Abstract
Low-grade nasopharyngeal papillary adenocarcinoma is an extremely rare tumor, with only a limited number of cases reported in the literature. Some published studies have paid more attention to the clinicopathological features of nasopharyngeal adenocarcinoma, while little effort has been made to study the optimal therapeutic strategies. We report about a woman diagnosed with low-grade nasopharyngeal papillary adenocarcinoma. She received the treatment approach that combined transnasal endoscopic surgery to remove the lesion with postoperative radiotherapy for nasal cavity. There was no evidence of recurrence after 4 months of surgery, and further follow-up is being continued. Through this example, we wanted to explore the optimal therapeutic strategies for primary nasopharyngeal adenocarcinomas.
Keywords: nasopharyngeal adenocarcinoma, immunohistochemistry, treatment policy, survival and prognosis
Introduction
Nasopharyngeal carcinoma (NPC) is commonly diagnosed in Southeastern Asia, particularly in southern China.1 It originates from the epithelial lining of the nasopharynx and has a variety of pathological subtypes. The vast majority of NPC cases are keratinizing or nonkeratinizing squamous cell carcinoma.2 Additional pathological types of NPC, which include adenocarcinoma, lymphoma, sarcoma, and minor salivary gland tumor, constitute <5% of all NPC cases.3,6 Therefore, primary nasopharyngeal adenocarcinomas (NPACs) are extremely rare, with only a limited number of cases reported in the literature.1–3 Having obtained written informed consent from the patient and approval of the research ethics board of Shangdong Cancer hospital, we report a case of primary low-grade nasopharyngeal papillary adenocarcinoma (LGNPPA) in a 36-year-old woman who was treated in our hospital. We also include a review of the literature in order to highlight what we know to date about this rare entity.
Case report
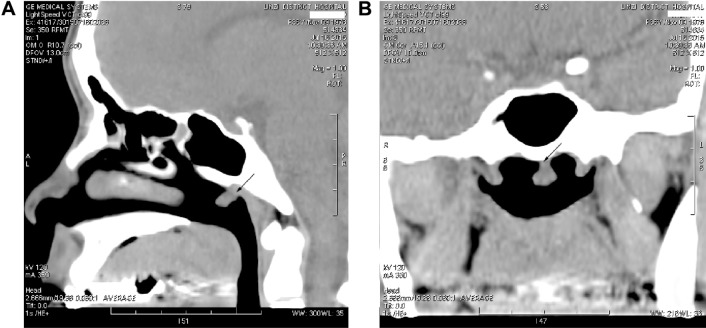
A 36-year-old Chinese woman presented with a 2-year history of bilateral nasal obstruction and mild postnasal drip. It did not vary in severity during this period. No bloody sputum, epistaxis, tinnitus, otitis media, facial pain, aural fullness, or any other complaint was mentioned. A computed tomography (CT) scan of the head and neck showed bilateral mucosal thickening in the maxillary sinus, a soft tissue density in the ethmoid and sphenoid sinuses, and a soft tissue mass in the nasopharynx (Figure 1). There was no additional relevant medical history. The cervical lymph nodes were not palpable, and the physical examination findings were also not remarkable, except for nasal septum deviation irregularly. Other investigations were negative, including CT scan and sonography of thyroid.
Figure 1.
CT scan: the tumor was identified on the roof of the nasopharynx (arrow).
Notes: (A) Sagittal view and (B) coronal view.
Abbreviation: CT, computed tomography.
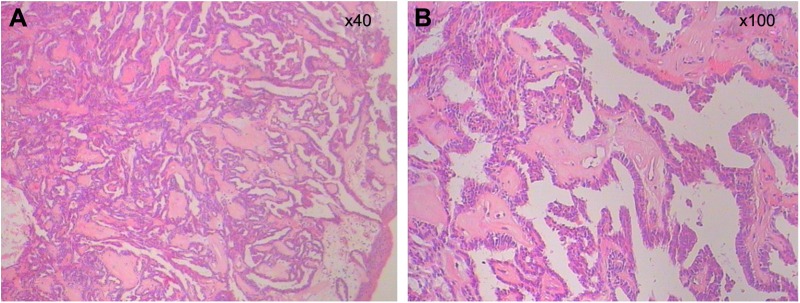
According to the localized nature of the lesion and the benign appearance, transnasal endoscopic resection was undertaken. In the surgical treatment, a papillary pedicle mass of size 0.7 cm, which was pedunculated and freely mobile, was found on the roof of the nasopharynx. Subsequently, the mass was resected with its stalk. The tumor was completely excised on endoscopy with an adequate surgical margin. Hematoxylin and eosin staining was performed for microscopic examination, and the lesion was characterized by papillary and glandular epithelial proliferation (Figure 2). Postoperative histopathology showed a low-grade papillary adenocarcinoma, and surgical margin was negative. According to the patient’s strong will after surgery and the cases reported in the literature, she had accepted postoperative radiotherapy (PORT) for nasal cavity with a total dose of 60 gray in 30 fractions over 6 weeks in our hospital. There was no evidence of recurrence after 4 months of surgery, and further follow-up is being carried out.
Figure 2.
The pictures show typical papillary growth pattern of glandular epithelial cells, which is usually seen in papillary thyroid carcinoma.
Notes: (A) H&E ×40 and (B) H&E ×100.
Abbreviation: H&E, hematoxylin and eosin.
Discussion
The most common malignancy in the nasopharynx is NPC. By and large, the most common nasopharyngeal neoplasm is nonkeratinizing and keratinizing squamous cell carcinomas without glandular differentiation.2 Primary NPACs as a group are extremely rare tumors, which are reported to occupy 0.48% of all types of NPC.3–5
Primary NPACs can be classified into two main categories: the conventional or mucosal surface origin type and the salivary gland type, with different morphological features and clinical behavior.2,3,7–10 Nasopharyngeal papillary adenocarcinoma, which belongs to the former category, has been reported in the literature.2 A retrospective study first described LGNPPAs as a distinct entity in a series of nine patients in 1988.7 There have been several case reports or small series in the literature about this entity after this seminal paper.7,9–12 Primary salivary gland-type nasopharyngeal carcinoma belongs to the latter category, which involves two main pathological subtypes: adenoid cystic carcinoma and mucoepidermoid carcinoma.2,9,13
The case we presented was LGNPPA, which according to the World Health Organization is defined LGNPPA as phyllodes papillary and glandular structures with exophytic growth characteristics of low-grade adenocarcinoma.8 Papillary NPAC is a special type of low-grade malignant carcinoma, and its morphology is similar to papillary thyroid carcinoma, which requires identification with the metastasis of papillary thyroid carcinoma. Thyroid transcription factor-1 (TTF-1) is generally deemed to be specific to the thyroid gland and the lung. However, some reports have occasionally shown positive immunohistochemical reactivity for TTF-1 in nasopharyngeal papillary adenocarcinoma.8,10 Therefore, TTF-1 protein might not be a suitable marker for distinguishing between primary or metastatic nasopharyngeal papillary adenocarcinoma. But the negative reactivity for thyroglobulin and CK20 promotes distinguishing metastatic thyroid papillary carcinoma from LGNPPA.10 Our case was diagnosed as LGNPPA because both derived from the epithelial surface and had a negative thyroid scan.
Recent and past studies are consistent in revealing the presence of Epstein–Barr virus (EBV) to be closely linked with the development and carcinogenesis of type II and III tumors.2,4 EBV DNA as a molecular marker is universally associated with the nonkeratinizing subtype of NPC.14 Some studies have detected low-expression levels of EBV markers in the NPAC tissue.2,15 Therefore, the relationship between NPACs and EBV is still uncertain. Further examination is still needed because of the extreme rarity of this tumor.
The treatment policy of primary NPACs is mainly chosen according to the clinical stage and histological grade of the tumor.6,16 Owing to the rarity of these cancers, the management of the lesions is usually empirically based, and no standard treatment exists for the tumors. Controversy exists as to whether radiotherapy alone, surgical treatment alone, or surgery combined with radiotherapy is the optimal therapeutic approach.
Several studies recommended that surgery combined with radiotherapy may be the main treatment policy for limited and resectable NPACs, such as adenoid cystic carcinoma, mucoepidermoid carcinoma, and well-differentiated adenocarcinoma.6,17 A retrospective study by Liu et al16 revealed that the overall survival in T1–T2 patients was significantly improved comparing combined surgical treatment group (n=12) with the nonsurgical (radiotherapy) treatment group (n=12; P=0.041). Multivariate analysis also indicated that surgical treatment was an independent predictor of survival in such patients (P=0.017), which is consistent with the results of Guo et al4 and Liu et al.18 However, some studies revealed that the addition of PORT could not bring survival benefit for early-stage (stages I and II) patients with clear margin and without adverse prognostic factors such as lymphovascular or perineural invasion, which is a set of conditions usually restricted to low-grade variants.7,19,20 On the basis of the patient’s strong will and the cases reported in the literature, she had accepted PORT. Therefore, large multicentric studies or studies with large sample size should be conducted to further evaluate whether PORT is necessary or not for early stage lesions.
For the poorly differentiated or unresectable primary NPACs, regardless of the conventional and salivary gland type, it is very difficult to achieve the purpose of radical cure with surgery alone. Indications for definitive radiotherapy included early-stage tumors with positive surgical margin, poorly differentiated carcinoma, perineural invasion, and deep muscle invasion. Also, high-grade (poorly differentiated) adenocarcinomas are relatively radiosensitive, and radiotherapy or chemoradiotherapy is currently considered as the primary treatment policy for such patients.17 Chemotherapy was also administered to patients, but no obvious benefit of treatment was found in some studies.16,21 No matter how rare this tumor is, further studies are still needed to ensure the efficacy of chemotherapy.
Our case presented as a T1N0M0 lesion was low-grade papillary NPAC, and the lesion was resected completely. Surgical resection is the mainstay of treatment for limited or resectable lesions, and complete resection can achieve excellent prognosis. In some studies, follow-up data have not reported local recurrence or metastatic disease ranging from 5 years to 20 years after complete surgical removal.3,7,9,10 In a study by Wenig et al,7 one patient failed primary radiotherapy treatment and subsequently underwent surgical resection as a salvage and had been free of disease over an 11-year period.7 The disease-free survival has no difference compared with those receiving surgery alone. It indicated the predominance of surgery in the treatment of LGNPPA. Therefore, although our case had only a 4-month of follow-up, we can still predict its excellent prognosis.
However, owing to anatomical limitations and poor exposure of the tumor in the nasopharynx, it is sometimes difficult to completely remove the tumor with an adequate safety margin, especially in cases of large infiltrative tumors. Therefore, adjuvant treatment was needed to manage such tumors, which cannot be removed completely. Unfortunately, low-grade papillary NPACs, which belong to the type of well-differentiated adenocarcinomas, have low sensitivity to conventional radiotherapy or chemoradiotherapy, and it became a problem to deal with incompletely removed tumors.
Several studies revealed that photodynamic therapy (PDT) is a new treatment modality for head and neck cancers, with satisfactory treatment responses and minimal complications.22–25 In a study by Wang et al,26 PDT combined with topical 5-aminolevulinic acid as an effective modality of the postoperative adjuvant therapy was successfully used in the patient to eradicate the residual disease, without compromising the quality of life of the patient. Primary papillary NPAC is able to become qualified candidate for PDT, due to the limited lesions and low incidence of lymph node metastasis.10,12 PDT may be a more suitable alternative method to be the adjuvant therapy for incompletely resectable tumors.
Controversy also exists regarding the prognosis about the two categories of primary NPACs. Pineda-Daboin et al9 reported that the common-type NPAC is usually maintained as a low-grade malignancy and is associated with a good prognosis, while the prognosis of salivary gland-type NPACs is weak overall. However, a study by Guo et al4 showed that patients with common-type or salivary gland-type NPAC had no significant differences in survival rates (63% versus 69%, respectively). A study by Liu et al27 showed that all patients with the conventional NPAC or salivary gland-type nasopharyngeal carcinoma, regardless of the early stage or advanced stage, had no significant differences in 5-year overall survival and disease-free survival. A possible reason for the difference in the results may be the small sample size enrolled in each of the three studies. Prospective studies with broad cross-section are needed to draw a definite conclusion.
Conclusion
Although primary NPACs are very rare, they should be included in the differential diagnosis of nasopharyngeal tumor, even in children. Inappropriate treatment will result in recurrence of the disease. Therefore, definitive diagnosis of primary NPACs, especially low-grade papillary NPACs, is of extraordinary significance. Moreover, it requires appropriate surgical management to ensure an excellent prognosis for the patient. For limited or resectable adenocarcinoma, combined surgery with radiotherapy may be the proper treatment policy. Radiotherapy or chemoradiotherapy is suitable for the poorly differentiated NPACs or unresectable tumors. PDT may be a more suitable alternative method to be the adjuvant therapy for incompletely resectable tumors. Therefore, large multicentric studies or similar meta-analyses are necessary to evaluate further the optimal treatment approaches and the most relevant prognostic factors for these lesions.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Wei WI, Sham JS. Nasopharyngeal carcinoma. Lancet. 2005;365(9476):2041–2054. doi: 10.1016/S0140-6736(05)66698-6. [DOI] [PubMed] [Google Scholar]
- 2.Kuo T, Tsang NM. Salivary gland type nasopharyngeal carcinoma: a histologic, immunohistochemical, and Epstein-Barr virus study of 15 cases including a psammomatous mucoepidermoid carcinoma. Am J Surg Pathol. 2001;25(1):80–86. doi: 10.1097/00000478-200101000-00009. [DOI] [PubMed] [Google Scholar]
- 3.He JH, Zong YS, Luo RZ, Liang XM, Wu QL, Liang YJ. Clinicopathological characteristics of primary nasopharyngeal adenocarcinoma. Ai Zheng. 2003;22(7):753–757. [PubMed] [Google Scholar]
- 4.Guo ZM, Liu WW, He JH. A retrospective cohort study of nasopharyngeal adenocarcinoma: a rare histological type of nasopharyngeal cancer. Clin Otolaryngol. 2009;34(4):322–327. doi: 10.1111/j.1749-4486.2009.01952.x. [DOI] [PubMed] [Google Scholar]
- 5.Tang Q, Hu QY, Piao YF, Hua YH, Fu AF. Clinical analysis of twenty-three nasopharyngeal adenocarcinoma patients. Chin J Cancer Prev Treat. 2009;14(21):1669–1672. [Google Scholar]
- 6.Schramm VL, Jr, Imola MJ. Management of nasopharyngeal salivary gland malignancy. Laryngoscope. 2001;111(9):1533–1544. doi: 10.1097/00005537-200109000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Wenig BM, Hyams VJ, Heffner DK. Nasopharyngeal papillary adenocarcinoma. A clinicopathologic study of a low-grade carcinoma. Am J Surg Pathol. 1988;12(12):946–953. [PubMed] [Google Scholar]
- 8.Ohe C, Sakaida N, Tadokoro C, et al. Thyroid-like low-grade nasopharyngeal papillary adenocarcinoma: report of two cases. Pathol Int. 2010;60(2):107–111. doi: 10.1111/j.1440-1827.2009.02480.x. [DOI] [PubMed] [Google Scholar]
- 9.Pineda-Daboin K, Neto A, Ochoa-Perez V, Luna MA. Nasopharyngeal adenocarcinomas: a clinicopathologic study of 44 cases including immunohistochemical features of 18 papillary phenotypes. Ann Diagn Pathol. 2006;10(4):215–221. doi: 10.1016/j.anndiagpath.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 10.Carrizo F, Luna MA. Thyroid transcription factor-1 expression in thyroid-like nasopharyngeal papillary adenocarcinoma: report of 2 cases. Ann Diagn Pathol. 2005;9(4):189–192. doi: 10.1016/j.anndiagpath.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 11.Fu CH, Chang KP, Ueng SH, Wu CC, Hao SP. Primary thyroid-like papillary adenocarcinoma of the nasopharynx. Auris Nasus Larynx. 2008;35(4):579–582. doi: 10.1016/j.anl.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 12.van Hasselt CA, Ng HK. Papillary adenocarcinoma of the nasopharynx. J Laryngol Otol. 1991;105(10):853–854. doi: 10.1017/s0022215100117542. [DOI] [PubMed] [Google Scholar]
- 13.Xu T, Li ZM, Gu MF, et al. Primary nasopharyngeal adenocarcinoma: a review. Asia Pac J Clin Oncol. 2012;8(2):123–131. doi: 10.1111/j.1743-7563.2011.01499.x. [DOI] [PubMed] [Google Scholar]
- 14.Lee AW, Ma BB, Ng WT, Chan AT. Management of nasopharyngeal carcinoma: current practice and future perspective. J Clin Oncol. 2015;33(29):3356–3364. doi: 10.1200/JCO.2015.60.9347. [DOI] [PubMed] [Google Scholar]
- 15.Petersson F, Vijayadwaja D, Loh KS, Tan KB. Reticular and myxoid non-keratinizing nasopharyngeal carcinoma: an unusual case mimicking a salivary gland carcinoma. Head Neck Pathol. 2014;8(3):364–368. doi: 10.1007/s12105-013-0512-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu TR, Chen FJ, Qian CN, et al. Primary salivary gland type carcinoma of the nasopharynx: therapeutic outcomes and prognostic factors. Head Neck. 2010;32(4):435–444. doi: 10.1002/hed.21203. [DOI] [PubMed] [Google Scholar]
- 17.Liu TR, Chen FJ, Zhang GP, Yang AK. Different therapeutic strategies in primary salivary gland-type nasopharyngeal carcinomas. Curr Opin Otolaryngol Head Neck Surg. 2011;19(2):87–91. doi: 10.1097/MOO.0b013e3283448402. [DOI] [PubMed] [Google Scholar]
- 18.Liu TR, Yang AK, Guo X, et al. Adenoid cystic carcinoma of the nasopharynx: 27-year experience. Laryngoscope. 2008;118(11):1981–1988. doi: 10.1097/MLG.0b013e3181801d23. [DOI] [PubMed] [Google Scholar]
- 19.Terhaard CH, Lubsen H, Rasch CR, et al. The role of radiotherapy in the treatment of malignant salivary gland tumors. Int J Radiat Oncol Biol Phys. 2005;61(1):103–111. doi: 10.1016/j.ijrobp.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 20.Terhaard CH, Lubsen H, Van der Tweel I, et al. Dutch Head and Neck Oncology Cooperative Group Salivary gland carcinoma: independent prognostic factors for locoregional control, distant metastases, and overall survival: results of the Dutch head and neck oncology cooperative group. Head Neck. 2004;26(8):681–692. doi: 10.1002/hed.10400. [DOI] [PubMed] [Google Scholar]
- 21.Li HW, Cao JZ, Zhang QX. Clinical study of primary nasopharyngeal adenocarcinoma. Chin J Radiat Oncol. 2006;15(3):195–196. [Google Scholar]
- 22.Lou PJ, Jager HR, Jones L, Theodossy T, Bown SG, Hopper C. Interstitial photodynamic therapy as salvage treatment for recurrent head and neck cancer. Br J Cancer. 2004;91(3):441–446. doi: 10.1038/sj.bjc.6601993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lou PJ, Jones L, Hopper C. Clinical outcomes of photodynamic therapy for head-and-neck cancer. Technol Cancer Res Treat. 2003;2(4):311–317. doi: 10.1177/153303460300200405. [DOI] [PubMed] [Google Scholar]
- 24.Biel MA. Photodynamic therapy and the treatment of head and neck neoplasia. Laryngoscope. 1998;108(9):1259–1268. doi: 10.1097/00005537-199809000-00001. [DOI] [PubMed] [Google Scholar]
- 25.D’Cruz AK, Robinson MH, Biel MA. mTHPC-mediated photodynamic therapy in patients with advanced, incurable head and neck cancer: a multicenter study of 128 patients. Head Neck. 2004;26(3):232–240. doi: 10.1002/hed.10372. [DOI] [PubMed] [Google Scholar]
- 26.Wang CP, Chang YL, Chen CT, Yang TH, Lou PJ. Photodynamic therapy with topical 5-aminolevulinic acid as a post-operative adjuvant therapy for an incompletely resected primary nasopharyngeal papillary adenocarcinoma: a case report. Lasers Surg Med. 2006;38(5):435–438. doi: 10.1002/lsm.20291. [DOI] [PubMed] [Google Scholar]
- 27.Liu LZ, Zhang YM, Chen Y, Li L. Spreading patterns, prognostic factors and treatment outcomes of nasopharyngeal papillary adenocarcinoma and salivary gland-type carcinomas. Clin Otolaryngol. 2016;41(2):160–168. doi: 10.1111/coa.12492. [DOI] [PubMed] [Google Scholar]