Abstract
Aim
Ischaemia‐reperfusion injury (IRI) causes impaired endothelial function and is a major component of the adverse effects of reperfusion following myocardial infarction. Rotigaptide increases gap junction conductance via connexin‐43. We tested the hypothesis that rotigaptide reduces experimental myocardial infarction size and ameliorates endothelial IRI in humans.
Methods
Myocardial infarction study: porcine myocardial infarction was achieved by catheter‐induced occlusion of the left anterior descending artery. In a randomized double‐blind study, rotigaptide (n = 9) or placebo (n = 10) was administered intravenously as a 10 min bolus prior to reperfusion and continuously during 2 h of reperfusion. Myocardial infarction size (IS) was assessed as proportion of the area at risk (AAR). Human translational study: forearm IRI was induced in the presence or absence of intra‐arterial rotigaptide. In a randomized double‐blind study, forearm arterial blood flow was measured at rest and during intra‐arterial infusion of acetylcholine (5–20 μg min–1; n = 11) or sodium nitroprusside (2–8 mg min–1; n = 10) before and after intra‐arterial infusion of placebo or rotigaptide, and again following IRI.
Results
Myocardial infarction study: Rotigaptide treatment was associated with a reduction of infarct size (IS/AAR[%]: 18.7 ± 4.1 [rotigaptide] vs. 43.6 ± 4.2 [placebo], P = 0.006). Human translational study: Endothelium‐dependent vasodilatation to acetylcholine was attenuated after ischaemia‐reperfusion in the presence of placebo (P = 0.007), but not in the presence of rotigaptide (P = NS). Endothelium‐independent vasodilatation evoked by sodium nitroprusside was unaffected by IRI or rotigaptide (P = NS).
Conclusions
Rotigaptide reduces myocardial infarction size in a porcine model and protects from IRI‐related endothelial dysfunction in man. Rotigaptide may have therapeutic potential in the treatment of myocardial infarction.
Keywords: blood flow, endothelium, myocardial infarction, pharmacology, reperfusion
What is Already Known about this Subject
Ischaemia‐reperfusion injury reflects the paradoxical injury associated with restoration of blood flow to an ischaemic organ.
Connexins may play a pivotal role in ischaemia‐reperfusion injury.
A means to prevent this paradoxical injury should translate into improved clinical outcomes for a wide range of patients including those treated for ischaemic stroke, myocardial infarction or for those undergoing solid organ transplantation.
What this Study Adds
Rotigaptide, a modulator of connexin 43 phosphorylation, is associated with a marked reduction in porcine myocardial infarction size when administered at the time of reperfusion.
Ischaemia‐reperfusion injury reduces endothelium‐dependent vasodilatation in the human forearm arterial circulation, but this effect is not seen in the presence of rotigaptide.
These findings provide important direction for the development of connexin modulators designed for the limitation of clinically important ischaemia‐reperfusion injury.
Introduction
Acute arterial occlusion may lead to end‐organ ischaemia and, ultimately, infarction. Although treatment is usually directed at prompt restoration of flow in the occluded artery, reperfusion may trigger further injury beyond that induced by ischaemia alone. Importantly, such ischaemia‐reperfusion injury (IRI) can markedly reduce the benefits of reperfusion therapies employed in a variety of clinical settings, including myocardial infarction and stroke 1.
Impaired endothelium‐dependent vasomotor function is an important manifestation of IRI 2, 3, 4. Disrupted endothelium‐dependent vasomotion may not just be a surrogate marker for IRI but also of prime importance in the pathophysiological process. Such reperfusion injury may be prevented by prior exposure to intermittent sublethal ischaemia 5, 6 but the mechanism underlying the preconditioning phenomenon remains incompletely defined. As such, despite being an obvious therapeutic target to protect from the important deleterious effects associated with vascular reperfusion, no single physical preconditioning strategy or pharmacologic agent has been identified to fulfill this role.
Gap junctions may be pivotal in IRI 7. They form an aqueous pore through which small hydrophilic molecules and ionic charge may pass between neighbouring cells. Each gap junction comprises two hemichannels, composed of connexin (Cx) subunits that allow the gap junction to open and close depending upon phosphorylation status 8. Cx43 is particularly abundant in myocardial tissue 9 and, in addition to a role in the mediation of vasodilator responses evoked by endothelium‐derived hyperpolarizing factor (EDHF) 10, Cx43 appears to be important in the mediation of ischaemia–reperfusion injury as well as the preconditioning process 11, 12.
Rotigaptide (ZP‐123) is a hexapeptide drug that was originally developed as an anti‐arrhythmic agent. It promotes electrical coupling between ventricular myocytes by increasing gap junction conductance 13, 14, potentially via alterations in the phosphorylation status of Cx43 15 and it increases the number of gap junctions in the ischaemic myocardium 16 The open‐state probability of Cx43 is depressed by acidosis 17 and during reperfusion after ischaemia 18 suggesting that rotigaptide's properties will be most suited to these environments. Indeed, rotigaptide's anti‐arrhythmic activity is more potent in conditions of metabolic stress 14.
We examined the effects of rotigaptide upon myocardial infarction size when administered during reperfusion in a porcine model. We subsequently assessed the potential protective effects of rotigaptide in the human forearm arterial circulation subjected to ischaemia and reperfusion.
Methods
Porcine myocardial infarction study
Animals and study design
Thirty Danish Landrace pigs (Paaskehoejgaardcentret, Aarhus, Denmark) weighing approximately 15 kg were studied. Animals were treated as humanely as possible according to the principles stated in Danish law on animal experiments and the recommendations of ARRIVE.
Animals were anaesthetized with midazolam 50 mg s.c. and ketamine 250 mg s.c., intubated and ventilated at 4.5 l min–1 with a 50/50 mixture of atmospheric air and oxygen. Anaesthesia was maintained with an infusion of pentobarbital 50 mg ml–1 at 5 ml h–1. Ventilatory alterations were made to maintain physiological levels of oxygenation and electrolyte balance and were guided by hourly blood gas measurements. Temperature was kept between 36.5°C and 38.0°C with the use of a heating blanket. Blood pressure was maintained at above 80 mmHg with epinephrine as needed. Ventricular fibrillation and sustained ventricular tachycardia were treated with DC cardioversion. Intravenous heparin 4000 IU at baseline followed by 2000 IU h–1 was administered to maintain anticoagulation.
Haemodynamic assessments
Pressure derived indices of ventricular function were acquired using a 5F pressure catheter positioned in the left ventricle. Pressures were sampled via high‐fidelity analogue to digital hardware at 500 Hz to dedicated data acquisition software (Notocord®, France). We used maximum pressure development over time (dP/dtmax, mmHg s−1), maximum negative pressure development over time (dP/dtmin, mmHg s−1) and end systolic pressure (ESP, mmHg) as indices of cardiac function.
Administration of rotigaptide
Pigs were randomized to intravenous infusion of either rotigaptide (1 μg kg–1 bolus at time of reperfusion +10 μg kg–1 h–1 infusion i.v. during 2 h of reperfusion) or placebo, (bolus + continuous infusion i.v.). Researchers were blinded to the presence of rotigaptide or placebo.
Induction of myocardial infarction
A standard 6F angioplasty guide catheter was used to introduce a guidewire into the left anterior descending artery (LAD) under fluoroscopic guidance. A 2 mm angioplasty balloon was positioned immediately distal to the first diagonal and inflated to achieve vessel occlusion for 40 min. LAD occlusion distal to the first diagonal was confirmed by angiography. After 40 min, the balloon was deflated. Reperfusion was for 120 min, after which median sternotomy was performed.
Assessment of myocardial infarction size and area at risk
A suture was applied immediately distal to the first diagonal artery and the heart was perfusion‐stained with intra‐atrial injection of 10% Evans blue dye before euthanasia and excision of the heart to determine the area at risk. Hearts were frozen at −80°C for 20 min and cut into 7 mm thick transverse sections parallel to the atrioventricular groove. Sections were subsequently incubated in 0.09 mol l–1 sodium phosphate buffer containing 1.0% triphenyl tetrazolium chloride (TTC; Sigma‐Aldrich Chemie Gmbh, Munich, Germany) and 8% dextran for 20 min at 37°C in order to demarcate viable from infarcted tissue. Slices were weighed before scanning on a flatbed scanner and traced to determine the final infarct size. The total slice area, the area at risk, and the infarcted area were measured by computer‐assisted planimetry. After normalization for the weight of the tissue slices, the size of the area at risk as percent of the left ventricle and the infarct size expressed as percent of the area at risk were calculated (Figure 1).
Figure 1.
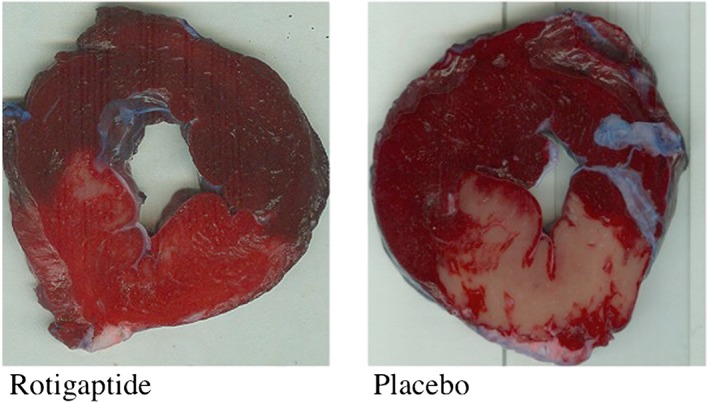
Left ventricular myocardial slices stained with Evans blue (area NOT at risk) and triphenyl tetrazolium chloride (TCC). Evans blue dye statining (dark red) delineates area not at risk from infarction (upper regions in these representative slices; left panel = rotigaptide treated animal, right panel = placebo). TTC staining bright red = viable tissue; white colour = infarcted tissue
Human translational study
This study was performed with the approval of the Lothian Research Ethics Committee (08/S1101/45) in accordance with the Declaration of Helsinki and with the written informed consent of each subject. The Clinical Trial registration was NCT00901563.
Subjects
Healthy non‐smokers were recruited into the study. Participants were excluded if they had clinically significant conditions including hypertension, hyperlipidaemia, diabetes mellitus, asthma or coagulopathy. No participant had suffered a recent infective or inflammatory condition, nor had they taken any medication in the 7 days prior to the study. On the day of study, participants fasted and abstained from caffeine for at least 4 h and from alcohol for 24 h. Subjects attended for two study visits, at least 2 weeks apart.
Drugs
Pharmaceutical grade acetylcholine (Novartis Ltd, Middlesex, UK), sodium nitroprusside (Hospira Inc., CA, USA) and rotigaptide (American Peptide Inc., CA., USA) were dissolved in physiological saline.
Forearm venous occlusion plethysmography and ischaemia
All subjects underwent cannulation of the brachial artery with a 27 standard wire gauge (SWG) steel needle under controlled conditions. All studies were performed with patients lying supine in a quiet, temperature controlled (22–24°C) room. The intra‐arterial infusion rate was kept constant at 1 ml min–1 throughout all studies. Forearm blood flow was measured in the infused and non‐infused arms by venous occlusion plethysmography using mercury‐in‐silastic strain gauges as described previously 19, 20. Supine heart rate and blood pressure were monitored at intervals throughout each study using a semi‐automated non‐invasive oscillometric sphygmomanometer.
Ischaemia reperfusion injury was induced in the non‐dominant arm by cuff inflation to 200 mmHg for 20 min in the presence or absence of intra‐arterial rotigaptide (25 nmol min–1 [15.4 μg min–1]). In protocol A bilateral arterial forearm blood flow was measured in 11 volunteers at rest and during intra‐arterial infusion of acetylcholine ([ACh] 5–20 μg min–1) before and after intra‐arterial infusion of placebo/rotigaptide and post IR injury (Figure 2). Baseline venous blood samples were collected for haematology characteristics. Protocol B was identical to protocol A except the endothelium‐independent vasodilator sodium nitroprusside ([SNP] 2–8 μg min–1) was used in place of acetylcholine (Figure 2). Ten volunteers completed this part of the study.
Figure 2.
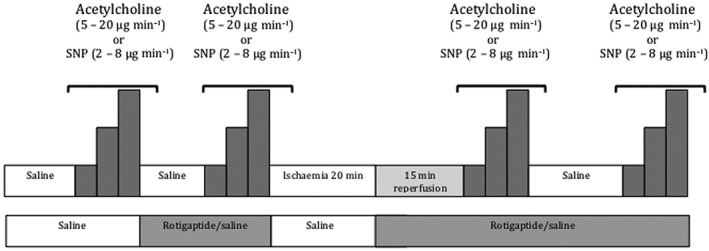
Human translational study schedule of drug administration and ischaemia/reperfusion timings. ACh: acetylcholine (protocol A); SNP: sodium nitroprusside (protocol B)
Data analysis and statistics
Forearm plethysmographic data were analyzed as described previously 19. Variables are reported as mean ± SEM and analyzed using repeated measures anova with post‐hoc Bonferroni corrections and two‐tailed Student's t‐test as appropriate. Statistical analysis was performed with GraphPad Prism (GraphPad Software Inc., La Jolla, CA, USA) and statistical significance taken at the 5% level. All analysis was performed by an investigator blinded to the treatment groups.
The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
Results
Porcine myocardial infarction study
Nineteen pigs completed the study protocol. Nine received rotigaptide and 10 received placebo. Eleven of the initial 30 pigs failed to complete the study protocol due to refractory arrhythmia following ischaemia–reperfusion and were not included in the analysis. The incidence of refractory arrhythmia was not specific to either group of animals (five in rotigaptide group, six in placebo group).
Heart rate, maximal rate of rise of left ventricular pressure (dP/dtmax) and maximum left ventricular pressure were not different between groups at baseline, during ischaemia and at any point following the administration of rotigaptide/placebo until the end of the study (Table 1).
Table 1.
Porcine haemodynamic measurements (mean (SD)).
| HR (beats min −1 ) | dP/dt max ( mmHg·s −1 ) | ESP (mmHg) | dP/dt min ( mmHg·s −1 ) | |||||
|---|---|---|---|---|---|---|---|---|
| Placebo | Rotigaptide | Placebo | Rotigatide | Placebo | Rotigaptide | Placebo | Rotigaptide | |
| Baseline | 66 (17) | 68 (13) | 1920 (367) | 2050 (504) | 127 (14) | 127 (14) | −2862 (835) | −2320 (562) |
| 0 min post‐ischaemia | 79 (26) | 66 (10) | 1655 (250) | 1503 (284) | 109 (12) | 108 (13) | −2284 (861) | −1846 (525) |
| 1 min post‐ischaemia | 152 (31) | 134 (23) | 1335 (358) | 1555 (817) | 108 (10) | 86 (22) | −1391 (541) | −1375 (789) |
| 5 min post‐ischaemia | 108 (28) | 114 (23) | 1736 (204) | 1502 (480) | 81 (15) | 93 (14) | −1898 (832) | −1676 (861) |
| 30 min post‐ischaemia | 104 (19) | 106 (20) | 1613 (197) | 1609 (383) | 86 (13) | 97 (10) | −1960 (665) | −1749 (559) |
| 60 min post‐ischaemia | 113 (14) | 99 (16) | 1515 (116) | 1623 (551) | 89 (11) | 100 (7) | −2056 (755) | −2129 (955) |
| 120 min post‐ischaemia | 88 (23) | 96 (21) | 1396 (266) | 1423 (338) | 101 (15) | 103 (8) | −2669 (1737) | −1888 (603) |
ESP, end‐systolic pressure; dP/dtmax, maximum positive pressure development over time; HR, heart rate; dP/dtmin, maximum negative pressure development over time. P = NS for all, placebo vs. rotigaptide (anova).
Rotigaptide treatment was associated with a marked reduction in myocardial infarction size as a percentage of area at risk (AAR) (18.7 ± 4.1% vs. 43.6 ± 4.2%, P = 0.006; in rotigaptide vs. controls, respectively) (Figure 3). The AAR as a percentage of the left ventricle (LV) was not different between the two groups (26.8 ± 2.1% vs. 28.4 ± 2.1%, P = NS,rotigaptide vs. controls).
Figure 3.
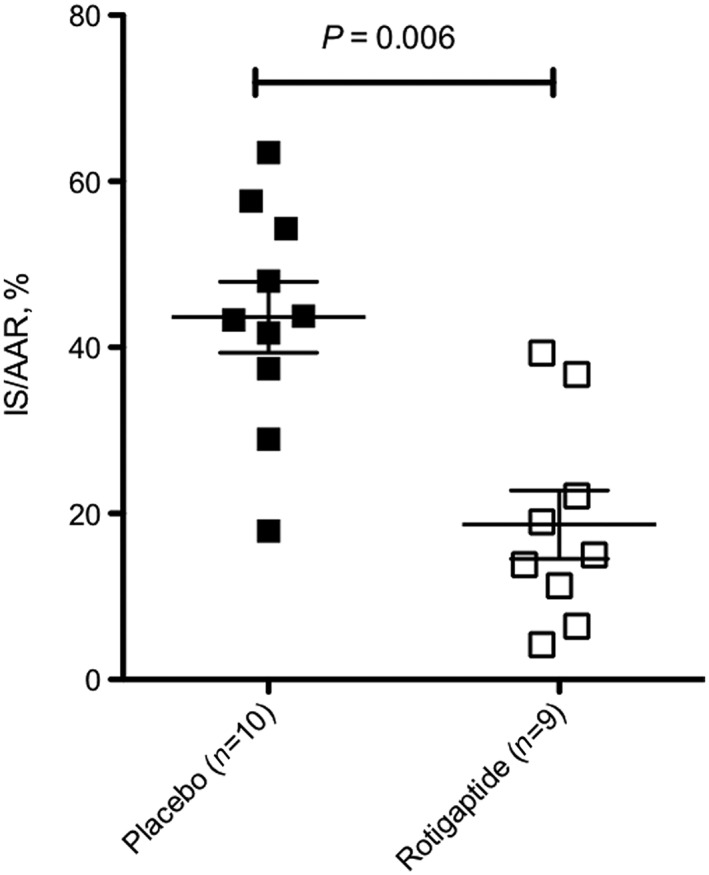
Final myocardial infarction size (IS) expressed as a proportion of the area at risk (AAR); P = 0.006, placebo vs. rotigaptide (anova)
Human translational study
IRI was well tolerated by all subjects with no adverse events. There were no differences in baseline clinical characteristics (including heart rate, blood pressure, haematocrit, haemoglobin and cholesterol) between volunteers in protocol A (assessment of endothelium‐dependent vasomotor responses to acetylcholine [ACh]) and protocol B (assessment of endothelium‐independent vasomotor responses to sodium nitroprusside [SNP]) (P = NS, data on file).
There was no difference in baseline forearm blood flow between visits in either protocol A or protocol B (P = NS, data on file). ACh and SNP evoked dose‐dependent forearm arterial vasodilatation (P = NS, for all, anova) that was not affected by co‐infusion of rotigaptide (P = NS, anova, data on file).
In protocol A, IR injury caused substantial impairment of ACh‐induced vasodilatation 15 min following reperfusion and 45 min after reperfusion (P = 0.007 and P = 0.05, respectively, anova) in the absence of rotigaptide (Figure 4). This impairment of vasomotor function, relative to baseline, was not seen in the presence of rotigaptide 15 min after reperfusion (P = NS, anova, Figure 4) and by 45 min after reperfusion blood flow responses to ACh in those receiving rotigaptide were greater than prior to ischaemia and reperfusion (P = 0.01, anova, Figure 4). In protocol B, endothelium‐independent vasodilatation evoked by SNP was unaffected by IR injury, both in the presence and the absence of rotigaptide (P = NS, anova, Figure 5).
Figure 4.
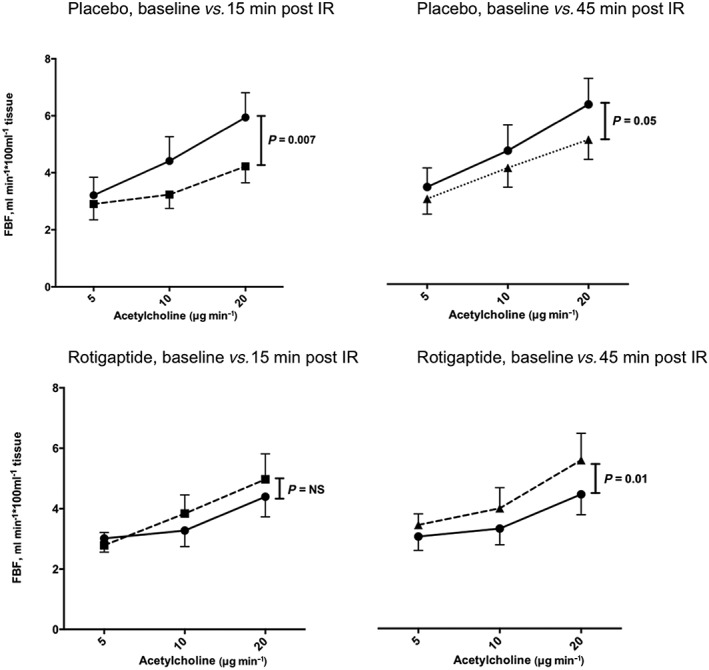
Forearm arterial vasomotor responses to intra‐arterial acetylcholine, in the presence and absence of rotigaptide, before and after ischaemia‐reperfusion injury. Two‐way anova baseline blood flow responses at baseline vs. 15 min (left panels) or 45 min (right panels) following reperfusion.  baseline,
baseline,  15 min and
15 min and  45 min
45 min
Figure 5.
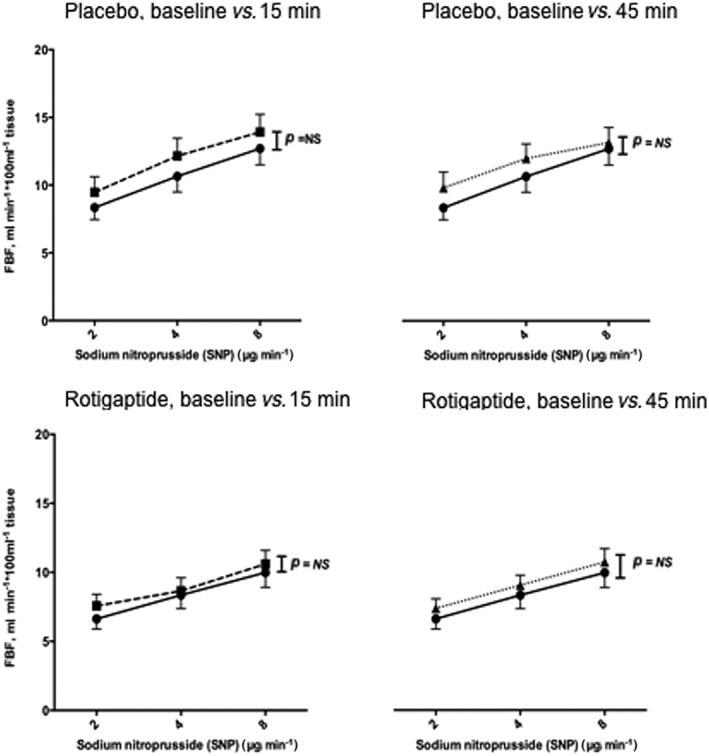
Forearm arterial vasomotor responses to intra‐arterial sodium nitroprusside, in the presence and absence of rotigaptide, before and after ischaemia‐reperfusion injury. Two‐way anova baseline blood flow responses at baseline vs. 15 min (left panels) or 45 min (right panels) following reperfusion.  baseline,
baseline,  15 min and
15 min and  45 min
45 min
Discussion
We have demonstrated that rotigaptide, a modulator of connexin 43 phosphorylation, is associated with a marked reduction in porcine myocardial infarction size when administered at the time of reperfusion. Furthermore, and for the first time in man, we have demonstrated the powerful protection that rotigaptide affords the human arterial vasculature at the time of IRI. These complementary studies highlight the pivotal role for healthy endothelial function in vascular homeostasis, including the limitation of ischaemic and reperfusion injury following acute arterial occlusion. Our findings provide important direction for the pharmaceutical development of connexin modulators designed for the limitation of clinically important IRI.
To examine the therapeutic utility of rotigaptide, we examined its effects in a clinically relevant animal model of myocardial infarction. In this closed‐chest porcine model, the administration of rotigaptide at the time of reperfusion was associated with a reduction in infarction size. Indeed we observed a reduction of infarction size by approximately 43% when expressed relative to the area at risk. Hennan and colleagues previously demonstrated the cardioprotective effect of rotigaptide administered to dogs subjected to a 1 h period of coronary artery occlusion in an open chest model. A bolus dose of rotigaptide was administered 10 min prior to reperfusion and then by continuous infusion over a 4 h period. They demonstrated a dose‐dependent reduction of infarct size as well as reduced ventricular arrhythmia burden 16. Porcine coronary anatomy is understood to reflect better that of humans with a less prominent network of collateral vessels than that found in dogs. Furthermore, it can be argued that the closed chest model better replicates spontaneous myocardial infarction and avoids the confounding effects of instrumentation of the chest and handling of the heart. Haugan and colleagues demonstrated that the administration of rotigaptide at the time of onset of myocardial ischaemia in rats reduced infarct size in a model of myocardial infarction without reperfusion assessed after 3 weeks 21. Whilst this study was the first to shed light upon the potential benefits of rotigaptide in myocardial infarction, it did not assess the effects of rotigaptide upon reperfusion injury. Furthermore, the administration of the drug at the onset of ischaemia cannot be considered a strategy easily translated to acute myocardial infarction but might rather inform its utility at the time of a more predictable ischaemia insult such as is encountered during solid organ transplantation. Danegaptide is an analogue of rotigaptide recently developed primarily to provide oral bio‐availability 22. Whilst our results compare favourably with those seen with the administration of this agent, albeit in an open‐chest study of reperfused porcine myocardial infarction 23, it remains to be seen whether there is a therapeutic advantage of one agent over the other. Indeed, in the acute setting the requirement for an orally available drug is probably not as pressing as it would be in a non‐emergent chronic setting.
Having demonstrated the effect of rotigaptide in porcine myocardial infarction we assessed the effects of rotigaptide in human ischaemia‐reperfusion injury. We have previously shown that Cx43 is required for the mediation of endothelium‐derived hyperpolarizing factor (EDHF) vasodilatation of human subcutaneous resistance vessels ex vivo 10 . Furthermore, we have also shown that, in the forearm arterial circulation of healthy men, rotigaptide has no effect on basal vascular tone, nor does it enhance endothelium‐dependent vasodilatation 24. However, in the in vivo healthy circulation, the open‐state probability of Cx43 is likely to be high and efforts directed to further opening of Cx43 may be futile. Phosphorylation is enhanced at times of physiologic stress and acidosis and Cx43 may thus be more likely to be in a closed state 25. During ischaemia, IRI and acidosis, rotigaptide may counteract the usual effects of this environment to re‐open channels of communication via Cx43. Therefore, in the current setting of IRI we hypothesized that the vascular effects of rotigaptide would be apparent and protective 26.
Consistent with the previous demonstration of IRI in the human forearm circulation, a 20 min period of ischaemia evoked a significant and specific impairment of endothelial vasomotor function 2. There was a decline in the vasodilator response to the endothelium‐dependent agonist acetylcholine. However, when the ischaemic insult was applied in the presence of rotigaptide, the deleterious effects of reperfusion on endothelial function were absent. Indeed, when assessed 15 and 45 min following reperfusion, endothelial vasomotor responses were preserved in the presence of rotigaptide. Neither rotigaptide nor IRI altered the blood flow response to the endothelium‐independent vasodilator, sodium nitroprusside, reinforcing the endothelial specificity of the IRI effect, and of rotigaptide.
In addition to its potential utility in the setting of acute myocardial infarction, the clinical use of such an agent could be extended to a wide range of other pathophysiologic processes, including in the treatment of acute stroke or for administration at the time of solid organ transplantation. We believe that the accumulating data, including that presented here, make a strong case for the assessment of rotigaptide's effects in a randomized controlled trial, particularly to assess its role in the reperfusion strategy for the treatment of acute myocardial infarction.
Limitations
We have demonstrated a clear beneficial effect of rotigaptide upon myocardial infarction size and have shown that rotigaptide has relevant protective effects in the human vasculature. These complementary studies do not, however, provide categorical proof that the limitation of infarct size is afforded by a protective endothelial effect or by some other protective non‐endothelial mechanism, either locally within the myocardium or via a paracrine process. We believe that the maintenance of healthy endothelial function including vasomotion, anti‐inflammatory activity and endogenous fibrinolysis is of central importance in rotigaptide's protective effects. Indeed, the capacity for vasodilatation to allow the rapid washout of toxic metabolites, diminished production of reactive oxygen species, the capacity for dissolution of micro‐thrombi and appropriate local anti‐inflammatory activity remain critical for the limitation of reperfusion injury. However, it is perhaps as important to note that rotigaptide's potential for myocardial protection is not offset by some otherwise unrecognised toxic effect upon human endothelial function.
In order to control for potential effects of rotigaptide upon vasomotor responses to agonists in the absence of IRI, the initial administration of rotigaptide was prior to the induction of ischaemia. Subsequent administration of rotgpatide/placebo was at the the time of reperfusion. By employing this design, we confirmed that rotigaptide does not have a non‐specific effect upon blood flow responses to acetylcholine or sodium nitroprusside in the absence of IRI. However, it might be argued that the protective effects of rotigaptide seen in this study include a contribution from pre‐ as well as post‐conditioning. Whilst we cannot fully exclude a contribution of pre‐conditioning to the protection afforded by rotigaptide in this context, our porcine study highlights the protective effects of rotigaptide when administered only at the time of reperfusion (post‐conditioning). Post‐ischaemic conditioning is most relevant to the treatment of acute myocardial infarction but pre‐conditioning may have a role in the protection of IRI at the time of solid organ transplatation or coronary bypass and is of clinical relevance. It is of note that physical (cuff‐induced remote ischaemia) pre‐conditioning has been disappointing in the setting of cardiopulmonary bypass 27, 28 but pharmacological strategies have, to date, received less attention in major clinical trials.
In conclusion, rotigaptide is associated with a marked reduction in myocardial infarction size after reperfusion in a porcine model. Furthermore, it provides important protection from the deleterious endothelial effects of ischaemia reperfusion therapy in man. The utility of rotgaptide and related agents holds clinical promise for use in the clinical treatment of acute myocardial infarction in man and warrants further clinical study in this setting.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare there are no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.
We are grateful to the staff of the Welcome Trust Clinical Research Facility at the Royal Infirmary of Edinburgh and to the Institute of Clinical Medicine, Aarhus University Hospital Skejby. DEN is supported by the British Heart Foundation (CH/09/002) and is the recipient of a Wellcome Trust Senior Investigator Award (WT103782AIA). CMP received funding from the Danish Agency for Science, Technology and Innovation, Region Midtjyllands Sundhedsvidenskabelige Forskningsfond, Det Classenske Fideicomis Jubilæumsfond, Snedkermester Sophus Jacobsen og hustru Astrid Jacobsen's Fond, Civilingeniør Stenild Hjorth's enke Else Hjorth's Fond, The A.P. Møller Foundation for the Advancement of Medical Science, Kirsten Antonius' Mindelegat and Institute of Clinical Medicine, University of Aarhus. RKK is funded by the NIHR Oxford Comprehensive Biomedical Research Centre.
Author Contributions
CMP, SV, HV, JAH and HC performed the research. NNL, RKK, DEN and NLC designed the research study. CMP, SV and JAH analysed the data. CMP and NNL wrote the paper. RKK, DEN, NCL, HEB and MRS critically revised the paper for important intellectual content.
Pedersen, C. M. , Venkatasubramanian, S. , Vase, H. , Hyldebrandt, J. A. , Contractor, H. , Schmidt, M. R. , Bøtker, H. E. , Cruden, N. L. , Newby, D. E. , Kharbanda, R. K. , and Lang, N. N. (2016) Rotigaptide protects the myocardium and arterial vasculature from ischaemia reperfusion injury. Br J Clin Pharmacol, 81: 1037–1045. doi: 10.1111/bcp.12882.
References
- 1. Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med 2007; 357: 1121–35. [DOI] [PubMed] [Google Scholar]
- 2. Kharbanda RK, Peters M, Walton B, Kattenhorn M, Mullen M, Klein N, Vallance P, Deanfield J, MacAllister R. Ischemic preconditioning prevents endothelial injury and systemic neutrophil activation during ischemia–reperfusion in humans in vivo . Circulation 2001; 103: 1624–30. [DOI] [PubMed] [Google Scholar]
- 3. Tiefenbacher CP, Chilian WM, Mitchell M, DeFily DV. Restoration of endothelium‐dependent vasodilation after reperfusion injury by tetrahydrobiopterin. Circulation 1996; 94: 1423–9. [DOI] [PubMed] [Google Scholar]
- 4. Pedersen CM, Barnes G, Schmidt MR, Botker HE, Kharbanda RK, Newby DE, Cruden NL. Ischaemia‐reperfusion injury impairs tissue plasminogen activator release in man. Eur Heart J 2012; 33: 1920–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kharbanda RK. Transient limb ischemia induces remote ischemic preconditioning in vivo . Circulation 2002; 106: 2881–3. [DOI] [PubMed] [Google Scholar]
- 6. Bøtker HE, Kharbanda R, Schmidt MR, Bøttcher M, Kaltoft AK, Terkelsen CJ, Munk K, Andersen NH, Hansen TM, Trautner S, Lassen JF, Christiansen EH, Krusell LR, Kristensen SD, Thuesen L, Nielsen SS, Rehling M, Sørensen HT, Redington AN, Nielsen TT. Remote ischaemic conditioning before hospital admission, as a complement to angioplasty, and effect on myocardial salvage in patients with acute myocardial infarction: a randomised trial. Lancet 2010; 375: 727–34. [DOI] [PubMed] [Google Scholar]
- 7. Morel S, Kwak BR. Roles of connexins in atherosclerosis and ischemia–reperfusion injury. Curr Pharm Biotechnol 2012; 13: 17–26. [DOI] [PubMed] [Google Scholar]
- 8. John SA, Kondo R, Wang SY, Goldhaber JI, Weiss JN. Connexin‐43 hemichannels opened by metabolic inhibition. J Biol Chem 1999; 274: 236–40. [DOI] [PubMed] [Google Scholar]
- 9. Verheule S, van Kempen MJ, Welscher te PH, Kwak BR, Jongsma HJ. Characterization of gap junction channels in adult rabbit atrial and ventricular myocardium. Circ Res 1997; 80: 673–81. [DOI] [PubMed] [Google Scholar]
- 10. Lang NN, Luksha L, Newby DE, Kublickiene K. Connexin 43 mediates endothelium‐derived hyperpolarizing factor‐induced vasodilatation in subcutaneous resistance arteries from healthy pregnant women. AJP: Heart and Circulatory Physiology. Am Physiol Soc 2007; 292: H1026–32. [DOI] [PubMed] [Google Scholar]
- 11. Ruiz‐Meana M, Rodríguez‐Sinovas A, Cabestrero A, Boengler K, Heusch G, Garcia‐Dorado D. Mitochondrial connexin43 as a new player in the pathophysiology of myocardial ischaemia‐reperfusion injury. Cardiovasc Res 2008; 77: 325–33. [DOI] [PubMed] [Google Scholar]
- 12. Schulz R. Connexin 43 and ischemic preconditioning. Cardiovasc Res 2004; 62: 335–44. [DOI] [PubMed] [Google Scholar]
- 13. Clarke TC, Thomas D, Petersen JS, Evans WH, Martin PEM. The antiarrhythmic peptide rotigaptide (ZP123) increases gap junction intercellular communication in cardiac myocytes and HeLa cells expressing connexin 43. Br J Pharmacol 2006; 147: 486–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Eloff BC. Pharmacological modulation of cardiac gap junctions to enhance cardiac conduction: evidence supporting a novel target for antiarrhythmic therapy. Circulation 2003; 108: 3157–63. [DOI] [PubMed] [Google Scholar]
- 15. Axelsen LN, Stahlhut M, Mohammed S, Larsen BD, Nielsen MS, Holstein‐Rathlou N‐H, Andersen S, Jensen ON, Hennan JK, Kjølbye A‐L. Identification of ischemia‐regulated phosphorylation sites in connexin43: A possible target for the antiarrhythmic peptide analogue rotigaptide (ZP123). J Mol Cell Cardiol 2006; 40: 790–8. [DOI] [PubMed] [Google Scholar]
- 16. Hennan JK. Rotigaptide (ZP123) prevents spontaneous ventricular arrhythmias and reduces infarct size during myocardial ischemia/reperfusion injury in open‐chest dogs. J Pharmacol Exp Ther 2005; 317: 236–43. [DOI] [PubMed] [Google Scholar]
- 17. Ek‐Vitorin JF, Calero G, Morley GE, Coombs W, Taffet SM, Delmar M. pH regulation of connexin43: molecular analysis of the gating particle. Biophys J 1996; 71: 1273–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang Y‐W, Zhang YW, Morita I, Morita I, Nishida M, Nishida M, Murota SI, Murota S‐I. Involvement of tyrosine kinase in the hypoxia/reoxygenation‐induced gap junctional intercellular communication abnormality in cultured human umbilical vein endothelial cells. J Cell Physiol 1999; 180: 305–13. [DOI] [PubMed] [Google Scholar]
- 19. Newby DE, Sciberras DG, Mendel CM, Gertz BJ, Boon NA, Webb DJ. Intra‐arterial substance P mediated vasodilatation in the human forearm: pharmacology, reproducibility and tolerability. Br J Clin Pharmacol 1997; 43: 493–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Newby DE, Wright RA, Labinjoh C, Ludlam CA, Fox KA, Boon NA, Webb DJ. Endothelial dysfunction, impaired endogenous fibrinolysis, and cigarette smoking: a mechanism for arterial thrombosis and myocardial infarction. Circulation 1999; 99: 1411–5. [DOI] [PubMed] [Google Scholar]
- 21. Haugan K, Marcussen N, Kjølbye AL, Nielsen MS, Hennan JK, Petersen JS. Treatment with the gap junction modifier rotigaptide (ZP123) reduces infarct size in rats with chronic myocardial infarction. J Cardiovasc Pharmacol 2006; 47: 236–42. [DOI] [PubMed] [Google Scholar]
- 22. Dobrev D, Carlsson L, Nattel S. Novel molecular targets for atrial fibrillation therapy. Nat Rev Drug Discov 2012; 11: 275–91. [DOI] [PubMed] [Google Scholar]
- 23. Skyschally A, Walter B, Schultz Hansen R, Heusch G. The antiarrhythmic dipeptide ZP1609 (danegaptide) when given at reperfusion reduces myocardial infarct size in pigs. Naunyn‐Schmiedeberg's Arch Pharmacol 2013; 386: 383–91. [DOI] [PubMed] [Google Scholar]
- 24. Lang NN, Myles RC, Burton FL, Hall DP, Chin YZ, Boon NA, Newby DE. The vascular effects of rotigaptide in vivo in man. Biochem Pharmacol 2008; 76: 1194–200. [DOI] [PubMed] [Google Scholar]
- 25. Lampe PD, Lau AF. The effects of connexin phosphorylation on gap junctional communication. Int J Biochem Cell Biol 2004; 36: 1171–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Haugan K, Olsen KB, Hartvig L, Petersen JS, Holstein‐Rathlou N‐H, Hennan JK, Nielsen MS. The antiarrhythmic peptide analog ZP123 prevents atrial conduction slowing during metabolic stress. J Cardiovasc Electrophysiol 2005; 16: 537–45. [DOI] [PubMed] [Google Scholar]
- 27. Hausenloy DJ, Candilio L, Evans R, Ariti C, Jenkins DP, Kolvekar S, Knight R, Kunst G, Laing C, Nicholas J, Pepper J, Robertson S, Xenou M, Clayton T, Yellon DM. Remote ischemic preconditioning and outcomes of cardiac surgery. N Engl J Med 2015; 373: 1408–17. [DOI] [PubMed] [Google Scholar]
- 28. Meybohm P, Bein B, Brosteanu O, Cremer J, Gruenewald M, Stoppe C, Coburn M, Schaelte G, Böning A, Niemann B, Roesner J, Kletzin F, Strouhal U, Reyher C, Laufenberg‐Feldmann R, Ferner M, Brandes IF, Bauer M, Stehr SN, Kortgen A, Wittmann M, Baumgarten G, Meyer‐Treschan T, Kienbaum P, Heringlake M, Schön J, Sander M, Treskatsch S, Smul T, Wolwender E, Schilling T, Fuernau G, Hasenclever D, Zacharowski K, RIPHeart Study Collaborators . A multicenter trial of remote ischemic preconditioning for heart surgery. N Engl J Med 2015; 373: 1397–407. [DOI] [PubMed] [Google Scholar]


