Abstract
Background. Neuropsychiatric symptoms are common in people with dementia, and pain is thought to be an important underlying factor. Pain has previously been associated with agitation, and pain treatment has been shown to ameliorate agitated behaviour. So far, the association between pain and psychosis and the effect of pain treatment on psychotic symptoms is unclear. Furthermore, the impact of opioid treatment on psychosis is not established. Aim. To investigate the efficacy of a stepwise protocol for treating pain (SPTP) on psychosis and agitation measured with the Neuropsychiatric Inventory, Nursing Home version, and to explore the impact of opioid analgesics on psychosis. Method. Secondary analyses are from a cluster-randomised controlled trial including 352 patients with advanced dementia and agitation from 18 nursing homes in Western Norway. The intervention group received pain treatment according to SPTP. Results. Pain was associated with disinhibition (adjusted OR: 1.21, 95% CI: 1.10–1.34) and irritability (adjusted OR: 1.10, 95% CI: 1.01–1.21) at baseline. Pain treatment reduced agitation (p < 0.001, df = 1; 300) and aberrant motor behaviour (p = 0.017, df = 1; 300). Psychosis was reduced in people with at least one symptom at baseline (p = 0.034, df = 1; 135). The use of opioid analgesics did not increase psychotic symptoms. Study Registration. This trial is registered with ClinicalTrials.gov (NCT01021696), Norwegian Medicines Agency, EudraCT (EudraCTnr: 2008-007490-20).
1. Introduction
Neuropsychiatric symptoms (NPS) are a feature in many neurodegenerative diseases, among other dementia, where over 90% of patients suffer from at least one NPS during the course of their disease [1]. NPS can be distressing for both patients and family alike and is often the main reason for admission to a nursing home (NH) [2]. NPS can be clustered in different ways. These clusters are most commonly defined by symptoms that present concurrently, like mood symptoms such as depression and anxiety, agitation symptoms such as aggression and irritability, and psychosis symptoms such as delusion and hallucination [3–6].
The aetiology of NPS is largely unknown, but factors like neuropathological changes in the brain, unmet psychosocial needs, and pain are thought to play a role [7]. Despite the multiple potential underlying factors, NPS are often treated with antipsychotic drugs with potential harmful side effects [8]. This highlights the importance of investigating the relationship between NPS and possible underlying treatable causes, such as pain, to avoid unnecessary antipsychotic drug use [9–11].
People in the later stages of dementia often reside in NHs and frequently experience pain, with 30–60% suffering daily from pain [12–14]. The cognitive decline with a subsequent loss of communicative abilities puts people with dementia at an increased risk of suffering from untreated pain [15, 16]. Research demonstrates that pain in people with dementia can act as a trigger for NPS such as agitation and mood symptoms [17, 18]. However, the relationship between pain and psychosis symptoms is less well studied, and only an association between pain and delusion has previously been described. Tosato et al. investigated the association between pain and NPS in NH patients with cognitive impairment and found pain to be associated with delusion [19]. In contrast, Cohen-Mansfield et al. found no association between pain and psychosis symptoms in an adult day care population (≥60 years old) residing in the community [20].
Our own research demonstrated the efficacy of individual pain treatment on behavioural disturbances in NH patients with advanced dementia and found that pain treatment ameliorated agitation as assessed by the Cohen-Mansfield Agitation Inventory (CMAI) [9]. Secondary analyses showed that pain treatment also reduced verbal aggression and restlessness [10]. Mood symptoms such as depression, sleep and appetite disturbances, measured with the Neuropsychiatric Inventory, Nursing Home version (NPI-NH) [11], and pain intensity assessed by the Mobilisation Observation Behaviour Intensity Dementia-2 (MOBID-2) Pain Scale [13] were also found to be reduced. The effect of pain treatment on psychosis and agitation symptoms measured by NPI-NH has, however, not yet been investigated.
Although there are no official guidelines for pain treatment in people with dementia, the use of opioid analgesics in pain treatment is recommended in guidelines for older people [21–23]. However, some physicians can be reluctant to prescribe these drugs, often due to the fear of possible side effects such as delirium, which also includes psychotic symptoms such as hallucination and delusion [24, 25]. The association between opioid analgesics and psychosis can therefore give relevant information regarding delirium as a potential side effect of opioid drug use.
The primary aim of this study was to investigate the efficacy of pain treatment on psychosis and agitation and the association between pain, psychosis, and agitation in people with advanced dementia. In addition, we investigated whether the use of opioid analgesics increased the prevalence of delusion and hallucination in people with dementia. We hypothesized an association between pain and agitation at baseline, but not between pain and psychosis, and suggested that pain treatment will reduce symptoms of agitation, but not symptoms of psychosis. We also hypothesized that the use of opioid analgesics does not increase the prevalence of hallucination and delusion.
2. Method
We conducted secondary analyses from a cluster-randomised controlled trial (RCT), investigating the efficacy of treating pain on behavioural disturbances in NH patients with advanced dementia from 18 NHs in Western Norway. For a more detailed description of the study procedure, we refer to previous publications [9, 11, 13]. In brief, patients included in this study had moderate to severe dementia as defined by the Diagnostic and Statistical Manual of mental disorders, 4th edition (DSM-IV); Functional Assessment Staging Test (FAST) score ≥ 4 [26]; Minimental State Examination (MMSE) score ≤ 20 [27], and clinically relevant behavioural disturbances as defined by a score ≥ 39 on CMAI [28]. Patients were excluded if they had an advanced medical disorder with expected survival ≤ 6 months, severe psychiatric or neurological disorder, hepatic or renal failure, a score ≥ 8 on the aggression item of the NPI-NH, with aggression as the predominant symptom [29], or allergy to paracetamol, morphine, buprenorphine, or pregabalin.
2.1. Study Design
Each NH unit was defined as a single cluster and was randomised to either intervention or control. Randomisation was performed by a statistician using Stata version 8, by generating a list of random numbers used for allocating each cluster to either intervention or control. The intervention group received individual pain treatment according to a stepwise protocol for treating pain (SPTP) for 8 weeks, followed by a 4-week washout period where analgesics were reverted back to preintervention treatment. The control group received treatment as usual. The SPTP was based on recommendations made by the American Geriatrics Society [22]. According to assessment of current medication and degree of pain, the patient was allocated to one of four steps, receiving either paracetamol (Paracetamol®), extended release morphine (Dolcontin®), buprenorphine transdermal patch (Norspan®) for patients with swallowing difficulties, or pregabalin (Lyrica®) for patients with suggested neuropathic pain. Physicians were instructed to keep the prescription unchanged if possible. Use of as-needed analgesics was not prohibited and was monitored during the study.
2.2. Outcome Measures
The primary outcome measure was NPS as measured by the NPI-NH [29]. The NPI-NH rates the frequency (F) and severity (S) of twelve different NPS. Frequency is rated on a scale from 1 to 4, where 1 represents occasionally (less than once a week) and 4 represents very frequent (daily or more often). Severity is measured on a scale from 1 to 3, where 1 represents mild (causes little stress for the patient) and 3 represents severe (puts very much stress on the patient and cannot easily be diverted by caregivers). The frequency and severity scores are multiplied (F × S) to give an item score for each NPS, where a score ≥ 4 was viewed as a clinically significant symptom [30].
The NPS measured by NPI-NH were clustered in three groups: agitation (aggression, disinhibition, irritability, and aberrant motor behaviour), psychosis (delusion, hallucination, and euphoria), and mood (depression, anxiety, apathy, and sleep and appetite disturbances), according to factor analyses by Cheng et al. [6].
Pain intensity was assessed by the MOBID-2 Pain Scale [31–33]. This is a nursing staff-administered pain tool, consisting of two parts. The first part assesses pain originating from the musculoskeletal system during five active guided movements. The second part assesses pain that might be related to internal organs, head, and skin based on the caregivers' observation during the last week. Taking all items into account, the caregiver rated the patients' pain on a Numerical Rating Scale (NRS) ranging from 0 to 10, where 0 represented no pain and 10 the worst pain imaginable. This tool has been thoroughly tested for its psychometric properties and showed good validity, reliability, and responsiveness [32, 33].
All assessments were conducted at baseline and Weeks 2, 4, 8, and 12 by the primary caregivers who knew the patient best in collaboration with a specialised study nurse.
2.3. Statistics
Differences in baseline characteristics were explored using an independent sample t-test for normally distributed variables; a Chi-squared test was used for categorical variables, and a Mann-Whitney U test was used for nonparametric variables. Associations between pain, psychosis, and agitation at baseline were investigated by using crude and adjusted logistic regression. Each symptom of psychosis and agitation represented the dependent variable, while total pain intensity, assessed by MOBID-2, represented the explanatory variable. Associations were adjusted for age, gender, dementia severity (assessed by MMSE and FAST), and activities of daily living (ADL) function assessed by Barthels ADL index [34]. The changes in F × S score between the intervention and control groups from baseline to Week 8 were compared using the Mann-Whitney U test. The association between opioid analgesics and delusion and hallucination was evaluated at baseline and Week 8 using logistic regression. Associations were adjusted for age, gender, dementia severity (MMSE and FAST), ADL function (Barthels ADL index), and pain intensity (MOBID-2). Statistic calculations were performed using the Statistical Package for Social Sciences (SPSS) version 22.
3. Ethics
Informed consent was obtained from patients who were cognitively able to understand the possible risks and benefits of the study. Consent was, if possible, obtained in a meeting where next of kin was present as well. A presumed consent was obtained from next of kin, or a legal guardian, if the patient was not able to give an informed consent. All consents were obtained in accordance with local law, approved by the Regional Ethical Committee for Medical Ethics in Western Norway (REK-Vest 248.08), and authorised by the participating institutions' review board.
4. Results
Three hundred and fifty-two patients from 60 NH units were included. Units were randomised to either intervention or control, generating 177 patients in the control group and 175 patients in the intervention group. With the exception of age (p = 0.022), we found no differences between the two groups. Baseline characteristics are described in Table 1. During the intervention period, 13 patients in the control and 25 in the intervention group were excluded, with no significant differences between the two groups [9]. At baseline, 71 people in the control group (40%) and 83 people in the intervention group (47%) had one or more symptoms of psychosis, while 128 people in the control group (72%) and 137 people in the intervention group (78%) had one or more symptoms of agitation. The most prevalent symptom was irritability (48%), while the least prevalent one was euphoria (9%).
Table 1.
Sample characteristics of patients at baseline.
| Control (n = 177) | Intervention (n = 175) | df | p | |
|---|---|---|---|---|
| Age (SD)a | 86.5 (6.7) | 84.9 (7.0) | 350 | 0.022 |
| Women (%)b | 131 (74.0) | 131 (74.9) | 1 | 0.856 |
| FAST (SD)c | 6.0 (0.7) | 6.1 (0.7) | 349 | 0.057 |
| MMSE (SD)c | 8.4 (6.7) | 7.5 (6.5) | 346 | 0.177 |
| Barthels ADL total score (SD)c | 8.6 (5.6) | 7.9 (5.7) | 339 | 0.216 |
| CMAI total score (SD)c | 56.2 (16.1) | 56.5 (15.2) | 349 | 0.487 |
| MOBID-2 (SD)c | 3.7 (2.5) | 3.8 (2.7) | 325 | 0.988 |
| Medications (SD)c | 3.6 (1.6) | 3.4 (2.1) | 318 | 0.146 |
| Analgesics (%)b | 122 (68.9) | 117 (66.9) | 1 | 0.404 |
| Paracetamol (%)b | 94 (53.1) | 99 (56.6) | 1 | 0.665 |
| Opioids (%)b | 51 (28.8) | 43 (24.6) | 1 | 0.292 |
| NSAIDS (%)b | 9 (5.1) | 13 (7.4) | 1 | 0.364 |
| Psycholeptics (%)b | 112 (63.3) | 104 (59.4) | 1 | 0.458 |
| Antipsychotics (%)b | 13 (7.3) | 17 (9.7) | 1 | 0.465 |
| Anxiolytics (%)b | 86 (48.6) | 80 (45.7) | 1 | 0.589 |
| Psychosis symptoms (%)b | 71 (20.2) | 83 (23.6) | 1 | 0.209 |
| Delusion (%)b | 49 (27.7) | 66 (37.7) | 1 | 0.056 |
| Hallucination (%)b | 29 (16.4) | 32 (18.3) | 1 | 0.690 |
| Euphoria (%)b | 15 (8.5) | 16 (9.1) | 1 | 0.864 |
| Agitation symptoms (%)b | 128 (36.4) | 137 (38.9) | 1 | 0.285 |
| Agitation/aggression (%)b | 74 (41.8) | 85 (48.6) | 1 | 0.253 |
| Disinhibition (%)b | 56 (31.6) | 59 (33.7) | 1 | 0.760 |
| Irritability (%)b | 84 (47.5) | 85 (48.6) | 1 | 0.956 |
| Aberrant motor behaviour (%)b | 57 (32.2) | 65 (37.1) | 1 | 0.388 |
aIndependent-samples t-test.
bPearson's Chi-squared test.
cMann-Whitney U test.
Related to symptoms of psychosis, no associations were found between pain and symptoms of psychosis at baseline. During the intervention period, no reduction in the psychosis cluster (p = 0.091, df = 1; 300), delusion (p = 0.052, df = 1; 300), hallucination (p = 0.832, df = 1; 300), and euphoria (p = 0.507, 1; 300) was observed in response to individual pain treatment compared to the control group from baseline and to Week 8 (Table 2, Figures 1 –3). However, for people with one or more symptoms of psychosis at baseline, a decrease was observed in the psychosis cluster (p = 0.034, df = 1; 135) and delusion (p = 0.031, df = 1; 135) in the intervention group compared with the control group (Table 3, Figure 7).
Table 2.
Efficacy of treating pain on psychosis and agitation.
| Baseline | 8 weeks | ||||||
|---|---|---|---|---|---|---|---|
| Control (n = 177) | Intervention (n = 175) | p a | Control (n = 157) | Intervention (n = 146) | p a | p changeb | |
| NPI total score | 31.4 (21.4) | 34.8 (21.9) | 0.132 | 26.6 (20.1) | 18.9 (17.5) | <0.001 | <0.001 |
| Psychosis cluster | 4.8 (5.8) | 6.1 (6.9) | 0.087 | 3.7 (4.9) | 3.9 (5.5) | 0.682 | 0.091 |
| Delusion | 2.6 (3.8) | 3.6 (4.3) | 0.030 | 2.0 (3.1) | 2.0 (3.2) | 0.813 | 0.052 |
| Hallucination | 1.5 (2.9) | 1.8 (3.2) | 0.427 | 1.1 (2.3) | 1.4 (2.7) | 0.405 | 0.832 |
| Euphoria | 0.7 (2.0) | 0.8 (2.2) | 0.887 | 0.6 (1.9) | 0.5 (1.8) | 0.123 | 0.507 |
| Agitation cluster | 13.4 (10.9) | 14.8 (10.9) | 0.155 | 11.3 (10.9) | 7.8 (8.3) | 0.007 | <0.001 |
| Agitation/aggression | 3.7 (3.9) | 4.2 (4.3) | 0.373 | 3.4 (3.8) | 2.1 (3.1) | 0.001 | 0.001 |
| Disinhibition | 3.0 (4.0) | 2.9 (3.8) | 0.922 | 2.6 (3.9) | 1.7 (3.0) | 0.061 | 0.293 |
| Irritability | 3.7 (3.7) | 4.2 (4.1) | 0.338 | 3.0 (3.4) | 2.3 (3.1) | 0.092 | 0.093 |
| Abb. motor behaviour | 3.0 (4.5) | 3.5 (4.7) | 0.328 | 2.4 (3.7) | 1.7 (3.6) | 0.052 | 0.017 |
aCalculated by analyzing the difference between the intervention group and control group at each measurement point using the Mann-Whitney U test.
bCalculated by analyzing the difference in change of NPI-NH score in the intervention group versus the control group from baseline to Week 8 using the Mann-Whitney U test.
Figure 1.
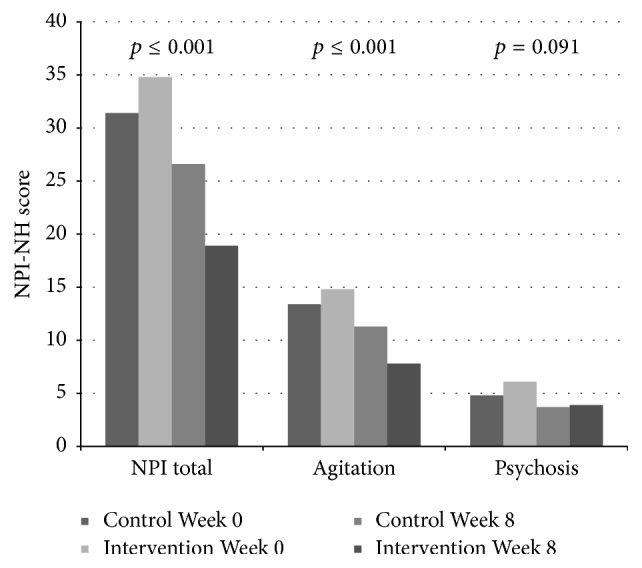
The efficacy of treating pain on psychosis and agitation.
Figure 2.
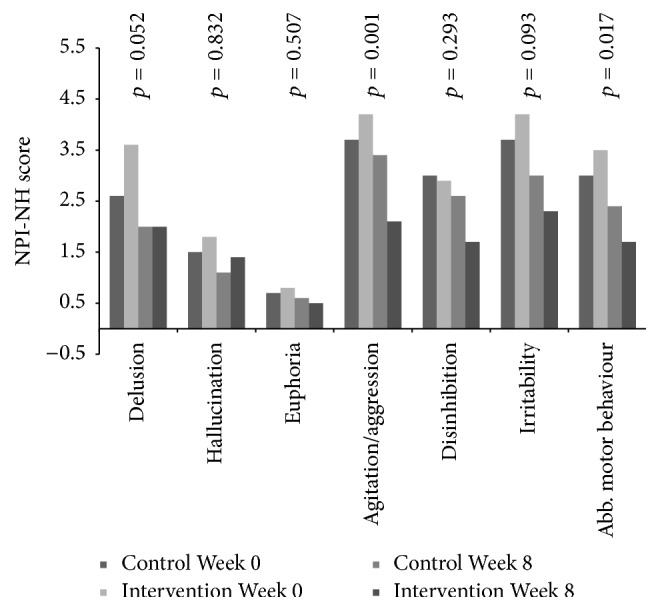
The efficacy of pain treatment on individual neuropsychiatric symptoms.
Figure 3.
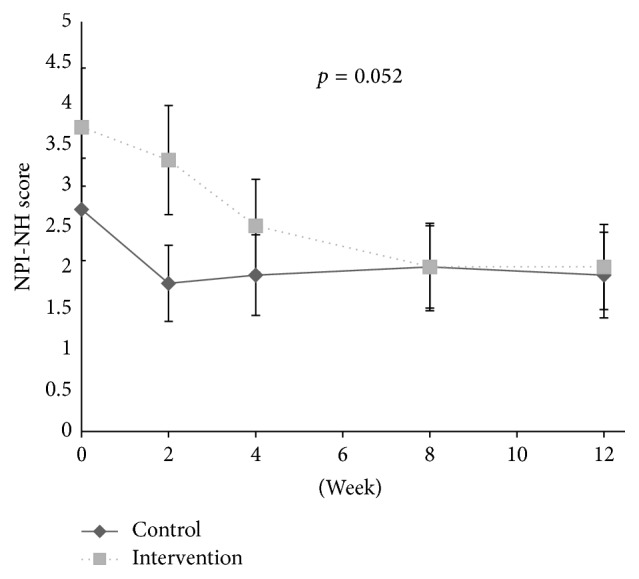
Development of delusion during the intervention and washout period.
Table 3.
Efficacy of treating pain on psychosis and agitation in patients presenting one or more clinically significant symptoms at baseline (NPI-NH ≥ 4).
| Baseline (SD) | 8 weeks (SD) | ||||||
|---|---|---|---|---|---|---|---|
| Control (n = 71) | Intervention (n = 83) | p a | Control (n = 67) | Intervention (n = 70) | p a | p changeb | |
| Psychosis cluster | 10.5 (4.7) | 11.6 (5.9) | 0.314 | 6.4 (5.3) | 5.6 (6.1) | 0.148 | 0.034 |
| Delusion | 5.6 (4.2) | 6.9 (4.0) | 0.043 | 3.2 (3.7) | 2.9 (3.6) | 0.770 | 0.031 |
| Hallucination | 3.2 (3.8) | 3.3 (4.0) | 0.813 | 2.1 (3.1) | 2.1 (3.3) | 0.987 | 0.925 |
| Euphoria | 1.7 (2.9) | 1.4 (3.1) | 0.211 | 1.0 (2.2) | 0.5 (1.9) | 0.027 | 0.758 |
|
| |||||||
| Control (n = 128) | Intervention (n = 137) | p a | Control (n = 117) | Intervention (n = 113) | p a | p changeb | |
| Agitation cluster | 17.4 (9.7) | 18.0 (9.6) | 0.422 | 14.0 (11.0) | 8.8 (8.8) | <0.001 | <0.001 |
| Agitation/aggression | 4.7 (4.0) | 5.1 (4.2) | 0.441 | 4.2 (4.0) | 2.5 (3.3) | 0.001 | 0.004 |
| Disinhibition | 3.9 (4.3) | 3.5 (4.0) | 0.618 | 3.3 (4.2) | 1.9 (3.2) | 0.008 | 0.211 |
| Irritability | 4.8 (3.6) | 5.1 (4.1) | 0.664 | 3.6 (3.6) | 2.6 (3.2) | 0.023 | 0.183 |
| Abb. motor behaviour | 4.0 (4.7) | 4.3 (4.9) | 0.639 | 2.9 (3.9) | 1.8 (3.5) | 0.008 | 0.007 |
aCalculated by analyzing the difference between the intervention group and control group at each measurement point using the Mann-Whitney U test.
bCalculated by analyzing the difference in change of NPI-NH score in the intervention group versus the control group from baseline to Week 8 using the Mann-Whitney U test.
Figure 7.
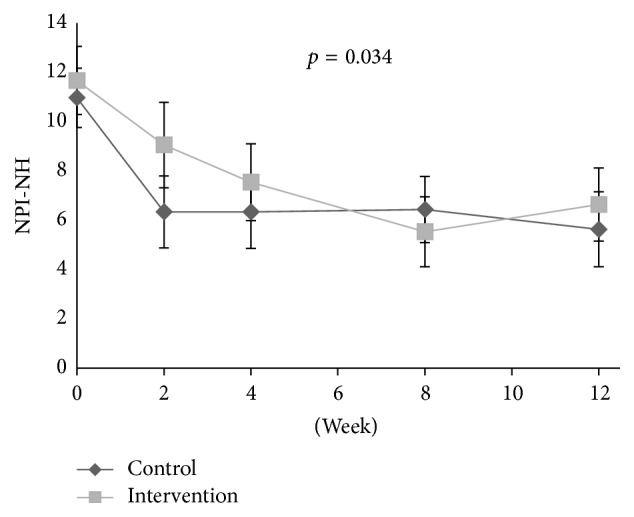
Development of the psychosis cluster in patients with one or more clinically significant NPS of psychosis at baseline (NPI-NH ≥ 4).
At baseline, the adjusted logistic regression analysis showed a positive association between disinhibition and level of pain (OR: 1.18, aOR: 1.21, 95% CI: 1.10–1.34, and p < 0.001) and between irritability and level of pain (OR: 1.11, aOR: 1.10, 95% CI: 1.01–1.21, and p = 0.032), adjusted for confounders. During the intervention period, a decrease in the agitation cluster (p < 0.001, df = 1; 301), agitation/aggression (p = 0.001, df = 1; 301), and aberrant motor behaviour (p = 0.017, df = 1; 301) was found in the treatment group compared to the control group (Table 2, Figures 1, 2, 4, 5, and 6). For people with one or more symptoms of agitation at baseline, a decrease during the intervention period was observed in the agitation cluster (p < 0.001, df = 1; 228), agitation/aggression (p = 0.004, df = 1; 228), and aberrant motor behaviour (p = 0.007, df = 1; 228) in the treatment group compared with the control group (Table 3, Figure 8).
Figure 4.
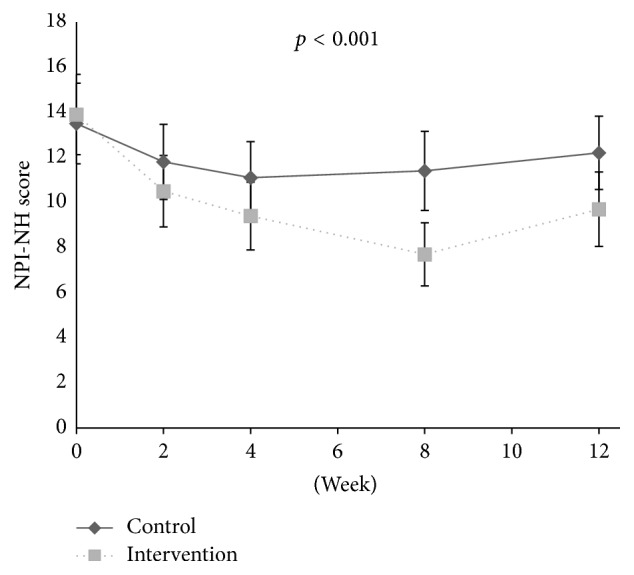
Development of agitation scores in clusters during intervention and washout period.
Figure 5.
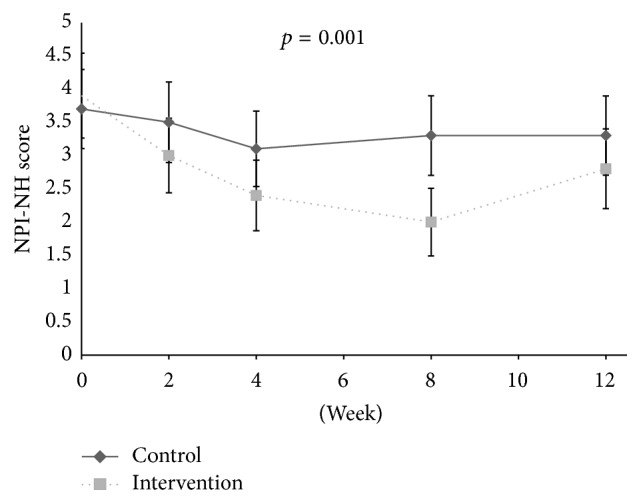
Development of agitation/aggression during the intervention and washout period.
Figure 6.
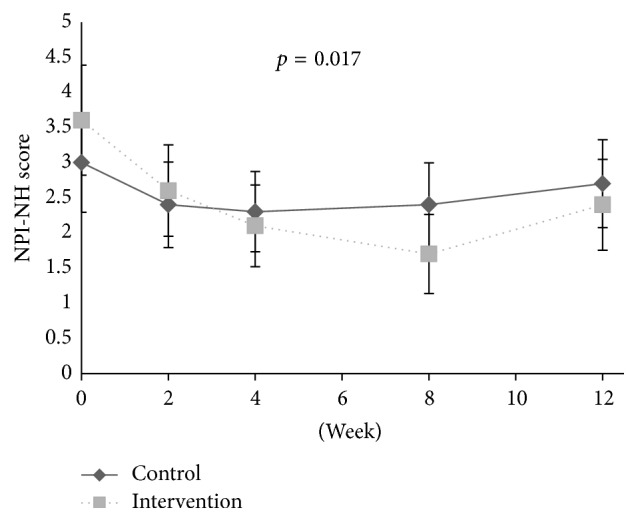
Development of aberrant motor behaviour during the intervention and washout period.
Figure 8.
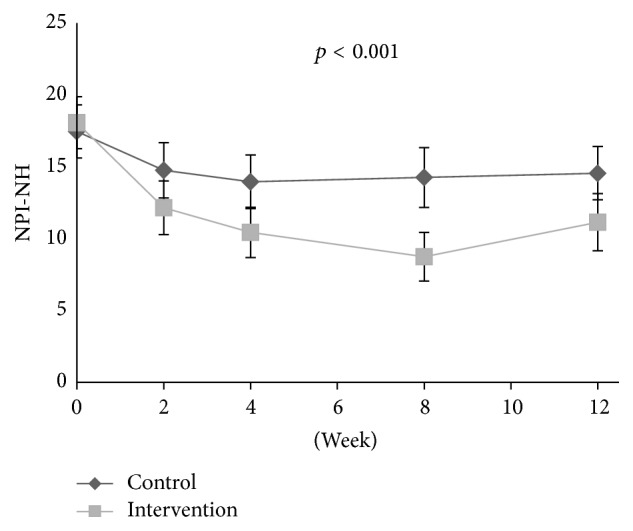
Development of the agitation cluster in patients with one or more clinically significant NPS of agitation at baseline (NPI-NH ≥ 4).
At baseline, the use of opioid analgesics was not associated with the prevalence of delusions (OR: 0.97, aOR: 0.96, 95% CI: 0.56–1.65, and p = 0.870) or hallucination (OR: 0.76, aOR: 0.69, 95% CI: 0.34–1.41, and p = 0.314). Following the intervention period at Week 8, opioids were not associated with the prevalence of delusion (OR: 1.90, aOR: 1.89, 95% CI: 0.72–4.98, and p = 0.200) or hallucination (OR: 1.05, aOR: 1.26, 95% CI: 0.39–4.09, and p = 0.700).
5. Discussion
This study aimed to investigate the relationship between pain, psychosis, and agitation, the efficacy of treating pain on psychosis and agitation, and the potential impact of opioid analgesics on the development of hallucination and delusion in NH patients with advanced dementia.
The study showed that treatment of pain ameliorates the prevalence of psychosis and delusion in people with dementia who presented at least one psychosis symptom at baseline. It is also established that, in this study, opioid analgesics did not increase the prevalence of hallucination or delusion. These findings confirmed the hypothesis that pain is a potential underlying cause for psychosis and that proper pain management is needed in order to avoid psychotic symptoms. This provides important information for clinicians when pharmacological treatment options for pain are to be evaluated. Some clinicians can be reluctant to prescribe opioid analgesics for pain treatment of people with dementia, often due to fear of anticholinergic side effects, such as delirium [24]. Finally, we found that pain treatment reduced agitation, aggression, and aberrant motor behaviour. This underlines previous findings where pain was found to be an important underlying cause for agitation assessed with CMAI in people with dementia. These findings highlight the fact that proper pain assessment should be a prerequisite when deciding treatment options for agitation in people with dementia.
The current study was the first parallel group-controlled trial investigating the efficacy of analgesics on psychotic symptoms in people with advanced dementia. Although individual pain treatment reduced psychosis in people with psychotic symptoms, pain was, interestingly, not cross-sectionally associated with hallucination and delusion at baseline. Tosato et al. used data from the Minimum Data Set (MDS) and investigated the relationship between pain and psychiatric symptoms in 2822 NH residents with cognitive impairment and found an association between pain and delusion but not between pain and hallucination [19], contrary to our results. In Tosato's study, the interRAI MDS 2.0 instrument for long-term facilities was used to measure psychosis and pain, while our study used the MOBID-2 Pain Scale to measure pain. Cohen-Mansfield et al. also investigated the association between pain, delusion, and hallucination in an adult day care population and found no association between pain and delusion or pain and hallucination [20]. However, in contrast to our study, these people were not residing in NHs and patients suffering from dementia were not analyzed as a separate group. The study used the Behavioural Pathology in Alzheimer's disease rating scale to measure psychosis and a questionnaire, based on the short form of the McGill Pain Questionnaire, distributed to family and caregivers to measure pain. Pain should be measured by a tool thoroughly tested for psychometric properties, and among the measurement tools used, only MOBID-2 has been tested for validity, reliability, and responsiveness [32, 33].
We used a symptom clustering largely based on a factor analyses of the NPI-NH by Cheng et al., where the symptoms were clustered in three main groups: agitation, mood, and psychosis [6]. This clustering makes “clinical sense” and is in line with other previous studies. Hollingworth et al. grouped delusion and hallucination in a psychosis cluster, aggression and irritability in an agitation cluster, and disinhibition, euphoria, and aberrant motor behaviour in a behavioural dyscontrol cluster [3]. In a four-factor solution, Selbæk and Engedal grouped hallucination and delusion as a psychosis cluster and aggression, irritability, disinhibition, and aberrant motor behaviour in an agitation cluster [4]. Overall, the clusters may be viewed as merely theoretical constructs and changes assessed over time [4].
The reduction in psychosis was largely attributed to the reduction of delusion, as neither hallucination nor euphoria was reduced in response to pain treatment. This indicates that hallucination and euphoria may not be associated with pain. Traditionally, antipsychotics are recommended for short-time treatment of psychosis, also in people with dementia, despite potential harmful side effects and increased mortality [8]. Our results suggested that hallucination and euphoria were not associated with pain, making the use of antipsychotics in treatment of hallucination and euphoria more warranted than in treatment of delusion.
The use of opioid analgesics did not increase the prevalence of delusion or hallucination at baseline, or after the 8-week intervention. This is of key importance, because opioid analgesics such as morphine or buprenorphine can have multiple side effects such as confusion and delirium caused by anticholinergic activity [24]. Notably, delirium, psychosis, and depression have several similarities in people with dementia, making them difficult to distinguish and diagnose. This highlights the importance of trained staff in order to discriminate between the more acute state delirium and more chronic symptoms in dementia [25].
The reduction of agitation in response to pain treatment was fairly expected, as previous analyses on the study population have shown a decrease in behavioural disturbances, especially agitation, as measured using CMAI [9, 10]. NPI-NH does however measure more specific symptoms in contrast to CMAI, which measures more specific behavioural items. Therefore, the efficacy of pain treatment on the specific symptom aberrant motor behaviour is an interesting finding, supported by previous studies which found that pain treatment may reduce agitation. An article by Flo et al. reviewed studies on pain management in people with dementia and found that pharmacological pain treatment could reduce agitation [17]. Achterberg et al. reviewed the efficacy of pain management in people with dementia and found that pain can be a possible underlying cause for agitation and that a thorough pain assessment and management can ameliorate agitation [16]. The present analyses also found that there was an association between pain and disinhibition and irritability at baseline. While previous studies have found an association between pain and agitation, the direct association between pain, disinhibition, and irritability has not previously been described [17, 18, 35]. Our results showed that NPS associated with pain at baseline, like irritability and disinhibition, were not reduced in response to pain treatment. Results also showed that NPS not associated with pain at baseline, like agitation and delusion, were reduced in response to pain treatment. This paradox simply highlights the complex aetiology of NPS of agitation, and a thorough assessment of all possible underlying causes is important when deciding on possible treatment options for neuropsychiatric symptoms in people with dementia. Pain and behaviour are strongly intertwined, and the efficacy of both behavioural interventions and pain medication can improve both pain and behaviour [36].
Strengths and Limitations. This is the first RCT investigating the efficacy of treating pain on psychosis. Results came from secondary analyses from a previous study where CMAI was the primary outcome and NPI-NH was a secondary outcome. Inclusion criteria were therefore based on behavioural disturbances measured using CMAI. The number of study participants was also a limitation, as the group of patients with psychosis at baseline were a subgroup of the original population and a small sample. Despite this, the study is still the largest RCT investigating the efficacy of treating pain on psychosis and agitation.
6. Conclusion
Pain seems to be an underlying cause of psychosis and especially delusion. In addition, pain seems to be an underlying cause of agitation, such as aberrant motor behaviour. Thus, proper pain assessment is needed when treating these symptoms in people with dementia. The use of opioid analgesics does not seem to increase the prevalence of delusion and hallucination; therefore, the reluctance to use them may not necessarily be to the benefit of the patient.
Acknowledgments
Torstein F. Habiger is financed by the Medical Student Research Programme at The Faculty of Medicine and Dentistry, University of Bergen, Norway. Elisabeth Flo was sponsored by the Research Council of Norway (Sponsor's Protocol Code: 222113/H10). Bettina S. Husebo would like to thank the Norwegian Government and the GC Rieber Foundation for supporting her time for this work. Research Council of Norway is a sponsor (Protocol code 189439).
Ethical Approval
The study was approved by the Regional Ethical Committee for Medical Research of Western Norway (248.08).
Competing Interests
The authors declare that there are no competing interests regarding the publication of this paper.
References
- 1.Selbæk G., Engedal K., Benth J. Š., Bergh S. The course of neuropsychiatric symptoms in nursing-home patients with dementia over a 53-month follow-up period. International Psychogeriatrics. 2014;26(1):81–91. doi: 10.1017/S1041610213001609. [DOI] [PubMed] [Google Scholar]
- 2.Zuidema S. U., Derksen E., Verhey F. R. J., Koopmans R. T. C. M. Prevalence of neuropsychiatric symptoms in a large sample of Dutch nursing home patients with dementia. International Journal of Geriatric Psychiatry. 2007;22(7):632–638. doi: 10.1002/gps.1722. [DOI] [PubMed] [Google Scholar]
- 3.Hollingworth P., Hamshere M. L., Moskvina V., et al. Four components describe behavioral symptoms in 1,120 individuals with late-onset Alzheimer's disease. Journal of the American Geriatrics Society. 2006;54(9):1348–1354. doi: 10.1111/j.1532-5415.2006.00854.x. [DOI] [PubMed] [Google Scholar]
- 4.Selbæk G., Engedal K. Stability of the factor structure of the Neuropsychiatric Inventory in a 31-month follow-up study of a large sample of nursing-home patients with dementia. International Psychogeriatrics. 2012;24(1):62–73. doi: 10.1017/S104161021100086X. [DOI] [PubMed] [Google Scholar]
- 5.Wetzels R. B., Zuidema S. U., de Jonghe J. F. M., Verhey F. R. J., Koopmans R. T. C. M. Course of neuropsychiatric symptoms in residents with dementia in nursing homes over 2-year period. The American Journal of Geriatric Psychiatry. 2010;18(12):1054–1065. doi: 10.1097/jgp.0b013e3181f60fa1. [DOI] [PubMed] [Google Scholar]
- 6.Cheng S.-T., Kwok T., Lam L. C. W. Neuropsychiatric symptom clusters of Alzheimer's disease in Hong Kong Chinese: prevalence and confirmatory factor analysis of the Neuropsychiatric Inventory. International Psychogeriatrics. 2012;24(9):1465–1473. doi: 10.1017/s1041610212000609. [DOI] [PubMed] [Google Scholar]
- 7.Ballard C., Corbett A., Chitramohan R., Aarsland D. Management of agitation and aggression associated with Alzheimer's disease: controversies and possible solutions. Current Opinion in Psychiatry. 2009;22(6):532–540. doi: 10.1097/yco.0b013e32833111f9. [DOI] [PubMed] [Google Scholar]
- 8.Ballard C., Hanney M. L., Theodoulou M., et al. The dementia antipsychotic withdrawal trial (DART-AD): long-term follow-up of a randomised placebo-controlled trial. The Lancet Neurology. 2009;8(2):151–157. doi: 10.1016/S1474-4422(08)70295-3. [DOI] [PubMed] [Google Scholar]
- 9.Husebo B. S., Ballard C., Sandvik R., Nilsen O. B., Aarsland D. Efficacy of treating pain to reduce behavioural disturbances in residents of nursing homes with dementia: cluster randomised clinical trial. British Medical Journal. 2011;343(7816) doi: 10.1136/bmj.d4065.d4065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Husebo B. S., Ballard C., Cohen-Mansfield J., Seifert R., Aarsland D. The response of agitated behavior to pain management in persons with dementia. The American Journal of Geriatric Psychiatry. 2014;22(7):708–717. doi: 10.1016/j.jagp.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 11.Husebo B. S., Ballard C., Fritze F., Sandvik R. K., Aarsland D. Efficacy of pain treatment on mood syndrome in patients with dementia: a randomized clinical trial. International Journal of Geriatric Psychiatry. 2014;29(8):828–836. doi: 10.1002/gps.4063. [DOI] [PubMed] [Google Scholar]
- 12.Achterberg W. P., Gambassi G., Finne-Soveri H., et al. Pain in European long-term care facilities: cross-national study in Finland, Italy and the Netherlands. Pain. 2010;148(1):70–74. doi: 10.1016/j.pain.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 13.Sandvik R. K., Selbaek G., Seifert R., et al. Impact of a stepwise protocol for treating pain on pain intensity in nursing home patients with dementia: a cluster randomized trial. European Journal of Pain. 2014;18(10):1490–1500. doi: 10.1002/ejp.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Husebo B. S., Strand L. I., Moe-Nilssen R., BorgeHusebo S., Aarsland D., Ljunggren A. E. Who suffers most? Dementia and pain in nursing home patients: a cross-sectional study. Journal of the American Medical Directors Association. 2008;9(6):427–433. doi: 10.1016/j.jamda.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 15.Corbett A., Husebo B., Malcangio M., et al. Assessment and treatment of pain in people with dementia. Nature Reviews Neurology. 2012;8(5):264–274. doi: 10.1038/nrneurol.2012.53. [DOI] [PubMed] [Google Scholar]
- 16.Achterberg W. P., Pieper M. J. C., van Dalen-Kok A. H., et al. Pain management in patients with dementia. Clinical Interventions in Aging. 2013;8:1471–1482. doi: 10.2147/CIA.S36739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flo E., Gulla C., Husebo B. S. Effective pain management in patients with dementia: benefits beyond pain? Drugs & Aging. 2014;31(12):863–871. doi: 10.1007/s40266-014-0222-0. [DOI] [PubMed] [Google Scholar]
- 18.van Dalen-Kok A. H., Pieper M. J., de Waal M. W., Lukas A., Husebo B. S., Achterberg W. P. Association between pain, neuropsychiatric symptoms, and physical function in dementia: a systematic review and meta-analysis. BMC Geriatrics. 2015;15(1, article 49) doi: 10.1186/s12877-015-0048-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tosato M., Lukas A., van der Roest H. G., et al. Association of pain with behavioral and psychiatric symptoms among nursing home residents with cognitive impairment: results from the SHELTER study. Pain. 2012;153(2):305–310. doi: 10.1016/j.pain.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 20.Cohen-Mansfield J., Taylor L., Werner P. Delusions and hallucinations in an adult day care population. A longitudinal study. The American Journal of Geriatric Psychiatry. 1998;6(2):104–121. [PubMed] [Google Scholar]
- 21.Abdulla A., Adams N., Bone M., et al. Guidance on the management of pain in older people. Age and ageing. 2013;42(supplement 1):i1–i57. doi: 10.1093/ageing/afs200. [DOI] [PubMed] [Google Scholar]
- 22.American Geriatrics Society. Pharmacological management of persistent pain in older persons. Pain Medicine. 2009;10(6):1062–1683. doi: 10.1111/j.1526-4637.2009.00699.x. [DOI] [PubMed] [Google Scholar]
- 23.Achterberg W. P., de Ruiter C. M., de Weerd-Spaetgens C. M., Geels P., Horikx A., Verduijn M. M. Multidisciplinary guideline ‘Recognition and treatment of chronic pain in vulnerable elderly people’. Nederlands Tijdschrift voor Geneeskunde. 2012;155(35)A4606 [PubMed] [Google Scholar]
- 24.Durán C. E., Azermai M., Vander Stichele R. H. Systematic review of anticholinergic risk scales in older adults. European Journal of Clinical Pharmacology. 2013;69(7):1485–1496. doi: 10.1007/s00228-013-1499-3. [DOI] [PubMed] [Google Scholar]
- 25.Siafarikas N. I., Preuss U. Delirium and dementia. Fortschritte der Neurologie Psychiatrie. 2014;82(9):492–501. doi: 10.1055/s-0034-1366626. [DOI] [PubMed] [Google Scholar]
- 26.Reisberg B. Functional assessment staging (FAST) Psychopharmacology Bulletin. 1988;24(4):653–659. [PubMed] [Google Scholar]
- 27.Folstein M. F., Folstein S. E., McHugh P. R. ‘Mini-mental state’. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 28.Cohen-Mansfield J., Marx M. S., Rosenthal A. S. A description of agitation in a nursing home. Journals of Gerontology. 1989;44(3):M77–M84. doi: 10.1093/geronj/44.3.m77. [DOI] [PubMed] [Google Scholar]
- 29.Selbæk G., Kirkevold Ø., Sommer O. H., Engedal K. The reliability and validity of the Norwegian version of the Neuropsychiatric Inventory, nursing home version (NPI-NH) International Psychogeriatrics. 2008;20(2):375–382. doi: 10.1017/s1041610207005601. [DOI] [PubMed] [Google Scholar]
- 30.Margallo-Lana M., Swann A., O'Brien J., et al. Prevalence and pharmacological management of behavioural and psychological symptoms amongst dementia sufferers living in care environments. International Journal of Geriatric Psychiatry. 2001;16(1):39–44. doi: 10.1002/1099-1166(200101)16:1<39::aid-gps269>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 31.Husebo B. S., Strand L. I., Moe-Nilssen R., Husebo S. B., Snow A. L., Ljunggren A. E. Mobilization-Observation-Behavior-Intensity-Dementia Pain Scale (MOBID): development and validation of a nurse-administered pain assessment tool for use in dementia. Journal of Pain and Symptom Management. 2007;34(1):67–80. doi: 10.1016/j.jpainsymman.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 32.Husebo B. S., Strand L. I., Moe-Nilssen R., Husebo S. B., Ljunggren A. E. Pain in older persons with severe dementia. Psychometric properties of the Mobilization-Observation-Behaviour-Intensity-Dementia (MOBID-2) pain scale in a clinical setting. Scandinavian Journal of Caring Sciences. 2010;24(2):380–391. doi: 10.1111/j.1471-6712.2009.00710.x. [DOI] [PubMed] [Google Scholar]
- 33.Husebo B. S., Ostelo R., Strand L. I. The MOBID-2 pain scale: Reliability and responsiveness to pain in patients with dementia. European Journal of Pain. 2014;18(10):1419–1430. doi: 10.1002/ejp.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mahoney F. I., Barthel D. W. Functional evaluation: the barthel index. Maryland State Medical Journal. 1965;14:61–65. [PubMed] [Google Scholar]
- 35.Corbett A., Husebo B. S., Achterberg W. P., Aarsland D., Erdal A., Flo E. The importance of pain management in older people with dementia. British Medical Bulletin. 2014;111(1):139–148. doi: 10.1093/bmb/ldu023. [DOI] [PubMed] [Google Scholar]
- 36.Pieper M. J. C., van Dalen-Kok A. H., Francke A. L., et al. Interventions targeting pain or behaviour in dementia: a systematic review. Ageing Research Reviews. 2013;12(4):1042–1055. doi: 10.1016/j.arr.2013.05.002. [DOI] [PubMed] [Google Scholar]


