Abstract
Aims
X‐linked adrenoleukodystrophy (X‐ALD) is a peroxisomal disorder, most commonly affecting boys, associated with increased very long chain fatty acids (C26:0) in all tissues, causing cerebral demyelination and adrenocortical insufficiency. Certain monounsaturated long chain fatty acids including oleic and erucic acids, known as Lorenzo's oil (LO), lower plasma C26:0 levels. The aims of this study were to characterize the effect of LO administration on plasma C26:0 concentrations and to determine whether there is an association between plasma concentrations of erucic acid or C26:0 and the likelihood of developing brain MRI abnormalities in asymptomatic boys.
Methods
Non‐linear mixed effects modelling was performed on 2384 samples collected during an open label single arm trial. The subjects (n = 104) were administered LO daily at ~2–3 mg kg−1 with a mean follow‐up of 4.88 ± 2.76 years. The effect of erucic acid exposure on plasma C26:0 concentrations was characterized by an inhibitory fractional Emax model. A Weibull model was used to characterize the time‐to‐developing MRI abnormality.
Results
The population estimate for the fractional maximum reduction of C26:0 plasma concentrations was 0.76 (bootstrap 95% CI 0.73, 0.793). Our time‐to‐event analyses showed that every mg l−1 increase in time‐weighted average of erucic acid and C26:0 plasma concentrations was, respectively, associated with a 3.7% reduction and a 753% increase in the hazard of developing MRI abnormality. However, the results were not significant (P = 0.5344, 0.1509, respectively).
Conclusions
LO administration significantly reduces the abnormally high plasma C26:0 concentrations in X‐ALD patients. Further studies to evaluate the effect of LO on the likelihood of developing brain MRI abnormality are warranted.
Keywords: erucic acid, inborn errors of metabolism, nonmem, population pharmacodynamics
What is Already Known about this Subject
Accumulation of C26:0 is believed to be the major cause of morbidity and mortality in X‐ALD patients.
Lorenzo's oil (LO) has been shown to reduce C26:0 plasma concentrations in X‐ALD patients.
The effect of LO on X‐ALD clinical outcome is controversial.
What this Study Adds
This is the first study to quantify the effect size of LO on C26:0 and the variability associated with it using a population pharmacodynamic modelling approach.
This study sets the foundation for optimizing LO dosage for future studies.
Introduction
X‐linked adrenoleukodystrophy (X‐ALD) is a genetic disorder due to a mutation in the ABCD1 gene, which codes for a peroxisomal membrane transporter. The defect in this protein results in accumulation of saturated very long chain fatty acids (SVLCFAs, mainly hexacosanoic acid or C26:0) in plasma and most tissues, particularly brain white matter and the adrenal cortex 1, 2. It is the most common peroxisomal disorder, with an incidence of 1 : 17 000 3.
There are four major phenotypes of X‐ALD, but none of these correlate with genotype. These are cerebral X‐ALD (CALD), adrenomyeloneuropathy (AMN), Addison only and an asymptomatic presentation. CALD, the most severe phenotype, is characterized by a progressive, irreversible inflammatory cerebral demyelination resulting in death within 2–3 years. Approximately 35–40% of children with X‐ALD mutations are at risk of developing childhood CALD (CCALD), with age at onset between 4 and 8 years 4. AMN is a slowly progressive, non‐inflammatory distal axonopathy that involves the spinal cord long tracts. Among children with X‐ALD, 10–15% develop the Addison only phenotype, which is characterized by primary adrenocortical insufficiency without demonstrable neurological deficit. The asymptomatic group has elevated plasma concentrations of SVLCFA without clinically evident neurological or endocrine abnormalities. Eventually all males with asymptomatic X‐ALD will develop either CALD or AMN at some point. For females, about 65% with the X‐ALD mutation will develop AMN and the rest remain asymptomatic throughout their lives 5.
Lorenzo's Oil (LO) is a 4 : 1 mixture of oleic and erucic acid in triglyceryl form 6. Erucic acid is considered the active component of LO and is believed to reduce the concentration of SVLCFAs via competitive inhibition of elongase, which is responsible for the elongation of long‐chain fatty acids, such as behenic acid (C22:0) and erucic acid (C22:1) into SVLCFAs and mono‐unsaturated VLCFAs, respectively 7. Several clinical investigations have reported that LO normalizes plasma SVLCFAs within 4–6 weeks 8. LO appears to have a relatively safe profile: 30–40% of patients develop a moderate, reversible reduction in platelet count without clinically significant bleeding, while 55% have a mild increase in transaminases without clinically significant hepatotoxicity 9, 10, 11. Despite the apparent benefits, clinical use of LO in X‐ALD have been controversial for more than 20 years. A few studies have indicated that LO can prevent CCALD only when treatment is administered prior to cerebral involvement, but without controlled clinical trials the results are inconclusive 11, 12.
Small sample sizes are common when evaluating drug treatment in rare diseases and the use of placebo‐controlled clinical trials presents ethical dilemmas. While both factors may contribute to conflicting results, it is also critical to note that LO dose–response relationships and the associated variability have not been studied. In this article we develop a population pharmacodynamic (PD) model to characterize the effect of erucic acid exposure on plasma C26:0 concentrations and determine the variability associated with this response. We also determine the effect of LO administration on the hazard of developing CCALD, characterized by developing brain magnetic resonance imaging (MRI) abnormalities, in asymptomatic X‐ALD children.
Methods
Subjects
Boys with X‐ALD were identified by screening at‐risk relatives of patients with X‐ALD or boys with Addison's disease at the John Hopkins Research Hospital from 2000 to 2014 under an expanded access trial (Clinical Trials.gov, NCT02233257). Diagnosis of X‐ALD was confirmed in these boys by a SVLCFAs assay. Only those with asymptomatic X‐ALD were included in our analysis. Subjects were excluded from the study if they had any demonstrable neurological or radiological abnormality at baseline (n = 1), had another disease associated with brain MRI abnormality such as a brain tumour or another peroxisomal disorder (n = 6), or if they were not adherent to LO dose (n = 19). The John Hopkins Research Hospital and the University of Minnesota Medical Center Institutional Review Boards approved the study. Subjects were followed until they developed any brain MRI abnormality. Written informed consent was obtained from the parents or guardians, with assent from the subjects as appropriate.
Treatment
All participants received a daily dose of approximately 2–3 mg kg−1 of LO. This dose was calculated to provide 20% of caloric intake as previously described 11. Supplements of essential fatty acids provided 5% of total caloric need. Fat intake from other sources was limited to 10 to 15% of total calories. When there was a clinically significant reduction of platelet counts, LO was halted and replaced by glyceryl trioleate at the same dosage. Once the platelet counts returned to normal value, LO was restarted at a lower dose and returned back to the target dose gradually.
Sampling and assays
The study design involved collection of blood samples at baseline (i.e. pretreatment) as well as every month for the first 6 months and then at 3–6 month intervals after LO administration. Children were asked to fast overnight and withhold their morning LO dose until blood collection in the morning. The samples were processed and analyzed to obtain a profile for 70 fatty acids (including C26:0 and erucic acid) according to a previously reported method 13.
Clinical outcome measures
Brain MRI studies were scheduled at 6–12 month intervals, and were used to define clinical outcome. Brain MRI results were assessed with the 34‐point loess score devised specifically for X‐ALD 14. The MRI results were considered abnormal if the loess score was 1 or higher.
Population PD model building
A population non‐linear mixed‐effect modelling approach was applied, using the software nonmem ® 7.3 (Icon Development Solutions, Ellicott City, Maryland, USA) 15. This approach involves estimation of two levels of effects, the fixed effect, which provides estimation of the population parameters and the random effects, which quantify the random variability. The first order conditional estimation (FOCE) method with interaction was used for parameter estimation. The choice of the structural model was based on the significant change of the objective function value (−2 log‐likelihood) for nested models. Specifically, two hierarchical models differing by one degree of freedom were considered significantly different if the objective function value decreased at least 3.84 units (P < 0.05). For non‐nested models, the model with lower Akaike information criterion (AIC) was considered superior. The goodness‐of‐fit plots, created by the Xpose 4.5.0 package in R 3.0.2 (The R Development Core Team, Vienna, Austria), were also considered during model selection 16, 17.
For the fixed effect, an inhibitory fractional Emax model was assumed according to the following:
where ER is the observed erucic acid plasma concentration, E0 is the predicted baseline concentration of C26:0 in the absence of erucic acid, Emax is the maximal fractional drop of C26:0 and EC 50 is the concentration of erucic acid at which 50% of the maximum effect of erucic acid on C26:0 occurs.
Two sources of variability composed the random effects, between‐subject variability (BSV) and residual errors. An exponential model was used to account for BSV, according to the following:
where Pi is the value of a parameter for an individual, is the population parameter value and ηi.is the individual deviation from .
ηi is defined as a normally distributed, zero‐mean random variable with a variance that is estimated as part of the model estimation. This assumption would allow Pi to be log‐normally distributed and therefore, preventing Pi to be less than zero. The correlations between different Pi were also evaluated.
For residual errors, the additive, the proportional and the combined additive and proportional error models were investigated.
Model evaluation
The final model was evaluated by prediction‐ and variability‐corrected visual predictive check (pvcVPC) 18 and bootstrap using Perl‐speaks‐nonmem (PsN 4.4.0, Uppsala University, Uppsala, Sweden) 27. For pvcVPC, a total of 1000 replicates of the original dataset were simulated using the final model. Both observations and the simulated data were normalized to the median population prediction as well as to the typical variability in each bin in order to account for the differences within a bin coming from independent variables (erucic acid concentrations and doses) and between subject variability. The 95% prediction intervals of the 5th, 50th and 95th percentiles of the prediction‐ and variability‐corrected (pvc) simulated data were calculated for relevant erucic acid concentration bins, and compared with the 5th, 50th and 95th percentiles of the pvc observed data within the same bin. Binning was done by count to provide eight bins with equal number of observations in each bin. X‐pose 4.5.0 was used for data visualization under the environment of the R 3.0.2 16, 17.
To evaluate the precision of final estimated parameters, a non‐parametric bootstrap approach was used. This approach involves random sampling with replacement from the original dataset to generate new datasets (n = 1000). The final model was fitted to each of these datasets and estimates of parameters were obtained. These estimates were used to generate 95% non‐parametric confidence intervals (CI) and median values for all parameter estimates.
Time‐to‐event analyses
We performed time‐to‐event analyses to analyze the relationship between LO administration and the time from study entry to the development of brain MRI abnormality. To account for interval censoring between the last visit and the actual time for development of brain MRI abnormality, we used a parametric survival model where the brain MRI abnormality onset was interval‐censored for those subjects who developed brain MRI abnormalities and right‐censored at the last disease‐free visit for subjects who did not develop MRI abnormalities. Of the several parametric models available (such as exponential, Weibull, normal, logistic, log‐logistic 19, 20), Weibull distribution was chosen as the most consistent distribution with our data. Model choice was based on the diagnostic probability plot provided as SAS® (SAS Institute, Cary, NC, USA) output from PROC LIFEREG and AIC (the lower the better).
We used the time‐weighted average since study entry (area under the curve of erucic acid adjusted for the duration of follow‐up as described in 11, [LAUCER]) as a predictor for clinical outcome. The LAUCER was calculated for each subject utilizing the AUC function in the MESS package in R 3.0.2 21. The AUC function utilizes a composite trapezoidal rule for calculation of area under concentration–time curve (AUC) from study entry until the last observation for each subject. To account for the differences in follow‐up period for each subject, the calculated AUC was normalized to the follow‐up time for each subject. Any observed erucic acid concentration greater than 30 mg l−1 was censored as it was considered to be not reflective of the steady‐state brain concentrations, but instead peak plasma concentrations of erucic acid that were collected immediately after LO administration. This reflects personal experience (by GVR) with individuals who received erucic acid in the inpatient setting where wide fluctuations in erucic acid concentrations have not been observed.
To assess the association between C26:0 in plasma and the development of brain MRI abnormalities, LAUCC26:0 (calculated in the same way as LAUCER but using C26:0 plasma concentrations) was used as a predictor. All analyses were done in SAS Studio® 3.1.
Power analyses
To assess the effect of the number of MRI abnormality events on the power of the study, we performed a post hoc power analysis. This was done through re‐simulating the original dataset 1000 times within R 3.0.2. The time‐to‐event (t i) was assumed to follow a Weibull distribution, while the censoring time (ci) was assumed to follow a truncated normal distribution with minimum time of zero. In symbols, our generation algorithm is:
where r is the Weibull shape parameter, μi is the Weibull scale, α is the intercept, β is the slope associated with the LAUC effect, LAUCi is the LAUC for either erucic acid (LAUCER) or C26:0 (LAUCC26:0) from the original dataset and μc and σc are the mean and the standard error of the truncated normal (TN) distribution, respectively.
For the time‐to‐event distribution parameters, values were based on the fitted Weibull model in SAS®, whereas for the censoring time distribution, μc and σc were chosen based on sensitivity analyses to provide values for the observed times and numbers of events similar to those in the original dataset. After generating independent time‐to‐event values (t i) and censoring times (ci), when the generated ci was less than t i, the event in the simulated dataset was assumed to be right‐censored and thus deleted.
To estimate our procedure's type I error, the true β value was fixed at zero, and the Weibull model was fitted within R 3.0.2 using the ‘survival’ package to each of the resulting 1000 simulated null datasets 22. The type I error and a corresponding 95% CI were estimated by Monte Carlo methods as:
Power was estimated in a similar manner from 1000 simulated ‘alternative’ datasets (where the true β was set equal to the values obtained from survival fitting using LAUCi from the original data in SAS®.
In addition to the abovementioned simulated scenario (scenario I), we simulated another scenario (scenario II) to estimate the power associated with 21 events for the same sample size. This was done by changing the values of μc and σc.
Results
Subject characteristics
A total of 104 boys with asymptomatic X‐ALD were included in the study. From this cohort 2384 paired C26:0 and erucic acid measurements were available to develop the PD model. The baseline demographics of the subjects are presented in Table 1. The median baseline age at study entry was 2.79 years (range 0.068–8.92). Pretreatment plasma concentrations of erucic acid and C26:0 were available for 97 individuals. We were not able to calculate the LAUC of both erucic and C26:0 plasma concentrations for one subject because only baseline concentrations were available. This subject was kept in the dataset to provide information on the baseline estimation of C26:0 (E0) and was excluded from the time‐to‐event analyses.
Table 1.
Subject's demographics and characteristics
| Demographic/Characteristic | Median (range) |
|---|---|
| Baseline weight (kg) | 14.90 (9.40–40.60)* |
| Baseline age (years) | 2.79 (0.068–8.92) |
| Follow‐up period (years) | 4.64 (0.00–10.26) |
| Pretreatment erucic acid plasma concentration (mg l −1 ) | 0.5 (0.22–1.94)* |
| Pretreatment C26:0 plasma concentration (mg l −1 ) | 1.06 (0.46–1.68)* |
| Post‐treatment erucic acid plasma concentration (mg l −1 ) | 18.63 (0.21–336.1) |
| Post‐treatment C26:0 plasma concentration (mg l −1 ) | 0.402 (0.194–1.21) |
| LAUC ER (mg l −1 ) | 13.80 (0.39–24.05)†,‡ |
| LAUC C26:0 (mg l −1 ) | 0.45 (0.23–1.66)† |
Based on 97 individuals only.
Based on 103 subjects.
Values of erucic acid plasma concentrations >30 mg l−1 were censored.
The follow‐up period differed from subject to subject, with a median of 4.64 years and a range from 0 to 10.26 years after diagnosis. Of the 103 subjects included in our time‐to‐event analyses, 10 developed a brain MRI abnormality event during the observation period. The median time until the first abnormality in these raw data, not taking censoring into account, was 1.84 years (range 0.51–6.83 years). Data from these subjects were analyzed as interval‐censored data. For the remaining 93 subjects who did not experience any brain abnormality, data were analyzed as right‐censored events. The median time for censoring was 4.94 years (range 0 to 10.26 years).
Population PD analyses
The fixed and random effects of the inhibitory fractional Emax model are shown in Table 2. We estimated a strong negative correlation between E0 and EC 50 (−0.877, Table 2). On the other hand, correlations between Emax and other fixed effect parameters were very small and, therefore, were fixed to zero in the final model. A proportional error model best described the residual errors for these data. The combined error model did not provide any significant improvement of fit (ΔOFV < 3.84, P value > 0.05), whereas the additive error model provided a worse fit as indicated by the AIC when compared with the proportional error model (Δ AIC ~ 671).
Table 2.
Pharmacodynamic parameter estimates
| Parameter | Estimate (RSE%) | Shrinkage % | Bootstrap median value | 95% bootstrap CI |
|---|---|---|---|---|
| Fixed effect | ||||
| E0 (mg l−1) | 1.44 (7) | — | 1.44 | 1.29, 1.66 |
| Emax | 0.76 (2) | — | 0.761 | 0.73, 0.793 |
| EC 50 (mg l−1) | 0.734 (20) | — | 0.733 | 0.488, 1.04 |
| Random effect | ||||
| BSVE0, CV%* | 31.5 (14) | 22 | 31.7 | 22.6, 40.4 |
| BSVEmax, CV%* | 6.2 (24) | 33 | 6.05 | 1.25, 8.89 |
| BSVEC50, CV%* | 171.3 (9) | 21 | 176.4 | 124.6, 261.3 |
| Correlation (E0 and EC 50) | −0.877 (6) | — | −0.891 | −0.981, −0.749 |
| Residual error, CV%† | 27.6 (6) | 3 | 27.4 | 24.7, 31 |
BSV, between subject variability; CI, confidence interval; CV, coefficient of variation; RSE, Relative standard error.
BSV, CV% was calculated as .
Residual error, CV% was calculated as .
All model‐fitted parameters were estimated with good precision and all relative standard errors were less than 25%. Except for Emax, shrinkages of both η and ε random effects were below 25% (Table 2, calculated according to 23). Model goodness of fit plots showed no reason to reject the model (Supplementary material, Figure S1). The pvcVPC on a log scale is shown in Figure 1. The pvc C26:0 observed data percentiles for most erucic acid concentration bins were within the prediction intervals of pvc simulations. Table 2 also shows the median and the 95% CIs obtained from the bootstrap datasets. All estimates obtained from the pharmacodynamic model lie within the 95% bootstrap CIs and are close to the median values obtained from the bootstrap datasets, providing support to our model findings.
Figure 1.
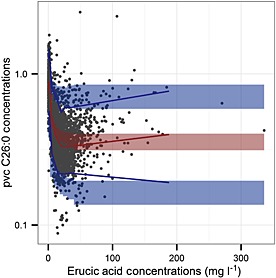
Prediction‐ and variability‐corrected visual predictive check (pvcVPC). Prediction‐ and variability‐corrected (pvc) C26:0 concentrations are displayed on a log scale. Circles represent pvc C26:0 observations, the red line represents the 50th percentiles of the pvc C26:0 observations, blue lines represent the 5th, and the 95th percentiles of the pvc C26:0 observations and shaded areas represent 95% prediction intervals of the 5th, 50th, and 95th percentiles of pvc simulated data
Time‐to‐event analyses
We performed a time‐to‐event analysis to characterize the hazard of developing brain MRI abnormalities and to assess the effect of LO administration on this hazard. Of all examined parametric models, the Weibull model was most consistent with the distribution of the time to development of brain MRI abnormalities data (Supplementary material, Figure S2). The shape parameter associated with the Weibull distribution was estimated to be less than 1, indicating that the baseline hazard was actually decreasing over time.
The hazard of developing brain MRI abnormalities was estimated to decrease by 3.7% for every unit increase in LAUCER. The hazard ratio was 0.963. Therefore, the observed median LAUCER (Table 1) is associated with a 40% reduction in this hazard. However, this result was not statistically significant (95% CI 0.85, 1.08, P = 0.5344).
For LAUCC26:0, the hazard was estimated to increase by 753% for every 1 mg l−1 increase in LAUCC26:0. The hazard ratio was 8.53. Therefore, an estimated fractional maximal effect (Emax) of 0.76 reduction of C26:0 plasma concentration is associated with 80% reduction in this hazard. This result was also not conclusive (95% CI 0.46, 157.25, P = 0.1509).
Values used in our post hoc power analyses were based on the results of fitting the Weibull model to the observed time‐to‐brain MRI abnormality data using observed LAUCER and LAUCC26:0 as predictors and are shown in Tables 3, 4, respectively. For scenario I, the estimated powers at a 0.05 significance level to detect a hazard ratios of 0.963 and 8.53 for every unit increase in LAUCER or LAUCC26:0 were 10% (95% CI 8%, 12%) and 43% (95% CI 40%, 46%), respectively. For scenario II, with the number of observed events doubled, the calculated powers at a 0.05 significance level to detect the same hazard ratios for every unit increase in LAUCER and LAUCC26:0 were 16% (95% CI 14%, 18%) and 65% (95% CI 62%, 68%), respectively. Results of our power analyses are shown in Table 5.
Table 3.
Parameter values used for power analysis (LAUCER)
| Parameter | Value | |
|---|---|---|
| Weibull shape (r) | 0.744 | |
| Weiubll intercept (α) | 6.49 | |
| Weibull coefficient (β) with LAUC ER | ||
| Null hypothesis | 0 | |
| Alternative hypothesis | 0.05 | |
| μc | ||
| Scenario I | 60 | |
| Scenario II | 150 | |
| σc | ||
| Scenario I | 30 | |
| Scenario II | 20 | |
μc, mean of truncated normal distribution for censoring time; σc , standard deviation of truncated normal distribution for censoring time.
Table 4.
Parameter values used for power analysis (LAUCC26:0)
| Parameter | Value | |
|---|---|---|
| Weibull shape (r) | 0.828 | |
| Weibull intercept (α) | 8.19 | |
| Weibull coefficient (β) with LAUC C26:0 | ||
| Null hypothesis | 0 | |
| Alternative hypothesis | −2.59 | |
| μ c | ||
| Scenario I | 60 | |
| Scenario II | 150 | |
| σc | ||
| Scenario I | 30 | |
| Scenario II | 20 | |
μc, mean of truncated normal distribution for censoring time; σc , standard deviation of truncated normal distribution for censoring time.
Table 5.
Power analyses results
| Coefficient parameter value (β) | Sample size | Target number of events | Simulated mean number of events | Target type I error | Actual type I error (95% CI) | Calculated power (95% CI) |
|---|---|---|---|---|---|---|
| LAUC ER | ||||||
| Scenario I | 103 | 10 | 10 | 0.05 | 0.056 (0.042, 0.07) | 0.098 (0.08, 0.116) |
| Scenario II | 103 | 21 | 19 | 0.05 | 0.05 (0.036, 0.064) | 0.158 (0.135, 0.181) |
| LAUC C26:0 | ||||||
| Scenario I | 103 | 10 | 10 | 0.05 | 0.041 (0.029, 0.053) | 0.426 (0.395, 0.457) |
| Scenario II | 103 | 21 | 21 | 0.05 | 0.034 (0.023, 0.045) | 0.645 (0.615, 0.675) |
CI, confidence interval.
Discussion
X‐ALD is a genetic disorder which is characterized by elevated SVLCFAs concentrations and a wide phenotypic spectrum. CCALD is the most severe, progressive neurodegenerative phenotype of X‐ALD resulting in death within 2–3 years. The current standard of care treatment for children with CCALD is haematopoietic stem cell transplant (HSCT). However, this treatment is only effective in the early stages of CCALD and is restricted to children with a matched donor 24. HSCT is expensive and has a high morbidity. LO administration has been shown to lower plasma SVLCFAs in several clinical trials, but its efficacy in X‐ALD is not established 6, 8, 25, 26.
Based on a review of the literature, this is the first population pharmacodynamic study characterizing the effect of chronic LO administration on plasma SVLCFAs (C26:0) in asymptomatic X‐ALD children. When the plasma erucic acid concentration is zero, the baseline C26:0 plasma concentration (E0) was estimated to be 1.44 mg l−1. In a normal population, the mean plasma C26:0 level is 0.29 ± 0.29 mg l−1, whereas in X‐ALD males, it is four‐fold greater (1.18 ± 0.53 mg l−1) 13. It should be noted that E0 estimated from our model represents an extrapolation of the C26:0 plasma concentration that would be seen at zero plasma concentration of erucic acid. However, there is an endogenous production and a limited dietary intake of erucic acid and we observed a median pretreatment erucic acid plasma concentration of 0.5 mg l−1 (Table 1). This pretreatment erucic acid plasma concentration predicts a pretreatment C26:0 plasma concentration of 0.997 mg l−1, estimated under our model parameters. This is consistent with the reported value for this population. Our model estimated a statistically significant normalization of plasma C26:0 with intake of LO. Study results indicate that, on average, erucic acid was associated with a maximum reduction of C26:0 plasma concentrations from 1.44 mg l−1 to 0.346 mg l−1. The fractional Emax was estimated as 0.76. This is consistent with a previous report on a similar cohort 11, where they observed that most patients who were administered LO reduced their pretreatment C26:0 concentrations by 0.4–0.6 mg l−1. Based on our PD parameter estimates, the observed median LAUCER of 13.08 mg l−1 (Table 1) is associated with reducing the C26:0 plasma concentration to 0.404 mg l−1 on average. We also found that the BSV associated with EC 50 is very high (CV% is estimated to be 171.3%) which indicates large differences in drug potency among individuals. The differences in drug potency among children could be ascribed to different elongase enzyme levels or a different affinity of the enzyme to erucic acid as a result of genetic variations.
An advantage of using an inhibitory fractional Emax model is the ease in interpreting the effect of LO on C26:0. For example, an estimated Emax of 0.76 would mean that on average, the maximum effect of LO in lowering C26:0 from its baseline concentration is 76%. In addition, this model takes into account the correlation between the absolute maximum effect that can be reached and the individual baseline. For example, individuals with large C26:0 plasma concentrations at baseline tend to have a lesser reduction in their C26:0 concentrations attributable to LO therapy. This is consistent with the disease pathophysiology, where higher C26:0 at baseline might indicate higher levels of elongase or a higher affinity of this enzyme to C22 fatty acids (whether it is C22:0 or erucic acid). This speculation was supported by the finding of a strong negative correlation between the baseline C26:0 concentration and EC 50.
Unlike published reports that assumed an exact time of the observed brain MRI abnormality in asymptomatic X‐ALD boys on LO, we adopted a parametric survival model approach that takes into account the interval censored nature of these data and can characterize the baseline hazard. In this study we found that a Weibull distribution best described the time to development of brain MRI abnormalities. The shape parameter associated with the Weibull distribution indicates that the hazard of development of brain MRI abnormalities decreases with time in subjects on LO therapy. This is consistent with a previous study in a similar group receiving LO, where LO was observed to reduce the risk of developing the childhood cerebral form by a factor of 2 11. Moreover the risk for development of CCALD decreases after 8 years of age 4. The unavailability of a placebo group in this study precluded us from describing the hazard of developing CCALD over time in the absence of LO therapy. It should be noted, however, that the confidence interval includes 1 for the scale parameter, which reduces the Weibull distribution to an exponential distribution for which the baseline hazard of developing brain MRI abnormalities is constant over time. Assessment of probability plots as well as AIC values made us reject the exponential distribution as inadequate.
The mechanism of LO's preventive effect on CCALD is not well understood. In previous studies of postmortem tissue of LO‐treated patients, the effect of C26:0 concentrations in brain pathology has been variable. Further, it is unclear if LO crosses the human blood–brain barrier 26, 27, although 14C‐labelled erucic acid does enter rodent brain tissue 28. We estimated the effect of erucic acid plasma exposure on the hazard of developing brain abnormalities in CCALD. Although the results indicated some beneficial signal for those who received LO, our results were not statistically significant. Our analyses showed that the power associated with this effect was very small (10%).
The pathogenesis of CALD is complex and not fully understood. It has been suggested that accumulation of SVLCFAs in the brain is the cause of development of inflammatory demyelination 29, 30. We assessed the association between C26:0 concentrations in plasma and the development of brain MRI abnormalities. We observed a trend of increasing the hazard of developing MRI abnormality as the C26:0 plasma concentrations increased. However, the result was not statistically significant. Again, this may be due to the small number of individuals who developed brain MRI abnormalities (only 10 individuals, vs. 21 individuals in Moser et al.’s study 11), limiting the power of this study. This was confirmed by our analyses, indicating that the power associated with our study was small (43%). When subjects were followed for a longer time (as in the case for Moser et al. 11, mean [range] follow‐up time was 6.9 years [0.6–15]), the calculated power increased (65%). This power calculation was based on our calculated effect, which might be an underestimate. A well‐powered study testing several different hypotheses was beyond the scope of this manuscript.
No pharmacological basis was considered for choosing LO doses in this study or in any previously reported study. This is mainly due to the absence of any information regarding LO pharmacokinetics (PK) in X‐ALD subjects. We were unable to determine erucic acid PK in the subjects in the present study because only one determination of erucic acid concentration was made per subject per visit and the absence of dosing and sampling times. Future studies will characterize the PK of LO in children and adults with X‐ALD.
In conclusion, we have provided evidence that LO administration significantly reduces plasma SVLCFAs. We have developed a population pharmacodynamic model that characterizes the effect of LO on C26:0. Some experts/clinicians recommend offering LO therapy to neurologically asymptomatic X‐ALD male subjects who are at risk of developing CALD as a preventive therapy 11, 12. Although our analyses only indicated a trend of LO's beneficial effect on CALD characterized by development of brain MRI abnormalities, our results are not statistically conclusive. However, our analyses indicated we have a limited power. Nonetheless, our results from the population pharmacodynamic model can be used to provide the effect size of LO on C26:0 plasma concentrations and the response variability in the design of future clinical trials.
Competing Interests
All authors have completed the Unified Competing Interest form at www.icmje.org/coi disclosure.pdf (available on request from the corresponding author) and declare no support from any organization for the submitted work.
The authors would like to thank the subjects and families for their cooperation, our research nurses at The John Hopkins Research Hospital and University of Minnesota Medical Center, and the staff at the Kennedy Krieger Institute.
Contributors
Designed the study/contributed to the concept: MAA, RVK, RB, JC, ROJ, ABM, AF and GVR. Collected and compiled the data: MAA, ROJ, ABM, AF and GVR. Analyzed and interpreted the data: MAA, RVK, CB, BC and RB. Drafted and revised the paper: MAA, RVK, BC, RB, JC and GVR. All authors have seen and approved the manuscript.
PI statement
JVR was the PI of this study.
Supporting information
Figure S1 Goodness of fit plots. Upper panel: the observed C26:0 concentrations (mg l−1) vs. the population predicted C26:0 concentrations (mg l−1), the observed C26:0 concentrations (mg l−1) vs. the individual predicted C26:0 concentrations (mg l−1); solid line represents line of identity, dashed red line represents loess. Lower panel: conditional weighted residuals vs. population predicted C26:0 concentrations (mg l−1) and vs. erucic acid concentrations (mg l−1); solid line represents line of y = 0, dashed red line represents loess. CWRES conditional weighted residuals
Figure S2 Probability plot for the Weibull model applied to the time‐to‐MRI abnormality data. The circles are the non‐parametric survival function estimates. The shaded bands are the 95% confidence bands predicted by the Weibull model. The solid red line is the line of linear regression of the non‐parametric survival function estimates
Supporting info item
Supporting info item
Ahmed, M. A. , Kartha, R. V. , Brundage, R. C. , Cloyd, J. , Basu, C. , Carlin, B. P. , Jones, R. O. , Moser, A. B. , Fatemi, A. , and Raymond, G. V. (2016) A model‐based approach to assess the exposure–response relationship of Lorenzo's oil in adrenoleukodystrophy. Br J Clin Pharmacol, 81: 1058–1066. doi: 10.1111/bcp.12897.
References
- 1. Kemp S, Valianpour F, Denis S, Ofman R, Sanders RJ, Mooyer P, Barth PG, Wanders RJ. Elongation of very long‐chain fatty acids is enhanced in X‐linked adrenoleukodystrophy. Mol Genet Metab 2005; 84: 144–51. [DOI] [PubMed] [Google Scholar]
- 2. Aubourg P. X‐linked adrenoleukodystrophy. Ann Endocrinol (Paris) 2007; 68: 403–11. [DOI] [PubMed] [Google Scholar]
- 3. Kaiser E, Kramar R. Clinical biochemistry of peroxisomal disorders. Clin Chim Acta 1988; 173: 57–80. [DOI] [PubMed] [Google Scholar]
- 4. Schaumburg HH, Powers JM, Raine CS, Suzuki K, Richardson EP. Adrenoleukodystrophy: a clinical and pathological study of 17 cases. Arch Neurol 1975; 32: 577–91. [DOI] [PubMed] [Google Scholar]
- 5. Kemp S, Berger J, Aubourg P. X‐linked adrenoleukodystrophy: clinical, metabolic, genetic and pathophysiological aspects. Biochim Biophys Acta 1822; 2012: 1465–74. [DOI] [PubMed] [Google Scholar]
- 6. Odone A, Odone M. Lorenzo's oil: a new treatment for adrenoleukodystrophy. J Pediatr Neurosci 1989; 5: 55–61. [Google Scholar]
- 7. Bourre J, Daudu O, Baumann N. Nervonic acid biosynthesis by erucyl‐CoA elongation in normal and quaking mouse brain microsomes. Elongation of other unsaturated fatty acyl‐CoAs (mono and polyunsaturated). Biochim Biophys Acta 1976; 424: 1–7. [DOI] [PubMed] [Google Scholar]
- 8. Rizzo WB, Leshner RT, Odone A, Dammann AL, Craft DA, Jensen ME, Jennings SS, Davis S, Jaitly R, Sgro JA. Dietary erucic acid therapy for X‐linked adrenoleukodystrophy. Neurology 1989; 39: 1415–22. [DOI] [PubMed] [Google Scholar]
- 9. Grice H, Heggtveit H. Relevance to humans of myocardial lesions induced in rats by marine and rapeseed oils In: High and low erucic acid rapeseed oils: production, usage, chemistry, and toxicological evaluation, eds Kramer JKG, Sauer FD, Pigden WJ, Toronto: Academic Press, 1983. [Google Scholar]
- 10. Kickler TS, Zinkham WH, Moser A, Shankroff J, Borel J, Moser H. Effect of erucic acid on platelets in patients with adrenoleukodystrophy. Biochem Mol Med 1996; 57: 125–33. [DOI] [PubMed] [Google Scholar]
- 11. Moser HW, Raymond GV, Lu SE, Muenz LR, Moser AB, Xu J, Jones RO, Loes DJ, Melhem ER, Dubey P, Bezman L, Brereton NH, Odone A. Follow‐up of 89 asymptomatic patients with adrenoleukodystrophy treated with Lorenzo's oil. Arch Neurol 2005; 62: 1073–80. [DOI] [PubMed] [Google Scholar]
- 12. Moser HW, Raymond GV, Koehler W, Sokolowski P, Hanefeld F, Korenke GC, Green A, Loes DJ, Hunneman DH, Jones RO. Evaluation of the preventive effect of glyceryl trioleate‐trierucate (“Lorenzo's oil”) therapy in X‐linked adrenoleukodystrophy: results of two concurrent trials In: Peroxisomal Disorders and Regulation of Genes. New York: Springer: Springer Science+ Business Media, 2003; 369–87. [DOI] [PubMed] [Google Scholar]
- 13. Moser AB, Kreiter N, Bezman L, Lu S, Raymond GV, Naidu S, Moser HW. Plasma very long chain fatty acids in 3,000 peroxisome disease patients and 29,000 controls. Ann Neurol 1999; 45: 100–10. [DOI] [PubMed] [Google Scholar]
- 14. Loes DJ, Hite S, Moser H, Stillman AE, Shapiro E, Lockman L, Latchaw RE, Krivit W. Adrenoleukodystrophy: a scoring method for brain MR observations. AJNR Am J Neuroradiol 1994; 15: 1761–6. [PMC free article] [PubMed] [Google Scholar]
- 15. Beal S, Sheiner L, Boeckmann A, Bauer R. NONMEM User's Guides (1989–2009). Ellicott City MD: Icon Development Solutions, 2009. [Google Scholar]
- 16. Jonsson EN, Karlsson MO. Xpose—an S‐PLUS based population pharmacokinetic/pharmacodynamic model building aid for NONMEM. Comput Methods Programs Biomed 1998; 58: 51–64. [DOI] [PubMed] [Google Scholar]
- 17. Pinheiro J, Bates D, DebRoy S, Sarkar D, R Development Core Team . 2010. nlme: linear and nonlinear mixed effects models. R package version 3.1–97. R Foundation for Statistical Computing, Vienna, 2011.
- 18. Bergstrand M, Hooker AC, Wallin JE, Karlsson MO. Prediction‐corrected visual predictive checks for diagnosing nonlinear mixed‐effects models. AAPS J 2011; 13: 143–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Allison PD. Survival analysis using SAS: a practical guide. Cary, North Carolina: SAS Institute, 2010. [Google Scholar]
- 20. Akaike H Likelihood of a model and information criteria. J Econ 1981; 16: 3–14. [Google Scholar]
- 21. Ekstrom C.. MESS: Miscellaneous Esoteric Statistical Scripts. R package version 0.3–2, 2014.
- 22. Therneau T. A package for survival analysis in S. R package version 2.37–4 [Online]. Available at http://CRAN.R‐project.org/package=survival. Box 2013; 980032: 23298–0032 (last accessed 9 June 2013).
- 23. Savic RM, Karlsson MO. Importance of shrinkage in empirical bayes estimates for diagnostics: problems and solutions. AAPS J 2009; 11: 558–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Berger J, Pujol A, Aubourg P, Forss‐Petter S. Current and future pharmacological treatment strategies in X‐linked adrenoleukodystrophy. Brain Pathol 2010; 20: 845–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Aubourg P, Adamsbaum C, Lavallard‐Rousseau M, Rocchiccioli F, Cartier N, Jambaque I, Jakobezak C, Lemaitre A, Boureau F, Wolf C. A two‐year trial of oleic and erucic acids (“Lorenzo's oil”) as treatment for adrenomyeloneuropathy. N Engl J Med 1993; 329: 745–52. [DOI] [PubMed] [Google Scholar]
- 26. Rasmussen M, Moser AB, Borel J, Khangoora S, Moser HW. Brain, liver, and adipose tissue erucic and very long chain fatty acid levels in adrenoleukodystrophy patients treated with glyceryl trierucate and trioleate oils (Lorenzo's oil). Neurochem Res 1994; 19: 1073–82. [DOI] [PubMed] [Google Scholar]
- 27. Poulos A, Gibson R, Sharp P, Beckman K, Rattan‐Smith PG. Very long chain fatty acids in X‐linked adrenoleukodystrophy brain after treatment with Lorenzo's oil. Ann Neurol 1994; 36: 741–6. [DOI] [PubMed] [Google Scholar]
- 28. Golovko MY, Murphy EJ. Uptake and metabolism of plasma‐derived erucic acid by rat brain. J Lipid Res 2006; 47: 1289–97. [DOI] [PubMed] [Google Scholar]
- 29. Paintlia AS, Gilg AG, Khan M, Singh AK, Barbosa E, Singh I. Correlation of very long chain fatty acid accumulation and inflammatory disease progression in childhood X‐ALD: implications for potential therapies. Neurobiol Dis 2003; 14: 425–39. [DOI] [PubMed] [Google Scholar]
- 30. Asheuer M, Bieche I, Laurendeau I, Moser A, Hainque B, Vidaud M, Aubourg P. Decreased expression of ABCD4 and BG1 genes early in the pathogenesis of X‐linked adrenoleukodystrophy. Hum Mol Genet 2005; 14: 1293–303. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Goodness of fit plots. Upper panel: the observed C26:0 concentrations (mg l−1) vs. the population predicted C26:0 concentrations (mg l−1), the observed C26:0 concentrations (mg l−1) vs. the individual predicted C26:0 concentrations (mg l−1); solid line represents line of identity, dashed red line represents loess. Lower panel: conditional weighted residuals vs. population predicted C26:0 concentrations (mg l−1) and vs. erucic acid concentrations (mg l−1); solid line represents line of y = 0, dashed red line represents loess. CWRES conditional weighted residuals
Figure S2 Probability plot for the Weibull model applied to the time‐to‐MRI abnormality data. The circles are the non‐parametric survival function estimates. The shaded bands are the 95% confidence bands predicted by the Weibull model. The solid red line is the line of linear regression of the non‐parametric survival function estimates
Supporting info item
Supporting info item


