Abstract
Aims
N‐acetylcysteine (NAC) may be useful in the management of non‐paracetamol drug‐induced liver injury (DILI). Our objective was to review systematically evidence for the use of NAC as a therapeutic option for non‐paracetamol DILI.
Methods
We searched for randomized controlled trials (RCTs) and prospective cohort studies. We searched several bibliographic databases, grey literature sources, conference proceedings and ongoing trials. Our pre‐specified primary outcomes were all cause and DILI related mortality, time to normalization of liver biochemistry and adverse events. Secondary outcomes were proportion receiving liver transplant, time to transplantation, transplant‐free survival and hospitalization duration.
Results
We identified one RCT of NAC vs. placebo in patients with non‐paracetamol acute liver failure. There was no difference in the primary outcomes of overall survival at 3 weeks between NAC [70%, 95% confidence interval (CI) = 60%, 81%, n = 81] and placebo (66%, 95% CI = 56%, 77%, n = 92). NAC significantly improved the secondary outcomes of transplant‐free survival compared with placebo: 40% NAC (95% CI = 28%, 51%) vs. 27% placebo (95% CI = 18%, 37%). A subgroup analysis according to aetiology found improved transplant‐free survival in patients with non‐paracetamol DILI, NAC (58%, n = 19) vs. placebo (27%, n = 26), odds ratio (OR) 0.27 (95% CI = 0.076, 0.942). Overall survival was similar, NAC (79%) vs. placebo (65%);, OR 0.50 (95% CI = 0.13, 1.98).
Conclusion
Current available evidence is limited and does not allow for any firm conclusions to be made regarding the role of NAC in non‐paracetamol DILI. We therefore highlight the need for further research in this area.
Keywords: drug‐induced, hepatitis, liver failure, N‐acetylcysteine, non‐acetaminophen, non‐paracetamol
Introduction
Drug‐induced liver injury (DILI) refers to acute or chronic liver injury that may occur as a consequence of using drugs and herbal or dietary supplements 1, 2. According to recent estimates, in the USA, it is the most common cause of acute liver failure (ALF), with 11% of cases due to idiosyncratic DILI 3. In South Africa it is the second highest cause of death due to adverse drug reactions in medical wards 4. Two recent studies estimated incidence at between 14 and 19 cases per 100 000 persons receiving prescription medication 5, 6. True incidence of DILI may be underestimated due to diagnostic difficulty as well as underreporting 2.
The general management of DILI consists of the discontinuation of the offending drug in combination with supportive treatment 2. Patients often require prolonged hospital stays which may be costly to both the patient and the health service. Therapeutic re‐challenge with the offending drug is generally not recommended but may be attempted in certain instances after a thorough consideration of the risks and potential benefits. Specific therapies available for DILI are limited to carnitine for valproic acid and N‐acetylcysteine (NAC) for paracetamol overdose 7, 8.
NAC was first used as a treatment for paracetamol overdose in 1979 9. Since then, it has been firmly established as an effective and safe treatment for this condition 8. NAC has also been shown to be safe and effective outside of paracetamol overdose. NAC has been evaluated as a treatment option for non‐paracetamol ALF in adults and paediatric patients. In a randomized clinical trial comparing NAC with placebo in adults with non‐paracetamol ALF, NAC was associated with an improvement in transplant‐free survival in a subgroup of patients with grade 1 and grade 2 encephalopathy 10. In a cohort study conducted in adults with non‐paracetamol ALF at a centre without the facility for transplantation, the use of NAC was associated with a mortality benefit 11. In a retrospective study in paediatric patients with non‐paracetamol ALF, NAC was associated with a shorter hospital stay and improved survival post‐transplantation 12. Furthermore, in a case series of patients with DILI secondary to Amanita phalloides mushroom poisoning, 10 of 11 patients recovered fully after receiving NAC in combination with other therapies 13.
NAC has also been evaluated for non‐liver related clinical indications. These indications include its use as a mucolytic agent in pulmonary diseases, in the prevention of radio‐contrast associated nephrotoxicity and for the treatment of certain ophthalmic conditions 14, 15, 16, 17.
In paracetamol overdose, a form of non‐idiosyncratic DILI, the pathogenesis underlying hepatotoxicity is fairly well understood. Here, the metabolism of paracetamol produces an excess of the hepatotoxic metabolite N‐acetyl‐p‐benzo‐quinone imine (NAPQI). NAPQI is normally inactivated by hepatic glutathione; however, glutathione is depleted in paracetamol overdose. This results in an accumulation of NAPQI with consequent hepatic cell injury and death. NAC is thought to replenish hepatic glutathione stores, which forms the basis for its efficacy in this condition 18. The administration of NAC early on in paracetamol‐induced liver injury is associated with more favourable outcomes, which is explained by its effect on replenishing hepatic glutathione. However, administration of NAC is also beneficial in patients with established paracetamol‐induced fulminant liver failure, where its beneficial effects are independent of glutathione replenishent, and include improved oxygen delivery to, and oxygen consumption by, the injured liver 19. These latter effects may also explain why NAC is of benefit in non‐paracetamol idiosyncratic DILI, wherein the mechanism underlying hepatotoxicity does not involve glutathione depletion. However, the precise pathogenesis in idiosyncratic DILI is not clearly defined 20. The proposed pathogenic mechanisms in idiosyncratic DILI include direct cell injury, immune mediated damage and mitochondrial injury. These mechanisms, especially those that lead to mitochondrial damage, have significant implications. Mitochondria are involved in protecting hepatocytes against oxidative stress from oxygen‐free radicals in the liver. The damage and loss of mitochondria leads to an accumulation of oxygen‐free radicals and subsequent oxidative cell damage. NAC may be of benefit in this context through its antioxidant effect 21, 22. Additional benefits of NAC in this context involve the improvement of systemic haemodynamics and tissue oxygen delivery, through the relaxation of vascular smooth muscle and reversal of vascular nitrate tolerance. Furthermore, NAC may be of benefit through an anti‐inflammatory effect by inhibiting leukocyte chemotaxis 19, 23, 24.
The aim of this systematic review was to synthesize the evidence of safety and effectiveness including improvement in time, if any, to normalization of liver function tests and of NAC in non‐paracetamol DILI. NAC has already been established as a safe and effective treatment for paracetamol‐induced liver injury. Recently, the research focus has shifted to investigating the use of NAC in non‐paracetamol drug‐induced liver injury. It is important to review the evidence of NAC safety and efficacy in this setting to determine if NAC may be considered as a treatment option in non‐paracetamol DILI. The evidence from this research may then be used to inform the decisions made by policymakers, health care practitioners, as well as researchers in this area.
Methods
This review is registered in the PROSPERO International Prospective Register of systematic reviews, registration number CRD42014008771. The protocol was peer reviewed 25.
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs) and prospective cohort studies.
Language and timing
No language and time restrictions applied.
Types of participants
Human participants of any age diagnosed with non‐paracetamol DILI, diagnosed according to recognized diagnostic criteria 26, 27, 28, 29.
Types of interventions
Intervention: NAC administered intravenously or orally. Control: placebo or standard of care (as described in the study) or alternative therapy.
There were no restrictions on dose, timing and route of administration of NAC.
Types of outcome measures
Primary outcomes
All‐cause mortality, mortality due to DILI, time to normalization of liver biochemistry (e.g. return of alanine transaminase to <100 U l−1 and/or International Normalized Ratio (INR) < 1.5), adverse events (graded using the Common Terminology Criteria for Adverse Events) 30.
Secondary outcomes
Transplantation rate, time to transplantation, transplant‐free survival, duration of hospitalization.
Search methods for identification of studies
We performed a comprehensive search in June 2015 of electronic databases and conference proceedings to identify all relevant studies, regardless of language or publication status. We searched both peer‐reviewed journal articles and grey literature (unpublished, internal or non‐reviewed papers and reports).
Electronic searches
We searched the following electronic databases: Cochrane Library, Medline via PubMed, SCOPUS, Web of Science (SciELO) and EBSCO (CINAHL, Africa‐Wide, Academic Search Premier). We used both text words and medical subject heading (MeSH) terms. The literature search strategy was adapted to suit each database. Briefly, we used a combination of the following terms: N‐acetylcysteine, acetylcysteine, drug‐induced, hepatitis, liver, liver failure, non‐paracetamol, non‐acetaminophen.
Conference proceedings
We conducted a manual search of relevant abstracts or proceedings of the following conferences (2000 to 2015): American Association for the Study of Liver Diseases (AASLD) Drug‐Induced Liver Injury Conference, AASLD‐FDA‐NIH‐PhRMA‐Hepatotoxicity Special Interest Group Conferences, European Association for the Study of Liver (EASL) and The International Liver Congress and Digestive Diseases Week (DDW).
Searching other sources
We searched Google Scholar and SCOPUS for conference proceedings and www.opengrey.eu and www.greylit.org for grey literature. For ongoing studies, we searched the Pan African National Clinical Trials Registry (PACTR), World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP), ClinicalTrials.gov and NHS Clinical Trials. Individuals and organizations working in the field of DILI were consulted for information regarding unpublished data and work in progress.
Data collection and analysis
The methods for data collection and analysis are based on the Cochrane Handbook of Systematic Reviews for Interventions 31.
Selection of studies
Two review authors (MFC and NK) independently reviewed all relevant material identified from the above search. After reading the titles and abstracts of the identified articles, we acquired the full text articles of all citations deemed to meet the inclusion criteria. These articles were independently inspected to verify that they met the pre‐specified inclusion criteria. We resolved disagreements between the two reviewers regarding study eligibility through discussion with a third author (KC).
Data extraction and management
Two authors (MFC and NK) used a standardized data extraction form to extract data from the included study and to assess study quality. Any discrepancies were resolved via discussion of the original article with a third author. We requested additional data from study authors. References were managed using Mendeley Desktop reference manager. We prepared our review using Review Manager 5.3 (RevMan5) software 32.
Assessment of risk of bias in included studies
MFC and NK independently assessed the risk of bias in the included study. The assessment included information on sequence generation, allocation concealment, blinding, incomplete outcome data or missing data, selective outcome reporting, other sources of bias and overall risk of bias. Each methodological component was assessed and the study was described as having a low, unclear or high risk of bias, as per the Cochrane Handbook of Systematic Reviews of Interventions 31. The two authors resolved disagreements in the assessment of risk of bias by discussion and consensus, consulting a third reviewer, KC, to resolve any persistent disagreements.
Measures of treatment effect
We planned to conduct our data analysis using Review Manager Version 5.3 32. We present the data from the included study with respect to overall survival and transplant‐free survival in the overall study population with acute liver failure. We proceeded to calculate the odds ratios and 95% CI for the outcomes of overall survival and transplant‐free survival for the DILI subgroup.
Dealing with missing data
We contacted the authors to assist with absent or incomplete data.
Data synthesis, assessment of heterogeneity and sensitivity analyses
In view of limited available data, we could not conduct a meta‐analysis, assess heterogeneity or sensitivity analyses.
Presenting and reporting of results
This systematic review is reported according to the Preferred Reporting Items for Systematic reviews and Meta‐Analyses (PRISMA) statement 33.
Results
Results of search
Figure 1 shows the flow diagram for study inclusion. A total of 691 records were identified through the search until June 2015. After screening titles and abstracts, we excluded 687 records leaving four articles for full text review. We excluded three further articles after full text review and identified one article from which we could extract data for inclusion in a qualitative analysis. We also identified one ongoing placebo‐controlled trial of NAC for DILI due to first line tuberculosis treatment, which is still recruiting 34.
Figure 1.
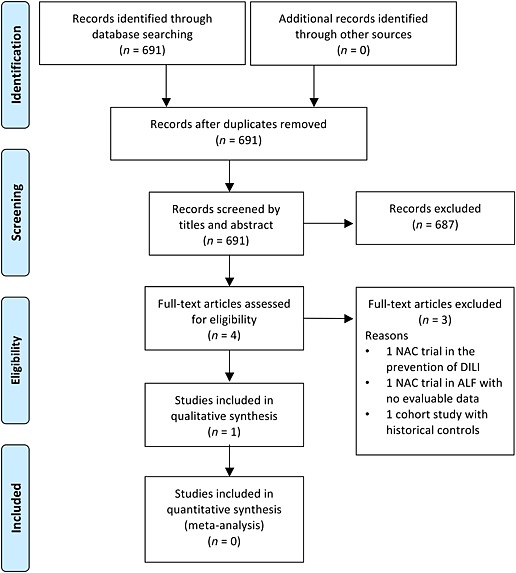
Flow diagram of screened, excluded and included publications
Characteristics of excluded studies (see Table 1)
Table 1.
Excluded studies with rationale
| Study | Reason for exclusion |
|---|---|
| Baniasadi et al. 2010 35 | Investigated the use of NAC in the prevention of DILI |
| Squires et al. 2013 36 | No evaluable data in patients with non‐paracetamol DILI |
| Mumtaz et al. 2009 11 | Not a prospective cohort study |
| Baniasadi et al. 2010 35 | |
| Methods | Randomized, open‐label trial |
| Participants | 60 patients with tuberculosis commencing antituberculous therapy |
| Interventions | Oral NAC for initial 2 weeks of antituberculous therapy vs. no NAC |
| Outcomes | Primary outcome was incidence of DILI, defined as: |
| 1. ALT/AST ≥ 5 times upper limit of normal | |
| 2. Raised serum total bilirubin >1.5 mg dl−1 | |
| 3. Any increase in AST and/or ALT above the pretreatment levels together with the hepatitis symptoms | |
| Reason for exclusion | Investigated the use of NAC in the prevention of DILI |
| Squires et al. 2013 36 | |
| Methods | Randomized, adaptive allocation, doubly mask, placebo‐controlled trial |
| Participants | 184 paediatric patients with non‐paracetamol ALF. |
| • ALF defined as biochemical evidence of acute liver injury and a liver‐based coagulopathy | |
| • DILI cases formed a subgroup of ALF cases | |
| • No description regarding diagnosis of DILI | |
| Interventions | Intravenous NAC or placebo infused for up to 7 days |
| Outcomes | Primary outcome was 1 year survival |
| Secondary outcomes included liver transplantation, survival without liver transplantation, length of hospital stay, maximum degree of hepatic encephalopathy and number of organ systems failing | |
| Reason for exclusion | No evaluable data in patients with non‐paracetamol DILI |
| Mumtaz et al. 2009 11 | |
| Methods | Retrospective cohort study |
| Participants | 91 patients with non‐paracetamol ALF |
| • ALF defined as rapid development of acute liver injury with impaired synthetic function and encephalopathy | |
| • DILI cases formed a subgroup of ALF cases | |
| • No description regarding diagnosis of DILI | |
| Interventions | Oral NAC vs. no NAC |
| Outcomes | Primary outcome was overall survival |
| Secondary outcomes included evaluation of factors related to survival and safety of NAC | |
| Reason for exclusion | Not a prospective cohort study |
ALF, acute liver failure; ALT, alanine transaminase; AST, aspartate transaminase; DILI, drug‐induced liver injury; NAC, N‐acetylcysteine.
The first excluded study was a small open label RCT conducted in Iran that investigated the hepatoprotective effect of NAC in antituberculous DILI 35. This study was excluded on the basis that it investigated the use of NAC in the prevention of DILI, as opposed to the treatment of DILI. Sixty participants newly diagnosed with tuberculosis were randomly assigned to group 1 (n = 32) where they received daily oral antituberculous therapy [isoniazid (5 mg kg−1), rifampicin (10 mg kg−1), pyrazinamide (25 mg kg−1), ethambutol (15 mg kg−1) only, or to group 2 (n = 28) where they received daily oral antituberculous therapy [isoniazid (5 mg kg−1), rifampicin (10 mg kg−1), pyrazinamide (25 mg kg−1), ethambutol (15 mg kg−1)] and NAC (oral 600 mg twice daily for the first 2 weeks of TB therapy). Patients were followed‐up for 2 weeks. The outcome of interest was the development of DILI, which occurred in 12/32 and 0/28 participants in group 1 and group 2, respectively. The mean duration of antituberculous therapy prior to onset of DILI was 4.67 ± 4.58 days. Although these findings suggested that NAC might be of potential benefit in the prevention of antituberculous DILI, the small sample size, lack of placebo comparison and short duration of follow‐up limited the study.
The second excluded study was a multicentre, randomized, double masked, placebo‐controlled trial investigating NAC as treatment for non‐paracetamol ALF in paediatric participants 36. Of the 184 participants who were enrolled, half of the participants (n = 92) were assigned to receive intravenous NAC (150 mg kg−1 day−1) in 5% dextrose (D5W) and water, while the other half (n = 92) were assigned to receive placebo. Study drugs were infused over 24 h for up to 7 consecutive days. The primary outcome was overall survival at 1 year after treatment allocation. Overall survival at 1 year was not significantly different between NAC and placebo, 73% vs. 82%, respectively (P = 0.19). Of relevance to our review is that this study included a subgroup analysis according to the aetiology of ALF. There was a high proportion of patients with indeterminate aetiology in this analysis. Non‐paracetamol DILI was included in the aetiology of ALF and although there were three cases of ALF secondary to non‐paracetamol DILI in the placebo group, there were no cases of ALF secondary to non‐paracetamol DILI amongst those who received NAC. Therefore, we excluded this study on the basis that it could not provide evaluable data for our review.
The third excluded study was a cohort study investigating NAC as treatment in non‐paracetamol ALF 11. Between 2004 and 2007, 47 adult participants with non‐paracetamol ALF were prospectively enrolled to receive oral NAC (group 1), and compared with 44 historical controls with ALF who did not receive NAC (group 2). The primary outcome was reduction in mortality. There was no statistically significant difference in mortality between the groups, 53.2% (group 1) and 72.7% (group 2), P = 0.05. This study was excluded on the basis that it included a comparison with retrospective controls and therefore did not meet our inclusion criteria of being a prospective cohort study.
Characteristics of included study (see Table 2)
Table 2.
Characteristics of included study
| Lee et al. 2009 10 | ||
|---|---|---|
| Methods | Randomized, double‐blind, placebo‐controlled trial | |
| Participants | 173 patients with non‐paracetamol ALF, defined as: | |
| • Evidence of acute liver failure (any degree of encephalopathy and coagulopathy: international normalized ratio [INR] ≥1.5) due to an illness of less than 24 weeks duration. | ||
| • DILI cases formed a subgroup of patients with ALF | ||
| • DILI diagnosis based on expert opinion of site principal investigator | ||
| Interventions | Intravenous NAC or placebo infused for 72 h. | |
| The NAC regimen was as follows: initial loading dose of 150 mg kg−1 h−1 of NAC over 1 h followed by 12.5 mg kg−1 h−1 for 4 h, then continuous infusions of 6.25 mg kg−1 h−1 NAC for the remaining 67 h. | ||
| Outcomes | Primary outcome was overall survival at 3 weeks | |
| Secondary outcomes included transplant‐free survival, rate of transplantation, length of hospital stay and number of organ systems failing | ||
| Results | Overall study population | Overall survival at 3 weeks: |
| 70% (95% CI = 60%, 81%) NAC group vs. 66% (95% CI = 56%, 77%) placebo group, P = 0.283. | ||
| Transplant‐free survival at 3 weeks: | ||
| 40% NAC group (95% CI = 28%, 51%) vs. 27% placebo group(95% CI = 18%, 37%), P = 0.043. | ||
| DILI subgroup | Overall survival at 3 weeks: | |
| 79% (95% CI = 58%, 100%) NAC group vs. 65% (95% CI = 45%, 86%) in the placebo group, odds ratio = 0.50 (95% CI = 0.13, 1.98, P = 0.33). | ||
| Transplant‐free survival at 3 weeks: | ||
| 58% (95% CI = 33%, 83%) NAC group vs. 27% (95% CI = 8%, 46%) placebo group, odds ratio = 0.27 (95% CI 0.076, 0.942, P = 0.04). | ||
| Notes | Subgroup of 45 patients with non‐paracetamol DILI provided data specific to our review question. Nineteen patients received NAC and 26 patients received placebo. | |
ALF, acute liver failure; DILI, drug‐induced liver injury; NAC, N‐acetylcysteine.
The included study was a randomized, double‐blind, placebo‐controlled trial investigating a 72 h intravenous infusion of NAC as treatment for adults with non‐paracetamol ALF, at multiple sites in the USA 10. ALF was defined in the study as encephalopathy with accompanying coagulopathy. Randomization was stratified by hepatic encephalopathy grade (1–2 vs. 3–4) and study site. The study enrolled 173 participants and randomly assigned 81 participants to receive NAC and 92 participants to receive placebo. After randomization, an infusion of either 5% dextrose with NAC or 5% dextrose only (placebo) was administered. The primary outcome was overall survival at 3 weeks. Although not listed as an outcome of interest, the study did report on rates of adverse events in study participants.
Effect of NAC on outcomes in overall study population (participants with non‐paracetamol ALF)
Overall survival at 3 weeks was similar for the NAC and placebo groups; 70% (95% CI = 60% to 81%) vs. 66% (95% CI = 56% to 77%), Chi squared P = 0.283. In contrast, transplant‐free survival was greater in the NAC group than the placebo group; 40% (95% CI = 28%, 51%) vs. 27% (95% CI = 18%, 37%), Chi squared P = 0.043. In a secondary analysis, transplant‐free survival was stratified by coma grade. In patients with coma grade I–II survival was higher in the NAC group than the placebo group; 52% (95% CI = 38% to 65%, n = 58) vs. 30% (95% CI = 17%, 43%, n = 56), P = 0.010, with an odds ratio (OR) of 2.46 (95% CI = 1.14 to 5.30). In contrast in participants with coma grades III–IV transplant‐free survival was lower in the NAC group but this did not reach statistical significance; 9% (95% CI = 0% to 22%, n = 23) vs. 22% (95% CI = 7% to 37%, n = 36), P = 0.912, OR 0.33 (95% CI = 0.06 to 1.74). The difference in odds ratios according to coma grade was statistically significant (P = 0.012). Transplantation rates were 32% (95% CI = 21%, 43%) in the NAC group and 45% (95% CI = 34%, 55%) in the placebo group, P = 0.09.). Rates of adverse events were similar between groups; nausea and vomiting was more common in the NAC than the placebo group, 14% (95% CI = 6%, 22%), vs. 4% (95% CI = 0%, 9%), P = 0.031. In total, there were five early discontinuations of therapy due to side effects possibly due to the drug, four due to NAC.
Effect of NAC on outcomes in subgroup with ALF due to non‐paracetamol DILI
A subgroup analysis by aetiology of ALF was conducted. Non‐paracetamol DILI was the largest aetiological subgroup with 45 participants, of whom 19 received NAC and 26 received placebo. Outcome data on the 45 DILI participants were limited to overall survival and transplant‐free survival. There were four deaths in the NAC arm compared with nine deaths in the placebo arm, which corresponded with an overall survival of 79% (n = 15) in the NAC arm and 65% (n = 17) in the placebo arm, with an odds ratio of 0.50 (95% CI = 0.13, 1.98, P = 0.33). Transplant‐free survival was higher in the participants with non‐paracetamol DILI treated with NAC than those treated with placebo, 58% (n = 11) vs. 27% (n = 7), with an odds ratio of 0.27 (95% CI 0.076, 0.942, P = 0.04). The study was not powered to detect differences within the DILI subgroups.
Risk of bias in included study
We graded the overall risk of bias in the study as ‘unclear’ (see Table 3 for further details regarding risk of bias assessment).
Table 3.
Risk of bias in included study
| Lee et al. 2009 10 | ||
|---|---|---|
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomization not mentioned in detail. Randomization was stratified by encephalopathy grade with a blocking factor of 4. |
| Allocation concealment (selection bias) | High risk | Not mentioned |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind. Participants and all study personnel, except biostatisticians and site pharmacist were blinded. |
| Blinding of outcome assessment (detection bias) | Low risk | Double‐blind. All study personnel except biostatisticians and site pharmacist were blinded. |
| Incomplete outcome data (attrition bias) | Low risk | Intention‐to‐treat analysis. All participants analyzed in the group they were randomized to. No missing data. |
| Selective reporting (reporting bias) | Low risk | No |
| Other bias | Low risk | No |
Discussion
After systematic review of published and unpublished literature, we identified only one study addressing the effectiveness and safety of NAC in non‐paracetamol DILI. Participants with non‐paracetamol DILI were a subgroup in this randomized controlled trial. This subgroup analysis only addressed one of our primary endpoints (overall survival) and found no difference in this subgroup, but was underpowered for this comparison. Based on this study's findings NAC may be of benefit in treatment of non‐paracetamol DILI in improving the secondary endpoint of transplant free survival. We cannot draw firm conclusions on the effectiveness of NAC in management of non‐paracetamol DILI, on the basis of limited outcome data confined to this small subgroup. Findings may not be generalizable to patients with less severe forms of liver injury. Patients with DILI present on a spectrum from mild liver injury to severe liver injury (ALF). We found no studies exploring the benefit of NAC in patients with less severe forms of liver injury. A final limitation concerns the methodological quality, with the study deemed as having an overall ‘unclear’ risk of bias.
The strengths of our review include the use of a comprehensive search, thereby limiting the likelihood that we missed any potentially relevant studies. In addition, eligibility for study inclusion, data extraction and the risk of bias assessment was carried out by two authors independently, thereby reducing the chances of bias in our review process.
This review has highlighted the need for further research to investigate the role of NAC in non‐paracetamol DILI. There is a clear need for prospective studies with sufficient sample sizes that enrol participants with varying grades of severity of DILI. However, there may be certain challenges in undertaking these studies such as the difficulty in enrolling sufficient numbers of participants as a consequence of diagnostic difficulty and under‐reporting of DILI 2.
We found an ongoing placebo‐controlled RCT currently enrolling in South Africa attempting to address this research gap, by investigating the role of NAC in participants with antituberculous DILI 34. Low resource settings such as South Africa have a high prevalence of tuberculosis with accompanying high rates of antituberculous DILI between 5 and 33% of patients 37. Death may be a consequence of DILI and this was highlighted in a recently published cross‐sectional survey in hospitalized patients, which found DILI to be the second most common adverse drug reaction contributing to death, with antituberculous drugs being implicated in the majority of DILI cases 4.
Conclusion
Our review has highlighted a paucity of data, limited to a single RCT in non‐paracetamol ALF suggesting a trend for improved transplant and overall survival in a subgroup of participants with non‐paracetamol DILI. However, the study was not powered to detect differences in this subgroup with DILI. Therefore, due to the limited available evidence, we are unable to determine conclusively if there is a role for NAC in patients with non‐paracetamol DILI. Thus, we are unable to make recommendations for clinical practice and emphasize the need for high quality prospective RCTs in this area.
Competing Interests
All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare no support from any organization for the submitted work, no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.
There was no specific funding source for this manuscript.
We thank Dr Sameera Allie for her support and valuable input throughout the review process.
Dr Mark Engel from the Evidence‐Based Medicine Research Support Unit, Faculty of Health Sciences, University of Cape Town was funded through the incentivising research in the Faculty of Health Sciences funding scheme.
Contributors
MFC conceived the idea for the review and drafted the manuscript. MFC and NK were involved in data acquisition. All authors contributed to the final manuscript. WS served as a content expert in the field of drug‐induced liver injury. MW provided input on methodology. KC served as the overall supervisor, content expert and provided input on methodology, data analysis and interpretation. All authors have given their approval for publication.
Author information
MFC is a clinical pharmacology registrar in the Department of Medicine, UCT. NK is a research pharmacist at the Clinical Research Centre, UCT. WS is a consultant hepatologist and is head of the Division of Hepatology, Department of Medicine, UCT. MW is a PhD Fellow, Division of Endocrinology and Diabetes, Chronic Disease Initiative for Africa, (CDIA) Department of Medicine, UCT. KC is a consultant clinical pharmacologist, Department of Medicine, UCT.
Chughlay, M. F. , Kramer, N. , Spearman, C. W. , Werfalli, M. , and Cohen, K. (2016) N‐acetylcysteine for non‐paracetamol drug‐induced liver injury: a systematic review. Br J Clin Pharmacol, 81: 1021–1029. doi: 10.1111/bcp.12880.
References
- 1. Davern TJ. Drug‐induced liver disease. Clin Liver Dis 2012; 16: 231–45. [DOI] [PubMed] [Google Scholar]
- 2. Leise MD, Poterucha JJ, Talwalkar JA. Drug‐induced liver injury. Mayo Clin Proc 2014; 89: 95–106. [DOI] [PubMed] [Google Scholar]
- 3. Navarro VJ, Senior JR. Drug‐related hepatotoxicity. N Engl J Med 2006; 354: 731–9. [DOI] [PubMed] [Google Scholar]
- 4. Mouton JP, Mehta U, Parrish AG, Wilson DP, Stewart A, Njuguna CW, Kramer N, Maartens G, Blockman M, Cohen K. Mortality from adverse drug reactions in adult medical inpatients at four hospitals in South Africa: a cross‐sectional survey. Br J Clin Pharmacol 2015; 80: 818–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation, and outcomes in patients with drug‐induced liver injury in the general population of Iceland . Gastroenterology 2013; 144: 1419–25. [DOI] [PubMed] [Google Scholar]
- 6. Sgro C, Clinard F, Ouazir K, Chanay H, Allard C, Guilleminet C, Lenoir C, Lemoine A, Hillon P. Incidence of drug‐induced hepatic injuries: a French population‐based study. Hepatology 2002; 36: 451–5. [DOI] [PubMed] [Google Scholar]
- 7. Lheureux PER, Hantson P. Carnitine in the treatment of valproic acid‐induced toxicity. Clin Toxicol (Phila) 2009; 47: 101–11. [DOI] [PubMed] [Google Scholar]
- 8. Brok J, Buckley N, Gluud C. Interventions for paracetamol (acetaminophen) overdose. Cochrane Database Syst Rev 2006. Apr 19; CD003328. [DOI] [PubMed] [Google Scholar]
- 9. Prescott LF, Illingworth RN, Critchley JA, Stewart MJ, Adam RD, Proudfoot AT. Intravenous N‐acetylcystine: the treatment of choice for paracetamol poisoning. Br Med J 1979; 2: 1097–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee WM, Hynan LS, Rossaro L, Fontana RJ, Stravitz RT, Larson AM, Davern TJ, Murray NG, McCashland T, Reisch JS, Robuck PR. Intravenous N‐acetylcysteine improves transplant‐free survival in early stage non‐acetaminophen acute liver failure. Gastroenterology 2009; 137: 856–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mumtaz K, Azam Z, Hamid S, Abid S, Memon S, Ali Shah H, Jafri W. Role of N‐acetylcysteine in adults with non‐acetaminophen‐induced acute liver failure in a center without the facility of liver transplantation. Hepatol Int 2009; 3: 563–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kortsalioudaki C, Taylor RM, Cheeseman P, Bansal S, Mieli‐Vergani G, Dhawan A. Safety and efficacy of N‐acetylcysteine in children with non‐acetaminophen‐induced acute liver failure. Liver Transpl 2008; 14: 25–30. [DOI] [PubMed] [Google Scholar]
- 13. Montanini S, Sinardi D, Praticò C, Sinardi A. Use of acetylcysteine as the life‐saving antidote in amanita phalloides (death cap) poisoning. Case report on 11 patients. Arzneimittelforschung 1999; 49: 1044–7. [DOI] [PubMed] [Google Scholar]
- 14. Nair GB, Ilowite JS. Pharmacologic agents for mucus clearance in bronchiectasis. Clin Chest Med 2012; 33: 363–70. [DOI] [PubMed] [Google Scholar]
- 15. Fishbane S. N‐acetylcysteine in the prevention of radiocontrast‐induced nephropathy. J Am Soc Nephrol 2004; 15: 251–60. [DOI] [PubMed] [Google Scholar]
- 16. Yalçin E, Altin F, Cinhüseyinoglue F, Arslan MO. N‐acetylcysteine in chronic blepharitis. Cornea 2002; 21: 164–8. [DOI] [PubMed] [Google Scholar]
- 17. Akyol‐Salman I, Azizi S, Mumcu U, Baykal O. Efficacy of topical N‐acetylcysteine in the treatment of meibomian gland dysfunction. J Ocul Pharmacol Ther 2010; 26: 329–33. [DOI] [PubMed] [Google Scholar]
- 18. Smilkstein MJ, Bronstein AC, Linden C, Augenstein WL, Kulig KW, Rumack BH. Acetaminophen overdose: a 48‐hour intravenous N‐acetylcysteine treatment protocol. Ann Emerg Med 1991; 20: 1058–63. [DOI] [PubMed] [Google Scholar]
- 19. Harrison PM, Wendon JA, Gimson AE, Alexander GJ, Williams R. Improvement by acetylcysteine of hemodynamics and oxygen transport in fulminant hepatic failure. N Engl J Med 1991; 324: 1852–7. [DOI] [PubMed] [Google Scholar]
- 20. Suk KT, Kim DJ. Drug‐induced liver injury: present and future. Clin Mol Hepatol 2012; 18: 249–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Smilkstein MJ, Knapp GL, Kulig KW, Rumack BH. Efficacy of oral N‐acetylcysteine in the treatment of acetaminophen overdose. Analysis of the national multicenter study (1976 to 1985). N Engl J Med 1988; 319: 1557–62. [DOI] [PubMed] [Google Scholar]
- 22. Harrison PM, Keays R, Bray GP, Alexander GJM, Williams R. Improved outcome of paracetamol‐induced fulminant hepatic failure by late administration of acetylcysteine. Lancet 1990; 335: 1572–3. [DOI] [PubMed] [Google Scholar]
- 23. Harrison P, Wendon J, Williams R. Evidence of increased guanylate cyclase activation by acetylcysteine in fulminant hepatic failure. Hepatology 1996; 23: 1067–72. [DOI] [PubMed] [Google Scholar]
- 24. Keays R, Harrison PM, Wendon JA, Forbes A, Gove C, Alexander GJ, Williams R. Intravenous acetylcysteine in paracetamol induced fulminant hepatic failure: a prospective controlled trial. BMJ 1991; 303: 1026–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chughlay MF, Kramer N, Werfalli M, Spearman W, Engel ME, Cohen K. N‐acetylcysteine for non‐paracetamol drug‐induced liver injury: a systematic review protocol. Syst Rev 2015; 4: 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Danan G, Benichou C. Causality assessment of adverse reactions to drugs—I. A novel method based on the conclusions of international consensus meetings: application to drug‐induced liver injuries. J Clin Epidemiol 1993; 46: 1323–30. [DOI] [PubMed] [Google Scholar]
- 27. Benichou C, Danan G, Flahault A. Causality assessment of adverse reactions to drugs—II. An original model for validation of drug causality assessment methods: case reports with positive rechallenge. J Clin Epidemiol 1993; 46: 1331–6. [DOI] [PubMed] [Google Scholar]
- 28. Maria VA, Victorino RM. Development and validation of a clinical scale for the diagnosis of drug‐induced hepatitis. Hepatology 1997; 26: 664–9. [DOI] [PubMed] [Google Scholar]
- 29. Agarwal VK, McHutchison JG, Hoofnagle JH. Important elements for the diagnosis of drug‐induced liver injury. Clin Gastroenterol Hepatol 2010; 8: 463–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. National Cancer Institute Common Terminology Criteria for Adverse Events v4.0 NCI, NIH, DHHS. May 29, 2009 NIH publication # 09–7473
- 31. Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011].
- 32. Review Manager (RevMan) [Computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
- 33. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000 Feb 29 ‐. Identifier NCT02182167, A Trial Of Intravenous N‐Acetylcysteine In The Management Of Antituberculous Drug‐Induced Hepatitis (NAC in TB DIH); 2014 Jun 18 [1 screen]. Available at https://clinicaltrials.gov/ct2/show/NCT02182167?term=N‐acetylcysteine+hepatitis&rank=5 (last accessed 20 Jun 2015).
- 35. Baniasadi S, Eftekhari P, Tabarsi P, Fahimi F, Raoufy MR, Masjedi MR, Velayati AA. Protective effect of N‐acetylcysteine on antituberculosis drug‐induced hepatotoxicity. Eur J Gastroenterol Hepatol 2010; 22: 1235–8. [DOI] [PubMed] [Google Scholar]
- 36. Squires RH, Dhawan A, Alonso E, Narkewicz MR, Shneider BL, Rodriguez‐Baez N, Olio DD, Karpen S, Bucuvalas J, Lobritto S, Rand E, Rosenthal P, Horslen S, Ng V, Subbarao G, Kerkar N, Rudnick D, Lopez MJ, Schwarz K, Romero R, Elisofon S, Doo E, Robuck PR, Lawlor S, Belle SH. Intravenous N‐acetylcysteine in pediatric patients with non‐acetaminophen acute liver failure: a placebo‐controlled clinical trial. Hepatology 2013; 57: 1542–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jong E, Conradie F, Berhanu R, Black A, John M‐A, Meintjes G, Menezes C. Consensus statement: management of drug‐induced liver injury in HIV‐positive patients treated for TB. South Afr J HIV Med 2013; 14: 113–9. [Google Scholar]


