INTRODUCTION
Malperfusion syndrome results from end-organ ischemia in the setting of an aortic dissection. Malperfusion can affect nearly all major vascular beds, including the carotid, spinal cord, visceral, renal, and lower extremity branch vessels with varying frequency and severity (Figure 1). While complete obstruction of these vessels often manifests with overt symptoms corresponding to the vascular distribution affected, it is important to recognize incomplete or subtotal vessel occlusion may produce intermittent symptoms of variable intensity. Furthermore, symptoms can occur over the course of days or weeks, complicating both the diagnosis and management of malperfusion syndrome in this setting. Prompt consideration of malperfusion syndrome following the diagnosis of aortic dissection is important, as the incidence approaches 25–30% despite improvements in medical therapy (1–4).
Figure 1.
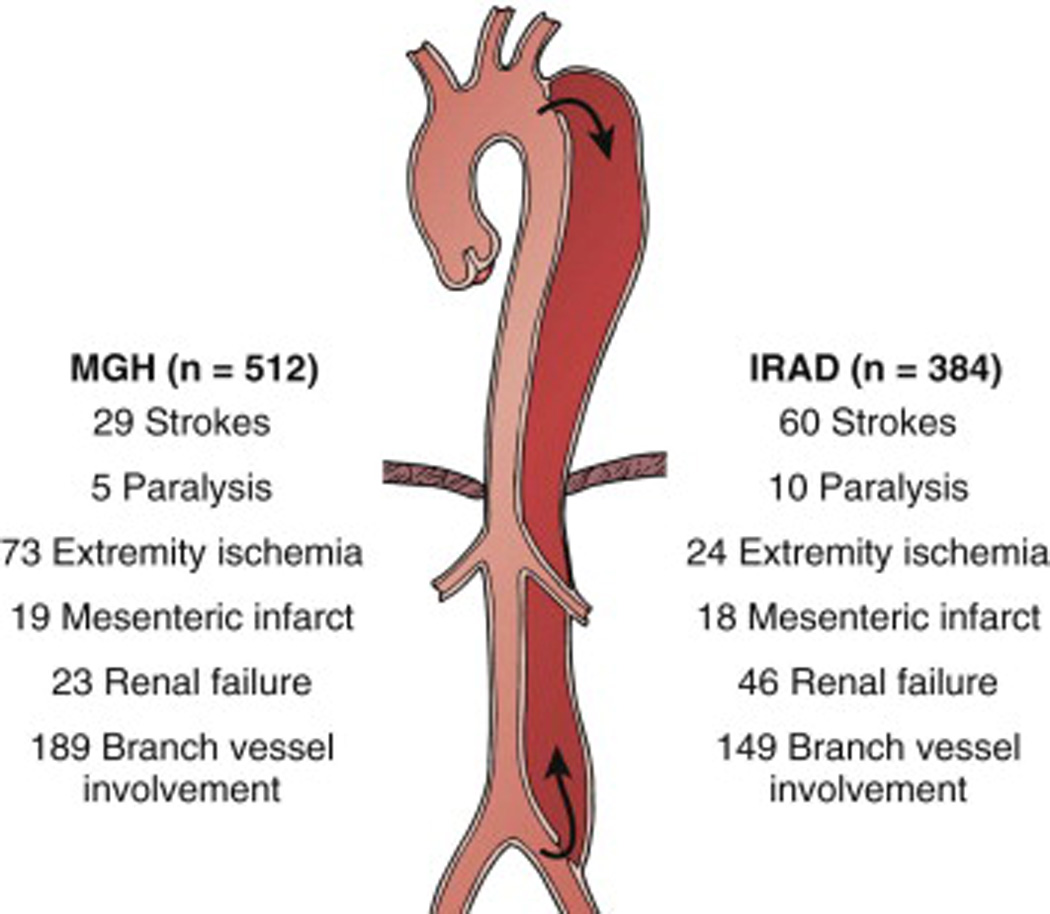
Frequency of distribution and symptoms associated with descending aortic dissection based on studies by MGH and IRAD (19, 55). Reprinted with permission.
The pathogenesis of malperfusion syndrome has been an area of scientific focus, as a thorough understanding of factors contributing to its development may aid in diagnosis and management. Over time, shear forces on the wall of the aorta lead to degeneration of elastin and smooth muscle cells of the tunica media (5, 6). This pathologic remodeling is exacerbated by increasing age, hypertension, and mutations in connective tissue proteins (7, 8). Dissection results when disruption of the intima facilitates propagation of blood through a cleavage plane into the outer portion of the diseased media. This blood-filled space within the media is known as the false lumen and is separated from the true lumen by an intimo-medial septum (i.e. the intimal flap). The balance of the hydrodynamic gradient between true and false lumens dictates whether the dissection progresses antegrade or retrograde (9). The stress produced by the blood column may give rise to additional intimal tears, which may potentiate further dissections or communication with the true lumen. These exit points for the false lumen are often found at aortic branch ostia, thus setting the stage for a malperfusion event (10).
Malperfusion syndrome itself perpetuates an inflammatory cascade stemming from end-organ ischemia which may significantly impair operative success (11). Previous studies have demonstrated increased myeloperoxidase production and complement consumption in the setting of visceral, renal, and limb ischemia (12, 13). Additionally, free radicals generated through neutrophil activation in ischemic tissue mediate endothelial injury and compromise membrane integrity. Upregulation of Tumor Necrosis Factor-alpha (TNF-α) and Interleukin-1(IL-1) catalyzes a positive feedback loop culminating in increased cellular adhesion molecule production that perpetuates leukocyte extravasation into ischemic tissue and further cytokine release (14, 15). While these inflammatory signaling molecules propagate local injury, the systemic effects of this cytokine release are evidenced by end organ injury in locations beyond the ischemic territories, most notably the lungs (16).
Knowledge of this systemic inflammatory cascade has undoubtedly created a dilemma on timing of operative intervention, especially in those with Type A dissection. Controversy over timing of aortic repair versus initial malperfusion-directed interventions remains. Groups favoring immediate aortic repair have demonstrated resolution of malperfusion syndrome with aortic repair alone in up to 75–80% of patients (4, 17, 18). Utilizing this management paradigm, mortality was essentially equivalent in patients with and without malperfusion at the time of presentation. Those advocating operative delay to correct malperfusion have previously noted a high mortality rate with immediate repair (11). Others have been more selective in identifying certain populations that may benefit from operative delay. Preoperative mesenteric malperfusion carries a dismal prognosis. There is some suggestion that mesenteric revascularization prior to definitive aortic repair may improve outcomes (19–21). Despite the theoretical benefits of delayed repair of Type A dissection, an impressive risk of rupture exists for those who definitive operation is delayed. In fact, in one study over 17% of patients expired from aortic rupture while awaiting resolution of malperfusion syndrome (20). In acutely managing Type A dissections, complex root and great vessel reconstruction undoubtedly prolongs operative time and may contribute to worse outcomes (11).
Certainly for patients that are managed with immediate operative intervention, unabated malperfusion appears to adversely impact survival. In the initial International Registry of Acute Aortic Dissection (IRAD) study, consisting of 12 international referral centers and 464 patients, the second most common cause of death among repaired Type A patients was mesenteric ischemia, with rupture being the most common cause (22).
Signs of malperfusion in Type B dissections are often more subtle. In these patients, hypertension may incite the initial event in up to 70% of patients and resistance to blood pressure control during early medical management may be one of the initial signs of a malperfusion event (22). As rupture of a Type B dissection is comparatively rare (versus a Type A dissection), the treatment priority controversy is less significant. However, controversies regarding diagnosis, timing of repair and type of repair still exist. When malperfusion persists despite optimal medical therapy, both open and endovascular surgical techniques have been employed to combat end-organ ischemia.
This review explores the latest understanding of factors contributing to malperfusion syndrome, useful diagnostic modalities that assist in guiding the timing of repair, and the role of endovascular techniques to combat this challenging clinical predicament.
STATIC VS DYNAMIC OBSTRUCTION
Historically, peripheral ischemia in conjunction with aortic dissection was attributed to propagation of the dissection flap into the branch vessel with resultant obstruction. However, real-time imaging has enabled researchers to capture the dynamic relationship between the true lumen and the mobile intimo-medial septum (23, 24). As ventricular contraction produces a fluid column that travels down both true and false lumens, the law of Laplace predicts ectasia of the false lumen as it is largely deficient in elastin and does not easily accommodate the wall tension generated by the systolic fluid wave (10). The pressure difference between the false lumen and true lumen may allow for the mobile intimo-medial septum to bulge into the ostia of aortic branches, giving rise to either transient or persistent (static) obliteration of branch vessel ostia (Figure 2). Several variables influence the degree to which this process unfolds. These include the percentage of aortic circumference involved in the dissection, the topography of branch vessel ostia to the true and false lumens and the presence of distal communication between the false and true lumen, a so-called reentrant focus (25). This process of intermittent branch vessel compromise is known as dynamic obstruction. As its manifestations may at times be subtle, early recognition of signs of end organ malperfusion, including metabolic acidosis, lactic acidemia, pulse deficits, persistent pain or sensorimotor disturbances may alert one to this diagnosis.
Figure 2.
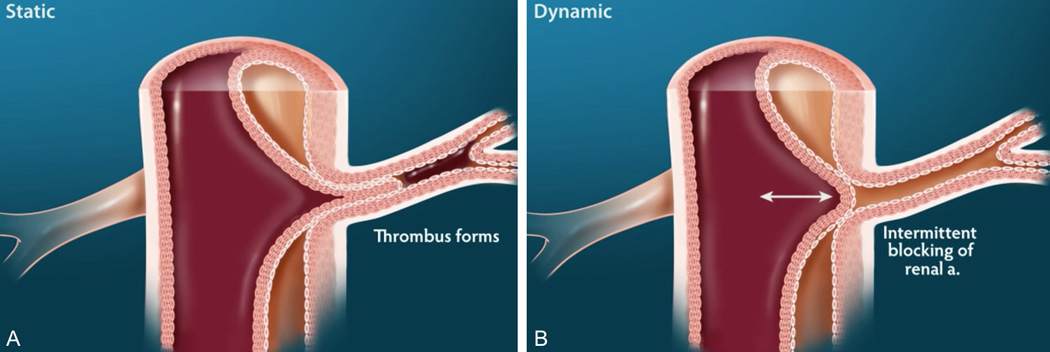
A. Static dissection seen as protrusion of the intimal flap into the ostium of the affected branch vessel causes subsequent thrombosis of the branch vessel with resulting perfusion impairments. B. Dynamic dissection demonstrates protrusion into the ostium of a branch vessel is the most common cause of malperfusion syndrome and results in variable symptoms given the dynamic nature of the occlusion.
Dynamic obstruction is more often the cause of malperfusion syndrome than static obstruction and is responsible for approximately 80% of cases (24). There are two distinct etiologies of dynamic malperfusion. First, insufficient flow through the true lumen may lead to hypoperfusion when branch vessel perfusion is maintained by the true lumen. The degree of hypoperfusion is related to the counterbalance of forces favoring collapse of the true lumen. As expected, a larger circumference of dissected aorta, increased systolic blood pressure, increased heart rate and diminished peripheral resistance to true lumen outflow may exacerbate this process (23). Given the variability in these factors early in the course of dissection, it is not surprising that pulse deficits may wax and wane. The second mechanism of dynamic obstruction reflects the mobility of the intimal flap. When the false lumen prolapses into a branch vessel ostium, flow is dynamically compromised (26). This relationship between the mobile false lumen and branch vessel lumen is depicted below (Figure 2). Dynamic obstruction, as suggested by its name, is intermittent in nature and importantly, reducing the number of events of flow obstruction is the theoretical benefit of dP/dT management with Beta-blocker medications.
Static obstruction, characterized by narrowing or occlusion of branch vessels, is the culmination of false lumen protrusion into the branch vessel with associated thrombosis (Figure 2). The combination of the hypercoagulable state of the false lumen, attributable to the exposed adventitial and medial layers, and stasis within the blind end of the false lumen renders the lumen particularly vulnerable to thrombosis (10, 26). Static obstruction leads to two scenarios: in the first, a shearing effect on the intimo-medial septum may permit perfusion of a branch vessel by the false lumen and thus, end organ ischemia is avoided. Alternatively, thrombosed false lumen prolapse into a branch vessel may compromise blood flow to the involved territory, although the incidence of this scenario is lower than the former situation (10). In these instances, endovascular or open surgical intervention is often necessary to revascularize the ischemic region.
DIAGNOSIS OF MALPERFUSION SYNDROME
Symptomatology of malperfusion can vary significantly by location of the affected vascular bed. Pain is often the first manifestation of malperfusion syndrome, and with many complicated aortic dissections, pain onset is typically abrupt (22). Depending on the anatomic distribution of pain, level of neurologic deficit, or pulse differential, localization of the compromised aortic branch vessel may be ascertained at the bedside. If a dissection flap compromises branches of the aortic arch, stroke or alteration in mental status may be the first clinical sign. A differential in brachial blood pressure measurements also suggests involvement of the brachiocephalic or subclavian vessels. Intra-abominal compromise secondary to dissections in the mesenteric bed may be evidenced by laboratory derangements including metabolic acidosis and a rising lactate, or as an elevated creatinine in the case of a dissection altering renal perfusion.
Computed Tomographic Angiography (CTA) has replaced conventional angiography as the gold standard in the diagnosis of aortic dissection (22). With the advent of three-dimensional reconstruction, topographic relationships of the true and false lumen may be elucidated (10). Intraluminal thrombus is useful in identification of the false lumen although not completely specific (27). In over 90% of dissections, the false lumen diameter is larger than the true lumen (27). CTA is useful not only for the diagnosis of dissection but also in identifying malperfusion syndrome. Radiographic evidence of compression of the true lumen raises concern for visceral, renal, or lower extremity malperfusion. Asymmetric kidney enhancement is also important to recognize as an indicator of a patient at risk for malperfusion. In patients with physiologic or biochemical derangements suggestive of ischemia, CTA alone may provide additional evidence to dictate the need for revascularization.
The kidneys remain one of the most common organ systems affected by aortic dissection-mediated malperfusion, yet its detection has proven to be the most challenging. Acute kidney injury is common in the setting of aortic dissection, owing to multiple predisposing factors. Acute tubular necrosis (ATN) may result from hypoperfusion perpetuated by an obstructive dissection flap, contrast-induced injury and even relative hypotension from strict blood pressure control and may significantly limit kidney function. Though iodinated contrast agents are nephrotoxic, they are often required to correctly delineate both true and false lumens as well as to identify ischemic segments of vasculature. In this setting, contrast-mediated nephropathy is relatively common, especially when administered to poorly perfused kidneys. The wide differential makes renal malperfusion an elusive diagnosis, thus providers must remains vigilant in attempting to identify ongoing renal malperfusion. Beyond its effect on long term morbidity, acute kidney injury is an independent predictor of increased operative mortality and steps to detect renal malperfusion and mitigate its influence are crucial (4).
Despite an aggressive disposition in yearning to diagnose renal malperfusion earlier in its course (based on impaired solute clearance, rising creatinine or refractory hypertension) and our improved ability to identify renal parenchymal heterogeneity on CTA, the positive predictive value for malperfusion remains imperfect and approaches only 70% (28). Here, renal artery duplex ultrasonography is a useful adjunct and supplements clinical and anatomic considerations with an assessment of renal perfusion. Increased proximal renal artery peak systolic velocity in combination with both a depressed distal peak systolic velocity and loss of diastolic flow have been shown to predict renal malperfusion (29) (Figure 3). Additionally, a normal distal renal artery waveform has been shown to carry a negative predictive value of 100% for malperfusion (29).
Figure 3.
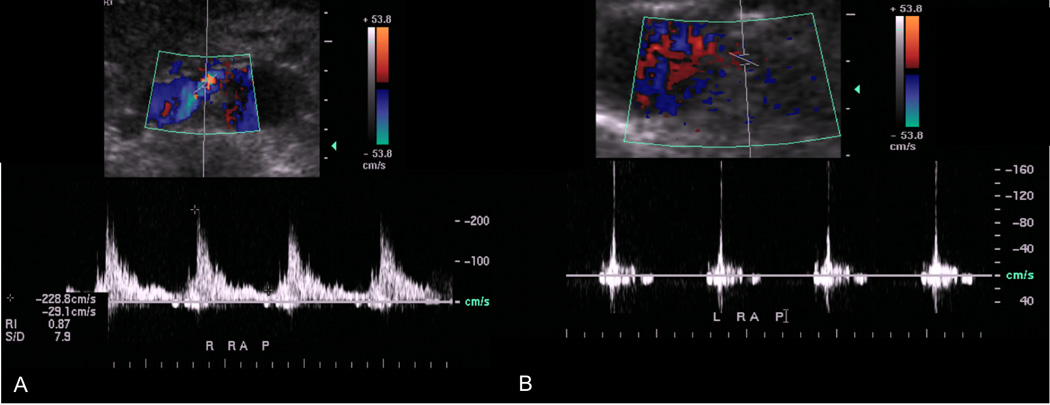
Ultrasound findings of renal artery malperfusion is manifested as A. increased proximal peak systolic velocities and B. absence of diastolic flow.
The presence of mesenteric ischemia, regardless of the success of endovascular interventions, conveys a poor prognosis. In fact, IRAD data suggest mesenteric ischemia is responsible for 15% of the mortality observed with acute aortic dissection (22). While improvements have been realized over time, aortic dissection leading to mesenteric compromise still carries an early mortality rate of over 30% (19). Delays in diagnosis may contribute to this grave condition, especially with Type A dissections as patients are rapidly triaged to the operating room for surgical repair. This delay is perpetuated post-operatively when sedation precludes accurate clinical assessment.
Lower extremity pulse deficits, pain, pallor, paresthesias, poikilothermia or paresis, in the absence of preexisting peripheral artery disease, are consistent with malperfusion syndrome and may be found in 30–50% of patients with thoracoabdominal or aortic arch involvement (1, 3). Limb ischemia is present in greater than 70% of patients with malperfusion syndrome and is frequently seen in conjunction with visceral ischemia (30). The presence of lower extremity malperfusion confers a significantly higher risk of in-hospital mortality (30).
ENDOVASCULAR THERAPY FOR MALPERFUSION SYNDROME
Despite improvements in endovascular stent grafting, open surgical intervention remains the standard of care for Type A dissections. Even with expeditious operative repair of the ascending aorta in Type A dissections, branch vessel ischemia persists in 25% of cases (17, 18, 31). A similar rate of sustained peripheral malperfusion is observed after endovascular repair of complicated descending aortic dissections despite optimal medical management (32). This is largely due to a persistent patent false lumen rate of approximately 50% (33, 34). In this setting, endovascular intervention may alleviate malperfusion at a more acceptable risk than open operation. Early reports regarding the efficacy of endovascular stent graft therapy for peripheral malperfusion have demonstrated a reduction in mortality rates to less than 30% (28, 35).
Endovascular stent grafting as an alternative to conventional surgical repair for complicated Type B aortic dissections was first advocated in 1999 (32). Since its inception, thoracic endovascular aortic repair (TEVAR) has emerged as a viable treatment in the management of Type B aortic dissection (19, 36). TEVAR has been demonstrated to treat malperfusion syndrome effectively and to promote favorable aortic remodeling without the morbidity of an open procedure. In fact, several studies have demonstrated superior outcomes when TEVAR is utilized as opposed to open surgical repair (37, 38). Three-dimensional CTA has furthered the utility of TEVAR, allowing for accurate assessment of aortic contour, measurements of aortic diameter, and adequacy of proximal and distal fixation zones for stent deployment. Early success rates in achieving thrombosis of the false lumen approached 80% when TEVAR was undertaken (32).
Importantly, stent graft design has evolved over time. Previous animal models of aortic dissection have yielded unsatisfactory outcomes when uncovered stent grafts were deployed (39, 40). This is largely related to the mechanism of deployment of uncovered stents. As stents were balloon-expanded, over-distention of the true lumen, while seeking to compress the false lumen, subjected the intimal flap to significant radial sheer force. This contributed to an increased risk of aortic rupture. Thus, self-expanding covered stents have largely gained favor in the endovascular management of aortic dissection (Figure 4).
Figure 4.
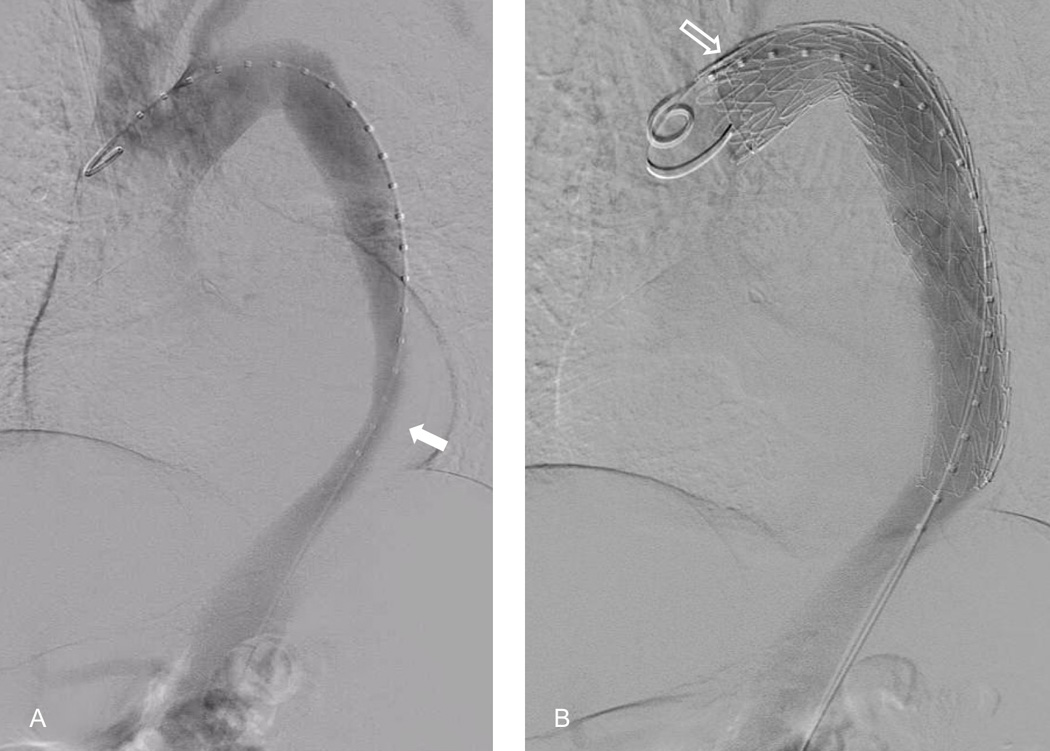
A. Aortic angiography of proximal descending aortic dissection with aortic narrowing due to dissection flap (solid arrow). B. Successful deployment of self-expanding stent into the proximal descending aorta for the management of a descending thoracic dissection. The takeoff of the left subclavian artery can be seen at the second position marker (outlined arrow).
Proximal coverage of the intimal tear with TEVAR does not always lead to thrombosis of the false lumen and in these instances, dynamic collapse of the true lumen may be observed (41, 42). Recently, aortic scaffolding with bare metal stents, known as the PETTICOAT technique or Povisional ExTension to Induce COmplete ATtachment, has been popularized to combat this predicament (43). In one series involving 25 patients with complicated Type B dissection, follow-up studies focused on aortic remodeling demonstrated a 100% increase in true lumen volume post-procedurally. At 1 year, true lumen volume had increased by 130% in comparison to pre-intervention measurements and the false lumen volume had decreased by 30% (44). Alternatively, a dual-construct design, combining a proximal covered stent graft with a bare metal stent in the distal thoracic aorta (Zenith Dissection Endovascular System, Cook), has emerged to afford adequate coverage of the intimal flap while maintaining patency of the aortic true lumen (41, 45).
Identification of the true lumen is fundamental to the success of endovascular therapy. Intravascular ultrasound (IVUS) is our preferred method of distinguishing true and false lumens and delineating the relationship between the intimal flap and the ostia of branch vessel arteries (46). Subsequent angiography helps to identify the false lumen and origin and mobility of the intimal flap which allows for access into this lumen. During angiography, consideration is given to the presence of equivalent intra-luminal pressures in the true and false lumens of the thoracic aorta, however this does not ensure adequate distal branch vessel perfusion (10). In this scenario, the combination of angiography and IVUS may also elucidate ongoing branch vessel malperfusion and hasten endovascular interventions. IVUS may also confirm expansion of the true lumen after successful coverage of the proximal intimal tear. A transbrachial approach may be utilized for Type B dissections as retrograde transit through the subclavian artery facilitates antegrade access to the true lumen of the aorta.
Despite the emergence of endovascular stent grafting as the standard of care in Type B dissections with associated malperfusion, complications related to stent grafting continue to challenge endovascular surgeons. Such challenges include cerebral and spinal cord malperfusion. Dissection involving the carotid or innominate arteries may lead to syncope, which is present in 5–10% of Type A dissections (47). Cerebral ischemia may also be iatrogenic in nature. Coverage of the left subclavian artery (LSA) during TEVAR has been independently associated with increased rates of stroke and peri-operative mortality (48). These findings were corroborated in the STABLE trial, a prospective multicenter study involving 40 patients with complicated Type B dissections, in which all patients who suffered a post-operative stroke had required coverage of the LSA (41). In a retrospective study involving 1010 patient that underwent TEVAR, coverage of the LSA independently increased the risk of stroke (49). However, pre-operative revascularization of the LSA prior to coverage during TEVAR significantly reduced the rate of stroke from 9.1% to 5.1% (49).
Spinal cord ischemia may be present due to the dissection flap’s disruption of intercostal vessels which feed the spinal arteries, but additionally often results from efforts to ensure patency of aortic branch vessels. In fact, it is one of the most devastating complications of successful endovascular thoracic aortic aneurysm repair. Risk factors for spinal cord ischemia following TEVAR include the extent of segmental artery compromise or sacrifice during the repair, involvement of the lumbar region of the aorta, peri-operative and post-operative hypotension and coverage of the LSA during stent deployment (50).
The LSA plays a substantial role in preserving blood flow to spinal arteries, especially the anterior spinal artery. Successful thoracic endovascular stent grafting often requires coverage of the LSA to facilitate an optimal proximal landing zone. As stent grafting additionally often requires covering intercostal branches that may provide collateral flow to spinal arteries, maintained perfusion of the LSA is imperative. Thus, performance of carotid-subclavian transposition or bypass, either pre-operatively or shortly after emergent endovascular repair, is important in preventing spinal cord ischemia when it is recognized that the LSA must be covered by the endograft (49, 51). Avoidance of aortic cross-clamping is a primary advantage of endovascular repair, especially in mitigating the risk of spinal cord injury. Intra-operative monitoring of spinal cord perfusion via somatosensory and motor evoked potentials have revolutionized peri-operative care and early detection of spinal cord ischemia (52). The use of lumbar drains for cerebrospinal fluid drainage and post-operative arterial blood pressure monitoring to optimize spinal cord perfusion are now standard practices during extensive thoracic aortic stent grafting or scaffolding (41).
Techniques to alleviate renal malperfusion, especially if a proximal TEVAR does not resolve renal ischemia, are well-described and include central aortic fenestration, branch artery stenting, or a combination of the two (28). Utilizing arteriography and IVUS, an aortorenal gradient can be measured and the relationship of the dissection flap to the lumen of the branch vessel can be further elucidated as static, dynamic, or combined obstruction. Endovascular fenestration is often a first step in the management of dynamic obstruction (28), although this is debated. Controversy exists as to whether fenestration should precede stent grafting, as aortic fenestration may lead to unpredictable alterations in aortic flow which can compromise subsequent endovascular access into the branch vessel in question (53).
Identification of the renal artery ostia with IVUS helps to target the region of fenestration. Using a combination of ultrasound and fluoroscopy, a small needle is used to puncture across the intimo-medial septum and gain access to the false lumen. A stiff wire is subsequently placed across the true lumen into the false lumen and a 5F catheter is then advanced over the wire. Angiography is then performed to confirm proper positioning. Next, an angioplasty balloon of a minimum 12–15mm in diameter is inflated to create a fenestration tear. Confirmation of a reduced aortorenal gradient after completion of fenestration suggests successful revascularization, and this should be corroborated with clinical improvement. Thoracic stent grafting may additionally be of value in the management of dynamic branch vessel occlusion, as it is more likely to resolve with elimination of flow through the false lumen.
Endovascular management of static obstruction of renal arteries typically requires endovascular stent grafting which is performed with a self-expanding bare stent or a balloon expandable stent-graft (28). In comparison to endovascular stenting for atherosclerotic renal stenosis, stenting for malperfusion often requires extension of the stent graft into the aorta to ensure patency (Figure 5). Barriers to successful stent grafting arise when the dissection flap extends to involve the lobar arteries or causes extensive thrombosis of the renal artery. In these situations, safe cannulation may be impeded and stenting is rendered unfeasible. Single institution experiences have demonstrated a peri-operative mortality rate of approximately 20–25% utilizing fenestration and stent graft techniques (17, 28).
Figure 5.
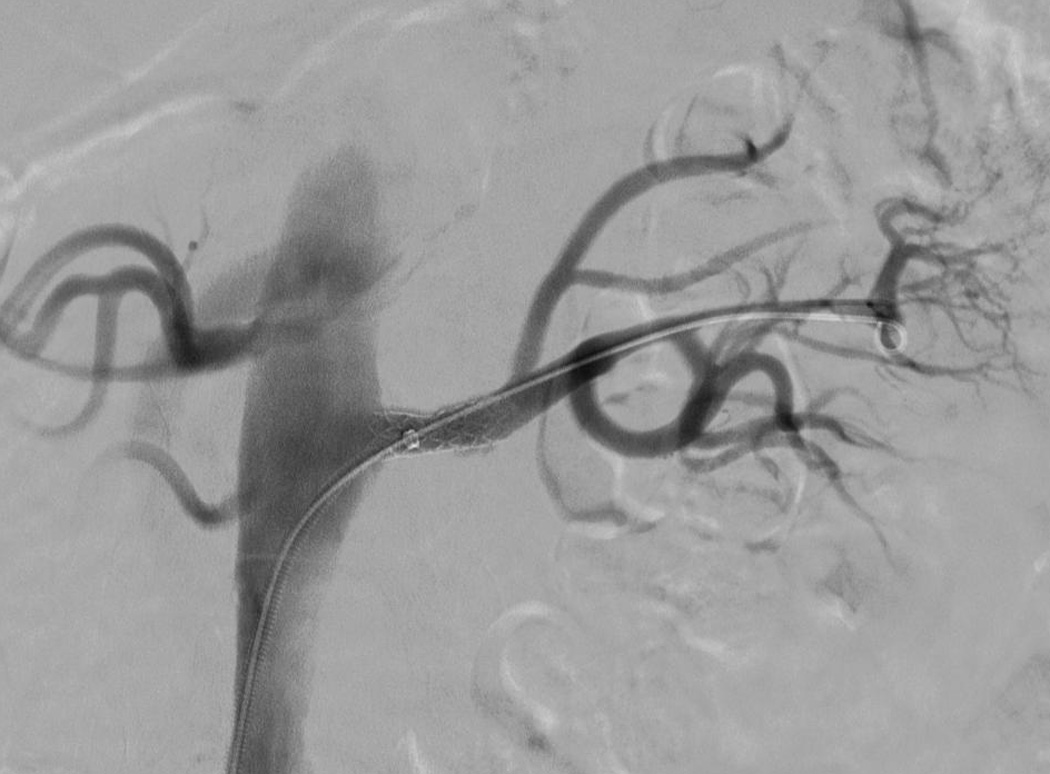
Completion angiogram after deployment of self-expanding stent into the proximal renal artery for management of dissection involving the left renal artery revealing unimpaired blood flow.
Similar revascularization techniques are employed for mesenteric and lower extremity malperfusion. A bi-femoral approach to cannulation can be implemented in the case of extremity malperfusion to create a distal aortic fenestration and reperfuse the affected iliac territory. Static obstruction often requires stent grafting to ensure patency. Resolution of ischemia with endovascular stent grafting has been achieved in greater than 90% of patients in a previous case series (48).
When aggressive medical therapy fails to relieve visceral or limb ischemia and the degree of branch vessel obstruction precludes endovascular intervention, open surgical fenestration or extra-anatomic bypass may be performed for visceral and limb ischemia. Open fenestration is preferred in the setting of concomitant visceral and lower extremity ischemia and typically requires supraceliac or suprarenal aortic cross-clamping. In high-risk patients with isolated limb ischemia whereby the morbidity of open fenestration would be poorly tolerated, extra-anatomic bypass is a superior revascularization strategy. In a recent systematic review including 138 patients with lower extremity malperfusion, open fenestration or extra-anatomic bypass via axillo-femoral or femoral-femoral bypass grafting were associated with 30-day failure and mortality rates of 27% and 14%, respectively (54).
Even with successful endovascular revascularization of mesenteric branch vessels, ischemia can often progress to infarct. Thus, the role of surgical intervention is rather complementary in these circumstances and may supplement initial percutaneous techniques in the management of mesenteric malperfusion. In patients with peritonitis, refractory lactic acidosis or hemodynamic instability, suggestive of ongoing intestinal malperfusion, immediate laparotomy should be performed with resection of all devitalized intestine. It is most often favorable to delay definitive abdominal closure in these situations to provide an opportunity for a second look to ensure the remaining bowel is viable.
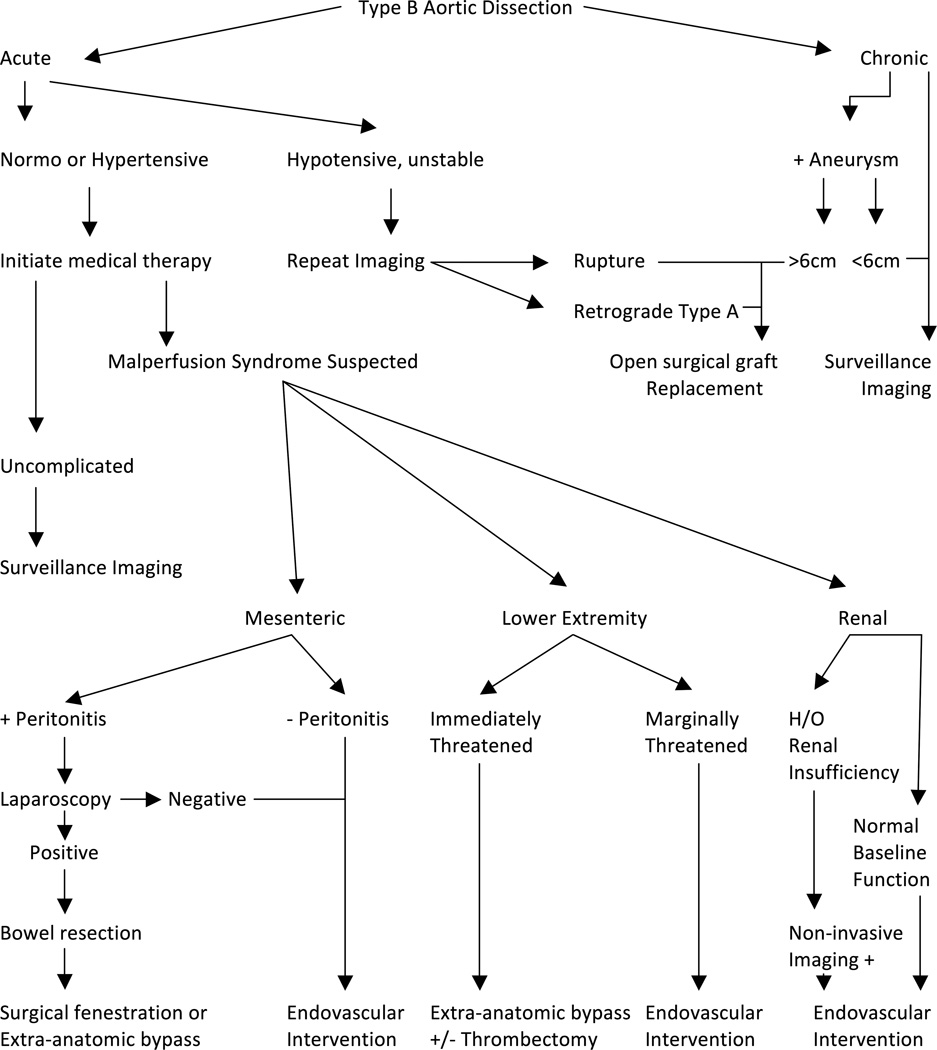
Successful management of complicated aortic dissections involving renal, mesenteric and extremity segments requires prompt recognition and aggressive combined medical and surgical management (Figure 6).
Figure 6.
Suggested guidelines for management of complicated type B aortic dissection with evidence of renal, mesenteric and extremity segment malperfusion.
FUTURE DIRECTIONS
While convincing evidence supports the role of endovascular stent grafting in the management of complicated Type B dissections with peripheral malperfusion, the utility of endovascular stenting in the management of uncomplicated Type B dissections remains uncertain. Results from the INSTEAD trial, a randomized controlled trial involving 140 patients at a minimum of two weeks post-dissection,failed to demonstrate a mortality benefit in those patients randomized to TEVAR as opposed to optimal medical therapy (55). Still, outcomes after uncomplicated Type B dissection are grim with a reported mortality rate approaching 30% at 5 years (30, 56). A non-trivial percentage of patients with uncomplicated dissections will develop aneurysmal degeneration leading to rupture or peripheral ischemia.
In a follow-up study to address outcomes at 5 years, INSTEAD XL demonstrated a significant survival benefit in patients randomized to TEVAR for uncomplicated aortic dissection with a reduction in aorta-related mortality after 2 years. Favorable aortic remodeling and a reduction in emergent crossover TEVARs were also observed in those randomized to early TEVAR (57). The results of the initial INSTEAD trial demonstrated that survival after Type B dissection improved with stringent blood pressure control and close surveillance. However, this treatment paradigm lacks the ability to prevent late complications. Perhaps a shift in management strategies towards endovascular repair of uncomplicated Type B dissections will emerge, though it remains an area of continued research.
Endovascular interventions possess the ability to facilitate advantageous aortic remodeling after Type B aortic dissections (58, 59). Identifying which patients are more likely to experience rapid aneurysmal degeneration will be integral to the future care of the patient with Type B dissection. Dake and colleagues have popularized a mnemonic, DISSECT, to provide a framework that better informs practitioners tasked with caring for these challenging patients (60). This classification system draws on both anatomic and clinical characteristics to integrate six features of dissection, and will help to individualize treatment strategies for Type B dissection.
Additional opportunities to broaden utilization of thoracic endovascular repair exist in the management of Type A dissections. Though the proximity to coronary and aortic arch vessels increases the risk of myocardial infarction and stroke, improvements in aortic contour mapping, endovascular stent graft design, and deployment techniques may offer endovascular surgeons a competitive advantage going forward.
CONCLUSION
Peripheral malperfusion complicates roughly 25–30% of cases of aortic dissection. While surgical repair remains the standard of care for Type A dissections in the United States, TEVAR has emerged as a reliable intervention in the management of Type B dissections with associated end-organ ischemia. Substantial improvements in morbidity and mortality have been realized when this technique is implemented for complicated Type B dissections. Endovascular interventions have additionally proven effective in revascularization of branch vessels when surgical repair of Type A dissections or TEVAR fails to relieve peripheral ischemia. As technological innovations give rise to more sophisticated endovascular stent graft design and deployment strategies, indications for stent grafting will most certainly expand and will hopefully impact malperfusion treatment strategies in a favorable manner.
References
- 1.Fann JI, Smith JA, Miller DC, et al. Surgical management of aortic dissection during a 30-year period. Circulation. 1995 Nov 1;92(9 Suppl):II113–II121. doi: 10.1161/01.cir.92.9.113. [DOI] [PubMed] [Google Scholar]
- 2.DeBakey ME, McCollum CH, Crawford ES, et al. Dissection and dissecting aneurysms of the aorta: twenty-year follow-up of five hundred twenty-seven patients treated surgically. Surgery. 1982 Dec;92(6):1118–1134. [PubMed] [Google Scholar]
- 3.Cambria RP, Brewster DC, Gertler J, et al. Vascular complications associated with spontaneous aortic dissection. J Vasc Surg. 1988 Feb;7(2):199–209. [PubMed] [Google Scholar]
- 4.Fann JI, Sarris GE, Mitchell RS, et al. Treatment of patients with aortic dissection presenting with peripheral vascular complications. Ann Surg. 1990 Dec;212(6):705–713. doi: 10.1097/00000658-199012000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O'Gara PT, DeSanctis RW. Acute aortic dissection and its variants. Toward a common diagnostic and therapeutic approach. Circulation. 1995 Sep 15;92(6):1376–1378. doi: 10.1161/01.cir.92.6.1376. [DOI] [PubMed] [Google Scholar]
- 6.Wheat MW., Jr Acute dissection of the aorta. Cardiovasc Clin. 1987;17(3):241–262. [PubMed] [Google Scholar]
- 7.Mehta RH, Manfredini R, Hassan F, et al. Chronobiological patterns of acute aortic dissection. Circulation. 2002 Aug 27;106(9):1110–1115. doi: 10.1161/01.cir.0000027568.39540.4b. [DOI] [PubMed] [Google Scholar]
- 8.Reed D, Reed C, Stemmermann G, et al. Are aortic aneurysms caused by atherosclerosis? Circulation. 1992 Jan;85(1):205–211. doi: 10.1161/01.cir.85.1.205. [DOI] [PubMed] [Google Scholar]
- 9.Wilson SK, Hutchins GM. Aortic dissecting aneurysms: causative factors in 204 subjects. Arch Pathol Lab Med. 1982 Apr;106(4):175–180. [PubMed] [Google Scholar]
- 10.Black JH, Cambria RP. Rutherford's Textbook of Vascular Surgery. 6. Philadelphia, PA: Elsevier Health Sciences; 2005. Aortic Dissection: Perspectives for the Vascular/Endovascular Surgeon. [Google Scholar]
- 11.Deeb GM, Williams DM, Bolling SF, et al. Surgical delay for acute type A dissection with malperfusion. Ann Thorac Surg. 1997 Dec;64(6):1669–1675. doi: 10.1016/s0003-4975(97)01100-4. discussion 75-7. [DOI] [PubMed] [Google Scholar]
- 12.Freischlag JA, Hanna D. Neutrophil (PMN) phagocytosis and chemotaxis after reperfusion injury. J Surg Res. 1992 Feb;52(2):152–156. doi: 10.1016/0022-4804(92)90297-d. [DOI] [PubMed] [Google Scholar]
- 13.Caty MG, Guice KS, Oldham KT, et al. Evidence for tumor necrosis factor-induced pulmonary microvascular injury after intestinal ischemia-reperfusion injury. Ann Surg. 1990 Dec;212(6):694–700. doi: 10.1097/00000658-199012000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seekamp A, Mulligan MS, Till GO, et al. Role of beta 2 integrins and ICAM-1 in lung injury following ischemia-reperfusion of rat hind limbs. Am J Pathol. 1993 Aug;143(2):464–472. [PMC free article] [PubMed] [Google Scholar]
- 15.Seekamp A, Warren JS, Remick DG, et al. Requirements for tumor necrosis factor-alpha and interleukin-1 in limb ischemia/reperfusion injury and associated lung injury. Am J Pathol. 1993 Aug;143(2):453–463. [PMC free article] [PubMed] [Google Scholar]
- 16.Welbourn CR, Goldman G, Paterson IS, et al. Neutrophil elastase and oxygen radicals: synergism in lung injury after hindlimb ischemia. Am J Physiol. 1991 Jun;260(6 Pt 2):H1852–H1856. doi: 10.1152/ajpheart.1991.260.6.H1852. [DOI] [PubMed] [Google Scholar]
- 17.Slonim SM, Miller DC, Mitchell RS, et al. Percutaneous balloon fenestration and stenting for life-threatening ischemic complications in patients with acute aortic dissection. J Thorac Cardiovasc Surg. 1999 Jun;117(6):1118–1126. doi: 10.1016/s0022-5223(99)70248-5. [DOI] [PubMed] [Google Scholar]
- 18.Girardi LN, Krieger KH, Lee LY, et al. Management strategies for type A dissection complicated by peripheral vascular malperfusion. Ann Thorac Surg. 2004 Apr;77(4):1309–1314. doi: 10.1016/j.athoracsur.2003.09.056. discussion 14. [DOI] [PubMed] [Google Scholar]
- 19.Lauterbach SR, Cambria RP, Brewster DC, et al. Contemporary management of aortic branch compromise resulting from acute aortic dissection. J Vasc Surg. 2001 Jun;33(6):1185–1192. doi: 10.1067/mva.2001.115377. [DOI] [PubMed] [Google Scholar]
- 20.Patel HJ, Williams DM, Dasika NL, et al. Operative delay for peripheral malperfusion syndrome in acute type A aortic dissection: a long-term analysis. J Thorac Cardiovasc Surg. 2008 Jun;135(6):1288–1295. doi: 10.1016/j.jtcvs.2008.01.026. discussion 95-6. [DOI] [PubMed] [Google Scholar]
- 21.Girdauskas E, Kuntze T, Borger MA, et al. Surgical risk of preoperative malperfusion in acute type A aortic dissection. J Thorac Cardiovasc Surg. 2009 Dec;138(6):1363–1369. doi: 10.1016/j.jtcvs.2009.04.059. [DOI] [PubMed] [Google Scholar]
- 22.Hagan PG, Nienaber CA, Isselbacher EM, et al. The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease. JAMA. 2000 Feb 16;283(7):897–903. doi: 10.1001/jama.283.7.897. [DOI] [PubMed] [Google Scholar]
- 23.Chung JW, Elkins C, Sakai T, et al. True-lumen collapse in aortic dissection: part II. Evaluation of treatment methods in phantoms with pulsatile flow. Radiology. 2000 Jan;214(1):99–106. doi: 10.1148/radiology.214.1.r00ja3499. [DOI] [PubMed] [Google Scholar]
- 24.Williams DM, Lee DY, Hamilton BH, et al. The dissected aorta: percutaneous treatment of ischemic complications--principles and results. J Vasc Interv Radiol. 1997 Jul-Aug;8(4):605–625. doi: 10.1016/s1051-0443(97)70619-5. [DOI] [PubMed] [Google Scholar]
- 25.Cambria RP. Surgical treatment of complicated distal aortic dissection. Semin Vasc Surg. 2002 Jun;15(2):97–107. doi: 10.1053/svas.2002.33439. [DOI] [PubMed] [Google Scholar]
- 26.Williams DM, Lee DY, Hamilton BH, et al. The dissected aorta: part III. Anatomy and radiologic diagnosis of branch-vessel compromise. Radiology. 1997 Apr;203(1):37–44. doi: 10.1148/radiology.203.1.9122414. [DOI] [PubMed] [Google Scholar]
- 27.LePage MA, Quint LE, Sonnad SS, et al. Aortic dissection: CT features that distinguish true lumen from false lumen. AJR Am J Roentgenol. 2001 Jul;177(1):207–211. doi: 10.2214/ajr.177.1.1770207. [DOI] [PubMed] [Google Scholar]
- 28.Barnes DM, Williams DM, Dasika NL, et al. A single-center experience treating renal malperfusion after aortic dissection with central aortic fenestration and renal artery stenting. J Vasc Surg. 2008 May;47(5):903–910. doi: 10.1016/j.jvs.2007.12.057. discussion 10-1. [DOI] [PubMed] [Google Scholar]
- 29.Williams TK, Call DM, Shah AS, et al. Accuracy of Duplex Ultrasonography to Evaluate Renal Malperfusion Syndromes and Late Renal Outcomes in Aortic Dissection. Unpublished. [Google Scholar]
- 30.Suzuki T, Mehta RH, Ince H, et al. Clinical profiles and outcomes of acute type B aortic dissection in the current era: lessons from the International Registry of Aortic Dissection (IRAD) Circulation. 2003 Sep 9;108(Suppl 1):II312–II317. doi: 10.1161/01.cir.0000087386.07204.09. [DOI] [PubMed] [Google Scholar]
- 31.Elefteriades JA, Hammond GL, Gusberg RJ, et al. Fenestration revisited. A safe and effective procedure for descending aortic dissection. Arch Surg. 1990 Jun;125(6):786–790. doi: 10.1001/archsurg.1990.01410180112018. [DOI] [PubMed] [Google Scholar]
- 32.Dake MD, Kato N, Mitchell RS, et al. Endovascular stent-graft placement for the treatment of acute aortic dissection. N Engl J Med. 1999 May 20;340(20):1546–1552. doi: 10.1056/NEJM199905203402004. [DOI] [PubMed] [Google Scholar]
- 33.Sasaki S, Yasuda K, Kunihara T, et al. Surgical results of Stanford type B aortic dissection. Comparisons between partial and subtotal replacement of the dissected aorta. J Cardiovasc Surg (Torino) 2000 Apr;41(2):227–232. [PubMed] [Google Scholar]
- 34.Lansman SL, Hagl C, Fink D, et al. Acute type B aortic dissection: surgical therapy. Ann Thorac Surg. 2002 Nov;74(5):S1833–S1835. doi: 10.1016/s0003-4975(02)04134-6. discussion S57–63. [DOI] [PubMed] [Google Scholar]
- 35.Greenberg RK, Haulon S, Khwaja J, et al. Contemporary management of acute aortic dissection. J Endovasc Ther. 2003 Jun;10(3):476–485. doi: 10.1177/152660280301000312. [DOI] [PubMed] [Google Scholar]
- 36.White RA, Miller DC, Criado FJ, et al. Report on the results of thoracic endovascular aortic repair for acute, complicated, type B aortic dissection at 30 days and 1 year from a multidisciplinary subcommittee of the Society for Vascular Surgery Outcomes Committee. J Vasc Surg. 2011 Apr;53(4):1082–1090. doi: 10.1016/j.jvs.2010.11.124. [DOI] [PubMed] [Google Scholar]
- 37.Nienaber CA, Fattori R, Lund G, et al. Nonsurgical reconstruction of thoracic aortic dissection by stent-graft placement. N Engl J Med. 1999 May 20;340(20):1539–1545. doi: 10.1056/NEJM199905203402003. [DOI] [PubMed] [Google Scholar]
- 38.Xenos ES, Minion DJ, Davenport DL, et al. Endovascular versus open repair for descending thoracic aortic rupture: institutional experience and meta-analysis. Eur J Cardiothorac Surg. 2009 Feb;35(2):282–286. doi: 10.1016/j.ejcts.2008.10.042. [DOI] [PubMed] [Google Scholar]
- 39.Kato N, Hirano T, Takeda K, et al. Treatment of aortic dissections with a percutaneous intravascular endoprosthesis: comparison of covered and bare stents. J Vasc Interv Radiol. 1994 Nov-Dec;5(6):805–812. doi: 10.1016/s1051-0443(94)71610-9. [DOI] [PubMed] [Google Scholar]
- 40.Moon MR, Dake MD, Pelc LR, et al. Intravascular stenting of acute experimental type B dissections. J Surg Res. 1993 Apr;54(4):381–388. doi: 10.1006/jsre.1993.1061. [DOI] [PubMed] [Google Scholar]
- 41.Lombardi JV, Cambria RP, Nienaber CA, et al. Prospective multicenter clinical trial (STABLE) on the endovascular treatment of complicated type B aortic dissection using a composite device design. J Vasc Surg. 2012 Mar;55(3):629–640. e2. doi: 10.1016/j.jvs.2011.10.022. [DOI] [PubMed] [Google Scholar]
- 42.Mossop PJ, McLachlan CS, Amukotuwa SA, et al. Staged endovascular treatment for complicated type B aortic dissection. Nat Clin Pract Cardiovasc Med. 2005 Jun;2(6):316–321. doi: 10.1038/ncpcardio0224. quiz 22. [DOI] [PubMed] [Google Scholar]
- 43.Nienaber CA, Kische S, Zeller T, et al. Provisional extension to induce complete attachment after stent-graft placement in type B aortic dissection: the PETTICOAT concept. J Endovasc Ther. 2006 Dec;13(6):738–746. doi: 10.1583/06-1923.1. [DOI] [PubMed] [Google Scholar]
- 44.Melissano G, Bertoglio L, Rinaldi E, et al. Volume changes in aortic true and false lumen after the "PETTICOAT" procedure for type B aortic dissection. J Vasc Surg. 2012 Mar;55(3):641–651. doi: 10.1016/j.jvs.2011.10.025. [DOI] [PubMed] [Google Scholar]
- 45.Melissano G, Bertoglio L, Kahlberg A, et al. Evaluation of a new disease-specific endovascular device for type B aortic dissection. J Thorac Cardiovasc Surg. 2008 Oct;136(4):1012–1018. doi: 10.1016/j.jtcvs.2008.06.021. [DOI] [PubMed] [Google Scholar]
- 46.Chavan A, Hausmann D, Dresler C, et al. Intravascular ultrasound-guided percutaneous fenestration of the intimal flap in the dissected aorta. Circulation. 1997 Oct 7;96(7):2124–2127. doi: 10.1161/01.cir.96.7.2124. [DOI] [PubMed] [Google Scholar]
- 47.Nallamothu BK, Mehta RH, Saint S, et al. Syncope in acute aortic dissection: diagnostic, prognostic, and clinical implications. Am J Med. 2002 Oct 15;113(6):468–471. doi: 10.1016/s0002-9343(02)01254-8. [DOI] [PubMed] [Google Scholar]
- 48.Ryan C, Vargas L, Mastracci T, et al. Progress in management of malperfusion syndrome from type B dissections. J Vasc Surg. 2013 May;57(5):1283–1290. doi: 10.1016/j.jvs.2012.10.101. discussion 90. [DOI] [PubMed] [Google Scholar]
- 49.Patterson BO, Holt PJ, Nienaber C, et al. Management of the left subclavian artery and neurologic complications after thoracic endovascular aortic repair. J Vasc Surg. 2014 Dec;60(6):1491–1497. e1. doi: 10.1016/j.jvs.2014.08.114. [DOI] [PubMed] [Google Scholar]
- 50.Etz CD, Weigang E, Hartert M, et al. Contemporary spinal cord protection during thoracic and thoracoabdominal aortic surgery and endovascular aortic repair: a position paper of the vascular domain of the European Association for Cardio-Thoracic Surgerydagger. Eur J Cardiothorac Surg. 2015 Jun;47(6):943–957. doi: 10.1093/ejcts/ezv142. [DOI] [PubMed] [Google Scholar]
- 51.Matsumura JS, Lee WA, Mitchell RS, et al. The Society for Vascular Surgery Practice Guidelines: management of the left subclavian artery with thoracic endovascular aortic repair. J Vasc Surg. 2009 Nov;50(5):1155–1158. doi: 10.1016/j.jvs.2009.08.090. [DOI] [PubMed] [Google Scholar]
- 52.Weigang E, Hartert M, Siegenthaler MP, et al. Neurophysiological monitoring during thoracoabdominal aortic endovascular stent graft implantation. Eur J Cardiothorac Surg. 2006 Mar;29(3):392–396. doi: 10.1016/j.ejcts.2005.11.039. [DOI] [PubMed] [Google Scholar]
- 53.Clair DG. Aortic dissection with branch vessel occlusion: percutaneous treatment with fenestration and stenting. Semin Vasc Surg. 2002 Jun;15(2):116–121. doi: 10.1053/svas.2002.33091. [DOI] [PubMed] [Google Scholar]
- 54.Gargiulo M, Bianchini Massoni C, Gallitto E, et al. Lower limb malperfusion in type B aortic dissection: a systematic review. Ann Cardiothorac Surg. 2014 Jul;3(4):351–367. doi: 10.3978/j.issn.2225-319X.2014.07.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nienaber CA, Rousseau H, Eggebrecht H, et al. Randomized comparison of strategies for type B aortic dissection: the INvestigation of STEnt Grafts in Aortic Dissection (INSTEAD) trial. Circulation. 2009 Dec 22;120(25):2519–2528. doi: 10.1161/CIRCULATIONAHA.109.886408. [DOI] [PubMed] [Google Scholar]
- 56.Svensson LG, Kouchoukos NT, Miller DC, et al. Expert consensus document on the treatment of descending thoracic aortic disease using endovascular stent-grafts. Ann Thorac Surg. 2008 Jan;85(1 Suppl):S1–S41. doi: 10.1016/j.athoracsur.2007.10.099. [DOI] [PubMed] [Google Scholar]
- 57.Nienaber CA, Kische S, Rousseau H, et al. Endovascular repair of type B aortic dissection: long-term results of the randomized investigation of stent grafts in aortic dissection trial. Circ Cardiovasc Interv. 2013 Aug;6(4):407–416. doi: 10.1161/CIRCINTERVENTIONS.113.000463. [DOI] [PubMed] [Google Scholar]
- 58.Nienaber CA, Kische S, Akin I, et al. Strategies for subacute/chronic type B aortic dissection: the Investigation Of Stent Grafts in Patients with type B Aortic Dissection (INSTEAD) trial 1-year outcome. J Thorac Cardiovasc Surg. 2010 Dec;140(6 Suppl):S101–S108. doi: 10.1016/j.jtcvs.2010.07.026. discussion S42–S46. [DOI] [PubMed] [Google Scholar]
- 59.Song JM, Kim SD, Kim JH, et al. Long-term predictors of descending aorta aneurysmal change in patients with aortic dissection. J Am Coll Cardiol. 2007 Aug 21;50(8):799–804. doi: 10.1016/j.jacc.2007.03.064. [DOI] [PubMed] [Google Scholar]
- 60.Dake MD, Thompson M, van Sambeek M, et al. DISSECT: a new mnemonic-based approach to the categorization of aortic dissection. Eur J Vasc Endovasc Surg. 2013 Aug;46(2):175–190. doi: 10.1016/j.ejvs.2013.04.029. [DOI] [PubMed] [Google Scholar]