Abstract
Background
Bridging therapy plays an increasingly important role in the management of patients with hepatocellular carcinoma (HCC) awaiting liver transplantation (LT). Combination therapy with drug-eluting bead transarterial chemoembolization (DEB-TACE) and percutaneous thermal ablation, such as radiofrequency ablation (RFA) or microwave ablation (MWA), has shown success at prolonging survival and bridging patients to LT. However, few studies have evaluated the two combination therapy regimens head-to-head at a single institution, and fewer have compared histopathology. This retrospective study compares tumor coagulation on explanted livers in patients with HCC treated with DEB-TACE sequentially combined with RFA versus MWA.
Methods
From 2005 to 2015, 42 sequential patients underwent combination therapy prior to LT by Milan criteria, with 11 patients (11 tumors; mean, 2.9 cm; range, 1.8–4.3 cm) in the DEB-TACE/RFA cohort and 31 patients (40 tumors; mean, 2.4 cm; range, 1.1–5.4 cm) in the DEB-TACE/MWA cohort. The mean TACE procedures in the RFA and MWA cohorts were 1.3 (range, 1–2) and 1.3 (range, 1–3), respectively. The mean thermal ablations in the RFA and MWA cohorts were 1.2 (range, 1–2) and 1.3 (range, 1–3), respectively. Tumor coagulation was evaluated on explanted livers.
Results
Mean tumor coagulation in the RFA and MWA cohorts were 88.9% (range, 0–100%) and 90.5% (range, 30–100%), respectively (P=0.82). Rates of complete tumor coagulation in the RFA and MWA cohorts were 45% and 53%, respectively (P=0.74). No difference in tumor coagulation was found between the cohorts when separating tumors <3 cm (P=0.21) and >3 cm (P=0.09). Among all 51 tumors, the 36 in complete response (CR) on imaging at LT demonstrated mean tumor coagulation of 95.8%. No correlation was found between tumor coagulation and initial tumor size or time interval to LT. No tumor seeding was seen along the ablation tracts.
Conclusions
RFA and MWA in sequential combination with DEB-TACE, used as a bridge to LT, are equally efficacious at inducing HCC tumor coagulation.
Keywords: Hepatocellular carcinoma (HCC), chemoembolization, radiofrequency, microwave, ablation
Introduction
Bridging therapy plays an increasingly important role in the management of patients with hepatocellular carcinoma (HCC) awaiting liver transplantation (LT), as wait list dropout rates from disease progression reach as high as 11% after 6 months and 38% after 12 months (1,2). Locoregional therapy such as drug-eluting bead transarterial chemoembolization (DEB-TACE) and percutaneous thermal ablation have been successful at prolonging survival in patients with HCC and at bridging patients to LT (1-4). However, both techniques as monotherapy have also demonstrated limitations, such as incomplete tumor necrosis, tumor recurrence, and inadequate control of medium to large size HCC (5-9). In this retrospective study, we evaluate the histopathologic efficacy of DEB-TACE combined with percutaneous thermal ablation, building upon prior studies demonstrating enhanced overall survival and recurrence-free survival with combination therapy (5,9-11).
Radiofrequency ablation (RFA) and microwave ablation (MWA) are the most commonly used thermal ablation techniques. This study extends upon the body of work evaluating each of these techniques individually by comparing both techniques head-to-head at a single institution when used as part of sequential combination therapy with DEB-TACE.
The primary endpoint of this study was to compare histopathologic coagulation necrosis of HCC on explanted livers, comparing DEB-TACE/RFA to DEB-TACE/MWA. Secondary endpoints included comparing tumor coagulation by tumor size, number of treatments, days to transplantation, and imaging response.
Methods
Institutional review board approval was obtained prior to the study and adherence to Health Insurance Portability and Accountability Act guidelines was maintained throughout. This study conforms to the provisions of the Declaration of Helsinki (12).
Patients
Forty-two consecutive patients with cirrhosis and HCC who underwent DEB-TACE in conjunction with RFA or MWA from May 2005 to March 2015 prior to LT at our institution were included for the present retrospective analysis. Demographic data and treatment information of both cohorts are listed in Table 1. The DEB-TACE/RFA cohort preceded the DEB-TACE/MWA cohort as the interventional radiology department transitioned from RFA to MWA at a mid-time point in 2009.
Table 1. Patient demographics & treatment characteristics.
| Characteristics | DEB-TACE/RFA | DEB-TACE/MWA | P value |
|---|---|---|---|
| Gender, N [%] | |||
| M | 6 [55] | 21 [68] | 0.48 |
| F | 5 [45] | 10 [32] | |
| Age (y) | |||
| Median | 62 | 57 | 0.09 |
| Range | 49–72 | 45–71 | |
| Primary cause of liver disease, N [%] | |||
| HBV | 0 [0] | 2 [6] | 0.79 |
| HCV | 8 [73] | 27 [87] | 0.89 |
| Alcohol | 0 [0] | 0 [0] | 1.00 |
| NASH/cryptogenic | 2 [18] | 2 [6] | 0.84 |
| Autoimmune | 1 [9] | 0 | 0.72 |
| Initial tumor size, cm | |||
| Mean | 2.9 | 2.4 | 0.20 |
| Range | 1.8–4.3 | 1.1–5.4 | |
| Tumor size, N [%] | |||
| <3 cm | 6 [55] | 32 [80] | 0.12 |
| >3 cm | 5 [45] | 8 [20] | |
| Tumors, per patient | 1.0 | 1.3 | 0.18 |
| BCLC stage, N [%] | |||
| A | 11 [100] | 29 [97] | 1.00 |
| B | 0 | 2 [3] | |
| DEB-TACE, per tumor | |||
| Mean | 1.3 | 1.3 | 0.73 |
| Range | 1–2 | 1–3 | |
| Thermal ablations, per tumor | |||
| Mean | 1.2 | 1.2 | 0.72 |
| Range | 1–2 | 1–3 | |
| Interval to LT, days | |||
| Median | 76 | 115 | 0.23 |
| Range | 29–297 | 8–982 | |
| Imaging response at LT, N [%] | |||
| Tumor(s) at CR | 10 [91] | 26 [65] | 0.14 |
| Tumor(s) at PR/SD | 0 [0] | 4 [10] | 0.57 |
| LT prior to follow-up CT/MR | 1 [9] | 10 [25] | 0.42 |
DEB-TACE, drug-eluting bead transarterial chemoembolization; RFA, radiofrequency ablation; MWA, microwave ablation; BCLC, Barcelona-Clinic Liver Cancer classification; LT, liver transplantation; CR, complete response; PR, partial response; SD, stable disease.
Eleven patients were treated with DEB-TACE/RFA (total 11 tumors; mean 1.0 tumors per patient), and 31 patients were treated with DEB-TACE/MWA (total, 40 tumors; mean, 1.3 tumors per patient) (P=0.18). No significant difference was seen between the cohorts regarding age (P=0.09), sex (P=0.48), etiology of liver cirrhosis, initial tumor size (P=0.20), BCLC staging (P=0.54), number chemoembolization procedures per tumor (P=0.73), or number of thermal ablations per tumor (P=0.72).
HCC was diagnosed on multi-phase CT with Omnipaque 350 (GE Healthcare, Amersham, UK) or MRI with MultiHance (Bracco Diagnostic Inc., Monroe Township, NJ, USA) by the presence of a hypervascular liver mass (Figure 1) greater than 1 cm in the arterial phase with contrast washout in the portal venous or delayed phase, in accordance with the American Association for the Study of Liver Diseases (AASLD) guidelines (13). Underlying disease burden was staged with the Child-Pugh criteria, United Network for Organ Sharing (UNOS), and the Barcelona-Clinic Liver Cancer (BCLC) classification (13). Patient demographics and assessment of portal venous hypertension and its sequelae were also taken into consideration prior to intervention.
Figure 1.
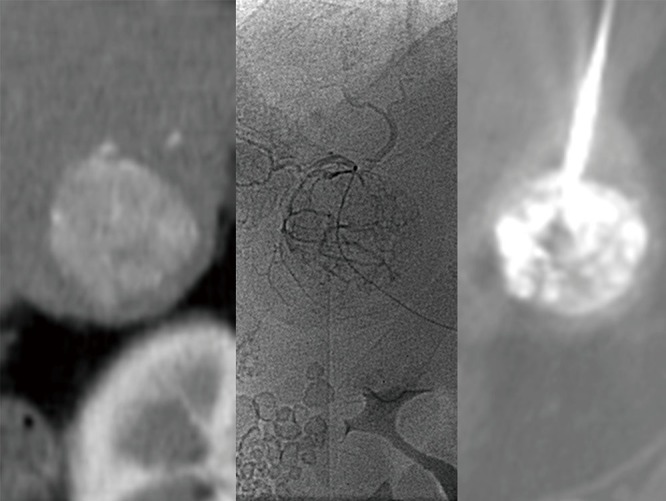
Hepatocellular carcinoma diagnosed by CT (left), identified on angiography prior to chemoembolization (middle), and localized by CT for thermal ablation (right).
Patients were chosen for combination therapy once discussed in a multi-disciplinary conference and meeting Milan criteria for LT. If the patient exceeded Milan criteria yet plan for transplantation listing existed, combination therapy was performed to downsize their tumor(s) to within Milan criteria prior to listing. Exclusion criteria included tumor locations unamenable to thermal ablation or alpha-fetoprotein (AFP) levels >1,000 ng/mL.
Sequential combination therapy
DEB-TACE/RFA and DEB-TACE/MWA were performed at a single tertiary care center by six physicians with certificates of added qualification for vascular interventions and 7–18 years of experience in performing the procedures. To ensure consistency in the reporting of results, this manuscript follows reporting standards on transcatheter therapy for hepatic malignancy and image-guided tumor ablation (14,15). Patients underwent DEB-TACE first, followed by RFA or MWA on the subsequent day. The procedures were performed with patients under moderate sedation using intravenous 50–100 mcg fentanyl and 1–2 mg midazolam with constant hemodynamic monitoring.
TACE was performed with a solution containing 50 mg of superabsorbent polymer drug-eluting embolic agent (Quadrasphere 50–100 micron diameter, 25 mg dry weight per vial, Merit Medical Systems, Utah, USA) that were loaded with either 50 mg of Epirubicin (Farmorubicin, Pfizer, New York, USA) or with 50–100 mg of Doxorubicin (Pfizer, New York, USA), depending on national and institutional pharmaceutical availability, and then reconstituted in 20 mL of 0.9% saline. After two hours of loading the chemotherapeutic agents into the spheres, the mixture was diluted with 20 mL of non-ionic contrast prior to administration. Of the 15 total DEB-TACE treatments in the RFA cohort and 52 total DEB-TACE treatments in the MWA cohort, 12 and 25 were performed with doxorubicin, and 3 and 27 with epirubicin, respectively (P=0.06). Chemoembolization material was administered under continuous fluoroscopic guidance (Figure 1) until visible stasis of flow to the sub-selected hepatic artery.
RFA was performed with a commercially available system (RF 3000 Radiofrequency Ablation System, Boston Scientific, Natick, MA, USA) to generate up to 200 W of energy to cause adequate coagulation necrosis of the target tumor and a 1-cm margin. A 10-tine monopolar electrode applicator with 15-gauge cannula (LeVeen Needle, 3–5 cm array, Boston Scientific, Natick, MA, USA) was advanced under ultrasound and/or CT guidance, with positioning confirmed by CT prior to expansion of the tines (Figure 1). Treatment began at a 50 W level, with wattage increasing 10 W every 2 minutes for 10 minutes or until tissue impedance rose and prevented further current flow. Two cycles with the impedance control algorithm were performed. As the probe was withdrawn, small amounts of energy were used to cauterize the hepatic tract.
A transition was made in 2009 to MWA at our institution based on preference of use and studies suggesting reduced heat sink effect, increased intratumoral temperatures, larger ablation zones, decreased tumoral charring (16). MWA was performed with a commercially available system with a 915-MHz generator and a maximum output of 45 W of energy (Evident MW Ablation System, Covidien, Boulder, CO, USA). A 13-gauge transcutaneous, water-cooled dipole antenna was placed under ultrasound guidance, and positioning was confirmed by CT. Tumors were treated for 5–10 minutes at 40 W to ensure adequate coagulation necrosis of the target tumor and a 1-cm margin of tissue. Of the 50 total MWA procedures, 4 were performed with an overlapping ablation or additional antenna to attain adequate ablative margins. This is compared to 7 of 13 ablations in the RFA cohort that required such measures (P<0.001).
Follow-up
Patients were followed using multi-phase CT or contrast-enhanced MRI one month after treatment, then at three-month intervals for the first year, and then at six-month intervals until LT. One patient (9%) in the RFA cohort was followed with MRI, versus eleven patients (28%) in the MWA cohort (P=0.26). Response status was assessed using European Association for the Study of the Liver (EASL) criteria (17). Local tumor response was determined as complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD).
Criterion for re-treatment was any response less than CR on follow-up imaging. Four patients in the RFA cohort and nine patients in the MWA cohort required re-treatment by this criterion (P=0.71). Re-treatment was standardized as the same combination therapy as the given patient’s initial treatment. Repeat combination therapy was attempted on all tumors until CR was achieved or LT was performed. In the RFA cohort, one patient (1 tumor) underwent transplantation prior to their follow-up imaging. In the MWA cohort, eight patients (10 tumors) underwent transplantation prior to their follow-up imaging, and six patients (6 tumors) underwent transplantation prior to the achieving CR (P=0.08).
Explant liver evaluation
Each explanted liver underwent pathological evaluation at the time of surgery by the Department of Pathology. Median time interval from last procedure to LT was 76 days (range, 29–297 days) in the RFA cohort and 115 days (range, 8–982 days) in the MWA cohort (P=0.23). Median time interval from last imaging to LT was 30 days (range, 7–67 days) in the RFA cohort and 24 days (range, 8–90 days) in the MWA cohort (P=0.53).
For macroscopic evaluation, all explanted livers underwent formalin fixation and were dissected into 5 mm sections. Liver tumors were identified, measured in three dimensions, and assessed for tumor coagulation. Macroscopic coagulation was identified as soft, granular, and friable tissue with yellowed discoloration. The ablation tracts were evaluated for potential seeding.
All identified tumors were embedded in paraffin and underwent microscopic evaluation. Three µm sections with hematoxylin and eosin-stained (H&E) slides were made through the center and boundaries of the tumor, with care to include the adjacent non-ablated liver parenchyma. Additional sections were performed as needed to ensure any areas of potentially viable tissue were evaluated. Microscopic coagulation was defined as showing evidence of cell death, which included nuclear fragmentation, ill-defined or absent cell borders, cellular debris, and altered/absent background tissue architecture. Secondary features such as tumor location within the ablative margin, poor cellular staining, paucicellular surrounding fibrosis, and lack of neovascularization were taken into account in tissue with suspected thermal fixation. Based upon the combination macroscopic 5 mm sections, select microscopic 3 µm sections, and additional sections of potentially viable tissue throughout the tumor, HCC coagulation was determined visually to the nearest 5% (i.e., 0%, 5%, …, 95%, 100%). If a lesion did not initially demonstrate any evidence of viable tumor by these means, the entire tumor was submitted for 3 µm sections to fully confirm 100% coagulation necrosis.
Our exclusion criteria included patients with tumors for which a percentage of coagulation was not determined at LT, leading to the exclusion of four patients from the DEB-TACE/RFA cohort. This could not be determined retroactively as the full specimens were no longer available.
Statistical analysis
Demographic data were reported as totals and percentage of totals. Fisher exact test and the t-test were used to compare demographic data between the two cohorts. Continuous data were reported as means, medians, and ranges. The t-test was used for comparison of tumor size and tumor coagulation percentage. Regression analyses were used to compare tumor coagulation percentage to initial tumor size and time interval to transplantation, with R2=1 indicating a correlation trend line of perfect fit. A two-tailed P value <0.05 was considered statistically significant. Data analyses were performed using Stata Version 13 (StataCorp., College Station, TX, USA).
Results
Local tumor response on imaging
Among the 11 tumors treated with DEB-TACE/RFA, 10 (91%) had achieved in CR by the time of LT, and 1 tumor (9%) underwent LT prior to any follow up imaging. Among the 40 tumors treated with DEB-TACE/MWA, 26 (67%) had achieved CR by the time of LT, 4 (10%) remained in PR or SD on last imaging prior to LT, and 10 tumors (25%) underwent LT prior to any follow up imaging. No tumors demonstrated PD after treatment in either cohort. Per tumor analysis did not show a significant difference between the two cohorts in CR vs. non-CR rate (P=0.15).
Liver histology evaluation at transplantation
Mean tumor coagulation in the RFA and MWA cohorts were 88.9% (range, 0–100%) and 90.5% (range, 30–100%), respectively (P=0.82). Rates of complete tumor coagulation in the RFA and MWA cohorts were 45% and 53%, respectively (P=0.74). No difference in tumor coagulation was found between the cohorts when separating tumors <3 cm (P=0.33) and >3 cm (P=0.09). Within each individual cohort, no difference was seen in tumor coagulation percentage between patients who received combination therapy once versus required re-treatment (RFA cohort: P=0.53, MWA cohort: P=0.16). These results are listed in Table 2.
Table 2. Histopathology results.
| Variables | DEB-TACE/RFA | DEB-TACE/MWA | P value |
|---|---|---|---|
| Tumor coagulation, % | |||
| Mean | 88.9 | 90.5 | 0.82 |
| Range | 0–100 | 30–100 | |
| 100% tumor coagulation, N [%] | 5 [45] | 21 [53] | 0.74 |
| Tumor coagulation, mean % [N] | |||
| <3 cm tumors | 80.8 [6] | 90.9 [30] | 0.33 |
| >3 cm tumors | 98.8 [5] | 88.9 [10] | 0.09 |
| Tumors treated once | 84.1 [7] | 92.8 [27] | 0.27 |
| Tumors re-treated | 97.3 [4] | 86.9 [13] | 0.26 |
| Ablation tract seeding, foci | 0 | 0 | N/A |
DEB-TACE, drug-eluting bead transarterial chemoembolization; RFA, radiofrequency ablation; MWA, microwave ablation; N/A, not available.
Final imaging response at the time of LT was compared to pathological response in all 51 tumors, combining both cohorts. These results are listed in Table 3. Of note, the tumors in PR/SD at LT and the tumors undergoing LT prior to any imaging demonstrated significantly lower mean coagulation of 80% (P<0.01) and 75.4% (P<0.01), respectively when compared to tumors in CR.
Table 3. Histopathology response compared to imaging response.
| Variables | Imaging response (all 51 tumors) |
||
|---|---|---|---|
| Tumors with CR | Tumors with PR/SD | LT prior to follow-up CT/MR | |
| Tumor coagulation, mean % [N] | 95.8 [36] | 80 [4] | 75.4 [11] |
| P value (vs. tumors at CR) | <0.01 | <0.01 | |
CR, complete response; PR, partial response; SD, stable disease; LT, liver transplantation.
Regression analyses showed no discernable correlation between initial tumor size and tumor coagulation percentage in either the RFA (R2=0.19, P=0.59) or MWA cohort (R2=0.03, P=0.88). Additionally, no discernable correlation was seen between time interval from treatment to transplantation and tumor coagulation percentage at LT in the RFA (R2=0.05, P=0.88) or MWA cohort (R2=0.07, P=0.72).
Among all 42 patients, additional foci of HCC were found in 17 patients (40%) at explantation, ranging from 0.3–1.5 cm, which were not detected previously by CT or MRI and thus not treated. No tumor seeding was seen along the ablation tracts in either cohort.
Complications
The combination therapy procedures were well tolerated without major complications in either cohort, as defined by the Society of Interventional Radiology clinical practice guidelines (18). In the RFA cohort, nominal therapy was provided for two patients (18%) experiencing abdominal pain. In the MWA cohort, nominal therapy was provided for eight patients (26%): five patients with abdominal pain, one patient with nausea, and two patients with shortness of breath (P=0.71).
Discussion
This study demonstrates that combination therapy of DEB-TACE with percutaneous thermal ablation is highly efficacious at inducing tumor coagulation, echoing previous studies demonstrating high rates of complete tumor coagulation in HCC tumors treated with combination therapy. Ashoori et al. demonstrated a complete tumor coagulation rate of 76.9% in 26 tumors (1.4–5.0 cm, median, 2.8 cm) treated with TACE-RFA (19). For comparison, with TACE alone, Herber et al. and Stampf et al. found complete necrosis rates of only 17% (105 tumors, 1.0–12.0 cm; mean, 4.1 cm; mean, 5.4 procedures) and 38% (104 tumors within Milan criteria; median, 3 procedures), respectively, despite a higher average number of procedures per tumor than in this study (20,21). This efficacy of combination therapy on histopathology mirrors prior studies demonstrating improved overall survival and disease-free survival in patients in patients treated with combination therapy versus monotherapy (5,6).
Combination therapy is safe and provides multiple proposed advantages to monotherapy, including reduced heat sink effect, larger uniform ablation zones, and control of micrometastases (5,6). From a procedural standpoint, DEB-TACE prior to ablation improves visualization of small tumors in locations poorly visualized otherwise by CT or ultrasound, ensuring effective localization for ablation. Additionally, the larger ablation zone and reduced heat sink effect of combination therapy assures adequate necrosis for tumors in locations otherwise technically problematic for thermal ablation alone. It is for these reasons our institution has maintained sequential combination therapy as first line treatment for HCC in patients being bridged to LT.
Additionally, this study specifically revealed no statistical difference between RFA and MWA in tumor coagulation percentage and complete tumor coagulation rate. This expands upon initial studies demonstrating equitable CR rates and equitable overall survival (4,22,23) and adds to the body of work supporting the use of combination therapy as a bridge to LT in patients with HCC.
Initial tumor size did not significantly influence coagulation necrosis percentage in this population of tumors, including tumors >3 cm, which is promising for downsizing patients exceeding Milan criteria in preparation for LT. Initial size of HCC tumors, particularly tumors >3 cm, have previously been shown to affect tumor necrosis with monotherapy (5,6,23), thus, this study demonstrates an additional benefit of combination therapy. This mirrors prior studies showing improved survival with combination therapy over monotherapy in lesions ranging up to 7 cm in size (5,6). However, it is acknowledged that the mean tumor size in this study was <3 cm and only 15 of 51 tumors (29%) measured equal to or greater than 3 cm, thus, supporting the need for further studies involving larger HCC lesions. Additionally, the length of time to transplantation also did not significantly influence coagulation necrosis percentage in this study, which is reassuring for patients awaiting transplantation for extended periods. This has been echoed in prior studies (24).
Tumor coagulation was found to correlate well with imaging response, with significantly higher coagulation necrosis in patients with confirmed CR on imaging. It was noted that 39% of patients with CR on imaging still demonstrated a portion of viable tumor on histopathology, however these amounts of viable tumor were microscopic. It is presumed that CT and MRI cannot detect microscopic tumor with perfect sensitivity, accounting for the discrepancy in CR rates by imaging and complete coagulation rates by pathology. This overestimation of response to therapy on imaging has been shown previously and was expected (24).
Among the 42 livers undergoing 63 ablation procedures in this study, no evidence of ablation tract seeding was found. Though ablation of the probe/antennae tract is routinely performed primarily to prevent bleeding, these findings suggest tract ablation may play a role in minimizing tract seeding. Additionally, 17 of the livers examined were found to have incidental HCC not seen on imaging, however, this is a common discovery expected at LT and has been shown to not adversely affect post-transplantation outcomes (25).
The following limitations with this study were recognized. First, this study was retrospective in nature and over a wide date range. As a result, the DEB-TACE/RFA cohort is notably smaller than the DEB-TACE/MWA cohort, as our institution did not begin uniformly performing combination therapy for HCC until approximately 2005, as literature suggesting its benefits over monotherapy began emerging. Also as a result of this extended date range, slight variations in chemoembolization regimens existed at our institution, particularly given that literature has yet to determine an optimal chemotherapeutic and embolic agent regimen for DEB-TACE (26). However, the chemoembolization regimens were not found to be significantly different between the two cohorts. Additionally, this extended time period led to loss of pathological material, making re-evaluation of some tumors infeasible. This resulted in the exclusion of four patients who underwent DEB-TACE/RFA from our study entirely. Second, as our institution is a transplantation center, one patient in the RFA cohort and eight patients in the MWA cohort rapidly underwent LT prior to scheduled follow-up imaging to detect residual enhancement (P=0.08). We believe this to be an unavoidable circumstance in the study of patients with HCC, as LT is the ultimate goal in treatment. Nonetheless, this undoubtedly resulted in patients with incompletely treated tumors, underestimating the histopathologic efficacy and CR rate of combination therapy, particularly of MWA in this study, though differences remained statistically insignificant. This was the case with the two patients’ tumors demonstrating coagulation necrosis of 30% or less, despite accurate localization confirmed on pathology. Confounding this, differing tumor biology has also been theorized in the past to contribute to variation in response to logoregional therapy (27). Third, follow-up imaging was predominantly with CT. Given the expected overestimation of CR by CT compared to histopathology seen in this study and others (27), MRI may theoretically provide improved detection of viable tumor for re-treatment and further improve the efficacy of bridging therapy. Our institution has since shifted towards use of MRI. As such, the higher rate of MRI use in the more recent MWA cohort vs. the RFA cohort (29% vs. 9%, P=0.26) may suggest an explanation for the slightly lower rate of CR in the MWA cohort (65% vs. 91%, P=0.14), though statistically insignificant. It is for these reasons we primarily focus upon pathological response rather than imaging response in this study. Fourth, immunohistochemical staining was not employed to assess tumor viability versus thermal fixation, as is the practice at some institutions. However, secondary findings of thermal fixation were well known to the interpreting pathologists in this study and have been readily recognized in prior studies (28,29). The diagnostic difficulty with thermal fixation on H&E staining occurs when tissue is evaluated acutely (1–3 days) after ablation, which was not the case in this study, as our cohorts’ median times from treatment to transplantation were 76 and 115 days. Accurate diagnosis was confidently made as the findings of cell death are known to increase recognizably over time (29).
In conclusion, this study provides a unique head-to-head histopathologic evaluation of patients with HCC treated with sequential combination therapy DEB-TACE/RFA versus DEB-TACE/MWA as a bridge to LT, demonstrating equivalent tumor coagulation on explanted livers between the two regimens.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: Resection versus transplantation. Hepatology 1999;30:1434-40. 10.1002/hep.510300629 [DOI] [PubMed] [Google Scholar]
- 2.Yao FY, Bass NM, Nikolai B, et al. Liver transplantation for hepatocellular carcinoma: analysis of survival according to the intention-to-treat principle and dropout from the waiting list. Liver Transpl 2002;8:873-83. 10.1053/jlts.2002.34923 [DOI] [PubMed] [Google Scholar]
- 3.Pompili M, Francica G, Ponziani FR, et al. Bridging and downstaging treatments for hepatocellular carcinoma in patients on the waiting list for liver transplantation. World J Gastroenterol 2013;19:7515-30. 10.3748/wjg.v19.i43.7515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang L, Wang N, Shen Q, et al. Therapeutic efficacy of percutaneous radiofrequency ablation versus microwave ablation for hepatocellular carcinoma. PLoS One 2013;8:e76119. 10.1371/journal.pone.0076119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peng ZW, Zhang YJ, Chen MS, et al. Radiofrequency ablation with or without transcatheter arterial chemoembolization in the treatment of hepatocellular carcinoma: a prospective randomized trial. J Clin Oncol 2013;31:426-32. 10.1200/JCO.2012.42.9936 [DOI] [PubMed] [Google Scholar]
- 6.Ni JY, Liu SS, Xu LF, et al. Meta-analysis of radiofrequency ablation in combination with transarterial chemoembolization for hepatocellular carcinoma. World J Gastroenterol 2013;19:3872-82. 10.3748/wjg.v19.i24.3872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu LF, Sun HL, Chen YT, et al. Large primary hepatocellular carcinoma: transarterial chemoembolization monotherapy versus combined transarterial chemoembolization-percutaneous microwave coagulation therapy. J Gastroenterol Hepatol 2013;28:456-63. 10.1111/jgh.12088 [DOI] [PubMed] [Google Scholar]
- 8.Fan WZ, Yang JY, Lü MD, et al. Transcatheter arterial chemoembolization plus percutaneous thermal ablation in large hepatocellular carcinoma: clinical observation of efficacy and predictors of prognostic factors. Zhonghua Yi Xue Za Zhi 2011;91:2190-4. [PubMed] [Google Scholar]
- 9.Tang ZY, Zhou XD, Ma ZC, et al. Multimodality treatment of hepatocellular carcinoma. Journal of Gastroenterology and Hepatology 1998;13:S315-S319. [DOI] [PubMed] [Google Scholar]
- 10.Zhang L, Yin X, Gan YH, et al. Radiofrequency ablation following first-line transarterial chemoembolization for patients with unresectable hepatocellular carcinoma beyond the Milan criteria. BMC Gastroenterol 2014;14:11. 10.1186/1471-230X-14-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo YJ, Huang WS, Zhou B, et al. Transarterial chemoembolization plus computed tomography-guided percutaneous radiofrequency ablation for small hepatocellular carcinoma in special locations. Zhonghua Yi Xue Za Zhi 2013;93:2627-30. [PubMed] [Google Scholar]
- 12.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013;310:2191-4. 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 13.Bruix J, Sherman M, American Association for the Study of Liver Diseases . Management of hepatocellular carcinoma: An update. Hepatology 2011;53:1020-2. 10.1002/hep.24199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown DB, Gould JE, Gervais DA, et al. Transcatheter Therapy for Hepatic Malignancy: Standardization of Terminology and Reporting Criteria. J Vasc Interv Radiol 2007;18:1469-78. 10.1016/j.jvir.2007.08.027 [DOI] [PubMed] [Google Scholar]
- 15.Ahmed M, Solbiati L, Brace CL, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria--a 10-year update. Radiology 2014;273:241-60. 10.1148/radiol.14132958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lubner MG, Brace CL, Hinshaw JL, et al. Microwave tumor ablation: mechanism of action, clinical results, and devices. J Vasc Interv Radiol 2010;21:S192-203. 10.1016/j.jvir.2010.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bruix J, Sherman M, Llovet JM, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol 2001;35:421-30. 10.1016/S0168-8278(01)00130-1 [DOI] [PubMed] [Google Scholar]
- 18.Cardella JF, Kundu S, Miller DL, et al. Society of Interventional Radiology Clinical Practice Guidelines. J Vasc Interv Radiol 2009;20:S189-91. 10.1016/j.jvir.2009.04.035 [DOI] [PubMed] [Google Scholar]
- 19.Ashoori N, Bamberg F, Paprottka P, et al. Multimodality Treatment for Early-Stage Hepatocellular Carcinoma: A Bridging Therapy for Liver Transplantation. Digestion 2012;86:338-48. 10.1159/000342813 [DOI] [PubMed] [Google Scholar]
- 20.Herber S, Biesterfeld S, Franz U, et al. Correlation of Multislice CT and Histomorphology in HCC Following TACE: Predictors of Outcome. Cardiovasc Intervent Radiol 2008;31:768-77. 10.1007/s00270-007-9270-8 [DOI] [PubMed] [Google Scholar]
- 21.Stampfl U, Bermejo JL, Sommer CM, et al. Efficacy and nontarget effects of transarterial chemoembolization in bridging of hepatocellular carcinoma patients to liver transplantation: a histopathologic study. J Vasc Interv Radiol 2014;25:1018-1026.e4. [DOI] [PubMed]
- 22.Shibata T, Iimuro Y, Yamamoto Y, et al. Small Hepatocellular Carcinoma: Comparison of Radio-frequency Ablation and Percutaneous Microwave Coagulation Therapy1. Radiology 2002;223:331-7. 10.1148/radiol.2232010775 [DOI] [PubMed] [Google Scholar]
- 23.Lu MD, Xu HX, Xie XY, et al. Percutaneous microwave and radiofrequency ablation for hepatocellular carcinoma: a retrospective comparative study. J Gastroenterol 2005;40:1054-60. 10.1007/s00535-005-1671-3 [DOI] [PubMed] [Google Scholar]
- 24.Bargellini I, Bozzi E, Campani D, et al. Modified RECIST to assess tumor response after transarterial chemoembolization of hepatocellular carcinoma: CT-pathologic correlation in 178 liver explants. Eur J Radiol 2013;82:e212-8. 10.1016/j.ejrad.2012.12.009 [DOI] [PubMed] [Google Scholar]
- 25.Adham M, Oussoultzoglou E, Ducerf C, et al. Results of orthotopic liver transplantation for liver cirrhosis in the presence of incidental and/or undetected hepatocellular carcinoma and tumour characteristics. Transpl Int 1998;11 Suppl 1:S197-200. 10.1007/s001470050460 [DOI] [PubMed] [Google Scholar]
- 26.Marelli L, Stigliano R, Triantos C, et al. Transarterial Therapy for Hepatocellular Carcinoma: Which Technique Is More Effective? A Systematic Review of Cohort and Randomized Studies. Cardiovasc Intervent Radiol 2007;30:6-25. 10.1007/s00270-006-0062-3 [DOI] [PubMed] [Google Scholar]
- 27.Wang ZL, Liang P, Dong BW, et al. Prognostic Factors and Recurrence of Small Hepatocellular Carcinoma after Hepatic Resection or Microwave Ablation: A Retrospective Study. J Gastrointest Surg 2008;12:327-37. 10.1007/s11605-007-0310-0 [DOI] [PubMed] [Google Scholar]
- 28.Coad JE, Kosari K, Humar A, et al. Radiofrequency ablation causes 'thermal fixation' of hepatocellular carcinoma: a post-liver transplant histopathologic study. Clin Transplant 2003;17:377-84. 10.1034/j.1399-0012.2003.00062.x [DOI] [PubMed] [Google Scholar]
- 29.Goldberg SN, Gazelle GS, Compton CC, et al. Treatment of intrahepatic malignancy with radiofrequency ablation: radiologic-pathologic correlation. Cancer 2000;88:2452-63. [DOI] [PubMed] [Google Scholar]


