Abstract
Objective
Low-energy fractures are frequent complications in Type 1 Diabetes Mellitus patients (T1DM). Modifications of bone intrinsic composition might be a potential cause of fragility observed in diabetic subjects. Advanced Glycation End-products (AGEs) were found in numerous connective tissues from T1DM patients. However, whether AGEs are present at high levels in bone matrix from diabetic subjects is unknown. Moreover, whether elevated AGEs in the bone matrix impair mineralization has not been addressed in humans. The purposes of this study were 1) to determine whether bone matrix from fracturing and non-fracturing T1DM contained more AGEs than bone from healthy patients (CTL), and 2) to compare the degree of mineralization of bone and hardness between fracturing and non-fracturing T1DM versus CTL.
Design and methods
We analyzed iliac crest bone biopsies from 5 fracturing (Type 1 Diabetes Mellitus) T1DM patients, 5 non-fracturing T1DM patients and 5 healthy subjects, all age- and sex-matched. AGEs (Pentosidine) in bone matrix was measured by high performance liquid chromatography separately in trabecular and cortical bone. The degree of mineralization of bone (DMB) was assessed by digitized microradiography, and mechanical properties by micro- and nanohardness tests.
Results
Trabecular bone from fracturing T1DM exhibited significantly higher levels of Pentosidine than CTL (p=0.04) and was more mineralized than non-fracturing T1DM (p=0.04) and CTL (p=0.04). Trabecular bone was not significantly different in pentosidine between non-fracturing T1DM and CTL. Cortical bone from non-fracturing T1DM was not significantly different from CTL. Positive correlations were found between HbA1c and Pentosidine (r′=0.79, p<0.003), and between HbA1c and DMB (r′=0.64, p<0.02).
Conclusion
Both modifications could lead to less flexible bone (reduced modulus of elasticity), and a tendency toward low-energy fractures in T1DM patients.
Keywords: Type 1 diabetes mellitus, Fracture, Bone quality, Pentosidine, mineralization
Introduction
Recently, researchers from the Mayo Clinic added bone deterioration to the list of diabetes complications [1]. Although, the type 1 diabetics (T1DM) are 12 times more likely to suffer a fracture than non-diabetics, whereas type 2 diabetics (T2DM) are 1.7 time more likely [2]. While a reduction of Bone Mineral Density (aBMD) measured by dual-energy X-ray absorptiometry (DXA) has been frequently observed in T1DM [3, 4], those patients have a markedly increased risk of hip fracture [5–7] that is not fully explained by low bone mass alone [6]. Moreover, low aBMD was not observed in all studies with T1DM patients [8–10]. It was thus hypothesized that modifications of bone quality in diabetic patients, rather than bone mass, could be the cause of their bone fragility. Advanced Glycation End-products (AGEs) induced by non-enzymatic reaction between proteins and sugar are considered to be harmful for bone collagen, and increase with ageing, osteoporosis, and diabetes [11, 12]. Pentosidine (PEN) is used as a surrogate marker of total AGEs formation [13]. High levels of PEN in serum and urine were reported to be a risk factor for fracture in type 2 diabetic patients [14, 15]. In type 1 diabetic patients, high levels of PEN in serum were also associated with prevalent fracture [16]. However, no data exist on the presence of PEN in bone from diabetic patients. Moreover, the impact of the presence of AGEs, in diabetic patients, on bone mineralization is unknown. Indeed, the degree of mineralization is a major intrinsic determinant of bone strength. An increase in degree of mineralization confers higher stiffness to bone, but a too high mineralization may be deleterious by increasing brittleness [17]. Therefore, the purposes of this study were to analyze the PEN content in bone from T1DM patients, along with the bone mineralization and micro- and nanohardness. Bone biopsies from T1DM-fracturing subjects (FX-T1DM), their age- and sex- matched T1DM non-fracturing subjects (non-FX-T1DM), and their age- and sex- matched healthy control subjects (CTL) were analyzed. Correlations between bone quality variables and clinical data were also performed.
Material and Methods
Subjects
These subjects were part of a larger cohort previously reported by Armas and colleagues [10] This was a cross-sectional, case-control study of bone material properties found in transiliac bone biopsies from subjects with T1DM and a prior fragility fracture as an adult, compared with healthy age- and sex- matched non-diabetic control subjects without history of fracture, and healthy age- and sex- matched diabetic control subjects without history of fracture. The fractures were self-reported and we sought to ascertain all fractures, the date or age at which they occurred and the circumstances surrounding the fracture. The 5 subjects reported here, sustained a low-energy fracture or fractures (defined as occurring from trauma equal to or less than that of falling from a standing height) while they were diabetic and as an adult (>18 yrs old at the time of fracture). The fragility fractures occurred in different areas including ankle, elbow, tibia, fibula, arm, knee or metatarsal. Interestingly, all but 1 of these subjects also reported previous fractures that had occurred in childhood or adolescence.
Analytical Methods
Laboratory Analytical Methods [10]
Glycated hemoglobin (HbA1c), serum creatinine, serum 25- hydroxyvitamin D [25(OH)D] and demographic data are shown in Table 1.
Table 1.
Subjects Demographics and Laboratory Dataa
| Controls | Diabetes Non-fracturing | Diabetes Fracturing | |
|---|---|---|---|
| No of patients | 5 | 5 | 5 |
| Sex (Male, Female) | 2,3 | 2,3 | 2,3 |
| Age (years) | 37 (21–45) | 38 (21–46) | 38 (22–46) |
| Years since diagnosis of T1DM | na | 25 (7–35) | 14 (10–25) |
| Height (cm) | 169 | 172 | 170 |
| Weight (kg) | 69 (48–73) | 76 (71–77) | 75 (69–101) |
| BMI | 24.2 (18.6–25.1) | 24.4 (22.7–29.8) | 27.1 (25.4–33.5) |
| Serum HbA1c(%) | 5.2 (4.8–5.5) | 7.1 (5.7–8.5)b | 8.1(6.3–10.1)b |
| Serum 25(OH)D (nmol/L) | 65 (49–93) | 74 (65–99) | 62 (47–76) |
| Serum creatinine (mg/dl) | 0.7 (0.5–0.8) | 0.9 (0.7–1.0) | 0.7 (0.6–0.9) |
| Histomorphometric data | |||
| BV/TV (%) | 19.98 (15.24–33.43) | 23.16 (12.13–31.31) | 16.04 (11.54–26.17) |
| Tb.Th | 114.73 (101.21–189.58) | 125.24 (102.50–167.87) | 102.02 (100.26–136.74) |
| Tb.N | 1.5 (1.29–1.88) | 1.77 (1.11–2.02) | 1.49 (1.13–1.98) |
| Tb.Sp | 654.12 (520.78–758.65) | 552.18 (479–888) | 660.37 (492.48–874.04) |
| OS/BS (%) | 10.43 (2.10–20.43) | 16.84 (10.11–27.34) | 4.12 (1.32–12.10)c |
| Ob.S/BS % (%) | 7.04 (0.69–14.39) | 8.58 (2.89–11.51) | 1.16 (0.74–8.92) |
| MS/BS (%) | 9.63 (0.97–15.39) | 9.8 (4.37–11.20) | 2.68 (1.59–11.63) |
| BFR/BS (μm3/μm2/d) | 5.3 (0.4–8.9) | 5.3 (2.2–7.5) | 1.3 (0.9–7) |
| Oc.S/BS (%) | 0.23 (0.06–0.85) | 0.46 (0.1–0.61) | 0.34 (0.24–0.88) |
| ES/BS (%) | 0.70 (0.40–2.36) | 1.66 (0.37–2.19) | 0.89 (0.72–2.08) |
| MAR (μm/d) | 0.55 (0.46–0.65) | 0.51 (0.48–0.72) | 0.56 (0.49–0.69) |
| Ac.F (N/y) | 0.6 (0.05–1.11) | 0.6 (0.27–0.85) | 0.19 (0.10–0.93) |
| MicroCT Data | |||
| Structure Model Index | 0.63 (−0.01–1.12) | 0.41 (−0.41–1.77) | 1.19 (0.34–1.75) |
| Tissue Mineral Density (g/cm3) | 782 (741–834) | 812 (765–867) | 820 (772–849) |
| DXA measurement | |||
| BMD Spine (g/cm2) | 1.059 (0.850–1.080) | 1.049 (0.960–1.150) | 0.973 (0.840–1.210) |
| T-score Spine | 0.1 (−2.2–0.3) | −0.2 (−0.8–1.0) | −0.7 (−2.3–1.1) |
| BMD Hip (g/cm2) | 0.965 (0.860–0.990) | 1.030 (0.900–1.040) | 0.862 (0.840–1.150) |
| T-score Hip | −0.3 (−0.7–0.4) | 0.0 (−0.3–0.7) | −0.8 (−1.1–0.8) |
Median (range)
p=0.0431 vs Control
p=0.0431 vs Diabetes Non-fracturing
na= not applicable
BMI= body mass index
HbA1c=Glycated hemoglobin
25(OH)D=25-hydroxyvitamin D
Transiliac Bone Biopsy
Histomorphometry and micro-computed tomography (μCT) analyses of a larger cohort were previously published [10]. For enzymatic and non-enzymatic cross-links measurements, ten consecutive slices of 10 μm-thickness were cut with a microtome (Reichert-Jung, Leica, Germany). For digitized microradiography, a 150 μm-thick section was cut with a precision diamond wire saw (Well, Escil, Chassieu, France) and were progressively ground (silicium carbides) to a thickness of 100 ±1 μm and polished with an alumina suspension (1 μm). For micro/nano-indentation, embedded blocks were polished and surfaced with alumina suspension.
Cross-links measurements by high performance liquid chromatography (HPLC)
From each 10 μm-thick slices (10 by sample), cortical and trabecular compartments were separated under a microscope, with a cutter and treated separately. To remove methylmethacrylate (MMA), bone sections were incubated at room temperature, under gently stirring in successive MMA and 2-methoxyethyl acetate baths, and then washed with ethanol and rehydrated progressively in graded alcohol/water baths. Then, bone sections were hydrolyzed by 6 M HCl at 110°C during 20 h and the hydrolysates were pre-fractioned.Separation of PYD, DPD and PEN was performed by HPLC on a C18 Atlantis® T3 column (Waters Corp., Milford, MA, USA) and cross-links were quantified by fluorimetry as described previously [18].
Digitized microradiography
Degree (DMB) and heterogeneity index (HI) of mineralization (grams of mineral/cm3 of bone) were assessed by digitized methodology with a Microfocus Hammamatsu L9421-02X-ray tube equipped with a copper anode, recently described [19].
Microindentation
Microhardness was measured using a Micromet 5104 (Buehler, Lake Bluff, Illinois, USA) equipped with a Vickers indenter [20]. For each sample, 60 indents (20 in cortical 1, 20 in cortical 2, and 20 in trabecular bone) were randomly performed on the surfaced blocks.
Nanoindentation
The nano-indentation testing was done by using a commercially-available nano-indentation system (Hysitron Inc., Minneapolis, MN) with a Berkovich diamond indenter tip, in dry conditions. The intrinsic material properties were determined from the measured force and displacement data for each indentation test. Both the cortical and trabecular bone sites were tested. Each bone site was subjected to at least 25 indents for a good average [21].
Statistical Analysis
The data of the fifteen patients (matched for sex and age) were extracted from the initial study, and the statistical analysis performed using the statistics package, PASW Statistics 21.0 (SPSS Inc., Chicago, Illinois). Nonparametric expressions were reported (median, minimum and maximum). Comparisons between fracturing cases and both T1DM and healthy controls were done using a Friedman test and posthoc comparisons were done using a Wilcoxon test. Correlations were performed with Spearman correlation.
Results
The bone samples used in this study came from a larger study whose aim was to describe the bone micro-architecture and bone remodeling in type 1 diabetic patients (n=29) compared to healthy age- and sex- matched non diabetic control subjects (n=29). Among the T1DM subjects, 5 had histories of low-energy fractures sustained after diabetic diagnosis. In the present study, the bone biopsies from these 5 type 1 fracturing-diabetic patients, comparing them to 5 age- and sex- matched non-fracturing type 1 diabetic patients and 5 age- and sex- matched healthy controls were analyzed. The demographic, laboratory, clinical and histomorphometric data for the 3 groups are described in Table 1. The type 1 diabetic patients (non-FX and FX) had a wide range of glucose control, with a serum HbA1c between 5.7–10.1%. No difference in creatinine was observed per study entry criteria. Three-dimensional micro-CT data showed no difference between the 3 groups, as in the initial study [10]. Although there was only one variable in histomorphometric analyses that reached statistical significance, there are some interesting trends. The FX-T1DM patients had generally lower bone volume (BV/TV), lower trabecular thickness (Tb.Th) than either non-FX-T1DM patients or CTL. The surface covered with osteoblasts (Ob.S/BS) and osteoid (OS/BS) was less and the mineralizing surface (MS/BS) was also lower in the FX-T1DM patients. This is reflected in a generally lower bone formation rate and activation frequency. However, mineral apposition rate (MAR) was similar between the 3 groups. DXA measurements in hip or spine were not statistically different between the 3 groups.
Non-Enzymatic crosslinks measurements
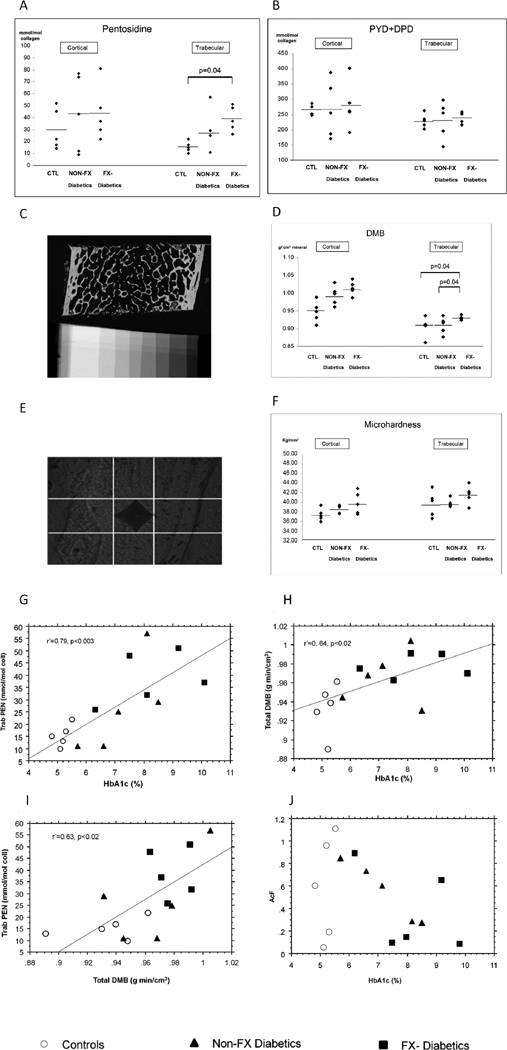
We found a significant higher PEN content (p=0.04) only in FX-T1DM compared CTL in trabecular bone (Fig. 1A). In cortical bone, PEN tended to be higher in FX-T1DM (Fig. 1A).
Figure 1.
Crosslinks contents (mmol/mol of collagen) measured in iliac bone biopsies [Control (CTL), non-fracturing (Non-FX-T1DM) and fracturing type 1 diabetic patients (FX-T1DM)] by High Performance Liquid Chromatography, in cortical and trabecular bone. (A) Non-enzymatic crosslinks (Pentosidine) content (B) Enzymatic crosslinks (PYD+DPD) content. (C) Degree of mineralization (DMB, g. min/cm3) measured by quantitative digitized microradiography, showing a microradiograph of iliac crest from fracturing-diabetic subject (22 year-old), and aluminum step-wedge. (D) DMB measured in cortical and trabecular in CTL, non-FX and FX-T1DM subjects. Trabecular bone is significantly more mineralized in FX-T1DM than CTL and Non-FX-T1DM patients. (E) Vickers indent in trabeculae from FX-T1DM subject (22 year-old). (F) Microhardness measured in cortical and trabecular bone in iliac bone biopsies. Spearman correlations between (G) HbA1c and trabecular pentosidine, trab PEN (H) HbA1c and total DMB, (I) Total Degree of mineralization (DMB) and trabecular PEN, (J) HbA1c and Activation Frequency (AcF).
Enzymatic crosslinks measurements
We did not find differences between CTL, Non-FX and FX-T1DM in PYD+DPD, either in cortical or trabecular bone (Fig. 1B).
Degree of mineralization (DMB)
We found significantly higher levels of DMB in FX-T1DM compared to both CTL (p=0.04) and non-FX-T1DM (p=0.04), in trabecular bone (Fig. 1C–D). No difference was found in HI between the 3 groups, either in cortical or trabecular bone.
Micro and nanoindentation
In microindentation, bone from FX and non-FX-T1DM were not significantly harder than CTL, either in cortical or trabecular bone (Fig. 1E–F).
In nanoindentation, the results show trends of greater hardness and modulus in FX-T1DM as compared to CTL (data not shown).
Correlations between variables
Interestingly, we found significant correlations between HbA1c and PENtrab bone (r′=0.79, p<0.003, Fig. 1G), and between HbA1c and DMBtotal (r′=0.64, p<0.02, Fig. 1H). Body mass index (BMI) was also significantly correlated with PENtrab (r′=0.70, p=0.004), DMBtotal (r′=0.66, p=0.008), DMBcort (r′=0.55, p=0.03) and DMBtrab (r′=0.63, p=0.01), PENcort (r′=0.58, p=0.02). After adjustment for BMI, the correlation between HbA1c and PENtrab bone remained significant (r′=0.68, p<0.01), whereas those between HbA1c and DMBtotal disappeared (r′=0.44, NS). A significant correlation between PENtrab and DMBtotal (r′=0.63, p<0.02, Fig. 1I) was also found. We did not find correlation between HbA1c and AcF, in the 3 groups (Fig. 1J). However, in the 2 diabetic groups only, a borderline correlation appeared (r′=−0.283, p=0.053).
Discussion
Type 1 diabetes mellitus is associated with up to 12 times the normal rate of fracture risk [2, 22]. Whether the fracture risk in diabetes is due to a deterioration in “bone quality” rather than a reduction in BMD (bone mass) is an important question and to date has been poorly investigated in human bone. The aim of this study was to analyze the bone matrix (organic and mineral) of iliac bone biopsies, from FX-T1DM (Fracturing) and non-FX-T1DM (Non-fracturing) patients, and to compare them to CTL, sex and age-matched.
We showed that trabecular bone from FX-T1DM contained a higher level of PEN compared to CTL. In addition, we also found a higher DMB in FX-T1DM patients compared to non-FX-T1DM patients and CTL. This is in conjunction with a generally lower rate of remodeling which suggests that the presence of excess glucose in the blood contributes to higher mineralization and that a low remodeling rate allows the bone to continue to be further mineralized without being replaced.
Interestingly, we found correlations between HbA1c and bone AGEs (PEN) content and between HbA1c and mineralization. Similar correlations were found with BMI. After adjustment for BMI, it appeared that the correlation between HbA1c and PEN remained significant, whereas those between HbA1c and mineralization disappeared. This suggests that the higher PENtrab in FX-T1DM patients is mainly due to T1DM, rather than to the trend to overweight/obesity in this group. However, the higher DMB in FX-T1DM may thus be due to either a higher BMI in this group or either insufficient statistical power. Yet, no relationship was found between BMI and mineralization in 152 human iliac crests in a recent study [23].
The main differences were found in trabecular bone, whereas only trends were found in cortical bone, for PEN content and degree of mineralization. It is possible that the presence of glucose and an inflammatory local environment in bone marrow influence the PEN formation more in trabecular rather than in cortical bone. Also, trabecular bone having a higher bone remodeling activity than cortical bone, it is possible that the trend to the lower rate of remodeling influence the increase in PEN and degree of mineralization first in trabecular bone.
Taken together, both high PEN content and mineralization could lead to a less flexible and more rigid bone matrix in FX-T1DM, explaining the bone fragility observed in those patients.
To conclude, we show, for the first time to our knowledge, that trabecular bone from fracturing T1DM subjects contains more PEN and was more mineralized than bone from non-fracturing T1DM subjects or controls. Those results have to be confirmed on a larger number of bone biopsies from T1DM.
Acknowledgments
This study was supported by NIH Grant K23 AR055542, The Health Future Foundation of Creighton University and the Gifford Foundation. The authors thank J. Turner and his team for the nanoindentation work.
Footnotes
Authors’ roles: Study Design: DF, LA, RR, GB. Data analysis and collection: DF, LA, RR, EG, MA, GB. Drafting manuscript: DF, LA. Reviewed/edited manuscript: LA, RR, EG, MA, GB. Approving final manuscript: DF, LA, EG, MA, RR, GB.
References
- 1.Farr JN, et al. In vivo assessment of bone quality in postmenopausal women with type 2 diabetes. J Bone Miner Res. 2014;29(4):787–95. doi: 10.1002/jbmr.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nicodemus KK, Folsom AR. Type 1 and type 2 diabetes and incident hip fractures in postmenopausal women. Diabetes Care. 2001;24(7):1192–7. doi: 10.2337/diacare.24.7.1192. [DOI] [PubMed] [Google Scholar]
- 3.Jehle PM, et al. Serum levels of insulin-like growth factor system components and relationship to bone metabolism in Type 1 and Type 2 diabetes mellitus patients. J Endocrinol. 1998;159(2):297–306. doi: 10.1677/joe.0.1590297. [DOI] [PubMed] [Google Scholar]
- 4.Joshi A, et al. A study of bone mineral density and its determinants in type 1 diabetes mellitus. J Osteoporos. 2013;2013:397814. doi: 10.1155/2013/397814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miao J, et al. Elevated hip fracture risk in type 1 diabetic patients: a population-based cohort study in Sweden. Diabetes Care. 2005;28(12):2850–5. doi: 10.2337/diacare.28.12.2850. [DOI] [PubMed] [Google Scholar]
- 6.Janghorbani M, et al. Systematic review of type 1 and type 2 diabetes mellitus and risk of fracture. Am J Epidemiol. 2007;166(5):495–505. doi: 10.1093/aje/kwm106. [DOI] [PubMed] [Google Scholar]
- 7.Vestergaard P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes--a meta-analysis. Osteoporos Int. 2007;18(4):427–44. doi: 10.1007/s00198-006-0253-4. [DOI] [PubMed] [Google Scholar]
- 8.Weber G, et al. Bone mass in young patients with type I diabetes. Bone Miner. 1990;8(1):23–30. doi: 10.1016/0169-6009(91)90137-o. [DOI] [PubMed] [Google Scholar]
- 9.Gallacher SJ, et al. An evaluation of bone density and turnover in premenopausal women with type 1 diabetes mellitus. Diabet Med. 1993;10(2):129–33. doi: 10.1111/j.1464-5491.1993.tb00029.x. [DOI] [PubMed] [Google Scholar]
- 10.Armas LA, et al. Trabecular bone histomorphometry in humans with Type 1 Diabetes Mellitus. Bone. 2012;50(1):91–6. doi: 10.1016/j.bone.2011.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bailey AJ, Paul RG, Knott L. Mechanisms of maturation and ageing of collagen. Mech Ageing Dev. 1998;106(1–2):1–56. doi: 10.1016/s0047-6374(98)00119-5. [DOI] [PubMed] [Google Scholar]
- 12.Vashishth D. The role of the collagen matrix in skeletal fragility. Curr Osteoporos Rep. 2007;5(2):62–6. doi: 10.1007/s11914-007-0004-2. [DOI] [PubMed] [Google Scholar]
- 13.Dong XN, et al. In situ accumulation of advanced glycation endproducts (AGEs) in bone matrix and its correlation with osteoclastic bone resorption. Bone. 2011;49(2):174–83. doi: 10.1016/j.bone.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwartz AV, et al. Pentosidine and increased fracture risk in older adults with type 2 diabetes. J Clin Endocrinol Metab. 2009;94(7):2380–6. doi: 10.1210/jc.2008-2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamamoto M, et al. Serum pentosidine levels are positively associated with the presence of vertebral fractures in postmenopausal women with type 2 diabetes. J Clin Endocrinol Metab. 2008;93(3):1013–9. doi: 10.1210/jc.2007-1270. [DOI] [PubMed] [Google Scholar]
- 16.Neumann T, et al. High serum pentosidine but not esRAGE is associated with prevalent fractures in type 1 diabetes independent of bone mineral density and glycaemic control. Osteoporos Int. 2014;25(5):1527–33. doi: 10.1007/s00198-014-2631-7. [DOI] [PubMed] [Google Scholar]
- 17.Currey JD. Tensile yield in compact bone is determined by strain, post-yield behaviour by mineral content. J Biomech. 2004;37(4):549–56. doi: 10.1016/j.jbiomech.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 18.Viguet-Carrin S, et al. Simple and sensitive method for quantification of fluorescent enzymatic mature and senescent crosslinks of collagen in bone hydrolysate using single-column high performance liquid chromatography. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877(1–2):1–7. doi: 10.1016/j.jchromb.2008.10.043. [DOI] [PubMed] [Google Scholar]
- 19.Montagner F, et al. Validation of a novel microradiography device for characterization of bone mineralization. Journal of X-Ray Science and Technology. 2015 doi: 10.3233/XST-150481. [DOI] [PubMed] [Google Scholar]
- 20.Boivin G, et al. The role of mineralization and organic matrix in the microhardness of bone tissue from controls and osteoporotic patients. Bone. 2008;43(3):532–8. doi: 10.1016/j.bone.2008.05.024. [DOI] [PubMed] [Google Scholar]
- 21.Polly BJ, et al. Intrinsic material properties of trabecular bone by nanoindentation testing of biopsies taken from healthy women before and after menopause. Calcif Tissue Int. 90(4):286–93. doi: 10.1007/s00223-012-9575-8. [DOI] [PubMed] [Google Scholar]
- 22.Zhukouskaya VV, et al. Prevalence of morphometric vertebral fractures in patients with type 1 diabetes. Diabetes Care. 2013;36(6):1635–40. doi: 10.2337/dc12-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koehne T, et al. Trends in trabecular architecture and bone mineral density distribution in 152 individuals aged 30–90 years. Bone. 66:31–8. doi: 10.1016/j.bone.2014.05.010. [DOI] [PubMed] [Google Scholar]