Abstract
Obesity/overweight is reported to affect MR-measured brain tissue volume and white matter (WM) signal intensity. This study investigated possible effects of fat on these measures, using pig fat on three participants at a 4 Tesla magnet. Gray matter volumes in the presence of fat were lower than baseline measures. Total WM volumes in the presence of fat were higher than baseline measures. WM hypo-intensities on T1-weighted images were higher in the presence of fat than baseline measures. Therefore physical effects of head fat of obese/overweight individual may at least, partly contribute to the association of obesity/overweight with MR structural measures.
Keywords: fat, magnetic resonance signal hypo-intensity, gray matter, white matter, brain, obesity
Several magnetic resonance imaging (MRI) studies reported an association of obesity/overweight with abnormalities in brain tissue volumes: smaller gray matter (GM) volumes [1 – 8], lower GM densities [9], and larger white matter (WM) volumes [10] than the corresponding measures for normal weight individuals. Similarly, increased incidence of WM signal hyper-intensities on T2-weighted images have been linked to obesity/overweight [11].
Generally the associations of obesity/overweight with the MR-observed abnormalities of brain tissue measures in these publications have been interpreted to reflect altered neurobiology in association with obesity/overweight, which constitutes a potentially increased risk for cognitive decline or development of dementia. However, physical effects of fat on MR-measured brain volumes and WM signal quality have not been assessed. This is imperative because obese/overweight individuals are expected to have more fat deposits on the head than normal weight individuals; and if fat has an adverse effect on these measures, that effect could be more pronounced in obese/overweight individuals as compared to normal weight individuals. In a recent report we demonstrated that fat tissue superficially placed in the vicinity of an MR spectroscopy voxel reduced metabolite signal strengths [12]. In this report, physical effects of the superficial fat on MR-measured GM and WM volume measures as well as on WM hypo-intensities on T1-weighted images (which is equivalent to hyper-intensities on T2-images) of the human brainare assessed.
The structural MRI data acquired for the MR spectroscopy experiments that demonstrated the physical effects of fat on metabolite signal strengths [12] were used for this report. The data were acquired at 4 Tesla (Bruker MedSpec system, Ettlingen, Germany), from three healthy male volunteers (aged 30, 31 and 55 years) with BMI of 25.9, 23.5 and 25.6 kg/m2, respectively. Each participant was scanned first without fat and then with two 0.7cm thick layers of pig back fat: one layer was placed beneath the occiput and the other on the forehead. All participants signed a formal written consent approved by the committee on human research at the University of California San Francisco.
T1-weighted images were segmented into GM, WM and cerebrospinal fluid (CSF) tissue volumes using two different methods: the expectation maximization segmentation (EMS) technique [13] and the publicly available volumetric segmentation and cortical surface reconstruction methods provided by FreeSurfer v 5.1 (e.g.,[14,15]).Total GM, WM, CSF volumes as well as intra-cranial volume (ICV) were estimated from the EMS data, while FreeSurfer reconstructed 72 small cortical regions of interest, which were appropriately combined to yield temporal, frontal, occipital, and parietal GM volumes. FreeSurfer also segmented WM hypo-intensities on the T1-weighted images; i.e., voxels within WM regions with signal intensities lower than the threshold level for WM.
The EMS segmented data of the three participants are shown in Table 1. The mean difference in total GM volume when subtracting the fat-layer-free (baseline) volumes from the volumes observed in the presence of fat was −0.8 ± 0.4%. On the other hand, the mean difference for total WM volumes was +1.3± 0.4% when subtracting total WM volumes at baseline from the total WM volumes observed in the presence of fat. The difference for CSF volumes was −0.2± 0.4% upon similar subtractions, but these changes were less consistent and much smaller across the three participants compared to GM and WM values. ICV remained essentially unchanged at 0.1± 0.2% on average.
Table 1.
Total GM, WM, CSF and ICV volumes from EMS segmentation (in mL)for scans with and without externally placed fat layers.
| Tissue | Participant 1 | Participant 2 | Participant 3 | Mean ± standard deviation across participants |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| W/o fat | With fat | % diff | W/o fat | With fat | % diff | W/o fat | With fat | % diff | W/o fat | With fat | |
| Total GM | 859.0 | 850.9 | −0.9 | 901.2 | 891.4 | −1.1 | 777.5 | 774.4 | −0.4 | 845.9 ± 62.9 | 838.9 ± 59.4 |
| Total WM | 447.4 | 454.5 | +1.6 | 477.3 | 481.3 | +0.8 | 414.5 | 420.7 | +1.5 | 446.4 ± 31.4 | 452.1 ± 30.3 |
| Total CSF | 463.4 | 462.8 | −0.1 | 442.2 | 442.9 | +0.2 | 347.5 | 345.1 | −0.7 | 417.7 ± 61.7 | 416.9 ± 63.0 |
| ICV | 1769.8 | 1768.1 | −0.1 | 1820.7 | 1815.5 | −0.3 | 1539.5 | 1540.2 | 0.0 | 1710.0 ± 149.9 | 1708.0 ± 147.2 |
ICV = intracranial volume, W/o = without
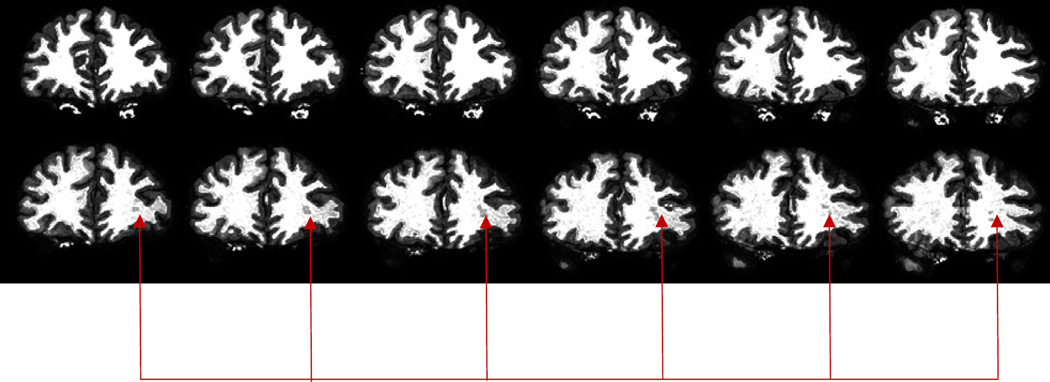
Data from the FreeSurfer segmentation showed smaller cortical GM measures for all participants in the presence of fat (1 – 11%) relative to the corresponding values of the baseline experiments; the temporal cortex was consistently affected the most in all participants (−9.3± 4.2%; see Table 2). In addition, the number of WM hypo-intensities detected on the T1-weighted images in the presence of fat were higher compared to the fat-layer-free baseline measures. Figure 1 shows segmented tissue maps for some image slices of one participant, illustrating the higher prevalence of WM hypo-intensities (shown by arrows) on segmented T1-weighted images in the presence of fat (bottom row).
Table 2.
Regional cortical GM volumes from FreeSurfer segmentation (in cubic millimeters) for scans with and without externally placed fat layers.
| Tissue | Participant 1 | Participant 2 | Participant 3 | Mean ± standard deviation across participants |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| W/o fat | With fat | % diff | W/o fat | With fat | % diff | W/o fat | With fat | % diff | W/o fat | With fat | |
| tGM | 93275 | 83071 | −11.0 | 105193 | 91954 | −13.0 | 93429 | 89243 | −9.0 | 97299.0 ± 6836.8 |
88089.3 ± 4552.5 |
| fGM | 139813 | 133378 | −4.6 | 145751 | 144917 | −0.6 | 115513 | 114862 | −0.5 | 133692.3 ± 16021.3 |
131052.3 ± 15161.9 |
| oGM | 52782 | 52184 | −1.0 | 62633 | 61927 | −1.0 | 50359 | 48889 | −3.0 | 55258.0 ± 6500.8 |
54333.3 ± 6779.5 |
| pGM | 117193 | 115865 | −1.0 | 134338 | 121965 | −9.0 | 107542 | 105816 | −2.0 | 119691.0 ± 13571.5 |
114548.7 ± 8154.6 |
tGM = temporal gray matter, fGM = frontal gray matter, oGM = occipital gray matter, pGM = parietal gray matter, W/o = without
Figure 1.
FreeSurfer segmented brain tissues of T1-weighted images: top: segmented baseline images; bottom: corresponding segmented images obtained with fat added to the head. The arrows point to a large hypo-intensity on images in the presence of fat.
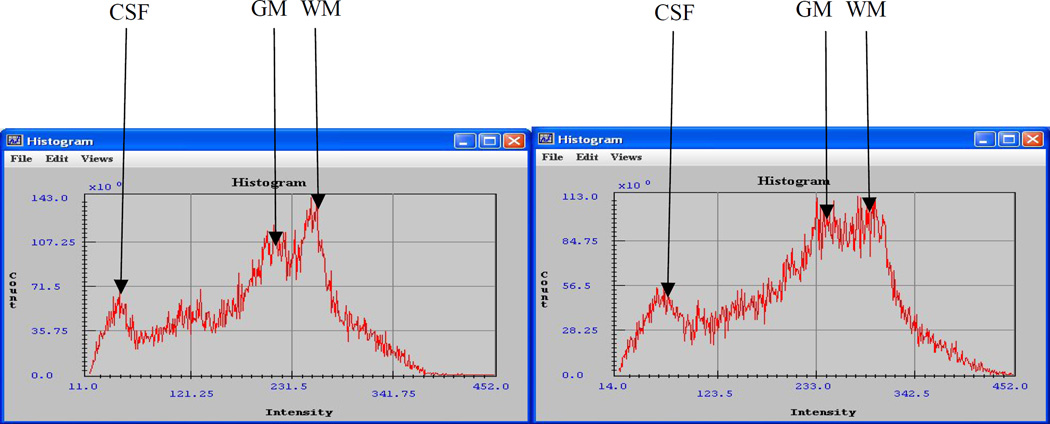
We examined signal histograms of the images of both baseline and the fat experiments. Figure 2 shows histograms of one slide of the brain unprocessed T1 image data (skull and added fat excluded) of one of the participants. a is the histogram of an image slide of a baseline experiment and b is the histogram of the corresponding image slide in the presence of fat. The arrows labeled ‘CSF’, ‘GM’ and ‘WM’ point to the signal intensity peaks for CSF, GM and WM, respectively. Notice the change in vertical scale of the overall signal magnitudes from (143.0 × 106) units at baseline down to (113.0 × 106) in the presence of fat. Also notice the horizontal shift in signal intensities of the tissues overall: intensities range from11 to 370 units in the baseline image and from 14 to 450 units in the image with fat layers on the head. The estimated maximal intensities for CSF signals shift from 36 units at baseline to 59 units in the presence of fat (+23 units), from 210 at baseline to 243 in the presence of fat for GM (+33 units), and from 251 at baseline to 293 in the presence of fat for WM (+42 units). Thus, there is an overall broadening of tissue signal distributions and a shift of the GM maximum signal into WM signal intensities region, leading to an increased overlap of GM signal with WM signal in the presence of fat. This change of signal distributions with the addition of fat layers to the head was apparent in the images from all participants, and could give insight to possible physical causes of the change in GM and WM volumes in the presence of fat.
Figure 2.
Skull and fat stripped image signal intensity histograms. a: baseline image signal intensity histogram; b: signal intensity histogram in the presence of fat on the head. Notice the change in vertical scale of the overall signal magnitudes from (143.0 × 106) in a down to (113.0 × 106) in b. Also, notice the horizontal shift of the entire histogram and of its signal maxima
In this study we investigated the effects of exogenous fat layers added to the head of three healthy volunteers on measures of brain tissue volumes and WM signal hypo-intensities derived from T1-weighted images. Using two different image segmentation methods, we observed that the measurement values for total and lobar cortical GM volumes were lower in the presence of added fat, while those for total WM volumes were larger in all participants in the presence of added fat. Furthermore, we detected a higher prevalence of WM hypo-intensities on T1-weighted images in the presence of fat.
The cause of the smaller GM and larger WM volume measures in the presence of fat is unclear; however, in the signal histograms we observed a substantial shift of GM signal intensities into WM intensities region in the presence of fat, which lead to a greater overlap with signal representing WM. This led to a lack of distinct features in the histogram and most likely led to misclassification of some GM tissue as WM tissue by the segmentation software, which in turn could have resulted in the altered tissue volume measures. The reason for the observed WM hypo-intensities on the T1-weighted images in the presence of fat is also not known, but B1 field intensity variations (i.e., radio-frequency field non-uniformities) due to the presence of fat could partly explain the results; non-uniformity of the B1 field gives rise to low MR signal intensities from regions that experience a lower B1 field. Thus, in the presence of fat, signal may decrease in certain WM regions below WM signal threshold and get misclassified as WM hypo-intense signal.
In recent years, several MRI volumetric studies linked obesity/overweight with smaller regional GM volumes, larger regional WM volumes and higher prevalence of WM signal hyper-intensities on T2-weighted images (which translate to hypo-intensities on T1-weighted images). These observations in the previous reports are similar to the observations in this report, where fat placed on the head led to apparent smaller GM volumes and larger WM volumes as well as higher prevalence of WM signal hypo-intensities on T1-weighted images. Regional GM [4] and total GM [3] reductions of up to 8% and 9% were observed in obese individuals, which are of the order of the regional GM volume reductions we observed with the FreeSurfer data when fat was placed on the head of a participant. Thus, our findings suggest that the associations of obesity/overweight with these MR measures may at least partly be attributable to potentially larger fat stores surrounding the brain in obese/overweight individuals giving rise to technical/physical issues that affect MR signal intensities. Diffusion tensor metrics, such as fractional anisotropy and diffusivity have also been reported to be affected by obesity/overweight [16, 17] and therefore warrant assessing for possible physical effects from fat surrounding the head. This work is rudimentary, calling for more detailed studies to confirm and extend these intriguing findings because of their clear relevance to interpreting MR findings in obesity/overweight.
Acknowledgments
This work was supported by the National Institutes of Health AA10788 (DJM), DA025202 (DJM), resources and facilities at the San Francisco Veterans Administration Medical Center (USA) and Koforidua Polytechnic (Ghana).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no disclosures or conflicts of interest to report.
References
- 1.Walther K, Birdsill AC, Glisky EL, Ran L. Structural brain differences and cognitive functioning related to body mass index in older females. Hum Brain Mapp. 2010;31:1052–1064. doi: 10.1002/hbm.20916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ward MA, Carlsson CM, Trivedi MA, Sager MA, Johnson SC. The effect of body mass index on global brain volume in middle-age adults: a crosssectional study. BMC Neurol. 2005;5:23. doi: 10.1186/1471-2377-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gunstad J, Paul RH, Cohen RA, Tate DF, Spitznagel MB, Grieve S, Gordon E. Relationship between body mass index and brain volume in healthy adults. Int J Neurosci. 2008;118:1582–1593. doi: 10.1080/00207450701392282. [DOI] [PubMed] [Google Scholar]
- 4.Raji CA, Ho AJ, Parikshak NN, Becker JT, Lopez OL, Kuller LH, Hua X, Leow AD, Toga AW, Thompson PM. Brain structure and obesity. Hum Brain Mapp. 2010;31:353–364. doi: 10.1002/hbm.20870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taki Y, Kinomura S, Sato K, Inoue K, Goto R, Okada K, Uchida S, Kawashima R, Fukuda H. Relationship between body mass index and gray matter volume in 1,428 healthy individuals. Obesity (Silver Spring) 2008;16(1):119–112. doi: 10.1038/oby.2007.4. [DOI] [PubMed] [Google Scholar]
- 6.Yokum S, Ng J, Stice E. Relation of regional gray and white matter volumes to current BMI and future increases in BMI: A prospective MRI study. Int J Obes. (Lond) 2012;36(5):656–664. doi: 10.1038/ijo.2011.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dommes E, Georgiewa P, Klingebiel R. Grey matter volume differences in obese as compared to normal-weight individuals: A voxel-based morphometric study. Arch Euromed. 2013;3(2):11–16. [Google Scholar]
- 8.Gustafson D, Lissner L, Bengtsson C, Bjorkelund C, Skoog L. A 24-year follow-up of body mass index and cerebral atrophy. Neurol. 2004a;63:1876–1881. doi: 10.1212/01.wnl.0000141850.47773.5f. [DOI] [PubMed] [Google Scholar]
- 9.Pannacciulli N, Del Parigi A, Chen K, Le DS, Reiman EM, Tataranni PA. Brain abnormalities in human obesity: A voxel-based morphometric study. Neuroim. 2006;31:1419–1425. doi: 10.1016/j.neuroimage.2006.01.047. [DOI] [PubMed] [Google Scholar]
- 10.Walther K, Birdsill AC, Glisky EL, Ryan L. Structural brain differences and cognitive functioning related to body mass index in older females. Hu Brai Mapp. 2010;31(7):1052–1064. doi: 10.1002/hbm.20916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gustafson D, Steen B, Skoog I. Body mass index and white matter lesions in elderly women. An 18-year longitudinal study. Int Psychogeriatr. 2004b;16:327–336. doi: 10.1017/s1041610204000353. [DOI] [PubMed] [Google Scholar]
- 12.Mon A, Abe C, Durazzo CT, Meyerhoff JD. Effects of fat on MR-measured metabolite signal strengths: implications for in vivo MRS studies of the human brain. NM Biomed. 2013;26(12):1768–1774. doi: 10.1002/nbm.3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Leemput K, Maes F, Vandermeulen D, Suetens P. Automated model-based tissue classification of MR images of the brain. IEEE Tran Med Imag. 1999;18(10):897–908. doi: 10.1109/42.811270. [DOI] [PubMed] [Google Scholar]
- 14.Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole Brain Segmentation: Automated Labeling of Neuroanatomical Structures in the Human Brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 15.Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat D, Busa E, Seidman L, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B, Dale AM. Automatically Parcellating the Human Cerebral. Cortex Cereb Cort. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- 16.Stanek KM, Grieve SM, Brickman AM, Korgaonkar MS, Paul RH, Cohen RA, Gunstad JJ. Obesity is associated with reduced white matter integrity in otherwise healthy adults. Obesity. 2011;19(3):500–504. doi: 10.1038/oby.2010.312. [DOI] [PubMed] [Google Scholar]
- 17.Verstynen TD, Weinstein AM, Schneider WW, Jakicic JM, Rofey DL, Erickson KI. Increased body mass index is associated with a global and distributed decrease in white matter microstructural integrity. Psycho Med. 2012;64:682–690. doi: 10.1097/PSY.0b013e318261909c. [DOI] [PMC free article] [PubMed] [Google Scholar]