Abstract
Acetazolamide is the standard carbonic anhydrase (CA) inhibitor used for acute mountain sickness (AMS), however some of its undesirable effects are related to intracellular penetrance into many tissues, including across the blood–brain barrier. Benzolamide is a much more hydrophilic inhibitor, which nonetheless retains a strong renal action to engender a metabolic acidosis and ventilatory stimulus that improves oxygenation at high altitude and reduces AMS. We tested the effectiveness of benzolamide versus placebo in a first field study of the drug as prophylaxis for AMS during an ascent to the Everest Base Camp (5340 m). In two other studies performed at sea level to test side effect differences between acetazolamide and benzolamide, we assessed physiological actions and psychomotor side effects of two doses of acetazolamide (250 and 1000 mg) in one group of healthy subjects and in another group compared acetazolamide (500 mg), benzolamide (200 mg) and lorazepam (2 mg) as an active comparator for central nervous system (CNS) effects. At high altitude, benzolamide‐treated subjects maintained better arterial oxygenation at all altitudes (3–6% higher at all altitudes above 4200 m) than placebo‐treated subjects and reduced AMS severity by roughly 50%. We found benzolamide had fewer side effects, some of which are symptoms of AMS, than any of the acetazolamide doses in Studies 1 and 2, but equal physiological effects on renal function. The psychomotor side effects of acetazolamide were dose dependent. We conclude that benzolamide is very effective for AMS prophylaxis. With its lesser CNS effects, benzolamide may be superior to acetazolamide, in part, because some of the side effects of acetazolamide may contribute to and be mistaken for AMS.
Keywords: Acetazolamide, acute mountain sickness, benzolamide, carbonic anhydrase inhibitor, high altitude, lorazepam, oxygen saturation, side effects
Abbreviations
- AMS
acute mountain sickness
- ANOVA
analysis of variance
- CA
carbonic anhydrase
- SaO2
arterial hemoglobin oxygen saturation
Introduction
Carbonic anhydrase (CA) inhibition with acetazolamide (DiamoxR) reduces the incidence and symptomatology of acute mountain sickness (AMS) (Forward et al., 1987; Wright et al., 1983; Birmingham Medical Research Expeditionary Society Acute Mountain Sickness Study Group [BMRES] 1981; Swenson, 2014a). These benefits are a result of improved arterial oxygenation arising from the well‐known ventilatory stimulation by this drug due largely to its inhibition of renal CA and generation of a metabolic acidosis (Kronenberg and Cain, 1968a; Larson et al. 1982; Swenson and Teppema 2007). Not surprisingly, acetazolamide has become the drug of choice for AMS prophylaxis (Bärtsch and Swenson, 2013; Luks et al. 2010). There is, however, an unavoidable disadvantage with acetazolamide and other clinically approved CA inhibitors, such as methazolamide, in that several of its side effects are the same as some AMS symptoms, particularly headache, drowsiness, and nausea or vomiting (Wang et al. 2013). Thus, it is plausible that the efficacy of acetazolamide in AMS may be underestimated (Ellsworth et al. 1987; Swenson 2014a) and that a CA inhibitor with fewer side effects by virtue of lesser central nervous system (CNS) penetrance would be more effective against AMS, avoid diagnostic confusion, and afford clearer evaluation of the benefits of targeted CA inhibition.
Benzolamide is a 10‐fold more potent CA inhibitor and is more hydrophilic and less lipophilic than acetazolamide (Travis 1969). These attributes lead to very limited membrane permeability, restricting its uptake into the central and peripheral nervous systems and other organs besides the kidneys. The kidneys are uniquely sensitive to any of the sulfonamide CA inhibitors, independent of lipid and water solubility, because organic acid transporters in the renal tubule concentrate the drugs intracellularly and in the tubular fluid to fully inhibit renal enzyme at low doses (Swenson, 2014a). On this basis, benzolamide should likely have less CNS side effects of acetazolamide, and thus be of greater value at high altitude. These predictions and conclusions have never been adequately tested, but in two small studies, benzolamide was shown to be effective in reducing AMS symptomatology in a 3‐day hypoxic chamber simulation (Kronenberg et al., 1968a) and against sleep‐related periodic breathing at high altitude (Swenson et al. 1991). Based on the work showing beneficial effects of benzolamide on subretinal fluid absorption in rabbits (Wolfensberger et al. 2000), benzolamide was substituted for acetazolamide in university age patients with macular edema. These students found examination work easier than when taking acetazolamide (A. C. Bird, Moorfields Eye Hospital, London, unpublished observations).
Despite a considerable literature on the efficacy of acetazolamide for the prophylaxis and treatment of AMS, there has been much controversy concerning its optimal dosing with advocacy of doses ranging from 250 to 1000 mg daily. There has been some resolution toward lower dosing recently with demonstration that doses as low as 125 mg bid are as effective as higher doses, at least for typical ascent profiles. With lower dosing there may be fewer side effects (Basnyat et al. 2006; Van Patot et al. 2008).
The hypotheses tested herein were that benzolamide, which remains an orphan drug since its first testing in humans more than 50 years ago and never tested under field conditions for AMS prevention, is effective in increasing arterial oxygenation and preventing AMS during a high‐altitude expedition. Underlying this proposed effectiveness and possible superiority of benzolamide over acetazolamide is the likelihood that benzolamide has fewer CNS side effects than acetazolamide, when tested in a rigorous double‐blinded placebo‐controlled fashion with an active comparator, lorazepam, known to alter CNS function (Dawson et al.2008). We found that benzolamide is effective in reducing AMS during a high‐altitude trek up to 5430 m and this efficacy is associated and correlated with improvements in arterial oxygenation. At sea level, benzolamide has fewer CNS side effects than acetazolamide at all doses studied.
Materials and Methods
Study 1: effectiveness of benzolamide during a high‐altitude trek
For the high‐altitude investigation (Study 1) examining the effectiveness of benzolamide on oxygenation and AMS during a Himalayan trek, volunteers were recruited from the membership of the British Mount Everest Medical Expedition. Data were collected and entered before the randomization codes were broken. All subjects gave written informed consent to these studies, following approval by the Ethics Committee of the City and Hackney Health Authority, London, United Kingdom. The high‐altitude study was conducted in accordance with the CONSORT guidelines on the performance and reporting of clinical trials (Begg et al. 1996).
During the high‐altitude study 25 volunteers each received either 100 mg benzolamide or matching placebo capsules twice daily in a double‐blind placebo‐controlled manner. Individual volunteers were assigned to benzolamide or placebo prior to leaving the U.K. A third party produced the allocation schedule, treatment was assigned randomly (without replacement) to the list of volunteers. Capsules of placebo were visually indistinguishable from benzolamide and were packed and labeled by an independent pharmacist. Tablets were begun 1 day before leaving Kathmandu (1250 m) for an internal air flight to Lukla (2800 m), and subjects then trekked to Everest base camp (5340 m) following a conservative ascent profile, climbing no more than 300 m per day above 3000 m, and taking a rest day every 2–3 days (Ward et al. 1995). Subjects were the members of different parties (separated in time) during the ascent to base camp.
All subjects completed the Lake Louise consensus questionnaire, a standardized AMS questionnaire (Hackett and Oelz, 1992), and measured and recorded their oxygen saturation by pulse oximetry (Nellcor N20, Minneapolis, MN, USA) each morning and evening. On arrival at base camp, 11 of the subjects had arterialized capillary blood sampled from a warmed thumb and analyzed using a Ciba‐Corning 248 (Medfield, MA, USA) blood gas analyzer. Of these 11 subjects, eight were later found to have taken placebo, and three benzolamide. This imbalance was not planned, but was simply a random result of the fact that blood gas analysis was not available for all subjects arriving at base camp due to an avalanche blast that temporarily halted some measurements dependent on instrumentation in the affected research tents. Although the study was not designed to compare side effects of benzolamide against those of placebo, all subjects were asked after arrival at base camp, what symptoms they had experienced. This was before the sealed code was broken. The code was broken at base camp (5340 m) after data collection was complete.
Studies 2 and 3: CNS effects of acetazolamide and benzolamide at sea level
We performed two placebo‐controlled, randomized, double‐blind, cross‐over studies with university‐level students (age range 18–32 years) at sea level (London, UK) to examine the physiological and psychometric effects of benzolamide and acetazolamide.
In the first study (Study 2), 15 fasting volunteers attended the laboratory on the same day each week for four consecutive weeks at 8:30 am. They emptied their bladders and underwent a series of psychometric and related tests described below.
Three matching treatments were given: a single dose of (1) placebo, (2) acetazolamide 250 mg, or (3) acetazolamide 1000 mg. The different tablets were identical and a test battery, including a urine collection, was performed at 0, 2, 4.5, and 6.5 h postdosing. For this study, arterialized capillary blood gas analysis was performed during each test battery from a warmed ear lobe using a Ciba‐Corning 248 analyzer. End‐tidal carbon dioxide tension was also measured, after 5 min of sitting rest, using an infra red carbon dioxide analyzer (PK Morgan Capnograph, Chatham, Kent, UK).
The second study (Study 3) was of similar design to the first and was performed over a 4‐week period of time. After subjects emptied their bladders, they were each witnessed taking a single dose of (1) placebo, (2) lorazepam 2 mg (a positive control for neuropsychological changes), (3) acetazolamide 500 mg, or (4) benzolamide 200 mg according to a Latin square design. Capsules containing each of the four preparations were of identical appearance, smell, and taste. Urine collections and the test battery were performed at 0, 2, 4.5, and 6.5 h after dosing. In both the studies, the subjects were randomized such that each took one of the four drugs each week so that at the end of the 4 weeks each subject had taken all four drugs in a randomized order.
The psychometric test battery for Studies 2 and 3 comprised a series of tests including critical flicker fusion (CFF), reaction time assessment (Study 3 only), peg and washer placement timing, postural sway assessed using a force platform, and vertical judgment error. Intraocular pressure (by noncontact tonometry) was also measured. Each of these tests was performed three times at each time interval and the arithmetic mean of the three results was used to describe subject performance in each test at each interval. A series of visual analog scales were also completed which assessed subjective sleepiness, clear headedness, and dizziness as well as control questions about hunger and feeling hot or cold. These objective and self‐reported measures were designed to elicit symptoms that could easily be confused with presentations of AMS as well as the known physiological effects of these drugs. Urinary pH and volume were determined at each time interval. Each subject was also asked about any side effects they had experienced while taking any of the drugs.
For Studies 2 and 3 (at sea level), the measures for each test were compared between the different control and treatment groups by means of a general linear model for analysis of variance (ANOVA). Dunnett's test was used to determine pairwise comparisons of data. For the high‐altitude study (Study 3), AMS scores and mean SaO2 values at various altitudes were compared using the paired t‐test and ANOVA. Arterialized capillary blood gas analysis results between the two groups at Everest base camp were compared using the unpaired t‐test. A linear regression model was calculated for the relationship between arterial oxygen saturation and AMS score, with ANOVA used to test significance; P < 0.05 was considered as significant. Statistics were calculated using Minitab.
Results
Study 1: effectiveness of benzolamide during a high‐altitude trek
Twenty‐five subjects completed the study, 13 with placebo and 12 with benzolamide. Three subjects inadvertently violated the protocol, one subject (D. J. C.) had to descend for a prolonged period to attend to logistical problems with research equipment and so had a grossly atypical ascent profile (placebo group). Another subject's flight from Kathmandu to Lukhla was delayed, but he had started his tablets nonetheless, and so ran out of tablets at 4930 m (Lobuche), and was without tablets for 2 days before arrival at base camp. The third subject had not declared any respiratory difficulties previously, but proved to have significant chronic obstructive pulmonary disease. These subjects were therefore excluded from the per‐protocol analysis, but were included in the primary intention‐to‐treat analysis to avoid bias – even though the protocol violations were all decided prior to unmasking the study. Subjects taking placebo reached base camp after a median of 13 days (mean = 12.6, SD = 2.1; range, 8–14 days), subjects on benzolamide took a median of 12 days (mean = 12.0, SD = 2.2; range, 9–15 days, P = 0.4, nonsignificant difference by Mann–Whitney U test).
Subjects taking benzolamide experienced significantly less AMS particularly during the higher elevations of the trek. The average AMS scores on arrival at each new altitude during the ascent are shown in Figure 1. Comparison of average AMS scores on placebo and benzolamide showed significantly lower values on the drug (paired t‐test; placebo average 2.18, benzolamide average 1.28, mean difference 0.90, SEM 0.37, t = 2.46, P = 0.025, one‐sided). It can be seen that benzolamide produced a significant reduction in AMS symptomatology. The median scores for placebo are higher than for benzolamide for the last two stopover sites and arrival at base camp (5340 m). This was significant at base camp (P < 0.025, per‐protocol analysis). There was also a significant difference for the worst scores (*P = 0.02, intention‐to‐treat analysis; P < 0.005, per‐protocol analysis). Considered overall by ANOVA, the reductions in AMS on benzolamide were significant (P = 0.02, intention‐to‐treat analysis) or highly significant (P = 0.003, per‐protocol analysis).
Figure 1.
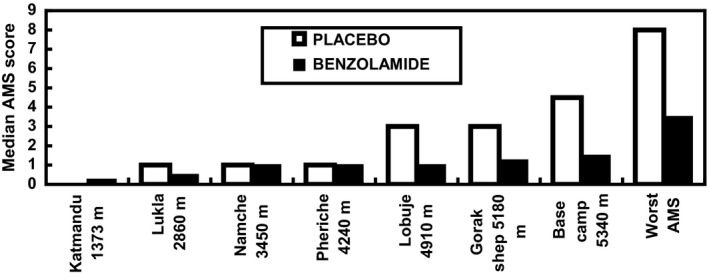
Median acute mountain sickness (AMS) scores on arrival at each new altitude during ascent with the median values of the worst AMS scores for comparison. The scores are given for placebo and benzolamide‐treated subjects at each altitude (placebo n = 13; benzolamide n = 12 intention‐to‐treat analysis).
Figure 2 shows the mean values of arterial oxygen saturations for placebo and benzolamide‐treated subjects on arrival at each altitude (stopover sites). At all sites from Pheriche (4240 m) and above up to Everest Base Camp, arterial oxygen saturation was statistically significantly higher in the subjects taking benzolamide.
Figure 2.
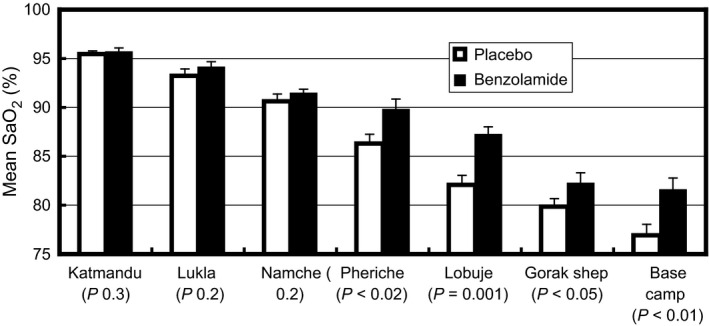
Mean values for arterial oxygen saturation for placebo and benzolamide‐treated subjects, measured by oximetry, on arrival at each altitude (stopover site). Values are mean ± SEM, per‐protocol analysis (placebo n = 12; benzolamide n = 10).
In the 11 volunteers who had arterialized capillary blood gas analysis on arrival at 5340 m, pH, PaCO2 and bicarbonate were all lower in those treated with benzolamide than for subjects on placebo (Table 1). With benzolamide the average pH was 7.41 (range 7.40–7.41), whereas on placebo average pH was 7.44 (range 7.41–7.47; P = 0.003, 95% CI = 0.016, 0.053: unpaired t‐test). Arterialized capillary PCO2 was lower on benzolamide, averaging 23.6 mmHg (range 22.8–24.3 mmHg), compared with 27.0 mmHg on placebo (range 24.3–32.0 mmHg; P < 0.01, 95% CI = 0.16, 0.77: unpaired t‐test). Mean bicarbonate concentration was lower, 17.7 mmol/L (range 17.5–17.8 mmol/L) in the benzolamide group and 20.6 mmol/L (range 19.5–22) in the placebo group (P < 0.0001, t = 9.0, 95% CI = 2.14, 3.62: unpaired t‐test). Although the mean arterialized PaO2 tended to be higher in the benzolamide group (45.7 mmHg) than in the placebo group (43.6 mmHg), the increase while of similar magnitude to the fall in PaCO2 did not reach statistical significance (P = 0.2).
Table 1.
Arterialized capillary blood gases in subjects taking benzolamide or placebo on arrival at 5340 m (Everest base camp)
| Subject | PaCO2 | PaO2 | |
|---|---|---|---|
| Units | pHa | mmHg | mmHg |
| Benzolamide | |||
| 42 | 7.404 | 24.3 | 44.8 |
| 5 | 7.411 | 22.8 | 44.3 |
| 28 | 7.409 | 23.5 | 46.1 |
| Mean | 7.408a | 23.6a | 45.1 |
| SD | 0.004 | 0.8 | 0.9 |
| SEM | 0.002 | 0.5 | 0.5 |
| Placebo | |||
| 1 | 7.447 | 26.7 | 46.4 |
| 7 | 7.41 | 29.6 | 41.3 |
| 26 | 7.433 | 32 | 41.6 |
| 37 | 7.463 | 25.1 | 41.6 |
| 53 | 7.435 | 25.7 | 46.1 |
| 67 | 7.464 | 24.3 | 47.6 |
| 69 | 7.466 | 26 | 42.1 |
| 63 | 7.419 | 26.9 | 42.7 |
| Mean | 7.442 | 27 | 43.6 |
| SD | 0.021 | 2.6 | 2.6 |
| SEM | 0.008 | 0.9 | 0.9 |
P < 0.01 compared to placebo.
Symptoms at base camp included paresthesia (8 of 12 subjects on benzolamide and 0 of 13 on placebo) and breathlessness (0 on benzolamide and 4 on placebo). One subject on benzolamide reported urinary frequency and another tinnitus (persisting for several days after stopping the drug). No subjects found the tingling troublesome. Two subjects on benzolamide appeared to develop significant AMS after stopping the drug (scores >10), one having run out of the drug and the other stopping (per‐protocol analysis) on arrival at Everest base camp.
Studies 2 and 3: CNS effects of acetazolamide and benzolamide at sea level
In both the sea level pharmacological studies a few subjects were unable to attend every weekly session. In the two sea level studies, we obtained completed data sets for 21 subjects taking placebo and acetazolamide, 20 subjects taking benzolamide, and 18 taking lorazepam.
In Study 2, the effects of the two different doses of acetazolamide are presented in Tables A1 and A2 in the Appendix and may be summarized as follows. Acetazolamide at a dosage of 250 mg caused an increase in urinary pH (P < 0.001) and a decrease in blood pH (P < 0.01), base excess (P < 0.05), and intraocular pressure (P < 0.05, combined left and right eye results). At a dose of 1000 mg, it caused an increase in urinary pH (P < 0 .001) and volume (P < 0.01), and a decrease in blood pH (P < 0.001), base excess (P < 0.001), blood bicarbonate (P < 0.01), and intraocular pressure (P < 0.001). Although PetCO2 appeared to fall with increasing acetazolamide dosage from 35.2 mmHg on placebo to 34.3 mmHg on 250 mg and 33.6 mmHg on 1000 mg acetazolamide the variation in responses was so large that the average PCO2 decrease (<2 mmHg) did not meet statistical significance. Visual analog scores (VAS) of sleepiness (P < 0.001) and dizziness (P < 0.01) were increased and clear headedness declined (P < 0.001) with 1000 mg acetazolamide. There were no significant differences in any of the testing measures between placebo and 250 mg acetazolamide.
In Study 3, the head‐to‐head comparison of placebo, benzolamide 200 mg, acetazolamide 500 mg, and lorazepam 2 mg, we obtained a complete set of data for 16 subjects. Inclusion of all data collected, rather than restriction to the 16 complete data sets does not alter the results significantly, and so all available data are included here. Although the capsules were identical in taste, smell, and appearance, some subjects (about 10%) guessed their allocation code during one of the four study days.
Side effects at any time point were reported in 2/21 (10%) taking placebo, 9/18 (50%) taking lorazepam 2 mg, 10/21 (48%) taking acetazolamide 500 mg, and 5/20 (25%) taking benzolamide 200 mg. The specific side effects are listed in Table 2. The physiological and psychometric results for each drug, for each time point, are given in Table A3 in Appendix. Compared to placebo, lorazepam caused a fall in CFF and number of pegs and washers placed correctly (both P < 0.001), an increase in postural sway (P < 0.01) and vertical error (P < 0.05). Subjectively, lorazepam led to an increase in self‐reported dizziness, drowsiness, and decrease in clear headedness (all, P < 0.001). Lorazepam had no significant effect on intraocular pressure, urinary pH, or volume.
Table 2.
Number of subjects complaining of specific side effects after administration of each drug in Study 3
| Effect | Placebo (21) | Lorazepam (18) | Acetazolamide (21) | Benzolamide (20) |
|---|---|---|---|---|
| Headachea | 2 | 5 (2 severe) | 4 | |
| Drowsiness | 1 | 8 | 4 | 2 |
| Dizzinessa | 9 | 2 | ||
| Tingling of lip or limb | 4 | 1 | ||
| Numbness of hands | 2 | |||
| Earache | 2 | |||
| Coldness | 1 | |||
| Vomitinga | 1 | |||
| Metallic taste | 1 | |||
| Sore throat | 1 | |||
| Dry eyes | 1 |
Symptoms common to acute mountain sickness.
Compared to placebo, acetazolamide 500 mg caused an increase in urinary pH and volume (P < 0.001), fall in intraocular pressure (P < 0.01), increase in postural sway and vertical error (P < 0.05), an increase in dizziness, and a decrease in clear headedness (both P < 0.001) and drowsiness (P < 0.05), but no significant effect on CFF or peg and washer placement.
Compared to placebo, benzolamide 200 mg caused an increase in urinary pH (P < 0.001) and volume (P < 0.01), but had no significant effect on CFF, postural sway, vertical error, peg and washer placement, intraocular pressure, drowsiness, dizziness, or clear headedness.
The time course and magnitude of the diuretic effects of acetazolamide and benzolamide are illustrated in Figure 3. Both drugs caused a statistically significant alkaline diuresis of equal magnitude and onset when compared to placebo.
Figure 3.
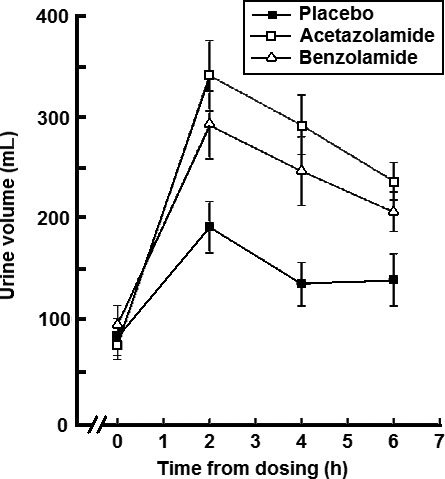
The time courses of urine output after dosing with either placebo, acetazolamide 500 mg, or benzolamide 200 mg. Mean values at each time point are shown ± SEM.
Discussion
Our altitude study, for subjects trekking to 5340 m (17,520 feet), confirms the effectiveness of benzolamide 100 mg bid as prophylaxis for AMS. The present study accords with findings of a chamber study (72 h elevation to the equivalent of 4600 m, 14,000 feet) that benzolamide has prophylactic properties in regard to AMS symptomatology (Kronenberg and Cain 1968a). The effectiveness of benzolamide on arterial blood gases appears equal to that of acetazolamide, studied in a similar chamber experiment (Kronenberg and Cain 1968b). Comparison of our findings with other studies suggests that benzolamide 100 mg bid has similar efficacy to acetazolamide at both 125 mg bid and 250 mg bid dosing schedules in preventing AMS symptoms under similar trekking conditions (Basnyat et al. 2003, 2006).
The other focus of our study was to critically assess and compare side effects and physiological effects of benzolamide and acetazolamide in a large number of healthy subjects at sea level so as to avoid any confounding effects of hypoxia and other stresses of traveling in a new environment, which may vary from subject to subject. Taken together the data across two groups of subjects showed that benzolamide at 200 mg is equally effective as all doses of acetazolamide (250, 500, 1000 mg) in causing an alkaline diuresis, the hallmark of renal CA inhibition. However, only acetazolamide causes evidence of other tissue CA inhibition, in this case easily and relatively noninvasively assessed as a reduction in intraocular pressure as a consequence of CA inhibition in the ciliary apparatus of the eye. In keeping with a lack of significant tissue penetrance, particularly into the CNS, we show that benzolamide has far fewer side effects than acetazolamide, even at the lowest dose of acetazolamide shown to be effective against AMS. This is unequivocally the case for benzolamide 200 mg versus acetazolamide 500 mg since the same subjects took both doses (Study 3). Although benzolamide was not tested in the same subjects taking the 250 and 1000 mg doses of acetazolamide (Study 2), these subjects in Study 2 were similar in age, health, and background to those in Study 3 and all subjects in both studies served as their own controls by taking placebo. When the change from placebo at 6.5 h after drug administration for the three VAS‐scored symptoms most relevant to AMS of dizziness, sleepiness, and decline in concentration are used (Table 3), benzolamide caused the least impact, with lorazepam roughly equivalent to the greater side effects of acetazolamide at 250 and 500 mg. The 1000 mg dose of acetazolamide had the greatest side effect impact. The data showed a very plausible dose–response relationship for acetazolamide across the three doses studied.
Table 3.
Change in VASa score from placebo at 6.5 h after drug ingestion
| ACTZ 250 | ACTZ 500 | ACTZ 1000 | BENZ 200 | LOR 2 | |
|---|---|---|---|---|---|
| Dizziness | 15 | 18 | 38 | −5 | 18 |
| Decline in concentration | 21 | 19 | 42 | 2 | 9 |
| Sleepiness | 15 | 22 | 28 | 0 | 15 |
Visual analog score of symptom severity: range 0–100.
Our findings of modest but definite CNS side effects of acetazolamide at 250 mg accord with the recent study by Wang et al. (2013) which suggests that the side effects of acetazolamide at this lowest dosing are not trivial. In particular, their study found significant neuropsychological decline in the areas of concentration, cognitive processing speed, reaction time, and short‐term memory with acetazolamide after 4 days even at the low dosage of 125 mg bid. We may indeed underestimate the benefits at high altitude of benzolamide over acetazolamide observed in our one time dose study. The greater lipid solubility of acetazolamide is more likely to cause problems as more drug reaches the CNS with repeated dosing over several days, as suggested by Wang et al. (2013). Because these side effects, which are similar to those that constitute the symptom complex of AMS, are detectable at sea level, and thus cannot be attributable to AMS, the full effectiveness of prophylaxis with CA inhibition at high altitude is not realized. Our results with benzolamide suggest that AMS prophylaxis with CA inhibitors might be most effectively achieved with less lipophilic, more hydrophilic soluble drugs that pass the blood–brain barrier with lesser capacity (Table 4).
Table 4.
PCO2 and pH data from the present study and the three other major studies
| PAPER | Present study | Swenson et al. 1991 | Kronenberg and Cain 1968a | Swenson and Hughes 1993 | ||
|---|---|---|---|---|---|---|
| Drug | Benz | Benz | Benz | Acet | ||
| Duration | Days | 24 h | 24 h | 72 h | Acute i.v. | 24 h |
| Condition | Altitude | Sea level | Chamber | Chamber | Sea level | Sea level |
| pHa | ||||||
| Placebo | 7.442 | 7.44 | 7.493 | 7.501 | 7.43 | 7.44 |
| Inhibitor | 7.408 | 7.39 | 7.437 | 7.445 | 7.44 | 7.40 |
| PaCO2 | ||||||
| Placebo | 27.0 | 39.4 | 28.5 | 27.3 | 41.2 | 39.9 |
| Inhibitor | 23.6 | 36.0 | 26.7 | 25.8 | 38.0 | 35.3 |
| PaCO2 fall | 3.4 | 3.4 | 1.8 | 1.5 | 3.2 | 4.6 |
Unfortunately, benzolamide has no patent protection and thus it is unlikely that any pharmaceutical company will ever market this drug. However, with an emerging realization that plasma membrane‐associated CA isozymes with extracellular orientation (IX and XII) are richly and almost uniquely expressed in cancer cells and critical to their growth and metastatic potential (Robertson et al. 2004; Swietach et al. 2010), clinical development of other membrane impermeant CA inhibitors which block CA IX are underway and have shown promising features. Drugs in this class would be well worth studying in AMS, since they too like benzolamide would be concentrated in the kidney at low doses by the same mechanism as for benzolamide and all sulfonamide CA inhibitors.
The basis by which acetazolamide and other CA inhibitors act to reduce AMS is overwhelmingly by improvement in arterial oxygenation (Roach et al. 1998; Kazunobu et al. 2001; Muza et al. 2004), although other mechanisms are emerging (Swenson 2014a). The improvement in arterial oxygenation arises from renal CA inhibition and the subsequent alkaline diuresis to decrease the magnitude of respiratory alkalemia and its braking action on the fullest expression of the ventilatory response to hypoxia (Swenson 2014a). This is shown in Tables 1, 2, 3; the latter compared our data with selected data of others and demonstrated that the increase in ventilation as evidenced by lowering of PaCO2 with benzolamide is equal to that of acetazolamide.
In support of a causal pathway between arterial oxygenation and AMS, we found in a recent study of two modest ascents (each of them to around 5000 m), following an initial 5 days acclimatization at 3324 m, a definite negative correlation between individual mean AMS scores and SaO2 value (Brierley et al. 2012). We therefore, additionally, have explored the relationship between AMS scores and SaO2 in this present study of benzolamide at altitude. When we plot mean values of the AMS scores against mean SaO2 values for all subjects at each altitude, there is a significant negative AMS–SaO2 correlation, both for the placebo and benzolamide‐treated subjects individually (Figure 4) and when combined (Figure 5). Numerous other studies have found a correlation (Faulhaber et al. 2014; Roach et al. 1998; Kazunobu et al. 2001; Tannheimer et al. 2002; Koehle et al. 2010; Roeggla et al. 1996), but not all (Roach et al. 1995; O'Connor et al. 2004). Because correlation highly suggests but does not prove causation, and AMS itself may alter lung function to cause further hypoxemia (Swenson 2014a,b) better studies with attention to what degree of hypoxemia precedes the onset of AMS along with investigations of prophylactic strategies that stimulate ventilation or act by other mechanisms, such as dexamethasone, are needed (Luks and Swenson 2011).
Figure 4.
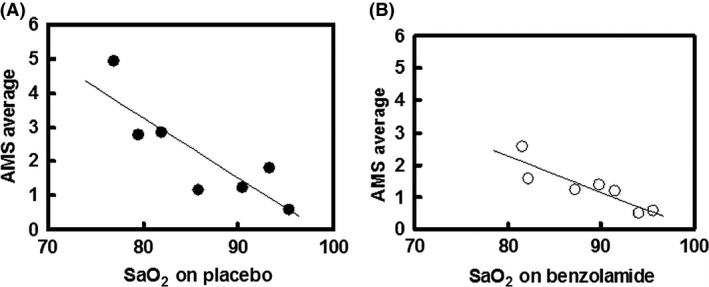
Average acute mountain sickness score for each altitude plotted against average SaO2 for subjects on placebo (A) and on benzolamide (B). For placebo: y = −0.1761x + 17.362, r = 0.850, P < 0.02; for benzolamide: y = −0.1117x + 11.201, r = 0.889, P < 0.01.
Figure 5.
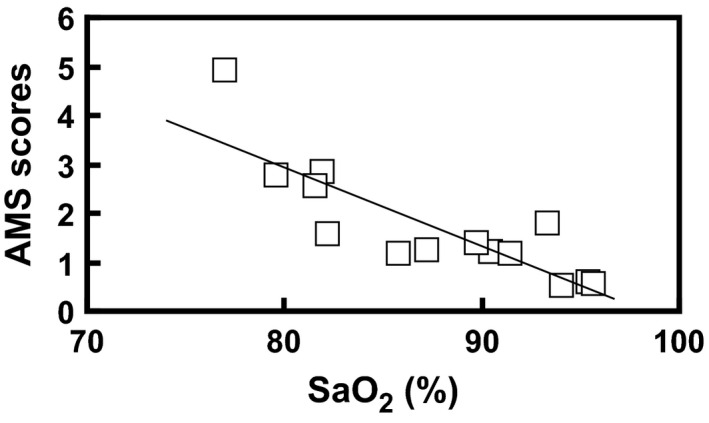
Average acute mountain sickness (AMS) scores at each altitude plotted against average SaO2 for all subjects. The regression line is AMS score = −0.161SaO2 + 15.82, r = 0.840, P < 0.02.
Our study has several limitations requiring some acknowledgment. We did not test benzolamide directly against acetazolamide at high altitude because we did not have sufficient volunteers to include another arm in Study 1. Such a trial in the future is clearly deserving of study, but it will require a greater number of subjects to prove either noninferiority or superiority due to the efficacy both drugs display. A second weakness of the study is that we did not compare benzolamide directly in the sea level study against the 250 and 1000 mg doses of acetazolamide (Study 2). However, we believe that the use of placebo groups in very similar subjects does permit us to combine results of the two studies as we did and show in Table 3. Future head to head studies of these doses would be useful. Finally, we chose to study the drugs for their side effect profile at sea level to minimize any differences due to variable influences of high altitude, cold, change in diet, and the stress of travel and trekking on our results. These results provide important background reference data for the interpretation of any future comparative study of these drugs at high altitude.
In conclusion, our investigation confirms that the membrane impermeant CA inhibitor, benzolamide, has prophylactic benefit against AMS and does so under field conditions of high‐altitude trekking. It acts as a ventilatory stimulant by virtue of its targeted action on the kidney and that the beneficial effect is due to the resultant higher arterial oxygen saturation. Extensive and well‐controlled psychometric testing shows that benzolamide has a considerably lesser side effect profile than acetazolamide at sea level, that theoretically should make it, or a similar membrane impermeant CA inhibitor, a better choice for AMS prophylaxis.
Author Contributions
D. J. C. and E. R. S. conceived the study designs; A. M. H. and D. J. C. ran the sea level studies; J. N. assisted with the altitude study; R. J. F. held the codes for the altitude studies; C. B. W., D. J. C., and E. R. S. drafted the manuscript and analyzed the data; and all authors were involved in drafting and revising the manuscript.
Disclosures
For all the authors of this paper there are no competing interests. All authors have completed the Unified Competing Interest form at www.icmje.org/coi disclosure.pdf (available on request from D. J. C.) and declare: We have had no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and have no other relationships or activities that could appear to have influenced the submitted work.
Acknowledgements
We thank (for the altitude study) the members of the British Mount Everest Medical Expedition, MEDEX (Simon Currin) and Andrew Pollard (Joint Research Lead with D. J. C.) and Medical Expeditions, the nonprofit making charity that organized and funded many aspects of the expedition. For the sea level studies we thank the William Harvey Research Institute, Centre of Clinical Pharmacology, and students from the London School of Pharmacy.
Acknowledgements
The Barts and The London National Institutes of Health (NIHR) are acknowledged for their support of this work.
Appendix 1.
Table A1.
Psychometric results from Study 2 (acetazolamide dosage)
| Test | Drug | Time 0 h | Time 2 h | Time 4.5 h | Time 6.5 h |
|---|---|---|---|---|---|
| CFF (hz) | Placebo | 31.4 (0.8) | 32.1 (0.7) | 31.1 (0.6) | 30.9 (0.6) |
| Acetazolamide 250 mg | 31.3 (1.1) | 31.5 (0.8) | 30.0 (0.7) | 31.7 (0.8) | |
| Acetazolamide 1000 mg | 32.2 (0.7) | 31.1 (0.5) | 32.5 (0.5) | 32.2 (0.5) | |
| Pegs correctly placed in 2 min | Placebo | 32.1 (1.5) | 33.5 (1.4) | 32.9 (0.9) | 30.8 (1.2) |
| Acetazolamide 250 mg | 33.1 (1.3) | 33.8 (1.2) | 35.1 (1.4) | 36.0 (1.4) | |
| Acetazolamide 1000 mg | 33.2 (1.0) | 32.9 (0.8) | 31.9 (0.9) | 33.8 (1.0) | |
| VAS wide awake | Placebo | 74.8 (5.4) | 81.1 (3.7) | 75.2 (6.3) | 69.0 (7.3) |
| Acetazolamide 250 mg | 77.2 (4.4) | 70.1 (4.9) | 59.6 (7.0) | 53.9 (8.1) | |
| Acetazolamide 1000 mg*** | 77.4 (5.2) | 51.6 (6.3) | 34.2 (5.3) | 41.3 (5.7) | |
| VAS decline in clear headedness | Placebo | 15.0 (4.3) | 11.3 (3.4) | 9.7 (4.1) | 16.6 (5.6) |
| Acetazolamide 250 mg | 21.5 (5.5) | 22.6 (4.0) | 30.5 (7.5) | 37.4 (7.7) | |
| Acetazolamide 1000 mg*** | 19.3 (5.6) | 45.4 (6.8) | 62.4 (4.1) | 58.4 (8.5) | |
| VAS dizzy | Placebo | 95.5 (1.8) | 95.5 (1.4) | 96.5 (1.1) | 85.8 (4.9) |
| Acetazolamide 250 mg | 95.5 (1.7) | 92.1 (2.3) | 73.1 (7.2) | 70.0 (8.0) | |
| Acetazolamide 1000 mg** | 96.4 (1.7) | 67.9 (7.4) | 48.6 (5.3) | 47.3 (8.3) | |
| VAS hungry | Placebo | 42.1 (5.1) | 55.4 (6.5) | 9.5 (1.7) | 39.4 (2.5) |
| Acetazolamide 250 mg | 37.8 (6.9) | 52.1 (6.6) | 12.1 (4.2) | 23.4 (4.6) | |
| Acetazolamide 1000 mg | 45.1 (7.1) | 66.9 (4.7) | 26.0 (7.1) | 44.7 (5.1) |
*P < 0.05, **P < 0.01, ***P < 0.001 (relative to placebo at and beyond 2 h). Values are mean ± SEM.
Table A2.
Physiological results from Study 2
| Test | Drug | Time 0 h | Time 2 h | Time 4.5 h | Time 6.5 h |
|---|---|---|---|---|---|
| Intraocular pressure (mmHg) | Placebo | 10.1 (0.9) | 9.6 (0.7) | 9.5 (0.6) | 9.2 (0.4) |
| Acetazolamide 250 mg* | 10.4 (0.8) | 8.6 (0.6) | 8.1 (0.3) | 7.8 (0.6) | |
| Acetazolamide 1000 mg*** | 10.1 (0.8) | 7.7 (0.5) | 8.4 (0.6) | 7.2 (0.5) | |
| End‐tidal PCO2 (mmHg) | Placebo | 34.0 (1.3) | 34.4 (1.0) | 35.2 (1.1) | 35.5 (1.1) |
| Acetazolamide 250 mg | 34.3 (0.9) | 34.0 (1.1) | 34.7 (1.1) | 32.0 (0.9) | |
| Acetazolamide 1000 mg | 36.7 (1.2) | 33.5 (1.2) | 33.8 (1.0) | 32.1 (1.7) | |
| Blood pH | Placebo | 7.44 (0.01) | 7.44 (0.01) | 7.45 (0.01) | 7.45 (0.01) |
| Acetazolamide 250 mg** | 7.43 (0.00) | 7.40 (0.01) | 7.41 (0.01) | 7.39 (0.01) | |
| Acetazolamide 1000 mg*** | 7.42 (0.01) | 7.38 (0.01) | 7.39 (0.00) | 7.40 (0.01) | |
| Blood PCO2 | Placebo | 35.2 (0.8) | 34.8 (0.9) | 33.9 (1.0) | 34.5 (1.0) |
| Acetazolamide 250 mg | 35.2 (1.2) | 35.7 (1.0) | 35.7 (1.0) | 33.9 (0.8) | |
| Acetazolamide 1000 mg | 35.4 (0.9) | 34.3 (0.8) | 35.2 (0.6) | 32.8 (0.7) | |
| Blood bicarbonate (calculated) | Placebo | 23.7 (0.5) | 23.2 (0.4) | 23.1 (0.6) | 23.2 (0.5) |
| Acetazolamide 250 mg | 23.8 (0.6) | 22.4 (0.8) | 22.5 (0.6) | 19.8 (0.4) | |
| Acetazolamide 1000 mg** | 22.9 (0.6) | 20.1 (0.5) | 21.2 (0.4) | 20.1 (0.6) | |
| Simple Reaction time | Placebo | 233.9 (9.5) | 226.8 (9.4) | 221.3 (9.9) | 228.2 (9.9) |
| Acetazolamide 250 mg | 236.9 (13.7) | 235.3 (11.5) | 213.6 (9.2) | 222.0 (6.7) | |
| Acetazolamide 1000 mg | 221.6 (10.4) | 227.8 (9.8) | 207.2 (9.7) | 224.8 (7.7) | |
| Base Excess (mEq/L) | Placebo | −0.17 (0.523) | −1.120 (0.509) | −1.233 (0.693) | −0.907 (0.591) |
| Acetazolamide 250 mg* | −0.930 (0.700) | −3.05 (0.509) | −2.163 (0.831) | −5.267 (0.537) | |
| Acetazolamide 1000 mg*** | −0.446 (0.975) | −4.860 (0.509) | −3.715 (0.501) | −4.914 (0.781) | |
| Urine pH | Placebo | 6.0 (0.1) | 6.6 (0.15) | 6.6 (0.2) | 6.8 (0.1) |
| Acetazolamide 250 mg*** | 5.7 (0.1) | 7.7 (0.06) | 7. 9 (0.0) | 7.7 (0.0) | |
| Acetazolamide 1000 mg*** | 6.2 (0.2) | 7.7 (0.06) | 7.9 (0.0) | 7.7 (0.0) | |
| Urine volume (mL) | Placebo | 159 (28) | 238 (44) | 143 (22) | 147 (19) |
| Acetazolamide 250 mg | 88 (18) | 401 (45) | 268 (24) | 345 (24) | |
| Acetazolamide 1000 mg** | 126 (20) | 527 (46) | 382 (46) | 364 (23) |
*P < 0.05, **P < 0.01 ***P < 0.001 (relative to placebo). Values shown are mean ± SEM.
Table A3.
Physiological and psychometric results in Study 3
| Test | Drug | Time 0 h | Time 2 h | Time 4.5 h | Time 6.5 h |
|---|---|---|---|---|---|
| Urine pH | Placebo | 5.5 (0.1) | 5.9 (0.1) | 5.7 (0.1) | 6.1 (0.1) |
| Lorazepam | 5.6 (0.1) | 6.2 (0.1) | 6.2 (0.1) | 6.5 (0.2) | |
| Acetazolamide*** | 5.6 (0.2) | 7.5 (0.1) | 7.7 (0.1) | 7.4 (0.2) | |
| Benzolamide*** | 5.7 (0.1) | 7.5 (0.1) | 7.7 (0.1) | 7.5 (0.1) | |
| Urine vol (mL) | Placebo | 92 (21) | 211 (30) | 142 (26) | 145 (31) |
| Lorazepam | 65 (15) | 225 (38) | 132 (22) | 132 (20) | |
| Acetazolamide*** | 81 (16) | 358 (38) | 301 (36) | 238 (21) | |
| Benzolamide** | 100 (21) | 321 (36) | 265 (37) | 210 (24) | |
| CFF (Hz) | Placebo | 33.9 (0.9) | 33.6 (0.9) | 32.8 (0.8) | 32.9 (0.8) |
| Lorazepam*** | 33.7 (0.7) | 30.4 (0.9) | 32.6 (0.9) | 31.9 (1.0) | |
| Acetazolamide | 32.9 (1.0) | 33.3 (1.0) | 33.5 (0.9) | 33.4 (0.9) | |
| Benzolamide | 33.6 (0.6) | 33.0 (0.7) | 33.1 (0.7) | 32.3 (0.9) | |
| Sway (degrees) | Placebo | 0.53 (0.03) | 0.57 (0.04) | 0.57 (0.03) | 0.58 (0.04) |
| Lorazepam** | 0.56 (0.04) | 0.83 (0.05) | 0.69 (0.05) | 066 (0.04) | |
| Acetazolamide* | 0.53 (0.04) | 0.62 (0.03) | 0.62 (0.04) | 0.68 (0.06) | |
| Benzolamide | 0.53 (0.03) | 0.52 (0.03) | 0.60 (0.05) | 0.63 (0.05) | |
| Vertical error (sum of steps) | Placebo | 21.0 (3.1) | 23.7 (3.0) | 36.5 (5.6) | 27.1 (5.5) |
| Lorazepam* | 29.7 (5.7) | 32.8 (7.2) | 32.1 (6.9) | 41.9 (8.4) | |
| Acetazolamide* | 30.2 (3.9) | 44.3 (5.6) | 31.1 (2.9) | 30.3 (5.5) | |
| Benzolamide | 29.6 (3.9) | 27.1 (3.0) | 26.9 (3.3) | 30.4 (3.6) | |
| Pegs correctly placed in 2 min | Placebo | 34.5 (1.1) | 36.1 (1.3) | 34.7 (1.1) | 36.6 (1.2) |
| Lorazepam*** | 36.3 (1.0) | 28.9 (1.4) | 35.7 (1.9) | 33.9 (1.2) | |
| Acetazolamide | 34.1 (1.1) | 34.8 (1.3) | 35.0 (1.2) | 34.5 (1.5) | |
| Benzolamide | 33.7 (1.0) | 34.0 (1.0) | 34.0 (1.0) | 34.8 (1.2) | |
| VAS sleepy | Placebo | 66.8 (6.5) | 60.5 (6.2) | 65.1 (5.4) | 64.2 (6.1) |
| Lorazepam*** | 72.1 (6.4) | 40.8 (7.5) | 43.6 (7.7) | 47.8 (6.6) | |
| Acetazolamide* | 64.8 (6.6) | 51.7 (6.0) | 47.3 (6.1) | 42.8 (8.3) | |
| Benzolamide | 75.8 (5.5) | 68.3 (6.0) | 58.3 (7.4) | 64.8 (6.5) | |
| VAS clear headedness | Placebo | 23.3 (5.0) | 25.9 (3.9) | 28.6 (5.4) | 28.4 (5.3) |
| Lorazepam*** | 28.8 (6.8) | 53.1 (6.7) | 45.6 (6.6) | 38.4 (7.2) | |
| Acetazolamide*** | 30.6 (5.9) | 58.4 (5.2) | 54.3 (6.0) | 46.9 (7.1) | |
| Benzolamide | 17.3 (4.7) | 28.8 (6.5) | 35.2 (7.0) | 30.6 (6.7) | |
| VAS dizzy | Placebo | 80.9 (6.3) | 80.9 (4.6) | 73.3 (6.4) | 73.9 (6.2) |
| Lorazepam*** | 79.9 (5.8) | 31.8 (6.3) | 33.9 (6.1) | 56.1 (8.4) | |
| Acetazolamide*** | 72.5 (7.2) | 58.8 (8.2) | 53.8 (8.4) | 55.8 (7.8) | |
| Benzolamide | 87.5 (4.5) | 82.5 (5.0) | 74.7 (6.6) | 79.0 (6.1) | |
| VAS hungry | Placebo | 25.8 (5.7) | 51.8 (6.9) | 25.1 (5.3) | 36.1 (6.0) |
| Lorazepam | 28.8 (6.5) | 54.0 (7.1) | 25.4 (5.5) | 36.1 (5.9) | |
| Acetazolamide | 33.7 (6.7) | 49.9 (7.5) | 20.4 (4.4) | 35.3 (7.1) | |
| Benzolamide | 26.5 (5.3) | 51.6 (5.3) | 24.4 (5.2) | 35.5 (5.5) | |
| Intraocular pressure (mmHg) | Placebo | 9.8 (0.7) | 10.4 (0.6) | 9.0 (0.4) | 9.1 (0.4) |
| Lorazepam | 9.9 (0.6) | 9.3 (0.5) | 9.0 (0.5) | 8.9 (0.5) | |
| Acetazolamide** | 9.0 (0.6) | 7.7 (0.7) | 7.7 (0.5) | 8.3 (0.5) | |
| Benzolamide | 9.4 (0.6) | 8.5 (0.4) | 8.6 (0.5) | 8.7 (0.5) |
*P < 0.05, **P < 0.01, ***P < 0.001 (relative to placebo). Values are mean ± SEM.
Collier D. J., Wolff C. B., Hedges A.‐M., Nathan J., Flower R. J., Milledge J. S., Swenson E. R.. Benzolamide improves oxygenation and reduces acute mountain sickness during a high‐altitude trek and has fewer side effects than acetazolamide at sea level, Pharma Res Per, 4(3), 2016, e00203, doi: 10.1002/prp2.203
References
- Bärtsch P, Swenson ER (2013). Clinical practice: acute high altitude illnesses. N Engl J Med 368: 2294–3027. [DOI] [PubMed] [Google Scholar]
- Basnyat B, Gertsch JH, Johnson EW, Castro‐Marin F, Inoue Y, Yeh C (2003). Efficacy of low‐dose acetazolamide (125 mg BID) for the prophylaxis of acute mountain sickness: a prospective, double‐blind, randomized, placebo‐controlled trial. High Alt Med Biol 4: 45–52. [DOI] [PubMed] [Google Scholar]
- Basnyat B, Gertsch JH, Holck PS, Johnson W, Luks AM, Donham BP, et al. (2006). Acetazolamide 125 mg BD is not significantly different from 375 mg BD in the prevention of acute mountain sickness: the prophylactic acetazolamide dosage comparison for efficacy (PACE) trial. High Alt Med Biol 7: 17–27. [DOI] [PubMed] [Google Scholar]
- Begg C, Cho M, Eastwood S, Horton R, Moher D, Olkin I, et al. (1996). Improving the quality of reporting of randomized controlled trials: the CONSORT statement. J Am Med Assoc 276: 637–639. [DOI] [PubMed] [Google Scholar]
- Birmingham Medical Research Expeditionary Society Acute Mountain Sickness Study Group . 1981. Acetazolamide in control of acute mountain sickness. Lancet i:180–183. [Google Scholar]
- Brierley G, Parks T, Wolff CB (2012). The relationship of acute mountain sickness to arterial oxygen saturation at altitudes of 3324 to 5176 m. Adv Exp Med Biol 737: 207–212. [DOI] [PubMed] [Google Scholar]
- Dawson J, Boyle J, Stanley N, Johnsen S, Hindmarch I, Skene DJ (2008). Benzodiazepine‐induced reduction in activity mirrors decrements in cognitive and psychomotor performance. Hum Psychopharmacol 23:605–613. [DOI] [PubMed] [Google Scholar]
- Ellsworth AJ, Larson EB, Strickland D (1987). A randomized trial of dexamethasone and acetazolamide for acute mountain sickness prophylaxis. Am J Med 83: 1024–1030. [DOI] [PubMed] [Google Scholar]
- Faulhaber M, Wille M, Gatterer H, heinrich D Burtscher M (2014). Resting arterial oxygenation and breathing frequency as predictors for acute mountain sickness: a prospective cohort study. Sleep Breath 18: 669–674. [DOI] [PubMed] [Google Scholar]
- Forwand SA, Landowne M, Follanshee JN, Hansen JE (1987). Effect of acetazolamide on acute mountain sickness. New Engl J Med 279: 839–845. [DOI] [PubMed] [Google Scholar]
- Hackett PH, Oeltz O (1992). The Lake Louis Consensus on the definition and quantification of altitude illness Pp. 327–330 in Sutton J. R., Coates G. and Houston C. S., eds. Hypoxia and Mountain Medicine. Queen City Printers, Burlington. [Google Scholar]
- Kazunobu O, Hiroshi E, Yumiko E, Katsumi A (2001). Acute mountain sickness and arterial oxygen saturation during altitude trekking. Comparisons between high school adolescents, young adults and middle‐older adults. Jap J Mountain Med 21: 83–88. [Google Scholar]
- Koehle MS, Guenette JA, Warburton DE (2010). Oximetry, heart rate variability and diagnosis of mid‐to moderate acute mountain sickness. Eur J Emerg Med 17: 119–122. [DOI] [PubMed] [Google Scholar]
- Kronenberg RS, Cain SM (1968a). Hastening respiratory acclimatisation to altitude with benzolamide (CL 11366). Aerospace Med 39: 296–300. [PubMed] [Google Scholar]
- Kronenberg RS, Cain SM (1968b). Effects of acetazolamide and hypoxia on cerebrospinal fluid bicarbonate. J Appl Physiol 24: 17–20. [DOI] [PubMed] [Google Scholar]
- Larson EB, Roach RC, Schoene RB, Hornbein TF (1982). Acute mountain sickness and acetazolamide. Clinical efficacy and effect on ventilation. J Am Med Assoc 248: 328–332. [PubMed] [Google Scholar]
- Luks AM, Swenson ER (2011). Clinician's Corner: pulse oximetery at high altitude. High Alt Med Biol 12: 109–119. [DOI] [PubMed] [Google Scholar]
- Luks AM, McIntosh SE, Grissom CK, Auerbach PS, Rodway GW, Schoene RB, et al. (2010). Wilderness Medical Society consensus guidelines for the prevention and treatment of acute altitude illness. Wilderness Environ Med. 21: 146–155. [DOI] [PubMed] [Google Scholar]
- Muza SR, Fulco CS, Cymerman A (2004). Altitude acclimatization guide. U.S Army Medical Research and Materiel Command, Technical note no. TN04‐05.
- O'Connor F, Dubowitz G, Bickler P (2004). Pulse oximetry in the diagnosis of acute mountain sickness. High Alt Med Biol 5: 341–348. [DOI] [PubMed] [Google Scholar]
- Roach RC, Houston CS, Honigman B, Nicholas RA, Yaron M, Grissom CK, et al. (1995). How well do older persons tolerate moderate altitude. West J Med 162: 32–36. [PMC free article] [PubMed] [Google Scholar]
- Roach RC, Greene ER, Schoene RB, Hackett PH (1998). Arterial oxygen saturation for prediction of acute mountain sickness. Aviat Space Environ Med 69: 1182–1185. [PubMed] [Google Scholar]
- Robertson N, Potter C, Harris AL (2004). Role of carbonic anhydrase IX in human tumor cell growth, survival, and invasion. Cancer Res 64: 6160–6165. [DOI] [PubMed] [Google Scholar]
- Roeggla G, Roeggla M, Podolsky A, Wagner A, Laggner AN (1996). How well can acute mountain sickness be quantified at high altitude? J Royal Soc Med 89: 141–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson ER (2014a). Carbonic anhydrase inhibitors and high altitude illnesses. Subcell Biochem 75: 361–386. [DOI] [PubMed] [Google Scholar]
- Swenson ER (2014b). The lung in acute mountain sickness: victim or perpetrator or both? Am J Med 127: 899–900. [DOI] [PubMed] [Google Scholar]
- Swenson ER, Hughes JMB (1993). Effects of acute and chronic acetazolamide on resting ventilation and ventilatory responses in men. J Appl Physiol 73: 230–237. [DOI] [PubMed] [Google Scholar]
- Swenson ER, Teppema LJ (2007). Prevention of acute mountain sickness by acetazolamide: as yet an unfinished story. J Appl Physiol 102: 1305–1307. [DOI] [PubMed] [Google Scholar]
- Swenson ER, Leatham KL, Roach RC, Schoene RB, Mills WJ, Hackett PH (1991). Renal carbonic anhydrase inhibition reduces high altitude sleep periodic breathing. Respir Physiol 86: 333–343. [DOI] [PubMed] [Google Scholar]
- Swietach P, Hulikova A, Vaughan‐Jones RD, Harris AL (2010). New insights into the physiological role of carbonic anhydrase IX in tumour pH regulation. Oncogene 29: 6509–6521. [DOI] [PubMed] [Google Scholar]
- Tannheimer M, Thomas A, Gerngross H (2002). Oxygen saturation course and altitude symptomatology during an expedition to Braod Peak (8047 m). Int J Sports Med 23: 329–335. [DOI] [PubMed] [Google Scholar]
- Travis DM (1969). Renal carbonic anhydrase inhibition by benzolamide (CL 11,366) in man. J Pharmacol Exp Therap 167: 253–264. [PubMed] [Google Scholar]
- Van Patot MC, Leadbetter G, Keyes LE, Maakestad KM, Olson S, Hackett PH (2008). Prophylactic low‐dose acetazolamide reduces the incidence and severity of acute mountain sickness. High Alt Med Biol 9: 289–293. [DOI] [PubMed] [Google Scholar]
- Wang J, Ke T, Chen Y, Liu M, Chen J, Luo W (2013). Effects of acetazolamide on cognitive performance during high altitude exposure. Neurotoxicol Teratol 35: 28–33. [DOI] [PubMed] [Google Scholar]
- Ward MP, Milledge JS, West J (1995). High altitude medicine and physiology. Chapman and Hall, London. [Google Scholar]
- Wolfensberger TJ, Chiang RK, Takeuchi A, Marmor MF (2000). Inhibition of membrane‐bound carbonic anhydrase enhances subretinal fluid absorption and retinal adhesiveness. Graefes Arch Clin Exp Ophthalmol 238: 76–80. [DOI] [PubMed] [Google Scholar]
- Wright AD, Bradwell AR, Fletcher RF (1983). Methazolamide and acetazolamide in acute mountain sickness. Aviat Space Environ Med 54: 619–621. [PubMed] [Google Scholar]


