Abstract
High levels of reactive oxygen species in the body and hyperlipidemia are key factors for the development of cardiovascular diseases such as atherosclerosis. The present study investigated the antioxidant and hypolipidemic activity of hydroethanolic extract of Curatella americana L. leaves (ExC). The antioxidant activity of ExC was assessed by 2,2-diphenyl-1-picrylhydrazyl free radical (DPPH) scavenging capacity and protection against hemolysis induced by 2,2′-azobis(2-amidinopropane) dihydrochloride (AAPH), followed by quantification of malondialdehyde (MDA). Wistar rats with hyperlipidemia induced by high-fructose diet (60%) were treated for 60 days with water, simvastatin (30 mg·Kg−1), ciprofibrate (2 mg·Kg−1), and ExC (200 mg·Kg−1). ExC revealed IC50 of 6.0 ± 0.5 μg·mL−1, an intermediary value among positive controls used in the assay of DPPH scavenging capacity. At all concentrations (50 to 125 μg·mL−1) and times (60 to 240 min) evaluated, ExC protected erythrocytes against AAPH-induced hemolysis, which was confirmed by lower MDA levels. In vivo tests showed a reduction of 34 and 45%, respectively, in serum concentration of cholesterol and triglycerides in hyperlipidemic rats treated with ExC, a similar effect compared to the reference drugs, simvastatin and ciprofibrate, respectively. Together, the results showed the antioxidant activity of ExC and its ability to improve the serum lipid profile in hyperlipidemic rats.
1. Introduction
Hyperlipidemia causes about 17 million deaths worldwide each year [1]; in addition, it is also a key factor for the development of heart and coronary diseases and atherosclerosis. Atherosclerosis is a chronic inflammatory disease triggered by multiple factors, with strong contribution of endothelial damage related to lipid peroxidation. This endothelial dysfunction increases the permeation of low-density lipoproteins (LDL) through the intima layer, resulting in oxidation and formation of atherosclerotic damage [2, 3]. In order to control this imbalance, the body has enzymatic and nonenzymatic antioxidant defense mechanisms [4] capable of preventing the deleterious effects of oxidation, inhibiting lipid peroxidation, free radicals scavenging, and maintaining redox balance in cells.
In addition to endogenous antioxidants, there are antioxidants from exogenous sources. The beneficial effects of foods have been linked to the presence of bioactive compounds and other nutrients. Examples of biomolecules that have antioxidant potential are phenolic compounds such as isoflavones, phenolic acids, catechins, chlorogenic acids, anthocyanins, and terpenes [5]. Thus, plants have been described as an alternative to the development of new drugs [6] applied to treatment of many diseases such as hypercholesterolemia, ulcers, depurative blood, and cancer [7–9].
Curatella americana L. is a member of the Dilleniaceae family, popularly known in Brazil as “lixa or lixeira” [10]. The beneficial effects of C. americana have been described in scientific research and indicated by its popular use. The anti-inflammatory, analgesic, antihypertensive, and vasodilator effects of the hydroethanolic extract of Curatella americana L. leaves have been evaluated in [11–13]. In folk medicine, leaf decoction is used as an antiseptic and astringent; bark infusion is used for the treatment of cold and healing wounds, ulcers, diabetes, and hypertension [14].
In this context, the aim of this study was to evaluate the antioxidant and hypolipidemic activity of the hydroethanolic extract of Curatella americana L. leaves (ExC) on rats with hyperlipidemia induced by high-fructose diet.
2. Material and Methods
2.1. Plant Material and Extract Preparation
C. americana L. leaves were collected in Mato Grosso do Sul, Brazil. The plant material was dried (45–50°C), crushed, and macerated in ethanol : water (80 : 20, v/v) at room temperature for seven days. After this period, the extract was filtered, concentrated in a rotary vacuum evaporator (FISATOM), and lyophilized. The lyophilized ExC was stored at 4°C and protected from light.
2.2. Dosage of Phenolic Compounds, Total Flavonoids, and Saponins
The concentration of phenolic compounds in samples was determined by the spectrophotometric method described by [15] using the Folin-Ciocalteu method. Three ExC measurements were performed, the average being presented in mg of gallic acid equivalents (GAE) per 100 g of sample.
The content of total flavonoids was determined according to methodology described by [16], with some adaptations, using 2% aluminum chloride solution in methanol as reagent. Extract solutions were prepared in methanol : water (1 : 1) at concentration of 10 mg·mL−1. About 0.5 mL ExC was added to 4.5 mL methanolic solution of 2% hydrate aluminum chloride. After 30 min at rest, the absorbance of solutions was read at 415 nm.
The presence of saponins was evaluated by preparing 10 mg of ExC dissolved in 2 mL of ethanol. Then, 5 mL of boiling water was added; the sample was vigorously shaken and allowed to stand for 20 min. According to [17], foaming indicates the presence of saponins.
2.3. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Free Radical Scavenging Activity
The antioxidant activity of the hydroethanolic extract of C. americana L. leaves was evaluated using technique described and adapted by [18] of 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging. DPPH solutions were prepared (0.11 mM) using ascorbic acid and butylhydroxytoluene (BHT) as positive controls and ExC at concentration of 20 mg·mL−1 in 80% ethanol solution. Serial dilutions were prepared based on these solutions in the concentrations investigated. To establish the half-maximal inhibitory concentration (IC50) of DPPH free radical scavenging, the samples were tested in serial dilutions (0.1, 1, 5, 10, 25, 50, 100, 500, and 1000 μg/mL) and analyzed by means of nonlinear regression using the Prism 5 GraphPad Software. Samples were assessed by spectrophotometer at 517 nm. The absorbance of each sample was divided by the absorbance of DPPH and multiplied by one hundred to represent the antioxidant activity in percentage. All independent experiments were performed in triplicate.
2.4. Protection against Hemolysis Induced by 2,2′-Azobis(2-amidinopropane) Dihydrochloride (AAPH)
Protection against lipid peroxidation of the extract was evaluated by hemolysis technique induced by 2,2-azobis 2-amidinopropane dihydrochloride (AAPH), described in [19]. About 5 mL of peripheral blood was collected from healthy donors, stored in tubes with sodium citrate (protocol approved by Ethics Research Committee: protocol number 123/12), and subsequently centrifuged at 2000 rpm for 5 min. The buffy coat was removed from plasma. The remaining erythrocytes underwent three washes with saline (0.9% NaCl) at 1500 rpm to remove possible interferences, with the supernatant discarded after each washing cycle. Subsequently, a 10% erythrocyte solution was prepared in saline.
The erythrocyte solution was incubated with distilled water (total hemolysis) and hemolysis induced by 2,2′-azobis(2-amidinopropane) dihydrochloride (AAPH; Sigma-Aldrich®) (50 mM) alone or concomitant with the standard antioxidant, ascorbic acid (AA), and ExC at concentrations of 50, 75, 100, and 125 µg·mL−1, reaching a final concentration of red blood cells of 2.5%. Aliquots were taken every 60 min after the start of incubation for 240 min, which were read in spectrophotometer at 540 nm. Three independent experiments were performed in duplicate.
2.5. Dosage of Malondialdehyde (MDA)
A 20% suspension was used to assess the protective effects of ExC against lipid peroxidation. The dosage of MDA was evaluated after 240 min of incubation at 37°C with and without addition of 500 µL of peroxyl radicals generated by thermal decomposition of 50 mM AAPH diluted in saline (0.9% NaCl). For this, after the incubation period, an aliquot of 0.5 mL of the reaction mixture was collected and was added to 0.5 mL of 20% trichloroacetic acid with subsequent homogenization. An aliquot of 0.5 mL was removed from this mixture and added into tubes previously pipetted with 1 mL of thiobarbituric acid reagent (TBA) 10 nM and incubated in water bath at 94°C for 45 min. After this period, samples were kept at room temperature for 15 min, followed by addition of 3 mL of butanol with subsequent agitation and centrifugation as described by [20]. Reading of the supernatant absorbance was carried out in spectrophotometer at 532 nm. Three independent experiments were performed in duplicate.
2.6. Hyperlipidemia Induced by High-Fructose Diet
The experimental procedures were approved by the Ethics Research Committee on Animal Experiments of UFGD under protocol number 022/2012. Wistar rats weighting approximately 156 ± 9 g were pretreated for 90 days with high-fructose diet (66%), prepared with 330 g of commercial chow (Labina) mixed with 660 g of fructose to induce hyperlipidemia. Concomitantly, normoglycemic rats were kept in commercial rodent chow (Labina) during all experimental period constituting the control group (control diet group, CD). All rats were kept in controlled light cycle and temperature with feed and water being offered ad libitum.
2.7. Experimental Design
Normoglycemic (n = 5) and hyperlipidemic Wistar rats (28) were assessed in this study. Hyperlipidemic rats were divided into four groups (n = 7 each) and daily provided by gavage for 60 days of water (control), simvastatin (30 mg·Kg−1 of body weight, simvastatin group), ciprofibrate (2 mg·Kg−1 of body weight, ciprofibrate group), and ExC (200 mg·Kg−1 of body weight, ExC group). At the end of treatment and after euthanasia, organs, tissue, and blood were collected for analysis.
2.8. Biochemical Analysis
The blood collected was centrifuged at 3000 rpm for 10 min and serum was used to measure total cholesterol, HDL-cholesterol, triglycerides, aminotransferases (AST and ALT), urea, and creatinine with support of Integra 400 Plus equipment (Roche™).
2.9. Statistical Analysis
Data are shown as mean ± standard error of the mean and were submitted to one-way analysis of variance (ANOVA) followed by Tukey posttest. The results were considered significant when P < 0.05.
3. Results
3.1. Chemical Profile
The concentration of total phenolic compounds and flavonoids was 391 ± 5.0 mg EAG·100 g−1 of ExC and 59 ± 3.6 mg EQ·100 g−1 of ExC, and analyses were positive for saponins.
3.2. Antioxidant Activity
Considering the presence of potentially antioxidant substances in ExC, an in vitro evaluation of DPPH free radical scavenging at different concentrations was performed. The 50% inhibitory concentration (IC50) and the maximum activity in assay of DPPH free radical scavenging of ExC were approximately one-third that of BHT and three times higher than that of ascorbic acid as shown in Table 1.
Table 1.
IC50 and maximum activity of DPPH free radical scavenging of standard antioxidants and the hydroethanolic extract of C. americana L. leaves (ExC).
| Treatment | IC50 (µg·mL−1) | n ∗ | Maximum activity | |
|---|---|---|---|---|
| % | µg·mL−1 | |||
| Ascorbic acid | 1.8 ± 0.4 | 2 | 92.3 ± 0.8 | 10 |
| BHT | 18.3 ± 4.5 | 2 | 93.7 ± 1.3 | 500 |
| ExC | 6.0 ± 0.5 | 3 | 96.5 ± 1.2 | 25 |
∗ n = number of independent experiments in triplicate. BHT = butylhydroxytoluene.
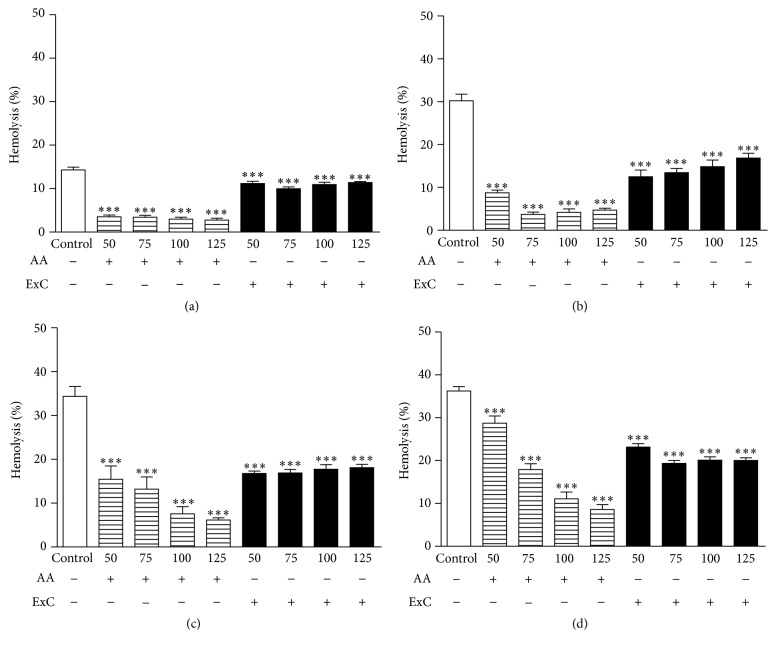
The antihemolytic potential of ExC was evaluated in erythrocytes submitted to lipid peroxidation and consequent hemolysis induced by 2,2′-azobis(2-amidinopropane) dihydrochloride (AAPH) for 240 min at different concentrations. ExC decreased the hemolysis of erythrocytes induced by AAPH in a time dependent manner, but independent of evaluated dose as shown in Figure 1.
Figure 1.
Hemolysis assessment at (a) 60, (b) 120, (c) 180, and (d) 240 min after addition of AAPH in erythrocytes at 2.5% (control) incubated with different concentrations (50–125 µg·mL−1) of ascorbic acid (AA) and hydroethanolic extract of C. americana L. leaves (ExC). ∗∗∗ P < 0.0001 versus control samples.
Thus, the release of malonaldehyde (MDA) that occurs during the lipid peroxidation process was assessed. Erythrocytes incubated with ExC for 240 min at 125 µg·mL−1 presented reduced MDA concentrations compared with control sample (P < 0.001) and similar to samples incubated with ascorbic acid (Figure 2).
Figure 2.
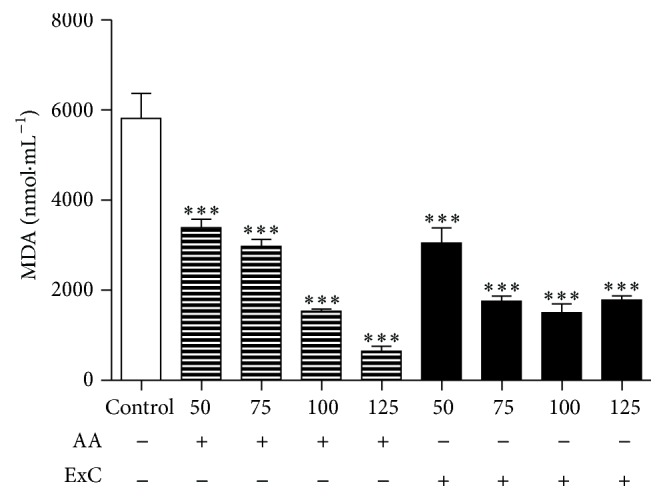
Malondialdehyde (MDA) concentration at 240 min after the addition of AAPH hemolysis inducer in 2.5% erythrocytes incubated with different concentrations (50–125 µg·mL−1) of ascorbic acid (AA) and hydroethanolic extract of C. americana L. leaves (ExC) compared with control samples. ∗∗∗ P < 0.001 versus control samples.
3.3. Hypolipidemic Effect
Rats treated with ExC showed decreased serum levels of total cholesterol and triglycerides, 34% and 45%, respectively, compared to control hyperlipidemic rats. Similar results were observed for the standard drugs, ciprofibrate, used to control cholesterol, and simvastatin, used to control triglycerides. Other biochemical parameters evaluated regarding the hepatic and renal functions were similar among groups investigated (Table 2). However, the analysis between hyperlipidemic and normolipidemic rats (t-test analyses) has shown an increase in the serum levels of AST in both groups treated with simvastatin and ciprofibrate, which did not occur in ExC group.
Table 2.
Serum lipid profile and hepatic and renal parameters of normolipidemic and hyperlipidemic Wistar rats induced by high-fructose diet (66%) treated with water (control), simvastatin (20 mg·kg−1 of body weight, simvastatin), ciprofibrate (2 mg·kg−1 of body weight, ciprofibrate), and hydroethanolic extract of C. americana L. leaves (ExC) (200 mg·kg−1 of body weight, ExC).
| Parameters | Normolipidemic | Hyperlipidemic | |||
|---|---|---|---|---|---|
| Control | Simvastatin | Ciprofibrate | ExC | ||
| Total cholesterol (mg·dL−1) | 80.0 ± 2.0a | 118.3 ± 13.0b | 91.3 ± 5.2ab | 86.4 ± 4.3a | 77.9 ± 4.1a |
| HDL-cholesterol (mg·dL−1) | 45.0 ± 2.4a | 58.0 ± 4.4b | 54.0 ± 3.1a | 47.0 ± 2.4a | 46.0 ± 2.3a |
| Triglycerides (mg·dL−1) | 138.3 ± 23.0a | 225.0 ± 33.0b | 119.0 ± 7.0a | 155.0 ± 14.0ab | 136.9 ± 7.5a |
| AST (U·L−1) | 174.0 ± 6.5 | 178.0 ± 14.5 | 220.0 ± 20.1 | 209.0 ± 9.4 | 191.8 ± 12.3 |
| ALT (U·L−1) | 53.2 ± 3.3 | 65.4 ± 7.0 | 64.9 ± 5.6 | 65.0 ± 4.0 | 64.3 ± 9.3 |
| Urea (U·L−1) | 23.0 ± 2.0 | 30.0 ± 3.1 | 21.0 ± 1.4 | 26.0 ± 3.7 | 24.0 ± 3.8 |
| Creatinine (U·L−1) | 0.28 ± 0.02 | 0.34 ± 0.03 | 0.32 ± 0.02 | 0.30 ± 0.02 | 0.27 ± 0.02 |
Mean values followed by different superscript letters indicate significant difference (P < 0.05).
4. Discussion
The aim of this study was to evaluate the antioxidant and hypolipidemic activities of the hydroethanolic extract of C. americana L. leaves related to both human health conditions and interest in the development of new drugs.
The oxidative balance in the body is regulated by endogenous and exogenous mechanisms, in which the excess of free radicals is related to many diseases [21]. The control of the excess of oxidative molecules includes especially exogenous intake of antioxidant molecules, which are largely found in plants [22]. The chemical composition of these plants has shown that same classes of polyphenols can exert such function such as flavonoids [23, 24]. The capacity of ExC of DPPH free radicals scavenging was intermediary among standard antioxidants and approximately three times higher than that of BHT. It is noteworthy that both controls used are isolated molecules, which stimulates new studies for the isolation of compounds from ExC, in which the presence of phenolic compounds and flavonoids was identified. In [25], investigating Dillenia suffruticosa, a plant of the Dilleniaceae family, identified the presence of polyphenols, although in lower amounts than those observed for C. americana L., and these compounds were correlated with antioxidant activity. In addition, another compound common to these two plants is saponin, which has shown hypocholesterolemic and anti-inflammatory activities; however, it has also been described to be able to promote destabilization of the cell membrane and induce hemolysis due to its emulsifying action [26–31], which was not observed for ExC at in vivo and in vivo studies.
ExC decreased lipid peroxidation induced by AAPH, protecting erythrocytes from cell death similarly to ascorbic acid, which is a vitamin with antioxidant capacity as demonstrated by decreased lipid peroxidation and malonaldehyde (MDA) production [32]. The lower levels of MDA produced during hemolysis induced by AAPH in samples incubated with ExC confirm the antioxidant action, also corroborated by [33] in the study of antioxidant activity of Toona sinensis leaves. The damage to the cell membrane resulting from lipid peroxidation induced by reactive species occurs in many diseases such as atherosclerosis, obesity, diabetes, hypertension, and cancer [21].
The importance of new products in the treatment and prevention of dyslipidemias becomes essential to reduce the mortality and morbidity due to cardiovascular complications. In addition, the search for less toxic drugs has increased the interest of the scientific community for natural products. The ExC showed to able to manage hyperlipidemia induced by high-fructose diet, reducing serum levels of total cholesterol and triglycerides, without signs of change in hepatic and renal function, suggesting that ExC is safe in the evaluated conditions. Ciprofibrate is a drug widely used to control cholesterol; however, it is contraindicated in patients with renal and hepatic disorders [34]. In this study, the ciprofibrate group showed an increase in liver and kidney weight (data not show), although the serum levels of ALT, creatinine, and urea remained unchanged. When compared to normolipidemic rats, the hyperlipidemic group treated with ciprofibrate presented an increase in serum level of AST.
The hypolipidemic activity of natural products can be correlated to the presence of flavonoids due to their properties of inhibiting cholesterol biosynthesis and absorption and modifying the activity of lipogenic and lipolytic enzymes, leading to reduced lipid metabolism [35–37], as observed in hyperlipidemic rats treated with ExC, which showed significant reduction in the levels of total cholesterol and triglycerides. Other molecules able to decrease the serum level of cholesterol are saponins [38], also present in ExC. It is very interesting that ExC was able to decrease both serum level of cholesterol and total triglycerides.
In conclusion, our results showed that Curatella americana L. leaves reduce oxidative stress by free radical scavenging and protect against lipid peroxidation and is also able to manage hyperlipidemia by decreasing serum level of cholesterol and triglycerides, similarly to standard drugs.
Acknowledgments
The authors would like to thank the Foundation to Support to Development of Education, Science and Technology of Mato Grosso do Sul State (FUNDECT), Brazilian National Research Council (CNPq), and Coordination of Improvement of Higher Level (CAPES). Edson Lucas dos Santos is recipient of fellowship from Brazilian National Research Council (CNPq), Brazil.
Competing Interests
The authors declare that they have no competing interests.
References
- 1.WHO. Global Atlas on Cardiovascular Disease Prevention and Control. Vol. 3. Geneva, Switzerland: World Health Organization; 2011. [Google Scholar]
- 2.Vogiatzi G., Tousoulis D., Stefanadis C. The role of oxidative stress in atherosclerosis. Hellenic Journal of Cardiology. 2009;50(5):402–409. [PubMed] [Google Scholar]
- 3.Li H., Horke S., Förstermann U. Vascular oxidative stress, nitric oxide and atherosclerosis. Atherosclerosis. 2014;237(1):208–219. doi: 10.1016/j.atherosclerosis.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 4.Bonomini F., Tengattini S., Fabiano A., Bianchi R., Rezzani R. Atherosclerosis and oxidative stress. Histology and Histopathology. 2008;23:381–390. doi: 10.14670/HH-23.381. [DOI] [PubMed] [Google Scholar]
- 5.Zanotti I., Dall'Asta M., Mena P., et al. Atheroprotective effects of (poly) phenols: a focus on cell cholesterol metabolism. Food and Function. 2015;6(1):13–31. doi: 10.1039/c4fo00670d. [DOI] [PubMed] [Google Scholar]
- 6.Calixto J. B. Twenty-five years of research on medicinal plants in Latin America: a personal view. Journal of Ethnopharmacology. 2005;100(1-2):131–134. doi: 10.1016/j.jep.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 7.de Souza P. M., de Sales P. M., Simeoni L. A., Silva E. C., Silveira D., de Oliveira Magalhães P. Inhibitory activity of α-amylase and α-glucosidase by plant extracts from the Brazilian cerrado. Planta Medica. 2012;78(4):393–399. doi: 10.1055/s-0031-1280404. [DOI] [PubMed] [Google Scholar]
- 8.De Toledo C. E. M., Britta E. A., Ceole L. F., et al. Antimicrobial and cytotoxic activities of medicinal plants of the Brazilian cerrado, using Brazilian cachaa as extractor liquid. Journal of Ethnopharmacology. 2011;133(2):420–425. doi: 10.1016/j.jep.2010.10.021. [DOI] [PubMed] [Google Scholar]
- 9.Melo e Silva F., de Paula J. E., Espindola L. S. Evaluation of the antifungal potential of Brazilian Cerrado medicinal plants. Mycoses. 2009;52(6):511–517. doi: 10.1111/j.1439-0507.2008.01647.x. [DOI] [PubMed] [Google Scholar]
- 10.Silva C. R., Vieira P. M., Santos S. C., Chen-Chen L. Assessment of genotoxicity and cytotoxicity of ‘lixeira’ (Curatella americana L.) using the prophage λ induction test (SOS inductest) Anais da Academia Brasileira de Ciências. 2012;84(1):149–156. doi: 10.1590/s0001-37652012000100015. [DOI] [PubMed] [Google Scholar]
- 11.Alexandre-Moreira M. S., Piuvezam M. R., Araújo C. C., Thomas G. Studies on the anti-inflammatory and analgesic activity of Curatella americana L. Journal of Ethnopharmacology. 1999;67(2):171–177. doi: 10.1016/s0378-8741(99)00009-4. [DOI] [PubMed] [Google Scholar]
- 12.Guerrero M. F., Puebla P., Carrón R., Martín M. L., Arteaga L., Román L. S. Assessment of the antihypertensive and vasodilator effects of ethanolic extracts of some Colombian medicinal plants. Journal of Ethnopharmacology. 2002;80(1):37–42. doi: 10.1016/s0378-8741(01)00420-2. [DOI] [PubMed] [Google Scholar]
- 13.Hiruma-Lima C. A., Rodrigues C. M., Kushima H., et al. The anti-ulcerogenic effects of Curatella americana L. Journal of Ethnopharmacology. 2009;121(3):425–432. doi: 10.1016/j.jep.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 14.de Medeiros P. M., Ladio A. H., Albuquerque U. P. Patterns of medicinal plant use by inhabitants of Brazilian urban and rural areas: a macroscale investigation based on available literature. Journal of Ethnopharmacology. 2013;150(2):729–746. doi: 10.1016/j.jep.2013.09.026. [DOI] [PubMed] [Google Scholar]
- 15.Meda A., Lamien C. E., Romito M., Millogo J., Nacoulma O. G. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chemistry. 2005;91(3):571–577. doi: 10.1016/j.foodchem.2004.10.006. [DOI] [Google Scholar]
- 16.Liberio S. A., Pereira A. L. A., Dutra R. P., et al. Antimicrobial activity against oral pathogens and immunomodulatory effects and toxicity of geopropolis produced by the stingless bee Melipona fasciculata Smith. BMC Complementary and Alternative Medicine. 2011;11, article 108:1–10. doi: 10.1186/1472-6882-11-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martucci M. E. P., De Vos R. C. H., Carollo C. A., Gobbo-Neto L. Metabolomics as a potential chemotaxonomical tool: application in the genus Vernonia Schreb. PLoS ONE. 2014;9(4) doi: 10.1371/journal.pone.0093149.e93149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Casagrande J. C., Macorini L. F., Antunes K. A., et al. Antioxidant and cytotoxic activity of hydroethanolic extract from Jacaranda decurrens leaves. PloS ONE. 2014;9(11) doi: 10.1371/journal.pone.0112748.e112748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Valente M. J., Baltazar A. F., Henrique R., Estevinho L., Carvalho M. Biological activities of Portuguese propolis: protection against free radical-induced erythrocyte damage and inhibition of human renal cancer cell growth in vitro . Food and Chemical Toxicology. 2011;49(1):86–92. doi: 10.1016/j.fct.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 20.Campos J. F., dos Santos U. P., Macorini L. F. B., et al. Antimicrobial, antioxidant and cytotoxic activities of propolis from Melipona orbignyi (Hymenoptera, Apidae) Food and Chemical Toxicology. 2014;65:374–380. doi: 10.1016/j.fct.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 21.Taniyama Y., Griendling K. K. Reactive oxygen species in the vasculate: molecular and cellular mechanisms. Hypertension. 2003;42:1075–1081. doi: 10.1161/01.hyp.0000100443.09293.4f. [DOI] [PubMed] [Google Scholar]
- 22.Liu R. H. Health-promoting components of fruits and vegetables in the diet. Advances in Nutrition. 2013;4(3):384S–392S. doi: 10.3945/an.112.003517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Middleton E. J. Flavonoids in the Living System. Vol. 439. Berlin, Germany: Springer; 1998. Effect of plant flavonoids on immune and inflammatory cell function; pp. 175–182. (Advances in Experimental Medicine and Biology). [DOI] [PubMed] [Google Scholar]
- 24.Dryden G. W., Song M., McClain C. Polyphenols and gastrointestinal diseases. Current Opinion in Gastroenterology. 2006;22(2):165–170. doi: 10.1097/01.mog.0000208463.69266.8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Armania N., Yazan L. S., Musa S. N., et al. Dillenia suffruticosa exhibited antioxidant and cytotoxic activity through induction of apoptosis and G2/M cell cycle arrest. Journal of Ethnopharmacology. 2013;146(2):525–535. doi: 10.1016/j.jep.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 26.Francis G., Kerem Z., Makkar H. P. S., Becker K. The biological action of saponins in animal systems: a review. British Journal of Nutrition. 2002;88(6):587–605. doi: 10.1079/bjn2002725. [DOI] [PubMed] [Google Scholar]
- 27.Bangham A. D., Horne R. W., Glauert A. M., Dingle J. T., Lucy J. A. Action of saponin on biological cell membranes. Nature. 1962;196:952–955. doi: 10.1038/196952a0. [DOI] [PubMed] [Google Scholar]
- 28.Nasri S., Ben Salem H. Effect of oral administration of Agave americana or Quillaja saponaria extracts on digestion and growth of Barbarine female lamb. Livestock Science. 2012;147(1–3):59–65. doi: 10.1016/j.livsci.2012.04.001. [DOI] [Google Scholar]
- 29.Monterrosas-Brisson N., Arenas Ocampo M. L., Jiménez-Ferrer E., et al. Anti-inflammatory activity of different agave plants and the compound Cantalasaponin-1. Molecules. 2013;18(7):8136–8146. doi: 10.3390/molecules18078136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gilabert-Oriol R., Mergel K., Thakur M., et al. Real-time analysis of membrane permeabilizing effects of oleanane saponins. Bioorganic and Medicinal Chemistry. 2013;21(8):2387–2395. doi: 10.1016/j.bmc.2013.01.061. [DOI] [PubMed] [Google Scholar]
- 31.Voutquenne L. L. C., Lavaud C., Massiot G., Le Men-Olivier L. Structure-activity relationships of haemolytic saponins. Pharmaceutical Biology. 2002;40(4):253–262. doi: 10.1076/phbi.40.4.253.8470. [DOI] [Google Scholar]
- 32.Abdollahzad H., Eghtesadi S., Nourmohammadi I., Khadem-Ansari M., Nejad-Gashti H., Esmaillzadeh A. Effect of vitamin C supplementation on oxidative stress and lipid profiles in hemodialysis patients. International Journal for Vitamin and Nutrition Research. 2009;79(5-6):281–287. doi: 10.1024/0300-9831.79.5.281. [DOI] [PubMed] [Google Scholar]
- 33.Hseu Y.-C., Chang W.-H., Chen C.-S., et al. Antioxidant activities of Toona sinensis leaves extracts using different antioxidant models. Food and Chemical Toxicology. 2008;46(1):105–114. doi: 10.1016/j.fct.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 34.Broeders N., Knoop C., Antoine M., Tielemans C., Abramowicz D. Fibrate-induced increase in blood urea and creatinine: is gemfibrozil the only innocuous agent? Nephrology Dialysis Transplantation. 2000;15(12):1993–1999. doi: 10.1093/ndt/15.12.1993. [DOI] [PubMed] [Google Scholar]
- 35.Borradaile N. M., de Dreu L. E., Barrett P. H. R., Behrsin C. D., Huff M. W. Hepatocyte ApoB-containing lipoprotein secretion is decreased by the grapefruit flavonoid, naringenin, via inhibition of MTP-mediated microsomal triglyceride accumulation. Biochemistry. 2003;42(5):1283–1291. doi: 10.1021/bi026731o. [DOI] [PubMed] [Google Scholar]
- 36.Whitman S. C., Kurowska E. M., Manthey J. A., Daugherty A. Nobiletin, a citrus flavonoid isolated from tangerines, selectively inhibits class A scavenger receptor-mediated metabolism of acetylated LDL by mouse macrophages. Atherosclerosis. 2005;178(1):25–32. doi: 10.1016/j.atherosclerosis.2004.07.034. [DOI] [PubMed] [Google Scholar]
- 37.Brusq J.-M., Ancellin N., Grondin P., et al. Inhibition of lipid synthesis through activation of AMP kinase: an additional mechanism for the hypolipidemic effects of berberine. Journal of Lipid Research. 2006;47(6):1281–1288. doi: 10.1194/jlr.m600020-jlr200. [DOI] [PubMed] [Google Scholar]
- 38.Patel S., Santani D., Shah M., Patel V. Anti-hyperglycemic and anti-hyperlipidemic effects of bryonia laciniosa seed extract and its saponin fraction in streptozotocin-induced diabetes in rats. Journal of Young Pharmacists. 2012;4(3):171–176. doi: 10.4103/0975-1483.100024. [DOI] [PMC free article] [PubMed] [Google Scholar]