Abstract
Aims
Several clinical trials have confirmed the therapeutic benefit of imipenem for treatment of lung infections. There is however no knowledge of the penetration of imipenem into the lung epithelial lining fluid (ELF), the site of action relevant for lung infections. Furthermore, although the plasma pharmacokinetics (PK) of imipenem has been widely studied, most studies have been based on selected patient groups. The aim of this analysis was to characterize imipenem plasma PK across populations and to quantify imipenem ELF penetration.
Methods
A population model for imipenem plasma PK was developed using data obtained from healthy volunteers, elderly subjects and subjects with renal impairment, in order to identify predictors for inter‐individual variability (IIV) of imipenem PK. Subsequently, a clinical study which measured plasma and ELF concentrations of imipenem was included in order to quantify lung penetration.
Results
A two compartmental model best described the plasma PK of imipenem. Creatinine clearance and body weight were included as subject characteristics predictive for IIV on clearance. Typical estimates for clearance, central and peripheral volume, and inter‐compartmental clearance were 11.5 l h–1, 9.37 l, 6.41 l, 13.7 l h–1, respectively (relative standard error (RSE) <8%). The distribution of imipenem into ELF was described using a time‐independent penetration coefficient of 0.44 (RSE 14%).
Conclusion
The identified lung penetration coefficient confirms the clinical relevance of imipenem for treatment of lung infections, while the population PK model provided insights into predictors of IIV for imipenem PK and may be of relevance to support dose optimization in various subject groups.
Keywords: antibiotics, epithelial lining fluid, imipenem, lung, pharmacokinetics
What is Already Known about this Subject
Imipenem has therapeutic benefit for treatment of lung infections.
Previous pharmacokinetic studies have mainly been based on small and specific patient populations.
What this Study Adds
This is the first integrated analysis of imipenem pharmacokinetics based on a large dataset of healthy volunteers, elderly subjects and subjects with renal impairment.
This is the first report on imipenem lung exposure into the epithelial lining fluid.
The developed model can be used to optimize imipenem dosing strategies further across patient populations and for treatment of lung infections.
Introduction
Imipenem is a potent β‐lactam antibiotic of the carbapenem class. It has a broad spectrum of activity against aerobic and anaerobic gram‐negative and gram‐positive bacteria 1. Imipenem is co‐formulated with cilastatin, an inhibitor of dehydropeptidase, which prevents the occurrence of renal tubular necrosis and prolongs the therapeutic effect of imipenem 1. In clinical practice, imipenem is used for treatment of mixed aerobic and anaerobic infections and serious hospital‐acquired infections.
The pharmacokinetic (PK) characteristics of imipenem have been described for healthy volunteers 2, 3, 4, pediatric patients 5, 6, critically ill patients 7, 8, 9, burn patients 10, febrile neutropenic cancer patients 11, 12, patients with renal impairment 13, and renal replacement therapy 14. Imipenem is primarily renally excreted and hence renal function is a key determinant for dose adjustment in patients with renal impairment 15.
Quantitative understanding of not only the typical PK characteristics of antibiotics, but also their inter‐individual variability (IIV) and patient‐derived predictors for such IIV, are of considerable relevance to optimize antibiotic treatment regimens across and within populations. Using population PK modelling, such understanding can be obtained in order to derive optimized dose regimens of antibiotics in specific patient populations and with respect to specific infections 16, 17, 18, 19, 20, 21. Furthermore, together with defined pharmacodynamic (PD) targets, population PK models can be used to perform stochastic simulations (also referred to as Monte Carlo simulations) to quantify the probability of target attainment for different dosing regimens being investigated 22. In the case of imipenem, despite the aforementioned availability of various PK studies, imipenem population PK models explicitly quantifying IIV in relation to PK parameters have been described only for critically ill patients 7, 9, burn patients 10 and patients with febrile neutropenia 12 (Table 1). However, all of these models were based on studies of limited sizes and were mostly based on retrospectively collected (i.e. sparsely sampled) data. A population PK model for imipenem based on a large group of individuals with a more informative distribution of potential predictors of IIV and with informatively sampled PK curves, could be of great value to guide model‐based optimization of imipenem dosing strategies further.
Table 1.
Previously published population pharmacokinetic analyses of imipenem in comparison with the current pooled analysis
| Model 1 | Model 2 | Model 3* | Model 4 | This analysis§ | |
|---|---|---|---|---|---|
| Reference | Dailly et al. 10 | Sakka et al. 7 | Couffignal et al. 9 | Lamoth et al. 12 | ‐ |
| Study design | Retrospective TDM data | Randomized controlled trial | Prospective trial | Retrospective TDM data | Pooled analysis of prospective trials |
| Patients | Burn patients | Critically ill patients | Critically ill patients | Cancer patients with febrile neutropenia | Healthy volunteers, elderly, RI patients |
| Number of patients | 47 | 20 | 51 | 57 | 149 |
| Samples per patient ¶ | 2.5 | 6 | 6 | 2 | 11 |
| Dose | 2 g** | 1–2 g† | 500–1000 mg every 8 h | 500 mg every 6 h | 250–500mg single dose or every6 h |
| PK model | Two compartment | Two compartment | Two compartment | One compartment | Two compartment |
| Clearance (l h –1 ) | 16.37 | 12.3 | 13.0 | 10.7 | 11.5 |
| Central volume (l) | 26.32‡ | 12.2 | 22.4 | 33.5 | 9.37 |
| Peripheral volume (l) | 39†† | 92†† | 9.90 | ‐ | 6.41 |
| Inter‐compartmental clearance (l h –1 ) | 7.8†† | 10.5†† | 10.1 | ‐ | 13.7 |
| Covariates | CLcr | ‐ | CLcr, WT, ALB | GFR, WT | CLcr, WT |
RI, renal impaired; CLcr, creatinine clearance; WT, body weight; ALB, serum albumin
Parameter estimates of base model without covariate effects.
Study of short term vs. continuous infusion.
Converted from l kg–1 to l based on bodyweight of 77.3 kg (i.e. same scaling as current study)
Excluding the lung study.
Mean or median (what was provided)
Short term infusion.
Converted from micro constants.
Plasma concentrations relative to the minimum inhibitory concentration (MIC) are widely used to predict the efficacy of antibiotics against the bacteria of interest. However, plasma concentrations may not be reflective of the concentration at the site of infection and may therefore not necessarily reach therapeutic levels if dose selection is based on plasma concentrations. Hence, it is important to quantify these differences in concentration in plasma and at the site of infection with respect to the therapeutic concentration attainment necessary for resolution of infection 23. The treatment of lung infections due to common extracellular invading respiratory pathogens is one such important therapeutic application area, where the epithelial lining fluid (ELF) is the relevant site of infection. Antibiotic concentrations in the ELF indicative of drug penetration can differ greatly from corresponding plasma concentrations 24, 25.
Even though several studies 26, 27, 28, 29 have confirmed the clinical relevance of imipenem for treatment of lung infections, there is no knowledge of the penetration of imipenem into the lung ELF. There is merely knowledge available regarding concentrations attained in whole lung tissue homogenates 30, 31, which are not reflective of the ELF concentration 32.
In this work we describe the development of a comprehensive population PK model for imipenem plasma PK based on a pooled analysis of three prospective clinical studies in healthy volunteers, renal impairment subjects and elderly subjects that may be of used to inform dose regimen optimization. Furthermore, we report for the first time results on a study that investigated the distribution of imipenem into the ELF space.
Methods
Clinical studies
This work concerns the analysis of four clinical studies in which imipenem concentrations in plasma (three studies) and ELF penetration (one study) were measured. All studies were performed to support the clinical development of the novel β‐lactamase inhibitor MK‐7655 33, which was administered together with imipenem and cilastatin. The current analysis focusses specifically on the analysis of the imipenem PK data. Initial results of these studies have been published as conference abstracts at the Interscience Conference on Antimicrobial Agents and Chemotherapy meeting 34, 35, 36. Based on internal in vitro and preclinical experiments, including known information about transporters, metabolism and elimination mechanisms, no interaction was expected. This was confirmed in study 1 where the PK differences between MK‐7655 or imipenem administered alone and in combination were negligible 37.
Subjects provided written informed consent prior to participation in the study and all studies where approved by an ethics review committee, with details provided below. An overview of demographics and clinical study design details is provided in Table 2.
Table 2.
Study designs and subject demographics of includes clinical studies
| Study (reference) | Study 1 34 | Study 2 34 | Study 3 35 | Study 4 33 |
|---|---|---|---|---|
| Study description | Safety, tolerability and PK of MK‐7655 in combination with imipenem | Safety, tolerability and PK of MK‐7655 in combination with imipenem | Plasma PK of MK‐7655 in combination with imipenem | Intrapulmonary PK of MK‐7655 in combination with imipenem |
| Population | Healthy volunteers | Elderly and gender study including young females | Subjects with renal impairment | Healthy volunteers |
| Subjects (n) | 83 | 18 | 48 | 16 |
| Imipenem dose regimen | Single dose 500 mg (study 1A) | Single dose 500 mg | Single dose 250 mg | Repeated dose of 500 mg every 6 h |
| Repeated dose of 500 mg every 6 h (study 1B) | ||||
| Body weight (kg)[median, range] | 78.0 (60.0–101.4) | 63.5 (57.3–78.6) | 82 (60.1–115.8) | 85.3 (58.8–107.6) |
| Creatinine clearance ‡ (ml min–1) [median, range] | 139.9 (75.7–199.6) | 86.8 (58.3–173.0) | 74 (7.9–153.4) | 133.3 (50.2–199.2) |
| Age (years)[median, range] | 27 (18–45) | 61 (26–75) | 64 (26–75) | 30 (24–42) |
| Ethnicity (n) | Caucasian 67 | Caucasian 17 | Caucasian 34 | Caucasian 10 |
| African American 7 | African American 1 | African American 14 | African American 1 | |
| Other 2 | ||||
| Other 3 | ||||
| Other 9 | ||||
| Gender (male/female) | 83/0 | 6/12 | 28/20 | 13/3 |
| Concentrations | Plasma | Plasma | Plasma | Plasma/ELF |
| Study occasions (n) | 3* | 1 | 1 | 1 |
| Samples per ID of one occasion (median, range) | 11 (2–13) | 11 (10–12) | 10 (9–13) | 1† |
| Protocol sampling times post‐start of infusion (h) | 0.083, 0.25, 0.5 0.75, 1,1.5, 2, 3, 4.5, 6, 8, 10, 14 (study 1A and 1B¶) | 0.083, 0.25, 0.5 0.75, 1,1.5, 2, 3, 4.5, 6, 8, 10, 14 | 0.083, 0.25, 0.5 0.75, 1,1.5, 2, 3, 4.5, 6, 3, 10, 14 | 0, 24.5, 25, 25.5, 27§ |
| Steady‐state or first dose | First dose, steady‐state | First dose | First dose | Steady‐state |
ELF, epithelial lining fluid; PK, pharmacokinetics.
At five additional occasions single PK samples were obtained.
Paired samples in plasma and ELF
At baseline.
Only one time per subject for plasma/ELF concentration pairs.
Study 1B, at day 6: 0.083, 0.25, 0.5 0.75, 1,1.5, 2, 3, 4.5, 6, 8, 10, 14, or a pre‐dose steady‐state samples at day 1, 2, 4, 6, 7, or, a pre‐dose steady‐state samples at day 1, 4, 8, 12 or 14.
Study 1 investigated the safety, tolerability and PK of MK‐7655 in combination with imipenem in healthy volunteers in 83 subjects receiving either a single dose of 500 mg imipenem (study 1A) or repeated doses of imipenem every 6 h (study 1B). This study was conducted at the site of Hammersmith Medicines Research, London, UK and ethics approval was obtained from Plymouth Independent Research Ethics Committee (PIREC), Plymouth, UK.
Study 2 investigated the safety, tolerability and PK of MK‐7655 in combination with imipenem in elderly subjects and bother male and female subjects (n = 18). This study was conducted at the site of Comprehensive NeuroScience, Inc., Miramar, Florida, USA and ethics approval was obtained from Independent Investigational Review Board, Inc., Plantation, Florida, USA.
Study 3 investigated the PK of MK‐7655 in combination with imipenem in subjects with renal impairment (n = 48). This study was conducted at the site of Orlando Clinical Research Center, Orlando, Florida, USA and ethics approval was obtained from Independent Investigational Review Board, Inc., Plantation, Florida, USA.
Study 4 investigated the intrapulmonary PK of MK‐7655 in combination with imipenem in healthy volunteers (n = 16). Here, single paired samples of the plasma and ELF concentration of imipenem were collected at four different time points (0.5, 1.0, 1.5 and 3 h respectively), obtained after five doses of imipenem administered every 6 h. This study was conducted at the site of Pulmonary Associates, Phoenix, Arizona, USA and ethics approval was obtained from Quorum Review IRB, Seattle, Washington, USA.
Bronchoalveolar lavage procedure
In study 4, ELF concentrations were determined using a bronchoalveolar lavage (BAL) procedure. Subjects received a topical anaesthetic and the BAL was then conducted using fibreoptic bronchoscopy. Four aliquots of saline (50 ml each) were instilled for no longer than 1 min into the right middle lobe and aspirated. First aspirate was discarded and the remainder pooled and volume measured. Aliquots for cell count and urea assay were obtained. The BAL samples were then centrifuged and cell pellets and supernatant separated for imipenem quantification in the ELF. ELF volumes recovered by BAL were determined using urea as an endogeneous marker for providing a dilution ratio by measurement of urea concentration in BAL and serum 38.
Bioanalysis
Total imipenem plasma and ELF concentrations were quantified using validated bioanalytical methods. The assays were based on hydrophilic liquid chromatography (HILIC) tandem mass spectrometry (LC‐MS/MS) with protein precipitation as the sample preparation technique. To ensure stability of imipenem, the clinical samples were diluted 1 to 1 with a preservative. The preservative was composed of equal parts ethylene glycol and a buffer (1 m 2‐(N‐morpholino) ethanesulfonic acid, pH 6.7, MES).
The LC‐MS/MS system consisted of a Waters Acquity UPLC (Milford, MA, USA) and an API 4000 or 5000 triple quadrupole tandem mass spectrometer (AB Sciex, Ontario, Canada). Data acquisition and analyses were performed using a Sciex Analyst and Watson LIMS system. The mass spectrometer was equipped with a turboionspray (TIS) interface and operated in the positive ionization mode. The TIS was set at 500 °C with ionization potential at 5000 V. The curtain gas was set at 40 and declustering potential (DP) was set at +40 V. Collision‐induced dissociation at the second quadrupole used nitrogen as the collision gas (set at 6), and collision energy (CE) was set at 35 V. Analyses were performed under unit mass resolution with multiple reaction monitoring (MRM), using the characteristic precursor→product ion transitions at m/z 300 →98 and 307 → 98 for imipenem and stable isotope labelled internal standard, [13C3 15N2 2H2]‐Imipenem (IS), respectively. Unknown sample concentrations were calculated from the equation (y = Ax2 + Bx + c) as determined by weighted (1/x2) quadratic regression of the standard curve.
The chromatographic separation of the analytes was achieved using a Waters Atlantis HILIC (50 x 2.1 mm x 3 μm) column kept at 35 °C and mobile phase consisting of 5 mM ammonium acetate (pH 4.5) in 80 : 20 acetonitrile : water. The flow rates and run times for the plasma and ELF assays were 0.45 ml min–1, 3.0 min and at 0.4 ml min–1, 4.0 min, respectively. Under these conditions, the retention time of imipenem and IS was 2.1 min.
Sample preparation was based on 96‐well format protein precipitation of analyte from stabilized plasma or ELF. Briefly, 50 μl of the sample was transferred to 2 ml 96‐well plate. MES buffer was added (35 μl for plasma assay, 25 μl for ELF assay) followed by the addition of 10 μl of working IS. Precipitation solvent (500 μl of 80 : 20 acetonitrile : water for plasma; 400 μl 90 : 10 acetonitrile : water for ELF) was added, and then the samples were vortexed and centrifuged. Two to three microliter aliquots of the extract were injected into the LC‐MS/MS system.
The lower limit of quantitation (LLOQ) for the imipenem plasma assay was 0.25 μg ml–1 with a calibration range from 0.25 to 100 μg ml–1 using a 50 μl sample. The intraday accuracy and precision ranges (for three core runs) of the LLOQ and quality control samples (QCs at low, mid, high concentrations) were 84 to 104% for LLOQ, 89 to 97% for QCs with corresponding precision ranges of 4.8 to 11.5% and 1.6 to 5.4% (expressed as coefficient of variation, CV%), respectively. For the ELF assay, the LLOQ was 5 ng ml–1 with a calibration range from 5 to 500 ng ml–1. The intraday accuracy and precision ranges (for three core runs) of the LLOQ and QCs (low, mid, high concentrations) were 96 to 109% for LLOQ; 99 to 106% for QCs with corresponding precision ranges of 2.6 to 9.1% and 0.8 to 4.6%, respectively.
Model development
The population PK model of imipenem was developed by pooling all available data from studies 1–3. Subsequent modeling of ELF concentrations was based on data from study 4. (Table 2).
The population PK analysis for imipenem plasma concentrations was performed using a non‐linear mixed effect modelling approach implemented in the software package nonmem (version 7.3) 39. The first order conditional estimation method was used throughout the analysis.
Previous modelling efforts have shown that the PK of imipenem are best explained by a two compartment model with linear elimination 7, 9, 10, 12. Hence, a two compartmental model parametrized in terms of clearance (CL), inter‐compartmental clearance (Q), central volume (V c) and peripheral volume (V p) was used in the current analysis. Random effects for IIV on parameter estimates were modelled according to a log‐normal distribution as follows for typical parameter P and individual i:
| (1) |
where ηi was distributed according to N(0,ΩP 2). Additive, proportional and combined residual error models (equation 2) were considered:
| (2) |
Here, C ij,pred and C ij,obs represent the predicted or observed concentration for individual i and time j, εadd,ij and εprop,ij are distributed according to N(0,σadd 2) or N(0,σprop 2), respectively. For a combined error model σadd and σprop are estimated, while σadd = 0 for a proportional error model and σprop = 0 for an additive model.
Model development, including the selection of random effect structure, was guided by the –2 log likelihood (–2LL), the precision of parameter estimates and visual inspection of residual diagnostics.
For development of the covariate model for imipenem plasma PK, creatinine clearance (CRCL), as computed using the Cockcroft‐Gault equation 40, was included a priori based on the established relevance of renal function for imipenem PK. Both linear (equation (3)) and power (equation (4)) relationships were considered.
| (3) |
| (4) |
Furthermore, in order to allow for more precise and unbiased estimation of other covariates, the effect of body size was included using a fixed allometric relationship with body weight (WT) 41 on all clearances (equation (5)) and volumes (equation (6)).
| (5) |
| (6) |
Other covariates that were explored in relation to clearance and volumes included the effect of age, gender and ethnicity. Additional covariates were evaluated and considered for possible inclusion in the model based on a significant drop in –2LL (P < 0.01, likelihood ratio test) together with a relevant drop in IIV variance, clear physiological plausibility and clinical relevance of the magnitude of effect. These additional covariates were evaluated using linear (equations (7) and 8) or power relationships (equation 9):
| (7) |
| (8) |
| (9) |
where COVi represents the individual covariate value, θCOV represents the covariate effect, P represent the typical parameter value and Pi the individual parameter values. All continuous covariates including CLcr and WT were scaled by their median values.
The relationship between plasma and ELF concentrations of imipenem was incorporated in the model as follows. First, the parameter estimates from the final population PK model were fixed. Then, the PK model was used to predict the observed pairs of plasma and ELF concentrations. The extent and dynamics of penetration into the ELF was investigated using three different approaches: 1) time‐independent ratio estimated as a factor impacting the covariate adjusted predicted plasma concentration, 2) as a model with bidirectional mass transfer between the ELF space while estimating a volume of distribution for the ELF compartment 42 and 3) as bi‐directional ELF compartment (e.g. similar to a classical effect compartment model) without mass transfer. Finally, both additive, proportional and combined residual errors models were evaluated separately for the ELF concentration data (equation 2).
The suitability of the selected models for plasma and ELF PK was also evaluated using a visual predictive check (VPC). The precision of parameter estimates of the final population PK model was evaluated using a non‐parametric bootstrap analysis (n = 1000).
Results
Plasma pharmacokinetics
As expected, the plasma PK of imipenem were best described by a two compartmental model with first‐order elimination. An overview of the parameter estimates is provided in Table 3. The effect of CLcr on clearance was best described using a power function. Limited effects of age and race on clearance were identified. These effects were not included in the final model as their effect magnitudes were small and the reduction in IIV variance was negligible. Inclusion of WT and CLcr in the base model substantially reduced IIV on CL from 27% to 16%, while it explained less than 3% of observed IIV of the central and peripheral volume.
Table 3.
Parameter estimates and bootstrap results for the final population pharmacokinetic model for imipenem pharmacokinetics in plasma and epithelial lining fluid (ELF) penetration
| Description | Parameter | Unit | Estimate | RSE (%) | Bootstrap * | |
|---|---|---|---|---|---|---|
| Median | 95% PI | |||||
| Model of plasma PK | ||||||
| Structural model | ||||||
| Clearance | θCL | h–1 | 11.5 | 1.4 | 11.5 | 11.1, 11.8 |
| Volume, central | θVc | l | 9.37 | 3.3 | 9.39 | 8.78, 10.1 |
| Volume, peripheral | θVp | l | 6.41 | 4.0 | 6.37 | 5.91, 6.91 |
| Inter‐compartmental clearance | θQ | l h–1 | 13.7 | 5.6 | 13.5 | 10.9, 16.3 |
| Covariate model | ||||||
| Weight on CL and Q | (WT/77.3)θWT1 | ‐ | 0.75 | ‐ (fix) | ‐ | ‐ |
| Weight on V c and V p | (WT/77.3)θWT2 | ‐ | 1 | ‐ (fix) | ‐ | ‐ |
| Creatinine clearance on CL | (CLcr/125.14)θCLcr | 0.36 | 8.2 | 0.36 | 0.29, 0.42 | |
| Inter‐individual variability | ||||||
| Clearance | ΩCL | CV% | 15.9 | 6.7 | 15.7 | 13.7, 17.6 |
| Volume, central | ΩV1 | CV% | 32.9 | 6.5 | 32.6 | 28.3, 36.5 |
| Volume, peripheral | ΩV2 | CV% | 20.1 | 29.1 | 17.8 | 6.32, 28.6 |
| Inter‐compartmental clearance | ΩQ | CV% | ‐ | ‐ | ‐ | ‐ |
| Residual error | ||||||
| Additive error (SD) | σA | mg l–1 | 0.30 | 43 | 1.02 | 0.05, 1.92 |
| Proportional error | σP | CV% | 15.5 | 12 | 15.2 | 11.4, 18.5 |
| Description | Parameter | Unit | Estimate | RSE (%) | ||
|---|---|---|---|---|---|---|
| Model of ELF distribution | ||||||
| Penetration coefficient | PELF | ‐ | 0.444† | 14 | ||
| Proportional residual error | σP,ELF | CV% | 12.7 | 59 |
CV, coefficient of variation; RSE, relative standard error; PI, prediction interval; SD, Standard deviation.
Non‐parametric bootstrap.
C ELF,predicted = C plasma,predicted * PELF
All model parameters could be estimated with good precision (Table 3). Fixed effects had relative standard errors (RSEs) below 8.2%. Precision of parameter estimates was further confirmed by the results of the non‐parametric bootstrap (Table 3). The VPC (Figure 1) and goodness of fit diagnostics (Figure 2) indicated adequate description of the concentration–time curves. The final parameter–covariate relationships in the final plasma PK model are as follows:
| (10) |
| (11) |
| (12) |
| (13) |
Figure 1.
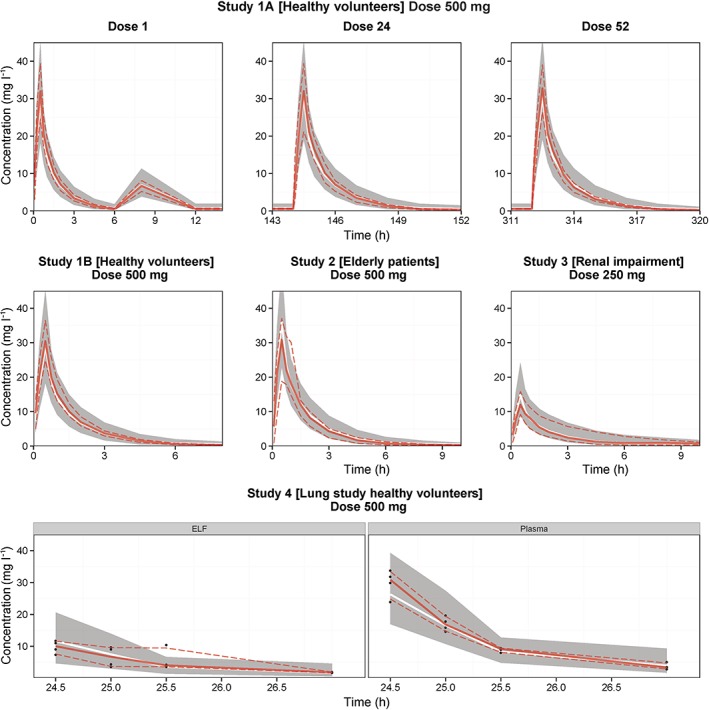
Visual predictive checks of the plasma and epithelial lining fluid (ELF) concentration–time profiles for imipenem, stratified by study. The solid lines represent the median of the observed data (red) and simulated data (white). The dashed lines and grey shaded areas represent the 5th and 95th percentiles of the observed and simulated data respectively. The design of these four studies is described in further detail in Table 2
Figure 2.
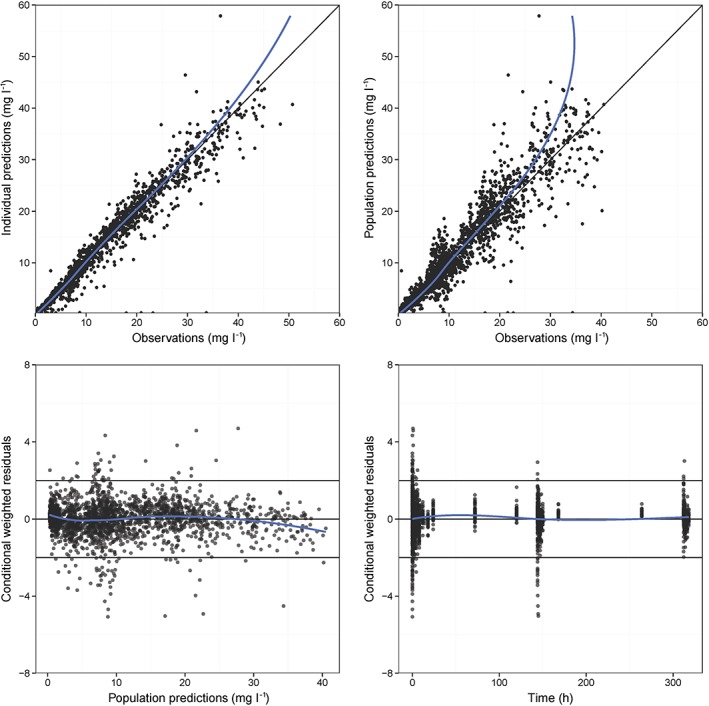
Goodness of fit plots for imipenem observed vs. individual and population predictions, and population predicted and time vs. conditional weighted residuals. The blue solid line represents a loess smoother
ELF data
The observed plasma : ELF ratios are provided in Table 4. The observed data and simulated values are depicted in the VPC (Figure 1). A limited trend over time was observed for the ratios reported here after receiving the fifth dose of imipenem. Approaches to describe the dynamics of the ELF concentrations (e.g. approach 2 and approach 3 (see Methods section) resulted in unstable models with high relative standard errors. Intermediate correlation matrices indicated high (r > 0.9) correlations between parameter estimates related to ELF distribution. Hence, a time‐independent penetration coefficient model (equation (14)) was selected to describe the relationship between plasma and ELF concentrations (Table 3).
| (14) |
Table 4.
Observed ratios of epithelial lining fluid/plasma including geometric mean ratio and its range
| Time (h) | Number of subjects | Geometric mean ratio | Range (minimum–maximum) |
|---|---|---|---|
| 0.5 | 4 | 0.325 | 0.302–0.369 |
| 1 | 4 | 0.360 | 0.245–0.508 |
| 1.5 | 4 | 0.554 | 0.429–1.103 |
| 3 | 4 | 0.504 | 0.386–0.592 |
This fixed penetration coefficient was estimated at 0.44 with good precision (RSE 14%). The plasma concentrations measured in this analysis were total imipenem plasma concentrations. However, only unbound imipenem will transfer to the ELF. The protein binding of imipenem is approximately 20% 43. Therefore, the unbound ratio would be increased from 0.44 to 0.55.
Discussion
In contrast to previous population PK analyses (Table 2), the developed population PK model for imipenem has been based on a relatively large number of subjects (n = 149), which allowed derivation of precise estimates of both typical values and IIV variances of parameter estimates. In contrast to other studies, we have included different populations (healthy, females, elderly, renally impaired) although these did not include subjects with lung infections. Combined analysis of PK data from healthy volunteers as well as elderly subjects and subject with renal impairment resulted in an informative distribution of both established and potentially relevant predictors for IIV in PK parameters. Our model provides precise estimates of CLcr as predictors for IIV in CL of imipenem. Moreover, this analysis provides a stronger basis to confirm the expected lack of clinical relevance of age, gender and ethnicity as possible clinically relevant predictors that can explain the IIV of the PK, which was previously based on studies of limited size 15.
In comparison with previously published population PK models in patients (Table 1), we found that the CL estimated for healthy volunteers in the current study is in line with typical CL estimates in patients. The typical estimates for distribution volumes and inter‐compartmental clearance were found lower compared with estimates described in patients. We expect that this is firstly related to physiological differences associated with conditions such as fluid loading and increases in haemodynamic blood flow in critically ill patients such as also reported for carbapenems 15 and other antibiotics. Secondly, early phase distribution of imipenem is very brief. Hence the central volume and inter‐compartmental clearance parameters are highly dependent on sampling designs used. Since our analysis used relatively densely sampled datasets, we expect that our estimates are less affected by sampling design issues in comparison with some of the other studies.
This is the first report of imipenem penetration into the ELF. A relatively high ELF : plasma ratio (EPR) of 0.44 (or 0.55 based on unbound concentration assuming a protein binding of 20%) was identified for unbound imipenem. No time‐dependent effects of ELF distribution could be reliably identified from the available data, even though a marginal change was apparent from the observed EPRs. This may be due to the inherent sparse design consisting of few time points for BAL studies in general, suggesting the need for potential design optimization of such studies. Additional BAL studies may benefit from the use of applying model‐based design optimization as described by Clewe et al. 44. Furthermore, various other technical challenges related to ELF collection complicate precise quantification of drug concentration in the ELF 45. Besides various technical challenges, the limited change in EPRs over time may also suggest that at least at the steady‐state (repeated dosing) conditions of our study, such time dependencies may not play a relevant role for imipenem, e.g. equilibration occurs rapidly. Previous reviews comparing EPRs in healthy volunteers and patients indicate relatively consistent values between these populations 25, 46. Still, in critically ill patients, EPRs may potentially be different due to the effect of inflammation of lung tissue among other factors. Although this ELF penetration analysis has been derived from a volunteer study and cannot be directly extrapolated to patients, it can serve as a first conservative estimate of imipenem lung penetration and a hypothesis generation tool.
A limitation of our analysis is that it did not involve patients but volunteers, which may result in decreased estimates of IIV. Potentially, a meta‐analysis of published population PK models for health volunteers and patients could help to further bridge this gap. In addition, ELF data were available only for a limited number of subjects out of the total number of 149 subjects, limiting the knowledge generation of dynamical and IIV characteristics for imipenem lung penetration.
Given that the identified EPR suggests antibiotic ELF concentrations substantially different than plasma concentrations, this ratio may be of relevance to inform simulation‐based dose regimen evaluation of imipenem in lung infections. The identified EPR can be combined with the population PK model described by us or with previously described population PK models. Using this approach, dose regimens of imipenem in lung infections for specific patient groups may be evaluated based on the target site concentration, rather than the plasma concentration.
A number of recent studies have investigated the use of imipenem for treatment of hospital‐ or ventilator‐associated pneumonia (HAP, VAP). These included trials evaluating single dose imipenem compared with doripenem 26, imipenem in combination with amikacin 27, imipenem compared with tigecycline 28 and imipenem compared with intravenous ciprofloxacin 29. These studies indicated therapeutic relevance for treatment of HAP/VAP, although it is not registered for these indications. While a dynamic penetration coefficient would have been preferable, the fixed penetration coefficient identified in this analysis is still of clinical relevance to inform the dose optimization of imipenem dosing regimens in patients with lung infections ensuring adequate drug penetration, while taking into consideration body size and renal function.
Specific simulation studies for various patient groups and dose regimens could be conducted to evaluate further currently used and potentially optimized dose regimens. Such an analysis was considered to fall outside the scope of the current work.
In conclusion, this is the first report of imipenem lung penetration, which is of relevance for further evaluation of lung infection treatment strategies with imipenem. At steady state, we identified a rapid equilibration resulting in a time‐independent ELF : plasma ratio. Furthermore, the developed population PK model based on several populations provided precise estimates for predictors in IIV. As such, the identified models for both ELF‐plasma penetration and plasma PK are of relevance to support the design of improved dosing strategies based on patient characteristics in specific patient populations or for specific infection strains. Ultimately, this may improve bactericidal exposure and may further minimize the risk of emergence of resistance.
Competing Interests
All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare PvdG, MD, GR had no support from any organization for the submitted work. JGCvH postdoctoral fellowship had support (co‐funding) from the independent research consortium PKPD platform 2.0 for the submitted work, Merck & Co. is a partner in the PKPD platform 2.0. JGCvH, GR, PHG, MD had no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and MLR, ML, CCE, SAGV, TK were employed by Merck & Co in the previous 3 years. All authors had no other relationships or activities that could appear to have influenced the submitted work.
This study was performed within the framework of Dutch Top Institute Pharma, PKPD PLATFORM 2.0 (project number D2‐501). This work was carried out on the Dutch national e‐infrastructure with the support of SURF Foundation.
van Hasselt, J. G. C. , Rizk, M. L. , Lala, M. , Chavez‐Eng, C. , Visser, S. A. G. , Kerbusch, T. , Danhof, M. , Rao, G. , and van der Graaf, P. H. (2016) Pooled population pharmacokinetic model of imipenem in plasma and the lung epithelial lining fluid. Br J Clin Pharmacol, 81: 1113–1123. doi: 10.1111/bcp.12901.
References
- 1. Clissold SP, Todd PA, Campoli‐Richards DM. Imipenem/cilastatin. A review of its antibacterial activity, pharmacokinetic properties and therapeutic efficacy. Drugs 1987; 33: 183–241. [DOI] [PubMed] [Google Scholar]
- 2. Norrby SR, Bjornegard B, Ferbert F, Jones KH. Pharmacokinetics of imipenem in healthy volunteers. J Antimicrob Chemother 1983; 12 (suppl D): 109–24. [DOI] [PubMed] [Google Scholar]
- 3. Norrby SR, Rogers JD, Ferber F, Jones KH, Zacchei AG, Weidner LL, Demetriades JL, Gravallese DA, Hsieh JY. Disposition of radiolabeled imipenem and cilastatin in normal human volunteers. Antimicrob Agents Chemother 1984; 26: 707–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lee LS, Kinzig‐Schippers M, Nafziger AN, Ma L, Sörgel F, Jones RN, Drusano GL, Bertino JS. Comparison of 30‐min and 3‐h infusion regimens for imipenem/cilastatin and for meropenem evaluated by Monte Carlo simulation. Diagn Microbiol Infect Dis 2010; 68: 251–8. [DOI] [PubMed] [Google Scholar]
- 5. Giannoni E, Moreillon P, Cotting J, Moessinger A, Bille J, Décosterd L, Zanetti G, Majcherczyk P, Bugnon D. Prospective determination of plasma imipenem concentrations in critically ill children. Antimicrob Agents Chemother 2006; 50: 2563–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yoshizawa K, Ikawa K, Ikeda K, Ohge H, Morikawa N. Population pharmacokinetic‐pharmacodynamic target attainment analysis of imipenem plasma and urine data in neonates and children. Pediatr Infect Dis J 2013; 32: 1208–16. [DOI] [PubMed] [Google Scholar]
- 7. Sakka SG, Glauner AK, Bulitta JB, Kinzig‐Schippers M, Pfister W, Drusano GL, Sörgel F. Population pharmacokinetics and pharmacodynamics of continuous versus short term infusion of imipenem‐cilastatin in critically ill patients in a randomized, controlled trial. Antimicrob Agents Chemother 2007; 51: 3304–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fish DN, Teitelbaum I, Abraham E. Pharmacokinetics and pharmacodynamics of imipenem during continuous renal replacement therapy in critically ill patients. Antimicrob Agents Chemother 2005; 49: 2421–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Couffignal C, Pajot O, Laouénan C, Burdet C, Foucrier A, Wolff M, Armand‐Lefevre L, Mentré F, Massias L. Population pharmacokinetics of imipenem in critically ill patients with suspected ventilator‐associated pneumonia and evaluation of dosage regimens. Br J Clin Pharmacol 2014; 78: 1022–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dailly E, Kergueris MF, Pannier M, Jolliet P, Bourin M. Population pharmacokinetics of imipenem in burn patients. Fundam Clin Pharmacol 2003; 17: 645–50. [DOI] [PubMed] [Google Scholar]
- 11. Drusano GL, Plaisance KI, Forrest A, Bustamante C, Devlin A, Standiford HC, Wade JC. Steady‐state pharmacokinetics of imipenem in febrile neutropenic cancer patients. Antimicrob Agents Chemother 1987; 31: 1420–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lamoth F, Buclin T, Csajka C, Pascual A, Calandra T, Marchetti O. Reassessment of recommended imipenem doses in febrile neutropenic patients with hematological malignancies. Antimicrob Agents Chemother 2009; 53: 785–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Verpooten GA, Verbist L, Buntinx AP, Entwistle LA, Jones KH, De Broe ME. The pharmacokinetics of imipenem (thienamycin‐formamidine) and the renal dehydropeptidase inhibitor cilastatin sodium in normal subjects and patients with renal failure. Br J Clin Pharmacol 1984; 18: 183–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mueller BA, Scarim SK, Macias WL. Comparison of imipenem pharmacokinetics in patients with acute or chronic renal failure treated with continuous hemofiltration. Am J Kidney Dis 1993; 21: 172–9. [DOI] [PubMed] [Google Scholar]
- 15. Mouton JW, Touw DJ, Horrevorts AM, Vinks AATMM. Comparative pharmacokinetics of the carbapenems. Clin Pharmacokinet 2000; 39: 185–201. [DOI] [PubMed] [Google Scholar]
- 16. van Hasselt JGC, Andrew MA, Hebert MF, Tarning J, Vicini P, Mattison DR. The status of pharmacometrics in pregnancy: Highlights from the 3rd American Conference on Pharmacometrics. Br J Clin Pharmacol 2012; 74: 932–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Derendorf H, Lesko LJ, Chaikin P, Colburn WA, Lee P, Miller R, Powell R, Rhodes G, Stanski D, Venitz J. Pharmacokinetic/pharmacodynamic modeling in drug research and development. J Clin Pharmacol 2000; 40: 1399–418. [PubMed] [Google Scholar]
- 18. van Hasselt JGC, Allegaert K, van Calsteren K, Beijnen JH, Schellens JHM, Huitema ADR. Semiphysiological versus empirical modelling of the population pharmacokinetics of free and total cefazolin during pregnancy. Biomed Res Int 2014; 2014: (Article ID 897216):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lo YL, van Hasselt JGC, Heng SC, Lim CT, Lee TC, Charles BG. Population pharmacokinetics of vancomycin in premature malaysian neonates: identification of predictors for dosing determination. Antimicrob Agents Chemother 2010; 54: 2626–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. van Hasselt JGC, Schellens JHM, Beijnen JH, Huitema ADR. Design of informative renal impairment studies: evaluation of the impact of design stratification on bias, precision and dose adjustment error. Invest New Drugs 2014; 32: 913–27. [DOI] [PubMed] [Google Scholar]
- 21. van Hasselt JGC, Gupta A, Hussein Z, Beijnen JH, Schellens JHM, Huitema ADR. Population pharmacokinetic‐pharmacodynamic analysis for eribulin mesilate associated neutropenia. Br J Clin Pharmacol 2013; 76: 2878–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ambrose PG. Monte Carlo Simulation in the evaluation of susceptibility breakpoints: predicting the future: insights from the Society of Infectious Diseases Pharmacists. Pharmacotherapy 2006; 26: 129–34. [DOI] [PubMed] [Google Scholar]
- 23. Liu P, Derendorf H. Antimicrobial tissue concentrations. Infect Dis Clin North Am 2003; 17: 599–613. [DOI] [PubMed] [Google Scholar]
- 24. Valitalo P, Griffioen K, Rizk M, Visser S, Danhof M, Rao M, van der Graaf P, van Hasselt J. Structure‐based prediction of anti‐infective drug concentrations in the human lung epithelial lining fluid. Pharm Res 2016; 33: 856–67. [DOI] [PubMed] [Google Scholar]
- 25. Rodvold KA, George JM, Yoo L. Penetration of anti‐infective agents into pulmonary epithelial lining fluid: focus on antibacterial agents. Clin Pharmacokinet 2011; 50: 637–64. [DOI] [PubMed] [Google Scholar]
- 26. Kollef MH, Chastre J, Clavel M, Restrepo MI, Michiels B, Kaniga K, Cirillo I, Kimko H, Redman R. A randomized trial of 7‐day doripenem versus 10‐day imipenem‐cilastatin for ventilator‐associated pneumonia. Crit Care 2012; 16: R218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pajot O, Burdet C, Couffignal C, Massias L, Armand‐Lefevre L, Foucrier A, Da Silva D, Lasocki S, Laouénan C, Mentec H, Mentré F, Wolff M. Impact of imipenem and amikacin pharmacokinetic/pharmacodynamic parameters on microbiological outcome of Gram‐negative bacilli ventilator‐associated pneumonia. J Antimicrob Chemother 2015; 70: 1487–94. [DOI] [PubMed] [Google Scholar]
- 28. Freire AT, Melnyk V, Kim MJ, Datsenko O, Dzyublik O, Glumcher F, Chuang YC, Maroko RT, Dukart G, Cooper CA, Korth‐Bradley JM, Dartois N, Gandjini H. Comparison of tigecycline with imipenem/cilastatin for the treatment of hospital‐acquired pneumonia. Diagn Microbiol Infect Dis 2010; 68: 140–51. [DOI] [PubMed] [Google Scholar]
- 29. Fink MP, Snydman DR, Niederman MS, Leeper KV, Johnson RH, Heard SO, Wunderink RG, Caldwell JW, Schentag JJ, Siami GA. Treatment of severe pneumonia in hospitalized patients: results of a multicenter, randomized, double‐blind trial comparing intravenous ciprofloxacin with imipenem‐cilastatin. The Severe Pneumonia Study Group. Antimicrob Agents Chemother 1994; 38: 547–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Benoni G, Cuzzolin L, Bertrand C, Puchetti V, Velo G. Imipenem kinetics in serum, lung tissue and pericardial fluid in patients undergoing thoracotomy. J Antimicrob Chemother 1987; 20: 725–8. [DOI] [PubMed] [Google Scholar]
- 31. Wise R, Donovan IA, Lockley MR, Drumm J, Andrews JM. The pharmacokinetics and tissue penetration of imipenem. J Antimicrob Chemother 1986; 18 (Suppl E): 93–101. [DOI] [PubMed] [Google Scholar]
- 32. Mouton JW, Theuretzbacher U, Craig WA, Tulkens PM, Derendorf H, Cars O. Tissue concentrations: Do we ever learn? J Antimicrob Chemother 2008; 61: 235–7. [DOI] [PubMed] [Google Scholar]
- 33. Blizzard TA, Chen H, Kim S, Wu J, Bodner R, Gude C, Imbriglio J, Young K, Park YW, Ogawa A, Raghoobar S, Hairston N, Painter RE, Wisniewski D, Scapin G, Fitzgerald P, Sharma N, Lu J, Ha S, Hermes J, Hammond ML. Discovery of MK‐7655, a β‐lactamase inhibitor for combination with Primaxin®. Bioorg Med Chem Lett 2014; 24: 780–5. [DOI] [PubMed] [Google Scholar]
- 34. Rhee E, Jumes P, Rizk M, Gotfried M, Mangin E, Bi S, Chavez‐Eng C, Butterton J. Intrapulmonary pharmacokinetics of MK‐7655, a novel β‐lactamase inhibitor, dosed in combination with imipenem/cilastatin in healthy subjects. In: Interscience Conference on Antimicrobial Agents and Chemotherapy2, 2013. p. A – 1028. [DOI] [PMC free article] [PubMed]
- 35. Jumes P, Rizk M, Gutierrez M, Li X, Stoch A, Wagner J, Butterton J. A phase I study evaluating the single‐dose safety, tolerability, and pharmacokinetics of an intravenous beta‐lactamase inhibitor in healthy elderly male, elderly female and young female volunteers. In: Interscience Conference on Antimicrobial Agents and Chemotherapy, 2012. p. A – 009.
- 36. Rizk M, Jumes P, Lasseter K, Marbury T, Mangin E, Liu Y, Wagner J, Butterton J. Pharmacokinetics of MK‐7655, a novel beta‐lactamase inhibitor (BLI), in combination with imipenem/cilastatin (IPM/ CIL) in subjects with impaired renal function. In: Interscience Conference on Antimicrobial Agents and Chemotherapy, 2012. p. A – 010.
- 37. Butterton J A phase I study evaluating the safety, tolerability, and pharmacokinetics of an intravenous beta‐lactamase inhibitor in healthy male volunteers. ICAAC Annu Meet 2010; Abstract: F1–1967. [Google Scholar]
- 38. Rennard SI, Basset G, Lecossier D, O’Donnell KM, Pinkston P, Martin PG, Crystal RG. Estimation of volume of epithelial lining fluid recovered by lavage using urea as marker of dilution. J Appl Physiol 1986; 60: 532–8. [DOI] [PubMed] [Google Scholar]
- 39. Beal SL, Boeckman AJ, Sheiner LB. NONMEM user guides. San Francisco, CA: University of California at San Francisco; 1988. [Google Scholar]
- 40. Cockroft D, Gault M. Prediction of creatinine clearance from serum creatinine. Nephron 1976; 16: 31–41. [DOI] [PubMed] [Google Scholar]
- 41. Holford NHG. A size standard for pharmacokinetics. Clin Pharmacokinet 1996; 30: 329–32. [DOI] [PubMed] [Google Scholar]
- 42. Lodise TP, Gotfried M, Barriere S, Drusano GL. Telavancin penetration into human epithelial lining fluid determined by population pharmacokinetic modeling and Monte Carlo simulation. Antimicrob Agents Chemother 2008; 52: 2300–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kahan BD. The potential role of rapamycin in pediatric transplantation as observed from adult studies. Pediatr Transplant [Internet]. 1999/09/16 ed1999; 3: 175–80. Available from: 10487276. [DOI] [PubMed] [Google Scholar]
- 44. Clewe O, Karlsson MO, Simonsson USH. Evaluation of optimized bronchoalveolar lavage sampling designs for characterization of pulmonary drug distribution. J Pharmacokinet Pharmacodyn 2015; 42: 699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Boselli E, Breilh D, Djabarouti S, Guillaume C, Rimmelé T, Gordien J‐B, Xuereb F, Saux M‐C, Allaouchiche B. Reliability of mini‐bronchoalveolar lavage for the measurement of epithelial lining fluid concentrations of tobramycin in critically ill patients. Intensive Care Med 2007; 33: 1519–23. [DOI] [PubMed] [Google Scholar]
- 46. Kiem S, Schentag JJ. Interpretation of antibiotic concentration ratios measured in epithelial lining fluid. Antimicrob Agents Chemother 2008; 52: 24–36. [DOI] [PMC free article] [PubMed] [Google Scholar]


