Abstract
Prototypical long-acting kappa opioid receptor (KOPR) antagonists [e.g., norbinaltorphimine (norBNI)] have been reported to exert anxiolytic-like effects in several commonly used anxiety tests in rodents including the novelty-induced hypophagia (NIH) and elevated plus maze (EPM) tests. It remains unknown if the short-acting KOPR antagonists (e.g., zyklophin and LY2444296) have similar effects. In this study effects of zyklophin and LY2444296 (s.c.) were investigated in the NIH and EPM tests in mice 1 h post-injection and compared with norBNI (i.p.) 48 h post-administration. In the NIH test, zyklophin at 3 and 1 mg/kg, but not 0.3 mg/kg, or LY2444296 at 30 mg/kg decreased the latency of palatable food consumption in novel cages, but had no effect in training cages, similar to norBNI (10 mg/kg). Zyklophin at 3 or 1 mg/kg increased or had a trend of increasing the amount of palatable food consumption in novel cages, with no effects in training cages, further indicating its anxiolytic-like effect, but norBNI (10mg/kg) and LY2444296 (30 mg/kg) did not. In the EPM test, norBNI (10 mg/kg) increased open arm time and % open arm entries or time, but zyklophin at all three doses and LY2444296 (30 mg/kg) had no effects. In addition, zyklophin at 3 mg/kg increased numbers of close and total arm entries on EPM, suggesting increased activity; however, norBNI and LY2444296 had no effects on close and total arm entries. Thus, all three KOPR antagonists had anxiolytic-like effects in the NIH test. However, only the long-acting one (norBNI), but not the short-acting ones (zyklophin and LY2444296), demonstrated anti-anxiety like effects in the EPM test. It remains to be investigated if the differences are due to the differences in their durations of action and/or pharmacodynamic properties.
Keywords: kappa opioid, anxiety, short-acting, antagonist
Introduction
The dynorphin/kappa opioid receptor (DYN/KOPR) system has been shown to mediate negative emotional states and stress-related behaviors [1]. KOPR agonists produce depression and dysphoria in humans [2] and conditioned place aversion in animals [3]. Several groups have demonstrated that KOPR antagonists norbinaltorphimine (norBNI), 5′-guanidinonaltrindole (5′-GNTI) and the JDTic reduced anxiety-/fear-like behaviors in rat and mouse tests, including the novelty-induced hypophagia (NIH), elevated plus maze (EPM), defensive burying, open field, and fear-potentiated startle tests [see [4;5] for reviews].
Despite few similarities in their chemical structures, the prototypical kappa opioid antagonists such as norBNI and JDTic have slow onsets of maximal KOPR antagonist actions (24-48 h) and their effects last for weeks in vivo [see [6] for a review]. It is not known whether the long-lasting effects of these prototypical KOPR antagonists are essential for their anti-fear/anxiety-like responses in animal models.
Zyklophin is a cyclic peptide [N-benzylTyr1,cyclo(D-Asp5,Dap8)]Dyn A-(1-11) amide] synthesized by Aldrich and colleagues [7]. It exhibits reasonable affinity and high selectivity for KOPR [Ki (KOPR) = 30 nM and Ki ratios (κ μ δ) of 1/194/>330 in radioligand binding assays]. It antagonizes KOPR-mediated inhibition of forskolin-stimulated adenylyl cyclase activity induced by dynorphin A(1-13) in vitro with a KB value of 84 nM [7]. Zyklophin has been shown to be systemically (s.c.) active with a much shorter duration (less than 12 h) than norBNI in antagonizing U50,488-induced antinociception and in inhibiting stress-induced reinstatement of cocaine-seeking behavior in mice [8]. Two short-acting KOPR antagonists, AZ-MTAB and LY-DMPF (also named LY2456302) [IC50 ratios (κ μ δ opioid receptors) of 1/37/440 and 1/40/490 in [35S]GTPγs binding assay, respectively] were reported to have anxiolytic-like activity in prenatally-stressed rats in the EPM test [9]. LY2456302 was recently revealed to alleviate the nicotine withdrawal syndromes including the associated anxiety in mice [10]. LY2444296, an analogue of LY2456302, is a selective short-acting KOPR antagonist with a Ki value of ∼1 nM for the KOPR and κ μ and κ δ selectivity of ∼60 and ∼350, respectively [compound 25 in [11]].
Here we determined the effects of zyklophin and LY2444296 in two commonly used anxiety tests and compared them to nor-BNI.
Materials and Methods
Animals
Male CD-1 mice (8 weeks) were purchased from Charles River Co. (Wilmington, MA). Mice were housed five per cage upon arrival in the animal facility in polycarbonate cages (11 × 7 × 5 inches) on a 12:12-h light/dark cycle (7 am-7pm) with ad libitum access to food and water. Mice weighed ∼32-36 g at the start of the study. Protocols were approved by the Institutional Animal Care and Use Committee of Temple University. Animal care and experimental procedures were conducted according to the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996). Animals were habituated for at least 1h before training or behavioral tests that were conducted between 12:30 pm and 6 pm.
Compounds
Zyklophin was synthesized as described previously [7]. LY2444296 was a generous gift from Eli Lilly and Co. (Indianapolis, IN). NorBNI and diazepam were provided by the National Institute on Drug Abuse (Bethesda, MD). Both zyklophin and norBNI were dissolved in deionized water. LY2444296 was dissolved in 85% DL- lactic acid (20 μl /mg compound), diluted with saline by vortex, and added 1N NaOH (150 μl per mg compound) with vortex to pH ∼5. Diazepam was moistened with a few drops of Tween 80 at a final concentration of 2% and then prepared as a water suspension using a mortar and a pestle. All solutions were freshly prepared on the day of use. Injections (zyklophin s.c, LY2444296 s.c, norBNI i.p., diazepam i.p. or water i.p. or s.c.) were carried out in a volume of 0.1 ml per 10 g of body weight. Doses used for zyklophin and norBNI were chosen following previous publications [5;8], and that for LY2444296 selected based on its dose responses in forced swim tests (our unpublished data).
NIH test
(see [12] for a review) was performed based on those used in Dr. Irwin Lucki's and Dr. Julie Blendy's laboratories with modifications [13;14]. Mice were allowed to acclimate to the animal facility for 2 nights prior to training. Training was done in the testing room, which was illuminated by a light (∼260 lux) similar to that in the holding room. The training consisted of daily sessions (20 min) in which each single mouse was placed in a training cage (11 × 7 × 5 inches), which is a clear polycarbonate cage identical to the home cage with a lid and bedding but without food and water supplies. Mouse was habituated for 5 min first and then given access to a highly palatable food (peanut butter chips; Nestle, Glendale, CA) for 15 min delivered in a clear plastic 60-mm petri dish. Mice from the same home cage were trained in one training cage sequentially. The latency to approach and initiate ingestion and the amount of food consumption during the session were measured. For CD-1 mice, a stable mean latency to approach the food was obtained by day 12, such that the variability of means over three sessions was <20%. Mice that did not meet this criterion (less than 10%) were not included in the study.
On day 13, mice were injected with drugs or H2O and returned to their home cages. One hour (for zyklophin or LY2444296) or 48 hours (for norBNI) after injection, testing in the novel environment was performed. Mice were removed from their home cage and placed in an empty, clear polycarbonate cage (15×12×7.5 inches) without bedding or lid in the same room. The novel cage was placed on a bench with white cardboard paper placed underneath and on two sides of the cage. Bright illumination (a 100W light bulb) was placed directly overhead in addition to the room lighting condition (∼260 lux). There was 5-min acclimation period prior to 15-min testing with access to peanut butter chips in the novel environment. Animals were videotaped and the latency to ingestion and the amount consumed during the test sessions in the novel environment were both examined as anxiety measures. On day 14 one hour after another zyklophin or H2O injection or 72 hr after the previous single norBNI or H2O injection, each mouse was tested in the same training cage with 5-min acclimation prior to 15-min testing.
EPM test
was carried out as we described previously [15] with modifications using a mouse EPM apparatus (San Diego Instruments, Inc.). Mice were tested under a consistent lighting condition (∼260 lux). To begin a test session, mice were placed in the center facing an open arm. The exploration of mice in the maze was videotaped, and entries into open and closed arms and center were recorded for the first 5 min of EPM exposure. The animal placing all four paws onto an arm was considered to be in the arm, otherwise the animal was in the center (see [16] for a review). Between each trial, the maze was wiped clean and dried. The measures of anxiety are the % of open arm entries and the % of time spent on open arms, both expressed as the % of the total entries into, or time spent on, the open and closed arms, whereas the numbers of total and closed arm entries are considered locomotor measures (see [16;17] for reviews). The absolute time spent on the open arms, which has been used as an indicator of anxiety by some researchers (e.g., [18]), was recorded in seconds (sec).
Locomotor activities
were measured with a Digiscan D Micro System (Accuscan, Columbus, OH, USA) and eight individual activity monitors according to our published procedures [19]. Total, ambulatory and stereotypic activities were measured over 1.5 h post injections in 5-min bins under a normal laboratory lighting condition.
Data analysis
Data are expressed as the mean±SEM, and analyzed for statistical significance by Student's t test or one-way analysis of variance (ANOVA) followed by Bonferroni post hoc tests (Prism v.5).
Results
Effects of zyklophin, LY2444296 and norBNI in the NIH test
Zyklophin (3 and 1 mg/kg, but not 0.3 mg/kg) 1h post-administration decreased the latency of palatable food consumption in novel cages (Fig. 1A), but had no effect in training cages (Fig. 1B), indicating its anxiolytic-like effect in the novel cages [F(3,64)=5.66, p=0.0017, one-way ANOVA]. NorBNI (10 mg/kg) 48h post-injection and LY2444296 (30 mg/kg) 1 h post-injection also had anti-anxiety effect in novel cages (p<0.01, Student's t test) with no effect in training cages (Figs. 1A and 1B). In addition, zyklophin 3 mg/kg, but not 1 or 0.3 mg/kg, significantly increased the amount of chip consumption in novel cages [F(3,71)=4.23, p=0.0082, one-way ANOVA] (Fig. 1C), but it had no effects in training cages (Fig. 1D), further indicating its anxiolytic-like effect. Zyklophin at 1 mg/kg had a trend to increase the amount of chip consumption (p=0.041 vs vehicle by Student's t test) in the novel cage (Fig. 1C). NorBNI (10 mg/kg) and LY2444296 (30 mg/kg) had no effects on palatable food consumption in either cages (Student's t test) (Figs.1C and 1D).
Fig. 1. Effects of zyklophin, LY2444296 and norBNI on latency to approach and ingest food and on amount of food consumed in the NIH test.
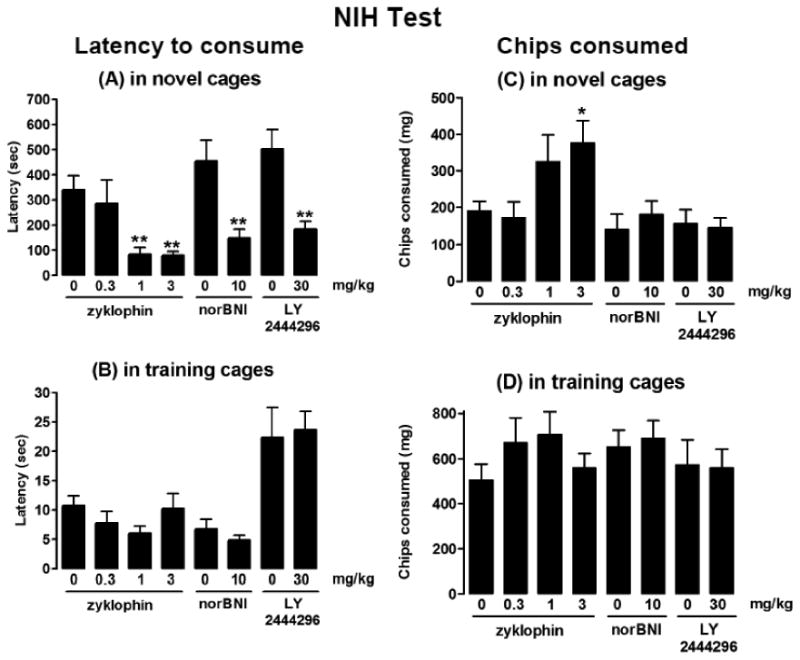
Latency in (A) novel and (B) training cages. Amount of food consumed in (C) novel and (D) training cages. Asterisks represent significant differences from the respective vehicle groups. Note the differences in the Y-axis scale between novel and training cages. **p<0.01,*p<0.05,one-way ANOVA followed by Bonferroni's multiple comparison test for zyklophin and Student's t test for norBNI and LY2444294. Data are expressed as mean ± SEM (n=13-15/group).
Effects of zyklophin, LY2444296 and norBNI in the EPM test
Zyklophin (3 and 1 mg/kg) or LY2444296 (30 mg/kg) 1 h after administration had no effects on open arm time and % open arm entries or time (Figs. 2A, 2B and 2C) (one-way ANOVA and Student's t test, respectively). Zyklophin at 3 mg/kg increased numbers of close and total arm entries on EPM, while at 1 mg/kg there was a trend of increase, but did not reach statistical significance (Figs. 2D and 2E) [F(2,39)=4.48, p=0.0177 and F(2,39)=4.47, p=0.0179 by one-way ANOVA]. LY2444296 had no effects on the numbers of close and total arm entries. In contrast, norBNI (10 mg/kg) 48h post-injection increased open arm time and % open arm entries or time (Figs. 2A, 2B and 2C) (*p<0.05, **p<0.01, Student's t test), and did not affect numbers of close and total arm entries (Student's t test)(Figs. 2D and 2E). As a positive control, diazepam (2.5 mg/kg) 1 h prior to the test increased open arm time and % open arm entries or time (Figs. 2A, 2B and 2C) (*p<0.05, Student's t test), and decreased the number of close arm entries (**p<0.01, Student's t test), indicating its sedative effect.
Fig. 2. Anxiety measures and activity measures for effects of zyklophin, LY2444296 and norBNI on EPM behaviors.
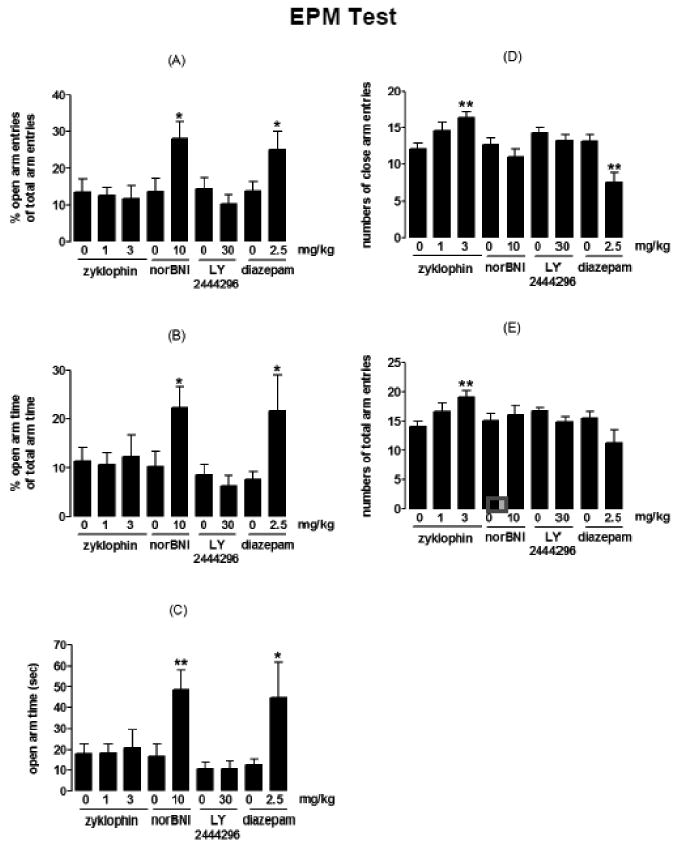
The anxiety measures: (A) % open arm entries, (B) % open arm time and (C) open arm time. The activity measures: (D) number of close arm entries and (E) number of total arm entries. Asterisks denote significant differences from the respective vehicle groups. **p<0.01,*p<0.05,one-way ANOVA followed by Bonferroni's multiple comparison test for zyklophin and Student's t test for norBNI, LY2444296 and diazepam. Data represent mean ± SEM (n=12-15/group).
Effects of zyklophin on locomotor activities
In the EPM test described above, zyklophin (3 mg/kg) increased close and total arm entries significantly; therefore, effects of the same dose of zyklophin on locomotor activities were further examined. The results demonstrated that zyklophin did increase total, ambulatory and stereotypic activities of mice (Fig.3).
Fig. 3. Effects of zyklophin (3 mg/kg) on locomotor activity in mice.
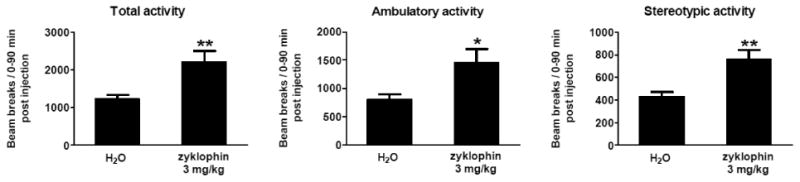
Mice were habituated in the locomotor chambers for 2 h first and then injected with water or zyklophin. Total (top pannel), ambulatory (middle pannel) and stereotypic (bottom pannel) activities were continuously monitored after injections for 90 min using automated locomotor chambers. Data are expressed as mean ± SEM beam breaks/90-min period (0-90 min post injections) and analyzed by Student's t test (**p<0.01,*p<0.05). n=11-13/group.
Discussion
Zyklophin, LY2444296 and norBNI had anxiolytic-like effects in the NIH test as manifested as a decrease in the latency to initiate palatable food ingestion. The anti-anxiety effect of zyklophin, but not norBNI and LY2444296, was further corroborated by an increase in palatable food consumption. However, norBNI, but not zyklophin or LY2444296, exhibited anti-anxiety-like effects in the EPM test. Zyklophin at 3 mg/kg increased mouse locomotor activities. To the best of our knowledge, this is the first demonstration that KOPR antagonists have different anxiolytic-like effects.
The anti-anxiety responses of norBNI in the NIH and EPM tests are in line with several publications on anxiolytic-like effects of the long-acting KOPR antagonists norBNI, JDTic, GNTI and DIPPA in rat or mouse tests of anxiety, including the EPM, NIH, defensive burying, open field and fear-potentiated startle tests (see [4;5] for reviews).
Short-acting KOPR antagonists have also been examined in anxiety tests. AZ-MTAB and LY-DMPF (also named LY2456302) were found to be anxiolytic in prenatally-stressed rats using a modified EPM test [9]. In addition, similar to norBNI and JDTic, LY2456302 attenuated nicotine withdrawal syndrome including associated anxiety-related behaviors in another modified EPM test in mice [10;20]. However, in the current study, zyklophin and LY2444296 did not exhibit anti-anxiety effects in the EPM test, in contrast to norBNI, even though zyklophin at a higher dose increased overall activities of mice on the EPM. It is noteworthy that in our studies mice displayed only acute anxiety-like responses evoked by exposure to the elevated open arms, while aforementioned rats or mice in the two modified EPM tests had more lasting or permanent increase in basal anxiety induced by the prenatal stress [9] or nicotine withdrawal [10], respectively. Thus, short-acting compounds will need to be further examined in animals pre-exposed to various prenatal or postnatal stressors to determine whether the stress will unmask the drug effects. In addition, more KOPR antagonists will need to be tested to determine whether NIH tests are more responsive to the anxiolytic effects of short-acting KOPR antagonists than the regular EPM test. Although the NIH and EPM tests are both considered tests of anxiety, they measure the behavioral construct in different ways. The NIH test is a conflict-based anxiety test where a trained behavioral response is suppressed by exposure to novelty, whereas the EPM test involves a readout of self-selection between different arms of the maze based on approach/avoidance behavior.
In contrast to the differences observed in this study, short-acting KOPR antagonists have been reported to have similar effects as long-acting ones in other behavioral end points. zyklophin [8], PF-04455242 [21] , AZ-ECPC [9], AZ-MTAB [9], and LY2456302 (called LY-DMPF) [9;22] were reported to reduce depression-like behaviors, inhibit reinstatement of seeking of drugs of abuse and antagonize KOPR agonist-induced antinociception and diuresis.
The observed differences among zyklophin, LY2444296 and norBNI in the EPM test may be attributed to differences in pharmacological specificity and pharmacokinetic properties. Although all compounds act on the KOPR, they each may have other targets. DiMattio et al. [23] recently reported that zyklophin injected into the nape of neck of mice caused scratching immediately, which was not blocked by 24-hour pretreatment with norBNI, and was not abolished in mutant mice lacking KOPR. These results indicate that zyklophin produces non-KOPR-mediated effects. Besides the KOPR, norBNI and LY2444296 might have effects on the MOPR and DOPR with lower affinities [11;24].
As a KOPR antagonist, zyklophin has a duration of action of <12 h [8], LY2444296, similar to LY2456302, did not block KOPR agonist-induced analgesia one week after administration, indicating lack of long-lasting effects (personal communications from L. Kehn-Rodrick of Eli Lilly and Co.), whereas norBNI shows a long duration of action of 2 weeks or more [25;26]. Whether the long duration of action is required for the anxiolytic-like effects in the EPM test is not known. The long duration of action of norBNI has been attributed to activation of c-Jun N-terminal kinase 1 leading to inhibition of signaling at the KOPR, while short-acting KOPR antagonists including LY2444296 (also called FP3FBZ) did not activate this kinase. [27;28]. These are consistent with a pharmacokinetic study [29], or long-lasting presence of norBNI in the brain following a single injection [30;31].
There are at least fifteen commonly used rat and mouse anxiety tests, besides the NIH and EPM tests, and more than seventy mouse or rat genetic models of anxiety disorders available, which are generated by single-gene engineering or selective breeding, and are supposed to produce abnormally elevated anxiety that more closely resemble the pathological nature of human anxiety disorders [32]. Thus, additional studies are also warranted to determine whether KOPR antagonists, in particular short-acting ones, elicit similar or different behavioral responses in other rat or mouse tests/models of anxiety as long-acting ones since short-acting ones are preferred for clinical development.
In conclusion, in two anxiety tests, short-acting KOPR antagonists (zyklophin and LY2444296) and long acting one (norBNI) showed similarities and differences. They all exhibited anxiolytic-like effects in the NIH test; however, the long-acting KOPR antagonist, but not the short-acting ones, displayed anti-anxiety effect in the EPM test. It remains to be investigated if the differences are due to the differences in their durations of action and/or pharmacodynamic properties.
Highlights.
Short-acting KOPR antagonists (zyklophin and LY2444296) and long acting one (norBNI) showed similarities and differences.
They all exhibited anxiolytic-like effects in the NIH test; however, the long-acting KOPR antagonist, but not the short-acting ones, displayed anti-anxiety effect in the EPM test.
Acknowledgments
This work was supported by National Institute of Health Grants R01 DA017302, DA036802 and P30DA013429 to LYLC and DA018832 and DA023924 to JVA. We thank Jennifer L. Onksen in Dr. Julie A Blendy's lab and Gregory V. Carr in Dr. Irwin Lucki's lab of Unviversity of Pennsylvania for their technical advice for the NIH test. We appreciate Dr. Lynn Kirby of Temple University for her advice and technical support on mouse behavioral tests.
Abbreviations
- 5′-GNTI
5′-Guanidinonaltrindole
- ANOVA
analysis of variance
- AZ-ECPC
Ethyl 4-(3-Carbamoylbenzyl)-1-(Cyclohexylmethyl)Piperidine-4-Carboxylate
- AZ-MTAB
3-((1R,3r,5S)-8-((5-Methylthiophen-2-yl)methyl)-8-azabicyclo[3.2.1]octan-3-yloxy)benzamide
- DOPR
delta opioid receptor
- EPM
elevated plus maze
- FST
forced swim test
- i.p
intraperitoneal(ly)
- JDTic
(3R)-7-Hydroxy-N-[(2S)-1-[(3R,4R)-4-(3-hydroxyphenyl)-3,4-dimethylpiperidin-1-yl]-3-methylbutan-2-yl]-1,2,3,4-tetrahydroisoquinoline-3-carboxamide
- KOPR
kappa opioid receptor
- LY2444296 (also called FP3FBZ)
(S)-3-fluoro-4-(4-((2-(3-fluorophenyl)pyrrolidin-1-yl)methyl)phenoxy)benzamide
- LY2456302 (also called LY-DMPF JSPA065 or CERC-501)
4-(4-{[(2S)-2-(3,5-Dimethylphenyl)-1-pyrrolidinyl]methyl}phenoxy)-3-fluorobenzamide
- LY-DMPF
see LY2456302
- MOPR
mu opioid receptor
- NIH
novelty-induced hypophagia
- norBNI
norbinaltorphimine
- PF-04455242
2-Methyl-N-((2′-(pyrrolidin-1-ylsulfonyl)biphenyl-4-yl)methyl)propan-1-amine
- s.c
subcutaneous(ly)
- zyklophin
N-Benzyl-L-tyrosylglycylglycyl-N-[(3S,6S,9S,12S)-6-[(2S)-2-butanyl]-3-({(2S)-5-carbamimidamido-1-[(2S)-2-{[(2S)-1,6-diamino-1-oxo-2-hexanyl]carbamoyl}-1-pyrrolidinyl]-1-oxo-2-pentanyl}carbamoyl)-9-(3-carbamimidamidopropyl)-5,8,11,14-tetraoxo-1,4,7,10-tetraazacyclotetradecan-12-yl]-L-phenylalaninamide or [N-benzylTyr1,cyclo(D-Asp5,Dap8)]Dyn A-(1-11) amide]
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bruchas MR, Land BB, Chavkin C. The dynorphin/kappa opioid system as a modulator of stress-induced and pro-addictive behaviors. Brain Res. 2010;1314:44–55. doi: 10.1016/j.brainres.2009.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pfeiffer A, Brantl V, Herz A, Emrich HM. Psychotomimesis mediated by kappa opiate receptors. Science. 1986;233:774–776. doi: 10.1126/science.3016896. [DOI] [PubMed] [Google Scholar]
- 3.Shippenberg TS, Herz A. Differential effects of mu and kappa opioid systems on motivational processes. NIDA Res Monogr. 1986;75:563–566. [PubMed] [Google Scholar]
- 4.Van't Veer A, Carlezon WA., Jr Role of kappa-opioid receptors in stress and anxiety-related behavior. Psychopharmacology (Berl) 2013;229:435–452. doi: 10.1007/s00213-013-3195-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hang A, Wang YJ, He L, Liu JG. The role of the dynorphin/kappa opioid receptor system in anxiety. Acta Pharmacol Sin. 2015;36:783–790. doi: 10.1038/aps.2015.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Metcalf MD, Coop A. Kappa opioid antagonists: past successes and future prospects. AAPS J. 2005;7:E704–E722. doi: 10.1208/aapsj070371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patkar KA, Yan X, Murray TF, Aldrich JV. [Nalpha-benzylTyr1,cyclo(D-Asp5,Dap8)]-dynorphin A-(1-11)NH2 cyclized in the “address” domain is a novel kappa-opioid receptor antagonist. J Med Chem. 2005;48:4500–4503. doi: 10.1021/jm050105i. [DOI] [PubMed] [Google Scholar]
- 8.Aldrich JV, Patkar KA, McLaughlin JP. Zyklophin, a systemically active selective kappa opioid receptor peptide antagonist with short duration of action. Proc Natl Acad Sci U S A. 2009;106:18396–18401. doi: 10.1073/pnas.0910180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peters MF, Zacco A, Gordon J, Maciag CM, Litwin LC, Thompson C, Schroeder P, Sygowski LA, Piser TM, Brugel TA. Identification of short-acting kappa-opioid receptor antagonists with anxiolytic-like activity. Eur J Pharmacol. 2011;661:27–34. doi: 10.1016/j.ejphar.2011.04.017. [DOI] [PubMed] [Google Scholar]
- 10.Jackson KJ, Jackson A, Carroll FI, Damaj MI. Effects of orally-bioavailable short-acting kappa opioid receptor-selective antagonist LY2456302 on nicotine withdrawal in mice. Neuropharmacol. 2015;97:270–274. doi: 10.1016/j.neuropharm.2015.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitch CH, Quimby SJ, Diaz N, Pedregal C, de la Torre MG, Jimenez A, Shi Q, Canada EJ, Kahl SD, Statnick MA, McKinzie DL, Benesh DR, Rash KS, Barth VN. Discovery of aminobenzyloxyarylamides as kappa opioid receptor selective antagonists: application to preclinical development of a kappa opioid receptor antagonist receptor occupancy tracer. J Med Chem. 2011;54:8000–8012. doi: 10.1021/jm200789r. [DOI] [PubMed] [Google Scholar]
- 12.Dulawa SC, Hen R. Recent advances in animal models of chronic antidepressant effects: the novelty-induced hypophagia test. Neurosci Biobehav Rev. 2005;29:771–783. doi: 10.1016/j.neubiorev.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 13.Balu DT, Hodes GE, Anderson BT, Lucki I. Enhanced sensitivity of the MRL/MpJ mouse to the neuroplastic and behavioral effects of chronic antidepressant treatments. Neuropsychopharmacology. 2009;34:1764–1773. doi: 10.1038/npp.2008.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Onksen JL, Brown EJ, Blendy JA. Selective deletion of a cell cycle checkpoint kinase (ATR) reduces neurogenesis and alters responses in rodent models of behavioral affect. Neuropsychopharmacology. 2011;36:960–969. doi: 10.1038/npp.2010.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang P, Liu-Chen LY, Kirby LG. Anxiety-like effects of SR141716-precipitated delta9-tetrahydrocannabinol withdrawal in mice in the elevated plus-maze. Neurosci Lett. 2010;475:165–168. doi: 10.1016/j.neulet.2010.03.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hogg S. A review of the validity and variability of the elevated plus-maze as an animal model of anxiety. Pharmacol Biochem Behav. 1996;54:21–30. doi: 10.1016/0091-3057(95)02126-4. [DOI] [PubMed] [Google Scholar]
- 17.File SE. Factors controlling measures of anxiety and responses to novelty in the mouse. Behav Brain Res. 2001;125:151–157. doi: 10.1016/s0166-4328(01)00292-3. [DOI] [PubMed] [Google Scholar]
- 18.Patel S, Hillard CJ. Pharmacological evaluation of cannabinoid receptor ligands in a mouse model of anxiety: further evidence for an anxiolytic role for endogenous cannabinoid signaling. J Pharmacol Exp Ther. 2006;318:304–311. doi: 10.1124/jpet.106.101287. [DOI] [PubMed] [Google Scholar]
- 19.Xu W, Wang Y, Ma Z, Chiu YT, Huang P, Rasakham K, Unterwald E, Lee DY, Liu-Chen LY. l-Isocorypalmine reduces behavioral sensitization and rewarding effects of cocaine in mice by acting on dopamine receptors. Drug Alcohol Depend. 2013;133:693–703. doi: 10.1016/j.drugalcdep.2013.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jackson KJ, Carroll FI, Negus SS, Damaj MI. Effect of the selective kappa-opioid receptor antagonist JDTic on nicotine antinociception, reward, and withdrawal in the mouse. Psychopharmacology (Berl) 2010;210:285–294. doi: 10.1007/s00213-010-1803-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grimwood S, Lu Y, Schmidt AW, Vanase-Frawley MA, Sawant-Basak A, Miller E, McLean S, Freeman J, Wong S, McLaughlin JP, Verhoest PR. Pharmacological characterization of 2-methyl-N-((2′-(pyrrolidin-1-ylsulfonyl)biphenyl-4-yl)methyl)propan-1-ami ne (PF-04455242), a high-affinity antagonist selective for kappa-opioid receptors. J Pharmacol Exp Ther. 2011;339:555–566. doi: 10.1124/jpet.111.185108. [DOI] [PubMed] [Google Scholar]
- 22.Rorick-Kehn LM, Witkin JM, Statnick MA, Eberle EL, McKinzie JH, Kahl SD, Forster BM, Wong CJ, Li X, Crile RS, Shaw DB, Sahr AE, Adams BL, Quimby SJ, Diaz N, Jimenez A, Pedregal C, Mitch CH, Knopp KL, Anderson WH, Cramer JW, McKinzie DL. LY2456302 is a novel, potent, orally-bioavailable small molecule kappa-selective antagonist with activity in animal models predictive of efficacy in mood and addictive disorders. Neuropharmacol. 2014;77:131–144. doi: 10.1016/j.neuropharm.2013.09.021. [DOI] [PubMed] [Google Scholar]
- 23.DiMattio KM, Yakovleva TV, Aldrich JV, Cowan A, Liu-Chen LY. Zyklophin, a short-acting kappa opioid antagonist, induces scratching in mice. Neurosci Lett. 2014;563:155–159. doi: 10.1016/j.neulet.2014.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Portoghese PS, Lipkowski AW, Takemori AE. Binaltorphimine and nor-binaltorphimine, potent and selective kappa-opioid receptor antagonists. Life Sci. 1987;40:1287–1292. doi: 10.1016/0024-3205(87)90585-6. [DOI] [PubMed] [Google Scholar]
- 25.Endoh T, Matsuura H, Tanaka C, Nagase H. Nor-binaltorphimine: a potent and selective kappa-opioid receptor antagonist with long-lasting activity in vivo. Arch Int Pharmacodyn Ther. 1992;316:30–42. [PubMed] [Google Scholar]
- 26.Horan P, Taylor J, Yamamura HI, Porreca F. Extremely long-lasting antagonistic actions of nor-binaltorphimine (nor-BNI) in the mouse tail-flick test. J Pharmacol Exp Ther. 1992;260:1237–1243. [PubMed] [Google Scholar]
- 27.Bruchas MR, Yang T, Schreiber S, Defino M, Kwan SC, Li S, Chavkin C. Long-acting kappa opioid antagonists disrupt receptor signaling and produce noncompetitive effects by activating c-Jun N-terminal kinase. J Biol Chem. 2007;282:29803–29811. doi: 10.1074/jbc.M705540200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Melief EJ, Miyatake M, Carroll FI, Beguin C, Carlezon WA, Jr, Cohen BM, Grimwood S, Mitch CH, Rorick-Kehn L, Chavkin C. Duration of action of a broad range of selective kappa-opioid receptor antagonists is positively correlated with c-Jun N-terminal kinase-1 activation. Mol Pharmacol. 2011;80:920–929. doi: 10.1124/mol.111.074195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Munro TA, Berry LM, Van't Veer A, Beguin C, Carroll FI, Zhao Z, Carlezon WA, Cohen BM. Long-acting kappa opioid antagonists nor-BNI, GNTI and JDTic: pharmacokinetics in mice and lipophilicity. BMC Pharmacol. 2012;12:5. doi: 10.1186/1471-2210-12-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patkar KA, Wu J, Ganno ML, Singh HD, Ross NC, Rasakham K, Toll L, McLaughlin JP. Physical presence of nor-binaltorphimine in mouse brain over 21 days after a single administration corresponds to its long-lasting antagonistic effect on kappa-opioid receptors. J Pharmacol Exp Ther. 2013;346:545–554. doi: 10.1124/jpet.113.206086. [DOI] [PubMed] [Google Scholar]
- 31.Kishioka S, Kiguchi N, Kobayashi Y, Yamamoto C, Saika F, Wakida N, Ko MC, Woods JH. Pharmacokinetic evidence for the long-lasting effect of nor-binaltorphimine, a potent kappa opioid receptor antagonist, in mice. Neurosci Lett. 2013;552:98–102. doi: 10.1016/j.neulet.2013.07.040. [DOI] [PubMed] [Google Scholar]
- 32.Griebel G, Holmes A. 50 years of hurdles and hope in anxiolytic drug discovery. Nat Rev Drug Discov. 2013;12:667–687. doi: 10.1038/nrd4075. [DOI] [PMC free article] [PubMed] [Google Scholar]


