Abstract
Aims
Proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors are an emerging class of low‐density lipoprotein cholesterol (LDL‐C)‐lowering agents. In spite of their known effects on lipids, the impact of these drugs on systemic inflammation is less known. We aimed to investigate the effect of PCSK9 inhibitors on high‐sensitivity C‐reactive protein (hs‐CRP) levels through a meta‐analysis of randomized controlled trials (RCTs).
Methods
A systematic literature search of Medline, SCOPUS and Google Scholar was conducted up to December 2015 to identify RCTs assessing changes in hs‐CRP concentrations during treatment with PCSK9 inhibitors. Quantitative data synthesis was performed using a random‐effects model, with weighed mean difference (WMD) and 95% confidence interval (CI) as summary statistics.
Results
Sixteen treatment arms, with a total of 2546 participants, were included. Random‐effects meta‐analysis did not show any significant effect of PCSK9 inhibitors on hs‐CRP levels (WMD: 0.002 mg l–1, CI: –0.017, 0.021; P = 0.807; I 2 = 37.26%). This effect size was robust, not sensitive to any single study, and not affected by the type of PCSK9 inhibitor (evolocumab: WMD: 0.002 mg l–1, CI: –0.02, 0.02; P = 0.855; alirocumab WMD: 0.15 mg l–1, CI: –0.11, 0.40; P = 0.259; I 2 = 0%), or dosing frequency (biweekly: WMD: 0.13 mg l–1, CI: –0.20, 0.46; P = 0.433; I 2 = 55.19%; monthly: WMD: 0.003 mg l–1, CI: –0.01, 0.01; P = 0.59; I 2 = 0%). Random‐effects meta‐regression did not suggest any association of changes in hs‐CRP levels with changes in plasma LDL‐C concentrations (P = 0.697) or cumulative dosage of the drug (P = 0.980).
Conclusions
This meta‐analysis of RCTs did not suggest an effect of PCSK9 inhibitors on hs‐CRP concentrations.
Keywords: atherosclerosis, hs‐CRP, inflammation, PCSK9 inhibitors, pleiotropic effect
Introduction
Atherosclerosis is the primary risk factor for several conditions, such as coronary artery disease, stroke and peripheral vascular disease, and is the leading cause of morbidity and mortality worldwide 1. Hypercholesterolaemia is a major cardiovascular (CV) risk factor that leads the process of atherosclerotic plaque formation 2. Besides being a disorder of lipid accumulation, atherosclerosis is known to have major inflammatory properties that contribute to plaque rupture and clinical events 3.
Low‐density lipoprotein cholesterol (LDL‐C) is the primary atherogenic lipoprotein, and LDL‐C reduction is the target of primary or secondary prevention of CV diseases (CVDs) 4. Currently, LDL reduction with statins is considered as the cornerstone of hypolipidaemic drug therapy to reduce CV risk 5, 6. Although statins were developed specifically to decrease cholesterol synthesis, many studies have suggested that their beneficial effects extend beyond lipid lowering. However, there is no evidence that they reduce the risk of additional CVD events, beyond that expected from the degree of LDL‐C lowering, beyond the reduction of CV risk expected from the reduction of LDL‐C levels 7, 8. The use of statins in primary and secondary prevention has suggested that the magnitude of their efficacy in the prevention of CV events is not linearly or simply related to their hypolipidaemic action; in addition, statin efficacy may be ascribed, in part, to their ability to attenuate systemic inflammation and high‐sensitivity C‐reactive protein (hs‐CRP) levels 9, 10.
Proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors have recently emerged as an elegant strategy to lower LDL‐C and other lipid parameters 11, 12, 13, 14, 15. They have been evaluated comprehensively in several phase 2 and ‐3 trials 16, 17, 18, 19, 20, 21, 22 exploring dose ranging, dose frequency and different patient populations, and tested as monotherapy or as add‐on therapy to conventional lipid‐lowering strategies. Furthermore, experimental studies showed that, beyond its role in LDL‐C homeostasis, PCSK9 is expressed in atherosclerotic plaques 23 and might promote atherosclerosis by stimulating inflammation, endothelial dysfunction and hypertension 13. Moreover, it has been observed that PCSK9 downregulates gene expression of the stress response and inflammation in liver cells, indicating that PCSK9 affects metabolic pathways beyond cholesterol metabolism 24.
Although an evaluation of systemic inflammation has been performed in most of the trials conducted to date, there has been no meta‐analysis to ascertain the overall effect size of PCSK9 inhibition on systemic inflammation. In this context, hs‐CRP has been the most widely used method for quantifying inflammation in CVDs 9, 25, 26. Therefore, the purpose of the present meta‐analysis was to investigate the impact of PCSK9 inhibitors on plasma hs‐CRP concentrations.
Methods
Search strategy
The present study was designed according to the guidelines of the 2009 Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) statement 27. SCOPUS (http://www.scopus.com), Medline (http://www.ncbi.nlm.nih.gov/pubmed) and Google Scholar (http://www.scholar.google.com) were searched using the following search terms in titles and abstracts (also in combination with MESH terms): (evolocumab OR alirocumab OR AMG145 OR ‘AMG 145’ OR SAR236553/REGN727 OR SAR236553 OR ‘SAR 236553’ OR REGN727 OR ‘REGN 727’ OR REGN727/SAR236553) AND (CRP OR C‐reactive protein OR ‘C reactive protein’ OR hsCRP OR hs‐CRP). The search was limited to human studies and the wild‐card term ‘*’ was used to increase the sensitivity of the search strategy. No language restriction was used in the literature search. The literature was searched from inception to 24 December 2015.
Study selection
Original studies were included if they met the following inclusion criteria: (i) being a randomized controlled trial (RCT) in a parallel, crossover or single‐arm design; (ii) investigating the impact of a PCSK9 inhibitor on plasma/serum concentrations hs‐CRP; (iii) having a treatment duration of at least 2 weeks; (iv) presenting sufficient information on hs‐CRP concentrations at the end of the treatment period or providing a value for net changes; and (v) including a control group. Exclusion criteria were: (i) nonclinical studies; (ii) observational studies with a case‐control, cross‐sectional or cohort design; iii) single‐arm trials; and (iv) lack of sufficient information on baseline or follow‐up PCSK9 concentrations.
Data extraction
Eligible studies were reviewed and the following data were abstracted: (i) first author's name; (ii) year of publication; (iii) study location; (iv) study design; (v) number of participants in the PCSK9 inhibitor and control groups; (vi) dose, frequency of injections, and duration of intervals between the injections of PCSK9 inhibitor; (vii) age, gender and body mass index (BMI) of study participants; (viii) baseline levels of total cholesterol, LDL‐C, high‐density lipoprotein cholesterol (HDL‐C), triglycerides and glucose; (ix) systolic and diastolic blood pressures; and (x) data regarding baseline and follow‐up concentrations of hs‐CRP.
Quantitative data synthesis
Meta‐analysis was conducted using Comprehensive Meta‐Analysis (CMA) V2 software (Biostat, NJ, USA) 28. hs‐CRP concentrations were collated in mg l–1. A division by 9.524 was used to convert hs‐CRP values expressed in nmol l–1 into mg l–1. Standard deviations (SDs) of the mean difference were calculated using the following formula: SD = square root [(SDpre‐treatment)2 + (SDpost‐treatment)2 – (2R × SDpre‐treatment × SDpost‐treatment)], assuming a correlation coefficient (R) = 0.5. In cases where the standard error of the mean (SEM) was reported, SD was estimated using the following formula: SD = SEM × square root (n), where n is the number of subjects. If the outcome measures were reported as the median and inter‐quartile range, the mean and SD values were estimated using the method described by Hozo et al. 29. To convert interquartile range into min–max range, the following equations were used: A = median + 2 × (Q3 – median) and B = median – 2 × (median – Q1), where a, b, Q1 and Q3 are upper and lower ends of the range, upper and lower ends of the interquartile range, respectively.
Net changes in measurements (change scores) were calculated for parallel and crossover trials, as follows: (measure at end of follow‐up in the treatment group − measure at baseline in the treatment group) − (measure at end of follow‐up in the control group − measure at baseline in the control group). A random‐effects model (using the DerSimonian–Laird method) and the generic inverse variance method were used to compensate for the heterogeneity of studies in terms of demographic characteristics of populations being studied and also differences in study design and type of fibrate being studied 30. Heterogeneity was assessed quantitatively using the I 2 index. Effect sizes were expressed as WMD and 95% confidence interval (CI). Interstudy heterogeneity was assessed using the Cochran Q test and I 2 index. In order to evaluate the influence of each study on the overall effect size, sensitivity analysis was conducted using the leave‐one‐out method – that is, removing one study each time and repeating the analysis [31, 32].
Meta‐regression
Random‐effects meta‐regression was performed using the unrestricted maximum likelihood method to evaluate the association between the changes in plasma hs‐CRP concentrations following treatment with PCSK9 inhibitors and respective reductions in LDL‐C concentrations and cumulative dosage of PCSK9 inhibitor received during the trial.
Publication bias
Potential publication bias was explored using visual inspection of Begg's funnel plot asymmetry, Begg's rank correlation and Egger's weighted regression tests. The Duval and Tweedie trim–and‐fill correction was used to adjust the analysis for the effects of publication bias [33].
Results
Flow and characteristics of included studies
A summary of the study selection process is shown in Figure 1. The initial literature search yielded 279 articles. The screening for potential relevance removed the articles for which the titles and/or abstracts were obviously irrelevant. Among the 27 full‐text articles that were assessed for eligibility, 21 studies were excluded: 18 because hs‐CRP was not measured, two because they were not original studies and one because it was not a clinical study. After careful assessment, seven articles, with 16 treatment arms, met the inclusion criteria and were selected for the final meta‐analysis 16, 17, 18, 19, 20, 21, 22.
Figure 1.
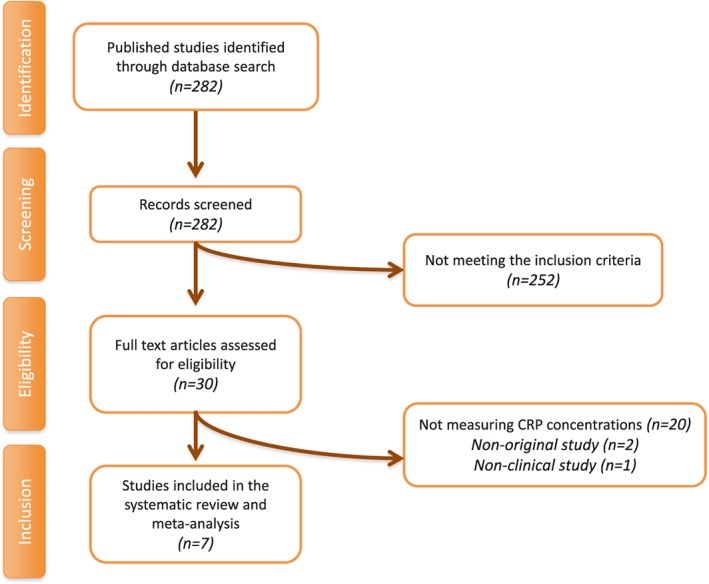
Flow chart of the studies identified and included into the meta‐analysis. CRP, C‐reactive protein
In total, 2546 participants were randomized, of whom 1707 were allocated to PCSK9 inhibitor intervention and 839 to control groups. The number of participants in these trials ranged from 49 to 901. All the included studies were published between 2012 and 2015, involving several countries worldwide. The following PCSK9 inhibitor doses were administered in the included trials: evolocumab 350 mg monthly, evolocumab 420 mg monthly, evolocumab 140 mg biweekly, alirocumab 150 mg biweekly, alirocumab 150 mg monthly, alirocumab 200 mg monthly, alirocumab 300 mg monthly and alirocumab 420 mg monthly. The duration of the intervention ranged between 3 months and 12 months. All studies were designed as multicentre, double‐blind RCTs, comprising a total of 16 treatment arms. Demographic and baseline laboratory parameters of the included studies are shown in Table 1.
Table 1.
Demographic characteristics of the included studies
| Study | Raal 2012, RUTHERFORD 16 | Raal 2014, RUTHERFORD‐2 17 | Raal 2014, TESLA Part B 18 | Stein 2012 19 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment arm | A | B | A | B | C | A | B | C | D | |||||
| Year | 2012 | 2014 | 2014 | 2012 | ||||||||||
| Jadad score | 5 | 5 | 5 | 5 | ||||||||||
| Location | Lipid clinics at 24 sites in North America, Western Europe, Hong Kong, Singapore, South Africa | 39 sites (mostly specialized lipid clinics, mainly attached to academic centres) in Australia, Asia, Europe, New Zealand, North America and South Africa | 17 sites in ten countries in North America, Europe, the Middle East and South Africa | 16 lipid clinics in the USA and Canada | ||||||||||
| Design | Phase 2, global, multicentre, double‐blind, placebo‐controlled | Phase 3, multicentre, randomized, double‐blind, placebo‐controlled | Phase 3, randomized, double‐blind, placebo‐controlled | Phase 2, randomized, double‐blind, placebo‐controlled | ||||||||||
| Length of trial | August 2011 –February 2012 | 7 February – 19 December 2013 | February 2013 – January 2014 | 18 January – 7 November 2011 | ||||||||||
| Inclusion criteria | M and F aged 18–75 years, with a diagnosis of heterozygous FH and LDL‐C ≥2.6 mmol l–1 (100 mg dl–1), TRIG ≤4.5 mmol l–1 (400 mg dl–1), at least 4 weeks of stable statin and other lipid‐lowering therapy | Patients 18–80 years of age with a diagnosis of heterozygous FH and on a stable dose of a statin with or without other approved lipid‐modifying therapy | M and F, aged ≥ 12 years, with homozygous FH and LDL‐C ≥3.4 mmol l–1 after at least 4 weeks of a stable, low‐fat diet and baseline lipid‐lowering therapies; TRIG ≤4.5 mmol l–1; and bodyweight ≥40 kg | M and F with a diagnosis of FH and LDL‐C ≥2.6 mmol l–1. at least 6 weeks of stable statin therapy | ||||||||||
| Treatment | Evolocumab 350 mg 4 weeks | Evolocumab 420 mg 4 weeks | Placebo 4 weeks | Evolocumab 140 mg 2 weeks | Placebo 2 weeks | Evolocumab 420 mg 4 weeks | Placebo 4 weeks | Evolocumab 420 mg 4 weeks | Placebo 4 weeks | Alirocumab 150 mg 2 weeks | Alirocumab 150 mg 4 weeks | Alirocumab 200 mg 4 weeks | Alirocumab 300 mg 4 weeks | Placebo 2 weeks |
| Length of treatment | 12 weeks | 12 weeks | 12 weeks | 12 weeks | 12 weeks | 12 weeks | 12 weeks | 12 weeks | 12 weeks | 12 weeks | 12 weeks | 12 weeks | 12 weeks | 12 weeks |
| N participants | 55 | 56 | 56 | 110 | 53 | 108 | 54 | 33 | 16 | 16 | 15 | 16 | 15 | 15 |
| Age (years) | 47.6 | 51.8 | 49.3 | 52.6 | 51.1 | 51.9 | 46.8 | 30 | 32 | 56.3 | 51.3 | 52.9 | 54.3 | 51.9 |
| Male (%) | 54.5 | 62.5 | 42.9 | 60 | 54 | 58 | 56 | 52 | 50 | 61 | 60 | 56 | 47 | 60 |
| BMI ( kg m –2 ) | 30,7 | 28,3 | 28,8 | 28,3 | 29,4 | |||||||||
| hs‐CRP baseline ( mg l–1 ) | 1.09 | 1.07 | 0.62 | 0.2 | 0.2 | 0.2 | 0.3 | 0.7 | 0.6 | 1.4 | 0.6 | 0.7 | 0,7 | 0.9 |
| hs‐CRP median change at the end of treatment ( mg l–1 ) | –0.06 | 0 | –0.08 | 0.01 | 0.01 | 0.01 | 0 | 0 | 0.02 | 1 | 0.4 | 0,7 | 0.6 | 0.7 |
| hs‐CRP median percentage change from baseline (%) | –7.99 | 0 | –20.34 | –7.1 | 9.2 | 4.3 | 1.4 | |||||||
| Study | Stroes 2014 , GAUSS‐2 20 | Blom 2014 21 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment arm | A | B | C | D | A | B | C | D | ||||
| Year | 2014 | 2014 | ||||||||||
| Jadad score | 5 | 5 | ||||||||||
| Location | 50 sites in North America, Europe, Asia, Africa and Australia | 88 centres in nine countries: USA, South Africa, Hungary, Denmark, Czech Republic, Canada, Belgium, Austria, Australia | ||||||||||
| Design | Phase 3, randomized, double‐blind, placebo‐ and ezetimibe‐controlled | Phase 3, randomized, double‐blind, placebo‐ and ezetimibe‐controlled | ||||||||||
| Length of trial | January – August 2013 | January 2012 – November 2013 | ||||||||||
| Inclusion criteria | Patients aged 18–80 years, on no or low‐dose statins, LDL‐C above their NCEP Adult Treatment Panel III goal, previous intolerance to ≥2 statins | Adults 18–75 years, LDL‐C ≥75 mg dl–1 (1.94 mmol l–1), TRIG ≤400 mg dl–1 (4.52 mmol l–1). On the basis of stratification according to the risk categories outlined by the ATP III of the NCEP, patients were started on background lipid‐lowering therapy with diet alone or diet plus atorvastatin at a dose of 10 mg daily, or a dose of 80 mg daily, or atorvastatin at a dose of 80 mg daily plus ezetimibe at a dose of 10 mg daily, for a run‐in period of 4–12 weeks. Patients with an LDL‐C level ≥75 mg dl–1 (1.9 mmol l–1) were then randomly assigned in a 2 : 1 ratio to receive either evolocumab (420 mg) or placebo every 4 weeks. | ||||||||||
| Treatment | Daily ezetimibe 10 mg + placebo 2 weeks | Evolocumab 140 mg 2 weeks + daily placebo | Daily ezetimibe 10 mg + placebo 4 weeks | Evolocumab 420 mg 4 weeks + daily placebo | Diet + evolocumab 420 mg 4 weeks | Diet + placebo 420 mg 4 weeks | Diet + atorvastatin 10 mg + evolocumab 420 mg 4 weeks | Diet + atorvastatin 10 mg + placebo 4 weeks | Diet + atorvastatin 80 mg + evolocumab 420 mg 4 weeks | Diet + atorvastatin 80 mg + placebo 4 weeks | Diet + atorvastatin 80 mg + ezetimibe 10 mg + evolocumab 420 mg 4 weeks | Diet + atorvastatin 80 mg + ezetimibe 10 mg + placebo 4 weeks |
| Length of treatment | 12 weeks | 12 weeks | 12 weeks | 12 weeks | 52 weeks | 52 weeks | 52 weeks | 52 weeks | 52 weeks | 52 weeks | 52 weeks | 52 weeks |
| N participants | 51 | 103 | 51 | 102 | 74 | 37 | 254 | 129 | 145 | 73 | 126 | 63 |
| Age (years) | 62 | 61 | 60 | 63 | 50.7 | 53.5 | 57.2 | 57 | 57.8 | 58.4 | 54.2 | 55.9 |
| Male (%) | 47 | 55 | 57 | 55 | 47.3 | 40.5 | 42.9 | 45.7 | 52.4 | 45.2 | 55.6 | 52.4 |
| BMI ( kg m –2 ) | 31.1 | 29.4 | 29.6 | 30.2 | 30.3 | 31.6 | 29.6 | 30.2 | ||||
| hs‐CRP baseline ( mg l–1 ) | 1.7 | 1.4 | 1.8 | 1.8 | 0.2 | 0.2 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| hs‐CRP median change at the end of treatment ( mg l–1 ) | 1.7 | 1.7 | 1.6 | 1.5 | 0.2 | 0.2 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| hs‐CRP median percentage change from baseline (%) | 13.1 | 27.4 | 0 | 0.7 | 0 | 0.9 | 0 | 10.2 | ||||
| Study | Cannon 2015 – ODYSSEY COMBO II | |
|---|---|---|
| Treatment arm | A | |
| Year | 2015 | |
| Jadad score | 5 | |
| Location | 126 sites (Europe, Israel, North America, South Africa, South Korea) | |
| Design | Double‐blind, double‐dummy, active‐controlled, parallel‐group | |
| Length of trial | August 2012 – May 2013 | |
| Inclusion criteria | High cardiovascular risk patients with inadequately controlled hypercholesterolaemia on maximally tolerated doses of statins | |
| Treatment | Alirocumab 75 mg biweekly. The dose in the alirocumab arm (only) was automatically increased, per protocol, at Week 12, to 150 mg biweekly if the Week‐8 LDL‐C value was ≥1.8 mmol l–1. | Ezetimibe 10 mg daily |
| Length of treatment |
52 weeks The study is ongoing at the time of writing, and randomized treatment will continue until Week 104, followed by an 8‐week post‐treatment observational period |
|
| N participants | 479 | 241 |
| Age (years) | 61.7 | 61.3 |
| Male (%) | 75.2 | 70.5 |
| BMI ( kg m –2 ) | 30.0 | 30.3 |
| CRP baseline ( mg l–1 ) | 0.8 | 0.9 |
| CRP median change at the end of treatment ( mg l–1 ) | 0.8 | 0.6 |
| CRP median percentage change from baseline (%) | ||
ATP, Adult Treatment Panel; BMI, body mass index; F, female; HF, familial hypercholesterolaemia; hs‐CRP, high‐sensitivity C‐reactive protein; LDL‐C, low‐density lipoprotein cholesterol; M, male; NCEP, National Cholesterol Education Program; PCSK9, proprotein convertase subtilisin/kexin type 9; TRIG, triglyceride.
Risk of bias assessment
The systematic assessment of bias in the included studies using Cochrane criteria 34 and the Jadad scale is shown in Table 2. Overall, studies included in the meta‐analysis provided sufficient data regarding the methods applied for random sequence generation, allocation concealment, blinding, as well other sources of bias. All studies reported detailed information about financial support from a commercial source.
Table 2.
Systematic assessment of bias in the included studies, using Cochrane criteria
| Random sequence generation | Allocation concealment | Blinding | Incomplete outcome data | Selective reporting | Free of other bias | |
|---|---|---|---|---|---|---|
| Raal et al. 16 , RUT HERFORD | YES | YES | YES | YES | YES | YES |
| Raal et al. 17 , RUTHERFORD‐2 | YES | YES | YES | YES | YES | YES |
| Raal et al. 17 , TESLA Part B | YES | YES | YES | YES | YES | YES |
| Stein et al. 19 | YES | YES | YES | YES | YES | YES |
| Str oes et al. 20 , GAUSS‐2 | YES | YES | YES | YES | YES | YES |
| Blom et al. 21 2014 | YES | YES | YES | YES | YES | YES |
| Cannon et al. 22 2015 ODISSE Y COMBO‐2 | YES | YES | YES | YES | YES | YES |
Effect of PCSK9 inhibitors on plasma hs‐CRP concentrations
Meta‐analysis of data from 16 randomized controlled treatment arms did not suggest any significant effect of PCSK9 inhibitors on plasma hs‐CRP concentrations (WMD: 0.002 mg l–1, CI: –0.017, 0.021; P = 0.807; I 2 = 37.26%) (Figure 2). This effect size was robust and not sensitive to any single study included in the analysis (Figure 2). When the included studies were stratified according to the type of PCSK9 inhibitor used, no significant difference was observed with either evolocumab (WMD: 0.002 mg l–1, CI: –0.02, 0.02; P = 0.855; I 2 = 50.98%) or alirocumab (WMD: 0.15 mg l–1, CI: –0.11, 0.40; P = 0.259; I 2 = 0%) (Figure 3). When the studies were categorized according to the number of PCSK9 inhibitor injections received during the course of the trial, there was no effect in the subgroup of trials with ≥6 injections (WMD: –0.01 mg l–1, CI: –0.02, 0.01; P = 0.531; I 2 = 45.07%) but a slight increase in plasma hs‐CRP was observed in the subgroup of trials with <6 PCSK9 inhibitor injections (WMD: 0.06 mg l–1, CI: 0.01, 0.11; P = 0.011; I 2 = 0%) (Figure 4). There was no effect of dosing strategy on the effect size as no significant alteration of hs‐CRP levels was seen in trials with either biweekly (WMD: 0.13 mg l–1, CI: –0.20, 0.46; P = 0.433; I 2 = 55.19%) or monthly (WMD: 0.003 mg l–1, CI: –0.01, 0.01; P = 0.59; I 2 = 0%) injection strategies (Figure 5). When the studies were categorized according to the studied population, no significant change in hs‐CRP was observed either in familial hypercholesterolaemia (FH) (WMD: 0.03 mg l–1, CI: –0.04, 0.11; P = 0.396; I 2 = 57.30%) or non‐FH (WMD: 0.0 mg l–1, CI: –0.01, 0.01; P = 0.979; I2 = 0%) populations (Figure 6).
Figure 2.
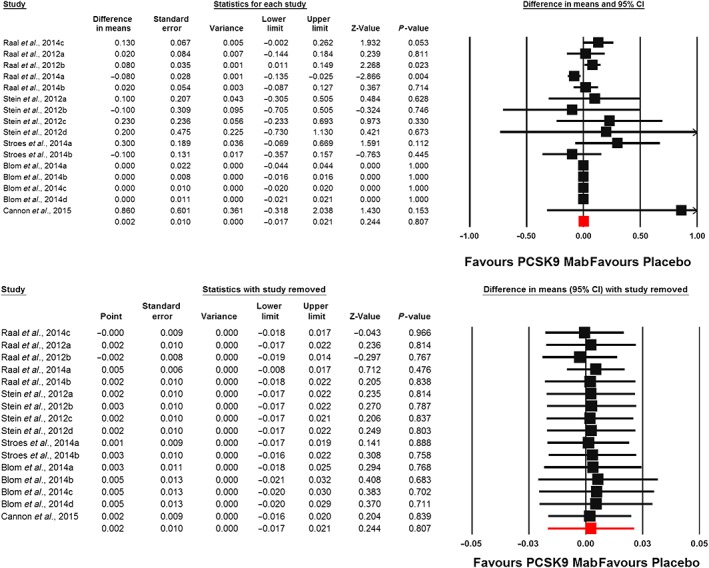
Forest plot detailing weighted mean difference and 95% confidence intervals (CIs) for the impact of proprotein convertase subtilisin/kexin type 9 inhibitors on plasma high‐sensitivity C‐reactive protein concentrations (upper plot). Lower plot shows the result of leave‐one‐out sensitivity analysis
Figure 3.

Forest plot detailing weighted mean difference and 95% confidence intervals (CIs) for the impact of evolocumab (upper plot) and alirocumab (lower plot) on plasma high‐sensitivity C‐reactive protein concentrations
Figure 4.
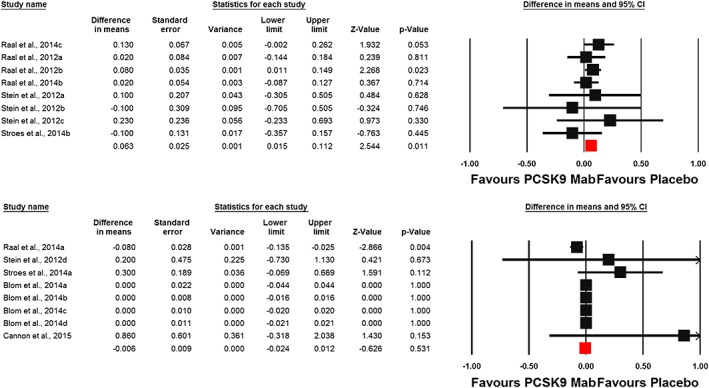
Forest plot detailing weighted mean difference and 95% confidence intervals (CIs) for the impact of proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors on plasma high‐sensitivity C‐reactive protein concentrations in trials with <6 (upper plot) and ≥6 (lower plot) injections of PCSK9 inhibitor
Figure 5.
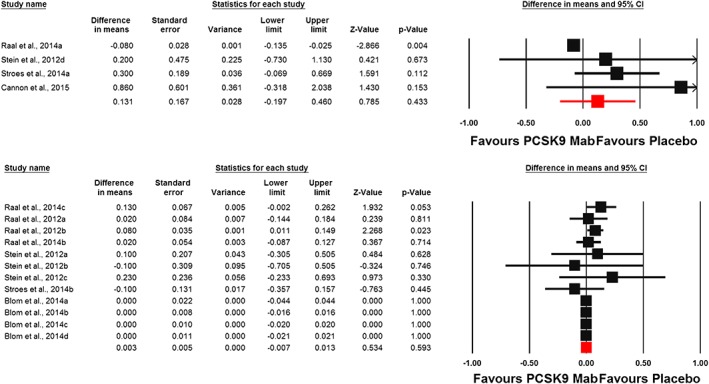
Forest plot detailing weighted mean difference and 95% confidence intervals (CIs) for the impact of proprotein convertase subtilisin/kexin type 9 inhibitors on plasma high‐sensitivity C‐reactive protein concentrations in trials with biweekly (upper plot) and monthly (lower plot) dosing strategy
Figure 6.
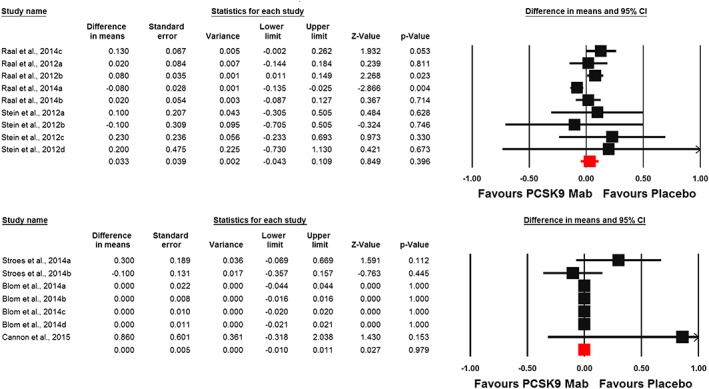
Forest plot detailing weighted mean difference and 95% confidence intervals (CIs) for the impact of proprotein convertase subtilisin/kexin type 9 inhibitors on plasma high‐sensitivity C‐reactive protein concentrations in trials in populations with (upper plot) and without (lower plot) familial hypercholesterolaemia
Meta‐regression
Random‐effects meta‐regression did not suggest an association between changes in plasma hs‐CRP and LDL‐C concentrations following treatment with PCSK9 inhibitors (slope: 0.0002; 95% CI: –0.001, 0.001; P = 0.698). Likewise, the impact of PCSK9 inhibitors on plasma hs‐CRP concentrations was not influenced by the cumulative dosage of drug received during the course of the trial (slope: 0; 95% CI: –0.00001, 0.00001; P = 0.980) (Figure 7).
Figure 7.
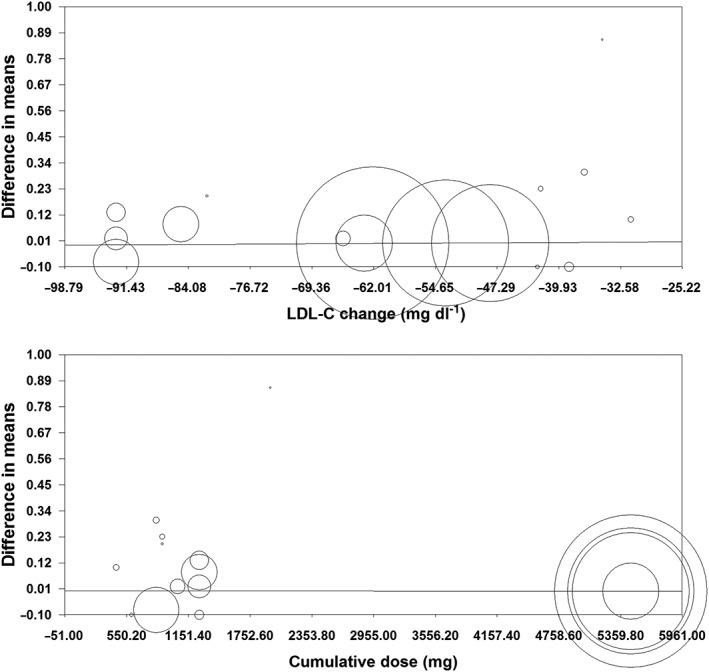
Meta‐regression plots for the association between mean changes in plasma high‐sensitivity C‐reactive protein concentrations with changes in plasma low‐density lipoprotein cholesterol (LDL‐C) concentrations (upper plot) and cumulative dose of proprotein convertase subtilisin/kexin type 9 inhibitor received during the course of the trial (lower plot)
Publication bias
The results of Begg's rank correlation (Kendall's tau with continuity correction = 0.26; Z = 1.40; two‐tailed P‐value = 0.163) and Egger's linear regression (intercept = 0.59; standard error = 0.39; 95% CI = –0.25, 1.43; t = 1.50; df = 14; two‐tailed P = 0.156) tests did not suggest publication bias in the meta‐analysis of the effect of PCSK9 inhibitors on plasma CRP concentrations. However, the funnel plot of study precision (inverse standard error) by effect size (mean difference) was asymmetric and indicative of potential publication bias. This potential bias was addressed using trim‐and‐fill correction, the results of which attributed this bias to four potentially missing studies on the left‐hand side of the funnel plot, yielding a corrected effect size of 0.001 mg l–1 (95% CI: –0.02, 0.02) (Figure 8).
Figure 8.
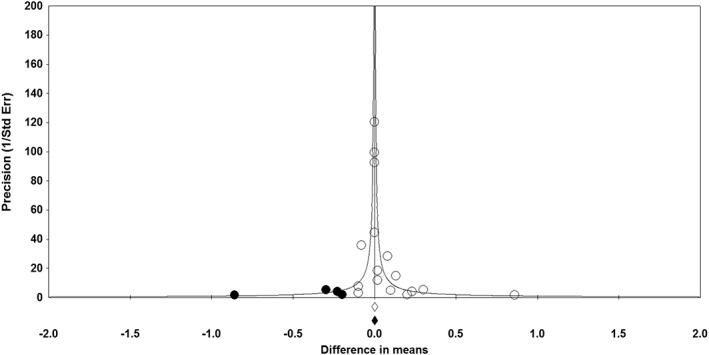
Funnel plot detailing publication bias in the studies reporting the impact of proprotein convertase subtilisin/kexin type 9 inhibitors on plasma sensitivity C‐reactive protein concentrations. Open circles represent observed published studies; closed circles represent imputed unpublished studies. Std Err, standard error
Discussion
RCTs have indicated that the PCSK9 inhibitors significantly reduced LDL‐C levels with or without other lipid‐lowering therapies 16, 17, 18, 19, 20, 21, 22. However, a possible pleiotropic effect of these agents, in terms of anti‐inflammatory actions, remains elusive. To the best of our knowledge, this meta‐analysis was the first with the aim of elucidating the role of PCSK9 inhibitors in reducing systemic inflammation. Overall, our findings clearly showed that PCSK9 inhibitors have no significant influence on hs‐CRP levels, independently from the type and dose of PCSK9 inhibitor, as well changes in LDL‐C levels. In spite of this observation, there are some issues that should be taken into account. Firstly, it is worth noting that the populations studied would be expected to have normal levels of hs‐CRP. In patients with FH, the marked increase in LDL‐C seems to be associated with vascular events, without an important underlying inflammatory state 35. Even patients with homozygous FH, who have extremely high levels of LDL‐C and therefore a very high risk of early atherosclerosis, were found to have only slightly increased levels of CRP compared with control subjects 36. It has also been argued that in FH patients, high LDL‐C levels cause atherosclerosis without a major inflammatory component, whereas an inflammatory response is driven by elevated remnant cholesterol concentrations 35. However, among subjects enrolled in the present meta‐analysis without FH – that is, statin‐intolerant patients – the design of the studies included a run‐in phase with statin therapy, which is known to mitigate the vascular inflammatory response. Taken together, the above observations could explain, at least in part, both the low hs‐CRP levels at baseline and the lack of reduction in this biomarker after treatment. Secondly, CRP has been the most widely used inflammatory biomarker in statin trials, in view of the fact that this is the only reproducible biomarker for which a standardized assay has been developed 37, 38. Although the association between elevated CRP levels and CVDs is well established, the lack of CRP‐lowering effect does not call into question the clinical benefit of PCSK9 inhibitors as these agents have been shown to reduce clinical outcomes and have been recently approved for patients with heterozygous FH 39, 40, 41. The recent success of these agents could be principally ascribed to the magnitude of the lipid‐lowering effect, and emphasizes the primacy of LDL‐C lowering as a strategy to prevent coronary heart disease.
It would be of paramount importance to elucidate further the role of PCSK9 beyond LDL‐C modulation and homeostasis. It is evident that PCSK9 is expressed in atherosclerotic plaques 23, and pathways connecting PCSK9 to vascular inflammation and apoptosis are being explored 42. Furthermore, an in vivo study revealed that infection and inflammation stimulate PCSK9 expression 43, and small interfering RNA‐mediated knockdown of PCSK9 in human macrophages has been found to reduce inhibitor of nuclear factor kappa B (NF‐κB)‐α degradation and NF‐κB nuclear translocation, thereby reducing the expression of proinflammatory genes 44. In patients with an acute myocardial infarction, plasma PCSK9 concentration is elevated compared with those with stable coronary artery disease 45. According to these findings, it is conceivable that PCSK9 inhibition might have a positive impact on plaque stability and growth, and the effects of these inhibitors on clinical outcomes have been recently established 39, 40, 41.
The present meta‐analysis had several limitations. Firstly, the trials tested different types and doses of PCSK9 inhibitors, which may have introduced heterogeneity to the results. However, a random‐effects model and subgroup analyses were conducted to minimize the impact of heterogeneity. Secondly, included the studies differed in terms of baseline characteristics, study design and duration. In particular, most of the subjects underwent a run‐in phase with high‐dose statin therapy, and this could have influenced the results in terms of hs‐CRP‐levels. In addition, most of the evolocumab trials lasted 12 weeks, so we cannot exclude a time‐dependent effect of these agents on hs‐CRP. Thirdly, none of the studies considered elevated baseline hs‐CRP as an inclusion criterion and none was conducted on patients with acute coronary syndromes, who might have been more prone to having high hs‐CRP levels and, in turn, to responding to the effects of lipid‐lowering agents on systemic inflammation.
In conclusion, the findings of the present meta‐analysis did not suggest an effect of PCSK9‐inhibitors in decreasing hs‐CRP levels. Further investigations are needed to elucidate whether these agents have pleiotropic actions that go beyond their established hypolipidaemic effects. However, as preliminary data suggest that PCSK‐9 inhibitors reduce cardiovascular events without lowering hs‐CRP levels, the question arises as to whether inflammation plays a major causal role in atherosclerosis. These findings underline the priority of LDL‐C lowering as a strategy for preventing cardiovascular events 46. Other studies, enrolling patients after acute coronary syndromes, are currently ongoing, and it will be important to evaluate the effect of PCSK9 inhibitors on the inflammatory response in populations with elevated baseline hs‐CRP levels, to shed light on their possible anti‐inflammatory actions.
Competing Interests
The authors declare no conflict of interests. All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf and declare no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
Supporting information
Appendix S1 Excluded trials' list with reason for exclusion
Supporting info item
Sahebkar, A. , Di Giosia, P. , Stamerra, C. A. , Grassi, D. , Pedone, C. , Ferretti, G. , Bacchetti, T. , Ferri, C. , and Giorgini, P. (2016) Effect of monoclonal antibodies to PCSK9 on high‐sensitivity C‐reactive protein levels: a meta‐analysis of 16 randomized controlled treatment arms. Br J Clin Pharmacol, 81: 1175–1190. doi: 10.1111/bcp.12905.
References
- 1. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics – 2015 update: a report from the American Heart Association. Circulation 2015; 131: e29–e322. [DOI] [PubMed] [Google Scholar]
- 2. Steinberg D Atherogenesis in perspective: hypercholesterolemia and inflammation as partners in crime. Nat Med 2002; 8: 1211–7. [DOI] [PubMed] [Google Scholar]
- 3. Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation 2002; 105: 1135–43. [DOI] [PubMed] [Google Scholar]
- 4. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) . Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002; 106: 3143–421. [PubMed] [Google Scholar]
- 5. Taylor FC, Huffman M, Ebrahim S. Statin therapy for primary prevention of cardiovascular disease. JAMA 2013; 310: 2451–2. [DOI] [PubMed] [Google Scholar]
- 6. Naci H, Brugts JJ, Fleurence R, Tsoi B, Toor H, Ades AE. Comparative benefits of statins in the primary and secondary prevention of major coronary events and all‐cause mortality: a network meta‐analysis of placebo‐controlled and active‐comparator trials. Eur J Prev Cardiol 2013; 20: 641–57. [DOI] [PubMed] [Google Scholar]
- 7. Robinson JG, Smith B, Maheshwari N, Schrott H. Pleiotropic effects of statins: benefit beyond cholesterol reduction? A meta‐regression analysis. J Am Coll Cardiol 2005; 46: 1855–62. [DOI] [PubMed] [Google Scholar]
- 8. Robinson JG. Models for describing relations among the various statin drugs, low‐density lipoprotein cholesterol lowering, pleiotropic effects, and cardiovascular risk. Am J Cardiol 2008; 101: 1009–15. [DOI] [PubMed] [Google Scholar]
- 9. Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ; JUPITER Study Group . Rosuvastatin to prevent vascular events in men and women with elevated C‐reactive protein. N Engl J Med 2008; 359: 2195–207. [DOI] [PubMed] [Google Scholar]
- 10. Ridker PM, Lüscher TF. Anti‐inflammatory therapies for cardiovascular disease. Eur Heart J 2014; 35: 1782–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gouni‐Berthold I, Berthold HK. PCSK9 antibodies for the treatment of hypercholesterolemia. Nutrition 2014; 6: 5517–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sahebkar A, Chew GT, Watts GF. Recent advances in pharmacotherapy for hypertriglyceridemia. Prog Lipid Res 2014; 56: 47–66. [DOI] [PubMed] [Google Scholar]
- 13. Giugliano RP, Sabatine MS. Are PCSK9 inhibitors the next breakthrough in the cardiovascular field? J Am Coll Cardiol 2015; 65: 2638–51. [DOI] [PubMed] [Google Scholar]
- 14. Sahebkar A, Watts GF. New LDL‐cholesterol lowering therapies: pharmacology, clinical trials, and relevance to acute coronary syndromes. Clin Ther 2013; 35: 1082–98. [DOI] [PubMed] [Google Scholar]
- 15. Sahebkar A, Watts GF. New therapies targeting apoB metabolism for high‐risk patients with inherited dyslipidaemias: what can the clinician expect? Cardiovasc Drugs Ther 2013; 27: 559–67. [DOI] [PubMed] [Google Scholar]
- 16. Raal F, Scott R, Somaratne R, Bridges I, Li G, Wasserman SM, Stein EA. Low‐density lipoprotein cholesterol‐lowering effects of AMG 145, a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 serine protease in patients with heterozygous familial hypercholesterolemia: the Reduction of LDL‐C with PCSK9 Inhibition in Heterozygous Familial Hypercholesterolemia Disorder (RUTHERFORD) randomized trial. Circulation 2012; 126: 2408–17. [DOI] [PubMed] [Google Scholar]
- 17. Raal FJ, Stein EA, Dufour R, Turner T, Civeira F, Burgess L, Langslet G, Scott R, Olsson AG, Sullivan D, Hovingh GK, Cariou B, Gouni‐Berthold I, Somaratne R, Bridges I, Scott R, Wasserman SM, Gaudet D; RUTHERFORD‐2 Investigators . PCSK9 inhibition with evolocumab (AMG 145) in heterozygous familial hypercholesterolaemia (RUTHERFORD‐2): a randomised, double‐blind, placebo‐controlled trial. Lancet 2015; 385: 331–40. [DOI] [PubMed] [Google Scholar]
- 18. Raal FJ, Honarpour N, Blom DJ, Hovingh GK, Xu F, Scott R, Wasserman SM, Stein EA; TESLA Investigators . Inhibition of PCSK9 with evolocumab in homozygous familial hypercholesterolaemia (TESLA Part B): a randomised, double‐blind, placebo‐controlled trial. Lancet 2015; 385: 341–50. [DOI] [PubMed] [Google Scholar]
- 19. Stein EA, Gipe D, Bergeron J, Gaudet D, Weiss R, Dufour R, Wu R, Pordy R. Effect of a monoclonal antibody to PCSK9, REGN727/SAR236553, to reduce low‐density lipoprotein cholesterol in patients with heterozygous familial hypercholesterolaemia on stable statin dose with or without ezetimibe therapy: a phase 2 randomised controlled trial. Lancet 2012; 380: 29–36. [DOI] [PubMed] [Google Scholar]
- 20. Stroes E, Colquhoun D, Sullivan D, Civeira F, Rosenson RS, Watts GF, Bruckert E, Cho L, Dent R, Knusel B, Xue A, Scott R, Wasserman SM, Rocco M; GAUSS‐2 Investigators . Anti‐PCSK9 antibody effectively lowers cholesterol in patients with statin intolerance: the GAUSS‐2 randomized, placebo‐controlled phase 3 clinical trial of evolocumab. J Am Coll Cardiol 2014; 63: 2541–8. [DOI] [PubMed] [Google Scholar]
- 21. Blom DJ, Hala T, Bolognese M, Lillestol MJ, Toth PD, Burgess L, Ceska R, Roth E, Koren MJ, Ballantyne CM, Monsalvo ML, Tsirtsonis K, Kim JB, Scott R, Wasserman SM, Stein EA; DESCARTES Investigators . A 52‐week placebo‐controlled trial of evolocumab in hyperlipidemia. N Engl J Med 2014; 370: 1809–19. [DOI] [PubMed] [Google Scholar]
- 22. Cannon CP, Cariou B, Blom D, McKenney JM, Lorenzato C, Pordy R, Chaudhari U, Colhoun HM; ODYSSEY COMBO II Investigators . Efficacy and safety of alirocumab in high cardiovascular risk patients with inadequately controlled hypercholesterolaemia on maximally tolerated doses of statins: the ODYSSEY COMBO II randomized controlled trial. Eur Heart J 2015; 36: 1186–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ferri N, Tibolla G, Pirillo A, Cipollone F, Mezzetti A, Pacia S, Corsini A, Catapano AL. Proprotein convertase subtilisin kexin type 9 (PCSK9) secreted by cultured smooth muscle cells reduces macrophages LDLR levels. Atherosclerosis 2012; 220: 381–6. [DOI] [PubMed] [Google Scholar]
- 24. Lan H, Pang L, Smith MM, Levitan D, Ding W, Liu L, Shan L, Shah VV, Laverty M, Arreaza G, Zhang Q, Murgolo NJ, Hernandez M, Greene JR, Gustafson EL, Bayne ML, Davis HR, Hedrick JA. Proprotein convertase subtilisin/kexin type 9 (PCSK9) affects gene expression pathways beyond cholesterol metabolism in liver cells. J Cell Physiol 2010; 224: 273–81. [DOI] [PubMed] [Google Scholar]
- 25. Ridker PM, Cannon CP, Morrow D, Rifai N, Rose LM, McCabe CH, Pfeffer MA, Braunwald E; Pravastatin or Atorvastatin Evaluation and Infection Therapy‐Thrombolysis in Myocardial Infarction 22 (PROVE IT‐TIMI 22) Investigators . C‐reactive protein levels and outcomes after statin therapy. N Engl J Med 2005; 352: 20–8. [DOI] [PubMed] [Google Scholar]
- 26. Ridker PM, Rifai N, Pfeffer MA, Sacks F, Braunwald E. Long‐term effects of pravastatin on plasma concentration of C‐reactive protein. The Cholesterol and Recurrent Events (CARE) Investigators. Circulation 1999; 100: 230–5. [DOI] [PubMed] [Google Scholar]
- 27. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ 2009; 339: b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Borenstein M, Hedges L, Higgins J, Rothstein Hl. Comprehensive Meta‐analysis Version 2, Biostat, Englewood NJ, 2005. [Google Scholar]
- 29. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005; 5: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sutton AJ, Abrams KR, Jones DR, Sheldon TA, Song F. Methods for Meta‐Analysis in Medical Research. West Sussex: John Wiley & Sons, 2000. [Google Scholar]
- 31. Ferretti G, Bacchetti T, Sahebkar A. Effect of statin therapy on paraoxonase‐1 status: a systematic review and meta‐analysis of 25 clinical trials. Prog Lipid Res 2015; 60: 50–73. [DOI] [PubMed] [Google Scholar]
- 32. Sahebkar A Are curcuminoids effective C‐reactive protein‐lowering agents in clinical practice? Evidence from a meta‐analysis. Phytother Res 2014; 28: 633–42. [DOI] [PubMed] [Google Scholar]
- 33. Duval S, Tweedie R. Trim and fill: a simple funnel‐plot‐based method of testing and adjusting for publication bias in meta‐analysis. Biometrics 2000; 56: 455–63. [DOI] [PubMed] [Google Scholar]
- 34. Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2. The Cochrane Collaboration, 2009. [Google Scholar]
- 35. Varbo A, Benn M, Tybjærg‐Hansen A, Nordestgaard BG. Elevated remnant cholesterol causes both low‐grade inflammation and ischemic heart disease, whereas elevated low‐density lipoprotein cholesterol causes ischemic heart disease without inflammation. Circulation 2013; 128: 1298–309. [DOI] [PubMed] [Google Scholar]
- 36. Panz V, Immelman A, Paiker J, Pilcher G, Raal F. High‐dose statin therapy does not induce insulin resistance in patients with familial hypercholesterolemia. Metab Syndr Relat Disord 2012; 10: 351–7. [DOI] [PubMed] [Google Scholar]
- 37. Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO 3rd, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, Rifai N, Smith SC Jr, Taubert K, Tracy RP, Vinicor F; Centers for Disease Control and Prevention; American Heart Association . Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 2003; 107: 499–511. [DOI] [PubMed] [Google Scholar]
- 38. Passacquale G, di Giosia P, Ferro A. The role of inflammatory biomarkers in developing targeted cardiovascular therapies: lessons from the cardiovascular inflammation reduction trials. Cardiovasc Res 2015; pii: cvv227. [DOI] [PubMed] [Google Scholar]
- 39. Navarese EP, Kolodziejczak M, Schulze V, Gurbel PA, Tantry U, Lin Y, Brockmeyer M, Kandzari DE, Kubica JM, D'Agostino RB Sr, Kubica J, Volpe M, Agewall S, Kereiakes DJ, Kelm M. Effects of proprotein convertase subtilisin/kexin type 9 antibodies in adults with hypercholesterolemia: a systematic review and meta‐analysis. Ann Intern Med 2015; 163: 40–51. [DOI] [PubMed] [Google Scholar]
- 40. Sabatine MS, Giugliano RP, Wiviott SD, Raal FJ, Blom DJ, Robinson J, Ballantyne CM, Somaratne R, Legg J, Wasserman SM, Scott R, Koren MJ, Stein EA; Open‐Label Study of Long‐Term Evaluation against LDL Cholesterol (OSLER) Investigators . Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med 2015; 372: 1500–9. [DOI] [PubMed] [Google Scholar]
- 41. Robinson JG, Farnier M, Krempf M, Bergeron J, Luc G, Averna M, Stroes ES, Langslet G, Raal FJ, el Shahawy M, Koren MJ, Lepor NE, Lorenzato C, Pordy R, Chaudhari U, Kastelein JJ; ODYSSEY LONG TERM Investigators . Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med 2015; 372: 1489–99. [DOI] [PubMed] [Google Scholar]
- 42. Kühnast S, van der Hoorn JW, Pieterman EJ, van den Hoek AM, Sasiela WJ ,Gusarova V, Peyman A, Schäfer HL, Schwahn U, Jukema JW, Princen HM. Alirocumab inhibits atherosclerosis, improves the plaque morphology, and enhances the effects of a statin. J Lipid Res 2014; 55: 2103–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Feingold KR, Moser AH, Shigenaga JK, Patzek SM, Grunfeld C. Inflammation stimulates the expression of PCSK9. Biochem Biophys Res Commun 2008; 374: 341–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tang Z, Jiang L, Peng J, Ren Z, Wei D, Wu C, Pan L, Jiang Z, Liu L. PCSK9 siRNA suppresses the inflammatory response induced by oxLDL through inhibition of NF‐κB activation in THP‐1‐derived macrophages. Int J Mol Med 2012; 30: 931–8. [DOI] [PubMed] [Google Scholar]
- 45. Almontashiri NA, Vilmundarson RO, Ghasemzadeh N, Dandona S, Roberts R, Quyyumi AA, Chen HH, Stewart AF. Plasma PCSK9 levels are elevated with acute myocardial infarction in two independent retrospective angiographic studies. PLoS One 2014; 9: e106294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jarcho JA, Keaney JF Jr Proof that lower is better – LDL cholesterol and IMPROVE‐IT. N Engl J Med 2015; 372: 2448–50. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Excluded trials' list with reason for exclusion
Supporting info item


