow cytometry sorting (Ai) were subcutaneously injected into nude mice (50,000 cells/mouse; n=5 mice/group) for tumorigenesis analysis. The representative tumor size and the average tumor weight in each group are shown (Aii). RT-PCR analysis of KLF4 expression in CD44-positive or -negative subpopulations of L3.7 and SW620 cells (Aiii).
(B) Addition of doxycycline (Dox) into tumorsphere culture containing single-cell suspension of primary spheroid-forming RF4-L3.7 cells (Bi) or RF4-SW620 cells (Bii) on KLF4 expression (left panel, WB assay) and their secondary spheroid formation, the representative images of spheroids formed and the quantitative data are shown, respectively.
(C) Addition of a low dose of Dox into the single-cell suspension of tumorsphere culture drastically sensitized the primary spheroid-forming RF4-SW620 and RF4-L3.7 cells to 5-fluorouracil (5-FU) and gemcitabine (GEM) treatment, as determined by spheroid formation assay. Sensitivity is expressed as the 50% inhibitory concentrations (IC50) of 5-FU and GEM.
(D) Administration of Dox in drinking water inhibited tumorigenesis and liver metastasis after subcutaneous or ileocolic vein injection of a single-cell suspension of primary RF4-L3.7SF cells into mice (n=8/group). Representative images of tumor growth in mice (Di) and average tumor weight (Dii) and number of liver metastases (Diii) are shown. (*P<0.05; **P<0.01).
To provide causal evidence of KLF4 regulation of cancer stem-like properties, we established the tetracycline-inducible KLF4 expression cell lines RF4-L3.7 and RF4-SW620 for spheroid formation experiments. After 3–4 weeks of culture, primary spheroids were subjected to doxycycline (Dox, 50ng/ml) treatment. KLF4 was significantly induced in those primary spheroid-forming (SF) cells and their secondary spheroid formation abilities were drastically reduced (Figure 1B; Figure S1B). Additionally, the typical spheres were broken and ghost-like cells can be seen when the primary spheroids were exposed to a higher dose of Dox (Figure S1C), whereas the same dose did not have any effect on parental L3.7 cells (Figure S1D). The addition of a very low dose of Dox significantly increased the sensitivity of RF4-L3.7SF and RF4-SW620SF cells to gemcitabine (GEM) and 5-fluorouracil (5-FU) treatment in spheroid colony formation experiments (Figure 1C), suggesting an effect of KLF4 overexpression on the chemoresistance of RF4-SW620SF and RF4-L3.7SF cells.
To determine whether KLF4 affects CSC properties in vivo, we performed tumorigenicity and metastasis analysis using mice injected with single cell suspension of RF4-L3.7SF cells. Our results showed that administration of Dox into the RF4-L3.7SF tumor-bearing mice significantly inhibited tumorigenesis and metastasis (Figure 1D; Figure S1E). These results indicate that KLF4 overexpression inhibits the stem-like properties of cancer cells in vitro and in vivo.
Genetic Ablation Uncovers the Stemness-Inhibitory Function of KLF4 in a Mouse Model of Cancer
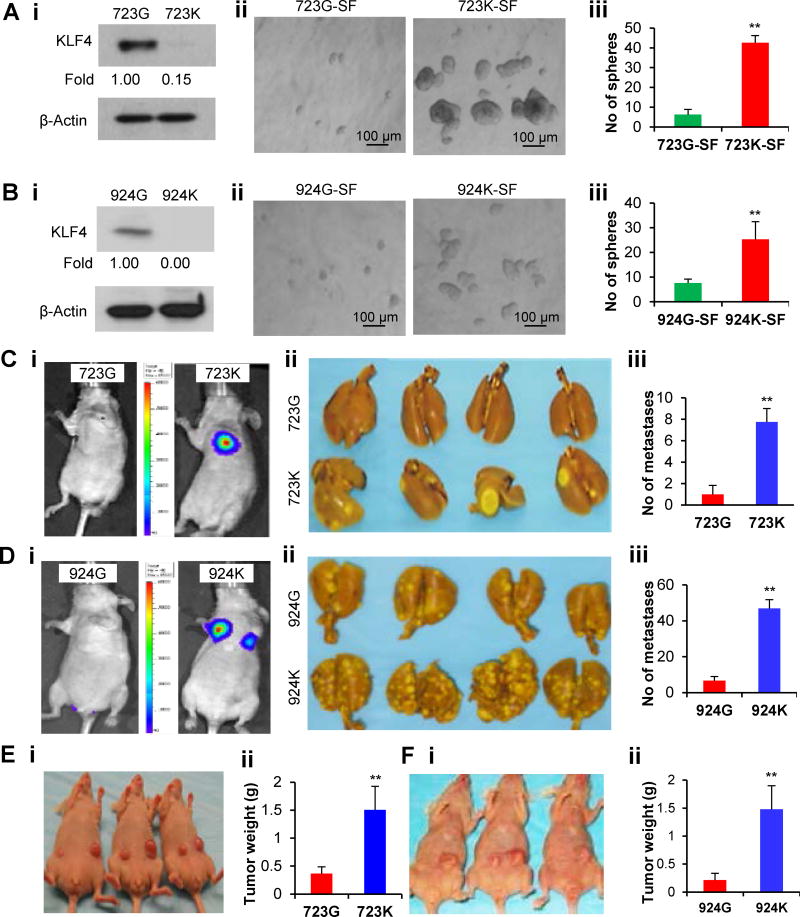
To provide definitive evidence of KLF4 regulation of cancer stem-like properties, we generated two parental PDA cell lines (723G and 924G) derived from Klf4loxp/loxp mice and then genetically engineered two corresponding cell lines with Klf4 gene knockout (723K and 924K) (Figure 2Ai & Bi; Figure S2). As shown in Figure 2 Aii–iii & 2Bii–iii, the cells with Klf4 gene ablation had greater primary SF abilities in terms of spheroid numbers and sizes than did their parental cells. Consistently, ablation of the Klf4 gene also drastically increased the metastatic and tumorigenic abilities of the cancer cells in mouse models (Figure 2C–F). Thus, our data clearly established that KLF4 deficiency increases tumorigenicity and metastasis, possibly because of a promoted stemness of the mouse cancer cells.
Figure 2. Genetic ablation uncovers the stemness-suppressive function of Klf4 in mouse model.
(A–B) WB analysis of KLF4 expression in parental 723G/924G (Klf4loxp/loxp) and derived 723K/924K (Klf4−/−) cells (Ai & Bi), and their spheroid-forming abilities were determined in tumorsphere culture (Aii–iii & Bii–iii).
(C–D) Paired 723G/723K and 924G/924K cells carrying the luciferase reporter gene were intravenously injected into mice through the tail vein (n=8/group), and lung tumor metastasis was monitored by in vivo bioluminescence imaging (representative photos, Ci & Di), and the metastatic nodules visible on the surface of the lungs were accounted at the end of experiment (representative images, Cii & Dii; quantitative data, Ciii & Diii).
(E–F) 723G and 723K cells were subcutaneously injected into the abdominal flanks of mice (n=5/group), respectively, and representative images of tumor growth (Ei) and the average tumor weight measured (Eii) at the end of experiment are showed. Similar results were obtained using the paired 924G/924K cells (Fi & Fii). (*P<0.05; **P<0.01).
CD44 Was Identified as an Important Downstream Target Gene of KLF4
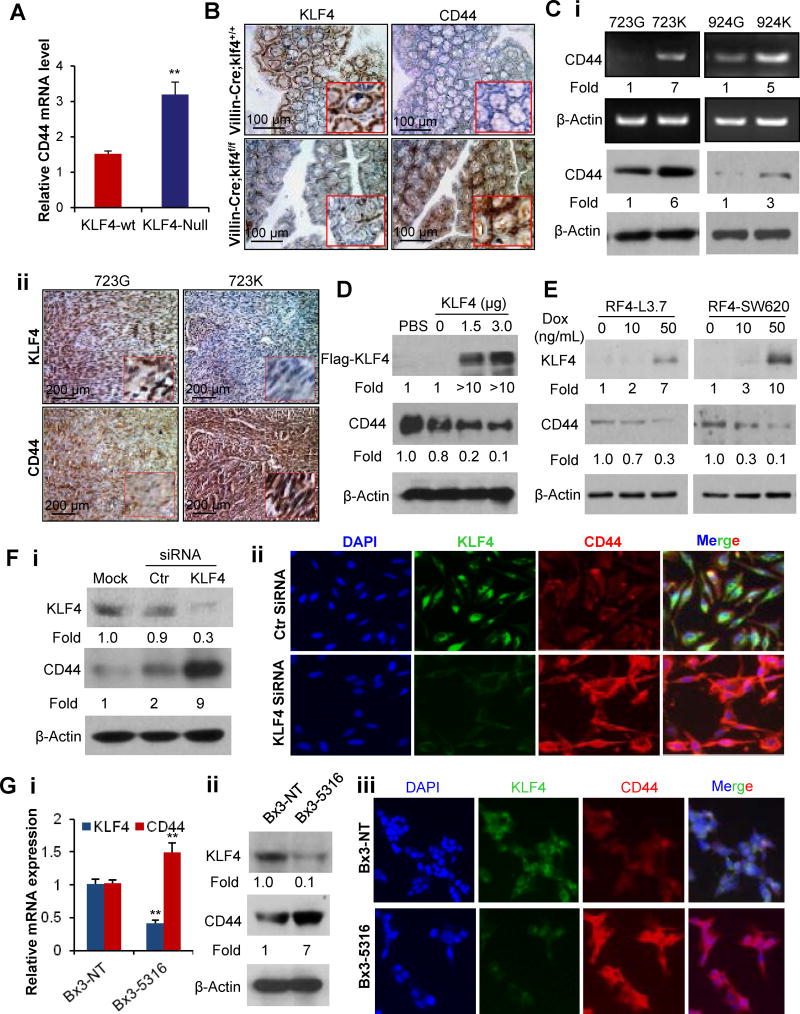
To investigate the mechanisms by which KLF4 regulates the stem-like properties and metastasis of cancer cells, we examined CD44 expression in Klf4 gene-modified cell lines and mice. First, we searched database of GEO profiles and found that the level of CD44 mRNA was significantly increased in Klf4-knockout (Klf4-null) mouse embryonic fibroblast (MEF) cells when compared with that in Klf4 wild-type MEF cells (Figure 3A). Consistently, genetic ablation of Klf4 increased CD44 expression in mouse colon and intestine tissues (Figure 3B; Figure S3) (32).
Figure 3. Identifying CD44 as a downstream target gene of KLF4.
(A) Relative CD44 mRNA expression in Klf4 wild-type (Klf4-wt) and Klf4 knockout (Klf4-null) mouse embryonic fibroblast cells detected in cDNA microarray analysis (GEO accession # GSE21768).
(B) IHC detection of Klf4 and CD44 expressions in colon tissues derived from Villin-Cre;Klf4+/+ or Villin-Cre;Klf4loxp/loxp mice.
(C) RT-PCR (Ci, upper panel) or WB (Ci, lower panel) analysis of CD44 expression in paired 723G/723K and 924G/924K cells. IHC staining of CD44 and KLF4 expressions in consecutive sections of xenograft tumors derived from 723G/723K cells (Cii).
(D) CD44 and KLF4 protein expressions in PANC-1 cells after transduced with pcDNA3.1 or pcDNA3.1-KLF4 vector.
(E) WB analysis of CD44 and KLF4 expressions in RF4-SW620 and RF4-L3.7 cells treated with different doses of Dox for 48 hours.
(F) CD44 and KLF4 expressions in PANC-28 cells at 48 hours after transduced with control small interfering RNA (Ctr), KLF4-siRNA, or mock transfection, as determined by WB (Fi) or immunofluorescent (IF) staining (Fii).
(G) KLF4 and CD44 expressions in BxPC3 PDA cells stably transduced with non-target shRNA (Bx3-NT) or KLF4-shRNA (Bx3-5316), as determined by real-time PCR (Gi), WB (Gii), or IF staining (Giii) assays. (*P<0.05, **P<0.01).
Next, we examined the relationship between KLF4 and CD44 expression in different cancer cells. Knockout of Klf4 significantly up-regulated CD44 expression in mouse cancer cell lines and tumor tissues derived from the cell lines (Figure 3C), respectively. Furthermore, forced or induced KLF4 expression inhibited CD44 expression in cancer cells (Figure 3D–3E), whereas knockdown of KLF4 expression by siRNA or shRNA induced CD44 expression in cancer cells (Figure 3F; 3G) (35). These results indicate that CD44 is a downstream target gene of KLF4.
CD44 Is Critical in Mediating the Inhibitory Effect of KLF4 on Cancer Cell Stemness
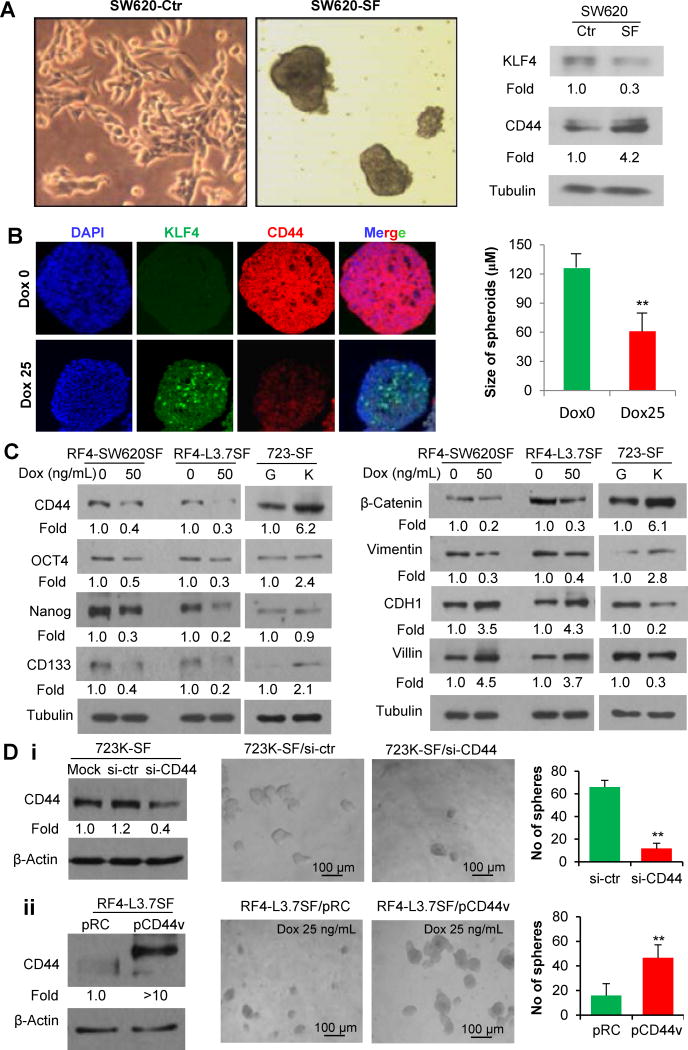
To investigate whether CD44 is required for KLF4’s regulatory effects on cancer stemness, we first compared the expressions of KLF4 and CD44 in adherent monolayer cells and spheroid forming (SF) cells. We found that SW620-SF cells had higher CD44 but lower KLF4 expression than adherent cancer cells (Figure 4A; Figure S4). Exposure of the spheroids to Dox treatment significantly induced KLF4 expression and resulted in decreased CD44 expression and much smaller spheroids when compared with the control group without Dox treatment (Figure 4B; Figure S1C). Dox treatment reduced stem-related markers Oct4, Nanog, CD133, β-Catenin, and Vimentin, but upregulated epithelial-related markers such as E-cadherin (CDH1) (Figure 4C) expressions in spheroid-forming (SF) cells. These results suggest that induction of KLF4 expression can restrain self-renewal and induce differentiation of stem-like cells, which is consistent with the findings that KLF4 primarily functions as an inducer of cell differentiation as demonstrated in germline or conditional Klf4 knockout mouse models or ES cells (22,36–38).
Figure 4. CD44 is critical in mediating the regulatory effect of KLF4 on cancer cell stemness.
(A) WB analysis of KLF4 and CD44 protein expressions in adherent monolayer SW620 (SW620-Ctr) and spheroid-forming SW620 (SW620-SF) cells.
(B) IF staining of KLF4 and CD44 expressions in RF4-SW620 spheroid sections prepared from tumorsphere culture with (25 ng/ml) or without Dox treatment for 4 days.
(C) WB analysis of stem-related protein marker expressions in 723G/723K-SF cells, or RF4-SW620SF and RF4-L3.7SF cells cultured with or without Dox treatment.
(D) 723K-SF cells in single-cell suspension were transfected with control small interfering RNA (si-ctr), CD44-siRNA (siCD44) or mock transfection and subjected to WB analysis of CD44 expression at 48 hours after transfection or for evaluation of spheroid formation on day 10 after transfection (Di). Similarly, RF4-L3.7SF cells in single-cell suspension were transfected with control (pRC) or pCD44v, and subjected for WB analysis of CD44 expression or for evaluation of spheroid formation (Dii). (*P<0.05; **P<0.001).
To further define the causal role of CD44 in mediating KLF4’s regulatory effect on cancer stemness, reciprocal rescue experiments were performed. Transduction of CD44 siRNA into 723K-SF cells significantly inhibited their spheroid colony formation (Figure 4Di). In contrast, when pCD44v-expressing vector was transfected into RF4-L3.7SF cells and exposed to Dox treatment, the number of secondary spheroid colonies significantly increased (Figure 4Dii). These gain and loss of CD44 function assays clearly demonstrated that CD44 plays a critical role in mediating KLF4’s effects on cancer cell stemness.
KLF4 Negatively Regulated the Expression of CD44 at Transcriptional Level
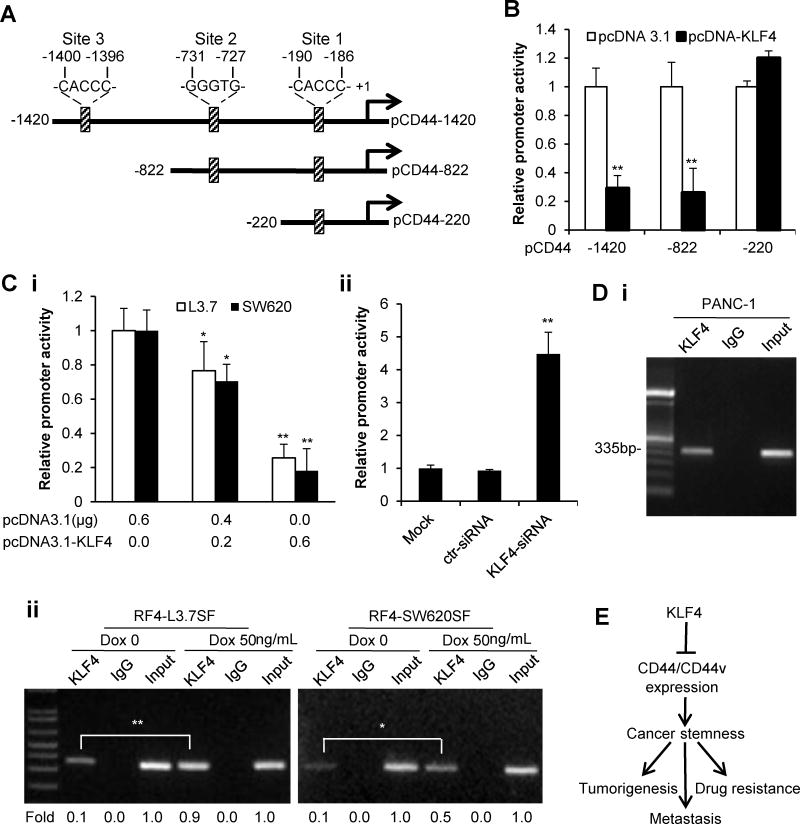
The inverse relationship between KLF4 and CD44 expression (Figure 1A; Figure 3A, 3Ci & Gi; Figure S1A & S4) suggests a transcriptional regulation of CD44 expression by KLF4. Indeed, we identified three potential KLF4 binding sites in the promoter region of CD44 by motif analysis (Figure 5A). CD44 promoter deletion mutation analysis indicated the region from −822 to −220 contains the response element(s) required for KLF4’s regulatory control of CD44 promoter activity (Figure 5B). Additionally, a dose-dependent inhibitory effect was observed when pCD44-822 vector was cotransfected with different doses of pcDNA3.1-KLF4-expressing vector into L3.7 and SW620 cells (Figure 5Ci). In contrast, when KLF4 siRNA was used for contransfection assay in PANC-28 cells, CD44 promoter activity was drastically increased (Figure 5Cii). Chromatin immunoprecipitation (ChIP) assay showed that endogenous KLF4 protein bound to the CD44 promoter region (Figure 5A, 5Di). When Dox was added to induce KLF4 expression, the binding of KLF4 to the CD44 promoter region significantly increased (Figure 5Dii). Therefore, KLF4 represses CD44 expression and stemness properties in cancer cells, thus suppressing tumorigenesis, metastasis and drug resistance (Figure 5E).
Figure 5. Negative regulation of CD44 expression by KLF4.
(A) Schematic structure of the full-length CD44 promoter reporter and its deletion-mutant constructs. Three potential KLF4 binding sites are indicated.
(B) CD44 promoter activity in PANC-1 cells at 48 hours after transfected with pcDNA3.1 (Ctr) or pcDNA3.1-KLF4 vector (Tr).
(C) Similarly, the pCD44-822 promoter construct was cotransfected with pcDNA3.1-KLF4 or pcDNA3.1 vector into L3.7 and SW620 cells (Ci) or with KLF4-siRNA or control siRNA (ctr-siRNA) into PANC-28 (Cii) cells for CD44 promoter activity assay.
(D) ChIP analysis of KLF4 binding to the CD44 promoter region with potential KLF4 binding site 2 in PANC-1 cells (Di) or in RF4-L3.7SF/RF4-SW620SF cells with or without doxycycline treatment, and the relative band intensities were quantified for statistical analysis (Dii).
(E) A proposed model for KLF4 regulation of CD44 expression and stemness properties in cancer cells. (*P<0.05; **P<0.01).
Additionally, we found that metastatic L3.7 and SW620 cells had higher CD44v and epithelial splicing regulatory protein 1 (ESPR1) at both mRNA and protein levels and knockdown or knockout of KLF4 expression significantly increased CD44v and ESPR1 expression, whereas increased KLF4 expression inhibited ESRP1 and CD44v expression (Figure S5). Thus, in addition to transcriptional suppression of CD44 expression, KLF4 can also negatively regulate CD44v expression, possibly through modulating ESRP1 expression given its critical function in regulation of CD44v expression and EMT (39,40).
Dysregulation of KLF4-CD44 Signaling Exhibited Clinical Significance
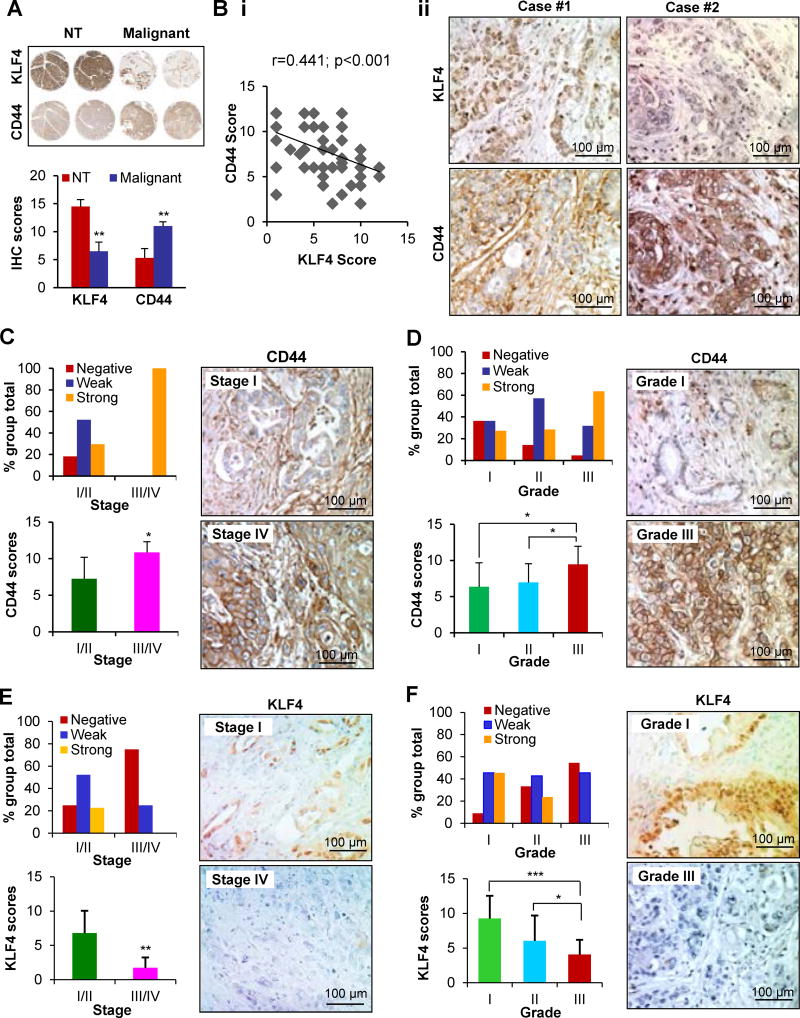
To investigate the clinical significance of KLF4-CD44 signaling in PDA, we first compared KLF4 and CD44 expression in a tissue microarray (TMA) containing six paired samples of PDA and adjacent non-tumor tissues by immunohistochemical (IHC) analysis. We found that, in general, adjacent non-tumor (NT) tissues had relatively high expression of KLF4 and very low expression of CD44; in contrast, PDA tissue had low KLF4 but high CD44 expression (Figure 6A). Then, we examined the overall KLF4 and CD44 expressions in a TMA containing 48 pancreatic primary cancer specimens and observed a significant inverse correlation between KLF4 and CD44 expression levels (Figure 6Bi–ii). Furthermore, CD44 expression was positively correlated with disease stage and was upregulated in late-stage tumors. The difference in CD44 expression was significant between stage I/II and stage III/IV tumors (P<0.05) (Figure 6C; Supplementary Table S1). In addition, increased CD44 expression correlated with higher tumor grades (Figure 6D; Supplementary Table S1). In contrast, KLF4 expression was inversely correlated with disease stage and tumor grade in human PDA tissues (Figures 6E and 6F; Supplementary Table S2). These findings indicate that dysregulated KLF4-CD44 signaling plays a critical role in PDA progression and is a valuable biomarker for this disease.
Figure 6. KLF4 and CD44 expressions in pancreatic normal and cancer tissues.
(A) IHC analysis of KLF4 and CD44 expressions in consecutive sections of a human pancreatic tissue microarray (TMA) containing PDA and adjacent non-tumor tissues (NT). The representative images of KLF4 and CD44 staining (upper panel, 5X) and the average scores of the staining (lower panel) are shown.
(B) IHC analysis of KLF4 and CD44 expressions in consecutive sections of a human PDA TMAs. An inverse correlation between KLF4 and CD44 expression scores was found (Bi), and the representative photos are shown (Bii).
(C) CD44 expression was positively correlated with disease stage, and the representative photos of stage I and IV tumors are shown.
(D) CD44 expression was positively correlated with tumor differentiation, and the representative photos of grade I and III tumors are shown.
(E) KLF4 expression was negatively correlated with disease stage, and the representative photos of stage I and IV tumors are shown.
(F) KLF4 expression was negatively correlated with tumor differentiation, and the representative photos of grade I and III tumors are shown. (*P< 0.05; **P< 0.01; ***P<0.001).
Dysregulation of KLF4-CD44 Signaling Significantly Impacted Metastasis
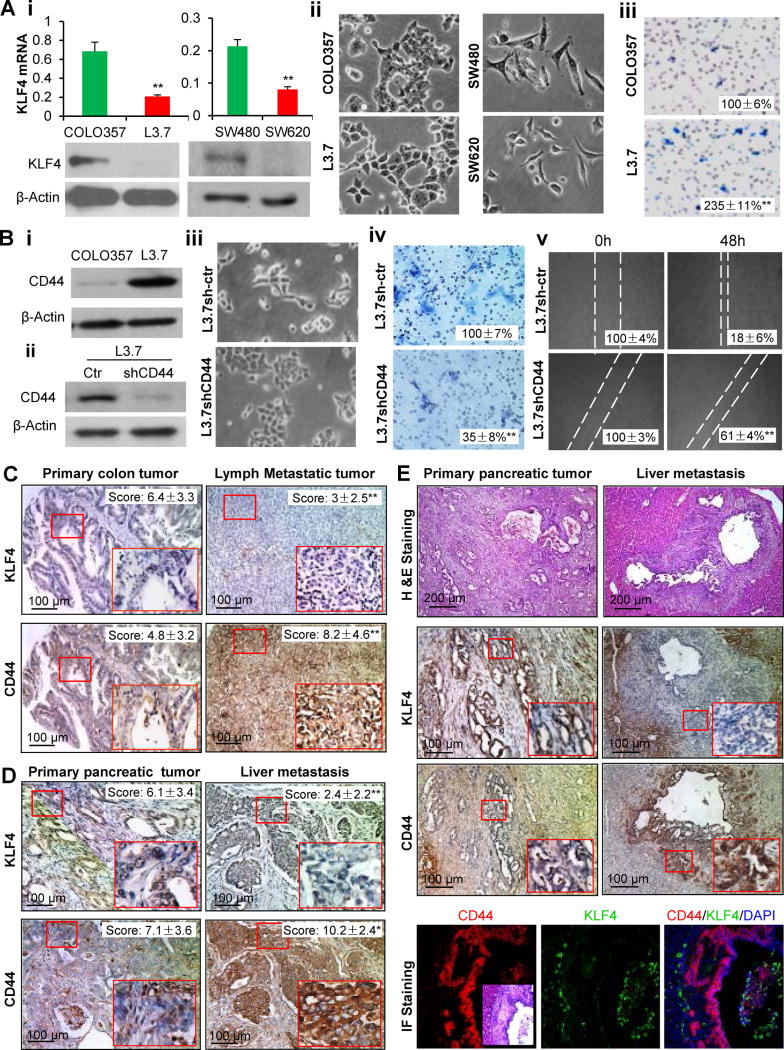
To further investigate whether dysregulated KLF4-CD44 signaling is responsible for cancer metastasis, we first examined the expression of KLF4 in two pairs of well characterized human pancreatic and colon cancer cell lines with low or high metastatic potential. Highly metastatic L3.7 cells had lower levels of KLF4 expression than did parental COLO357 cells and exhibited typical mesenchymal morphology and EMT gene expression pattern, including reduced expression of E-cadherin and increased expression of Vimentin and β-catenin, which are the direct targets of KLF4 in ChIP/sequencing analyses (41). Consistently, L3.7 cells had increased in vitro migration and invasion abilities and in vivo metastatic potential (Figure 7A; Figure S6). Similarly, lower KLF4 expression and more mesenchymal morphology were observed in highly metastatic SW620 cells when compared with SW480 cells (Figure 7A). In contrast to KLF4 expression, CD44 expression was higher in metastatic L3.7 cells than in parental COLO357 cells (Figure 7Bi). When CD44 shRNA was used to knock down CD44 expression (Figure 7Bii), L3.7shCD44 cells exhibited epithelial-like morphology and had reduced invasion and migration abilities (Figure 7Biii–Bv). These experimental data indicate that dysregulation of KLF4-CD44 signaling is a biological feature of metastatic cancer cells.
Figure 7. KLF4-CD44 signaling in cancer metastasis.
(A) KLF4 expression in paired metastatic and non-metastatic parental cell lines of L3.7/COLO357 or SW620/SW480 (Ai, upper panels, real-time PCR; lower panels, Western blotting); and the representative morphologic photos of L3.7/COLO357 and SW620/SW480 cells are shown (Aii). Boyden chamber method analysis of the invasion of L3.7/COLO357 cells, with representative photos shown in (Aiii).
(B) WB analysis of CD44 expression in L3.7/COLO357 cells (Bi) or L3.7 cells stably transduced with control or CD44shRNA (Bii); the representative photos of cell morphology (Biii), cell invasion and migration abilities (Biv–Bv) are shown.
(C) IHC analysis of KLF4 and CD44 expressions in consecutive sections of a human colon cancer tissue microarray (TMA) containing both primary tumor (PT) and metastatic tumor (MT) tissues. The IHC staining was scored and the overall quantitative data are expressed as Mean±SD, and the representative photos are shown.
(D) Similarly, the overall KLF4 and CD44 expressions in consecutive sections of a human PDA tissue microarray (TMA) containing both primary tumor (PT) and metastatic tumor (MT) tissues were analyzed by IHC assay and the related results are presented.
(E) Representative photos of hematoxylin and eosin (HE) staining of paired invasive pancreatic primary tumor (PT) and its liver metastatic tumor (MT) derived from a mouse strain of Pdx-1-Cre;LSL-KrasG12D (upper two panels), and the representative photos of KLF4 and CD44 staining in consecutive sections of PT and MT tissues (middle four panels). IF double staining of CD44 and KLF4 expressions in liver metastatic lesion (lower three panels), showing that metastatic PDA cells drastically lost KLF4 and gained CD44 expression, whereas adjacent liver cells and immune cells in the lumen (insert: HE staining of the selected area) expressed KLF4. (*P<0.05; **P<0.01).
We also analyzed the overall expressions of KLF4 and CD44 in a human cancer TMA containing 30 cases of primary tumor and 28 cases of lymph and liver metastatic tumor tissues. We found that lower KLF4 but higher CD44 expressions were observed in metastatic tissues compared with primary colon cancer tissues (Figure 7C, P<0.01). Similarly, metastatic pancreatic tumors had lower KLF4 but higher CD44 expression than primary pancreatic tumors (Figure 7D, P<0.01 and P<0.05, respectively). These results are in line with our data obtained from two pairs of low/high metastatic human cancer cell lines, and paired Klf4+/+/Klf4−/− mouse cancer cell lines (Figure 2; Figure 7A).
To specifically investigate the change of KLF4-CD44 signaling during tumor progression from invasive tumor to metastasis, a genetically engineered PDA progression mouse model (Pdx1-Cre;LDS-KrasG12D/+) was used (34), from which paired pancreatic primary tumor/liver metastatic tumor tissues were collected from individual mice (Figure S7A) and used for IHC and IF analysis. Strikingly, we found that metastatic PDA cells drastically lost KLF4 and gained CD44 expressions when compared with pancreatic primary tumor cells (Figure 7E, Case #1; Figure S7B, Case #2). Consistently, KLF4 expression was further decreased in liver metastatic lesions when compared to that in pancreatic xenograft tumors derived from orthotopic mouse model of human PDA using the highly metastatic L3.7 cells (Figure S7C). Again, CD44 expression in pancreatic tumor was much higher than that in adjacent normal tissues (Figure S7D). These data clearly demonstrated that KLF4-CD44 signaling dysregulation is critical for cancer cells to become metastatic.
DISCUSSION
Normal stem cells and cancer stem cells differ significantly in many aspects, while subtle defects in cancer stem cells alter the homeostatic equilibrium and result in unbalanced cell growth and the formation of heterogeneous tumors (2,42). Currently, several lines of evidence support the notion that KLF4 functions differently in normal and cancer stem cells. For example, loss of klf4 in the stomachs of adult mice led to precancerous lesions through imbalanced cell proliferation and differentiation in gastric pit glands, suggesting that KLF4 is critical for coordinated regulation of the self-renewal and differentiation of gastric progenitor cells (37), thus preventing progenitor cell transformation and tumor initiation (32). These results are in line with the previous studies showing that KLF4 is essential for maintaining genomic stability and has tumor-suppressive function in many cancers. Similarly, repression of KLF4 by NUMBL, a factor required for neural progenitor cells, maintained progenitor-like cells in lung cancer, and restoration of KLF4 expression was able to eradicate the progenitor-like population, and inhibit the colony formation and tumorigenicity of lung cancer cells (43). In contrast, roles of KLF4 in breast and colon cancer stem cells remain controversial (17,18,29,44).
The discrepancies in the function of KLF4 in cancer stemness may be due to differences in experimental and technical approaches as well as to the genetic backgrounds of the cancer cells. In the present study, both the definitive genetic knockout and tetracycline-inducible gene expression systems were used to manipulate the expression of KLF4 and define its causal impact on cancer stem-like properties in pancreatic and colon cancer cells. Our data clearly show that KLF4 has a stemness-inhibitory function in terms of spheroid formation, drug resistance, tumorigenesis and metastasis, and induction of epithelial differentiation.
Mechanistically, we identified, for the first time, CD44 as a direct downstream target gene of KLF4, which negatively regulated CD44 transcription and CD44v expression. Tremendous effort has been put into defining the biological functions of CD44 and its various splicing variants and the underlying signaling (4,39,40,45–48). But few studies examined its transcriptional regulation, even though the magnitude of CD44 transcription is critical for CD44 expression. Interestingly, Godar et al. reported that p53 transcriptionally suppressed CD44 expression and tumor growth, whereas p63, a paralogue of p53 did the opposite (49). Functionally, this study is consistent with our findings and also in line with previous studies showing that KLF4 is an essential mediator of p53 in response to cellular stress (50) and has the opposite function of p63 in controlling the proliferation and differentiation of epidermal cells (51). Given that frequent p53 mutation and loss of KLF4 expression have been found in many cancers, it is not surprising to find CD44 overexpression in various cancers that is associated with poor prognosis (48,52), and targeting CD44 may have potential as a strategy for blocking tumor formation and post-therapy recurrence (7).
The critical role of KLF4 in regulation of cancer stemness properties was not only clearly demonstrated by our results using multiple reliable experimental approaches but also attested to by its regulation of and dependence on CD44. CD44 is the most common cancer stem cell marker identified so far and has multiple functions in cell adhesion, migration, homing, proliferation, survival, and apoptosis (4,5). CD44 can act as a ligand–binding receptor by interacting with hyaluronan, osteopontin, extracellular matrix, and other extracellular components to form stem cell niches, which support stem cell maintenance, and metastatic colonization (52,53). It has been shown that HA-CD44 binding can induce Nanog activity and lead to the up-regulation of miR-21, and Nanog and miR-21 are critical for cancer stem cell self-renewal and maintenance (54,55). CSCs frequently express CD44 variants, and these variants initiate alternative signaling pathways, provide CSCs with survival advantages, and promote growth and metastasis (4,48,56). Thus, CD44 is a central component in the cross-talk between CSCs and their respective niches and has many essential functions in cancer stem cells.
Metastasis is the primary cause of tumor lethality in cancer patients. One of the prominent findings in this study is that dysregulated KLF4-CD44 signaling plays a critical role in tumor metastasis, which was demonstrated by three lines of evidence obtained from Klf4 knockout cancer cell lines, human colon and pancreatic primary/metastatic tumor tissue samples, and especially, a mouse PDA progression model. Our finding is supported by many previous studies showing that KLF4 is a critical negative regulator of EMT (13,17), an event that is critical for gains in stem-like features and tumor metastasis (1,24), and has metastasis-suppressive functions in lung, colon, breast, and gastric cancers (15,17,30,35,57). Our results are also supported by the promotion of the multistage metastasis process of cancer cell adhesion, migration, homing, survival, and in particular, metastatic colonization by CD44 (53), which is substantiated by the extremely high level of CD44 expression in the metastatic frontier in the livers of our PDA progression mouse model. The drastic loss of KLF4 expression in the metastatic foci further confirmed KLF4’s suppressive function on CD44 expression and metastasis.
In summary, we demonstrated for the first time that dysregulated KLF4-CD44 signaling plays a critical role in regulating the stemness and metastasis of pancreatic and colon cancers. Our study also established a strong rationale for developing KLF4 as a therapeutic target to control CSCs and metastasis. Excitingly, targeted activation of KLF4 for therapeutic intervention of advanced solid tumors has been approved for clinical trial and a recent Phase 1 study showed that APTO-253 HCl, an inducer of KLF4, was found to be well tolerated and treatment was associated with stable disease in 5 of 21 (24 %) patients with advanced or metastatic solid tumors (12). However, further investigations are warranted to evaluate the effectiveness of APTO-253 HCl in clinical trials and whether activation of KLF4 and suppression of cancer stemness are indeed responsible for the anti-tumor activity of APTO-253.
Supplementary Material
Acknowledgments
The authors thank Dawn Chalaire for editorial comments. The authors thank Dr. Robert Weinberg (Whitehead Institute for Biomedical Research, MIT) and Dr. Osamu Nagano (Division of Gene Regulation, Keio University, Japan) for plasmids.
Footnotes
Conflicts of interest
The authors disclose no conflicts.
References
- 1.Oskarsson T, Batlle E, Massague J. Metastatic Stem Cells: Sources, Niches, and Vital Pathways. Cell stem cell. 2014;14(3):306–21. doi: 10.1016/j.stem.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8(10):755–68. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- 3.Clevers H. The cancer stem cell: premises, promises and challenges. Nat Med. 2011;17(3):313–9. doi: 10.1038/nm.2304. [DOI] [PubMed] [Google Scholar]
- 4.Williams K, Motiani K, Giridhar PV, Kasper S. CD44 integrates signaling in normal stem cell, cancer stem cell and (pre)metastatic niches. Exp Biol Med (Maywood) 2013;238(3):324–38. doi: 10.1177/1535370213480714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zoller M. CD44: can a cancer-initiating cell profit from an abundantly expressed molecule? Nat Rev Cancer. 2011;11(4):254–67. doi: 10.1038/nrc3023. [DOI] [PubMed] [Google Scholar]
- 6.Li Y, Zhang Q, Yin X, Yang W, Du Y, Hou P, et al. Generation of iPSCs from mouse fibroblasts with a single gene, Oct4, and small molecules. Cell research. 2011;21(1):196–204. doi: 10.1038/cr.2010.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li L, Hao X, Qin J, Tang W, He F, Smith A, et al. Antibody Against CD44s Inhibits Pancreatic Tumor Initiation and Postradiation Recurrence in Mice. Gastroenterology. 2014;146(4):1108–18. e12. doi: 10.1053/j.gastro.2013.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Misra S, Heldin P, Hascall VC, Karamanos NK, Skandalis SS, Markwald RR, et al. Hyaluronan-CD44 interactions as potential targets for cancer therapy. FEBS J. 2011;278(9):1429–43. doi: 10.1111/j.1742-4658.2011.08071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wei D, Kanai M, Huang S, Xie K. Emerging role of KLF4 in human gastrointestinal cancer. Carcinogenesis. 2006;27(1):23–31. doi: 10.1093/carcin/bgi243. [DOI] [PubMed] [Google Scholar]
- 10.Tetreault MP, Yang Y, Katz JP. Kruppel-like factors in cancer. Nat Rev Cancer. 2013;13(10):701–13. doi: 10.1038/nrc3582. [DOI] [PubMed] [Google Scholar]
- 11.Faber K, Bullinger L, Ragu C, Garding A, Mertens D, Miller C, et al. CDX2-driven leukemogenesis involves KLF4 repression and deregulated PPARgamma signaling. J Clin Invest. 2013;123(1):299–314. doi: 10.1172/JCI64745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cercek A, Wheler J, Murray PE, Zhou S, Saltz L. Phase 1 study of APTO-253 HCl, an inducer of KLF4, in patients with advanced or metastatic solid tumors. Invest New Drugs. 2015;33(5):1086–92. doi: 10.1007/s10637-015-0273-z. [DOI] [PubMed] [Google Scholar]
- 13.Liu YN, Abou-Kheir W, Yin JJ, Fang L, Hynes P, Casey O, et al. Critical and reciprocal regulation of KLF4 and SLUG in transforming growth factor beta-initiated prostate cancer epithelial-mesenchymal transition. Mol Cell Biol. 2012;32(5):941–53. doi: 10.1128/MCB.06306-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yori JL, Johnson E, Zhou G, Jain MK, Keri RA. Kruppel-like factor 4 inhibits epithelial-to-mesenchymal transition through regulation of E-cadherin gene expression. J Biol Chem. 2010;285(22):16854–63. doi: 10.1074/jbc.M110.114546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen HY, Lin YM, Chung HC, Lang YD, Lin CJ, Huang J, et al. miR-103/107 promote metastasis of colorectal cancer by targeting the metastasis suppressors DAPK and KLF4. Cancer Res. 2012;72(14):3631–41. doi: 10.1158/0008-5472.CAN-12-0667. [DOI] [PubMed] [Google Scholar]
- 16.Okuda H, Xing F, Pandey PR, Sharma S, Watabe M, Pai SK, et al. miR-7 suppresses brain metastasis of breast cancer stem-like cells by modulating KLF4. Cancer Res. 2013;73(4):1434–44. doi: 10.1158/0008-5472.CAN-12-2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yori JL, Seachrist DD, Johnson E, Lozada KL, Abdul-Karim FW, Chodosh LA, et al. Kruppel-like factor 4 inhibits tumorigenic progression and metastasis in a mouse model of breast cancer. Neoplasia. 2011;13(7):601–10. doi: 10.1593/neo.11260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu F, Li J, Chen H, Fu J, Ray S, Huang S, et al. Kruppel-like factor 4 (KLF4) is required for maintenance of breast cancer stem cells and for cell migration and invasion. Oncogene. 2011;30(18):2161–72. doi: 10.1038/onc.2010.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 20.Robinton DA, Daley GQ. The promise of induced pluripotent stem cells in research and therapy. Nature. 2012;481(7381):295–305. doi: 10.1038/nature10761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hou P, Li Y, Zhang X, Liu C, Guan J, Li H, et al. Pluripotent stem cells induced from mouse somatic cells by small-molecule compounds. Science. 2013;341(6146):651–4. doi: 10.1126/science.1239278. [DOI] [PubMed] [Google Scholar]
- 22.Jiang J, Chan YS, Loh YH, Cai J, Tong GQ, Lim CA, et al. A core Klf circuitry regulates self-renewal of embryonic stem cells. Nature cell biology. 2008;10(3):353–60. doi: 10.1038/ncb1698. [DOI] [PubMed] [Google Scholar]
- 23.Chen J, Liu J, Yang J, Chen Y, Ni S, Song H, et al. BMPs functionally replace Klf4 and support efficient reprogramming of mouse fibroblasts by Oct4 alone. Cell research. 2011;21(1):205–12. doi: 10.1038/cr.2010.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu S, Li W, Zhou H, Wei W, Ambasudhan R, Lin T, et al. Reprogramming of human primary somatic cells by OCT4 and chemical compounds. Cell stem cell. 2010;7(6):651–5. doi: 10.1016/j.stem.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoffmeyer K, Raggioli A, Rudloff S, Anton R, Hierholzer A, Del Valle I, et al. Wnt/beta-catenin signaling regulates telomerase in stem cells and cancer cells. Science. 2012;336(6088):1549–54. doi: 10.1126/science.1218370. [DOI] [PubMed] [Google Scholar]
- 26.Wong CW, Hou PS, Tseng SF, Chien CL, Wu KJ, Chen HF, et al. Kruppel-like transcription factor 4 contributes to maintenance of telomerase activity in stem cells. Stem Cells. 2010;28(9):1510–7. doi: 10.1002/stem.477. [DOI] [PubMed] [Google Scholar]
- 27.Sellak H, Wu S, Lincoln TM. KLF4 and SOX9 transcription factors antagonize beta-catenin and inhibit TCF-activity in cancer cells. Biochim Biophys Acta. 2012;1823(10):1666–75. doi: 10.1016/j.bbamcr.2012.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Evans PM, Chen X, Zhang W, Liu C. KLF4 interacts with beta-catenin/TCF4 and blocks p300/CBP recruitment by beta-catenin. Mol Cell Biol. 2010;30(2):372–81. doi: 10.1128/MCB.00063-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leng Z, Tao K, Xia Q, Tan J, Yue Z, Chen J, et al. Kruppel-like factor 4 acts as an oncogene in colon cancer stem cell-enriched spheroid cells. PLoS One. 2013;8(2):e56082. doi: 10.1371/journal.pone.0056082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vaira V, Faversani A, Martin NM, Garlick DS, Ferrero S, Nosotti M, et al. Regulation of lung cancer metastasis by Klf4-Numb-like signaling. Cancer Res. 2013;73(8):2695–705. doi: 10.1158/0008-5472.CAN-12-4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wei D, Wang L, Kanai M, Jia Z, Le X, Li Q, et al. KLF4alpha up-regulation promotes cell cycle progression and reduces survival time of patients with pancreatic cancer. Gastroenterology. 2010;139(6):2135–45. doi: 10.1053/j.gastro.2010.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Q, Jia Z, Wang L, Kong X, Guo K, Tan D, et al. Disruption of Klf4 in villin-positive gastric progenitor cells promotes formation and progression of tumors of the antrum in mice. Gastroenterology. 2012;142(3):531–42. doi: 10.1053/j.gastro.2011.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takaishi S, Okumura T, Tu S, Wang SS, Shibata W, Vigneshwaran R, et al. Identification of gastric cancer stem cells using the cell surface marker CD44. Stem Cells. 2009;27(5):1006–20. doi: 10.1002/stem.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, Jacobetz MA, et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4(6):437–50. doi: 10.1016/s1535-6108(03)00309-x. [DOI] [PubMed] [Google Scholar]
- 35.Wei D, Kanai M, Jia Z, Le X, Xie K. Kruppel-like factor 4 induces p27Kip1 expression in and suppresses the growth and metastasis of human pancreatic cancer cells. Cancer Res. 2008;68(12):4631–9. doi: 10.1158/0008-5472.CAN-07-5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Segre JA, Bauer C, Fuchs E. Klf4 is a transcription factor required for establishing the barrier function of the skin. Nature genetics. 1999;22(4):356–60. doi: 10.1038/11926. [DOI] [PubMed] [Google Scholar]
- 37.Katz JP, Perreault N, Goldstein BG, Actman L, McNally SR, Silberg DG, et al. Loss of Klf4 in mice causes altered proliferation and differentiation and precancerous changes in the adult stomach. Gastroenterology. 2005;128(4):935–45. doi: 10.1053/j.gastro.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 38.Katz JP, Perreault N, Goldstein BG, Lee CS, Labosky PA, Yang VW, et al. The zinc-finger transcription factor Klf4 is required for terminal differentiation of goblet cells in the colon. Development (Cambridge, England) 2002;129(11):2619–28. doi: 10.1242/dev.129.11.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yae T, Tsuchihashi K, Ishimoto T, Motohara T, Yoshikawa M, Yoshida GJ, et al. Alternative splicing of CD44 mRNA by ESRP1 enhances lung colonization of metastatic cancer cell. Nat Commun. 2012;3:883. doi: 10.1038/ncomms1892. [DOI] [PubMed] [Google Scholar]
- 40.Brown RL, Reinke LM, Damerow MS, Perez D, Chodosh LA, Yang J, et al. CD44 splice isoform switching in human and mouse epithelium is essential for epithelial-mesenchymal transition and breast cancer progression. J Clin Invest. 2011;121(3):1064–74. doi: 10.1172/JCI44540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tiwari N, Meyer-Schaller N, Arnold P, Antoniadis H, Pachkov M, van Nimwegen E, et al. Klf4 is a transcriptional regulator of genes critical for EMT, including Jnk1 (Mapk8) PLoS One. 2013;8(2):e57329. doi: 10.1371/journal.pone.0057329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kreso A, Dick JE. Evolution of the cancer stem cell model. Cell stem cell. 2014;14(3):275–91. doi: 10.1016/j.stem.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 43.Hu W, Hofstetter WL, Li H, Zhou Y, He Y, Pataer A, et al. Putative tumor-suppressive function of Kruppel-like factor 4 in primary lung carcinoma. Clin Cancer Res. 2009;15(18):5688–95. doi: 10.1158/1078-0432.CCR-09-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dang DT, Chen X, Feng J, Torbenson M, Dang LH, Yang VW. Overexpression of Kruppel-like factor 4 in the human colon cancer cell line RKO leads to reduced tumorigenecity. Oncogene. 2003;22(22):3424–30. doi: 10.1038/sj.onc.1206413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ishimoto T, Nagano O, Yae T, Tamada M, Motohara T, Oshima H, et al. CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc(−) and thereby promotes tumor growth. Cancer Cell. 2011;19(3):387–400. doi: 10.1016/j.ccr.2011.01.038. [DOI] [PubMed] [Google Scholar]
- 46.Liu C, Kelnar K, Liu B, Chen X, Calhoun-Davis T, Li H, et al. The microRNA miR-34a inhibits prostate cancer stem cells and metastasis by directly repressing CD44. Nat Med. 2011;17(2):211–5. doi: 10.1038/nm.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saini S, Majid S, Shahryari V, Arora S, Yamamura S, Chang I, et al. miRNA-708 control of CD44(+) prostate cancer-initiating cells. Cancer Res. 2012;72(14):3618–30. doi: 10.1158/0008-5472.CAN-12-0540. [DOI] [PubMed] [Google Scholar]
- 48.Todaro M, Gaggianesi M, Catalano V, Benfante A, Iovino F, Biffoni M, et al. CD44v6 Is a Marker of Constitutive and Reprogrammed Cancer Stem Cells Driving Colon Cancer Metastasis. Cell stem cell. 2014;14(3):342–56. doi: 10.1016/j.stem.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 49.Godar S, Ince TA, Bell GW, Feldser D, Donaher JL, Bergh J, et al. Growth-inhibitory and tumor- suppressive functions of p53 depend on its repression of CD44 expression. Cell. 2008;134(1):62–73. doi: 10.1016/j.cell.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yoon HS, Chen X, Yang VW. Kruppel-like factor 4 mediates p53-dependent G1/S cell cycle arrest in response to DNA damage. J Biol Chem. 2003;278(4):2101–5. doi: 10.1074/jbc.M211027200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cordani N, Pozzi S, Martynova E, Fanoni D, Borrelli S, Alotto D, et al. Mutant p53 subverts p63 control over KLF4 expression in keratinocytes. Oncogene. 2011;30(8):922–32. doi: 10.1038/onc.2010.474. [DOI] [PubMed] [Google Scholar]
- 52.Pietras A, Katz AM, Ekstrom EJ, Wee B, Halliday JJ, Pitter KL, et al. Osteopontin-CD44 Signaling in the Glioma Perivascular Niche Enhances Cancer Stem Cell Phenotypes and Promotes Aggressive Tumor Growth. Cell stem cell. 2014;14(3):357–69. doi: 10.1016/j.stem.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Malanchi I, Santamaria-Martinez A, Susanto E, Peng H, Lehr HA, Delaloye JF, et al. Interactions between cancer stem cells and their niche govern metastatic colonization. Nature. 2012;481(7379):85–9. doi: 10.1038/nature10694. [DOI] [PubMed] [Google Scholar]
- 54.Niu Z, Goodyear SM, Rao S, Wu X, Tobias JW, Avarbock MR, et al. MicroRNA-21 regulates the self-renewal of mouse spermatogonial stem cells. Proc Natl Acad Sci U S A. 2011;108(31):12740–5. doi: 10.1073/pnas.1109987108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bourguignon LY, Spevak CC, Wong G, Xia W, Gilad E. Hyaluronan-CD44 interaction with protein kinase C(epsilon) promotes oncogenic signaling by the stem cell marker Nanog and the Production of microRNA-21, leading to down-regulation of the tumor suppressor protein PDCD4, anti-apoptosis, and chemotherapy resistance in breast tumor cells. J Biol Chem. 2009;284(39):26533–46. doi: 10.1074/jbc.M109.027466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lau WM, Teng E, Chong HS, Lopez KA, Tay AY, Salto-Tellez M, et al. CD44v8-10 Is a Cancer-Specific Marker for Gastric Cancer Stem Cells. Cancer Res. 2014 doi: 10.1158/0008-5472.CAN-13-2309. [DOI] [PubMed] [Google Scholar]
- 57.Wei D, Gong W, Kanai M, Schlunk C, Wang L, Yao JC, et al. Drastic down-regulation of Kruppel-like factor 4 expression is critical in human gastric cancer development and progression. Cancer Res. 2005;65(7):2746–54. doi: 10.1158/0008-5472.CAN-04-3619. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.