Abstract
Nonalcoholic fatty liver disease (NAFLD) is the most common chronic liver disease in the world, and it comprises a spectrum of hepatic abnormalities from simple hepatic steatosis to steatohepatitis, fibrosis, cirrhosis, and liver cancer. While the pathogenesis of NAFLD remains incompletely understood, a multihit model has been proposed that accommodates causal factors from a variety of sources, including intestinal and adipose proinflammatory stimuli acting on the liver simultaneously. Prior cellular and molecular studies of patient and animal models have characterized several common pathogenic mechanisms of NAFLD, including proinflammation cytokines, lipotoxicity, oxidative stress, and endoplasmic reticulum stress. In recent years, gut microbiota has gained much attention, and dysbiosis is recognized as a crucial factor in NAFLD. Moreover, several genetic variants have been identified through genome-wide association studies, particularly rs738409 (Ile748Met) in PNPLA3 and rs58542926 (Glu167Lys) in TM6SF2, which are critical risk alleles of the disease. Although a high-fat diet and inactive lifestyles are typical risk factors for NAFLD, the interplay between diet, gut microbiota, and genetic background is believed to be more important in the development and progression of NAFLD. This review summarizes the common pathogenic mechanisms, the gut microbiota relevant mechanisms, and the major genetic variants leading to NAFLD and its progression.
1. Introduction
Nonalcoholic fatty liver disease (NAFLD) is the most common chronic liver disease in the world. It is present in 30% of the general adult population and found predominantly in obese people with high-fat diets and inactive lifestyles. In reality, NAFLD comprises a spectrum of hepatic abnormalities that are observable in liver histological slides, from a simple intrahepatic accumulation of fat (steatosis or nonalcoholic fatty liver, NAFL) to various degrees of necrotic inflammation (nonalcoholic steatohepatitis, NASH) [1–3]. Simple steatosis (i.e., NAFL) rarely progresses to advanced disease whereas, in approximately 20% of patients with NASH, it progresses to fibrosis and cirrhosis and potentially to hepatocellular carcinoma over a 15-year time period [4, 5]. The majority of patients with NAFLD are obese or even morbidly obese and have accompanying insulin resistance that plays a central role in the metabolic syndrome [6–9]. Thus, NAFLD is also deemed to be hepatic manifestation of metabolic syndrome which is a cluster of complex conditions including central obesity, hypertension, hyperglycaemia, hypertriglyceridemia, and low HDL (high density lipoprotein) that are predictive risk factors of cardiovascular disease, stroke, and diabetes [10, 11].
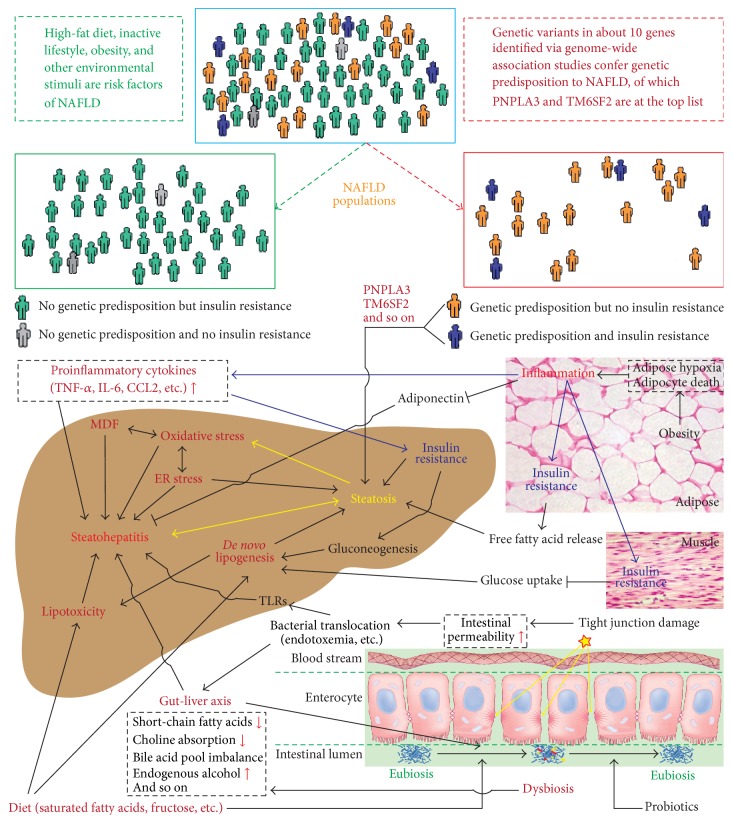
NAFLD has been considered a condition with a “two-hit” process of pathogenesis since 1998 when Day and James first proposed this hypothesis [12] with evidence from the Berson et al. study describing the role of lipid peroxidation in the liver injury [13]. Essentially, the first hit is the development of hepatic steatosis via accumulation of triglycerides in hepatocytes, which increases the vulnerability of the liver to various possible “second hits” that in turn lead to the inflammation, fibrosis, and cellular death characteristics of NASH. The second hit can be a variety of factors, such as oxidative stress, endoplasmic reticulum stress, proinflammatory cytokines, and gut-derived bacterial endotoxin. As it has evidently emerged that (1) accumulation of triglycerides in hepatocytes may be a protective mechanism from liver damage and (2) hepatic inflammation can precede the simple hepatic steatosis and can also be a cause of steatosis, it has been believed that many “hit” factors may act simultaneously leading to the development of NAFLD, which supports the multihit model proposed in 2010 [14]. Indeed, among the proposed hit factors, many can interact with each other, forming a vicious circle. Recent advances in metagenomics complicate the understanding of the pathogenesis of NAFLD further in that dysbiosis and host-microbiota interactions are now also implicated. Moreover, genome-wide association studies have discovered several promising candidate genes, serving as the genetic background for the disease. These genetic players appear to distinguish subgroups of NFLD patients from obese and insulin resistance associated populations. Although a high-fat diet and inactive lifestyles are typical risk factors for NAFLD, the interplay between diet, gut microbiota, and genetic background can play a crucial role in the development and progression of NAFLD. This review summarizes the common pathogenic mechanisms, the gut microbiota relevant mechanisms, and the major genetic variants leading to NAFLD and its progression (Figure 1).
Figure 1.
Overview at the pathogenesis of nonalcoholic fatty liver disease (NAFLD). The interplay between diet, microbiota, and host genetic variants plays a crucial role in the complex pathogenesis of NAFLD through a variety of mechanisms. The NAFLD patients can now be categorized into different populations based on their insulin sensitivity and genetic predisposition. Insulin resistance is at the center of the NAFLD pathogenic process, and a number of key factors are involved in the development of NAFLD, such as diet, dysbiosis, gut-liver axis, genetic predisposition genes (PNPLA3 and TM6SF2), oxidative stress, MDF (mitochondrial dysfunction), endoplasmic reticulum (ER) stress, de novo lipogenesis, lipotoxicity, and proinflammatory cytokines.
2. Common Pathogenic Mechanisms of NAFLD
Hepatic steatosis is a prerequisite to making a histological diagnosis of NAFLD [2]. Several mechanisms may lead to steatosis, including (1) increased fat supply such as high-fat diet and excess adipose lipolysis; (2) decreased fat export in the form of very low density lipoprotein-triglyceride; (3) decreased free fatty β-oxidation; and (4) increased de novo lipogenesis (DNL) [2]. Molecular mechanisms responsible for the accumulation of fat in the liver are not fully understood; however, certain cytokines derived from inflammation sites, particularly from extrahepatic adipose tissues, can trigger this process. In addition, the enhancement of hepatic DNL is deemed to be a unique feature in steatosis. More importantly, insulin resistance appears to be at center stage for the massive metabolic dysregulations of NAFLD that initiate and aggravate hepatic steatosis. At a certain point, the simple steatosis transforms to steatohepatitis in about 20–30% of NAFLD patients. This breakthrough-like process is mediated by the interplay of multiple hit factors. Pathological features of NASH include simple hepatic steatosis and, more characteristically, liver cell damage and accompanying inflammation and/or fibrosis. Currently, a number of common pathogenic mechanisms have been proposed and characterized for the transition from simple steatosis to NASH, such as lipotoxicity, oxidative stress, mitochondrial dysfunction, and endoplasmic reticulum stress.
2.1. Adipose Tissue Inflammation
What exactly initiates adipose tissue inflammation in obesity is uncertain; but hypoxia and death of rapidly expanding adipocytes are believed to play a role [15]. Adipocytes under inflammation secrete cytokines and chemokines, particularly tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and CC-chemokine ligand-2 (CCL2) [15, 16]. TNF-α was the first proinflammatory cytokine detected in adipose tissue and is involved in the regulation of insulin resistance. Studies indicated that neutralization of TNF-α activity by an anti-TNF-α monoclonal antibody improves insulin resistance and fatty liver disease in animals [17]. IL-6 is derived from many cells throughout the body including adipocytes. Serum levels of these cytokines correlate remarkably well with the presence of insulin resistance, and adipose tissue-derived TNF-α and IL-6 have been shown to regulate hepatic insulin resistance via upregulation of SOCS3, a suppressor of cytokine signaling [17]. CCL2 recruits macrophages to the adipose tissue, resulting in even more local cytokine production and perpetuating the inflammatory cycle; TNF-α and IL-6 induce a state of insulin resistance in adipocytes, which stimulates triglyceride lipolysis and fatty acid release into the circulation. At the same time, extrahepatic adipocytes are compromised in their natural ability to secrete adiponectin, an anti-inflammatory adipokine that facilitates the normal partitioning of lipid to adipocytes for storage [18]. Circulating adiponectin regulates hepatic fatty β-oxidation through AMP-activated protein kinase (AMPK) and acetyl-CoA carboxylase (ACC) signaling [19]. Together, these abnormalities accentuate fat loss from adipocytes and promote ectopic fat accumulation.
2.2. De Novo Lipogenesis (DNL)
Presumably lipogenesis in liver could be increased due to the steatotic nature of NAFLD. A number of prior studies have shown that diets enriched in both saturated fat and simple sugar carry a high risk of hepatic steatosis, at least in part, through enhanced DNL [20–23]. The role of DNL in the development of hepatic steatosis is further supported by a recent study in subjects with metabolic syndrome and a high content of liver fat [24]. A 3-fold higher rate of de novo fatty acid synthesis is seen in these subjects. In addition, specific dietary compositions may have different effects. Basically since carbohydrates are substrates for DNL, the amount of carbohydrate in the diet will positively influence the amount of DNL in the liver. Simple sugars are converted to fatty acids more readily than complex carbohydrates [25, 26], and fructose is a more potent inducer of DNL than glucose [27, 28]. This is also supported by epidemiologic evidence linking dietary fructose to hepatic steatosis and NASH [20, 29]. It is worth noting that dietary fat, particularly saturated fat, stimulates DNL by upregulating SREBP-1 (sterol responsive element binding protein), a key regulator of the lipogenic genes in the liver [30]. However, not all individuals with hepatic steatosis had increased DNL nor upregulated SREBP-1 expression, as observed in the Mancina et al. study showing a paradoxical dissociation between hepatic DNL and hepatic fat content due to the PNPLA3 148M allele [31].
2.3. Insulin Resistance
Studies have highlighted the fact that insulin resistance is a characteristic feature of NAFLD [7–9] and is caused by a variety of factors, including soluble mediators derived from immune cells and/or adipose tissue, such as TNF-α and IL-6 [32]. Serine phosphorylation of insulin receptor substrates by inflammatory signal transducers such as c-jun N-terminal protein kinase 1 (JNK1) or inhibitor of nuclear factor-κB kinase-β (IKK-β) is considered one of the key aspects that disrupts insulin signaling [14]. On the other hand, insulin resistant subjects with NAFLD show reduced insulin sensitivity, not only at the level of the muscle, but also at the level of the liver and adipose tissue [7–9, 33], which can lead to a far more complex metabolic disturbance of lipid and glucose. However, not all people with NAFLD have increased insulin resistance, and NAFLD, per se, cannot be considered a cause for insulin resistance but rather a consequence as shown by studies in subjects genetically predisposed to NAFLD. Mutations in PNPLA3 (patatin-like phospholipase domain containing 3) [34, 35], TM6SF2 (transmembrane 6 superfamily member 2) [6, 36], DGAT1 (diacylglycerol O-acyltransferase 1) [37], or hypobetalipoproteinemia [38, 39] genes are not related to increased insulin resistance except for severely obese individuals in which it is associated [40]. It is worth noting that insulin resistance is characterized not only by increased circulating insulin levels but also by increased hepatic gluconeogenesis, impaired glucose uptake by muscle, and increased release of free fatty acids and inflammatory cytokines from peripheral adipose tissues [41], which are the key factors promoting accumulation of liver fat and progression of hepatic steatosis (Figure 1).
2.4. Lipotoxicity
Studies have indicated that certain lipids can be harmful to hepatocytes in NAFLD. This is particularly true of the long-chain saturated fatty acids (SFAs) such as palmitate (C16:0) and stearate (C18:0), which are abundant in animal fat and dairy products and produced in the liver from dietary sugar. Under physiological conditions, SFAs are transported to mitochondria for β-oxidation or esterified for either excretion in the form of VLDL (very low density lipoproteins) or storage as lipid droplets. In the pathophysiology of NASH, multiple mechanisms are concurrently operative to produce liver injury in hepatocytes overwhelmed by SFA and by free cholesterol (FC) from de novo synthesis [42, 43]. FC accumulation leads to liver injury through the activation of intracellular signaling pathways in Kupffer cells (KCs), hepatic stellate cells (HSCs), and hepatocytes. The activation of KCs and HSCs promotes inflammation and fibrogenesis [44]. These lipids, including FC, SFA, and certain lipid intermediates from excessive SFA, can activate a variety of intracellular responses such as JNK1 and a mitochondrial death pathway, resulting in lipotoxic stress in the endoplasmic reticulum and mitochondria, respectively [42, 43, 45]. In addition, the toll-like receptor 4 (TLR4) is a pattern recognition receptor that activates a proinflammatory signaling pathway in response to excessive SFAs. This pathway is initiated by recruiting adaptor molecules such as toll/IL-1 receptor domain containing adaptor protein (TIRAP) and myeloid differentiation factor 88 (MyD88) that ultimately lead to activation of nuclear factor κB with production of TNF-α [46].
2.5. Mitochondrial Dysfunction
Mitochondria are the most important energy suppliers of the cell and play a pivotal role in fatty acid metabolism. Fatty acid oxidation is able to be upregulated to compensate for some degree of increased deposition of fat; however, multiple studies have shown that liver ATP levels are decreased in NAFLD [47–49]. This discrepancy implicates mitochondrial dysfunction in the state of liver fat overload that is characteristic of NAFLD. Although the mechanisms responsible for the mitochondrial dysfunction remain poorly understood in NAFLD, reduced enzymatic activities of mitochondrial electron transport chain (ETC) complexes may be attributed to increased generation of reactive oxygen species (ROS) as a result of ETC leakage during mitochondrial β-oxidation in energy production (in the form of ATP) [50]. Studies have found that ROS can damage the ETC [51] and even cause mutations in the mitochondria DNA [52].
2.6. Oxidative Stress
In the context of increased supply of fatty acids to hepatocytes, oxidative stress can occur and be attributable to raised levels of reactive oxygen/nitrogen species (ROS/RNS) and lipid peroxidation that are generated during free fatty acid metabolism in microsomes, peroxisomes, and mitochondria [53–55]. Peroxidation of plasma and intracellular membranes may cause direct cell necrosis/apoptosis and megamitochondria, while ROS-induced expression of Fas-ligand on hepatocytes may induce fratricidal cell death. Recent studies support the idea that oxidative stress may be a primary cause of liver fat accumulation and subsequent liver injury, and ROS may play a part even in fibrosis development [56, 57]. Importantly, these species can initiate lipid peroxidation by targeting polyunsaturated fatty acids (PUFAs), resulting in the formation of highly reactive aldehyde products, such as 4-hydroxy-2-nonenal (4-HNE) and malondialdehyde (MDA). These reactive lipid derivatives have the potential to amplify intracellular damage by mediating the diffusion of ROS/RNS into the extracellular space, thus causing tissue damage.
2.7. Endoplasmic Reticulum (ER) Stress
The ER is a vast dynamic and tubular network responsible for the synthesis, folding/repair, and trafficking of a wide range of proteins [58]. Under pathological and/or stressful conditions such as NASH, it has been observed that ER efficiency in the protein-folding, repairing, and/or trafficking machinery is decreased while the demand of protein synthesis and folding/repair is increased [58, 59]. Such an imbalance between the load of needed protein-folding and the response-related capability of the ER is termed ER stress, which can lead to the accumulation of unfolded and/or misfolded proteins within the ER lumen. This type of cellular stress usually triggers an adaptive response, aimed at resolving ER stress, called unfolded protein response (UPR) [60–62]. The UPR is mediated by at least three different stress-sensing pathways: protein kinase RNA-like ER kinase (PERK), inositol-requiring protein 1α (IRE1α), and activating transcription factor 6 (ATF6) [62]. Coupled with inflammation, oxidative stress, insulin resistance, and apoptosis signaling, hepatic ER stress seems to play an important role in regulating the composition and size of lipid droplets as well as lipid synthesis, including cholesterol metabolism [58, 59, 63], through SREBP.
3. Microbiota Associated Mechanisms of NAFLD
Gut microbiota was first found to be altered in patients with chronic liver disease more than 80 years ago. Derangement of the gut flora, in particular small intestinal bacterial overgrowth (SIBO), occurs in a large percentage (20–75%) of patients with chronic liver disease. In recent years, the gut microbiome has gained much more attention due to the advancement of the high-throughput next-generation sequencing (NGS) technology. Prior studies of gut flora relied on culture dependent techniques, which were labor intensive and limited only to a countable number of species, as over 80% of the gut microbes are not cultivatable [64]. In contrast, NGS-based taxonomic assignments of the uncultured, undefined microbes into operational taxonomic units (OTUs) represent an effective and revolutionary approach for studies on highly complex gut microbiota, which is based on clustering of the 16S rRNA sequences derived from the NGS platforms. This approach allows the characterization of both composition and diversity of the intestinal microbiota. According to evidence from relevant studies, the gut microbiota may contribute to the pathogenesis of NAFLD through several mechanisms, including (1) increased production and absorption of gut short-chain fatty acids; (2) altered dietary choline metabolism by the microbiota; (3) altered bile acid pools by the microbiota; (4) increased delivery of microbiota-derived ethanol to liver; (5) gut permeability alterations and release of endotoxin; and (6) interaction between specific diet and microbiota. Most recently, Musso et al. brought up a new mechanism by that chronic kidney disease may mutually aggravate NAFLD and associated metabolic disturbances through multiple paths including altered intestinal barrier function and microbiota composition [65]. In fact, diet can affect the composition and diversity of gut microbiota; thus any changes of gut microbiota that are observed in a diet-stratified study should be interpreted with caution because these changes could be either a direct effect of specific diets or an indirect effect of the gut-liver interactive axis which has been proposed and observed recently [66, 67].
3.1. Short-Chain Fatty Acids (SCFAs) Relevant Mechanisms
In the intestine, SCFAs are produced in the distal small intestine and colon where nondigestible carbohydrates like resistant starch, dietary fiber, and other low-digestible polysaccharides are fermented by saccharolytic bacteria which include the phyla Bacteroidetes, Firmicutes, and Actinobacteria. Acetate and propionate are the main products of Bacteroidetes phylum and butyrate is mainly produced by Firmicutes phylum. As an energy precursor, SCFAs are implicated in the pathogenesis of NAFLD because of their possible contribution to obesity. The first evidence regarding SCFAs was from Turnbaugh et al. study [68] showing that the cecum of ob/ob mice has an increased concentration of SCFAs and that transplantation of germ-free mice with the gut microbiome from ob/ob mice caused greater fat gain than transplants from lean animals. In humans, increased production of SCFAs by the gut microbiota was also observed in overweight and obese people, compared to lean subjects [69]. In metagenomics analysis, the majority of studies showed that ob/ob mice [70] and obese patients [71] exhibit reduced abundance of Bacteroidetes and proportionally increased abundance of Firmicutes. However, how these ratio changes affect energy imbalance leading to obesity and its complications including NAFLD needs further functional and species-level analyses. In fact, SCFAs have more beneficial effects than their obesity-causing effects in general [72]. Beneficial effects of SCFAs are through several ways, such as immunoregulation, enhanced intestinal barrier function, acting as a histone deacetylase 1 (HDAC) inhibitor to decrease expression of lipogenic genes and to increase carnitine palmitoyltransferase 1A expression [72], and a peroxisome proliferator-activated receptor-γ- (PPARγ-) dependent mechanism, shifting metabolism in adipose and liver tissue from lipogenesis to fatty acid oxidation [73].
3.2. Dietary Choline Mechanism
Dietary choline is required for very low density lipoprotein synthesis and hepatic lipid export; and dietary choline-deficiency has been linked with a variety of conditions including hepatic steatosis. Buchman et al. [74] found that, in patients with parenteral nutrition, diets deficient in choline can lead to increased hepatic steatosis, which can be reversed with choline supplementation. This study suggests a role of choline in fat export out of the hepatocytes. Recent studies indicate a role of the intestinal microbiota in the conversion of dietary choline to toxic methylamine, a substance that not only mimics a choline-deficient diet by decreasing effective choline levels but also exposes the host to an inflammatory toxic metabolite [75]. Very recently, a metagenomic analysis of the microbial communities living in the intestinal tracts of 15 women with a choline-depleted diet revealed that increased Gammaproteobacteria abundance and decreased Erysipelotrichi abundance were protective against developing steatosis [76].
3.3. Bile Acid Pool Related Mechanisms
Within hepatocytes, bile acids are synthesized from cholesterol through enzymatic pathways and then conjugated with either glycine or taurine before secretion into bile and released into the small intestine. In the small intestine, conjugated bile acids not only assist in lipid absorption and transport but have also been increasingly recognized to function as nuclear receptor binders and to have a putative role in altering the microbiome [77]. On the other hand, bacteria within the intestine can also chemically modify bile acids and thereby alter the composition of the bile acid pool [78, 79]. Besides the classic role as detergents to facilitate fat absorption, bile acids have also been recognized as important cell signaling molecules regulating lipid metabolism, carbohydrate metabolism, and inflammatory response [80]. These molecular functions are mediated through their binding and activation of the nuclear hormone receptor, farnesoid X receptor (FXR), and the G protein coupled cell surface receptor TGR5 [81]. Intestinal FXR activity upregulates endocrine FGF19 expression, which inhibits hepatic bile acid synthesis via CYP7A1 signaling [82]. McMahan et al. showed that activation of bile acid receptors with a receptor agonist was able to improve NAFLD histology in an obese mouse model [83]. Due to the nature of the complex interplay between the microbiome and the host bile acid pool, further studies are required in the context of risk for NAFLD and NASH.
3.4. Endogenous Alcohol Theory
The possible role for endogenous alcohol in NAFLD was first implicated in ob/ob mice. Cope et al. found that alcohol in the breath of obese animals is higher than that of lean animals [84], but they could not find any difference in the breath alcohol concentration between NASH patients and lean controls in a human study [85]. Recently, Zhu et al. found that NASH patients exhibited significantly elevated blood ethanol levels, while similar blood ethanol concentrations were observed between healthy subjects and obese non-NASH patients [86]. Further, in this metagenomics study, the composition of NASH microbiomes was found to be distinct from those of healthy and obese microbiomes, and Escherichia stood out as the only abundant genus that differed between NASH and obese patients. Because Escherichia are ethanol producers, this finding is in agreement with their previous report that alcohol-metabolizing enzymes are upregulated in NASH livers [87]. However, Engstler et al. provided evidence against the alcohol theory [88]. In their study, ethanol levels were similar in portal vein and chyme obtained from different parts of the GI tract between groups, while ethanol levels in vena cava plasma were significantly higher in ob/ob mice, suggesting that more ethanol was not metabolized in the liver due to a significantly lower ADH activity observed in these ob/ob mice. They proposed that increased blood ethanol levels in patients with NAFLD may result from insulin-dependent impairments of ADH activity in liver tissue, rather than from an increased endogenous ethanol synthesis. Thus, the alcohol theory currently faces conflicting results from different investigators. To clarify these conflicting results, de Medeiros and de Lima have provided an interesting mechanistic framework explaining how NAFLD might be an endogenous alcohol fatty liver disease (EAFLD) [89]. However, this framework requires experimental evidence to be validated.
3.5. Intestinal Permeability and Endotoxemia
The gut microbiota plays a part in maintaining the integrity of the intestinal barrier [90]; and changes in the composition of microbiota can lead to increased intestinal permeability and subsequent overflow of harmful bacterial by-products to the liver that in turn triggers hepatic inflammation and metabolic disorders. Endotoxin, that is, lipopolysaccharide (LPS), is derived from Gram-negative bacteria, and it has long been implicated in chronic liver diseases. The first evidence in support of a role for LPS in the pathogenesis of NASH was the observation that endotoxemia readily induces steatohepatitis in obese rats and mice [91]. Further, murine NAFLD models of bacterial overgrowth develop compositional changes of the gut microbiota and present increased intestinal permeability, with a concurrent reduction in the expression of tight junction proteins [92]. In human studies, Miele et al. found evidence of a disruption in the intestinal barrier of biopsy-proven NAFLD patients, along with an increased rate of small bowel bacteria overgrowth, suggesting that alterations in the microbiome may have contributed to disruption of gut barrier integrity [93]. In addition, high-fat diets may facilitate LPS uptake through elevated chylomicron production in intestinal epithelial cells [94]. On the other hand, Yuan et al. did not find the correlation between Gram-negative bacteria abundance and the concentration of serum endotoxin and there was no endotoxemia in the majority of pediatric NASH patients [95], highlighting the multihit hypothesis for the pathogenesis of NASH. Nonetheless, LPS and other exogenous stimuli are responded to first by innate immunity through pattern recognition receptors such as toll-like receptors (TLRs) and NOD-like receptors (NLRs). Although TLRs might respond to nutritional lipids such as free fatty acids [96], studies have implicated the importance of LPS-TLR4/TLR9 signaling in the pathogenesis of NAFLD. Both TLR4- and TLR9-deficient mice are protected from high-fat diet-induced inflammation and insulin resistance [97, 98], while mice deficient in TLR5 develop all features of metabolic syndrome including hyperphagia, obesity, insulin resistance, pancreatic inflammation, and hepatic steatosis [99]. Metagenomic analysis indicated that TLR5 deficiency affected the composition of the gut microbiota and, remarkably, transfer of the microbiota from TLR5−/− mice to healthy mice resulted in transfer of disease [99]. Moreover, Wlodarska et al. found that NOD-like receptor family pyrin domain containing 6 (NLRP6) inflammasome deficiency leads to an altered transmissible, colitogenic gut microbiota [100]. When fed with a methionine and choline-deficient diet (MCDD), these inflammasome deficient mice developed NASH with significantly higher severity than wild-type animals [101].
3.6. Saturated Fatty Acids
It has been well known that animal meats are rich in saturated fatty acids (SFAs) which are highly correlated to an increased risk of obesity, diabetes, and cardiovascular diseases. Many studies have indicated that saturated fatty acids are more toxic than their unsaturated counterparts [102, 103]. It is worth noting that SFAs are protective in alcohol induced fatty liver disease [104–106]. However, in liver and hepatocytes not exposed to alcohol, SFAs appear to promote apoptosis and liver injury [107, 108]. It has been shown that SFAs increase the saturation of membrane phospholipids, thus initiating unfolded protein response (UPR) and leading to ER stress [108, 109]. SFAs also affect mitochondrial metabolism and promote ROS accumulation [23]. Furthermore, hepatocyte apoptosis has been shown to be dependent on the activation of JNK stress signaling pathways that respond to prolonged ER and oxidative stress [109]. In addition, SFAs can interact with gut microbiota to affect the progression of liver injury. For instance, by analyzing changes in the intestinal metagenome and metabolome of alcohol-fed mice, Chen et al. recently found that synthesis of saturated long-chain fatty acid is significantly reduced when compared with normal-chew mice and that supplementation of saturated long-chain fatty acids recovers intestinal eubiosis and reduces ethanol-induced liver injury in mice [110]. Moreover, de Wit et al. observed an overflow of SFAs to the distal intestine in mice on a high-SFA diet, which, rather than obesity itself, reduced microbial diversity and increased the Firmicutes-to-Bacteroidetes ratio in the intestine. Such a typical obesity microbiota profile stimulated by SFAs favors the development of obesity and hepatic steatosis [103].
3.7. Fructose
Fructose has been utilized as artificial sweetener in many commercial soft drinks that are consumed largely by adolescents and in a variety of social circumstances. A number of studies have found that excess fructose consumption is involved in the pathogenesis of NAFLD and that upregulated de novo lipogenesis and inhibited fatty acid β-oxidation are distinct metabolic processes for the development of hepatic steatosis in individuals with NAFLD [20, 24, 111–113]. Further, Abdelmalek et al. observed that increased fructose consumption is associated with a higher fibrosis stage in patients with NAFLD, independent of age, sex, BMI, and total calorie intake [29]. Using a fructose-induced NAFLD mouse model, recent studies with metagenomics analysis found that fructose significantly decreased Bifidobacterium and Lactobacillus and tended to increase endotoxemia [114, 115]. Several probiotic bacterial strains of Lactobacillus protect mice from the development of high-fructose-induced NAFLD [116–118]. In addition, increased expression of TLRs has been implicated in the development of fructose-induced hepatic steatosis [119].
4. Genetic Background of NAFLD
Genomic variations that have a causative effect on the development of human diseases can be divided into two groups: ones in rare diseases and ones in common diseases. The former follow Mendelian inheritance patterns that are characterized by a single, highly penetrant but uncommon mutation in a specific gene being necessary and sufficient to cause the disease. The latter consist of causative mutations that are not subject to negative selection pressures, and disease susceptibility is due to the combined effects of multiple relatively common causative polymorphisms (minor allele frequency 1–5%) that are carried by affected individuals. Like most common diseases, NAFLD has been implicated in an inherited component to susceptibility, meaning that genetic variation does influence disease risk. As reviewed by Macaluso et al. in 2015 [120], dozens of genes with multiple polymorphisms have been discovered in genome-wide association studies (GWAS) that may be responsible for risk of NAFLD in certain populations. It is believed that as more large scale GWAS are complete, more genes could be identified. For instance, in early 2016 while we were preparing this review, a novel variant MBOAT7 rs641738 was reported to be associated with the development and severity of NAFLD in individuals of European descent [121]. Among all reported genes, only two of them (PNPLA3 and TM6SF2) have been identified as potential genetic modifiers in more than one large scale study [120, 122], which are the focus of our review. According to genotypes in those key genes and sensitivity to insulin, NAFLD patients can be categorized into different subpopulations (Figure 1).
4.1. PNPLA3 (Patatin-Like Phospholipase Domain Containing 3)
The PNPLA3 gene (adiponutrin) encodes a transmembrane polypeptide chain exhibiting triglyceride hydrolase activity [123], which is highly expressed on the endoplasmic reticulum and lipid membranes of hepatocytes and adipose tissue [124]. It is also reported that PNPLA3 is highly expressed in human stellate cells. The encoded protein has retinyl esterase activity and allows retinol secretion from hepatic stellate cells while the mutation causes intracellular retention of this compound [125–127]. As the first genome-wide association study with strong evidence for NAFLD, a report from Romeo et al. in 2008 showed that a genetic variant, an allele in PNPLA3 (rs738409[G], encoding Ile148Met), confers susceptibility to the disease in individuals of several western populations [128]. This genetic variant was associated with increased liver fat and hepatic inflammation and fibrosis. This finding has subsequently been reproduced with solid evidence as shown in a meta-analysis comprising 16 studies [129]. Compared with noncarriers, homozygous carriers of the variant had a 73% higher liver fat content, a 3.2-fold greater risk of high necroinflammatory scores, and a 3.2-fold greater risk of developing fibrosis. The association between the PNPLA3 variant and steatosis or severity of histological liver disease has been widely observed in the majority of subsequent genome-wide association studies [130] and several case-control studies, including those in Chinese, Korean, and Japanese populations [131–133]. It is worth noting that the link between PNPLA3 I148M variant and NAFLD is independent of metabolic syndrome (MS) and its features; that is, most of patients carrying this variant are not associated with obesity, diabetes, and atherogenic dyslipidemia, as demonstrated in the recent meta-analysis [129]. Furthermore, the PNPLA3 genotype seems to also influence steatosis development in patients with hepatitis B and hepatitis C and alcohol abuse, and it has been independently associated with the progression of hepatitis, including fibrosis, cirrhosis, and HCC occurrence [134–136]. The association between the PNPLA3 variant I148M and the risk of HCC development has been robustly validated in patients with NAFLD [137, 138], and it has been estimated that the homozygous carriers of the p.148M mutation carry a 12-fold increased HCC risk as compared to p.I148 homozygotes [139]. Finally, as described earlier, subpopulations of NAFLD patients with PNLA3 mutation are not associated with insulin resistance, a hallmark of metabolic syndrome. Collectively, it seems that a distinct entity might exist in which the PNPLA3 risk allele appears to be a major driver of disease progression in combination with viral infection, alcohol abuse, lifestyle (unhealthy diet and inactivity), and/or nonlifestyle (cryptogenic) causes, for example, PNPLA3-associated steatohepatitis (“PASH”) [140].
4.2. TM6SF2 (Transmembrane 6 Superfamily Member 2)
Another widely validated and intriguing genetic player in NAFLD is the nonsynonymous variant rs58542926 (c.449 C>T) within the TM6SF2 gene at the 19p13.11 locus, which encodes an E167K amino acid substitution. The role of variant E167K in TM6SF2 was first described by Kozlitina et al. [36] in an exome-wide association study in a multiethnic, population-based cohort, highlighting the association of the TM6SF2 variant with higher serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels—as surrogates for NASH—and with reduced plasma levels of triglycerides and low density lipoprotein- (LDL-) cholesterol. In addition, they performed a functional analysis for TM6SF2 in mouse models by silencing the gene via adeno-associated viral vectors. Silencing of the gene showed a 3-fold increase in hepatic triglycerides levels and a decrease in plasma levels of triglycerides, LDL- and high density lipoprotein- (HDL-) cholesterols, and very low density lipoprotein (VLDL). Overall, their results demonstrated that the TM6SF2 gene regulated hepatic triglyceride secretion and that the functional impairment of TM6SF2 promoted NAFLD. An association between the TM6SF2 rs58542926 SNP and the severity of liver disease has also been found in patients with biopsy-proven NAFLD in a recent study reported by Liu et al. [141]. More intriguingly, the E167K variant in TM6SF2 seems able to disconnect the risk of NAFLD/NASH progression from cardiovascular risk, which is supported mainly by the Dongiovanni et al. study [142] showing that 188 (13%) out of 1201 subjects who underwent liver biopsy for suspected NASH were carriers of the E167K variant and that these carriers had lower serum lipid levels than noncarriers, more severe steatosis, necroinflammation, ballooning, and fibrosis and were more likely to have NASH and advanced fibrosis after adjusting for metabolic factors and the PNPLA3 I148M risk variant. In addition, E167K carriers had lower risk of developing carotid plaque; and in Swedish obese subjects assessed for cardiovascular outcomes, E167K carriers had higher ALT and lower lipid levels but also a lower incidence of cardiovascular events. Consequently, carriers of the TM6SF2 E167K variant seem to be more at risk for progressive NASH, but at the same time they could be protected against cardiovascular diseases [143].
5. Interplay between Diet, Microbiota, and Host Genetics
One of the biggest lessons we learned from the metagenomic studies so far is that constitutive profiles of gut microbiota can determine liver pathology in response to a high-fat diet (HFD) in mice, reflecting a kind of interactive effect between diet and gut microbiota, that is, a net effect after the interplay. For instance, in a transplantation experiment [144], Le Roy et al. selected donor mice at first, based on their responses to a HFD. The “responders” developed hyperglycaemia and had a high plasma concentration of proinflammatory cytokines, and the “nonresponders” were normoglycaemic and had a lower level of systemic inflammation, although both developed comparable obesity on the HFD. Germ-free mice were then colonized with intestinal microbiota from either the responder or the nonresponder mice and then fed the same HFD. The responder-receiver (RR) group developed fasting hyperglycaemia and insulinaemia, whereas the nonresponder-receiver (NRR) group remained normoglycaemic. In contrast to NRR mice, RR mice developed hepatic macrovesicular steatosis, which was confirmed by a higher liver concentration of triglycerides and increased expression of genes involved in de novo lipogenesis. Pyrosequencing of the 16S ribosomal RNA genes revealed that RR and NRR mice had distinct gut microbiota including differences at the phylum, genera, and species levels. These results suggest that the gut microbiota can contribute to the development of NAFLD, independent of obesity but acting like a constitutional background of a host organ system. The interrelationship between diet, gut microbiota, and host genetics has been unraveled further in a recent study reported by Ussar and coworkers [145]. In this study, they utilized three commonly used inbred strains of mice: obesity/diabetes-prone C57Bl/6J, obesity/diabetes-resistant 129S1/SvImJ, and obesity-prone but diabetes-resistant 129S6/SvEvTac mice. Analysis of metabolic parameters and gut microbiota in all strains and their environmentally normalized derivatives revealed strong interactions between microbiota, diet, breeding site, and metabolic phenotype. More intriguingly, environmental reprogramming of microbiota resulted in obesity-prone 129S6/SvEvTac mice becoming obesity resistant. This study suggests that development of obesity/metabolic syndrome is the result of interactions between gut microbiota, host genetics, and diet.
6. Conclusions
NAFLD is best considered a multietiology disease trait, meaning that it is not caused by a single gene mutation genetically and is not associated with only a single factor environmentally; but it is the outcome of genetic variant-environmental factor interplay determining disease phenotype and progression. The genetic variants in PNPLA3 and TM6SF2 are only responsible for ~50% of NAFLD patients [120], and majority of PNPLA3-associated NAFLD patients are not obese and have no insulin resistance and its related diabetes and cardiovascular diseases [140]. In fact, like many common diseases, NAFLD is polygenic, where the heritable component to susceptibility variously accounts for up to 30–50% of relative risk [130]. Moreover, individual environmental factors, particularly the specific diets, interact with gut microbiota up front before a final beneficial or damaging signal is sent. Whether environmental factors, including lifestyle, are the cause of NAFLD will be steered by the interaction with the host genetics as well as the constitutional profile of gut microbiota. Thus, careful, multifaceted study designs are warranted in future analysis in order to “catch” the true causes to NAFLD.
Acknowledgments
Authors thank Dr. Richard D. Head, the Director of Genome Technology Access Center at Department of Genetics in Washington University School of Medicine in St. Louis, Missouri, United States, for his guidance and support of this work.
Competing Interests
No competing interests are declared by the authors.
References
- 1.Demir M., Lang S., Steffen H. Nonalcoholic fatty liver disease: current status and future directions. Journal of Digestive Diseases. 2015;16(10):541–557. doi: 10.1111/1751-2980.12291. [DOI] [PubMed] [Google Scholar]
- 2.Tiniakos D. G., Vos M. B., Brunt E. M. Nonalcoholic fatty liver disease: pathology and pathogenesis. Annual Review of Pathology: Mechanisms of Disease. 2010;5:145–171. doi: 10.1146/annurev-pathol-121808-102132. [DOI] [PubMed] [Google Scholar]
- 3.Abu-Shanab A., Quigley E. M. M. The role of the gut microbiota in nonalcoholic fatty liver disease. Nature Reviews Gastroenterology and Hepatology. 2010;7(12):691–701. doi: 10.1038/nrgastro.2010.172. [DOI] [PubMed] [Google Scholar]
- 4.Angulo P. Long-term mortality in nonalcoholic fatty liver disease: is liver histology of any prognostic significance? Hepatology. 2010;51(2):373–375. doi: 10.1002/hep.23521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soderberg C., Stål P., Askling J., et al. Decreased survival of subjects with elevated liver function tests during a 28-year follow-up. Hepatology. 2010;51(2):595–602. doi: 10.1002/hep.23314. [DOI] [PubMed] [Google Scholar]
- 6.Zhou Y., Llauradó G., Orešič M., Hyötyläinen T., Orho-Melander M., Yki-Järvinen H. Circulating triacylglycerol signatures and insulin sensitivity in NAFLD associated with the E167K variant in TM6SF2. Journal of Hepatology. 2015;62(3):657–663. doi: 10.1016/j.jhep.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 7.Lomonaco R., Ortiz-Lopez C., Orsak B., et al. Effect of adipose tissue insulin resistance on metabolic parameters and liver histology in obese patients with nonalcoholic fatty liver disease. Hepatology. 2012;55(5):1389–1397. doi: 10.1002/hep.25539. [DOI] [PubMed] [Google Scholar]
- 8.Pagano G., Pacini G., Musso G., et al. Nonalcoholic steatohepatitis, insulin resistance, and metabolic syndrome: further evidence for an etiologic association. Hepatology. 2002;35(2):367–372. doi: 10.1053/jhep.2002.30690. [DOI] [PubMed] [Google Scholar]
- 9.Sanyal A. J., Campbell-Sargent C., Mirshahi F., et al. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology. 2001;120(5):1183–1192. doi: 10.1053/gast.2001.23256. [DOI] [PubMed] [Google Scholar]
- 10.Dumas M.-E., Kinross J., Nicholson J. K. Metabolic phenotyping and systems biology approaches to understanding metabolic syndrome and fatty liver disease. Gastroenterology. 2014;146(1):46–62. doi: 10.1053/j.gastro.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Schuppan D., Schattenberg J. M. Non-alcoholic steatohepatitis: pathogenesis and novel therapeutic approaches. Journal of Gastroenterology and Hepatology. 2013;28(supplement 1):68–76. doi: 10.1111/jgh.12212. [DOI] [PubMed] [Google Scholar]
- 12.Day C. P., James O. F. W. Steatohepatitis: a tale of two ‘hits’? Gastroenterology. 1998;114(4 I):842–845. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 13.Berson A., De Beco V., Letteron P., et al. Steatohepatitis-inducing drugs cause mitochondrial dysfunction and lipid peroxidation in rat hepatocytes. Gastroenterology. 1998;114(4):764–774. doi: 10.1016/s0016-5085(98)70590-6. [DOI] [PubMed] [Google Scholar]
- 14.Tilg H., Moschen A. R. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology. 2010;52(5):1836–1846. doi: 10.1002/hep.24001. [DOI] [PubMed] [Google Scholar]
- 15.Johnson A. R., Milner J. J., Makowski L. The inflammation highway: metabolism accelerates inflammatory traffic in obesity. Immunological Reviews. 2012;249(1):218–238. doi: 10.1111/j.1600-065x.2012.01151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matherly S. C., Puri P. Mechanisms of simple hepatic steatosis: not so simple after all. Clinics in Liver Disease. 2012;16(3):505–524. doi: 10.1016/j.cld.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 17.Barbuio R., Milanski M., Bertolo M. B., Saad M. J., Velloso L. A. Infliximab reverses steatosis and improves insulin signal transduction in liver of rats fed a high-fat diet. Journal of Endocrinology. 2007;194(3):539–550. doi: 10.1677/JOE-07-0234. [DOI] [PubMed] [Google Scholar]
- 18.Tilg H. The role of cytokines in non-alcoholic fatty liver disease. Digestive Diseases. 2010;28(1):179–185. doi: 10.1159/000282083. [DOI] [PubMed] [Google Scholar]
- 19.Hasenour C. M., Berglund E. D., Wasserman D. H. Emerging role of AMP-activated protein kinase in endocrine control of metabolism in the liver. Molecular and Cellular Endocrinology. 2013;366(2):152–162. doi: 10.1016/j.mce.2012.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ouyang X., Cirillo P., Sautin Y., et al. Fructose consumption as a risk factor for non-alcoholic fatty liver disease. Journal of Hepatology. 2008;48(6):993–999. doi: 10.1016/j.jhep.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Assy N., Nasser G., Kamayse I., et al. Soft drink consumption linked with fatty liver in the absence of traditional risk factors. Canadian Journal of Gastroenterology. 2008;22(10):811–816. doi: 10.1155/2008/810961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zelber-Sagi S., Nitzan-Kaluski D., Goldsmith R., et al. Long term nutritional intake and the risk for non-alcoholic fatty liver disease (NAFLD): a population based study. Journal of Hepatology. 2007;47(5):711–717. doi: 10.1016/j.jhep.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 23.Machado M. V., Ravasco P., Jesus L., et al. Blood oxidative stress markers in non-alcoholic steatohepatitis and how it correlates with diet. Scandinavian Journal of Gastroenterology. 2008;43(1):95–102. doi: 10.1080/00365520701559003. [DOI] [PubMed] [Google Scholar]
- 24.Lambert J. E., Ramos-Roman M. A., Browning J. D., Parks E. J. Increased de novo lipogenesis is a distinct characteristic of individuals with nonalcoholic fatty liver disease. Gastroenterology. 2014;146(3):726–735. doi: 10.1053/j.gastro.2013.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hudgins L. C., Parker T. S., Levine D. M., Hellerstein M. K. A dual sugar challenge test for lipogenic sensitivity to dietary fructose. Journal of Clinical Endocrinology and Metabolism. 2011;96(3):861–868. doi: 10.1210/jc.2010-2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lecoultre V., Egli L., Carrel G., et al. Effects of fructose and glucose overfeeding on hepatic insulin sensitivity and intrahepatic lipids in healthy humans. Obesity. 2013;21(4):782–785. doi: 10.1002/oby.20377. [DOI] [PubMed] [Google Scholar]
- 27.Parks E. J., Skokan L. E., Timlin M. T., Dingfelder C. S. Dietary sugars stimulate fatty acid synthesis in adults. Journal of Nutrition. 2008;138(6):1039–1046. doi: 10.1093/jn/138.6.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stanhope K. L., Schwarz J. M., Keim N. L., et al. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. The Journal of Clinical Investigation. 2009;119(5):1322–1334. doi: 10.1172/jci37385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abdelmalek M. F., Suzuki A., Guy C., et al. Increased fructose consumption is associated with fibrosis severity in patients with nonalcoholic fatty liver disease. Hepatology. 2010;51(6):1961–1971. doi: 10.1002/hep.23535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin J., Yang R., Tarr P. T., et al. Hyperlipidemic effects of dietary saturated fats mediated through PGC-1β coactivation of SREBP. Cell. 2005;120(2):261–273. doi: 10.1016/j.cell.2004.11.043. [DOI] [PubMed] [Google Scholar]
- 31.Mancina R. M., Matikainen N., Maglio C., et al. Paradoxical dissociation between hepatic fat content and de novo lipogenesis due to PNPLA3 sequence variant. Journal of Clinical Endocrinology and Metabolism. 2015;100(5):E821–E825. doi: 10.1210/jc.2014-4464. [DOI] [PubMed] [Google Scholar]
- 32.Tilg H., Moschen A. R. Inflammatory mechanisms in the regulation of insulin resistance. Molecular Medicine. 2008;14(3-4):222–231. doi: 10.2119/2007-00119.tilg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gastaldelli A., Cusi K., Pettiti M., et al. Relationship between hepatic/visceral fat and hepatic insulin resistance in nondiabetic and type 2 diabetic subjects. Gastroenterology. 2007;133(2):496–506. doi: 10.1053/j.gastro.2007.04.068. [DOI] [PubMed] [Google Scholar]
- 34.Sevastianova K., Kotronen A., Gastaldelli A., et al. Genetic variation in PNPLA3 (adiponutrin) confers sensitivity to weight loss—induced decrease in liver fat in humans. The American Journal of Clinical Nutrition. 2011;94(1):104–111. doi: 10.3945/ajcn.111.012369. [DOI] [PubMed] [Google Scholar]
- 35.Kantartzis K., Peter A., Machicao F., et al. Dissociation between fatty liver and insulin resistance in humans carrying a variant of the patatin-like phospholipase 3 gene. Diabetes. 2009;58(11):2616–2623. doi: 10.2337/db09-0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kozlitina J., Smagris E., Stender S., et al. Exome-wide association study identifies a TM6SF2 variant that confers susceptibility to nonalcoholic fatty liver disease. Nature Genetics. 2014;46(4):352–356. doi: 10.1038/ng.2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kantartzis K., Machicao F., Machann J., et al. The DGAT2 gene is a candidate for the dissociation between fatty liver and insulin resistance in humans. Clinical Science. 2009;116(6):531–537. doi: 10.1042/CS20080306. [DOI] [PubMed] [Google Scholar]
- 38.Visser M. E., Lammers N. M., Nederveen A. J., et al. Hepatic steatosis does not cause insulin resistance in people with familial hypobetalipoproteinaemia. Diabetologia. 2011;54(8):2113–2121. doi: 10.1007/s00125-011-2157-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Amaro A., Fabbrini E., Kars M., et al. Dissociation between intrahepatic triglyceride content and insulin resistance in familial hypobetalipoproteinemia. Gastroenterology. 2010;139(1):149–153. doi: 10.1053/j.gastro.2010.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Palmer C. N. A., Maglio C., Pirazzi C., et al. Paradoxical lower serum triglyceride levels and higher type 2 diabetes mellitus susceptibility in obese individuals with the PNPLA3 148M variant. PLoS ONE. 2012;7(6) doi: 10.1371/journal.pone.0039362.e39362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gaggini M., Morelli M., Buzzigoli E., DeFronzo R. A., Bugianesi E., Gastaldelli A. Non-alcoholic fatty liver disease (NAFLD) and its connection with insulin resistance, dyslipidemia, atherosclerosis and coronary heart disease. Nutrients. 2013;5(5):1544–1560. doi: 10.3390/nu5051544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fuchs M., Sanyal A. J. Lipotoxicity in NASH. Journal of Hepatology. 2012;56(1):291–293. doi: 10.1016/j.jhep.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 43.Simonen P., Kotronen A., Hallikainen M., et al. Cholesterol synthesis is increased and absorption decreased in non-alcoholic fatty liver disease independent of obesity. Journal of Hepatology. 2011;54(1):153–159. doi: 10.1016/j.jhep.2010.05.037. [DOI] [PubMed] [Google Scholar]
- 44.Arguello G., Balboa E., Arrese M., Zanlungo S. Recent insights on the role of cholesterol in non-alcoholic fatty liver disease. Biochimica et Biophysica Acta (BBA)—Molecular Basis of Disease. 2015;1852(9):1765–1778. doi: 10.1016/j.bbadis.2015.05.015. [DOI] [PubMed] [Google Scholar]
- 45.Malhi H., Bronk S. F., Werneburg N. W., Gores G. J. Free fatty acids induce JNK-dependent hepatocyte lipoapoptosis. The Journal of Biological Chemistry. 2006;281(17):12093–12101. doi: 10.1074/jbc.m510660200. [DOI] [PubMed] [Google Scholar]
- 46.Sharifnia T., Antoun J., Verriere T. G. C., et al. Hepatic TLR4 signaling in obese NAFLD. American Journal of Physiology—Gastrointestinal and Liver Physiology. 2015;309(4):G270–G278. doi: 10.1152/ajpgi.00304.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Serviddio G., Bellanti F., Tamborra R., et al. Alterations of hepatic ATP homeostasis and respiratory chain during development of non-alcoholic steatohepatitis in a rodent model. European Journal of Clinical Investigation. 2008;38(4):245–252. doi: 10.1111/j.1365-2362.2008.01936.x. [DOI] [PubMed] [Google Scholar]
- 48.Jiang Y., Zhao M., An W. Increased hepatic apoptosis in high-fat diet-induced NASH in rats may be associated with downregulation of hepatic stimulator substance. Journal of Molecular Medicine. 2011;89(12):1207–1217. doi: 10.1007/s00109-011-0790-y. [DOI] [PubMed] [Google Scholar]
- 49.Jin X., Yang Y.-D., Chen K., et al. HDMCP uncouples yeast mitochondrial respiration and alleviates steatosis in L02 and hepG2 cells by decreasing ATP and H2O2 levels: a novel mechanism for NAFLD. Journal of Hepatology. 2009;50(5):1019–1028. doi: 10.1016/j.jhep.2008.10.034. [DOI] [PubMed] [Google Scholar]
- 50.Day C. P. Pathogenesis of steatohepatitis. Best Practice & Research Clinical Gastroenterology. 2002;16(5):663–678. doi: 10.1053/bega.2002.0333. [DOI] [PubMed] [Google Scholar]
- 51.Sadek H. A., Szweda P. A., Szweda L. I. Modulation of mitochondrial complex I activity by reversible Ca2+ and NADH mediated superoxide anion dependent inhibition. Biochemistry. 2004;43(26):8494–8502. doi: 10.1021/bi049803f. [DOI] [PubMed] [Google Scholar]
- 52.Kujoth C. C., Hiona A., Pugh T. D., et al. Medicine: mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science. 2005;309(5733):481–484. doi: 10.1126/science.1112125. [DOI] [PubMed] [Google Scholar]
- 53.Day C. P. Non-alcoholic fatty liver disease: current concepts and management strategies. Clinical Medicine. 2006;6(1):19–25. doi: 10.7861/clinmedicine.6-1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Koek G. H., Liedorp P. R., Bast A. The role of oxidative stress in non-alcoholic steatohepatitis. Clinica Chimica Acta. 2011;412(15-16):1297–1305. doi: 10.1016/j.cca.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 55.Nowicka B., Kruk J. Occurrence, biosynthesis and function of isoprenoid quinones. Biochimica et Biophysica Acta (BBA)—Bioenergetics. 2010;1797(9):1587–1605. doi: 10.1016/j.bbabio.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 56.Pessayre D. Role of mitochondria in non-alcoholic fatty liver disease. Journal of Gastroenterology and Hepatology. 2007;22(supplement 1):S20–S27. doi: 10.1111/j.1440-1746.2006.04640.x. [DOI] [PubMed] [Google Scholar]
- 57.Novo E., Busletta C., Bonzo L. V. D., et al. Intracellular reactive oxygen species are required for directional migration of resident and bone marrow-derived hepatic pro-fibrogenic cells. Journal of Hepatology. 2011;54(5):964–974. doi: 10.1016/j.jhep.2010.09.022. [DOI] [PubMed] [Google Scholar]
- 58.Hotamisligil G. S. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010;140(6):900–917. doi: 10.1016/j.cell.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Özcan U., Cao Q., Yilmaz E., et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306(5695):457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 60.Ron D., Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nature Reviews Molecular Cell Biology. 2007;8(7):519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 61.Lee J., Ozcan U. Unfolded protein response signaling and metabolic diseases. The Journal of Biological Chemistry. 2014;289(3):1203–1211. doi: 10.1074/jbc.r113.534743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nature Reviews Molecular Cell Biology. 2012;13(2):89–102. doi: 10.1038/nrm3270. [DOI] [PubMed] [Google Scholar]
- 63.Zambo V., Simon-Szabo L., Szelenyi P., et al. Lipotoxicity in the liver. World Journal of Hepatology. 2013;5(10):550–557. doi: 10.4254/wjh.v5.i10.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Langendijk P. S., Schut F., Jansen G. J., et al. Quantitative fluorescence in situ hybridization of Bifidobacterium spp. with genus-specific 16S rRNA-targeted probes and its application in fecal samples. Applied and Environmental Microbiology. 1995;61(8):3069–3075. doi: 10.1128/aem.61.8.3069-3075.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Musso G., Cassader M., Cohney S., et al. Emerging liver-kidney interactions in nonalcoholic fatty liver disease. Trends in Molecular Medicine. 2015;21(10):645–662. doi: 10.1016/j.molmed.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 66.Kirpich I. A., Marsano L. S., McClain C. J. Gut-liver axis, nutrition, and non-alcoholic fatty liver disease. Clinical Biochemistry. 2015;48(13-14):923–930. doi: 10.1016/j.clinbiochem.2015.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lin P., Lu J., Wang Y., et al. Naturally occurring stilbenoid TSG reverses non-alcoholic fatty liver diseases via gut-liver axis. PLoS ONE. 2015;10(10) doi: 10.1371/journal.pone.0140346.e0140346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Turnbaugh P. J., Ley R. E., Mahowald M. A., Magrini V., Mardis E. R., Gordon J. I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 69.Schwiertz A., Taras D., Schäfer K., et al. Microbiota and SCFA in lean and overweight healthy subjects. Obesity. 2010;18(1):190–195. doi: 10.1038/oby.2009.167. [DOI] [PubMed] [Google Scholar]
- 70.Ley R. E., Bäckhed F., Turnbaugh P., Lozupone C. A., Knight R. D., Gordon J. I. Obesity alters gut microbial ecology. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(31):11070–11075. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ley R. E., Turnbaugh P. J., Klein S., Gordon J. I. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444(7122):1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 72.den Besten G., van Eunen K., Groen A. K., Venema K., Reijngoud D.-J., Bakker B. M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. Journal of Lipid Research. 2013;54(9):2325–2340. doi: 10.1194/jlr.r036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.den Besten G., Bleeker A., Gerding A., et al. Short-chain fatty acids protect against high-fat diet–induced obesity via a PPARγ-dependent switch from lipogenesis to fat oxidation. Diabetes. 2015;64(7):2398–2408. doi: 10.2337/db14-1213. [DOI] [PubMed] [Google Scholar]
- 74.Buchman A. L., Dubin M. D., Moukarzel A. A., et al. Choline deficiency: a cause of hepatic steatosis during parenteral nutrition that can be reversed with intravenous choline supplementation. Hepatology. 1995;22(5):1399–1403. doi: 10.1016/0270-9139(95)90143-4. [DOI] [PubMed] [Google Scholar]
- 75.Dumas M.-E., Barton R. H., Toye A., et al. Metabolic profiling reveals a contribution of gut microbiota to fatty liver phenotype in insulin-resistant mice. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(33):12511–12516. doi: 10.1073/pnas.0601056103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Spencer M. D., Hamp T. J., Reid R. W., Fischer L. M., Zeisel S. H., Fodor A. A. Association between composition of the human gastrointestinal microbiome and development of fatty liver with choline deficiency. Gastroenterology. 2011;140(3):976–986. doi: 10.1053/j.gastro.2010.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Russell D. W. The enzymes, regulation, and genetics of bile acid synthesis. Annual Review of Biochemistry. 2003;72:137–174. doi: 10.1146/annurev.biochem.72.121801.161712. [DOI] [PubMed] [Google Scholar]
- 78.Ridlon J. M., Kang D.-J., Hylemon P. B. Bile salt biotransformations by human intestinal bacteria. Journal of Lipid Research. 2006;47(2):241–259. doi: 10.1194/jlr.R500013-JLR200. [DOI] [PubMed] [Google Scholar]
- 79.Sayin S. I., Wahlström A., Felin J., et al. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metabolism. 2013;17(2):225–235. doi: 10.1016/j.cmet.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 80.Schaap F. G., Trauner M., Jansen P. L. M. Bile acid receptors as targets for drug development. Nature Reviews Gastroenterology and Hepatology. 2014;11(1):55–67. doi: 10.1038/nrgastro.2013.151. [DOI] [PubMed] [Google Scholar]
- 81.Li Y., Jadhav K., Zhang Y. Bile acid receptors in non-alcoholic fatty liver disease. Biochemical Pharmacology. 2013;86(11):1517–1524. doi: 10.1016/j.bcp.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Inagaki T., Choi M., Moschetta A., et al. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metabolism. 2005;2(4):217–225. doi: 10.1016/j.cmet.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 83.McMahan R. H., Wang X. X., Cheng L. L., et al. Bile acid receptor activation modulates hepatic monocyte activity and improves nonalcoholic fatty liver disease. The Journal of Biological Chemistry. 2013;288(17):11761–11770. doi: 10.1074/jbc.m112.446575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cope K., Risby T., Diehl A. M. Increased gastrointestinal ethanol production in obese mice: implications for fatty liver disease pathogenesis. Gastroenterology. 2000;119(5):1340–1347. doi: 10.1053/gast.2000.19267. [DOI] [PubMed] [Google Scholar]
- 85.Nair S., Cope K., Terence R. H., Diehl A. M. Obesity and female gender increase breath ethanol concentration: potential implications for the pathogenesis of nonalcoholic steatohepatitis. American Journal of Gastroenterology. 2001;96(4):1200–1204. doi: 10.1016/s0002-9270(01)02265-1. [DOI] [PubMed] [Google Scholar]
- 86.Zhu L., Baker S. S., Gill C., et al. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: a connection between endogenous alcohol and NASH. Hepatology. 2013;57(2):601–609. doi: 10.1002/hep.26093. [DOI] [PubMed] [Google Scholar]
- 87.Baker S. S., Baker R. D., Liu W., Nowak N. J., Zhu L. Role of alcohol metabolism in non-alcoholic steatohepatitis. PLoS ONE. 2010;5(3) doi: 10.1371/journal.pone.0009570.e9570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Engstler A. J., Aumiller T., Degen C., et al. Insulin resistance alters hepatic ethanol metabolism: studies in mice and children with non-alcoholic fatty liver disease. Gut. 2015 doi: 10.1136/gutjnl-2014-308379. [DOI] [PubMed] [Google Scholar]
- 89.de Medeiros I. C., de Lima J. G. Is nonalcoholic fatty liver disease an endogenous alcoholic fatty liver disease?—a mechanistic hypothesis. Medical Hypotheses. 2015;85(2):148–152. doi: 10.1016/j.mehy.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 90.Artis D. Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nature Reviews Immunology. 2008;8(6):411–420. doi: 10.1038/nri2316. [DOI] [PubMed] [Google Scholar]
- 91.Yang S. Q., Lin H. Z., Lane M. D., Clemens M., Diehl A. M. Obesity increases sensitivity to endotoxin liver injury: implications for the pathogenesis of steatohepatitis. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(6):2557–2562. doi: 10.1073/pnas.94.6.2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Brun P., Castagliuolo I., Di Leo V., et al. Increased intestinal permeability in obese mice: new evidence in the pathogenesis of nonalcoholic steatohepatitis. American Journal of Physiology—Gastrointestinal and Liver Physiology. 2007;292(2):G518–G525. doi: 10.1152/ajpgi.00024.2006. [DOI] [PubMed] [Google Scholar]
- 93.Miele L., Valenza V., La Torre G., et al. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology. 2009;49(6):1877–1887. doi: 10.1002/hep.22848. [DOI] [PubMed] [Google Scholar]
- 94.Laugerette F., Vors C., Géloën A., et al. Emulsified lipids increase endotoxemia: possible role in early postprandial low-grade inflammation. Journal of Nutritional Biochemistry. 2011;22(1):53–59. doi: 10.1016/j.jnutbio.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 95.Yuan J., Baker S. S., Liu W., et al. Endotoxemia unrequired in the pathogenesis of pediatric nonalcoholic steatohepatitis. Journal of Gastroenterology and Hepatology. 2014;29(6):1292–1298. doi: 10.1111/jgh.12510. [DOI] [PubMed] [Google Scholar]
- 96.Medzhitov R. Toll-like receptors and innate immunity. Nature Reviews Immunology. 2001;1(2):135–145. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- 97.Tsukumo D. M. L., Carvalho-Filho M. A., Carvalheira J. B. C., et al. Loss-of-function mutation in toll-like receptor 4 prevents diet-induced obesity and insulin resistance. Diabetes. 2007;56(8):1986–1998. doi: 10.2337/db06-1595. [DOI] [PubMed] [Google Scholar]
- 98.Miura K., Kodama Y., Inokuchi S., et al. Toll-like receptor 9 promotes steatohepatitis by induction of interleukin-1β in mice. Gastroenterology. 2010;139(1):323–334.e7. doi: 10.1053/j.gastro.2010.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Vijay-Kumar M., Aitken J. D., Carvalho F. A., et al. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science. 2010;328(5975):228–231. doi: 10.1126/science.1179721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wlodarska M., Thaiss C. A., Nowarski R., et al. NLRP6 inflammasome orchestrates the colonic host-microbial interface by regulating goblet cell mucus secretion. Cell. 2014;156(5):1045–1059. doi: 10.1016/j.cell.2014.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Henao-Mejia J., Elinav E., Jin C., et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature. 2012;482(7384):179–185. doi: 10.1038/nature10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Leamy A. K., Egnatchik R. A., Young J. D. Molecular mechanisms and the role of saturated fatty acids in the progression of non-alcoholic fatty liver disease. Progress in Lipid Research. 2013;52(1):165–174. doi: 10.1016/j.plipres.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.de Wit N., Derrien M., Bosch-Vermeulen H., et al. Saturated fat stimulates obesity and hepatic steatosis and affects gut microbiota composition by an enhanced overflow of dietary fat to the distal intestine. American Journal of Physiology—Gastrointestinal and Liver Physiology. 2012;303(5):G589–G599. doi: 10.1152/ajpgi.00488.2011. [DOI] [PubMed] [Google Scholar]
- 104.Ronis M. J. J., Korourian S., Zipperman M., Hakkak R., Badger T. M. Dietary saturated fat reduces alcoholic hepatotoxicity in rats by altering fatty acid metabolism and membrane composition. Journal of Nutrition. 2004;134(4):904–912. doi: 10.1093/jn/134.4.904. [DOI] [PubMed] [Google Scholar]
- 105.Nanji A. A., Jokelainen K., Tipoe G. L., Rahemtulla A., Dannenberg A. J. Dietary saturated fatty acids reverse inflammatory and fibrotic changes in rat liver despite continued ethanol administration. Journal of Pharmacology and Experimental Therapeutics. 2001;299(2):638–644. [PubMed] [Google Scholar]
- 106.Kirpich I. A., Feng W., Wang Y., et al. The type of dietary fat modulates intestinal tight junction integrity, gut permeability, and hepatic toll-like receptor expression in a mouse model of alcoholic liver disease. Alcoholism: Clinical and Experimental Research. 2012;36(5):835–846. doi: 10.1111/j.1530-0277.2011.01673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wei Y., Wang D., Topczewski F., Pagliassotti M. J. Saturated fatty acids induce endoplasmic reticulum stress and apoptosis independently of ceramide in liver cells. American Journal of Physiology—Endocrinology and Metabolism. 2006;291(2):E275–E281. doi: 10.1152/ajpendo.00644.2005. [DOI] [PubMed] [Google Scholar]
- 108.Wang D., Wei Y., Pagliassotti M. J. Saturated fatty acids promote endoplasmic reticulum stress and liver injury in rats with hepatic steatosis. Endocrinology. 2006;147(2):943–951. doi: 10.1210/en.2005-0570. [DOI] [PubMed] [Google Scholar]
- 109.Solinas G., Naugler W., Galimi F., Lee M.-S., Karin M. Saturated fatty acids inhibit induction of insulin gene transcription by JNK-mediated phosphorylation of insulin-receptor substrates. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(44):16454–16459. doi: 10.1073/pnas.0607626103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chen P., Torralba M., Tan J., et al. Supplementation of saturated long-chain fatty acids maintains intestinal eubiosis and reduces ethanol-induced liver injury in mice. Gastroenterology. 2015;148(1):203–214.e16. doi: 10.1053/j.gastro.2014.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Moore J. B., Gunn P. J., Fielding B. A. The role of dietary sugars and de novo lipogenesis in non-alcoholic fatty liver disease. Nutrients. 2014;6(12):5679–5703. doi: 10.3390/nu6125679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.O'Sullivan T. A., Oddy W. H., Bremner A. P., et al. Lower fructose intake may help protect against development of nonalcoholic fatty liver in adolescents with obesity. Journal of Pediatric Gastroenterology and Nutrition. 2014;58(5):624–631. doi: 10.1097/mpg.0000000000000267. [DOI] [PubMed] [Google Scholar]
- 113.Mager D. R., Patterson C., So S., Rogenstein C. D., Wykes L. J., Roberts E. A. Dietary and physical activity patterns in children with fatty liver. European Journal of Clinical Nutrition. 2010;64(6):628–635. doi: 10.1038/ejcn.2010.35. [DOI] [PubMed] [Google Scholar]
- 114.Jegatheesan P., Beutheu S., Ventura G., et al. Effect of specific amino acids on hepatic lipid metabolism in fructose-induced non-alcoholic fatty liver disease. Clinical Nutrition. 2016;35(1):175–182. doi: 10.1016/j.clnu.2015.01.021. [DOI] [PubMed] [Google Scholar]
- 115.Jin R., Willment A., Patel S. S., et al. Fructose induced endotoxemia in pediatric nonalcoholic fatty liver disease. International Journal of Hepatology. 2014;2014:8. doi: 10.1155/2014/560620.560620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ritze Y., Bárdos G., Claus A., et al. Lactobacillus rhamnosus GG Protects against non-alcoholic fatty liver disease in mice. PLoS ONE. 2014;9(1) doi: 10.1371/journal.pone.0080169.e80169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wagnerberger S., Spruss A., Kanuri G., et al. Lactobacillus casei Shirota protects from fructose-induced liver steatosis: a mouse model. Journal of Nutritional Biochemistry. 2013;24(3):531–538. doi: 10.1016/j.jnutbio.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 118.Hsieh F.-C., Lee C.-L., Chai C.-Y., Chen W.-T., Lu Y.-C., Wu C.-S. Oral administration of Lactobacillus reuteri GMNL-263 improves insulin resistance and ameliorates hepatic steatosis in high fructose-fed rats. Nutrition and Metabolism. 2013;10(1, article 35) doi: 10.1186/1743-7075-10-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wagnerberger S., Spruss A., Kanuri G., et al. Toll-like receptors 1-9 are elevated in livers with fructose-induced hepatic steatosis. British Journal of Nutrition. 2012;107(12):1727–1738. doi: 10.1017/S0007114511004983. [DOI] [PubMed] [Google Scholar]
- 120.Macaluso F. S., Maida M., Petta S. Genetic background in nonalcoholic fatty liver disease: a comprehensive review. World Journal of Gastroenterology. 2015;21(39):11088–11111. doi: 10.3748/wjg.v21.i39.11088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mancina R. M., Dongiovanni P., Petta S., et al. The MBOAT7-TMC4 variant rs641738 increases risk of nonalcoholic fatty liver disease in individuals of European descent. Gastroenterology. 2016;150(5):1219–1230. doi: 10.1053/j.gastro.2016.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Anstee Q. M., Day C. P. The genetics of nonalcoholic fatty liver disease: spotlight on PNPLA3 and TM6SF2 . Seminars in Liver Disease. 2015;35(3):270–290. doi: 10.1055/s-0035-1562947. [DOI] [PubMed] [Google Scholar]
- 123.Pingitore P., Pirazzi C., Mancina R. M., et al. Recombinant PNPLA3 protein shows triglyceride hydrolase activity and its I148M mutation results in loss of function. Biochimica et Biophysica Acta—Molecular and Cell Biology of Lipids. 2014;1841(4):574–580. doi: 10.1016/j.bbalip.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 124.He S., McPhaul C., Li J. Z., et al. A sequence variation (I148M) in PNPLA3 associated with nonalcoholic fatty liver disease disrupts triglyceride hydrolysis. The Journal of Biological Chemistry. 2010;285(9):6706–6715. doi: 10.1074/jbc.m109.064501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mondul A., Mancina R. M., Merlo A., et al. PNPLA3 I148M variant influences circulating retinol in adults with nonalcoholic fatty liver disease or obesity. Journal of Nutrition. 2015;145(8):1687–1691. doi: 10.3945/jn.115.210633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kovarova M., Konigsrainer I., Konigsrainer A., et al. The genetic variant I148M in PNPLA3 is associated with increased hepatic retinyl-palmitate storage in humans. The Journal of Clinical Endocrinology & Metabolism. 2015;100(12):E1568–E1574. doi: 10.1210/jc.2015-2978. [DOI] [PubMed] [Google Scholar]
- 127.Pirazzi C., Valenti L., Motta B. M., et al. PNPLA3 has retinyl-palmitate lipase activity in human hepatic stellate cells. Human Molecular Genetics. 2014;23(15):4077–4085. doi: 10.1093/hmg/ddu121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Romeo S., Kozlitina J., Xing C., et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nature Genetics. 2008;40(12):1461–1465. doi: 10.1038/ng.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sookoian S., Pirola C. J. Meta-analysis of the influence of I148M variant of patatin-like phospholipase domain containing 3 gene (PNPLA3) on the susceptibility and histological severity of nonalcoholic fatty liver disease. Hepatology. 2011;53(6):1883–1894. doi: 10.1002/hep.24283. [DOI] [PubMed] [Google Scholar]
- 130.Anstee Q. M., Day C. P. The genetics of NAFLD. Nature Reviews Gastroenterology and Hepatology. 2013;10(11):645–655. doi: 10.1038/nrgastro.2013.182. [DOI] [PubMed] [Google Scholar]
- 131.Li Y., Xing C., Tian Z., Ku H.-C. Genetic variant I148M in PNPLA3 is associated with the ultrasonography-determined steatosis degree in a Chinese population. BMC Medical Genetics. 2012;13, article 113 doi: 10.1186/1471-2350-13-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lee S. S., Byoun Y.-S., Jeong S.-H., et al. Role of the PNPLA3 I148M polymorphism in nonalcoholic fatty liver disease and fibrosis in korea. Digestive Diseases and Sciences. 2014;59(12):2967–2974. doi: 10.1007/s10620-014-3279-z. [DOI] [PubMed] [Google Scholar]
- 133.Kawaguchi T., Sumida Y., Umemura A., et al. Genetic polymorphisms of the human PNPLA3 gene are strongly associated with severity of non-alcoholic fatty liver disease in Japanese. PLoS ONE. 2012;7(6) doi: 10.1371/journal.pone.0038322.e38322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Petta S., Vanni E., Bugianesi E., et al. PNPLA3 rs738409 I748M is associated with steatohepatitis in 434 non-obese subjects with hepatitis C. Alimentary Pharmacology and Therapeutics. 2015;41(10):939–948. doi: 10.1111/apt.13169. [DOI] [PubMed] [Google Scholar]
- 135.Viganò M., Valenti L., Lampertico P., et al. Patatin-like phospholipase domain-containing 3 I148M affects liver steatosis in patients with chronic hepatitis B. Hepatology. 2013;58(4):1245–1252. doi: 10.1002/hep.26445. [DOI] [PubMed] [Google Scholar]
- 136.Trepo E., Guyot E., Ganne-Carrie N., et al. PNPLA3 (rs738409 C>G) is a common risk variant associated with hepatocellular carcinoma in alcoholic cirrhosis. Hepatology. 2012;55(4):1307–1308. doi: 10.1002/hep.25518. [DOI] [PubMed] [Google Scholar]
- 137.Liu Y.-L., Patman G. L., Leathart J. B. S., et al. Carriage of the PNPLA3 rs738409 C>G polymorphism confers an increased risk of non-alcoholic fatty liver disease associated hepatocellular carcinoma. Journal of Hepatology. 2014;61(1):75–81. doi: 10.1016/j.jhep.2014.02.030. [DOI] [PubMed] [Google Scholar]
- 138.Burza M. A., Pirazzi C., Maglio C., et al. PNPLA3 I148M (rs738409) genetic variant is associated with hepatocellular carcinoma in obese individuals. Digestive and Liver Disease. 2012;44(12):1037–1041. doi: 10.1016/j.dld.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 139.Krawczyk M., Stokes C. S., Romeo S., Lammert F. HCC and liver disease risks in homozygous PNPLA3 p.I148M carriers approach monogenic inheritance. Journal of Hepatology. 2015;62(4):980–981. doi: 10.1016/j.jhep.2014.10.048. [DOI] [PubMed] [Google Scholar]
- 140.Krawczyk M., Portincasa P., Lammert F. PNPLA3-associated steatohepatitis: toward a gene-based classification of fatty liver disease. Seminars in Liver Disease. 2013;33(4):369–379. doi: 10.1055/s-0033-1358525. [DOI] [PubMed] [Google Scholar]
- 141.Liu Y.-L., Reeves H. L., Burt A. D., et al. TM6SF2 rs58542926 influences hepatic fibrosis progression in patients with non-alcoholic fatty liver disease. Nature Communications. 2014;5, article 4309 doi: 10.1038/ncomms5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Dongiovanni P., Petta S., Maglio C., et al. Transmembrane 6 superfamily member 2 gene variant disentangles nonalcoholic steatohepatitis from cardiovascular disease. Hepatology. 2015;61(2):506–514. doi: 10.1002/hep.27490. [DOI] [PubMed] [Google Scholar]
- 143.Musso G., Cassader M., Paschetta E., Gambino R. TM6SF2 may drive postprandial lipoprotein cholesterol toxicity away from the vessel walls to the liver in NAFLD. Journal of Hepatology. 2016;64(4):979–981. doi: 10.1016/j.jhep.2015.11.036. [DOI] [PubMed] [Google Scholar]
- 144.Le Roy T., Llopis M., Lepage P., et al. Intestinal microbiota determines development of non-alcoholic fatty liver disease in mice. Gut. 2013;62(12):1787–1794. doi: 10.1136/gutjnl-2012-303816. [DOI] [PubMed] [Google Scholar]
- 145.Ussar S., Griffin N. W., Bezy O., et al. Interactions between gut microbiota, host genetics and diet modulate the predisposition to obesity and metabolic syndrome. Cell Metabolism. 2015;22(3):516–530. doi: 10.1016/j.cmet.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]