Abstract
Fasiglifam (TAK‐875) is a free fatty acid receptor 1 (FFAR1)/G‐protein–coupled receptor 40 (GPR40) agonist that improves glycemic control in type 2 diabetes with minimum risk of hypoglycemia. Fasiglifam potentiates glucose‐stimulated insulin secretion (GSIS) from pancreatic β‐cells glucose dependently, although the precise mechanism underlying the glucose dependency still remains unknown. Here, we investigated key cross‐talk between the GSIS pathway and FFAR1 signaling, and Ca2+ dynamics using mouse insulinoma MIN6 cells. We demonstrated that the glucose‐dependent insulinotropic effect of fasiglifam required membrane depolarization and that fasiglifam induced a glucose‐dependent increase in intracellular Ca2+ level and amplification of Ca2+ oscillations. This differed from the sulfonylurea glimepiride that induced changes in Ca2+ dynamics glucose independently. Stimulation with cell‐permeable analogs of IP3 or diacylglycerol (DAG), downstream second messengers of Gαq‐FFAR1, augmented GSIS similar to fasiglifam, indicating their individual roles in the potentiation of GSIS pathway. Intriguingly, the IP3 analog triggered similar Ca2+ dynamics to fasiglifam, whereas the DAG analog had no effect. Despite the lack of an effect on Ca2+ dynamics, the DAG analog elicited synergistic effects on insulin secretion with Ca2+ influx evoked by an L‐type voltage‐dependent calcium channel opener that mimics glucose‐dependent Ca2+ dynamics. These results indicate that the Gαq signaling activated by fasiglifam enhances GSIS pathway via dual potentiating mechanisms in which IP3 amplifies glucose‐induced Ca2+ oscillations and DAG/protein kinase C (PKC) augments downstream secretory mechanisms independent of Ca2+ oscillations.
Keywords: Ca2+ oscillation, fasiglifam (TAK‐875), FFAR1/GPR40, glucose‐stimulated insulin secretion (GSIS), type 2 diabetes mellitus (T2DM)
Abbreviations
- AM
acetoxymethyl
- AUC
area under the curve
- Bt3IP3/AM
D‐myo‐inositol 1,3,5‐trisphosphate hexakisacetoxymethyl ester, 2,4,6‐tri‐O‐butyryl
- DAG
diacylglycerol
- DPP‐4
dipeptidyl peptidase 4
- ER
endoplasmic reticulum
- FFAs
free fatty acids
- FFAR1
free fatty acid receptor 1
- FFT
fast Fourier transform
- γ‐LA
γ‐linolenic acid
- GLP‐1
glucagon‐like peptide‐1
- GPR40
G‐protein–coupled receptor 40
- GSIS
glucose‐stimulated insulin secretion
- IP3
inositol 1,4,5‐triphosphate
- KATP
ATP‐sensitive potassium channel
- KRBH
Krebs‐Ringer‐bicarbonate‐HEPES
- OAG
1‐oleoyl‐2‐acetyl‐sn‐glycerol
- PKC
protein kinase C
- PKD
protein kinase D
- PSD
power spectrum density
- qRT‐PCR
quantitative real‐time PCR
- SGLT‐2
sodium/glucose cotransporter‐2
- SU
sulfonylurea
- TRPM5
transient receptor potential cation channel, subfamily M, member 5
- T2DM
type 2 diabetes
- VDCC
voltage‐dependent calcium channel
Introduction
Type 2 diabetes mellitus (T2DM) is a chronic and progressive disease with two major causes; insulin resistance and pancreatic β‐cell dysfunction. Several drug classes have been developed for the treatment of T2DM including sulfonylureas (SUs), biguanides (i.e., metformin), α‐glucosidase inhibitors, glitazones, dipeptidyl peptidase‐4 (DPP‐4) inhibitors, glucagon‐like peptide‐1 (GLP‐1) analogs, and recently approved sodium/glucose cotransporter‐2 (SGLT‐2) inhibitors (Inzucchi et al. 2012; American Diabetes Association, 2015). Among these drugs, SUs are utilized frequently after initial lifestyle intervention and metformin therapy. However, the use of SUs is accompanied by an inherent risk of uncontrollable hypoglycemia because they stimulate insulin secretion independently of the blood glucose concentration. DPP‐4 inhibitors and GLP‐1 analogs can overcome this drawback of SUs, but they are unable to achieve sufficient blood glucose control in many patients (American Diabetes Association, 2015). Therefore, a novel drug with less risk of inducing hypoglycemia is desirable.
Fasiglifam (TAK‐875) is an orally absorbed novel antidiabetic agent that enhances insulin secretion in a glucose‐dependent manner (Negoro et al. 2010, 2012). Fasiglifam is an ago‐allosteric modulator of free fatty acid receptor 1 (FFAR1; also known as G‐protein–coupled receptor 40, (GPR40)) and potentiates insulin secretion in synergy with FFAs (Yabuki et al. 2013), supported by the result of the X‐ray cocrystal structure demonstrating the unique binding of fasiglifam on FFAR1 (Srivastava et al. 2014). Both the preclinical and clinical studies achieved the improved glycemic control in rodents and human patients with a minimal risk of hypoglycemia (Tsujihata et al. 2011; Burant et al. 2012; Kaku et al. 2015). Although such a satisfactory benefit–risk profile of fasiglifam is brought by its strict glucose‐dependent action on insulin release, the underlying mechanism of the dependence remains unknown.
FFAR1 couples mainly with Gαq protein, and the resulting signal cascade is important for insulin secretion, as is the case with muscarinic receptor subtype M3 (Gilon and Henquin 2001; Sassmann et al. 2010). Inositol 1,4,5‐triphosphate (IP3) and diacylglycerol (DAG) are well‐known second messengers that activate different signaling pathways; that is, IP3 interacts with its receptor on the endoplasmic reticulum (ER) increasing the intracellular Ca2+ concentration, whereas DAG activates protein kinase C (PKC). But their individual contribution and interdependence in potentiating insulin secretion are not fully described.
In this study, we investigated interactions between Gαq signaling activated by fasiglifam and insulin secretory pathways, focusing on insulin release and Ca2+ oscillations. Pancreatic β‐cells exhibit Ca2+ oscillations of variable frequency and amplitude, triggering pulsatile insulin release (Liu et al. 1998; Nunemaker et al. 2006). We thus compared different oscillatory patterns when stimulated with fasiglifam and a SU glimepiride by decoding the signals using fast Fourier transforms (FFT). We previously reported that IP3 production by fasiglifam was not dependent on glucose concentration but intracellular Ca2+ concentration was tangibly glucose dependent (Tsujihata et al. 2011), thereby extending the efficacy of IP3 on insulin secretion and Ca2+ oscillations using cell‐permeable IP3 analog. Also, roles of DAG on insulin secretion and Ca2+ dynamics are examined by DAG analog combined with Ca2+ influx using an L‐type voltage‐dependent calcium channels (VDCC) opener.
Materials and Methods
Reagents
Fasiglifam
([(3S)‐6‐({2′,6′‐dimethyl‐4′‐[3‐(methylsulfonyl)propoxy]biphenyl‐3‐yl}methoxy)‐2,3‐dihydro‐1‐benzofuran‐3‐yl]acetic acid hemihydrate) was synthesized at Takeda Pharmaceutical Company (Osaka, Japan). Diazoxide, KCl, and glimepiride were purchased from Wako Pure Chemical Industries (Osaka, Japan). Sterile D‐(+)‐glucose solution (45% in water), γ‐linolenic acid (γ‐LA), and (S)‐(‐)‐Bay K8644 were purchased from Sigma‐Aldrich (St. Louis, MO). D‐myo‐inositol 1,3,5‐trisphosphate hexakisacetoxymethyl ester, 2,4,6‐tri‐O‐butyryl (Bt3IP3), a cell‐permeable derivative of D‐myo‐inositol 1,3,5‐trisphosphate, was purchased from Merck Millipore (Tokyo, Japan). A cell‐permeable analog of DAG, 1‐oleoyl‐2‐acetyl‐sn‐glycerol (OAG), was purchased from Cayman Chemical (Ann Arbor, MI). Nifedipine was purchased from MP Biomedicals (Tokyo, Japan). Krebs‐Ringer‐bicarbonate‐HEPES (KRBH) buffer (116 mmol/L NaCl, 4.7 mmol/L KCl, 1.17 mmol/L KH2PO4, 1.17 mmol/L MgSO4, 25 mmol/L NaHCO3, 2.52 mmol/L CaCl2, and 24 mmol/L HEPES (pH 7.4)) containing 0.2% bovine serum albumin (BSA) was freshly prepared before each experiment. Fura‐2 acetoxymethyl ester was purchased from Dojindo Laboratories (Kumamoto, Japan).
Cell lines
MIN6 cells (Miyazaki et al. 1990) were provided by Dr. Miyazaki (Osaka University) and grown in Dulbecco's Modified Eagle Medium containing 10% heat‐inactivated fetal bovine serum, 100 IU/mL penicillin, 100 μg/mL streptomycin, and 55 μmol/L 2‐mercaptoethanol (Life Technologies, Tokyo, Japan). Cells were cultured at 37°C in a humidified atmosphere containing 5% CO2/95% air.
Insulin secretion assay
MIN6 cells were seeded at a density of 6 × 104 cells/well in 96‐well plates and cultured for 2 days before each experiment. After the medium was discarded, cells were preincubated for 2 h at 37°C with KRBH buffer containing 0.2% BSA and 1 mmol/L glucose. The preincubation buffer was then discarded and KRBH‐containing 0.2% BSA, various concentrations of glucose, and stimulators/inhibitors as shown were added. The plate was then incubated for 2 h at 37°C. Supernatants from each well were collected and the insulin concentration was measured using AlphaLISA Human Insulin Immunoassay kit (PerkinElmer, Waltham, MA) following the manufacturer's instructions.
Ca2+ imaging assay
MIN6 cells were seeded at a density of 1.5 × 105 cells/well in an eight‐well Nunc Lab‐Tek Chamber Slide System (Thermo Scientific, Tokyo, Japan), and cultured for 3–5 days. After removing the medium, cells were loaded with 5 μmol/L fura‐2 acetoxymethyl (AM) ester in 1× Hank's Balanced Salt Solution (prepared in our laboratory according to standard protocols, and supplemented with various concentrations of glucose and 20 mmol/L HEPES buffer) for 45 min at 37°C, and washed three times with Hank's Balanced Salt Solution. Chambers were then mounted on the microscope stage (Eclipse Ti, Nikon, Tokyo, Japan). Cells were imaged for 5 min with an Aquacosmos ratio imaging system (Hamamatsu, Shizuoka, Japan). Ligand was added at 2.5 min after recording started. Using excitation wavelengths of 340 and 380 nm, changes in fluorescence intensity of fura‐2 AM were acquired every 0.5 sec at 535 nm. The fluorescence intensity ratios (340/380 nm) were calculated using Aquacosmos software (Hamamatsu, Shizuoka, Japan). Changes in the intracellular Ca2+ level were determined using the ratio of the area under the curve (AUC) between 1 min before and 1 min after ligand stimulation (“Post/Pre”). For spectral analysis, FFT was used to change the time unit to frequency. To obtain the power spectrum density (PSD), the Fourier transform was squared and normalized by dividing by N (128 time points). These methods were modified from a previous report (Uhlen 2004).
Data analysis and statistics
To determine statistical significance for potentiation, Welch's t‐test or ANOVA followed by Dunnett's multiple‐comparison test were performed at the significance level of P < 0.05. To show synergistic interaction between two factors, we conducted a two‐way ANOVA with a significance level of P < 0.05 followed by a post hoc test using Williams’ test. To test the dose–response relationships, Bartlett's test was conducted at P < 0.05 and Williams or Shirley–Williams tests were conducted at the one‐tailed significance level of P < 0.025. All statistical analyzes were performed using Statistical Analysis System version 9.3 (SAS Institute, Cary, NC).
Results
Membrane depolarization of β‐cells is necessary for the insulinotropic effect of fasiglifam
Membrane depolarization is a fundamental event in the integration of excitatory signals during glucose‐induced insulin secretion (MacDonald and Rorsman 2006; Drews et al. 2010). Thus, we examined whether the glucose‐dependent insulinotropic effect of fasiglifam was affected by the KATP channel opener diazoxide or exogenous high K+ that prevents or induces membrane depolarization. In the presence of 100 μmol/L diazoxide, the potentiating effect of fasiglifam on glucose‐stimulated insulin secretion (GSIS) was completely abolished in MIN6 cells (Fig. 1A). In contrast, in the presence of 10 mmol/L KCl without glucose, fasiglifam augmented insulin secretion (Fig. 1B). These results reinforce the significance of membrane depolarization for the insulinotropic action of fasiglifam.
Figure 1.
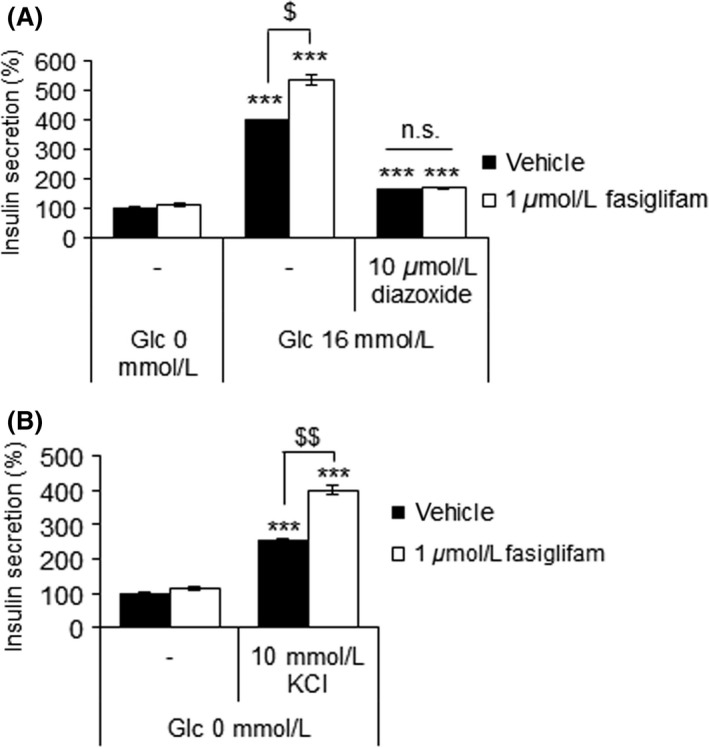
Membrane depolarization is essential and sufficient for fasiglifam to exert its insulinotropic effect. (A) Effects of diazoxide on the glucose‐stimulated insulin secretion (GSIS)‐potentiating effect of fasiglifam in mouse MIN6 cells. Data are normalized to the vehicle (0 mmol/L glucose) group and shown as means ± SEM (n = 3). ***P < 0.001 versus 0 mmol/L glucose by Dunnett's test. $ P < 0.05 by Welch's t‐test. (B) Effect of fasiglifam on KCl‐induced depolarization‐mediated insulin secretion in MIN6 cells. Data are normalized to the vehicle (no KCl) group and shown as means ± SEM (n = 3). ***P < 0.001 versus vehicle group by Dunnett's test. $$ P < 0.01 by Welch's t‐test.
Both the IP3/Ca2+ and DAG/PKC pathways downstream of FFAR1 contribute to the insulinotropic effect of fasiglifam
The roles of second messengers downstream of FFAR1 on the insulin potentiating effects of fasiglifam were examined. Consistent with our previous reports (Tsujihata et al. 2011; Yabuki et al. 2013), fasiglifam, alone and in combination with γ‐LA, enhanced GSIS in MIN6 cells in a glucose‐dependent manner (Fig. 2A). Because stimulation of Gαq‐coupled FFAR1 activates the IP3/Ca2+ and DAG/PKC pathways, we next investigated the roles of these two signaling branches on the potentiation of GSIS. Both Bt3IP3/AM and OAG augmented GSIS (Fig. 2B and C), indicating that two distinct pathways could play a role in the potentiation of insulin secretion.
Figure 2.
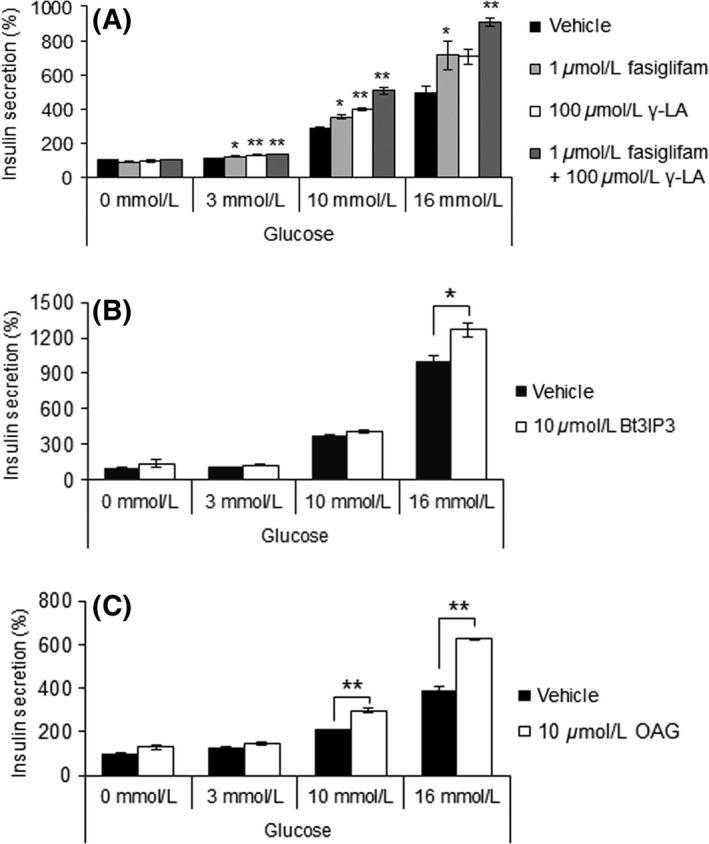
Both IP3‐mediated Ca2+ release from ER, and protein kinase C (PKC) activation downstream of FFAR1 contribute to the glucose‐stimulated insulin secretion (GSIS) potentiating effect of fasiglifam. (A) Potentiating effects of fasiglifam and γ‐linolenic acid on GSIS in MIN6 cells. Data are normalized to the vehicle (0 mmol/L glucose) group and shown as means ± SEM (n = 3). **P < 0.01, *P < 0.05, by Dunnett's test. (B) Effect of IP3 receptor activation by Bt3IP3 on GSIS in MIN6 cells. Data are normalized to the vehicle (0 mmol/L glucose) group and shown as means ± SEM (n = 3). Two‐way ANOVA: main effect of glucose: P < 0.01; main effect of Bt3IP3: P < 0.01; interaction of glucose and Bt3IP3: P < 0.01. *P < 0.05 by Welch's t‐test. (C) Effect of PKC activation by OAG on GSIS in MIN6 cells. Data are normalized to the vehicle (0 mmol/L glucose group) and shown as means ± SEM (n = 3). Two‐way ANOVA: main effect of glucose: P < 0.01; main effect of OAG: P < 0.01; interaction of glucose and OAG: P < 0.01. **P < 0.01 by Welch's t‐test.
Fasiglifam induces a glucose‐dependent increase in intracellular Ca2+ and amplified Ca2+ oscillations
Ionic or action potential dynamics are determinants of pulsatile insulin secretion (Bergsten 1995; MacDonald and Rorsman 2006). Thus, we investigated the effect of fasiglifam on Ca2+ dynamics. Addition of fasiglifam elevated the intracellular Ca2+ level in MIN6 cells in a glucose‐dependent manner (Fig. 3A and B). In addition, fasiglifam enhanced the amplitude of Ca2+ oscillation waves in a glucose‐dependent manner (Fig. 3C). Differences in bandwidths of Ca2+ waves affect the distinct phenotype of β‐cells; that is, short bandwidths couple mainly to β‐cells metabolism such as glycolytic activity, whereas long bandwidths couple to ion channel activity required for Ca2+‐triggered exocytosis (Liu et al. 1998; Bergsten 2002). Moreover, some genes, such as those coding for the transient receptor potential cation channel, subfamily M, member 5 (TRPM5), and the β3 subunit of L‐type VDCC, have been linked to certain oscillatory bandwidths (Berggren et al. 2004; Colsoul et al. 2010). Therefore, we analyzed the variability in Ca2+ waves using FFT by subdividing them into three bandwidths (~100, ~200, and ~1000 mHz). The results showed that fasiglifam affected the amplitude modulation in all three bandwidths, and had no effects on the modulation of certain frequencies (Fig. 3C). We then addressed the effects of glimepiride that augments insulin secretion independently of glucose, on intracellular Ca2+ dynamics. Intriguingly, glimepiride induced robust increases in Ca2+ levels and oscillations irrespective of the glucose concentration (Fig. 3D–F). These results clearly demonstrated that the glucose‐dependent regulation of intracellular Ca2+ by fasiglifam was distinct from glimepiride.
Figure 3.

Effect of fasiglifam and glimepiride on glucose‐dependent and ‐independent increases in intracellular Ca2+ level and Ca2+ oscillations. Ca2+ dynamics were determined in MIN6 cells loaded with fura‐2 AM and stimulated with (A–C) fasiglifam or (D–E) glimepiride. (A), (D) Representative traces in 21 regions of interest in one experiment at the indicated glucose concentrations. (B), (E) Changes in the intracellular Ca2+ response quantified by the area under the curve 1 min before and 1 min after stimulation. Data are presented as means ± SEM of 63 regions of interest within three experiments. (C), (F) Power spectrum density profiles describing the power of the oscillations at different frequencies generated by fast Fourier transform. Power is proportional to amplitude squared. The results were subclassified into three groups: low (1–100 mHz), middle (101–200 mHz), and high (201–1000 mHz). The Nyquist frequency in these experiments is about 1000 mHz. Data are expressed as means ± SEM of 63 regions of interest within three experiments. #P < 0.025 versus 0 mmol/L glucose by one‐tailed Williams’ test. *P < 0.05, **P < 0.01 by Welch's t‐test.
An IP3 analog enhances Ca2+ oscillations similar to fasiglifam, whereas a DAG analog has no effect
Intracellular Ca2+ dynamics in pancreatic β‐cells are regulated by two main sources: (1) Ca2+ release from intracellular Ca2+ stores, and (2) extracellular Ca2+ influx through Ca2+ channels. Both sources can occur in response to an extracellular stimulus. IP3, a downstream messenger of FFAR1, elicits Ca2+ release from the ER (Gwiazda et al. 2009). To investigate the role of IP3 on Ca2+ dynamics, we monitored the effect of its analog, Bt3IP3/AM, on MIN6 cells. Bt3IP3/AM augmented Ca2+ dynamics in a glucose‐dependent way, similar to fasiglifam (Fig. 4A–C). These results demonstrate a critical role of the IP3/Ca2+ pathway in increasing intracellular Ca2+ levels and enhancing the amplitude of Ca2+ oscillations, both of which may contribute to enhanced insulin secretion.
Figure 4.
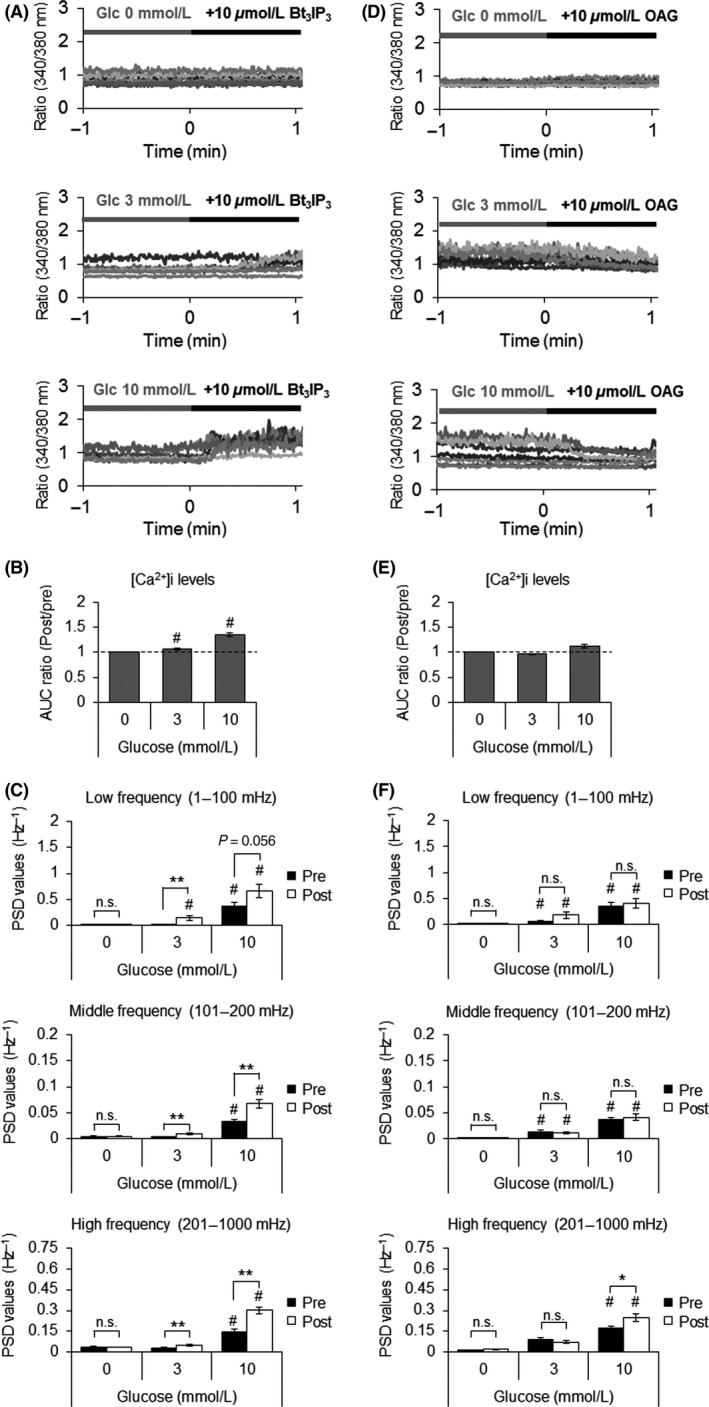
Effects of Bt3IP3 and OAG on glucose‐dependent intracellular Ca2+ dynamics. Detailed analyzes of Ca2+ dynamics were performed in MIN6 cells loaded with fura‐2 AM and stimulated with (A–C) Bt3IP3 or (D–E) OAG. (A), (D) Representative traces in 21 regions of interest in one experiment at the indicated glucose concentrations. (B), (E) Changes in the intracellular Ca2+ response quantified by the area under the curve 1 min before and 1 min after stimulation. Data are presented as means ± SEM of 63 regions of interest within three experiments. (C), (F) Power spectrum density profiles describing the power of the oscillations at different frequencies generated by fast Fourier transform. The mathematical conditions are the same as in Figure 3. Data are expressed as means ± SEM of 63 regions of interest within three experiments. # P < 0.025 versus 0 mmol/L glucose by one‐tailed Williams’ test. *P < 0.05, **P < 0.01 by Welch's t‐test.
The effect of the DAG/PKC pathway on Ca2+ dynamics was assessed by using the DAG analog, OAG. In contrast to fasiglifam and Bt3IP3/AM, OAG did not affect either the intracellular Ca2+ concentration or Ca2+ oscillations (Fig. 4, D–F). Because OAG augmented GSIS glucose dependently (Fig. 2C), the DAG/PKC pathway may potentiate GSIS by mechanisms independent of intracellular Ca2+.
Ca2+ influx and PKC activation elicit synergistic effects on insulin secretion with and without glucose
Our results indicated that IP3‐mediated Ca2+ release from ER downstream of FFAR1 acted as an amplifier of glucose‐induced Ca2+ oscillations, and that the effect of IP3 alone on GSIS was less potent than the effect of fasiglifam (Figs. 2B and 3). We also found that PKC activated by DAG could affect insulin secretion independent of Ca2+ dynamics (Fig. 4). We next investigated the importance of extracellular Ca2+ influx via an L‐type VDCC. The glucose‐dependent insulinotropic effect of fasiglifam in MIN6 cells was partially attenuated by 10 μmol/L nifedipine, an L‐type VDCC blocker (Fig. 5A). This result implied that Ca2+ released from the ER, as well as Ca2+ coming from extracellular fluid through opened‐phase L‐type VDCC, might be critical for insulin secretion evoked by fasiglifam. This hypothesis was further studied by using (S)‐(‐)‐Bay K 8644, an L‐type VDCC opener, and examining its interaction with OAG on insulin secretion in the absence of glucose. Although there was only a slight increase in insulin secretion with either (S)‐(‐)‐Bay K 8644 or OAG alone, coadministration provoked a synergistic increase even in the absence of glucose (Fig. 5B and C). This result indicated that both elevated Ca2+ influx via opened‐phase L‐type VDCC, and PKC activation, may be critical for the overall insulinotropic effects of fasiglifam.
Figure 5.
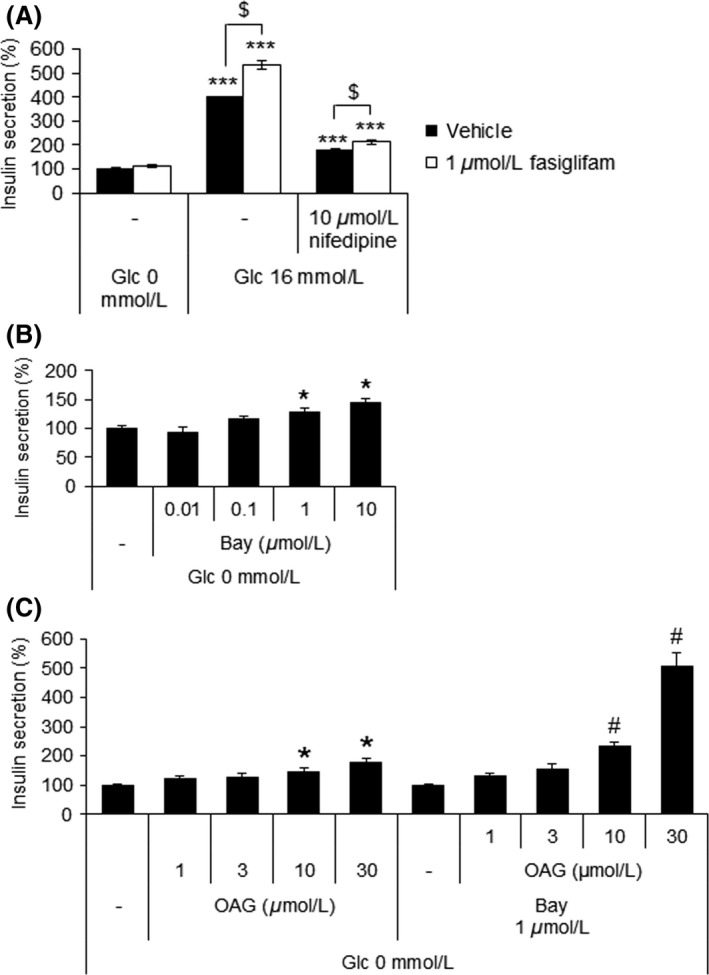
Synergistic effects on insulin secretion elicited by the induction of Ca2+ influx and activation of protein kinase C. (A) Effects of nifedipine on the glucose‐stimulated insulin secretion potentiating effect of fasiglifam. Data are normalized to the vehicle (0 mmol/L glucose) group and shown as means ± SEM (n = 3). ***P < 0.001 versus 0 mmol/L glucose by Dunnett's test, $ P < 0.05 by Welch's t‐test. (B) Dose–response effect of (S)‐(‐)‐Bay K 8644 (Bay) on insulin secretion in the absence of glucose in MIN6 cells. Data are normalized to the vehicle group and shown as means ± SEM (n = 3). *P < 0.025 versus vehicle by one‐tailed Williams’ test. (C) Dose–response effect of OAG, with and without 1 μmol/L Bay, on insulin secretion in the absence of glucose in MIN6 cells. Data are normalized to the vehicle (neither OAG nor Bay) and shown as means ± SEM (n = 3). *P < 0.025 versus vehicle by one‐tailed Williams’ test. # P < 0.025 versus 1 μmol/L Bay alone by one‐tailed Williams’ test. Two‐way ANOVA: main effect of OAG: P < 0.01; main effect of Bay: P < 0.01; interaction of OAG and Bay: P < 0.01.
Discussion
Pancreatic β‐cells are equipped with various ion channels, pumps, transporters, and intracellular machinery to transduce nutrient signals into orchestrated responses that trigger insulin secretion (Rorsman and Braun 2013; Gilon et al. 2014). In the case of glucose, glucose uptake induces an increased ATP/ADP ratio, closure of the KATP channel, followed by plasma membrane depolarization, opening of L‐type calcium channels, and the subsequent influx of Ca2+ from the extracellular environment, which is the main pathway leading to insulin secretion. On the other hand, Gαq‐mediated signals are well‐known to enhance GSIS (Fujiwara et al. 2005; Shapiro et al. 2005). Activation of Gαq‐coupled receptors, such as FFAR1 and the muscarinic M3 receptor, stimulates the IP3/Ca2+ and DAG/PKC pathways and potentiates GSIS (Gilon and Henquin 2001; Fujiwara et al. 2005). FFAR1 selective agonists, such as fasiglifam, augment insulin secretion in a glucose‐dependent manner (Tsujihata et al. 2011; Yabuki et al. 2013), but the underlying mechanism behind the glucose dependency is not fully understood.
In this study, we first indicated the requirement of membrane depolarization for the insulinotropic action of fasiglifam (Fig. 1). Even in the absence of glucose, fasiglifam augmented insulin secretion when the membrane potential was forcibly depolarized. Yang et al. made a similar observation in which GW9508, a FFAR1/FFAR4 dual agonist, enhanced insulin secretion triggered by a SU in the absence of glucose (Yang et al. 2010). These results demonstrate that membrane depolarization induced by glucose metabolism, and subsequent events are essential for fasiglifam to enhance GSIS.
Amplitude and frequency are two key components of Ca2+ oscillations to evoke pulsatile insulin secretion in β‐cells (Smedler and Uhlen 2014). As it has not yet determined that the effect of fasiglifam or Gαq signaling on Ca2+ oscillations, we analyzed it in Ca2+ imaging experiments. Frequency decoding of Ca2+ oscillations revealed that fasiglifam enhanced not only intracellular Ca2+ concentrations but also the amplitude of Ca2+ oscillations in a glucose‐dependent manner (Fig. 3A and B). However, the amplitude modulation of fasiglifam was common to all bandwidths examined, indicating that fasiglifam had no effects on the frequency modulation (Fig. 3C). The most likely physiological meaning of the amplitude enhancement by fasiglifam is the high secretory efficiency of insulin, but they can also affect Ca2+ homeostasis on various cellular organelles. For example, mitochondria, interacting with ER, contain several Ca2+‐dependent enzymes involved in the TCA cycle and ATP synthesis (Alam et al. 2012; Gilon et al. 2014). Thus, the enhanced amplitude by fasiglifam may affect glucose metabolism and promote ATP synthesis in mitochondria, leading to larger insulin secretion in the long term.
In contrast to fasiglifam, the SU drug, glimepiride, strongly mobilized Ca2+ even in the absence of glucose (Fig. 3D–F). SUs are often used as insulin secretagogues for the treatment of T2DM, but they have direct toxicity to β‐cells and the hypoglycemic risk in patients (Maedler et al. 2005). Our data can explain those drawbacks, in conjunction with the previous findings that sustained or excessive intracellular Ca2+ from the chronic use of SUs causes β‐cell apoptosis (Iwakura et al. 2000; Maedler et al. 2005).
To elucidate how the two major second messengers of FFAR1 affect Ca2+ dynamics and insulin secretion, we compared the effects of fasiglifam and analogs of IP3 or DAG. As we reported previously, the amount of IP3 produced in response to fasiglifam is independent of glucose concentrations (Tsujihata et al. 2011). Stimulation with the exogenous IP3 analog enhanced GSIS, intracellular Ca2+ levels, and the amplitude of Ca2+ oscillations similar to fasiglifam (Fig. 2A and B, Fig. 4A–C). These results indicate that the IP3 generated by FFAR1 plays a role in intracellular Ca2+ dynamics, which may contribute to augmented insulin secretion by fasiglifam.
Similar to fasiglifam, an exogenous DAG analog, OAG enhanced GSIS in a glucose‐dependent manner (Fig. 2A and C), consistent with a previous report using isolated mouse islets (Ferdaoussi et al. 2012). Importantly, OAG did not affect the glucose‐dependent Ca2+ oscillatory response unlike IP3 (Fig. 4D–F), indicating that the different mechanisms underlie the insulinotropic action of DAG. DAG activates PKC that then amplifies insulin granule exocytosis (Eliasson et al. 1996). Among 10 isoforms, PKCα and PKCε are most likely to play a role in DAG‐activated insulin secretion, supported by our findings that both abundantly expressed in MIN6 cells (Fig. S1). Recently, it has been reported that PKD, that is activated by DAG directly or indirectly via PKC, plays a role in FFAR1‐induced insulin secretion through its function on F‐actin depolymerization and cytoskeleton rearrangement (Kong et al. 2010; Ferdaoussi et al. 2012). Thus, PKD may also be involved in DAG‐induced insulin secretion in this study.
As shown in Figure 5A, Ca2+ influx from extracellular sites via an L‐type Ca2+ channel plays important roles in fasiglifam‐induced insulin secretion. Interestingly, the insulinotropic effects of (S)‐(‐)‐Bay K 8644 were synergistically stimulated with OAG in the absence of glucose (Fig. 5B and C). This observation indicates that DAG can potentiate the downstream insulin secretory pathways induced by Ca2+ influx. In addition, Ca2+ influx possibly enhances DAG‐mediated activation of Ca2+‐sensitive conventional PKCs including PKCα (Schmitz‐Peiffer and Biden 2008). Although the interaction between fasiglifam‐mediated IP3/Ca2+ pathway and Ca2+ influx from extracellular sites needs further investigation, the results of this study may provide insights into the entire process of FFAR1‐mediated insulin secretion.
In conclusion, we reinforce the roles of conventional Gαq signal branches behind the glucose‐dependent action of fasiglifam, in parallel with recent reports regarding the roles of FFA metabolism and β‐arrestin signaling via FFAR1 (Kristinsson et al. 2015; Mancini et al. 2015). The pathways defined in this study are illustrated in Figure 6. We propose a dual potentiating model in which glucose‐induced extracellular Ca2+ influx through L‐type VDCC is augmented by both IP3‐mediated amplification of Ca2+ oscillations and PKC‐mediated augmentation of downstream secretory pathways, followed by FFAR1/Gαq activation. Recently, state‐of‐the‐art designer receptors exclusively activated by designer drug (DREADD) technology has proven that activation of Gαq signaling maintains its effectiveness on β‐cells in a severe diabetic mouse model (Jain et al. 2013). Although fasiglifam was withdrawn from clinical trials due to liver safety concerns in December 2013, our findings are expected to help better characterize the utility of FFAR1 agonist therapy for the treatment of T2DM.
Figure 6.
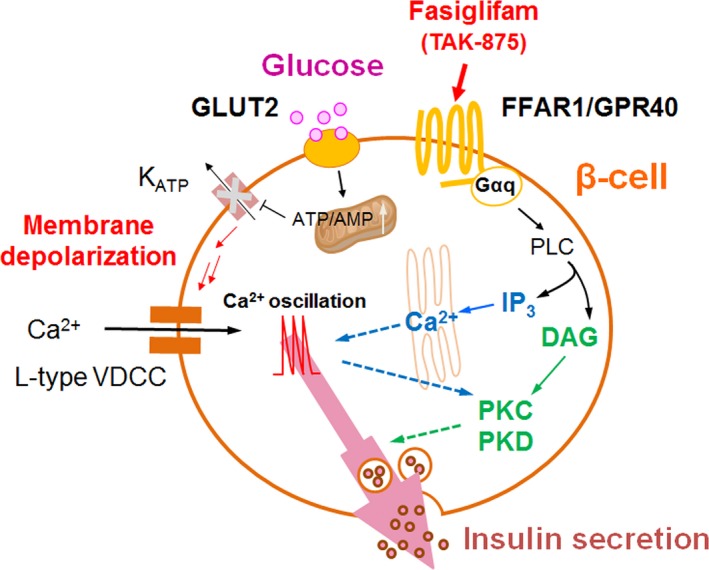
Schematic dual potentiating model for fasiglifam‐induced FFAR1 signaling. Fasiglifam, a selective FFAR1 ago‐allosteric modulator, potentiates glucose‐stimulated insulin secretion in depolarized β‐cells via at least two distinct Gαq‐mediated mechanisms: (1) fasiglifam‐induced IP3 production amplifies glucose‐induced Ca2+ oscillations, and (2) fasiglifam‐induced protein kinase C (PKC)/(PKD) protein kinase D activation augments downstream secretory mechanisms independent of Ca2+ oscillations.
Author Contributions
Participated in research design: Sakuma, Yabuki, Abiru, Maruyama, Komatsu, Tsujihata, Takeuchi, Habata, and Mori. Conducted experiments: Sakuma, Yabuki, Abiru, Maruyama, and Komatsu. Contributed new reagents or analytic tools: Negoro. Performed data analysis: Sakuma. Wrote or contributed to the writing of the manuscript: Sakuma, Maruyama, and Tsujihata.
Disclosure
All authors herein declare that there are no conflicts of interest associated with this manuscript.
Supporting information
Figure S1. mRNA levels of PKC and PKD family enzymes in MIN6 cells. Data S1. Method.
Acknowledgements
We thank Dr. Jun‐ichi Miyazaki (Osaka University, Japan) for kindly providing MIN6 cells. We also thank Drs. Yukio Yamada, Masakuni Noda, Ryo Ito, Shin‐ichi Abe, Matthew Ericson, and Kenichi Nagatome for helpful discussions, suggestions, and support for this study. This study was financially supported by the Takeda Pharmaceutical Company Limited. The authors, as employees of Takeda, were involved in the study design, data collection and analysis, decision to publish, and preparation of the manuscript.
Sakuma K. , Yabuki C. , Maruyama M. , Abiru A. , Komatsu H. , Negoro N. , Tsujihata Y. , Takeuchi K. , Habata Y. , Mori M.. Fasiglifam (TAK‐875) has dual potentiating mechanisms via Gαq‐GPR40/FFAR1 signaling branches on glucose‐dependent insulin secretion, Pharma Res Per, 4(3), 2016, e00237, doi: 10.1002/prp2.237
References
- Alam MR, Groschner LN, Parichatikanond W, Kuo L, Bondarenko AI, Rost R, et al. (2012). Mitochondrial Ca2+ uptake 1 (MICU1) and mitochondrial Ca2+ uniporter (MCU) contribute to metabolism‐secretion coupling in clonal pancreatic beta‐cells. J Biol Chem 287: 34445–34454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Diabetes Association (2015). Standards of medical care in diabetes–2015: 7. Approaches to Glycemic Treatment. Diabetes care 38(Suppl. 1): S41–48. [DOI] [PubMed] [Google Scholar]
- Berggren PO, Yang SN, Murakami M, Efanov AM, Uhles S, Kohler M, et al. (2004). Removal of Ca2+ channel beta3 subunit enhances Ca2+ oscillation frequency and insulin exocytosis. Cell 119: 273–284. [DOI] [PubMed] [Google Scholar]
- Bergsten P (1995). Slow and fast oscillations of cytoplasmic Ca2+ in pancreatic islets correspond to pulsatile insulin release. Am J Physiol 268: E282–E287. [DOI] [PubMed] [Google Scholar]
- Bergsten P (2002). Role of oscillations in membrane potential, cytoplasmic Ca2+, and metabolism for plasma insulin oscillations. Diabetes 51(Suppl 1): S171–S176. [DOI] [PubMed] [Google Scholar]
- Burant CF, Viswanathan P, Marcinak J, Cao C, Vakilynejad M, Xie B, et al. (2012). TAK‐875 versus placebo or glimepiride in type 2 diabetes mellitus: a phase 2, randomised, double‐blind, placebo‐controlled trial. Lancet 379: 1403–1411. [DOI] [PubMed] [Google Scholar]
- Colsoul B, Schraenen A, Lemaire K, Quintens R, Van Lommel L, Segal A, et al. (2010). Loss of high‐frequency glucose‐induced Ca2+ oscillations in pancreatic islets correlates with impaired glucose tolerance in Trpm5‐/‐ mice. Proc Natl Acad Sci USA 107: 5208–5213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drews G, Krippeit‐Drews P, Dufer M (2010). Electrophysiology of islet cells. Adv Exp Med Biol 654: 115–163. [DOI] [PubMed] [Google Scholar]
- Eliasson L, Renstrom E, Ammala C, Berggren PO, Bertorello AM, Bokvist K, et al. (1996). PKC‐dependent stimulation of exocytosis by sulfonylureas in pancreatic beta cells. Science 271: 813–815. [DOI] [PubMed] [Google Scholar]
- Ferdaoussi M, Bergeron V, Zarrouki B, Kolic J, Cantley J, Fielitz J, et al. (2012). G protein‐coupled receptor (GPR)40‐dependent potentiation of insulin secretion in mouse islets is mediated by protein kinase D1. Diabetologia 55: 2682–2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara K, Maekawa F, Yada T (2005). Oleic acid interacts with GPR40 to induce Ca2+ signaling in rat islet beta‐cells: mediation by PLC and L‐type Ca2+ channel and link to insulin release. Am J Physiol Endocrinol Metab 289: E670–E677. [DOI] [PubMed] [Google Scholar]
- Gilon P, Henquin JC (2001). Mechanisms and physiological significance of the cholinergic control of pancreatic beta‐cell function. Endocr Rev 22: 565–604. [DOI] [PubMed] [Google Scholar]
- Gilon P, Chae HY, Rutter GA, Ravier MA (2014). Calcium signaling in pancreatic beta‐cells in health and in Type 2 diabetes. Cell calcium 56: 340–361. [DOI] [PubMed] [Google Scholar]
- Gwiazda KS, Yang TL, Lin Y, Johnson JD (2009). Effects of palmitate on ER and cytosolic Ca2+ homeostasis in beta‐cells. Am J Physiol Endocrinol Metab 296: E690–E701. [DOI] [PubMed] [Google Scholar]
- Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, et al. (2012). Management of hyperglycemia in type 2 diabetes: a patient‐centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 35: 1364–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwakura T, Fujimoto S, Kagimoto S, Inada A, Kubota A, Someya Y, et al. (2000). Sustained enhancement of Ca2+ influx by glibenclamide induces apoptosis in RINm5F cells. Biochem Biophys Res Commun 271: 422–428. [DOI] [PubMed] [Google Scholar]
- Jain S, Ruiz de Azua I, Lu H, White MF, Guettier JM, Wess J (2013). Chronic activation of a designer G(q)‐coupled receptor improves beta cell function. J Clin Investig 123: 1750–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaku K, Enya K, Nakaya R, Ohira T, Matsuno R (2015). Efficacy and safety of fasiglifam (TAK‐875), a G protein‐coupled receptor 40 agonist, in Japanese patients with type 2 diabetes inadequately controlled by diet and exercise: a randomized, double‐blind, placebo‐controlled, phase III trial. Diabetes Obes Metab 17: 675–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong KC, Butcher AJ, McWilliams P, Jones D, Wess J, Hamdan FF, et al. (2010). M3‐muscarinic receptor promotes insulin release via receptor phosphorylation/arrestin‐dependent activation of protein kinase D1. Proc Natl Acad Sci USA 107: 21181–21186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristinsson H, Bergsten P, Sargsyan E (2015). Free fatty acid receptor 1 (FFAR1/GPR40) signaling affects insulin secretion by enhancing mitochondrial respiration during palmitate exposure. Biochimica et biophysica acta 1853: 3248–3257. [DOI] [PubMed] [Google Scholar]
- Liu YJ, Tengholm A, Grapengiesser E, Hellman B, Gylfe E (1998). Origin of slow and fast oscillations of Ca2+ in mouse pancreatic islets. The Journal of physiology 508(Pt 2): 471–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald PE, Rorsman P (2006). Oscillations, intercellular coupling, and insulin secretion in pancreatic beta cells. PLoS Biol 4: e49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maedler K, Carr RD, Bosco D, Zuellig RA, Berney T, Donath MY (2005). Sulfonylurea induced beta‐cell apoptosis in cultured human islets. J Clin Endocrinol Metab 90: 501–506. [DOI] [PubMed] [Google Scholar]
- Mancini AD, Bertrand G, Vivot K, Carpentier E, Tremblay C, Ghislain J, et al. (2015). Beta‐arrestin recruitment and biased agonism at free fatty acid receptor 1. J Biol Chem 290: 21131–21140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki J, Araki K, Yamato E, Ikegami H, Asano T, Shibasaki Y, et al. (1990). Establishment of a pancreatic beta cell line that retains glucose‐inducible insulin secretion: special reference to expression of glucose transporter isoforms. Endocrinology 127: 126–132. [DOI] [PubMed] [Google Scholar]
- Negoro N, Sasaki S, Mikami S, Ito M, Suzuki M, Tsujihata Y, et al. (2010). Discovery of TAK‐875: a potent, selective, and orally bioavailable GPR40 agonist. ACS Med Chem Lett 1: 290–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negoro N, Sasaki S, Mikami S, Ito M, Tsujihata Y, Ito R, et al. (2012). Optimization of (2,3‐dihydro‐1‐benzofuran‐3‐yl)acetic acids: discovery of a non‐free fatty acid‐like, highly bioavailable G protein‐coupled receptor 40/free fatty acid receptor 1 agonist as a glucose‐dependent insulinotropic agent. J Med Chem 55: 3960–3974. [DOI] [PubMed] [Google Scholar]
- Nunemaker CS, Bertram R, Sherman A, Tsaneva‐Atanasova K, Daniel CR, Satin LS (2006). Glucose modulates [Ca2+]i oscillations in pancreatic islets via ionic and glycolytic mechanisms. Biophys J 91: 2082–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorsman P, Braun M (2013). Regulation of insulin secretion in human pancreatic islets. Annu Rev Physiol 75: 155–179. [DOI] [PubMed] [Google Scholar]
- Sassmann A, Gier B, Grone HJ, Drews G, Offermanns S, Wettschureck N (2010). The Gq/G11‐mediated signaling pathway is critical for autocrine potentiation of insulin secretion in mice. J Clin Investig 120: 2184–2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz‐Peiffer C, Biden TJ (2008). Protein kinase C function in muscle, liver, and beta‐cells and its therapeutic implications for type 2 diabetes. Diabetes 57: 1774–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro H, Shachar S, Sekler I, Hershfinkel M, Walker MD (2005). Role of GPR40 in fatty acid action on the beta cell line INS‐1E. Biochem Biophys Res Commun 335: 97–104. [DOI] [PubMed] [Google Scholar]
- Smedler E, Uhlen P (2014). Frequency decoding of calcium oscillations. Biochim Biophys Acta 1840: 964–969. [DOI] [PubMed] [Google Scholar]
- Srivastava A, Yano J, Hirozane Y, Kefala G, Gruswitz F, Snell G, et al. (2014). High‐resolution structure of the human GPR40 receptor bound to allosteric agonist TAK‐875. Nature 513: 124–127. [DOI] [PubMed] [Google Scholar]
- Tsujihata Y, Ito R, Suzuki M, Harada A, Negoro N, Yasuma T, et al. (2011). TAK‐875, an orally available G protein‐coupled receptor 40/free fatty acid receptor 1 agonist, enhances glucose‐dependent insulin secretion and improves both postprandial and fasting hyperglycemia in type 2 diabetic rats. J Pharmacol Exp Ther 339: 228–237. [DOI] [PubMed] [Google Scholar]
- Uhlen P (2004). Spectral analysis of calcium oscillations. Sci STKE 2004:pl15. [DOI] [PubMed] [Google Scholar]
- Yabuki C, Komatsu H, Tsujihata Y, Maeda R, Ito R, Matsuda‐Nagasumi K, et al. (2013). A novel antidiabetic drug, fasiglifam/TAK‐875, acts as an ago‐allosteric modulator of FFAR1. PLoS ONE 8: e76280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Chisholm JW, Soelaiman S, Shryock JC (2010). Sulfonylureas uncouple glucose‐dependence for GPR40‐mediated enhancement of insulin secretion from INS‐1E cells. Mol Cell Endocrinol 315: 308–313. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. mRNA levels of PKC and PKD family enzymes in MIN6 cells. Data S1. Method.


