Abstract
Mitochondria are morphologically dynamic organelles constantly undergoing processes of fission and fusion that maintain integrity and bioenergetics of the organelle: these processes are vital for cell survival. Disruption in the balance of mitochondrial fusion and fission is thought to play a role in several pathological conditions including ischemic heart disease. Proteins involved in regulating the processes of mitochondrial fusion and fission are therefore potential targets for pharmacological therapies. Mdivi‐1 is a small molecule inhibitor of the mitochondrial fission protein Drp1. Inhibiting mitochondrial fission with Mdivi‐1 has proven cytoprotective benefits in several cell types involved in a wide array of cardiovascular injury models. On the other hand, Mdivi‐1 can also exert antiproliferative and cytotoxic effects, particularly in hyperproliferative cells. In this review, we discuss these divergent effects of Mdivi‐1 on cell survival, as well as the potential and limitations of Mdivi‐1 as a therapeutic agent.
Keywords: Cell death, Mdivi‐1, mitochondrial fission, mitochondrial fusion
Abbreviations
- AMPK
5′ adenosine monophosphate‐activated protein kinase
- CREB
cAMP response element binding
- MOMP
mitochondrial outer membrane permeabilization
- PLD
phosphatidylcholine hydrolyzing phospholipase D
- RISK
reperfusion injury salvation kinase
- ROS
reactive oxygen species
- SENP3
SUMO1/Sentrin/SMT3‐specific peptidase 3
- SLP2
stomatin‐like‐protein 2
Introduction
Under both physiological and pathological conditions, mitochondria change their shape through fusion and fission. These processes play central roles in quality control of mitochondria and are important for maintaining various cellular functions and viability, as well as regulating bioenergetic metabolism. Mitochondrial fusion is required for appropriate distribution of mitochondrial DNA, lipids, and proteins across all mitochondria. The main purpose of fusion is to ensure optimal conditions for mitochondria to carry out key cellular processes, such as energy metabolism, cellular differentiation, and calcium homeostasis (Palmer et al. 2011). A homogeneous mix of mitochondrial matrix proteins, mitochondrial DNA, and maintenance of optimal pH and mitochondrial membrane potential are essential for successful mitochondrial fusion (Kane and Youle 2010). Mitochondrial fusion is thus a complex sequential process which involves integration of the outer mitochondrial membrane, inner mitochondrial membrane, and matrix content. The main regulators of these processes are the GTP‐ase dynamin‐related proteins: mitofusin 1 (Mfn1), mitofusin 2 (Mfn2), and optical atrophy 1 (Opa1). Other profusion proteins include prohibitin 2, stomatin‐like‐protein 2 (SLP2), and the phosphatidylcholine hydrolyzing phospholipase D (PLD) (Fig. 1). The integration of theses enzymatic processes has been reviewed elsewhere and will not be detailed here (Palmer et al. 2011; Da Silva et al. 2014; Kasahara and Scorrano 2014).
Figure 1.
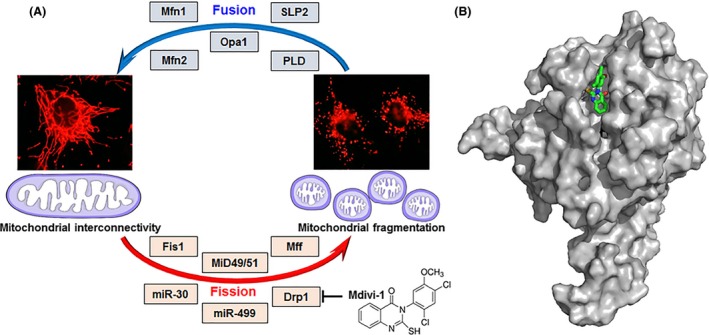
Mitochondrial fusion and fission cycle and its key players. (A) Mitochondrial interconnectivity is maintained by fusion which is regulated by proteins such as Mfn1, Mfn2, Opa1, SLP2, and PLD. Mitochondrial fragmentation follows fission, governed by several factors including Fis1, MiD49/51, Mff, miR‐30, miR‐499, and Drp1. Mitochondrial fission is suppressed via inhibition of Drp1 by the synthetic small molecule Mdivi‐1. (B) Proposed binding orientation of Mdivi‐1 (green carbons, sticks) to Drp1 (gray, surface rendered). Mdivi‐1 was computationally docked onto the Drp1 crystal structure (Wenger et al. 2013) using default conditions for the Geom‐dock module in Sybyl‐X 2.1.1 (Certara L.P.). Shown is a representative of the highest scoring cluster of solutions. Drp1, dynamin‐related protein 1; Fis1, fission 1; Mff, mitochondrial fission factor; Mfn1, mitofusin 1; Mfn2, mitofusin 2; MiD49/51, mitochondrial dynamics protein‐49/51; miR30/499, micro‐RNA 30/499; Opa1, optic atrophy protein; PLD, phosphatidylcholine hydrolyzing phospholipase D; SLP2, stomatin like protein‐2.
The opposite process, mitochondrial fission, plays an important role in mitochondrial proliferation following mitosis and is involved in removing damaged mitochondria from the cells through mitophagy (Otera and Mihara 2012). Mitochondrial fission is regulated by the large GTP‐ase dynamin‐related protein, Drp1 (the human homolog of the yeast mitochondrial dynamin, Dnm1). Similar to other dynamin‐related proteins, Drp1 has a GTP‐ase effector domain which is important for its GTP‐ase activity. However, it lacks membrane binding domains and thus is heavily dependent on proteins at the outer mitochondrial membrane for anchorage to the mitochondrion (Chan 2012; Dorn 2013). These profission docking proteins include Fis1 (mitochondrial fission 1), Mff (mitochondrial fission factor), MiD49 (mitochondrial dynamic protein of 49 kDa), MiD51 (mitochondrial dynamic protein of 51 kDa, also known as mitochondrial elongation factor 1, MIEF1), miR‐30, and miR‐499 (Fig. 1). Again these mechanisms have been thoroughly reviewed recently (Chan 2012; Da Silva et al. 2014; Lee and Yoon 2014).
Drp1 predominantly localizes in the cytosol as a tetramer and translocates to the outer mitochondrial membrane during mitochondrial fission, where it polymerizes into ring‐like structures around the mitochondria to induce fission (Shin et al. 1999; Cassidy‐Stone et al. 2008; Bossy et al. 2010). Translocation of Drp1 from the cytosol to the mitochondria is regulated by multiple posttranslational modifications including phosphorylation, ubiquitination, SUMOylation, and S‐nitrosylation (Karbowski et al. 2007; Taguchi et al. 2007; Wasiak et al. 2007; Cho et al. 2009). Phosphorylation is the most well‐studied mechanism, with known sites for phosphorylation being the serine residues 616 and 637 (equivalent to serine 585 and 656 in rats) which promote and inhibit translocation of Drp1 into the mitochondria, respectively (Taguchi et al. 2007; Qi et al. 2011).
Mitochondrial dynamics have been implicated in determining survival of many cell types including cardiomyocytes and neurons. Mitochondria fragmented as a result of fission are associated with apoptosis and autophagy (Ong et al. 2010; Chan 2012). Shifting the balance of mitochondrial morphology toward fission enhances susceptibility to death in various cell types. In contrast, fused mitochondria are energetically more active, preserve cell functions, and can better withstand oxidative stress (Ong et al. 2010). The discovery of Mdivi‐1, a small molecule that selectively and reversibly inhibits the mitochondrial fission protein Drp1 (Cassidy‐Stone et al. 2008), has led to a better understanding of the role of mitochondrial dynamics in the survival of various cell types under different pathophysiological conditions.
Mdivi‐1 as an Inhibitor of Drp1
Mdivi‐1 (mitochondrial fission inhibitor‐1) is the first selective inhibitor of the mitochondrial fission protein Drp1 (Cassidy‐Stone et al. 2008). It contains a quinazolinone core substituted with a thiol moiety and an aryl (2,4‐dichloro‐5‐methoxyphenyl) side chain attached to the N3 position (Figs. 1, 2) (Cassidy‐Stone et al. 2008; Qian et al. 2015). Structure–activity relationship analysis has shown that Mdivi‐1 is a mixture of two atropisomers which arise due to hindered rotation at its chiral axis around the nitrogen‐phenyl bond. The axial chirality at the aryl side chain greatly influences the selectivity of Mdivi‐1 for Drp1 (Cassidy‐Stone et al. 2008; Qian et al. 2015).
Figure 2.
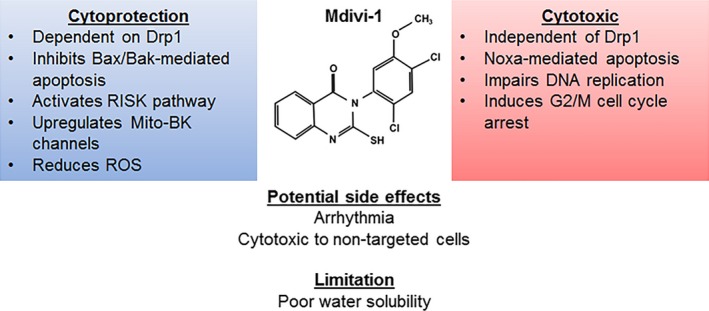
Pharmacodynamic profile of Mdivi‐1. Mdivi‐1 confers cytoprotection by employing a Drp1‐dependent inhibition of Bax/Bak‐mediated apoptosis, activating the RISK pathway, upregulating mitochondrial large conductance Ca2+and voltage activated K+ (Mito‐BK) channel as well as reducing ROS. Its cytotoxic effect is exerted independent of Drp1 and through activation of Noxa‐mediated apoptosis. Mdivi‐1 also exerts an inhibitory effect on hyperproliferative cells by inducing G2/M cell cycle arrest and impairs mitosis. Bak, Bcl2‐antagonist/killer 1; Bax, Bcl2‐associated X protein; DNA, deoxyribonucleic acid; Drp1, dynamin‐related protein 1; G2/M, second gap/mitosis; Mito‐BK, mitochondrial big potassium channel; RISK, reperfusion injury salvation kinase; ROS, reactive oxygen species.
Mdivi‐1 has been shown to target Drp1 selectively in mammalian cells by binding at an allosteric site and suppressing Drp1 capacity to catalyze GTP hydrolysis as well as self‐assembly into ring‐like structures around the mitochondria. Mdivi‐1 can induce rapid and reversible formation of interconnected mitochondria without affecting other cellular structures such as the cytoskeleton and endoplasmic reticulum, suggesting selectivity for mitochondrial fission. The half maximal inhibitory concentration of Mdivi‐1 ranges from 1 to 50 μmol/L depending on the cell and assay types (Cassidy‐Stone et al. 2008; Qian et al. 2015). Since its discovery in 2008 (Cassidy‐Stone et al. 2008), Mdivi‐1 has been widely employed as an inhibitor of Drp1 in multiple cell types (Table 1) and organs in different disease settings (Table 2). Interestingly, Mdivi‐1 exerts divergent effects on cell survival depending on the cell type and experimental setting.
Table 1.
In vitro studies of Mdivi‐1
| Cell types | Models | Treatment regimens | Findings | References | |
|---|---|---|---|---|---|
| Doses | Protocols | ||||
| Cardiovascular cells | |||||
|
Cardiomyocytes (mouse) |
Simulated ischemia‐reperfusion injury | 10 & 50 μmol/L | Started 45 min before ischemia |
↓Cell death ↑Mitochondrial membrane potential |
(Ong et al. 2010) |
|
Cardiomyocytes (mouse) |
Simulated ischemia‐reperfusion injury | 5 μmol/L | Started 30 min before ischemia |
↓Cell death ↓ROS ↓Cytosolic Ca2+ ↑Oxygen consumption rate No significant increase in ATP |
(Sharp et al. 2014) |
|
Cardiomyocytes (rat) |
Doxorubicin toxicity | 1 μmol/L | Cotreatment with doxorubicin |
Delayed mitochondrial depolarization Delayed hypercontracture |
(Gharanei et al. 2013) |
|
HL‐1 cells (mouse) |
Simulated ischemia‐reperfusion injury | 10 & 50 μmol/L | Started 40 min before ischemia |
↓Cell death ↑Mitochondria elongation Delayed mitochondrial depolarization |
(Ong et al. 2010) |
|
HL‐1 cells (mouse) |
— | 1–100 μmol/L | — |
↑Firing rate & duration of spontaneous action potential ↓amplitude of IKr
↓Opening probability of KAch |
(So et al. 2012) |
|
Vascular smooth muscle cells (human, pulmonary artery) |
Idiopathic pulmonary artery hypertension | 5, 10 & 25 μmol/L | — |
↓Proliferation G2/M cell cycle arrest |
(Marsboom et al. 2012) |
|
Vascular smooth muscle cells (human & rabbit, ductus arteriosus) |
O2 challenge on hypoxic cells | 20 μmol/L | During O2 challenge |
↓Proliferation ↓Oxygen consumption rate ↓Pyruvate dehydrogenase activity ↓mitochondrial H2O2 production ↓Ca2+ release from mitochondria & ER |
(Hong et al. 2013) |
|
Vascular smooth muscle cells (rat, aorta) |
Angiotensin II or H2O2 treatment | 1, 10 & 20 μmol/L |
Cotreatment with Angiotensin or H2O2
Pretreatment for 60 min |
↓Proliferation G2/M cell cycle arrest ↓Migration ↑Mitochondrial membrane potential ↓ROS ↓Phosphorylated ERK1/2, MEK1/2 |
(Lim et al. 2015) |
|
Vascular endothelial cells (human, umbilical cord vein) |
— | 30 μmol/L | 24 h |
↑Cell senescence ↓Cell migration ↓Angiogenic Tube formation ↑autophagosomes ↓Autolysosomes ↑ROS |
(Lin et al. 2015) |
| Neurons | |||||
|
Neurons (rat, hippocampus) |
Simulated ischemia‐reperfusion injury | 50 μmol/L | Pretreatment for 40 min |
↓Cell death ↓ROS |
(Wang et al. 2014) |
|
Neurons (rat, hippocampus) |
Simulated epilepsy with magnesium‐free culture | 10, 25 & 50 μmol/L | Pretreatment for 30 min |
↓Apoptosis ↓ROS ↓Endoplasmic reticulum stress |
(Xie et al. 2016) |
|
Neurons (human, embryonic stem cell‐derived) |
Propofol‐induced cell death | 25 μmol/L | Pretreatment for 60 min |
↓Apoptosis Delayed mitochondrial depolarization |
(Twaroski et al. 2015) |
|
Neurons (rat, spinal cord) |
Glutamate toxicity | 10 μmol/L | Cotreatment with glutamate |
↓Apoptosis ↓ROS ↑Mitochondrial membrane potential ↑Antioxidant activity ↑Expression of large‐conductance Ca2+‐activated K+ channel |
(Liu et al. 2015) |
|
Neurons (rat, cortex) |
Glutamate toxicity or simulated ischemia‐reperfusion injury | 25 μmol/L | Cotreatment with glutamate or during simulated ischemia | ↓Apoptosis | (Grohm et al. 2012) |
|
Neurons (rat, cortex) |
Simulated ischemia‐reperfusion injury | 25 μmol/L |
During 4 h of ischemia (cotreatment), without reperfusion During 24 h of reperfusion (posttreatment), after 2 h of ischemia |
↓Cell death (cotreatment) ↑Cell death (posttreatment) |
(Zhang et al. 2013a) |
|
Astrocytes (mouse) |
Hypoxia | 5‐30 μmol/L | 1–24 h |
↑Exogenous ATP metabolism ↑Extracellular adenosine ↑CD39 ↑cAMP levels & PKA activity ↑CREB expression |
(Cui et al. 2016) |
|
HT22 cells (mouse, hippocampal neuronal cells) |
Glutamate toxicity | 50 & 75 μmol/L | Cotreatment with glutamate or 2–12 h after glutamate challenge |
↓Apoptosis ↑Mitochondrial Membrane potential ↓ROS & lipid peroxidation Prevent ATP depletion |
(Grohm et al. 2012) |
|
N27 cells (rat, mesencephalic dopaminergic cells) |
PINK1‐induced mitochondrial fragmentation | 10 & 30 μmol/L | Cotreatment with ponasterone |
↑Mitochondrial membrane potential ↑ATP levels |
(Cui et al. 2010) |
| Skeletal myoblasts | |||||
|
C2C12 (mouse, skeletal muscle) |
Palmitate treatment | 50 & 150 μmol/L | Cotreatment with palmitate for 6 h |
↑Glucose uptake ↑Mitochondrial membrane potential ↓ROS |
(Jheng et al. 2012) |
|
C2C12 (mouse, skeletal muscle) |
Myogenic differentiation | 1, 10 & 20 μmol/L | 24 h |
↑Apoptosis Impaired myotube formation ↓Mitochondrial membrane potential ↓Mitochondrial mass & DNA ↓Expression of myogenic regulatory factors, MHC I & creatinine kinase activity |
(Kim et al. 2013) |
|
C2C12 (mouse, skeletal muscle) |
Oxidative stress | 25 μmol/L | After H2O2 exposure; for 1 h | ↓H2O2‐induced mitochondrial fragmentation | (Iqbal and Hood 2014) |
|
L6 (rat, skeletal muscle) |
Glucocorticoid‐induced muscle atrophy | 1 μmol/L | Cotreatment with dexamethasone for 6 and 24 h |
↓Dexamethasone‐induced mitochondrial fission & mitophagy ↓oxygen consumption ↓autophagic flux |
(Troncoso et al. 2014) |
| Cancer cells | |||||
|
A2780 cells (human, ovarian cancer) |
TRAIL‐induced apoptosis | 10, 20 & 50 μmol/L | Cotreatment with TRAIL for 16 h |
↑Apoptosis ↓Bid expression |
(Wang et al. 2015a) |
|
A2780cis cells (human, cisplatin‐resistant ovarian cancer) |
Cisplatin‐induced apoptosis | 20 & 50 μmol/L | Cotreatment with cisplatin for 20–72 h |
↑apoptosis ↑Bax, Bak & Noxa expression |
(Qian et al. 2014) |
|
A2780cis cells (human, cisplatin‐resistant ovarian cancer) |
TRAIL‐induced apoptosis | 10, 20 & 50 μmol/L | Cotreatment with TRAIL for 16 h | ↑Apoptosis | (Wang et al. 2015a) |
|
983A cells (human, melanoma) |
10–50 μmol/L | Cotreatment with cisplatin for 20 h | ↑Apoptosis | (Qian et al. 2014) | |
|
Cal33 cells (human, head, and neck squamous cell carcinoma) |
10–50 μmol/L | Cotreatment with cisplatin for 20 h | ↑Apoptosis | (Qian et al. 2014) | |
| Epithelial ovarian cancer cells (human) | Cisplatin‐induced apoptosis | 20 & 50 μmol/L | Cotreatment with cisplatin for 72 h | ↑Apoptosis | (Qian et al. 2014) |
|
HeLa cells (human, cervical cancer) |
Staurosporin‐induced apoptosis | 50 μmol/L | Cotreatment with staurosporine for 4 h | ↓Apoptosis | (Cassidy‐Stone et al. 2008) |
|
LN‐428 cells (human, glioblastoma) |
10–50 μmol/L | Cotreatment with cisplatin for 20 h | ↑Apoptosis | (Qian et al. 2014) | |
| MDA‐MB‐231 cells (human, breast carcinoma) | — | 20 & 50 μmol/L | 48 h | G2/M cell cycle arrest & aneuploidy | (Qian et al. 2012) |
| MDA‐MB‐231 cells (human, breast carcinoma) | Cisplatin or carboplatin‐induced apoptosis | 10–50 μmol/L | Cotreatment with cisplatin or carboplatin for 2–72 h |
↑Apoptosis ↓cell proliferation |
(Qian et al. 2014) |
|
MDA‐MB‐231 cells (human, breast carcinoma) |
— | 10–50 μmol/L | 16–48 h |
M phase cell cycle arrest Abnormal karyokinesis Impaired cytokinesis Hyperploidy |
(Wang et al. 2015b) |
|
SH‐SY5Y cells (human, neuroblastoma) |
Simulated ischemia‐reperfusion injury | 5, 10 & 20 μmol/L | Started 5 min before ischemia |
↑Cell viability ↑Mitochondrial membrane potential ↑Cellular ATP |
(Zhao et al. 2014) |
|
SH‐SY5Y cells (human, neuroblastoma) |
3NP‐induced autophagy | 10 μmol/L | Cotreatment with 3NP for 5 h | No effect on 3NP‐induced autophagy, ROS production, mitochondrial fragmentation, and Bax translocation to mitochondria | (Solesio et al. 2013) |
|
MCF7 cells (human, breast carcinoma) |
Mitosis | 50 μmol/L | 16 h |
M phase cell cycle arrest Impaired assembly of mitotic spindle & cytokinesis |
(Wang et al. 2015b) |
|
H1299 cells (human, nonsmall‐cell lung carcinoma) |
Cisplatin‐induced apoptosis | 10–50 μmol/L | Cotreatment with cisplatin or carboplatin for 2–20 h |
↑Apoptosis ↑Cleavage of caspase−9 & −3 ↑Cytochrome c release ↑Noxa expression |
(Qian et al. 2014) |
|
H1299 cells (human, nonsmall‐cell lung carcinoma) |
Mitosis | 50 μmol/L | 8–24 h |
↑Apoptosis M phase cell cycle arrest Impaired assembly of mitotic spindle & cytokinesis |
(Wang et al. 2015b) |
| Malignant mesothelioma cells (human) | PRX3‐deficiency model | G2/M cell cycle arrest | (Cunniff et al. 2014) | ||
| Brain tumor initiating cells of glioblastoma (human) | — | 10 & 20 μmol/L | 2–4 days |
↑Apoptosis ↑AMPK activation |
(Xie et al. 2015) |
|
GH3 cells (rat, pituitary tumor) |
— | 30 μmol/L | — | ↓Amplitude of IKr(ERG) | (So et al. 2012) |
|
HL‐60 cells (human, leukemia) |
Doxorubicin toxicity | 1 μmol/L | Cotreatment with doxorubicin | No effect on doxorubicin‐induced toxicity | (Gharanei et al. 2013) |
|
U2OS cells (human, osteosarcoma) |
Mitosis | 50 μmol/L | 8–24 h |
↑Apoptosis M phase arrest Impaired assembly of mitotic spindles & cytokinesis |
(Wang et al. 2015b) |
|
A375 & A2058 cells (human, melanoma) |
Death receptor‐induced apoptosis | 50 μmol/L | Cotreatment with αDR4 | ↑Apoptosis | (Suzuki‐Karasaki et al. 2015) |
|
SK‐N‐SH cells (human, neuroblastoma) |
High glucose treatment | 10 μmol/L | — |
↑Mitochondrial density ↑complex I activity Maintained mitochondrial length |
(Huang et al. 2015) |
|
PC12 cells (rat, pheochromocytoma) |
Ischemia‐reperfusion injury | 25, 50 & 100 μmol/L | Pretreatment for 30 min |
↓Cell death ↓ros ↑Mitochondrial membrane potential ↓Mitochondrial Ca2+ uptake & ER Ca2+ release |
(Tian et al. 2014) |
| Others | |||||
|
Immortalized fibroblasts (mouse, embryo) |
Cisplatin‐induced apoptosis | 50 μmol/L | Cotreatment with cisplatin for 20 h | ↑Apoptosis (Drp1 independent) | (Qian et al. 2014) |
|
Immortalized fibroblasts (mouse, embryo) |
— | 20 μmol/L | 20 h | ↑Apoptosis (Bax/Bak independent) | (Qian et al. 2015) |
|
Immortalized fibroblasts (mouse, embryo) |
Fas‐induced apoptosis | 50 μmol/L | 16 h | ↑Apoptosis (Drp1 & Bax/Bak independent) | (Wang et al. 2015a) |
|
Immortalized fibroblasts (mouse, embryo) |
— | 50 μmol/L | 6–48 h |
↑Apoptosis (Bax/Bak‐dependent) M phase cell cycle arrest (Drp1 independent) |
(Wang et al. 2015b) |
|
Immortalized fibroblasts (human, foreskin) |
— | 50 μmol/L | 6–24 h |
↑Apoptosis M phase cell cycle arrest |
(Wang et al. 2015b) |
|
COS cells (monkey, kidney cell line) |
Stausporine‐induced apoptosis | 1–200 μmol/L | Cotreatment with staurosporine for 4 h | ↓Apoptosis | (Cassidy‐Stone et al. 2008) |
|
Immortalized small airway epithelial cells (human) |
Irradiation | 50 μmol/L | Started 2 h before irradiation | No effect on irradiation‐induced mitochondrial respiratory dysfunction | (Zhang et al. 2013a) |
|
HEK293 cells (human embryo, kidney cell line) |
Simulated ischemia‐reperfusion injury | 50 μmol/L | 4 h | ↓SENP3‐induced cytochrome c release | (Guo et al. 2013) |
3NP, 3‐nitropropionic acid; AMPK, AMP‐activated protein kinase; ATP, adenosine triphosphate; Bak, bcl2‐antagonist/killer‐1; Bax, bcl2‐associated X protein; Bid, BH3‐interacting domain death agonist; cAMP, cyclic adenosine monophosphate; Chk1, checkpoint kinase 1; CREB, cAMP response element binding; Drp1, dynamin‐related protein 1; ER, endoplasmic reticulum; IK(erg), erg‐mediated K+ current; IKr, rapidly activating delayed‐rectifier K+ current; INa, Na+ current; Itail, amplitude of tail current; KAch, muscarinic K+ channel; MHC I, myosin heavy chain I; MPTP, mitochondrial membrane permeability transition pore; Noxa, latin for damage, alternative name for immediate‐early‐response protein APR; PKA, protein kinase A; PRX3, Peroxiredoxin 3; ROS, reactive oxygen species; SENP3, SUMO1/Sentrin/SMT3 Specific Peptidase 3; SOD, superoxide dismutase; TRAIL, tumor necrosis factor‐related apoptosis‐inducing ligand.
Table 2.
In vivo and ex vivo studies of Mdivi‐1
| Organ/Tissue | Model | Regimen | Findings | Reference | |
|---|---|---|---|---|---|
| Dose | Mode of treatment | ||||
|
Heart (mouse) |
Ischemia‐reperfusion injury | 0.24 & 1.2 mg/kg | Intravenous; 15 min before ischemia | ↓Infarct size | (Ong et al. 2010) |
|
Heart (mouse) |
Pressure overload‐induced heart failure | 50 mg/kg | Intraperitoneal; daily for 7 days |
↓Apoptosis ↓LV Dysfunction ↓Fibrosis ↑Angiogenesis ↑Mitochondrial density ↓Mitophagy ↓Expression of antiangiogenic factors, MMP‐9 & TIMP‐3 |
(Givvimani et al. 2012) |
|
Heart (mouse) |
Ischemia‐reperfusion injury and doxorubicin toxicity (ex vivo) | 1 μmol/L | Perfusion; Cotreatment with doxorubicin during 120 min reperfusion |
↓Infarct size ↑Coronary flow ↓Heart rate ↑phosphorylated Akt expression ↓Phosphorylated Erk1/2 & p53 expression |
(Gharanei et al. 2013) |
|
Heart (rat) |
Ischemia‐reperfusion injury (ex vivo) | 5 & 25 μmol/L | Perfusion; 10 min before ischemia or during 20 min reperfusion | ↑Diastolic function | (Sharp et al. 2014) |
|
Heart (mouse) |
Potassium‐induced cardiac arrest | 0.24 mg/kg | Intravenous; cotreatment with epinephrine; after cardiopulmonary resuscitation |
↑Animal survival ↑Heart rate, stroke volume & neurological outcomes ↓Myocardial lactate production |
(Sharp et al. 2015) |
|
Pulmonary artery (rat) |
Pulmonary artery hypertension (PAH) | 50 mg/kg |
Intraperitoneal; weekly for 4 weeks (CoCl2 study), biweekly for 4 weeks (chronic hypoxia study), or 5 daily injections 3 weeks after monocrotaline‐induced PAH |
↑Exercise capacity ↑RV function ↓RV hypertrophy ↑Hematocrit ↓Small pulmonary artery thickness |
(Marsboom et al. 2012) |
|
Ductus arteriosus (human & rabbit) |
O2‐induced constriction (ex vivo) | 20 μmol/L |
30 min before exposure O2 (PO2 120 mmHg); 6–14 days in normoxic culture (PO2 140 mmHg); |
Prevents O2‐induced constriction Prevent closure of ductus arteriosus ↓Smooth muscle cell proliferation ↓Fibrosis |
(Hong et al. 2013) |
|
Aortic ring (rat) |
Carotid artery balloon injury | 50 mg/kg/day | 7 days |
↓Neointimal formation ↓Smooth muscle cell proliferation |
(Lim et al. 2015) |
|
Brain (mouse) |
Ischemia‐reperfusion injury | 3 mg/kg | Intraperitoneal; prior to ischemia | ↓Infarct volume | (Grohm et al. 2012) |
|
Brain (mouse) |
Ischemia‐reperfusion injury | 10 & 20 mg/kg | Intraperitoneal |
↑Neurological outcome ↓Infarct volume ↓Brain edema ↓apoptosis |
(Zhao et al. 2014) |
|
Brain (mouse) |
Ischemia‐reperfusion injury | 10 & 20 mg/kg | Intraperitoneal; 4 h before ischemia and every 12 h for 10 days after reperfusion |
↑Animal survival ↓Infarct volume ↑cerebral blood flow ↓Extracellular ATP ↑Extracellular adenosine ↑CD39 expression ↑Phosphorylation of CREB protein |
(Cui et al. 2016) |
|
Brain (mouse) |
Implantation of brain tumor initiating cells of glioblastoma | 2.5 mg/kg | Intravenous; 3 days after tumor implantation, administered for 5 days |
↑Survival ↑Tumor latency |
(Xie et al. 2015) |
|
Brain (mouse) |
Traumatic brain injury | 3 mg/kg | Intraperitoneal; 10 min after injury |
↑Motoric and cognitive recovery ↓Infarct volume ↓Brain edema ↓Apoptosis |
(Wu et al. 2016) |
|
Brain (rat) |
Ischemia‐reperfusion injury | 0.24 & 1.2 mg/kg | Intravenous; 15 min prior to ischemia | ↓Apoptosis of neuron | (Zhang et al. 2013b) |
|
Brain (rat) |
Ischemia‐reperfusion injury | 3 mg/kg |
Intraperitoneal; During 24 h of focal ischemia (cotreatment), without reperfusion During 24 h of reperfusion, after 1 h of focal ischemia (posttreatment) |
No change of infarct volume (cotreatment) ↑Infarct volume (posttreatment) |
(Zhang et al. 2013c) |
|
Brain (rat) |
Pilocarpine‐induced seizure | 0.25 & 1.25 mg/kg | Intravenous; 15 min prior to pilocarpine injection | ↓Apoptosis | (Xie et al. 2013) |
|
Brain (rat) |
Pilocarpine‐induced seizure | 1.2 mg/kg | Intraperitoneal; 30 min prior to pilocarpine injection |
↓Apoptosis ↓ROS ↑SOD activity No effect on latency and intensity of seizure |
(Qiu et al. 2013) |
|
Brain (rat) |
Cardiac arrest | 0.24 & 1.2 mg/kg | Intravenous; after 1 min of restoration of spontaneous circulation |
↑Survival ↑Neurological outcome ↓Apoptosis |
(Li et al. 2015b) |
|
Hippocampus (mouse) |
Type 2 diabetes | 10 & 25 mg/kg | Intravenous; daily for 2 weeks |
↑Hippocampal long‐term potentiation ↑ATP levels ↑Complex I activity |
(Huang et al. 2015) |
|
Spinal cord (rat) |
Ischemia‐reperfusion injury | 1 mg/kg | Intravenous; at the beginning of ischemia |
↑Neurological outcome ↓Spinal cord edema ↑Expression of large‐conductance Ca2+ & voltage‐activated K+ channels |
(Liu et al. 2015) |
|
Spinal cord (rat) |
Acute spinal cord injury (Modified Allen's method) |
0.24 & 1.2 mg/kg | Intravenous; 15 min prior to injury |
↑Hind limb motor function ↓Apoptosis ↑Mitochondrial membrane potential ↓ROS ↑Reduced glutathione ↑ATP levels |
(Li et al. 2015a) |
|
Spinal dorsal horn (rat) |
Perineural HIV‐1 gp120‐induced neuropathic pain | 0.3, 1 & 3 μg/10 μL | Intrathecal |
↑Mechanical allodynia threshold ↓Mitochondrial superoxide |
(Kanda et al. 2016) |
|
Retina (mouse) |
Ischemia‐reperfusion injury | 50 mg/kg | Intraperitoneal; 60 min prior to & 6 h after ischemia |
↓Apoptosis of retinal ganglion cells ↓Glial fibrillary acidic protein expression |
(Park et al. 2011) |
|
Kidney (rat) |
Rhabdomyolysis‐induced acute kidney injury | 50 mg/kg | Intraperitoneal; 1 h or 12 h prior to rhabdomyolysis induction |
↓Apoptosis of tubular epithelial cells ↓ROS ↑ATP ↑Creatinin kinase |
(Tang et al. 2013) |
|
Liver (rat) |
Sepsis | 50 mg/kg | Intraperitoneal; 1 h prior to cecal ligation and puncture |
↓Apoptosis of hepatocytes ↑Mitochondrial elongation ↑Respiratory complex activity |
(Gonzalez et al. 2014) |
| Skeletal muscle (mouse) | Leptin deficiency | 44 mg/kg | Intraperitoneal; 16 h and 1 h prior to insulin/glucose injection |
↓Insulin resistance index (systemic) ↓Phosphorylated Erk1/2 and p38 MAPK |
(Jheng et al. 2012) |
8‐oHdG: 8‐Oxo‐2‐deoxyguanosine; ADP, adenosine diphosphate; AIF, apoptosis‐inducing factor; Akt, AKT8 virus oncogene cellular homolog; ATP, adenosine triphosphate; Bax, bcl2‐associated X protein; Bcl2, B‐cell lymphoma 2; CD39, cluster of differentiation 39; CREB, cAMP response element binding; Drp1, dynamin‐related protein 1; Erk 1/2, extracellular signal‐regulated kinase‐1/2; Fis1, fission 1; gp120, glycoprotein 120; LV, left ventricle; MMP 9, matrix metallopeptidase 9; p53, protein 53; PCNA, proliferating cell nuclear antigen; PO2, oxygen pressure; ROS, reactive oxygen species; RV, right ventricle; SOD, superoxide dismutase; TIMP 3, tissue inhibitor of metalloproteinases‐3.
Divergent Effects of Mdivi‐1 on Cell Survival
The cytoprotective effect of Mdivi‐1 was first demonstrated by Cassidy‐Stone et al. (2008). They showed that treatment with Mdivi‐1 significantly reduced mitochondrial fragmentation and apoptosis induced by staurosporine, to an extent similar to that observed in cells expressing the dominant negative Drp1K38A mutant (Cassidy‐Stone et al. 2008). Furthermore, Mdivi‐1 has been shown to attenuate Bax/Bak‐dependent mitochondrial outer membrane permeabilization (MOMP) induced by caspase 8‐cleaved recombinant Bid (Cassidy‐Stone et al. 2008). These findings suggested that Mdivi‐1 inhibits Drp1‐mediated mitochondrial fragmentation and the intrinsic apoptotic pathway. Other researchers have since confirmed the cytoprotective effect of Mdivi‐1 in various cell types, particularly in cardiovascular cells and neurons. In contrast, Mdivi‐1 has been shown to exert antiproliferative and cytotoxic effects in hyperproliferative cells such as in tumors and immortalized cells (Table 1).
Cardiomyocytes
Studies of Mdivi‐1 in cardiomyocytes utilized a wide array of injury models to simulate pathological conditions such as ischemia‐reperfusion injury and doxorubicin‐induced cardiotoxicity (Ong et al. 2010; Gharanei et al. 2013; Sharp et al. 2014). Death of cardiomyocytes and HL‐1 cells (a cardiac cell line derived from a mouse atrial tumor) was moderately repressed when pretreated with Mdivi‐1 prior to ischemic insult (Ong et al. 2010; Sharp et al. 2014). The cytoprotective effect of Mdivi‐1 was associated with increased phosphorylation of Drp1 at serine 637, thus preventing translocation of Drp1 into the mitochondria and consequently attenuating mitochondrial fragmentation (Ong et al. 2010; Sharp et al. 2014). Mdivi‐1 has also been shown to confer cytoprotection by reducing production of reactive oxygen species (ROS), attenuating cytosolic calcium overload, restoring mitochondrial membrane potential, and delaying hypercontracture of cardiomyocytes in ischemia‐reperfusion injury and doxorubicin‐induced cardiotoxicity (Ong et al. 2010; Gharanei et al. 2013; Sharp et al. 2014).
Interestingly, So et al. (2012) recently reported that Mdivi‐1 can alter the electrical activity of HL‐1 cells. Treatment with Mdivi‐1 prolonged the duration of the action potential, but increased the firing rate of spontaneous action potentials, inhibited the rapidly activating, delayed‐rectifier K+ current (IKr) and reduced the open probability of the muscarinic inward rectifier K+ channels (KAch) (So et al. 2012). The inhibitory effect of Mdivi‐1 on IKr was shown to be concentration‐dependent with a half maximal concentration of 11.6 μmol/L, similar to that which showed cytoprotection in other studies (Ong et al. 2010; So et al. 2012; Gharanei et al. 2013; Sharp et al. 2014). This raises a concern that Mdivi‐1 may have arrhythmogenic side effects.
It is important to note that outcomes of studies in HL‐1 cells and neonatal cardiomyocytes require cautious interpretation for these cells are not truly representative of primary adult cardiomyocytes. Differences in cell morphology, electrophysiology, and biogenesis can contribute to their individual resistance or susceptibility toward pathological stimuli and pharmacological agents (Bass et al. 2001; Milerova et al. 2010; Kuznetsov et al. 2015). Mitochondria in adult cardiomyocytes have spatio‐temporal restraint as well as slower rates of fusion–fission cycle when compared with neonatal cardiomyocytes and HL‐1 cells (Beraud et al. 2009; Chen et al. 2011; Piquereau et al. 2013). The relatively short and discrete mitochondria in adult cardiomyocytes are arranged in a regular pattern between myofibrils alongside the sarcomere. They do not form an interconnected network which might otherwise impose biomechanical restriction during cardiomyocyte contraction (Chen et al. 2011; Dorn and Kitsis 2015). In contrast, neonatal cardiomyocytes and HL‐1 cells have relatively longer and more dynamic mitochondria which usually form interconnected networks throughout the cell (Amchenkova et al. 1988; Anmann et al. 2006). Furthermore, cellular metabolism in adult cardiomyocytes is more dependent on oxidative phosphorylation, whereas neonatal rat cardiomyocytes and HL‐1 cells rely more on glycolysis (Bass et al. 2001; Anmann et al. 2006; Monge et al. 2009). Taken together, the morphological and bioenergetic differences between these cell types could lead to different outcomes in cell survival within similar experimental settings (Bass et al. 2001; Milerova et al. 2010; Kuznetsov et al. 2015).
Vascular cells
Mitochondrial fission is essential in smooth muscle cells for their proliferation and migration, processes that are relevant to several pathophysiological conditions such as premature closure of ductus arteriosus and pulmonary hypertension (Marsboom et al. 2012; Hong et al. 2013; Lim et al. 2015). Under oxidative stress and angiotensin II stimulation, ROS‐induced smooth muscle cell proliferation and migration have been attributed to activation of protein kinase Cδ which phosphorylates Drp1, resulting in translocation of Drp1 to the mitochondria and fission (Hong et al. 2013; Qi et al. 2013; Lim et al. 2015). Thus, Mdivi‐1 has been shown to suppress smooth muscle cell proliferation and migration through attenuation of ROS production and Drp1 phosphorylation (Hong et al. 2013). In arterial smooth muscle cells derived from subjects with pulmonary arterial hypertension, Mdivi‐1 was shown to suppress cell proliferation in a dose‐dependent manner, an effect attributed to G2/M cell cycle arrest and shown to be independent of cyclin B1/CDK1‐mediated phosphorylation of Drp1 at Serine 616 (Marsboom et al. 2012).
In addition to regulating proliferation and migration of smooth muscle cells, Drp1‐mediated mitochondrial fission plays an important role in metabolism. Oxygen‐induced mitochondrial fission in smooth muscle cells derived from ductus arteriosus has been shown to increase oxidative metabolism, oxygen consumption, and cytosolic calcium levels, which were all effectively prevented by Mdivi‐1 (Hong et al. 2013).
In endothelial cells, inhibition of Drp1 with Mdivi‐1 has been reported to induce premature senescence and impair the angiogenic function of human umbilical cord vein endothelial cells by increasing mitochondrial ROS production and reducing autophagic flux (Lin et al. 2015). These studies suggest a key regulatory role of Drp1 in maintaining vascular homeostasis and angiogenesis, and therefore may be a therapeutic target for vascular repair.
Neurons
Similar to cardiomyocytes, neurons contain metabolically active mitochondria and are susceptible to bioenergetic dysfunction and cell death. Therefore, preservation of normal mitochondrial function through manipulation of mitochondrial morphology is a potential therapeutic approach to neuroprotection. The cytoprotective effect of Mdivi‐1 in neurons has been well illustrated in several experimental injury models such as simulated ischemia‐reperfusion and toxicity of glutamate and propofol (Zhang et al. 2013b; Wang et al. 2014; Liu et al. 2015; Twaroski et al. 2015). Mechanistic insights include delayed mitochondrial permeability transition pore opening, preserved mitochondrial membrane potential, increased adenosine levels, attenuated oxidative stress, and reduced endoplasmic reticulum stress (Zhang et al. 2013b; Wang et al. 2014; Liu et al. 2015; Twaroski et al. 2015; Xie et al. 2016). Cui et al. (2016) demonstrated that Mdivi‐1 increases release of the neuroprotective agent, adenosine, through the cAMP/PKA/CREB pathway. Under oxidative stress, ROS trigger apoptotic cell death by increasing intracellular calcium levels and promoting outer mitochondrial membrane permeabilization, leading, in turn, to the release of cytochrome c and activation of the caspase cascade (Cardoso et al. 2004; Bajić et al. 2013). Treatment with Mdivi‐1 has been shown to reduce ROS levels partly by augmenting the activity of intracellular antioxidant enzymes such as superoxide dismutase and catalase (Liu et al. 2015). The cytoprotective effect of Mdivi‐1 in neurons has also been attributed to the opening of the large‐conductance calcium‐ and voltage‐activated potassium channels (Liu et al. 2015), which have long been implicated in cytoprotection against ischemic injury in the heart and are found in abundance in the central nervous system (Xu et al. 2002; Bentzen et al. 2014). The influx of potassium through these channels in the inner mitochondrial membrane can cause mild uncoupling of oxidative phosphorylation, ultimately inhibiting ROS production via Complex I (Kulawiak et al. 2008).
In a glutamate toxicity model, Mdivi‐1 has been reported to protect primary rat cortical neurons and HT‐22 cells (immortalized hippocampal neurons), from apoptosis. Moreover, Mdivi‐1 remains protective when given 2–8 h after the onset of glutamate challenge (Grohm et al. 2012). Using a different injury model, Zhang et al. (2013c) has suggested that the therapeutic window of Mdivi‐1 in protecting rat cortical neurons against simulated ischemia‐reperfusion injury is limited to the ischemic period, for Mdivi‐1 fails to confer protection when given during reperfusion. They showed that mitophagy‐mediated mitochondrial clearance during reperfusion after ischemia is neuroprotective and inhibition of mitochondrial fission by Mdivi‐1 may suppress mitophagy and aggravate ischemia‐induced injury (Zhang et al. 2013c). These studies have demonstrated the importance of precise temporal regulation of the mitochondrial fission protein Drp1, in neurons under different pathophysiological conditions. Whether a similar therapeutic window for Mdivi‐1 is applicable to other types of neurons and other cell types or in other injury models remains unclear and warrants further investigation.
Skeletal myoblasts
Mitochondrial dynamics play an important role in mitochondrial quality control and skeletal muscle homeostasis. Dysregulation of mitochondrial dynamics has been implicated in various pathological conditions of muscular dysfunction (Jheng et al. 2015). Inhibiting mitochondrial fission with Mdivi‐1 has been shown to attenuate palmitate‐induced mitochondrial dysfunction and insulin resistance in C2C12 skeletal myoblasts (Jheng et al. 2012). In L6 rat skeletal muscle cells, Mdivi‐1 suppressed dexamethasone‐induced autophagic flux and enhanced expression of muscle atrophy‐related genes. This suggests a regulatory role for mitochondrial fission in mitochondrial quality control in skeletal muscles via activation of autophagy (Troncoso et al. 2014). Mitochondrial dynamics also play a significant role in the myogenic differentiation of myoblasts. Inhibition of Drp1‐mediated mitochondrial fission with Mdivi‐1 impaired myotube formation in both C2C12 myoblasts and primary murine myoblasts, which was accompanied by increased apoptosis and impaired mitochondrial biogenesis (Kim et al. 2013).
Cancer cells
In contrast to the cytoprotective effect in cardiovascular cells and neurons, Mdivi‐1 exerts a cytodestructive effect in most hyperproliferative cancer and immortalized cell lines (Table 1). A hallmark of cancer cells is their unregulated proliferation and Drp1‐mediated mitochondrial fission has been shown to play an important role in cancer cell growth (Rehman et al. 2012; Xie et al. 2015). Inhibiting mitochondrial fission with Mdivi‐1 has been reported to exert a cytotoxic effect on cancer cells by reducing progression of mitosis and inducing apoptosis (Qian et al. 2014, 2015; Suzuki‐Karasaki et al. 2015; Wang et al. 2015a,b). As demonstrated in several cancer cell lines, Mdivi‐1 induced G2/M cycle arrest by interfering with DNA replication and synthesis, and activating checkpoint kinase‐1 (Qian et al. 2014; Wang et al. 2015b). The mitotic phase specifically is halted by Mdivi‐1 as a result of impaired assembly of mitotic spindles and cytokinesis, consequently disrupting chromosome segregation leading to aneuploidy (Wang et al. 2015a). Importantly, the proapoptotic and antiproliferative effects of Mdivi‐1 were absent in nontransformed normal human cells such as fibroblasts and epithelial cells, suggesting this effect is selective for tumor cells (Qian et al. 2014, 2015; Wang et al. 2015a,b; Xie et al. 2015).
Mdivi‐1 has also been shown to enhance the cytotoxic effect of the anticancer compound cisplatin; it does so by triggering Noxa‐dependent mitochondrial outer membrane permeabilization, bypassing the usual Bax/Bak‐dependency (Qian et al. 2014). However, whether the cytotoxic effect of Mdivi‐1 actually involves Drp1 remains controversial. Studies which suggest a Drp1‐independent pathway have been conducted in Drp1‐deficient, mouse immortalized embryonic fibroblasts without direct evidence in cancer cells (Qian et al. 2014, 2015; Wang et al. 2015a,b). There is other indirect evidence suggesting the involvement of the Bax/Bak pathway in the cytotoxic effect of Mdivi‐1, using mouse embryonic fibroblasts deficient in Bax/Bak (Qian et al. 2014, 2015; Wang et al. 2015a,b). However, just one study in brain tumor initiating cells showed support for the involvement of Drp1 in proliferation and survival of cancer cells, for Drp1 knockdown or treatment with Mdivi‐1 significantly reduced the tumorigenicity of the cells both in vitro and in vivo (Xie et al. 2015). In this study, the anticancer effect of Mdivi‐1 was suggested to involve upregulation of AMP‐activated protein kinase (AMPK), a downstream enzyme mediator of Drp1 (Xie et al. 2015).
Therapeutic Potential of Mdivi‐1
Several studies in animal disease models have highlighted the therapeutic potential of Mdivi‐1 in settings of ischemia‐reperfusion injury (Table 2). In myocardial ischemia‐reperfusion injury, treatment with Mdivi‐1 increased animal survival rate, reduced myocardial infarct size, and improved heart function (Gharanei et al. 2013; Sharp et al. 2014, 2015). Consistent with in vitro findings, the cardioprotective effect of Mdivi‐1 has been associated with activation of Akt signaling, a component of the reperfusion injury salvation kinase (RISK) pathway, and delayed the opening of mitochondrial permeability transition pores (Gharanei et al. 2013; Ong et al. 2015). Therapeutic benefits of Mdivi‐1 have also been found in other cardiovascular conditions such as pressure overload‐induced heart failure (Givvimani et al. 2012), cardiac arrest (Sharp et al. 2015) and pulmonary artery hypertension (Marsboom et al. 2012). Regarding vascular diseases, Mdivi‐1 prevented premature ductus arteriosus closure (Hong et al. 2013) and reduced neointima formation after carotid artery balloon injury (Lim et al. 2015) by suppressing proliferation of smooth muscle cells and fibrosis (Hong et al. 2013; Lim et al. 2015).
Disruption of mitochondrial dynamics has been associated with impaired mitochondrial biogenesis in the brain, which contributes to several neuropathologies. The lipophilic nature of Mdivi‐1 enables the small molecule to penetrate the blood–brain barrier, reaching its peak concentration in brain tissue 4 h after intravenous injection (Cui et al. 2016), and treatment has enabled cytoprotection against neuronal loss following ischemia‐reperfusion injury, diabetes‐induced neuropathy, virus‐sensory neuropathy, and seizures (Qiu et al. 2013; Xie et al. 2013; Zhang et al. 2013b; Zhao et al. 2014; Huang et al. 2015; Cui et al. 2016; Kanda et al. 2016). The neuroprotective effect of Mdivi‐1 is manifest as improved brain hemodynamics and neurological outcome (Zhao et al. 2014; Li et al. 2015a,b; Liu et al. 2015), and the beneficial effects were associated with reduced ROS levels (Qiu et al. 2013; Li et al. 2015a; Kanda et al. 2016), enhanced activity of antioxidant enzymes (Qiu et al. 2013; Li et al. 2015a), preserved mitochondrial function (Huang et al. 2015; Cui et al. 2016), and increased expression of large‐conductance Ca2+ and voltage‐activated K+ channels (Liu et al. 2015).
The therapeutic potential of Mdivi‐1 has also been reported in other organs such as retina (Park et al. 2011), kidney (Tang et al. 2013) and liver (Gonzalez et al. 2014), where in vivo administration of Mdivi‐1 conferred cytoprotection of important cell types in these organs (i.e., retinal ganglion cells, renal tubular epithelial cells, and hepatocytes, respectively). Despite these promising results, many challenges (as discussed in the next section) await before Mdivi‐1 might be suitable for patients.
Future Perspectives
In addition to cell survival, mitochondrial fission plays important roles in related cellular functions such as proliferation and differentiation which are particularly important in organ development. Permanent alterations of mitochondrial dynamics are detrimental, often leading to mitochondrial diseases such as autosomal dominant optical atrophy (heterozygous mutation in Opa1), Charcot‐Marie‐Tooth type 2A neuropathy (heterozygous mutations in Mfn2) and abnormal brain development (A395D mutation in Drp1). The physiological importance of mitochondrial dynamics in the heart has also been demonstrated in various mouse models with genetic deletion of mitochondrial fusion (Mfn1, Mfn2, and Opa1) or fission (Drp1, Mff, and Fis1) proteins, exhibiting developmental cardiac defects and increased susceptibility to cardiac injury (Chan 2012; Babbar and Sheikh 2013). Therefore, pharmacological agents such as Mdivi‐1, that allow timely and reversible manipulation of mitochondrial morphology in different pathological conditions may have therapeutic potential. However, effective application of Mdivi‐1 to manipulate mitochondrial dynamics will require further studies to identify the optimal therapeutic window through a better understanding of the temporal correlation between disease progression and changes in mitochondrial morphology, which can often be disease‐specific.
The divergent effects of Mdivi‐1 on cell survival is likely to be dependent on cell type. While Mdivi‐1 exerts protection of cardiovascular cells and neurons, this small molecule is toxic to hyperproliferative cells such as cancer cells and most immortalized cell lines (Table 1). The differential effects of Mdivi‐1 on cell survival could also be attributed to the duration of treatment. Most in vitro studies showing the cytotoxic effect of Mdivi‐1 were conducted for longer than 16 h of treatment, whereas studies reporting the cytoprotective effect of Mdivi‐1 were performed in much shorter duration (≤8 h treatment) (Table 1). This suggests that chronic inhibition of Drp1 with Mdivi‐1 might well be detrimental to cell function and survival.
Although the precise mechanisms underlying the differential effects of Mdivi‐1 on cell survival remain unclear, Drp1 has been shown to interact with various proteins, such as Cdk1/cyclin B, SUMO1/Sentrin/SMT3 Specific Peptidase 3 (SENP3), Bax/Bak, Noxa, protein kinase A, AMPK, Akt, and Erk2, depending on its posttranslational modification (Chang and Blackstone 2007; Taguchi et al. 2007; Wasiak et al. 2007; Guo et al. 2013; Jheng et al. 2015; Kashatus et al. 2015). In this regard, further investigation of functional outcomes which result from different posttranslational modifications of Drp1 will provide more mechanistic insights on the cytoprotective and cytotoxic effects of Mdivi‐1.
The pharmacokinetics and cytotoxic profile of Mdivi‐1 remain poorly understood. Cui et al. (2016) is the only in vivo pharmacokinetic profile of Mdivi‐1 conducted to date, and they found intraperitoneal administration of Mdivi‐1 at 20 mg/kg resulted in peak plasma and brain concentrations 2 and 4 h later, respectively, with a half‐life estimated at 12 h (Cui et al. 2016). Future studies should also characterize the pharmacokinetics of Mdivi‐1 via intravenous injection, a more common route of drug administration for patients. Furthermore, the toxicological profile of Mdivi‐1 is yet to be fully established. An in vitro study in the HL‐1 cardiac cell line has shown that Mdivi‐1 can inhibit potassium channels which resulted in longer duration and increased firing rate of action potentials, suggesting a potential arrhythmogenic effect of Mdivi‐1 (So et al. 2012). However, the relevance of this observation should be confirmed in primary cardiomyocytes and in vivo by electrophysiological studies of the heart muscle. Future studies should also investigate the physiological effect of the active metabolites of Mdivi‐1 to ensure that they are devoid of undersirable biological effects, as aprerequisite to advance Mdivi‐1 closer to clinical application.
In summary, current preclinical studies have demonstrated therapeutic potential of Mdivi‐1 as a cytoprotective, as well as an anticancer agent. However, many challenges and uncertainties remain to be addressed before such drugs might be applied clinically. The mechanism of action by which Mdivi‐1 affects cell survival also remains unclear. The pharmacokinetics (absorption, distribution, metabolism, and excretion) and toxicology profiles of Mdivi‐1 await further study before clinical translation. Moreover, the lipophilicity (i.e., poor water solubility) of Mdivi‐1 may limit its utility, and new Drp1 inhibitors with better specificity, potency, and solubility are highly desirable.
Disclosures
None declared.
Acknowledgements
This work was supported by grants from the National Health and Medical Research Council of Australia, St Vincent's Hospital (Melbourne) Research Endowment Fund and Stafford Fox Medical Research Foundation. Ayeshah Rosdah is supported by Australia Awards Scholarship. Greg Dusting is a National Health and Medical Research Council Principal Research Fellow. Jessica Holien is a Cure Cancer/Leukaemia Foundation Postgraduate Fellow. The O'Brien Institute Department, St Vincent's Institute of Medical Research, and the Centre for Eye Research Australia receive Operational Infrastructure Support from the Victorian State Government.
Rosdah A. A., Holien J., Delbridge L. M. D., Dusting G. J., Lim S. Y., Mitochondrial fission – a drug target for cytoprotection or cytodestruction?, Pharma Res Per, 4(3), 2016, e00235, doi: 10.1002/prp2.235
References
- Amchenkova AA, Bakeeva LE, Chentsov YS, Skulachev VP, Zorov DB (1988). Coupling membranes as energy‐transmitting cables. I. Filamentous mitochondria in fibroblasts and mitochondrial clusters in cardiomyocytes. J Cell Biol 107: 481–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anmann T, Guzun R, Beraud N, Pelloux S, Kuznetsov AV, Kogerman L, et al. (2006). Different kinetics of the regulation of respiration in permeabilized cardiomyocytes and in HL‐1 cardiac cells: importance of cell structure/organization for respiration regulation. Biochim Biophys Acta 1757: 1597–1606. [DOI] [PubMed] [Google Scholar]
- Babbar M, Sheikh M (2013). Metabolic stress and disorders related to alterations in mitochondrial fission or fusion. Mol Cell Pharmacol 5: 109–133. [PMC free article] [PubMed] [Google Scholar]
- Bajić A, Spasić M, Andjus PR, Savić D, Parabucki A, Nikolić‐Kokić A, et al. (2013). Fluctuating vs. continuous exposure to H2O2: the effects on mitochondrial membrane potential, intracellular calcium, and NF‐κB in astroglia. PLoS ONE 8: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass A, Stejskalova M, Stieglerova A, Ost adal B Samanek M, (2001). Ontogenetic development of energy‐supplying enzymes in rat and guinea‐pig heart. Physiol Res 50: 237–246. [PubMed] [Google Scholar]
- Bentzen BH, Olesen S‐P, Rønn LCB, Grunnet M (2014). BK channel activators and their therapeutic perspectives. Front Physiol 5: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beraud N, Pelloux S, Usson Y, Kuznetsov AV, Ronot X, Tourneur Y, et al. (2009). Mitochondrial dynamics in heart cells: very low amplitude high frequency fluctuations in adult cardiomyocytes and flow motion in non beating HL‐1 cells. J Bioenerg Biomembr 41: 195–214. [DOI] [PubMed] [Google Scholar]
- Bossy B, Petrilli A, Klinglmayr E, Chen J, Lütz‐Meindl U, Knott AB, et al. (2010). S‐nitrosylation of DRP1 does not affect enzymatic activity and is not specific to Alzheimer's disease. J Alzheimers Dis 20: S513–S526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso SM, Rego AC, Penacho N, Oliveira CR (2004). Apoptotic cell death induced by hydrogen peroxide in NT2 parental and mitochondrial DNA depleted cells. Neurochem Int 45: 693–698. [DOI] [PubMed] [Google Scholar]
- Cassidy‐Stone A, Chipuk JE, Ingerman E, Song C, Yoo C, Kuwana T, et al. (2008). Chemical inhibition of the mitochondrial division dynamin reveals its role in Bax/Bak‐dependent mitochondrial outer membrane permeabilization. Dev Cell 14: 193–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan DC (2012). Fusion and fission: interlinked processes critical for mitochondrial health. Annu Rev Genet 46: 265–287. [DOI] [PubMed] [Google Scholar]
- Chang CR, Blackstone C (2007). Cyclic AMP‐dependent protein kinase phosphorylation of Drp1 regulates its GTPase activity and mitochondrial morphology. J Biol Chem 282: 21583–21587. [DOI] [PubMed] [Google Scholar]
- Chen Y, Liu YQ, Dorn GW (2011). Mitochondrial fusion is essential for organelle function and cardiac homeostasis. Circ Res 109: 1327–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho DH, Nakamura T, Fang J, Cieplak P, Godzik A, Gu Z, et al. (2009). S‐nitrosylation of Drp1 mediates β‐amyloid‐related mitochondrial fission and neuronal injury. Science 324: 102–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui M, Tang X, Christian WV, Yoon Y, Tieu K (2010). Perturbations in mitochondrial dynamics induced by human mutant PINK1 can be rescued by the mitochondrial division inhibitor mdivi‐1. J Biol Chem 285: 11740–11752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui M, Ding H, Chen F, Zhao Y, Yang Q, Dong Q (2016). Mdivi‐1 protects against ischemic brain injury via elevating extracellular adenosine in a cAMP/CREB‐CD39‐dependent manner. Mol Neurobiol 53: 240–253. [DOI] [PubMed] [Google Scholar]
- Cunniff B, Wozniak AN, Sweeney P, DeCosta K, Heintz NH (2014). Peroxiredoxin 3 levels regulate a mitochondrial redox setpoint in malignant mesothelioma cells. Redox Biol 3: 79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silva AF, Mariotti FR, Maximo V, Campello S (2014). Mitochondria dynamism: of shape, transport and cell migration. Trends Cell Biol 24: 761–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn GW IIII (2013). Mitochondrial dynamism and cardiac fate: a personal perspective. Circ J 77: 1370–1379. [DOI] [PubMed] [Google Scholar]
- Dorn GW, Kitsis RN (2015). The mitochondrial dynamism‐mitophagy‐cell death interactome multiple roles performed by members of a mitochondrial molecular ensemble. Circ Res 116: 167–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharanei M, Hussain A, Janneh O, Maddock H (2013). Attenuation of doxorubicin‐induced cardiotoxicity by Mdivi‐1: a mitochondrial division/mitophagy inhibitor. PLoS ONE 8: e77713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Givvimani S, Munjal C, Tyagi N, Sen U, Metreveli N, Tyagi SC (2012). Mitochondrial division/mitophagy inhibitor (Mdivi) ameliorates pressure overload induced heart failure. PLoS ONE 7: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez AS, Elguero ME, Finocchietto P, Holod S, Romorini L, Miriuka SG, et al. (2014). Abnormal mitochondrial fusion‐fission balance contributes to the progression of experimental sepsis. Free Radic Res 48: 769–783. [DOI] [PubMed] [Google Scholar]
- Grohm J, Kim S, Mamrak U, Tobaben S, Cassidy‐Stone A, Nunnari J, et al. (2012). Inhibition of Drp1 provides neuroprotection in vitro and in vivo. Cell Death Differ 19: 1446–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo C, Hildick KL, Luo J, Dearden L, Wilkinson KA, Henley JM (2013). SENP3‐mediated deSUMOylation of dynamin‐related protein 1 promotes cell death following ischaemia. EMBO J 32: 1514–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Z, Kutty S, Toth PT, Marsboom G, Hammel JM, Chamberlain C, et al. (2013). Role of dynamin‐related protein 1 (Drp1)‐mediated mitochondrial fission in oxygen sensing and constriction of the ductus arteriosus. Circ Res 112: 802–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Wang Y, Gan X, Fang D, Zhong C, Wu L, et al. (2015). Drp1‐mediated mitochondrial abnormalities link to synaptic injury in diabetes model. Diabetes 64: 1728–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal S, Hood DA (2014). Oxidative stress‐induced mitochondrial fragmentation and movement in skeletal muscle myoblasts. Am J Physiol Cell Physiol 306: C1176–C1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jheng H‐F, Tsai P‐J, Guo S‐M, Kuo L‐H, Chang C‐S, Su I‐J, et al. (2012). Mitochondrial fission contributes to mitochondrial dysfunction and insulin resistance in skeletal muscle. Mol Cell Biol 32: 309–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jheng HF, Huang SH, Kuo HM, Hughes MW, Tsai YS (2015). Molecular insight and pharmacological approaches targeting mitochondrial dynamics in skeletal muscle during obesity. Ann N Y Acad Sci 1350: 82–94. [DOI] [PubMed] [Google Scholar]
- Kanda H, Liu S, Iida T, Yi H, Huang W, Levitt RC, et al. (2016). Inhibition of mitochondrial fission protein reduced mechanical allodynia and suppressed spinal mitochondrial superoxide induced by perineural human immunodeficiency virus gp120 in rats. Anesth Analg 122: 264–272. [DOI] [PubMed] [Google Scholar]
- Kane LA, Youle RJ (2010). Mitochondrial fission and fusion and their roles in the heart. J Mol Med 88: 971–979. [DOI] [PubMed] [Google Scholar]
- Karbowski M, Neutzner A, Youle RJ (2007). The mitochondrial E3 ubiquitin ligase MARCH5 is required for Drp1 dependent mitochondrial division. J Cell Biol 178: 71–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasahara A, Scorrano L (2014). Mitochondria: from cell death executioners to regulators of cell differentiation. Trends Cell Biol 24: 761–770. [DOI] [PubMed] [Google Scholar]
- Kashatus JA, Nascimento A, Myers LJ, Sher A, Byrne FL, Hoehn KL, et al. (2015). Erk2 phosphorylation of Drp1 promotes mitochondrial fission and MAPK‐driven tumor growth. Mol Cell 57: 537–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B, Kim J‐S, Yoon Y, Santiago MC, Brown MD, Park J‐Y (2013). Inhibition of Drp1‐dependent mitochondrial division impairs myogenic differentiation. Am J Physiol Regul Integr Comp Physiol 305: R927–R938. [DOI] [PubMed] [Google Scholar]
- Kulawiak B, Kudin AP, Szewczyk A, Kunz WS (2008). BK channel openers inhibit ROS production of isolated rat brain mitochondria. Exp Neurol 212: 543–547. [DOI] [PubMed] [Google Scholar]
- Kuznetsov AV, Javadov S, Sickinger S, Frotschnig S, Grimm M (2015). H9c2 and HL‐1 cells demonstrate distinct features of energy metabolism, mitochondrial function and sensitivity to hypoxia‐reoxygenation. Biochim Biophys Acta 1853: 276–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Yoon Y (2014). Mitochondrial fission: regulation and ER connection. Mol Cells 37: 89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Jia Z, Cao Y, Wang Y, Li H, Zhang Z, et al. (2015a). Mitochondrial division Iinhibitor 1 ameliorates mitochondrial injury, apoptosis, and motor dysfunction after acute spinal cord injury in rats. Neurochem Res 40: 1379–1392. [DOI] [PubMed] [Google Scholar]
- Li Y, Wang P, Wei J, Fan R, Zuo Y, Shi M, et al. (2015b). Inhibition of Drp1 by Mdivi‐1 attenuates cerebral ischemic injury via inhibition of the mitochondria‐dependent apoptotic pathway after cardiac arrest. Neuroscience 311: 67–74. [DOI] [PubMed] [Google Scholar]
- Lim S, Lee SY, Seo HH, Ham O, Lee C, Park JH, et al. (2015). Regulation of mitochondrial morphology by positive feedback interaction between PKC and Drp1 in vascular smooth muscle cell. J Cell Biochem 116: 648–660. [DOI] [PubMed] [Google Scholar]
- Lin JR, Shen WL, Yan C, Gao PJ (2015). Downregulation of dynamin‐related protein 1 contributes to impaired autophagic flux and angiogenic function in senescent endothelial cells. Arterioscler Thromb Vasc Biol 35: 1413–1422. [DOI] [PubMed] [Google Scholar]
- Liu J‐M, Yi Z, Liu S‐Z, Chang J‐H, Dang X‐B, Li Q‐Y, et al. (2015). The mitochondrial division inhibitor mdivi‐1 attenuates spinal cord ischemia‐reperfusion injury both in vitro and in vivo: involvement of BK channels. Brain Res 1619: 155–165. [DOI] [PubMed] [Google Scholar]
- Marsboom G, Toth PT, Ryan JJ, Hong Z, Wu X, Fang YH, et al. (2012). Dynamin‐related protein 1–mediated mitochondrial mitotic fission permits hyperproliferation of vascular smooth muscle cells and offers a novel therapeutic target in pulmonary hypertension. Circ Res 110: 1484–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milerova M, Charvatova Z, Skarka L, Ostadalova I, Drahota Z, Fialova M, et al. (2010). Neonatal cardiac mitochondria and ischemia/reperfusion injury. Mol Cell Biochem 335: 147–153. [DOI] [PubMed] [Google Scholar]
- Monge C, Beraud N, Tepp K, Pelloux S, Chahboun S, Kaambre T, et al. (2009). Comparative analysis of the bioenergetics of adult cardiomyocytes and nonbeating HL‐1 cells: respiratory chain activities, glycolytic enzyme profiles, and metabolic fluxes. Can J Physiol Pharmacol 87: 318–326. [DOI] [PubMed] [Google Scholar]
- Ong SB, Subrayan S, Lim SY, Yellon DM, Davidson SM, Hausenloy DJ (2010). Inhibiting mitochondrial fission protects the heart against ischemia/reperfusion injury. Circulation 121: 2012–2022. [DOI] [PubMed] [Google Scholar]
- Ong S‐B, Hall AR, Dongworth RK, Kalkhoran S, Pyakurel A, Scorrano L, et al. (2015). Akt protects the heart against ischaemia‐reperfusion injury by modulating mitochondrial morphology. Thromb Haemost 113: 513–521. [DOI] [PubMed] [Google Scholar]
- Otera H, Mihara K (2012). Mitochondrial dynamics: functional link with apoptosis. Int J Cell Biol 2012: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer CS, Osellame LD, Stojanovski D, Ryan MT (2011). The regulation of mitochondrial morphology: intricate mechanisms and dynamic machinery. Cell Signal 23: 1534–1545. [DOI] [PubMed] [Google Scholar]
- Park SW, Kim KY, Lindsey JD, Dai Y, Heo H, Nguyen DH, et al. (2011). A selective inhibitor of drp1, mdivi‐1, increases retinal ganglion cell survival in acute ischemic mouse retina. Invest Ophthalmol Vis Sci 52: 2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piquereau J, Caffin F, Novotova M, Lemaire C, Veksler V, Garnier A, et al. (2013). Mitochondrial dynamics in the adult cardiomyocytes: which roles for a highly specialized cell? Front Physiol 4: 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi X, Disatnik M‐H, Shen N, Sobel RA, Mochly‐Rosen D (2011). Aberrant mitochondrial fission in neurons induced by protein kinase Cδ under oxidative stress conditions in vivo. Mol Biol Cell 22: 256–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi X, Qvit N, Su Y‐C, Mochly‐Rosen D (2013). A novel Drp1 inhibitor diminishes aberrant mitochondrial fission and neurotoxicity. J Cell Sci 126: 789–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian W, Choi S, Gibson GA, Watkins SC, Bakkenist CJ, Van Houten B (2012). Mitochondrial hyperfusion induced by loss of the fission protein Drp1 causes ATM‐dependent G2/M arrest and aneuploidy through DNA replication stress. J Cell Sci 125: 5745–5757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian W, Wang J, Roginskaya V, McDermott LA, Edwards RP, Stolz DB, et al. (2014). Novel combination of mitochondrial division inhibitor 1 (mdivi‐1) and platinum agents produces synergistic pro‐apoptotic effect in drug resistant tumor cells. Oncotarget 5: 4180–4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian W, Salamoun J, Wang J, Roginskaya V, Van Houten B, Wipf P (2015). The combination of thioxodihydroquinazolinones and platinum drugs reverses platinum resistance in tumor cells by inducing mitochondrial apoptosis independent of Bax and Bak. Bioorg Med Chem Lett 25: 856–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu X, Cao L, Yang X, Zhao X, Liu X, Han Y, et al. (2013). Role of mitochondrial fission in neuronal injury in pilocarpine‐induced epileptic rats. Neuroscience 245: 157–165. [DOI] [PubMed] [Google Scholar]
- Rehman J, Zhang HJ, Toth PT, Zhang Y, Marsboom G, Hong Z, et al. (2012). Inhibition of mitochondrial fission prevents cell cycle progression in lung cancer. FASEB J 26: 2175–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp WW, Fang YH, Han M, Zhang HJ, Hong Z, Banathy A, et al. (2014). Dynamin‐related protein 1 (Drp1)‐mediated diastolic dysfunction in myocardial ischemia‐reperfusion injury: therapeutic benefits of Drp1 inhibition to reduce mitochondrial fission. FASEB J 28: 316–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp WW, Beiser DG, Fang YH, Han M, Piao L, Varughese J, et al. (2015). Inhibition of the mitochondrial fission protein dynamin‐related protein 1 improves survival in a murine cardiac arrest model. Crit Care Med 43: e38–e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin HW, Takatsu H, Mukai H, Munekata E, Murakami K, Nakayama K (1999). Intermolecular and interdomain interactions of a dynamin‐related GTP‐binding protein, Dnm1p/Vps1p‐like protein. J Biol Chem 274: 2780–2785. [DOI] [PubMed] [Google Scholar]
- So EC, Hsing CH, Liang CH, Wu SN (2012). The actions of mdivi‐1, an inhibitor of mitochondrial fission, on rapidly activating delayed‐rectifier K⁺ current and membrane potential in HL‐1 murine atrial cardiomyocytes. Eur J Pharmacol 683: 1–9. [DOI] [PubMed] [Google Scholar]
- Solesio ME, Saez‐Atienzar S, Jordan J, Galindo MF (2013). 3‐Nitropropionic acid induces autophagy by forming mitochondrial permeability transition pores rather than activating the mitochondrial fission pathway. Br J Pharmacol 168: 63–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki‐Karasaki Y, Fujiwara K, Saito K, Suzuki‐Karasaki M, Ochiai T, Soma M (2015). Distinct effects of TRAIL on the mitochondrial network in human cancer cells and normal cells: role of plasma membrane depolarization. Oncotarget 6: 21572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi N, Ishihara N, Jofuku A, Oka T, Mihara K (2007). Mitotic phosphorylation of dynamin‐related GTPase Drp1 participates in mitochondrial fission. J Biol Chem 282: 11521–11529. [DOI] [PubMed] [Google Scholar]
- Tang WX, Wu WH, Qiu HY, Bo H, Huang SM (2013). Amelioration of rhabdomyolysis‐induced renal mitochondrial injury and apoptosis through suppression of Drp‐1 translocation. J Nephrol 26: 1073–1082. [DOI] [PubMed] [Google Scholar]
- Tian Y, Li B, Shi W, Chang M, Zhang G, Di Z, et al. (2014). Dynamin‐related protein 1 inhibitors protect against ischemic toxicity through attenuating mitochondrial Ca2 + uptake from endoplasmic reticulum store in PC12 cells. Int J Mol Sci 15: 3172–3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troncoso R, Paredes F, Parra V, Gatica D, Vásquez‐Trincado C, Quiroga C, et al. (2014). Dexamethasone‐induced autophagy mediates muscle atrophy through mitochondrial clearance. Cell Cycle 13: 2281–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twaroski DM, Yan Y, Zaja I, Clark E, Bosnjak ZJ, Bai X (2015). Altered mitochondrial dynamics contributes to propofol‐induced cell death in human stem cell–derived neurons. Anesthesiology 123: 1067–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Wang P, Li S, Wang S, Li Y, Liang N, et al. (2014). Mdivi‐1 prevents apoptosis induced by ischemia–reperfusion injury in primary hippocampal cells via inhibition of reactive oxygen species–activated mitochondrial pathway. J Stroke Cerebrovasc Dis 23: 1491–1499. [DOI] [PubMed] [Google Scholar]
- Wang J, Hansen K, Edwards R, Van Houten B, Qian W (2015a). Mitochondrial division inhibitor 1 (mdivi‐1) enhances death receptor‐mediated apoptosis in human ovarian cancer cells. Biochem Biophys Res Commun 456: 7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Li J, Santana‐Santos L, Shuda M, Sobol RW, Van Houten B, et al. (2015b). A novel strategy for targeted killing of tumor cells: induction of multipolar acentrosomal mitotic spindles with a quinazolinone derivative mdivi‐1. Mol Oncol 9: 488–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasiak S, Zunino R, McBride HM (2007). Bax/Bak promote sumoylation of DRP1 and its stable association with mitochondria during apoptotic cell death. J Cell Biol 177: 439–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenger J, Klinglmayr E, Frohlich C, Eibl C, Gimeno A, Hessenberger M, et al. (2013). Functional mapping of human dynamin‐1‐like GTPase domain based on x‐ray structure analyses. PLoS ONE 8: e71835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Xia SX, Li QQ, Gao Y, Shen X, Ma L, et al. (2016). Mitochondrial division inhibitor 1 (Mdivi‐1) offers neuroprotection through diminishing cell death and improving functional outcome in a mouse model of traumatic brain injury. Brain Res 1630: 134–143. [DOI] [PubMed] [Google Scholar]
- Xie N, Wang C, Lian Y, Zhang H, Wu C, Zhang Q (2013). A selective inhibitor of Drp1, mdivi‐1, protects against cell death of hippocampal neurons in pilocarpine‐induced seizures in rats. Neurosci Lett 545: 64–68. [DOI] [PubMed] [Google Scholar]
- Xie Q, Wu Q, Horbinski CM, Flavahan WA, Yang K, Zhou W, et al. (2015). Mitochondrial control by DRP1 in brain tumor initiating cells. Nat Neurosci 18: 501–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie N, Wang C, Wu C, Cheng X, Gao Y, Zhang H, et al. (2016). Mdivi‐1 protects epileptic hippocampal neurons from apoptosis via inhibiting oxidative stress and endoplasmic reticulum stress in vitro. Neurochem Res DOI 10.1007/s11064‐016‐1835‐y. [DOI] [PubMed] [Google Scholar]
- Xu W, Liu Y, Wang S, McDonald T, Van Eyk JE, Sidor A, et al. (2002). Cytoprotective role of Ca2 + ‐activated K+ channels in the cardiac inner mitochondrial membrane. Science 298: 1029–1033. [DOI] [PubMed] [Google Scholar]
- Zhang B, Davidson MM, Zhou H, Wang C, Walker WF, Hei TK (2013a). Cytoplasmic irradiation results in mitochondrial dysfunction and DRP1‐dependent mitochondrial fission. Cancer Res 73: 6700–6710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Wang S, Li Y, Che L, Zhao Q (2013b). A selective inhibitor of Drp1, mdivi‐1, acts against cerebral ischemia/reperfusion injury via an anti‐apoptotic pathway in rats. Neurosci Lett 535: 104–109. [DOI] [PubMed] [Google Scholar]
- Zhang X, Yan H, Yuan Y, Gao J, Shen Z, Cheng Y, et al. (2013c). Cerebral ischemia‐reperfusion‐induced autophagy protects against neuronal injury by mitochondrial clearance. Autophagy 9: 1321–1333. [DOI] [PubMed] [Google Scholar]
- Zhao YX, Cui M, Chen SF, Dong Q, Liu XY (2014). Amelioration of ischemic mitochondrial injury and Bax‐dependent outer membrane permeabilization by Mdivi‐1. CNS Neurosci Ther 20: 528–538. [DOI] [PMC free article] [PubMed] [Google Scholar]


