Abstract
Mitochondria can undergo autophagic elimination for differing reasons, e.g. as part of a cell-wide macroautophagic response, as part of mitochondrial turnover during metabolic remodeling, or in the case of selective mitophagic destruction of dysfunctional mitochondria, during mitochondrial quality control. Multiple mechanistically distinct pathways converge upon, and activate, mitochondrial autophagy. Here, the evidence supporting a role for the prototypical mitochondrial quality control pathway, PINK1-Parkin mediated mitophagy, in cardiac homeostasis and heart disease is reviewed. Contrary to popular wisdom based on findings from non-cardiac systems, current data do not support a major role for Parkin-mediated mitophagy as a mechanism for constitutive mitochondrial house keeping, and instead suggest that this pathway primarily functions in adult hearts as an inducible cardiac stress-response mechanism. Recent findings have also uncovered an unsuspected role for Parkin-mediated mitochondrial turnover in the normal perinatal transformation of myocardial metabolism.
Introduction
It is axiomatic that the heart is critically dependent upon mitochondria to generate ATP that fuels cardiac contraction. Moreover, it is essential that those mitochondria function optimally. Preserving the overall functional integrity of the cardiomyocyte mitochondrial pool requires identification and removal of individual organelles that are senescent, damaged, or diseased. Collectively, these processes are grouped under the general term “mitochondrial quality control”. The most completely described process for mitochondrial quality control co-opts the cell autophagy apparatus to consume damaged or dysfunctional mitochondria, and is therefore called “mitophagy”. The best understood mechanism for targeted mitophagy of damaged or dysfunctional mitochondria is triggered by interactions between two Parkinson's disease factors, the mitochondrial kinase PINK1 and the normally cytosolic E3 ubiquitin ligase, Parkin. As reviewed below, PINK1 and Parkin co-functionality in mitochondrial quality control was initially described in Drosophila fruit fly lines carrying mutants of the respective fly genes, and has subsequently been observed in various types of cultured mammalian cells. Over the past decade we have come to understand important molecular details of mitophagy activation by these proteins, including how mitochondrial damage controls PINK1 kinase levels, and how PINK1 kinase regulates Parkin E3 ubiquitin ligase activity. Notwithstanding increased knowledge of these and other subcellular mechanistic details, the role of the PINK1-Parkin mitophagy pathway specifically in cardiomyocyte mitochondrial quality control in vivo remains uncertain. Indeed, the more we learn about Parkin and other factors promoting Parkin-mediated mitophagy, the less clear its role in normal mitochondrial homeostasis appears. Accumulating evidence supports multifunctionality of Parkin and its partner proteins that was not anticipated from the seminal Drosophila studies. Involvement of Parkin in cellular processes other than mitophagy and absence of definable and unambiguous mitophagy defects in Parkinson's disease have led some experts to question whether impaired mitophagy is the primary defect underlying neuronal degeneration provoked by loss-of-function mutations of PINK1 and Parkin [1]. Thus, the association between Parkin, mitophagy, and Parkinson's disease might be a case of “true, true, and not causally related”. Along the same reasoning, here I examine evidence both in support of, and that seemingly contests, the notion that Parkin-mediated mitophagy plays a meaningful role in helping to maintain homeostatic mitochondrial quality in normal (i.e. unstressed, undamaged and relatively young) vertebrate hearts.
The evolution of current concepts of Parkin-mediated mitophagy
To better understand ambiguities pertaining to a physiologically significant role of Parkin-mediated mitophagy in the heart, here I briefly describe the history of research in this area. Over time, greater mechanistic understanding of mitophagy pathways has prompted revision of original concepts that were seemingly straightforward in ways that suggest striking biological complexity. Thus, biological determinism has, after consideration of multi-functionality of Parkin pathway proteins and parallel or alternate mitophagy mechanisms, given way to a more probabilistic understanding of mitochondrial quality control. For similar reasons, currently accepted concepts are certain to evolve further according to future findings. Thus, what we currently think we “know” about Parkin-mediated mitophagy is an approximation. Accordingly, healthy skepticism seems appropriate when faced with conventional wisdom in this area, just as an open mind can be useful when challenged with unconventional findings.
The idea that PINK1 and Parkin interact derives from results of Drosophila studies designed to functionally interrogate genes implicated in autosomal recessive Parkinson's disease. It is important to recognize that most cases of Parkinson's disease do not have a monogenic cause, and only ~10% of cases are familial. Loss of function mutations in Park2 (encoding Parkin) and Park6 (encoding PINK1) are the most commonly associated genetic defects [2]. Notwithstanding strong genetic associations in autosomal recessive Parkinson's disease, it was not intuitively obvious how the mitochondrial-localized kinase PINK1 interacted with the cytosolic E3 ubiquitin ligase Parkin to evoke similar clinical syndromes. Indeed, a functional connection between the two proteins was not really considered until ~10 years ago when two simultaneous reports in Nature described genetic complementation between PINK1 and Parkin in mutant Drosophila fruit fly lines [3, 4]. The early studies identified mitochondrial abnormalities in flight muscles of Drosophila lacking Parkin [5, 6], and subsequently in flies with PINK1 deficiency [3, 4, 7], implicating both factors in mitochondrial homeostasis. In crossover rescue studies it was observed that transgenic overexpression of PINK1 did not alter phenotypes of Parkin mutant flies, whereas transgenic expression of Parkin fully compensated for absence of PINK1 [3, 4, 7]. These results supported a mitochondrial quality control mechanism wherein PINK1 and Parkin interact in the same disease pathway, and Parkin is downstream of PINK1. There is, however, a complicating observation that challenges the conclusion that genetic complementation of PINK1 and Parkin in flies identified interrupted mitophagy as a cause of Parkinson's disease: Parkin does not translocate effectively to mitochondria of PINK1-deficient mammalian cells [8, 9]. If Parkin is likewise dependent upon PINK1 for translocation to mitochondria in Drosophila, then Parkin overexpression should not repair mitophagy in PINK1 mutant flies, and the pathophysiological underpinnings of cellular dysfunction and clinical and experimental phenotypes provoked by PINK1 and Parkin mutations remain unclear [1]. Nevertheless, subsequent studies in flies [10] and cultured mammalian cells [11] have largely confirmed the original observations that PINK1 and Parkin can interact to promote mitophagy, have revealed evolutionary conservation of this mechanism, and have identified additional mechanistic details as recently reviewed [12-14].
The author was relatively unfamiliar with these subtleties when, approximately 3 years ago, our laboratory first began asking questions about the role of mitophagy in mammalian and invertebrate cardiac homeostasis and heart disease. Accordingly, we evaluated available data without preconceptions about prevailing concepts, and without any biases for or against particular outcomes. From this perspective, here I succinctly review aspects of Parkin biology in which I feel that the published data support a high degree of confidence. This is not intended to be a comprehensive mechanistic overview, but a highlighting of important mechanisms.
After the discovery that PINK1 and Parkin act sequentially to promote mitophagy in Drosophila and vertebrate cells, the central question to be answered was how these factors collaborate to identify and selectively target dysfunctional mitochondria. This was vexing because PINK1 protein is constitutively mitochondrial, but present in such small amounts in healthy cells as to stretch the limits of detection [8]. On the other hand, Parkin protein can be detected in many cell types, but is largely cytosolic. Thus, the mitophagy trigger mechanism had to explain how a kinase that is vanishingly rare and exclusively present in mitochondria “talked” to an E3 ubiquitin ligase located in the cytosol. The breakthrough in understanding was made by Narendra et al, who observed that PINK1 is almost immediately degraded upon entrance into normal, healthy mitochondria (accounting for its virtual absence), but that its proteolytic degradation is suppressed in pharmacologically impaired mitochondria [8]. An overview of the elegant molecular mechanism of PINK1 import and degradation (or not, depending upon the functional status of the mitochondrion) as described by Youle and colleagues is provided in one of their recent reviews [12]. The major point is that PINK1 can only be an effective mitophagy signaling kinase in damaged mitochondria because in healthy mitochondria it is immediately degraded. The development of Parkin tagged with fluorescent markers, such as maraschino cherry (mc) red (mcParkin), made it possible to directly observe functional interactions between PINK1 and Parkin. Thus, depolarization of mitochondria with the uncoupling agents FCCP and CCCP (the original model for PINK1 stabilization [8]) promotes translocation of Parkin from the cytosol to mitochondria; localization of Parkin to depolarized mitochondria is massively impaired in PINK1-deficient cells [8, 9].
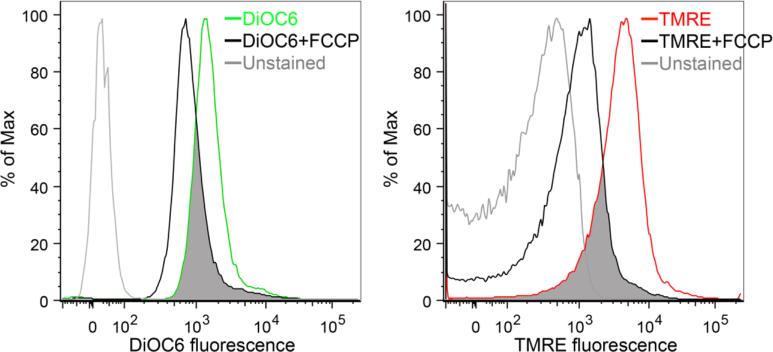
It is important to recognize that the physiological stimuli that provoke mitochondrial PINK1 stabilization are not precisely known. Because dissipation of the mitochondrial inner membrane electrochemical potential by pharmacologically poisoning the mitochondrial electron transport system (as with FCCP, CCCP and antimycin A) was used in initial studies describing mitochondrial accumulation of PINK1 and localization of Parkin, “mitochondrial depolarization” is frequently considered to be the relevant stimulus. The most compelling evidence to support this notion is the observation that the smaller product of an asymmetric mitochondrial fission event is hypopolarized compared to its larger hyperpolarized sibling, and is preferentially targeted for mitophagy [15]. However, healthy mitochondria exhibit a range of mitochondrial membrane potential, in comparison with pharmacologically depolarized mitochondria (Figure 1). Accordingly, terms such as “depolarized” that infer a binary state of mitochondria (polarized or not) can be misleading. Indeed, normal tissue must have a basal rate of mitophagy for homeostatic mitochondrial quality control, but standard experimental methods only recognize severely damaged mitochondria because artificial models have trained us to look for extremes of mitochondrial hypopolarization that should not normally occur. By extension, unless mitophagy is impaired, mitochondria that achieve the physiological threshold for their homeostatic elimination will be promptly removed. For this reason (and whether or not mitochondrial hypopolarization is the most relevant stimulus for housekeeping mitophagy), mitochondria appear normally polarized when mitophagy is functioning normally or is accelerated [16, 17]. Importantly, Youle's group has demonstrated that PINK1 can accumulate in mitochondria with apparently normal inner membrane potential, in response to misfolded mitochondrial proteins [18]. Thus, there appear to be multiple triggers of the PINK1-Parkin pathway under differing pathophysiological conditions, and those triggers that are the most relevant to housekeeping mitochondrial quality control (verses for the reactive elimination of damaged mitochondria after injury and in diseased hearts) have not yet been clearly defined.
Figure 1. Flow cytometry tracings demonstrating a range of mitochondrial membrane polarization in normal hearts.
Membrane polarization was measured either by staining mitochondria isolated from normal mouse hearts with green 3,3’-Dihexyloxacarbocyanine Iodide (DiOC6; left) or red Tetramethylrhodamine ethyl ester (TMRE; right). Colored tracings are at baseline and black tracings are after mitochondrial depolarization with 50 nM FCCP (20 min @ room temperature). Overlap, indicating hypopolarized mitochondria, is shaded grey.
Having developed an understanding of how PINK1-Parkin mediated mitophagy is specifically triggered by dysfunctional mitochondria, the next question was how mitochondrial PINK1 communicates with cytosolic Parkin to stimulate its translocation to individual damaged organelles. Because Parkin functionality as an E3 ubiquitin ligase requires that it bind to its substrate proteins, of which there are a hundred or more [19], it is not surprising that numerous Parkin binding proteins have been identified. Our group determined that the mitochondrial outer membrane fusion protein, mitofusin (Mfn) 2, was phosphorylated in cells overexpressing PINK1, and that this phosphorylation event provoked Mfn2-Parkin binding [20]. We further observed that Parkin fails to efficiently translocate to the depolarized mitochondria of cardiomyocytes isolated from cardiac-specific Mfn2 knockout mice [20]; a similar Parkin translocation defect was reported in Mfn2-deficient neurons [21]. We identified two critical phosphorylated residues on Mfn2 and found that their mutation to prevent phosphorylation (alanine substitution) suppressed PINK1-stimulated Mfn2-Parkin binding, whereas their mutation to mimic phosphorylation (glutamate substitution) promoted PINK1-independent Mfn2-Parkin binding. Accordingly, we proposed that PINK1-phosphorylated Mfn2 acts as a “receptor” for Parkin, attracting it to damaged mitochondria in which PINK1 has been stabilized [22, 23]. This central role for Mfn2 in Parkin-mediated mitophagy was unexpected and has been viewed by some as controversial. One concern is that Mfn2 may only bind Parkin in the heart [24], but this seems unlikely based on similar Parkin translocation defects in Mfn2-deficient neurons [21]. It has also been noted that Parkin can undergo FCCP-mediated mitochondrial translocation in murine fibroblasts lacking Mfn2 [11], which we have confirmed [9]. We do not view Parkin translocation to Mfn2-deficient mitochondria as proof that PINK1-phosphorylated Mfn2 does not function as a Parkin receptor. Rather, the more conservative conclusion is that parallel pathways or functional redundancies can compensate when Mfn2 in congenitally absent. In other words, Mfn2 is not the ONLY Parkin receptor, just as Parkin is not the ONLY mediator of mitophagy (vide infra). Finally, in our studies we inferred from manipulating PINK1, Mfn2, and Parkin that PINK1 directly phosphorylates Mfn2 in mitochondria. However, it was possible that PINK1 acted indirectly through one or more third parties, i.e. that PINK1 phosphorylated something else that did something further that resulted in Mfn2 being phosphorylated on the two critical residues. We recently resolved this uncertainty by demonstrating direct PINK1-mediated phosphorylation of Mfn2 in a cell-free system [9].
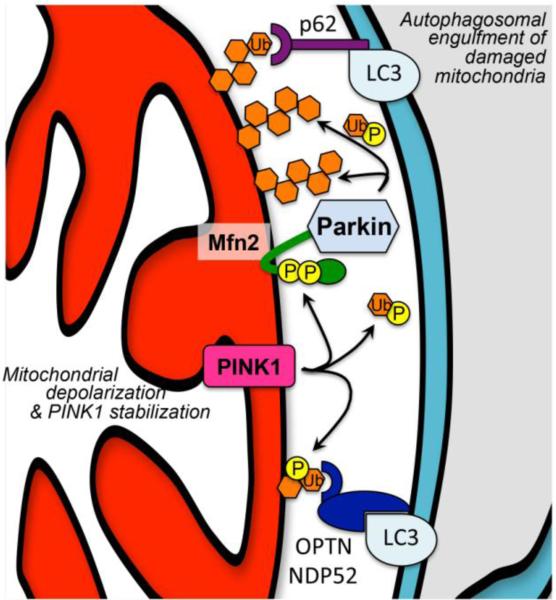
To summarize, damaged mitochondria exhibit impaired PINK1 proteolysis, permitting PINK1 to accumulate and phosphorylate Mfn2 that attracts Parkin, which ubiquitinates outer membrane proteins that mark the organelle for autophagosomal engulfment (Figure 2). A remaining question was how Parkin could exhibit robust ubiquitin ligase activity for mitochondrial proteins, but not for proteins in the cytosol where Parkin normally resides. A series of recent studies have revealed that PINK1-phosphorylated ubiquitin is the preferred substrate for Parkin [25-27]. Thus, PINK1 kinase activity not only drives Parkin localization to mitochondria by phosphorylating Mfn2, but it controls Parkin ubiquitin ligase activity for mitochondrial proteins by phosphorylating ubiquitin itself. Another new report indicates that two xenophagy pathway proteins, NDP52 and optineurin, can promote mitophagy independent of Parkin by functioning as autophagy receptors in HeLa cells after being tagged with PINK1-phosphorylated ubiquitin [28] (Figure 2) The relationship of this PINK1-mediated Parkin-independent mitophagy pathway to PINK1-Mfn2-Parkin signaling is unclear. Can the two processes occur simultaneously, or are they mutually exclusive? Is it possible that one pathway acts preferentially as a mitophagy trigger, whereas the other is an amplifier? Moreover, processes and pathways described in cultured HeLa or other cells need to be confirmed and extended to in vivo cardiac myocytes and neurons before their pathophysiological relevance can be understood in human heart and neurological diseases. We believe that in vivo studies are essential to the full understanding of the consequences of molecular interactions between PINK1, Parkin, Mfn2, and ubiquitin in different tissues and under conditions of both normal homeostasis and abnormal or diseased organ functioning.
Figure 2. Parkin-dependent and – independent mechanisms of mitophagy.
PINK1 stabilization initiates both pathways. Upper: phosphorylation of Mfn2 recruits cytosolic Parkin, and of ubiquitin activates Parkin, provoking ubiquitination of outer mitochondrial membrane proteins that attract atuophagosomal LC3 via docking protein p62. Lower: phosphorylation of ubiquitinated membrane proteins attracts autophagosomal LC3 via autophagy receptors optineurin (OPTN) or nuclear dot protein 52 (NDP52). The relative roles of each pathway in hearts and elsewhere are unknown.
Lessons learned from in vivo genetic models of Parkin deficiency
It is notable that many of the seminal scientific discoveries about the Parkin pathway for mitophagy derived not from genetic mouse models, but from fruit flies [29]. This is not because flies are intrinsically better models of either basic cell biology or human disease, but because mouse models failed to develop expected phenotypes. Identification of loss-of-function mutations in the Parkin (Park2) and PINK1 (Park6) genes as causative for hereditary autosomal recessive Parkinson's disease prompted the creation of analogous mouse models, in which Parkin or PINK1 were ablated in the mouse germ-line. As a brief summary of the results of dozens of studies, mice lacking these Parkinson's disease genes do not develop obvious Parkinson's disease-like phenotypes. Not only don't they develop full-blown Parkinson's disease, but one has to look very carefully [30] or compound mitochondrial injury as by combining Parkin null and mito-mutator (mitochondrial DNA polymerase G proofreading mutant) mice [31], to uncover histopathological or functional evidence for neurological damage. Does absence of Parkinson's disease phenotypes in Parkin knockout mice mean that the clinical studies linking Parkin mutations to human Parkinson's disease were erroneous (and likewise for PINK1)? Obviously not: partial loss of function of a protein is not identical to complete genetic ablation of the protein (a distinction that has sometimes been overlooked). Consider that mutational loss of function in nature is generally incomplete; a mutant protein with impaired functionality continues to be expressed. Conventional germ-line gene ablation in mice, typically involving insertion of an antibiotic resistance cassette with or without deletion of a coding exon, rarely results in protein expression because the abnormal mRNA undergoes nonsense-mediated decay. Thus, with engineered gene ablation there is zero functionality. Paradoxically, complete abrogation of function of a widely expressed protein is more likely than limitation or alteration of function to provoke developmental compensation (and if compensation is not successful the result is likely to be early embryonic lethality). In mice, developmental compensation in germ-line knockout models can often be avoided by restricting the gene deletion to specific non-critical tissues or by deleting the gene after developmental plasticity wanes using Cre-Lox or similar systems [32]. For this reason, when Parkin floxed alleles were specifically deleted in dopaminergic neurons of the substantia nigra of mouse brains (using stereotaxically injected viral Cre) the mice developed features of Parkinson's disease [33].
Germ-line Parkin ablation in mice has few effects on normal hearts. Gustafsson's group examined cardiac function in germ-line Parkin knockout mice and uncovered mitochondrial dysmorphology without any adverse effects on cardiac function in mice under 1 year of age [34, 35]. It is possible that these studies of Parkin knockout mice suffered from the limitations described above, i.e. developmental induction of ancillary or redundant mitophagy pathways that can opportunistically compensate for absent Parkin. Indeed, RNA sequening of adult germ-line Parkin knockout hearts revealed hundreds of functionally connected counter-regulated genes [37]. However, a recent report describing cardiomyocyte-specific ablation of Parkin in adult mice, which should have avoided developmental compensation, likewise found no adverse effects in otherwise normal younger mice [16]. Thus, the totality of evidence to date suggests that Parkin-mediated mitophagy is of secondary importance for homeostatic mitochondrial quality control in normal (unstressed) young adult hearts. On the other hand, evidence is accumulating that Parkin can play an important conditional role after cardiac or mitochondrial injury. Indirect evidence for a protective role of Parkin in overcoming age-dependent cardiac dysfunction was provided by studies of Parkin-overexpressing senescent mouse hearts and in Drosophila with cardiomyocyte-specific Parkin deficiency [36, 37]. Moreover, Kubli et al described diminished mitophagy, exaggerated tissue damage, and increased lethality after experimental myocardial infarction of germ-line Parkin knockout mice [35], implicating Parkin in tissue protection after ischemic injury.
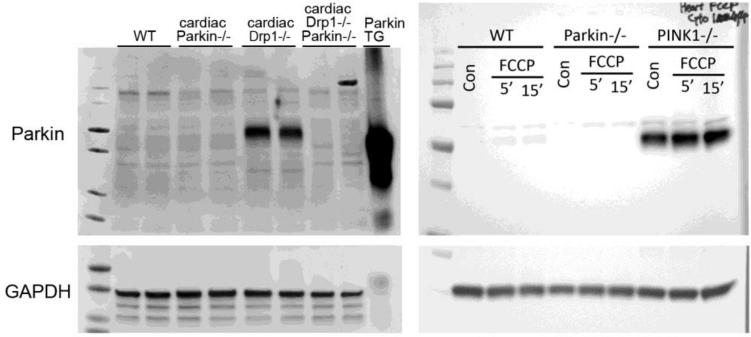
In both hearts and brains congenital systemic absence of Parkin did little to alter basal function, but in hearts Parkin ablation impeded recovery from myocardial injury [35]. The apparent discrepancy is resolved if Parkin is primarily a stress-activated factor in hearts: Parkin ablation would not be expected to have a significant effect on healthy hearts if it is not normally expressed at levels necessary for constitutive mitophagic activity. On the other hand, Parkin deletion could still alter the cardiac response to injury if it is a stress-inducible factor in hearts. Consistent with this notion, Kubli et al described marked Parkin upregulation after myocardial infarction [35]. More recently, Parkin mRNA and protein expression were also observed to be induced in mouse hearts deficient in the mitochondrial fission protein, dynamin-related protein 1 (Drp1) [16], and in hearts deficient in PINK1 [38]. In these reports, basal cardiac Parkin protein levels were at or below the level of detection using standard immunoblotting techniques (Figure 3). Moreover, RNA sequencing data from multiple independent sources indicate that Parkin mRNA abundance in normal adult mouse and human hearts is at levels that correspond to only 1-2 transcripts per cell, compared to dozens or hundreds of transcripts per cell for Pink1/Park6 and Mfn2 (Table 1). Thus, absence of a meaningful cardiac phenotype in otherwise normal adult hearts deficient in Parkin, either from germ-line gene ablation or conditional cardiomyocyte-specific gene ablation, may best be explained not by induction of alternate mitophagy pathways, but by absence of functionally meaningful levels of Parkin mRNA and protein in normal unstressed adult hearts. Likewise, adverse outcomes induced by Parkin deficiency after myocardial infarction (wherein mitophagy is needed to clear dysfunctional mitochondria; [35]) and improved outcomes induced by conditional Parkin deficiency in Drp1 deficient hearts (wherein accelerated mitophagy provoked by interruption of mitochondrial fission contributes to mitochondrial depletion and cardiomyopathy [16, 17], is explained by induction of Parkin in response to cardiac injury and mitochondrial stress.
Figure 3. Uncropped immunoblots of cytosolic Parkin in different types of mouse hearts.
On the left are original data from reference 16 demonstrating Parkin upregulation in hearts rendered acutely defective in mitochondrial fission by conditional ablation of Drp1 (30 μg protein/lane). On the right are original data from reference 38 demonstrating Parkin upregulation in PINK1 knockout (KO) mouse hearts (21.5 μg protein/lane). In both sets of blots WT is wild-type (normal adult) heart. Parkin−/− is conditional cardiac-specific Parkin KO (left) and germ-line Parkin KO (right). Parkin transgenic (TG) mouse heart on left blot serves as positive control (1 μg protein). GAPDH is protein loading control. Note minimal expression of Parkin in WT compared to Parkin−/− hearts in both studies, and marked increase in Parkin protein with mitochondrial stress evoked by Drp1 or PINK1 ablation. Immunoblots on the right were provided by, and used with permission of, Dr. Asa Gustafsson (UCSD).
Table 1.
Cardiac Parkin, PINK1, and Mfn2 mRNA levels from public RNA-Seq data
| Source tissue | Park2 FPKM | Pink1 FPKM | Mfn2 FPKM | NCBI GEO |
|---|---|---|---|---|
| Nonfailing human heart (8) | 5.2 | 79.1 | 117.0 | GSE46224 |
| Illumina Human Body Map 2.0, human heart HCT20143 (1) | 5.9 | 66.1 | 97.2 | GSE30611 |
| Illumina Human Body Map 2.0, human brain HCT20160 (1) | 3.5 | 64.7 | 26.3 | GSE30611 |
| E13.5 mouse heart (7) | 0.6 | 35.3 | 63.4 | GSE58455 |
| P1 mouse heart (6) | 1.4 | 86.2 | 163.7 | GSE68921 |
| P21 mouse heart (3) | 1.3 | 229.6 | 265.5 | GSE68921 |
| 5 week-old mouse heart (4) | 1.6 | 272.1 | 292.5 | GSE68921 |
| Sham-operated mouse heart (5) | 1.6 | 247.2 | 241.0 | GSE29446 |
| Sham-operated mouse heart (4) | 1.5 | 182.3 | 207.4 | GSE35350 |
| 17 week-old mouse heart (6) | 1.4 | 88.8 | 66.7 | GSE73909 |
| Adult mouse heart (pooled) | 1.3 | 146.4 | 158.4 | GSE49906 |
| Isolated adult mouse cardiac myocytes (pooled) | 1.1 | 325.7 | 265.3 | GSE49906 |
| Isolated adult mouse cardiac fibroblasts (pooled) | 0.9 | 15.9 | 7.0 | GSE49906 |
Data are RNA-Seq expression in FPKM (fragments per kilobase of exon per million fragments mapped); FPKM of 3 = approximately 1 mRNA copy/cell. NCBI Gene Expression Omnibus data set sources for each interrogation are provided. Numerals in parentheses indicate number of biological replicates. Pink1 is formerly known as Park6. Mfn2 is phosphorylated by PINK1 to become a mitochondrial outer membrane receptor for Parkin.
Parkin-mediated mitochondrial turnover and cardiac metabolic maturation
Together, the above data suggest either that absence of Parkin in normal hearts (wherein basal Parkin levels are already low) is not sufficient to stimulate compensatory induction of alternate mitophagy pathways, or that alternate mitophagy pathways induced to promote homeostatic mitochondrial quality control are distinct from, or inadequate to fully compensate for, mitophagy pathways induced by cardiac injury. Recent results support the latter: whereas germ-line Parkin knockout mice exhibit normal cardiac development through adulthood [35], cardiomyocyte-specific deletion of Parkin on the first day of life proved rapidly lethal and provoked abnormal retention of embryonic mitochondria [9].
Early lethality of perinatal cardiac-specific Parkin knockout mice exposed, but did not delineate specific characteristics of, an essential role for Parkin in hearts. Because cardiac Parkin deletion from adult mice of the same line is well tolerated [16], we considered that perinatal lethality must reflect loss of a temporally-restricted developmental effect of Parkin. However, the aggressive phenotype confounded mechanistic interrogations. Accordingly, we engineered mice in which conditional cardiomyocyte-specific expression of the PINK1 non-phosphorylatable Mfn2 AA mutant (a normal mitochondrial fusion protein, but a dominant inhibitor of Parkin localization to mitochondria; [9]) was used to specifically interdict Parkin-mediated mitophagy in the heart at various times after birth. Cardiomyocyte expression of wild-type Mfn2 from birth (controlling for increased cardiac Mfn2 levels) had no effect on mitochondrial or cardiac function. Likewise, expression of Mfn2 AA in young adult mice (8 weeks age) or at weaning (3 weeks age) had no effect on the heart or its mitochondria. Strikingly however, expressing Mfn2 AA in hearts from birth largely recapitulated the phenotype of perinatal cardiac Parkin ablation, including retention of embryonic mitochondria [9]. Although Parkin has roles in processes other than mitophagy [1], Mfn2 AA is functionally distinguished from normal Mfn2 only in that it lacks the ability to recruit Parkin to mitochondria. Thus, we concluded that absence of mitochondrial maturation in perinatal cardiac Parkin knockout and perinatal Mfn2 AA transgenic mice was, in both instances, the consequence of interrupting Parkin-mediated mitophagy. Moreover, comprehensive transcriptome analyses and metabolic profiling of Mfn2 AA hearts revealed that mitophagic impairment prevented normal maturation of myocardial metabolism, i.e. transitioning from preferential utilization of carbohydrates in the fetal heart to fatty acids and amino acids in the adult [39, 40]. Together, these results confirm that Parkin-mediated mitophagy has little role in homeostatic mitochondrial quality control in fully developed normal hearts (because neither cardiac-specific Parkin ablation nor Mfn2-AA expression in adult hearts Is damaging). However, the findings revealed a central role for Parkin-mediated mitophagy in multiple aspects of perinatal cardiomyocyte mitochondrial maturation. The preeminence of mitophagy in perinatal mitochondrial maturation suggests that glycolytic embryonic mitochondria are not simply transcriptionally reprogrammed for fatty acid metabolism, but that embryonic organelles must be removed and then replaced with biogenically derived mature mitochondria having a substrate utilization profile tuned for extra-uterine life [9]. Whether Parkin-mediated mitochondrial turnover has a similar role in the reverse metabolic transition (from fatty acids to carbohydrates) that occurs in diseased hearts [41] is not known.
Parkin-independent mitophagy and the heart
While the PINK1-Mfn2-Parkin pathway is the best understood mechanism for the targeted elimination of dysfunctional mitochondria at the molecular level, other mechanisms exist. At a phenomenological level, Parkin-deficient cells retain a limited ability for lysosomal engulfment of depolarized mitochondria [11]. Moreover, mice in which the PINK1-Parkin pathway is interrupted either by genetic deletion of Parkin or of its Mfn2 receptor nevertheless are capable of mounting a mitophagic response [42, 43]. Thus, there appear to be multiple mitophagy pathways, many of which are poorly or only recently characterized [44]. Perhaps equally as important as identifying the specific molecular events that can mediate mitophagy will be developing a more complete understanding of the functioning of generalized and targeted mitochondrial elimination by the autophagosomal apparatus, and more fully delineating the normal and pathological conditions under which distinct mitophagy pathways are activated.
If mitophagy is defined as mitochondrial elimination by autophagy, then it is obvious that general macroautophagy activated by starvation or nutrient deprivation will involve mitochondria. Thus, the experimental or clinical finding of mitochondria inside of autophagosomes or lysosomes will occur during cellular autophagy [45]. For the same reasons, mitophagy marked by co-localization of immunoreactive mitochondrial and autophagosomal proteins in a given subcellular fraction will be detected during generalized autophagy. However, this form of non-specific “mitochondrial autophagy” during general cellular autophagy is not a mechanism for targeted elimination of damaged organelles. It is important to distinguish between mitochondrial autophagy as a component of a generalized autophagic response and mitophagy as a mechanism for mitochondrial quality control via the specific identification, functional sequestration, and removal of damaged organelles.
Mitochondrial death proteins Nix/Bnip3L and Bnip3 are abundant in mouse hearts [46-49] and can act as targeting molecules and adaptor proteins that attract autophagosomes to mitochondria, either in concert with or independent of Parkin [50]. Accumulation of abnormal cardiomyocyte mitochondria and progressive cardiac dilatation with contractile dysfunction in mice with cardiac-specific combined ablation of Nix and Bnip3 [51] supports significant roles for these two factors in homeostatic mitochondrial quality control in normal hearts.
Less well-defined Parkin-independent mechanisms of mitophagy can be inferred from data revealing their effects in the absence of a fully functional PINK1-Parkin pathway. The observation that Parkin null mice exhibit an intact mitophagy response [42] is unambiguous evidence supporting the existence of one or more mitochondrial quality control mechanisms able to substitute or compensate for absent Parkin. Our laboratory interrogated mitochondrial quality control in mice lacking Mfn2, which promotes Parkin recruitment to depolarized mitochondria. Mitochondrial fitness deteriorated over a period of 16-40 weeks in hearts lacking Mfn2, exhibiting multiple abnormalities including increased production of reactive oxygen species (ROS) [20, 43]; ROS were a likely candidate for functional impairment of older Mfn2 null hearts. Indeed, and as expected if ROS elaborated by dysfunctional mitochondria that escape mitophagic elimination are ultimately cardio-toxic, reduction of mitochondrial ROS back to normal with a mitochondrial localized catalase transgene delayed the progressive cardiomyopathy induced by cardiomyocyte-specific Mfn2 ablation [43]. Surprisingly however, so-called “super-suppression” of ROS to levels that markedly below normal had the opposite effect, aggravating the cardiomyopathy and further impairing mitochondrial fitness. We concluded that mitochondrial ROS can function as a signal for one or more as-yet-unspecified Parkin-Mfn2 independent mitochondrial quality control mechanisms [43]. Other reports support the notion that mitochondrial ROS play a role in autophagy signaling [52].
Parkin knockout mice have been bred to mice deficient in the mitochondrial fission protein Drp1 by two groups interrogating Parkin-dependent mitophagy as a mechanism for cardiomyopathy induced by cardiac-specific Drp1 gene deletion. There are notable differences in experimental design and conclusions between the two studies [53]. Kageyama et al determined that mitophagy was triggered, but interrupted, in a small cohort of mice that developed early cardiomyopathy after floxed Drp1 alleles were homozygously deleted in cardiomyocytes from approximately the time of birth (using a Myh6 promoter-driven Cre transgene) [54]. Crossing these cardiac Drp1 knockout mice with germ-line Parkin knockout mice did not rescue the cardiomyopathy, leading the authors to conclude that the mitophagy triggered by Drp1 deletion is Parkin-independent. As detailed above, germ-line Parkin ablation may be problematic as a model for defining in vivo Parkin functioning.
Our group interrogated mitophagy mechanisms in the cardiomyopathy provoked by cardiac Drp1 deficiency using conditional, tissue-specific gene manipulation. Like Kageyama, we also found that perinatal deletion of Drp1 caused early cardiac disease [17]. In our hands the cardiomyopathy was so aggressive that it was not possible to distinguish between direct and indirect disease-related consequences of Drp1 deletion. Accordingly, we abandoned the conventional Myh6-Cre system in favor of a tamoxifen-inducible Myh6 promoter-modified estrogen receptor Cre chimeric protein to delete Drp1 in adult hearts: mice were permitted to grow for 8 weeks with normal Drp1, at which time tamoxifen was administered to delete the Drp1 gene specifically in cardiac myocytes [17]. Under these conditions the dilated cardiomyopathy provoked by interrupting mitochondrial fission progressed for 6 weeks and was ultimately fatal. Mitochondria of adult Drp1-deficient hearts exhibited the expected morphological enlargement (from unopposed fusion), and their respiratory function was normal. However, cardiac mitochondrial mass markedly decreased over time whereas markers of mitochondrial engulfment by autophagosomes (mitophagy) increased, suggesting that mitochondrial depletion from enhanced mitophagy contributed to cardiomyocyte dropout and lethal heart failure [17].
In a follow-up study designed to determine if Parkin transduced the accelerated mitophagy observed in Drp1 deficient hearts, we used a combined conditional cardiac-specific gene deletion approach to simultaneously delete Drp1 and Parkin in adult mice, reasoning that the tissue-specific Parkin deletion approach would avoid confounding developmental compensation. As predicted, conditional Parkin deletion in this manner prevented Parkin upregulation induced by Drp1 deletion (see Figure 3), and both improved and delayed the resulting cardiomyopathy [16]. These studies not only demonstrate that stress-inducible Parkin-mediated mitophagy contributes to mitochondrial depletion and cardiomyopathy in hearts with an acutely imposed defect in mitochondrial fission, but further demonstrate advantages of tissue-specific Parkin deletion over germ-line Park2 ablation for revealing otherwise latent Parkin functioning in vivo.
What the future may hold...
Many mechanistic assumptions have been made about Parkin and mitophagy based on early studies in Drosophila and follow-up studies in cultured mammalian cells. However, the relevance of these mechanisms to cardiac health, and even to human Parkinson's disease, is uncertain. Germ-line Parkin ablation in mice has proven to be an imperfect experimental model that, in part because of compensation from alternative pathways and in part because Parkin is a stress-inducible factor, does not always or accurately reflect the role of Parkin in vertebrate pathophysiology. Tissue-specific Parkin ablation or suppression, especially in a conditional manner, is showing promise as a better approach for revealing occult and pathophysiologically relevant Parkin functions in hearts, neurons, and other tissues [9, 35, 44]. In adult mouse hearts, Parkin in expressed at very low levels but is markedly induced by injury and mitochondrial stress. Absence of any acutely deleterious effect of conditional cardiac-specific Parkin deletion in otherwise normal adult hearts, and the paucity of Parkin protein and mRNA in healthy adult hearts, indicate that it has a limited role in normal homeostatic cardiac mitochondrial quality control. Instead, Parkin expression and its PINK1/Mfn2-mediated mitochondrial localization and activation appear to be components of a broader reactive response to cardiac injury or stress. Finally, it is often overlooked that Parkin does more than just mediate mitophagy. As nicely reviewed [1, 56], Parkin regulates mitochondrial biogenesis [33, 55], mitochondrial dynamism (fission and fusion) [57-61], and selective protein extraction from mitochondria via vesicle formation [62]. Parkin multifunctionality constitutes an unavoidable confounder for in vivo Parkin knockout studies that cannot be clarified by its conditional or tissue-specific genetic deletion. Until we have results of more in vivo studies using tools that selectively interrupt some Parkin functionality while maintaining others, it is advisable to maintain an open mind regarding the relative roles of Parkin-dependent and -independent mitophagy in maintaining normal heart health, and the consequence of mitophagic dysfunction on experimental and clinical pathological entities affecting the heart.
Highlights.
This review highlights recent findings and new interpretations of older data that challenge conventional views of the physiologically relevant role for Parkin in hearts.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Scarffe LA, Stevens DA, Dawson VL, Dawson TM. Parkin and PINK1: much more than mitophagy. Trends Neurosci. 2014;37:315–24. doi: 10.1016/j.tins.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klein C, Westenberger A. Genetics of Parkinson's disease. Cold Spring Harb Perspect Med. 2012;2:a008888. doi: 10.1101/cshperspect.a008888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clark IE, Dodson MW, Jiang C, Cao JH, Huh JR, Seol JH, et al. Drosophila pink1 is required for mitochondrial function and interacts genetically with parkin. Nature. 2006;441:1162–6. doi: 10.1038/nature04779. [DOI] [PubMed] [Google Scholar]
- 4.Park J, Lee SB, Lee S, Kim Y, Song S, Kim S, et al. Mitochondrial dysfunction in Drosophila PINK1 mutants is complemented by parkin. Nature. 2006;441:1157–61. doi: 10.1038/nature04788. [DOI] [PubMed] [Google Scholar]
- 5.Greene JC, Whitworth AJ, Kuo I, Andrews LA, Feany MB, Pallanck LJ. Mitochondrial pathology and apoptotic muscle degeneration in Drosophila parkin mutants. Proc Natl Acad Sci U S A. 2003;100:4078–83. doi: 10.1073/pnas.0737556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pesah Y, Pham T, Burgess H, Middlebrooks B, Verstreken P, Zhou Y, et al. Drosophila parkin mutants have decreased mass and cell size and increased sensitivity to oxygen radical stress. Development. 2004;131:2183–94. doi: 10.1242/dev.01095. [DOI] [PubMed] [Google Scholar]
- 7.Yang Y, Gehrke S, Imai Y, Huang Z, Ouyang Y, Wang JW, et al. Mitochondrial pathology and muscle and dopaminergic neuron degeneration caused by inactivation of Drosophila Pink1 is rescued by Parkin. Proc Natl Acad Sci U S A. 2006;103:10793–8. doi: 10.1073/pnas.0602493103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Narendra DP, Jin SM, Tanaka A, Suen DF, Gautier CA, Shen J, et al. PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol. 2010;8:e1000298. doi: 10.1371/journal.pbio.1000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gong G, Song M, Csordas G, Kelly DP, Matkovich SJ, Dorn GW., 2nd. Parkin-mediated mitophagy directs perinatal cardiac metabolic maturation in mice. Science. 2015 doi: 10.1126/science.aad2459. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ziviani E, Tao RN, Whitworth AJ. Drosophila parkin requires PINK1 for mitochondrial translocation and ubiquitinates mitofusin. Proc Natl Acad Sci U S A. 2010;107:5018–23. doi: 10.1073/pnas.0913485107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Narendra D, Tanaka A, Suen DF, Youle RJ. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol. 2008;183:795–803. doi: 10.1083/jcb.200809125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pickrell AM, Youle RJ. The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson's disease. Neuron. 2015;85:257–73. doi: 10.1016/j.neuron.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dorn GW, 2nd, Kitsis RN. The mitochondrial dynamism-mitophagy-cell death interactome: multiple roles performed by members of a mitochondrial molecular ensemble. Circ Res. 2015;116:167–82. doi: 10.1161/CIRCRESAHA.116.303554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shirihai OS, Song M, Dorn GW., 2nd. How mitochondrial dynamism orchestrates mitophagy. Circ Res. 2015;116:1835–49. doi: 10.1161/CIRCRESAHA.116.306374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Twig G, Elorza A, Molina AJ, Mohamed H, Wikstrom JD, Walzer G, et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 2008;27:433–46. doi: 10.1038/sj.emboj.7601963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song M, Gong G, Burelle Y, Gustafsson AB, Kitsis RN, Matkovich SJ, et al. Interdependence of Parkin-Mediated Mitophagy and Mitochondrial Fission in Adult Mouse Hearts. Circ Res. 2015;117:346–51. doi: 10.1161/CIRCRESAHA.117.306859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song M, Mihara K, Chen Y, Scorrano L, Dorn GW., 2nd. Mitochondrial fission and fusion factors reciprocally orchestrate mitophagic culling in mouse hearts and cultured fibroblasts. Cell Metab. 2015;21:273–85. doi: 10.1016/j.cmet.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jin SM, Youle RJ. The accumulation of misfolded proteins in the mitochondrial matrix is sensed by PINK1 to induce PARK2/Parkin-mediated mitophagy of polarized mitochondria. Autophagy. 2013;9:1750–7. doi: 10.4161/auto.26122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sarraf SA, Raman M, Guarani-Pereira V, Sowa ME, Huttlin EL, Gygi SP, et al. Landscape of the PARKIN-dependent ubiquitylome in response to mitochondrial depolarization. Nature. 2013;496:372–6. doi: 10.1038/nature12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Y, Dorn GW., 2nd. PINK1-phosphorylated mitofusin 2 is a Parkin receptor for culling damaged mitochondria. Science. 2013;340:471–5. doi: 10.1126/science.1231031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee S, Sterky FH, Mourier A, Terzioglu M, Cullheim S, Olson L, et al. Mitofusin 2 is necessary for striatal axonal projections of midbrain dopamine neurons. Hum Mol Genet. 2012;21:4827–35. doi: 10.1093/hmg/dds352. [DOI] [PubMed] [Google Scholar]
- 22.Dorn GW., 2nd. Mitochondrial dynamism and cardiac fate--a personal perspective. Circ J. 2013;77:1370–9. doi: 10.1253/circj.cj-13-0453. [DOI] [PubMed] [Google Scholar]
- 23.Song M, Dorn GW., 2nd. Mitoconfusion: noncanonical functioning of dynamism factors in static mitochondria of the heart. Cell Metab. 2015;21:195–205. doi: 10.1016/j.cmet.2014.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pallanck L. Mitophagy: mitofusin recruits a mitochondrial killer. Curr Biol. 2013;23:R570–2. doi: 10.1016/j.cub.2013.05.032. [DOI] [PubMed] [Google Scholar]
- 25.Koyano F, Okatsu K, Kosako H, Tamura Y, Go E, Kimura M, et al. Ubiquitin is phosphorylated by PINK1 to activate parkin. Nature. 2014;510:162–6. doi: 10.1038/nature13392. [DOI] [PubMed] [Google Scholar]
- 26.Kane LA, Lazarou M, Fogel AI, Li Y, Yamano K, Sarraf SA, et al. PINK1 phosphorylates ubiquitin to activate Parkin E3 ubiquitin ligase activity. J Cell Biol. 2014;205:143–53. doi: 10.1083/jcb.201402104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wauer T, Simicek M, Schubert A, Komander D. Mechanism of phospho-ubiquitin-induced PARKIN activation. Nature. 2015;524:370–4. doi: 10.1038/nature14879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lazarou M, Sliter DA, Kane LA, Sarraf SA, Wang C, Burman JL, et al. The ubiquitin kinase PINK1 recruits autophagy receptors to induce mitophagy. Nature. 2015;524:309–14. doi: 10.1038/nature14893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guo M. Drosophila as a model to study mitochondrial dysfunction in Parkinson's disease. Cold Spring Harb Perspect Med. 2012:2. doi: 10.1101/cshperspect.a009944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee Y, Dawson VL, Dawson TM. Animal models of Parkinson's disease: vertebrate genetics. Cold Spring Harb Perspect Med. 2012:2. doi: 10.1101/cshperspect.a009324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pickrell AM, Huang CH, Kennedy SR, Ordureau A, Sideris DP, Hoekstra JG, et al. Endogenous Parkin Preserves Dopaminergic Substantia Nigral Neurons following Mitochondrial DNA Mutagenic Stress. Neuron. 2015;87:371–81. doi: 10.1016/j.neuron.2015.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Song M, Matkovich SJ, Zhang Y, Hammer DJ, Dorn GW., 2nd. Combined cardiomyocyte PKCdelta and PKCepsilon gene deletion uncovers their central role in restraining developmental and reactive heart growth. Sci Signal. 2015;8:ra39. doi: 10.1126/scisignal.aaa1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shin JH, Ko HS, Kang H, Lee Y, Lee YI, Pletinkova O, et al. PARIS (ZNF746) repression of PGC-1alpha contributes to neurodegeneration in Parkinson's disease. Cell. 2011;144:689–702. doi: 10.1016/j.cell.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kubli DA, Quinsay MN, Gustafsson AB. Parkin deficiency results in accumulation of abnormal mitochondria in aging myocytes. Commun Integr Biol. 2013;6:e24511. doi: 10.4161/cib.24511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kubli DA, Zhang X, Lee Y, Hanna RA, Quinsay MN, Nguyen CK, et al. Parkin protein deficiency exacerbates cardiac injury and reduces survival following myocardial infarction. J Cell Biol. 2013;288:915–26. doi: 10.1074/jbc.M112.411363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoshino A, Mita Y, Okawa Y, Ariyoshi M, Iwai-Kanai E, Ueyama T, et al. Cytosolic p53 inhibits Parkin-mediated mitophagy and promotes mitochondrial dysfunction in the mouse heart. Nat Commun. 2013;4:2308. doi: 10.1038/ncomms3308. [DOI] [PubMed] [Google Scholar]
- 37.Bhandari P, Song M, Chen Y, Burelle Y, Dorn GW., 2nd. Mitochondrial contagion induced by Parkin deficiency in Drosophila hearts and its containment by suppressing mitofusin. Circ Res. 2014;114:257–65. doi: 10.1161/CIRCRESAHA.114.302734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kubli DA, Cortez MQ, Moyzis AG, Najor RH, Lee Y, Gustafsson AB. PINK1 Is Dispensable for Mitochondrial Recruitment of Parkin and Activation of Mitophagy in Cardiac Myocytes. PLoS One. 2015;10:e0130707. doi: 10.1371/journal.pone.0130707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lopaschuk GD, Spafford MA, Marsh DR. Glycolysis is predominant source of myocardial ATP production immediately after birth. Am J Physiol. 1991;261:H1698–705. doi: 10.1152/ajpheart.1991.261.6.H1698. [DOI] [PubMed] [Google Scholar]
- 40.Bartelds B, Knoester H, Smid GB, Takens J, Visser GH, Penninga L, et al. Perinatal changes in myocardial metabolism in lambs. Circulation. 2000;102:926–31. doi: 10.1161/01.cir.102.8.926. [DOI] [PubMed] [Google Scholar]
- 41.Stanley WC, Recchia FA, Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiol Rev. 2005;85:1093–129. doi: 10.1152/physrev.00006.2004. [DOI] [PubMed] [Google Scholar]
- 42.Williams JA, Ni HM, Haynes A, Manley S, Li Y, Jaeschke H, et al. Chronic Deletion and Acute Knockdown of Parkin Have Differential Responses to Acetaminophen-induced Mitophagy and Liver Injury in Mice. J Biol Chem. 2015;290:10934–46. doi: 10.1074/jbc.M114.602284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Song M, Chen Y, Gong G, Murphy E, Rabinovitch PS, Dorn GW., 2nd. Super-suppression of mitochondrial reactive oxygen species signaling impairs compensatory autophagy in primary mitophagic cardiomyopathy. Circ Res. 2014;115:348–53. doi: 10.1161/CIRCRESAHA.115.304384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yun J, Puri R, Yang H, Lizzio MA, Wu C, Sheng ZH, et al. MUL1 acts in parallel to the PINK1/parkin pathway in regulating mitofusin and compensates for loss of PINK1/parkin. Elife. 2014;3:e01958. doi: 10.7554/eLife.01958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ikeda Y, Shirakabe A, Maejima Y, Zhai P, Sciarretta S, Toli J, et al. Endogenous Drp1 mediates mitochondrial autophagy and protects the heart against energy stress. Circ Res. 2015;116:264–78. doi: 10.1161/CIRCRESAHA.116.303356. [DOI] [PubMed] [Google Scholar]
- 46.Diwan A, Krenz M, Syed FM, Wansapura J, Ren X, Koesters AG, et al. Inhibition of ischemic cardiomyocyte apoptosis through targeted ablation of Bnip3 restrains postinfarction remodeling in mice. J Clin Invest. 2007;117:2825–33. doi: 10.1172/JCI32490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Diwan A, Wansapura J, Syed FM, Matkovich SJ, Lorenz JN, Dorn GW., 2nd. Nix-mediated apoptosis links myocardial fibrosis, cardiac remodeling, and hypertrophy decompensation. Circulation. 2008;117:396–404. doi: 10.1161/CIRCULATIONAHA.107.727073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Diwan A, Matkovich SJ, Yuan Q, Zhao W, Yatani A, Brown JH, et al. Endoplasmic reticulum-mitochondria crosstalk in NIX-mediated murine cell death. J Clin Invest. 2009;119:203–12. doi: 10.1172/JCI36445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen Y, Lewis W, Diwan A, Cheng EH, Matkovich SJ, Dorn GW., 2nd. Dual autonomous mitochondrial cell death pathways are activated by Nix/BNip3L and induce cardiomyopathy. Proc Natl Acad Sci U S A. 2010;107:9035–42. doi: 10.1073/pnas.0914013107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ding WX, Ni HM, Li M, Liao Y, Chen X, Stolz DB, et al. Nix is critical to two distinct phases of mitophagy, reactive oxygen species-mediated autophagy induction and Parkin-ubiquitin-p62-mediated mitochondrial priming. J Biol Chem. 2010;285:27879–90. doi: 10.1074/jbc.M110.119537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dorn GW., 2nd. Mitochondrial pruning by Nix and BNip3: an essential function for cardiac-expressed death factors. J Cardiovasc Transl Res. 2010;3:374–83. doi: 10.1007/s12265-010-9174-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yuan H, Perry CN, Huang C, Iwai-Kanai E, Carreira RS, Glembotski CC, et al. LPS-induced autophagy is mediated by oxidative signaling in cardiomyocytes and is associated with cytoprotection. Am J Physiol Heart Circ Physiol. 2009;296:H470–9. doi: 10.1152/ajpheart.01051.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dorn GW., 2nd. Gone fission...: diverse consequences of cardiac Drp1 deficiency. Circ Res. 2015;116:225–8. doi: 10.1161/CIRCRESAHA.114.305672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kageyama Y, Hoshijima M, Seo K, Bedja D, Sysa-Shah P, Andrabi SA, et al. Parkin-independent mitophagy requires Drp1 and maintains the integrity of mammalian heart and brain. EMBO J. 2014;33:2798–813. doi: 10.15252/embj.201488658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stevens DA, Lee Y, Kang HC, Lee BD, Lee YI, Bower A, et al. Parkin loss leads to PARIS-dependent declines in mitochondrial mass and respiration. Proc Natl Acad Sci U S A. 2015;112:11696–701. doi: 10.1073/pnas.1500624112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sugiura A, McLelland GL, Fon EA, McBride HM. A new pathway for mitochondrial quality control: mitochondrial-derived vesicles. EMBO J. 2014;33:2142–56. doi: 10.15252/embj.201488104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Poole AC, Thomas RE, Andrews LA, McBride HM, Whitworth AJ, Pallanck LJ. The PINK1/Parkin pathway regulates mitochondrial morphology. Proc Natl Acad Sci U S A. 2008;105:1638–43. doi: 10.1073/pnas.0709336105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Deng H, Dodson MW, Huang H, Guo M. The Parkinson's disease genes pink1 and parkin promote mitochondrial fission and/or inhibit fusion in Drosophila. Proc Natl Acad Sci U S A. 2008;105:14503–8. doi: 10.1073/pnas.0803998105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Journal of Biological Chemistry. Lutz AK, Exner N, Fett ME, Schlehe JS, Kloos K, Lammermann K, et al. Loss of parkin or PINK1 function increases Drp1-dependent mitochondrial fragmentation. J Biol Chem. 2009;284:22938–51. doi: 10.1074/jbc.M109.035774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Poole AC, Thomas RE, Yu S, Vincow ES, Pallanck L. The mitochondrial fusion-promoting factor mitofusin is a substrate of the PINK1/parkin pathway. PLoS One. 2010;5:e10054. doi: 10.1371/journal.pone.0010054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang H, Song P, Du L, Tian W, Yue W, Liu M, et al. Parkin ubiquitinates Drp1 for proteasome-dependent degradation: implication of dysregulated mitochondrial dynamics in Parkinson disease. J Biol Chem. 2011;286:11649–58. doi: 10.1074/jbc.M110.144238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McLelland GL, Soubannier V, Chen CX, McBride HM, Fon EA. Parkin and PINK1 function in a vesicular trafficking pathway regulating mitochondrial quality control. EMBO J. 2014;33:282–95. doi: 10.1002/embj.201385902. [DOI] [PMC free article] [PubMed] [Google Scholar]