Abstract
Background
Resveratrol is a Sirt-1-specific activator, which also exerts cardioprotective effects that regulate redox signalling during oxidative stress and autophagy during cardiovascular disease (CVD).
Objective
This study investigated the protective effects of resveratrol against hydrogen peroxide-induced damage in cardiomyocytes.
Design
In this article, hydrogen peroxide-induced autophagy and apoptosis in H9c2 cardiomyoblasts were studied at an increasing concentration from 0 to 100 µM.
Results
Resveratrol pretreatment with concentrations of 10, 20, and 50 µM inhibits autophagic apoptosis by increasing p-Akt and Bcl-2 protein levels in H9c2 cells. Interestingly, resveratrol treatment activates the Beclin-1, LC3, p62, and the lysosome-associated protein LAMP2a within 24 h of administration.
Conclusions
These results suggest that resveratrol-regulated autophagy may play a role in degrading damaged organelles in H9c2 cells rather than causing apoptosis, and this may be a possible mechanism by which resveratrol protects the heart during CVD.
Keywords: resveratrol, oxidative stress, apoptosis, autophagy
Oxidative stress caused by reactive oxygen species (ROS) is usually more than endogenous antioxidant defence mechanisms, which protect DNA, proteins, and carbohydrates against oxidation. Atherosclerosis, hypertension, diabetes heart failure, and the pathogenesis of cardiovascular disease (CVD) are related to oxidative stress (1). ROS, including hydrogen peroxide (H2O2) and peroxynitrite, are likely involved in the pathogenesis of myocardial ischemia-reperfusion injury (2). In 1966, Christian de Duve discovered that autophagy could eliminate damaged organelles, such as endoplasmic reticulum (ER) and mitochondria, through lysosome-dependent degradation (3). Simultaneously, autophagy provides energy that enhances survival under nutrient starvation by utilizing a dynamic recycling system (4).
Autophagy is triggered by inflammation, hypoxia, oxidized lipoprotein, ER stress, and ROS (5–7). When mammalian target of rapamycin (m-TOR) activity is inhibited by rapamycin, the autophagy activator ULK1 forms complexes (8). Next, LC3B-I and the phosphatidylethanolamine (PE) membrane combine to form LC3B-II (9, 10). Exogenous ROS can also induce autophagy, and ROS-generating drugs can promote autophagy. However, the lysosomal pathway of self-degradation has essential pro-survival functions (11). Autophagy in cardiomyocytes results in metabolic profit and loss (12). Furthermore, the regulation of autophagy occurs via metabolic and stress signalling pathways in the heart; therefore, autophagy has important functions in the myocardium, and its dysregulation is implicated in a wide variety of cardiovascular pathologies (13, 14).
Resveratrol, which is considered to be a cardioprotective compound, is a polyphenolic compound found in natural products (15). Recently, it was shown that resveratrol can prevent or treat cancer, heart disease, ischemic injuries, diabetes, pathological inflammation, and oxidative stress injuries (16–19). In this study, the cardiac myoblast H9c2 cell line was used to investigate the cardioprotective effects of resveratrol heart during H2O2-induced oxidative stress. The results show that the H2O2 treatment reduces Sirt-1 expression directly in H9c2 cells and causes H9c2 cells apoptosis. Resveratrol treatments attenuated H2O2-induced damage, enhanced Sirt-1 expression, and reduced autophagic flux in H9c2 cells.
Methods and materials
Cell culture
H9c2 cells were purchased from the Bioresource Collection and Research Center (BCRC, Taipei, Taiwan) and were cultured in Dulbecco's modified essential medium supplemented with 10% fortified bovine calf serum and incubated at 85% humidity in a 5% CO2 at 37°C. The H9c2 cells were starved in serum-free medium for 12 h and then pretreated with or without resveratrol for 2 h prior to the H2O2 treatments. The dorsomorphin (compound C) and the other inhibitors were used to pretreat H9c2 cells for 1 h before H2O2 stimulation.
Mitochondrial emergence potential stain (JC-1)
H9c2 cells were pre-seeded at 2×104 cells/well in 24-well plates for each test. After the indicated treatments, the media in the plates were removed, and the cells were washed with PBS. Next, JC-1 stain solution was added to each well at 37°C for 20 min, after which the JC-1 stain solution was removed, and the cells were washed with wash buffer. Fluorescence was visualized using a fluorescence microscope coupled with an image analysis system.
Western blotting analysis
The protein concentration of each sample was determined using the Lowry protein assay. Protein samples for the western blotting assay were separated by 12% SDS polyacrylamide gel electrophoresis (SDS-PAGE) with a constant voltage of 75 V for 120 min. The proteins were then transferred to Hybond-C membranes (GE healthcare UK Ltd., Little Chalfont, Buckinghamshire, UK) at 50 V for 3 h. Hybond-C membranes with protein were incubated in 3% bovine serum albumin (BSA) in tricine buffer solution (TBS). Next, primary antibodies, including β-Actin (sc-47778, Santa Cruz Biotechnology, Dallas, TX, USA), Sirt-1 (sc-74465, Santa Cruz Biotechnology), p-Akt (sc-7985, Santa Cruz Biotechnology), m-TOR (#2983, Cell Signalling, Danvers, MA, USA), p-AMPK (AMP-activated protein kinase) (#2535, Cell Signalling), ULK-1 (#4773, Cell SignallingDanvers, MA,), p62 (#5114, Cell Signalling), Beclin-1 (#3738, Cell Signalling), Atg5 (#12994, Cell Signalling), LC3B I/II (#2775, Cell Signalling), LAMP2a (ab18528, Abcam, Cambridge, UK), cytochrome c (sc-13560, Santa Cruz Biotechnology), caspase 3 (sc-7148, Santa Cruz Biotechnology), Bax (sc-526, Santa Cruz Biotechnology), and Bcl-2 (sc-7382, Santa Cruz Biotechnology), were added to the membranes to recognize the proteins. Following primary antibody incubation, the membranes were incubated with horseradish peroxidase–linked secondary antibodies at room temperature for 1 h. Anti-rabbit, anti-mouse, or anti-goat IgG were used as the secondary antibodies, and the membranes were washed with TBS for 1 h. The blotting results were imaged with Fujifilm LAS-4000 (GE Healthcare UK Ltd.).
Statistical analysis
All results were obtained from individual duplicates, and the experiments were performed at least three times. The results are presented as the group mean±standard deviation (SD). A one-way analysis of variance was used to determine the overall statistical significance for the means of the four experimental groups. A p-value less than 0.05 was considered to be significant. Statistical analyses were performed using SigmaPlot v.10.0 software.
Results
Hydrogen peroxide-induced autophagy and apoptosis in H9c2 cells
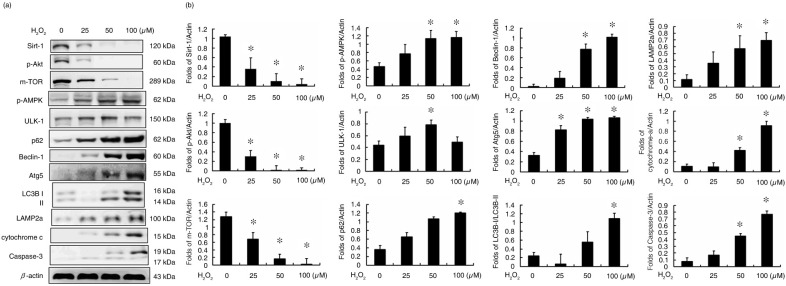
In this study, the protein analysis determined that the expression levels of Sirt-1, p-Akt, and an m-TOR were dose dependently reduced in H2O2-treated H9c2 cells (Fig. 1). In addition, the levels of autophagy-related proteins, such as p-AMPK, unc-51, autophagy activating kinase 1 (ULK1), nucleoporin p62 (p62), Beclin-1, autophagy protein 5 (ATG5), LC3B, and LAMP2a, were also affected by H2O2 treatment of the H9c2 cells, suggesting that autophagy is induced by H2O2 treatment (Fig. 1). Further analysis indicated that caspase-3 expression was also increased by H2O2-treatments, which resulted in the release of cytochrome c (Fig. 1).
Fig. 1.
Hydrogen peroxide-induced autophagy and apoptosis in H9c2 cells. (a) The protein markers of survival, autophagy, and cell apoptosis pathways. (b) The fold change in the expression of each protein in hydrogen peroxide-treated H9c2 cells. (*P<0.01 compared with the control group).
Hydrogen peroxide-induced autophagy is regulated by the AMPK-ULK signalling pathway
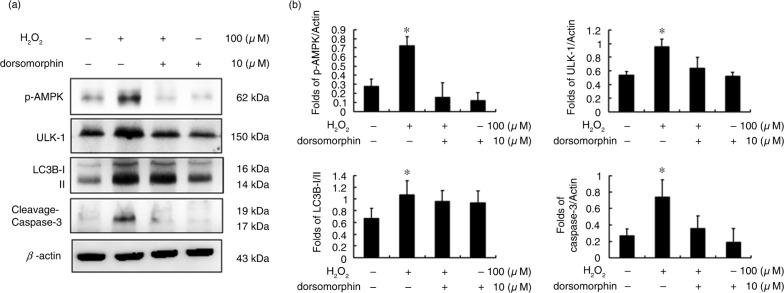
To identify the key pathway involved in H2O2-induced autophagy in H9c2 cells, dorsomorphin (compound C) was used to block AMPK activity. Indeed, p-AMPK protein levels were reduced after 10 µM dorsomorphin treatment (Fig. 2). The ULK-1 and LC3B proteins were downregulated after AMPK inhibition. Moreover, caspase-3 was also inhibited in dorsomorphin-treated H9c2 cells.
Fig. 2.
Hydrogen peroxide-induced autophagy is regulated by the AMPK-ULK signalling pathway. (a) Protein markers of the AMPK-ULK regulated pathway. (b) The fold change in expression for each protein in H9c2 cells with the indicated treatments. (*P<0.01 compared with the control group).
H9c2 cardiomyocyte cell apoptosis via autophagy
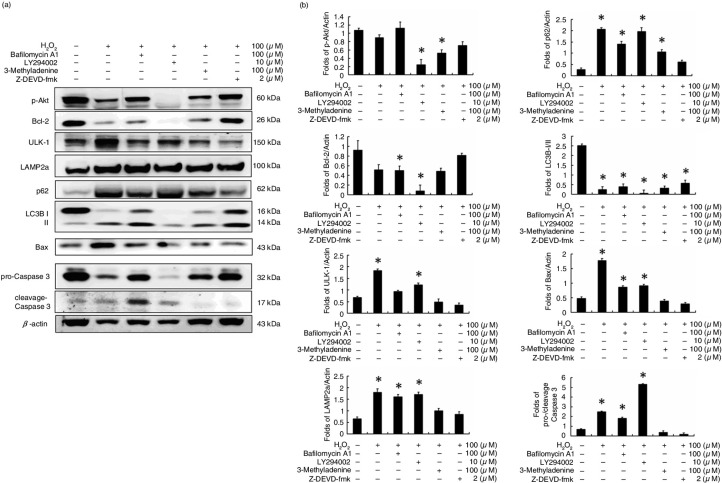
Based on the results of Fig. 2, caspase-3 expression was reduced in a manner dependent on AMPK inhibition. This suggests a relationship between autophagy and cell apoptosis in H2O2-treated H9c2 cells. Here, bafilomycin A1 (100 µM) was used to co-treat H9c2 cells with H2O2. The results show that bafilomycin A1 inhibits degradation of the autophagosome and causes more significant cell apoptosis in H2O2 co-treated H9c2 cells (Fig. 3). Furthermore, a PI3K-Akt inhibitor (LY294002, 10 µM) was used to co-treat H9c2 cells with H2O2 (Fig. 3). The results showed not only autophagy but also cell apoptosis in H9c2 cells. However, when H9c2 cells were co-treated with a specific autophagy inhibitor (3-methyladenine, 100 µM) and H2O2, the levels of autophagy biomarker proteins, such as ULK-1, p62, and LC3B, were reduced. Furthermore, cell apoptosis was reduced for H9c2 cells co-treated with 3-methyladenine (100 µM) and H2O2, which was similar to the results of caspase-3 inhibitor (z-DEVD-fmk, 2 µM) and H2O2 co-treatment of the H9c2 cells (Fig. 3). These results suggest that increased autophagy will lead to cell apoptosis in H2O2-treated H9c2 cells.
Fig. 3.
H9c2 cardiomyocyte cell apoptosis via autophagic pathway. (a) Protein markers of survival, autophagy, and cell apoptosis pathways. (b) The fold change in expression for each protein in hydrogen peroxide-treated H9c2 cells. (*P<0.01 compared with the control group).
Hydrogen peroxide-induced mitochondrial membrane potential decrease and protection by resveratrol treatment in H9c2 cells
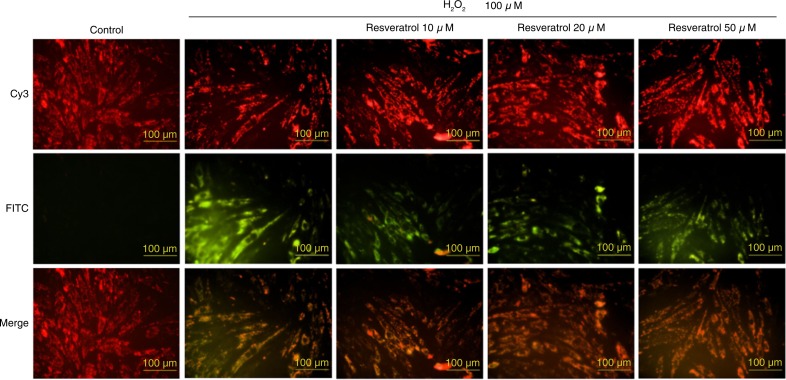
The decrease in mitochondrial membrane potential can be visualized using a JC-1 staining assay. In the H2O2 treatment only group, red represents the normal mitochondria and green represents H9c2 cells with mitochondrial membrane potential instability (Fig. 4). After 4 h of H2O2 pretreatment, resveratrol (10, 20 and 50 µM) stabilized the mitochondrial membrane potential of the H9c2 cells.
Fig. 4.
Hydrogen peroxide-induced mitochondrial membrane potential decrease and protection by resveratrol treatment in H9c2 cells.
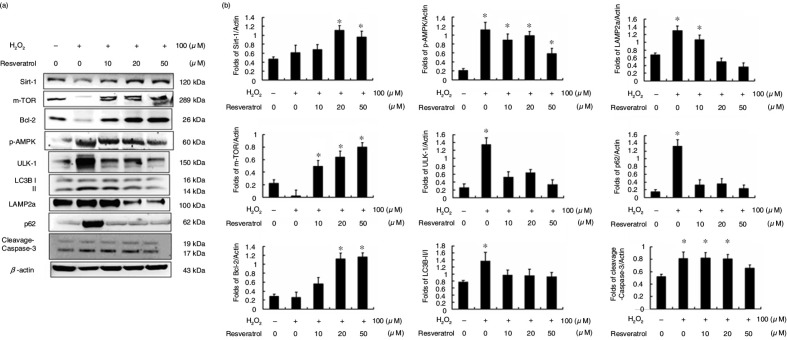
Resveratrol reduces hydrogen peroxide-induced autophagy and cell apoptosis in H9c2 cells
In this study, H9c2 cells were treated with or without H2O2 for 4 h and were then treated with or without resveratrol at different concentrations (0, 10, 20, and 50 µM). Protein analysis showed that autophagy and apoptosis were induced by H2O2 treatment only in H9c2 cells (Fig. 5). After resveratrol treatment, H2O2-induced autophagy and cell apoptosis were reduced in a dose-dependent manner.
Fig. 5.
Resveratrol reduces hydrogen peroxide-induced autophagy and cell apoptosis in H9c2 cells. (a) Protein markers of survival, autophagy, and cell apoptosis pathways. (b) The fold change of expression for each protein in hydrogen peroxide-treated H9c2 cells. (*P<0.01 compared with the control group).
Discussion
Previous studies revealed that the H2O2 treatment induces cell apoptosis through caspase-3 activation and cytochrome c expression in H9c2 cells (20). Past evidence indicates that AMPK is an important physiological energy sensor that balances energy supply and demand, and regulates cellular processes (21). Treatment with H2O2 will cause a significant increase in the concentration of p-AMPK. Therefore, H2O2-activated, autophagy-related proteins may inhibit m-TOR activity through p-AMPK activation. The study also found that the apoptotic proteins cytochrome c and caspase-3 also play significant roles, indicating that H2O2-induced autophagy may affect the activity of apoptotic proteins (Fig. 1).
AMPK is activated when the ATP yield decreases intracellularly. AMPK plays key roles in regulating growth and is also involved in cell functions and processes associated with autophagy (22). Our previous studies showed that H2O2 leads to AMPK phosphorylation and inhibits m-TOR activity; therefore, we hypothesized that autophagy activation occurs through the AMPK pathway. Here, we used the AMPK inhibitor dorsomorphin (compound C) to determine whether H2O2 induced autophagy by activation of the AMPK pathway (Fig. 2). After treatment with dorsomorphin, H2O2-induced autophagy proteins, such as ULK1 and LC3B, were significantly decreased, and the expression of the pro-apoptotic protein caspase-3 was reduced after compound C treatment (Fig. 2). This demonstrates that H2O2-induced autophagy may also affect the activity of downstream apoptotic proteins.
It was recently found that autophagy exerts cell protective effects against doxorubicin-induced toxicity, hypoxia-reoxygenation, and ischemia-reperfusion injury (23, 24). However, the mechanisms underlying these effects remain complex. The cardioprotective effects of resveratrol may be mediated by Sirt-1 activation. In our previous study, Sirt-1 activation was highly associated with the PI3K-Akt pathway (25). In this study, we used the PI3K specific inhibitor LY294002 to block p-Akt expression in H2O2-treated H9c2 cells, which increased the apoptosis of these cells (Fig. 3). Moreover, the autophagy inhibitor 3-methyladenine decreased H2O2-induced cell apoptosis in H9c2 cells, similar to the caspase-3 specific inhibitor z-DEVD-fmk.
These results are consistent with those of a previous study that showed that resveratrol has multiple protective functions, particularly functions related to antioxidant activity in vivo (26). Further elucidation of the role of resveratrol in protecting cells and decreasing death will increase the effectiveness of its administration. H2O2 is extensively used as an inducer of oxidative stress in vitro (27). The JC-1 staining assay showed that H2O2 treatment of H9c2 cells resulted in H2O2-induced damage to the mitochondrial membrane potential and unstable green fluorescence (Fig. 4). Furthermore, resveratrol significantly protects H9c2 cells (Fig. 5).
In conclusion, our current findings show that resveratrol treatment may reduce H2O2-induced autophagy and cell apoptosis in H9c2 cells through Sirt-1 and p-Akt to regulate cardiac survival pathways. Our results suggest that resveratrol treatment may be beneficial for CVD.
Conflict of interest and funding
The authors declare no conflict of financial interest. This study is supported in part by the Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence MOHW105-TDU-B-212-133019 and MOST-103-2410-H-029-037.
References
- 1.Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res. 2000;87:840–4. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- 2.Zhu X, Liu B, Zhou S, Chen YR, Deng Y, Zweier JL, et al. Ischemic preconditioning prevents in vivo hyperoxygenation in postischemic myocardium with preservation of mitochondrial oxygen consumption. Am J Physiol Heart Circ Physiol. 2007;293:H1442–50. doi: 10.1152/ajpheart.00256.2007. [DOI] [PubMed] [Google Scholar]
- 3.De Duve C, Wattiaux R. Functions of lysosomes. Annu Rev Physiol. 1966;28:435–92. doi: 10.1146/annurev.ph.28.030166.002251. [DOI] [PubMed] [Google Scholar]
- 4.Yorimitsu T, Klionsky DJ. Autophagy: molecular machinery for self-eating. Cell Death Differ. 2005;2:1542–52. doi: 10.1038/sj.cdd.4401765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ouimet M. Autophagy in obesity and atherosclerosis: interrelationships between cholesterol homeostasis, lipoprotein metabolism and autophagy in macrophages and other systems. Biochim Biophys Acta. 2013;1831:1124–33. doi: 10.1016/j.bbalip.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 6.Margariti A, Li H, Chen T, Martin D, Vizcay-Barrena G, Alam S, et al. Xbp1 mrna splicing triggers an autophagic response in endothelial cells through beclin-1 transcriptional activation. J Biol Chem. 2013;288:859–72. doi: 10.1074/jbc.M112.412783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsieh SR, Cheng WC, Su YM, Chiu CH, Liou YM. Molecular targets for anti-oxidative protection of green tea polyphenols against myocardial ischemic injury. BioMedicine. 2014;4:7–16. doi: 10.7603/s40681-014-0023-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jung CH, Jun CB, Ro SH, Kim YM, Otto NM, Cao J, et al. Ulk-atg13-fip200 complexes mediate motor signaling to the autophagy machinery. Mol Boil Cell. 2009;20:1992–2003. doi: 10.1091/mbc.E08-12-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lerena C, Calligaris SD, Colombo MI. Autophagy: for better or for worse, in good times or in bad times. Curr Mol Med. 2008;8:92–101. doi: 10.2174/156652408783769634. [DOI] [PubMed] [Google Scholar]
- 10.Kabeya Y, Mizushima N, Yamamoto A, Oshitani-Okamoto S, Ohsumi Y, Yoshimori T. Lc3, GABARAP and gate16 localize to autophagosomal membrane depending on form-ii formation. J Cell Sci. 2004;117:2805–12. doi: 10.1242/jcs.01131. [DOI] [PubMed] [Google Scholar]
- 11.Dewaele M, Maes H, Agostinis P. Ros-mediated mechanisms of autophagy stimulation and their relevance in cancer therapy. Autophagy. 2010;6:838–54. doi: 10.4161/auto.6.7.12113. [DOI] [PubMed] [Google Scholar]
- 12.Gluba A, Bielecka-Dabrowa A, Mikhailidis DP, Wong ND, Franklin SS, Rysz J, et al. An update on biomarkers of heart failure in hypertensive patients. J Hypertens. 2012;30:1681–9. doi: 10.1097/HJH.0b013e3283569a9c. [DOI] [PubMed] [Google Scholar]
- 13.Lee Y, Lee HY, Gustafsson AB. Regulation of autophagy by metabolic and stress signaling pathways in the heart. J Cardiovasc Pharmacol. 2012;60:118–24. doi: 10.1097/FJC.0b013e318256cdd0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mahgoub MA, Abd-Elfattah AS. Diabetes mellitus and cardiac function. Mol Cell Biochem. 1998;180:59–64. [PubMed] [Google Scholar]
- 15.Richard T, Pawlus AD, Iglesias ML, Pedrot E, Waffo-Teguo P, Merillon JM, et al. Neuroprotective properties of resveratrol and derivatives. Ann N Y Acad Sci. 2011;1215:103–8. doi: 10.1111/j.1749-6632.2010.05865.x. [DOI] [PubMed] [Google Scholar]
- 16.Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 17.Hung LM, Chen JK, Huang SS, Lee RS, Su MJ. Cardioprotective effect of resveratrol, a natural antioxidant derived from grapes. Cardiovasc Res. 2000;47:549–55. doi: 10.1016/s0008-6363(00)00102-4. [DOI] [PubMed] [Google Scholar]
- 18.Wang CH, Lin WD, Bau DT, Chou IC, Tsai CH, Tsai FJ. Appearance of acanthosis nigricans may precede obesity: An involvement of the insulin/IGF receptor signaling pathway. BioMedicine. 2013;3:28–87. [Google Scholar]
- 19.Wang B, Yang Q, Sun YY, Xing YF, Wang YB, Lu XT, et al. Resveratrol-enhanced autophagic flux ameliorates myocardial oxidative stress injury in diabetic mice. J Cell Mol Med. 2014;18:1599–611. doi: 10.1111/jcmm.12312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaushal GP, Liu L, Kaushal V, Hong X, Melnyk O, Seth R, et al. Regulation of caspase-3 and -9 activation in oxidant stress to rte by forkhead transcription factors, bcl-2 proteins, and map kinases. Am J Physiol Renal Physiol. 2004;287:F1258–68. doi: 10.1152/ajprenal.00391.2003. [DOI] [PubMed] [Google Scholar]
- 21.Inoki K, Kim J, Guan KL. Ampk and motor in cellular energy homeostasis and drug targets. Annu Rev Pharmacol Toxicol. 2012;52:381–400. doi: 10.1146/annurev-pharmtox-010611-134537. [DOI] [PubMed] [Google Scholar]
- 22.Mihaylova MM, Shaw RJ. The ampk signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol. 2011;13:1016–23. doi: 10.1038/ncb2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dutta D, Xu J, Dirain ML, Leeuwenburgh C. Calorie restriction combined with resveratrol induces autophagy and protects 26-month-old rat hearts from doxorubicin-induced toxicity. Free Radic Biol Med. 2014;74:252–62. doi: 10.1016/j.freeradbiomed.2014.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gurusamy N, Lekli I, Mukherjee S, Ray D, Ahsan MK, Gherghiceanu M, et al. Cardioprotection by resveratrol: a novel mechanism via autophagy involving the mtorc2 pathway. Cardiovasc Res. 2010;86:103–12. doi: 10.1093/cvr/cvp384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin CH, Lin CC, Ting WJ, Pai PY, Kuo CH, Ho TJ, et al. Resveratrol enhanced FOXO3 phosphorylation via synergetic activation of SIRT1 and PI3K/Akt signaling to improve the effects of exercise in elderly rat hearts. Age (Dordr) 2014;36:9705. doi: 10.1007/s11357-014-9705-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Das S, Das DK. Resveratrol: a therapeutic promise for cardiovascular diseases. Recent Pat Cardiovasc Drug Discov. 2007;2:133–8. doi: 10.2174/157489007780832560. [DOI] [PubMed] [Google Scholar]
- 27.Duarte TL, Jones GD. Vitamin c modulation of H2O2-induced damage and iron homeostasis in human cells. Free Radic Biol Med. 2007;43:1165–75. doi: 10.1016/j.freeradbiomed.2007.07.017. [DOI] [PubMed] [Google Scholar]