Abstract
Background
Non-small cell lung cancer (NSCLC) represents 85% of all lung cancers. It is estimated that 60% of patients with NSCLC at time of diagnosis have advanced disease. The aim of this study was to investigate clinical and demographic prognostic factors of long term survival in patients with unresectable NSCLC.
Methods
We retrospectively reviewed data of 1,156 patients with NSCLC stage IIIB or IV who survived more than 60 days from the time of diagnosis and treated from August 1987 until March 2013 in the Oncology Department of Pulmonary Clinic of the General Hospital Papanikolaou. Initially univariate analysis using the log-rank test was conducted and then multivariate analysis using the proportional hazards model of Cox. Also Kaplan Meier curves were used to describe the distribution of survival times of patients. The level of significance was set at 0.05.
Results
The mean age at diagnosis was 62 years. About 11.9% of patients were women and 88.1% were male. The majority of cases were adenocarcinomas (42.2%), followed squamous (33%) and finally the large cell (6%). Unlike men, most common histological type among women was adenocarcinoma rather than squamous (63% vs. 10.9%). In univariate analysis statistically significant factors in the progression free survival (PFS) and overall survival (OS) were: weight loss ≥5%, histological type, line 1 drugs, line 1 combination, line 1 cycles and radio lung. Specifically radio lung gives clear survival benefit in the PFS and OS in stage IIIB (P=0.002) and IV (P<0.001). On the other hand, the number of distant metastases in stage IV patients did not affect OS, neither PFS. In addition patients who received platinum and taxane had better PFS (P=0.001) and OS (P<0.001) than those who received platinum without taxane. Also the third drug administration proved futile, since survival (682.06±34.9) (P=0.023) and PFS (434.93±26.93) (P=0.012) of patients who received less than three drugs was significantly larger. Finally, large cell carcinoma recorded the shortest OS and PFS compared with adenocarcinoma (P=0.043 and P=0.016 respectively) and squamous cell carcinoma (P=0.021 and P=0.004 respectively). In multivariate analysis the same predictors were statistically significant except for line 1 drugs.
Conclusions
This study confirms the increased incidence of adenocarcinoma in women than in men and the aggressiveness of large cell carcinoma. It also underlines the vitality of factors such as weight loss, radio lung and doublet platinum-based. On the other hand, it excludes significant factors such as gender, age and smoking.
Keywords: Non-small cell lung cancer (NSCLC), targeted therapies, prognostic factors
Introduction
Lung cancer is nowadays a global threat, since it is estimated that 6.94% of men and women born today will be diagnosed with lung cancer at some time in their lives (1). Indeed it is estimated that 2.86% of males and 2.24% of females will be diagnosed with cancer between 50−70 years (2,3).
In particular non-small cell lung cancer (NSCLC) represents 85% of all lung cancers (4). The 5-year survival rate in Europe is 11%, while in the United States (US) at 16.8%. It is estimated that 60% of patients with NSCLC at time of diagnosis suffer from advanced disease (5). The overall incidence for advanced NSCLC in the US in 2014 amounted to 143,488 patients.
The reasonable question that arises is that patients with the same disease characteristics differ significantly in terms of survival. Perhaps specific factors contribute to the prognosis. Many researchers have dealt with the prognosis in lung cancer. Thus, it is a fact that lung cancer is more common in men, as the probability for men to develop lung cancer is 1:13 and 1:17 for women regardless of smoking (6,7). However, recent years have seen a dramatic increase in cases in the female population (8). Moreover, female patients tend to be younger, more sensitive to the carcinogenic effects of tobacco, while they fare much better in terms of survival (8,9). Adenocarcinoma in particular seems to be more frequent than squamous cell carcinoma and is associated with smoking more often in men than in women (10,11).
On the basis of current data, there are four general categories of prognostic factors in unresectable NSCLC (12-20). Those are related to patient, tumor, treatment and laboratory parameters. The first category includes features such as: weight loss, comorbidities, body mass index (obesity paradox), gender, age, smoking habits, and ethnicity. The second category includes histological type, number and type of metastasis, tumor size, paraneoplastic syndromes such as superior vena cava syndrome, and pleural effusion. The third category concerns the treatment such as type of chemotherapy, concurrent radiotherapy and response rate. Finally, laboratory parameters that may contribute to the prognosis are: LDH, alkaline phosphatase, albumin, hemoglobin, leukocytosis, CRP, ratio of neutrophils—lymphocytes and thrombocytes—lymphocytes.
The purpose of this study was to discover factors that play a major role in the survival of patients with unresectable NSCLC or even in finding prognostic indicators to guide treatment protocols of the oncology department.
Methods
Patient population
We isolated 1,542 patients with unresectable NSCLC (stage IIIB and IV) among patients diagnosed with NSCLC in the period from Jan 8, 1987 to Jan 3, 2013 and treated in our department. Long-term survival was defined as the one exceeding 60 days. Thus, our final sample was 1,156 patients.
Factors analyzed
Potential prognostic factors studied were: gender, age, smoking habit, packets per year, stage (IIIB or IV), weight loss ≥5%, within the last three months, dyspnea, comorbidity, histological type (adenocarcinoma, squamous cell, large cell or other), the existence of metastases, the number of drugs administered in first-line treatment (2 or >2), the type of drugs in first line chemotherapy (platinum based regimen and taxane, platinum based regimen with no taxane, no platinum based regimen in combination with taxane, no platinum based regimen with no taxane), malignant pleural effusion (yes, no), the thoracic radiotherapy (yes, no) and the number of cycles of chemotherapy.
The progression free survival (PFS) defined was the period from the date the treatment ended to the date of confirmed aggravation or the date of death. The aggravation of the patient may have been clinical or radiological after the imaging evaluation. The date of aggravation, treatment and monitoring of the patient was recorded in his file.
The overall survival (OS) defined was the period from the start of treatment until the date of death from any cause or date of last contact with the patient.
Statistical analysis
Kaplan Meier curves were used for describing the distribution of survival times of our patients. The effect of each individual variable was studied by performing univariate analysis using the log-rank test. The level of significance was set at 0.05. Then a multivariate analysis of variables that potentially affect the survival of patients with advanced NSCLC was conducted using the Cox proportional hazards model. Statistical analysis was performed using the statistical program SPSS 21.0 (Statistical Package for the social Sciences, IBM Inc., Armonk, USA).
Results
Of the 1,156 patients 138 (11.9%) were females and 1,018 (88.1%) were male. The median diagnosis age was 62 years. IIIB patients were 152 (13.1%), far fewer than the 1,004 stage IV patients (86.9%).
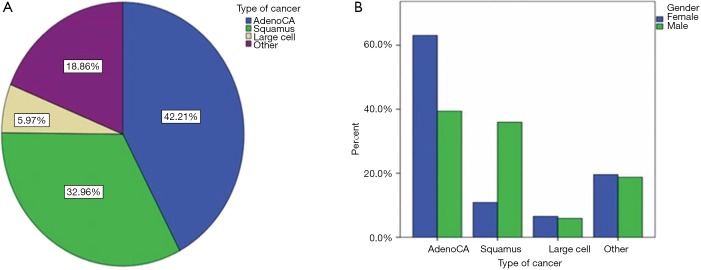
As regards the histological type of the total sample, adenocarcinoma (488 cases) was the most frequent accounting for 42.2%, followed by squamous cell (381 persons) and finally by large cell lung cancer (69 patients) with 33% and 6% respectively (Figure 1). This fact complies with the general current conviction that adenocarcinoma has surpassed squamous cell carcinoma as the most common type (21,22).
Figure 1.
Histology information. (A) Histological type; (B) histological type and gender.
However, while adenocarcinoma was clearly more predominant in women compare to the squamous cell carcinoma (63% vs. 10.9%, respectively), in men adenocarcinoma and squamous cell carcinoma were equally frequent (39.4% vs. 36%, respectively) (Figure 2).
Figure 2.
Number of metastases does not affect statistically significantly the OS of stage IV patients. OS, overall survival.
The results of the univariate analysis for the OS and the PFS are shown in the Table 1. Variables that were identified to have statistical significance for OS were: weight loss (P<0.001), histological type (P=0.032), number of drugs in first line chemotherapy (P=0.023), the first line chemotherapy (P<0.001) and thoracic radiotherapy (P=0.001). Specifically the survival of patients with large cell (464.23±61.54) is significantly shorter than the survival of patients with adenocarcinoma (648.78±46.15) (P=0.043).
Table 1. Patient characteristics.
| Characteristics | Patient number (%) | OS | P value | PFS | P value |
|---|---|---|---|---|---|
| Sex | 0.458 | 0.744 | |||
| Male | 1,018 (88.1) | 659.71±34.33 | 422.5±26.80 | ||
| Female | 138 (11.9) | 648.69±63.06 | 391.01±49.94 | ||
| Smoking | 0.479 | 0.372 | |||
| Non-smokers | 203 (17.6) | 623.69±64.52 | 395.85±55.13 | ||
| Smokers | 953 (82.4) | 666.36±35.30 | 422.54±26.53 | ||
| Stage | 0.979 | 0.946 | |||
| IIIB | 152 (13.1) | 666.23±88.92 | 381.85±49.11 | ||
| IV | 1004 (86.9) | 658.82±33.35 | 423.99±26.81 | ||
| Weight loss | 0.001 | 0.001 | |||
| 0 | 776 (67.1) | 700.82±38.21 | 446.84±29.16 | ||
| 1 | 380 (32.9) | 558.51±47.25 | 360.63±42.83 | ||
| Dyspnoea | 0.058 | 0.058 | |||
| 0 | 876 (75.8) | 679.33±37.08 | 427.36±27.93 | ||
| 1 | 280 (24.2) | 582.62±52.02 | 386.75±48.68 | ||
| Comorbidity | 0.198 | 0.106 | |||
| 0 | 675 (67.3) | 453.49±18.15 | 264.16±15.11 | ||
| 1 | 328 (32.7) | 495.06±30.55 | 302.28±26.21 | ||
| Histology | |||||
| Adenocarcinoma* | 488 (42.2) | 648.77±46.15 | − | 406.27±34.41 | − |
| Squamous cell | 381 (33.0) | 708.62±58.41 | 0.537 | 456.07±45.27 | 0.446 |
| Large cell | 69 (6.0) | 464.23±61.54 | 0.043 | 244.65±43.05 | 0.016 |
| Other | 218 (18.9) | 622.47±62.26 | 0.228 | 431.32±58.68 | 0.445 |
| Meta (yes, no) | 0.935 | 0.927 | |||
| 0 | 165 (14.3) | 662.11±79.8 | 385.19±46.09 | ||
| 1 | 991 (85.7) | 659.73±34.01 | 424.78±27.33 | ||
| No. of drugs in first line chemotherapy | 0.023 | 0.012 | |||
| 1–2 | 996 (86.2) | 682.06±34.9 | 434.93±26.93 | ||
| >2 | 160 (13.8) | 519.27±58.84 | 325.47±56.64 | ||
| Type of first line chemotherapy | |||||
| Platin + taxane* | 897 (77.6) | 714.85±36.79 | − | 459.59±29.68 | − |
| Platinum. no taxane | 184 (15.9) | 431.96±44.95 | <0.001 | 258.36±40.49 | <0.001 |
| Taxane + no platinum | 40 (3.5) | 617.09±179.06 | 0.093 | 388.57±109.23 | 0.294 |
| No platinum and no taxane | 35 (3.0) | 328.17±110.28 | <0.001 | 219.17±106.45 | <0.001 |
| Pleural effusion | 0.990 | 0.986 | |||
| 0 | 811 (70.2) | 676.32±40.59 | 425.80±30.68 | ||
| 1 | 345 (29.8) | 622.64±44.14 | 407.88±40.46 | ||
| Thoracic radiotherapy | 0.001 | 0.001 | |||
| 0 | 717 (62.0) | 595.20±35.65 | 368.86±26.00 | ||
| 1 | 439 (38.0) | 889.04±63.88 | 598.50±57.50 | ||
*, reference group. OS, overall survival; PFS, progression free survival.
Survival of patients with large cell (464.23±61.54) is significantly shorter than the survival of patients with squamous cell (708.62±58.41) (P=0.021).
Also, survival of patients administered with platinum based regimen and taxane regimen is significantly longer (714.85±36.79) than the survival of patients administered with platinum based regimen but without taxane regimen (431.96±44.95), P<0.001.
Survival of patients administered with platinum based regimen and taxane regimen is significantly longer (714.85±36.79) than the survival of patients administered with no platinum based regimen and no taxane regimen (328.17±110.28) (P<0.001).
The results were similar for the PFS, since statistically significant factors were: weight loss (P<0.001), histological type (P=0.013), number of drugs in first line chemotherapy (P=0.012), the type of first line chemotherapy (P<0.001) and thoracic radiotherapy (P=0.001).
Specifically, the PFS of patients with large cell (244.65±43.05) is significantly shorter than the PFS of patients with adenocarcinoma (406.27±34.41) (P=0.016).
The PFS of patients with large cell (244.65±43.05) is significantly shorter than the PFS of patients with squamous cell (456.07±45.27) (P=0.004).
The PFS of patients administered with platinum based regimen and taxane regimen is significantly higher (459.59±29.68) in PFS of patients administered with platinum based regimen but not taxane regimen (258.36±40.49) (P=0.001).
The PFS of patients administered with platinum based regimen and taxane regimen is significantly higher (459.59±29.68) in PFS of patients administered with no platinum based regimen and no taxane regimen (219.17±106.45) (P<0.001).
The quantitative variables (packets per year, number of cycles in the first line of chemotherapy and years of age) were studied separately. Table 2 shows that only the number of cycles was statistically significant.
Table 2. Number of cycles.
| Variables | P | HR | 95% CI for HR |
|---|---|---|---|
| Pack/years | |||
| PFS | 0.239 | 0.999 | 0.998−1.001 |
| OS | 0.248 | 0.999 | 0.998−1.001 |
| Age | |||
| PFS | 0.394 | 0.997 | 0.990−1.004 |
| OS | 0.520 | 0.998 | 0.991−1.005 |
| Line 1 cycles | |||
| PFS | <0.001 | 0.806 | 0.783−0.829 |
| OS | <0.001 | 0.881 | 0.857−0.906 |
PFS, progression free survival; OS, overall survival.
Specifically for each unit increase in the number of cycles in the first line of chemotherapy, the risk of death is reduced by 19.4% (HR =0.806; 95% CI, 0.783−0.829; P<0.001) and the risk of relapse by 11.9% (HR =0.881; 95% CI, 0.857−0.906; P <0.001).
We examined whether the number of distant metastases in stage IV patients affects the PFS and OS. Thus, 548 stage IV patients had one distant metastasis, 310 had two and 132 more than two (data on 14 patients were incomplete and they were not included in the sample).As shown the number of metastases does not affect statistically significantly the OS of stage IV patients (Figure 2), or the PFS (Figure 3).
Figure 3.
Number of metastases does not affect statistically significantly the PFS of stage IV patients. PFS, progression free survival.
We then studied the variable thoracic radiotherapy on PFS and OS by stage (IIIB and IV). 797 out of 1,004 stage IV patients received thoracic radiotherapy and 207 did not. 48 out of 152 stage IIIB patients received thoracic radiotherapy and 104 did not.
Thus, in stage IIIB the OS of patients irradiated (817.60±114.94) is significantly longer than the OS of non-irradiated patients (577.86±105.81) (P=0.002) and PFS of patients irradiated (555.79±103.50) is longer than the PFS of non-irradiated patients (279.47±42.12) (P=0.002).
In stage IV the OS of patients irradiated (907.11±73.46) is significantly longer than the OS of non-irradiated patients (593.95±36.93) (P<0.001) and PFS of patients irradiated (606.03±65.63) is significantly longer than the PFS of non-irradiated patients (375.22±28.09) (P<0.001).
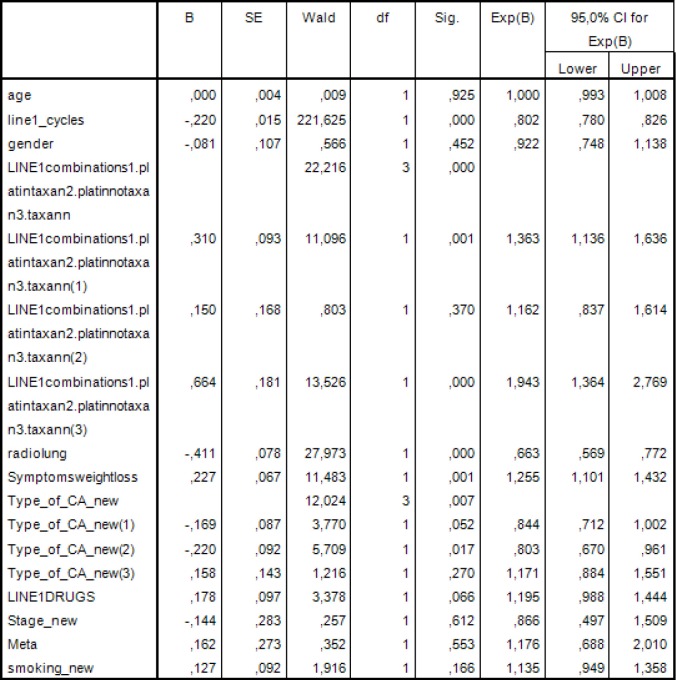
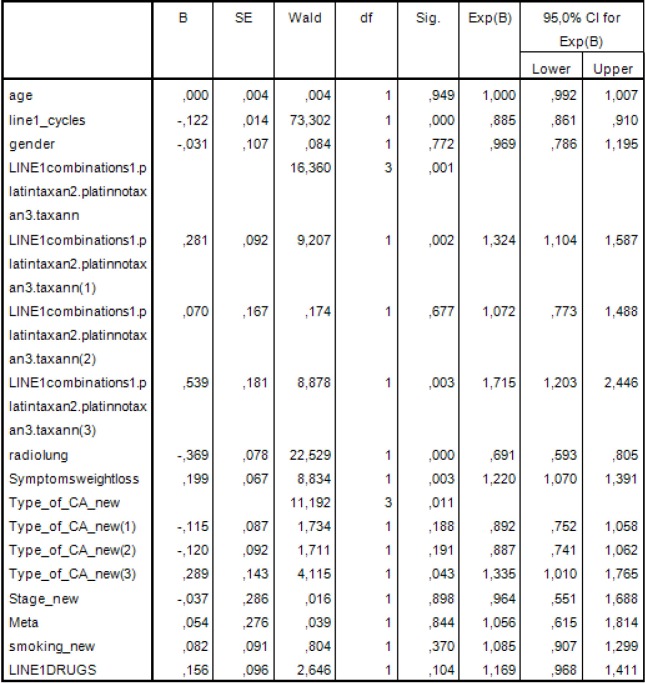
Then a multivariate analysis was performed that included clinically important variables or variables with statistical significance P<0.05 by the univariate analysis. Thus, in this model we examined the following: years of age, sex, type of first line chemotherapy, thoracic radiotherapy, number of cycles in the first line of chemotherapy, weight loss, histological type, number of drugs in first line chemotherapy, stage of disease, number of metastases and smoking. The results are shown in Figure 4.
Figure 4.
Multivariate analysis was performed that included clinically important variables or variables with statistical significance P<0.05 by the univariate analysis.
Statistically significant factors were the number of cycles in the first line of chemotherapy (P<0.001), type of first line chemotherapy (P<0.001), thoracic radiotherapy (P<0.001), weight loss (P=0.001) and histological type (P=0.007).
Specifically the risk of death for patients administered with platinum based regimen without taxane regimen is 1.363 times greater than those who received platinum based regimen and taxane regimen (HP =1.363; 95% CI, 1.136−1.636; P=0.001).
The risk of death for patients not administered with platinum or taxane is 1.943 times greater than those who received platinum based regimen and taxane regimen (HR =1.943; 95% CI, 1.364−2.769; P<0.001).
Also, the risk of death of patients with squamous cell is 19.7% times smaller than in those with another type of NSCLC cancer (HR =0.803; 95% CI, 0.670−0.961; P=0.017).
The other type defined was the bronchoalveolar and mixed type.
In considering the same variables on PFS the following Cox model was established as it seems in Figure 5.
Figure 5.
Variables on PFS, following Cox model was established with several variables. PFS, progression free survival.
As in the OS the statistically significant factors were the number of cycles in the first line of chemotherapy (P<0.001), type of first line chemotherapy (P=0.001), thoracic radiotherapy (P<0.001), weight loss (P=0.003) and histology (P=0.011).
Specifically, the risk of relapse for patients administered with platinum based regimen without taxane regimen is 1.324 times greater than those who received platinum based regimen and taxane regimen (HR =1.363; 95% CI, 1.104−1.587; P=0.002).
The risk of relapse for patients not administered with platinum based regimen or taxane regimen is 1.715 times greater than those who received platinum based regimen and taxane regimen (HR =1.715; 95% CI, 1.203−2.446; P=0.003).
Also, the risk of relapse of those with squamous cell is 1,335 times greater than those with another type of NSCLC cancer (HR =1.335; 95% CI, 1.010−1.765; P=0.043).
Conclusions
Large cell carcinoma recorded the shortest survival compared with adenocarcinoma and squamous cell, which demonstrates the aggressiveness of that tumor (23,24). Many studies have been conducted on the neuroendocrine tumor (LCNEC) and the poor prognosis that accompanies this type of large cell lung cancer (23,25).
The importance of weight loss as a prognostic indicator in the survival of patients with NSCLC is confirmed both in the univariate and multivariate analysis. Many researchers maintain that weight loss is associated with more aggressive disease (26-28). Specifically, Ross et al. examined 418 NSCLC patients and found that those who lost weight completed less frequently three cycles of chemotherapy or their treatment was postponed more often. Also, the same patients were more likely to develop severe anemia as toxicity (29). The conclusions of Topkan et al. examining the effect of weight loss on the survival of 425 patients with stage IIIB NSCLC who received modern chemotherapy and radiotherapy, were similar (30). Also, in patients with advanced NSCLC, and palliative chemotherapy, identified as a significant prognostic factor, the muscle mass increase and not the sarcopenia (21).
Patients who received platinum based regimen and taxane regimen recorded a statistically significant advantage for survival compared to those treated with platinum based regimen-free taxane, therefore, the use of taxane offered a clear survival benefit, both in the univariate and the multivariate model. The same conclusion was drawn by Belani and Liu et al. who compared platinum based regimen—docetaxel regimen doublets with platinum based regimen—vinorelbine regimen doublets (31,32). In both studies, the first doublet recorded a clear advantage. On the other hand, Zhu et al. indicate significantly lower side effects of platinum based regimen and taxane regimen doublet, compared with platinum based regimen—free taxane regimen (33).
Also the use platinum based regimen and taxane regimen was superior to TKIs. Of course, the validity of this result is contested, as the recording of patients for our sample was started in 1987 and completed in 2013, while the widespread use of TK’s started after 2012. Therefore, the percentage of those who were able to receive TK’s was very small (only 35 patients). So, numerous current studies have examined the efficacy of gefitinib compared with platinum based regimen and taxane regimen doublet in patients with lung adenocarcinoma. A minority of them result in non-statistical difference between the two treatment options regarding efficacy and survival of non-smokers with adenocarcinoma (34). Most clinical trials in patients with adenocarcinoma result in superiority of gefitinib in terms of survival rates and the relief of symptoms (35,36).
Although not statistically significant in the multivariate analysis, the number of drugs in first line chemotherapy had a major role in the survival of patients in the univariate analysis. Thus, it was found that the survival of patients who received up to 2 drugs (682.06±34.9) was significantly greater than the survival of patients who received more than two drugs (519.27±58.84) (P=0.023). The same conclusion was also drawn by Delbaldo et al. (37) and Paz-Ares et al. (38), as the third drug had no effect on survival. Specifically, Hainsworth et al. emphasize the increased incidence of severe leukopenia, as a result of adding vinorelbine in the platinum based regimen and taxane regimen doublet (39). Similarly according to Zhu et al. adding Bevacizumab in platinum based regimen and taxane regimen doublet in elderly patients with advanced NSCLC, imparts no survival benefit (40). In contrast, Shimizu et al. suggest adding it as a safe and effective option for patients with nonsquamous cell NSCLC and ILD (41). Also, Zhou et al. recommend adding bevacizumab to carboplatin based regimen and paclitaxel regimen, since it was well tolerated in patients with advanced non squamous cell NSCLC (42).
In addition, a clear survival benefit resulted from the use of thoracic radiotherapy. Many researchers studied the effect of this factor on the survival of patients with NSCLC. Thus, Mac Manus et al. found that a small number of patients with confirmed NSCLC who received palliative radiotherapy managed to survive more than 5 years (43). In a similar study, it was found that 1.3% of patients who received palliative thoracic radiotherapy managed to survive more than 5 years (44). Also, Fairchild et al. compared low dose with high dose and found annual survival rates statistically greater in the second group, although there was no difference in the relief of symptoms (45). Similarly, Stevens et al. demonstrate the role of palliative radiotherapy in relieving chest symptoms and suggest lower doses. This is because high doses do not seem to be superior in relieving symptoms and in the long-term survival (46). Ma et al. detected no difference in symptom relief and the rates of 1- and 2-year OS between low and high dose in patients with locally advanced lung cancer (47). In contrast, Walasek et al. associated the palliative radiotherapy with a worse prognosis and propose modern supportive care as an alternative method of treatment (48). Similarly, Strand et al. demonstrated that the palliative radiotherapy is a statistically insignificant factor for survival in multivariate analysis (49). When we studied separately by stage the effect of RT we found that it was statistically significant in both stage IIIB and IV patients (50).
The number of distant metastases in stage IV patients was not proved to be statistically significant factor in the OS, or in the PFS. Therefore, one or multiple metastases do not affect the OS. The results from similar studies comply with ours (13,51). Of interest is the cohort study of Hendriks et al. who study the effect of single against multiple organic metastases in stage IV patients (52). It was shown that the group with single distant metastasis had a significantly better prognosis than those who had multiple metastases. Therefore, the former group should be recognized as a distinct subcategory of stage IV.
Comorbidities also did not seem to affect survival (53). For this reason, elderly patients who typically have co-morbidities should not be excluded from a chemotherapy treatment. The same conclusion was drawn by Janssen-Heijnen et al. (54). Those researchers studied the incidence of comorbidity in patients over the age of 70 and found that it reached 73% in men and 61% in women. In fact, the most frequent concomitant diseases in men were cardiovascular (31%) and chronic obstructive pulmonary disease (COPD) (29%), whereas in women cardiovascular (22%), hypertension (22%) and COPD (20%). Despite the high frequency, comorbidity had no effect on the treatment’s efficacy. Only in stage IIIA patients aged 70−79 years, the presence of COPD reduce the likelihood of surgery. Similarly, Firat et al. identify comorbidity as an independent prognostic factor in stage III patients (55).
This study confirms the increased incidence of adenocarcinoma in women than in men and the aggressiveness of large cell carcinoma. It also underlines the vitality of factors such as weight loss, thoracic radiotherapy and doublet platinum-based. Undoubtedly the size of our sample is quite high (1,156 people), which guarantees reliable conclusions Also, the period in which it was collected reflects the course of the disease over 26 years (from 01/08/1987 to 01/03/2013). However, despite the international literature, it is remarkable that factors such as gender, years of age and smoking did not seem to play a statistically significant role (56-59).
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Lampaki S, Kioumis I, Pitsiou G, et al. Lung cancer and eye metastases. Med Hypothesis Discov Innov Ophthalmol 2014;3:40-4. [PMC free article] [PubMed] [Google Scholar]
- 2.Zarogoulidis P, Lampaki S, Turner JF, et al. mTOR pathway: a current, up-to-date mini-review (Review). Oncol Lett 2014;8:2367-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lampaki S, Lazaridis G, Zarogoulidis K, et al. Defining the role of tyrosine kinase inhibitors in early stage non-small cell lung cancer. J Cancer 2015;6:568-74. 10.7150/jca.11893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin 2009;59:225-49. 10.3322/caac.20006 [DOI] [PubMed] [Google Scholar]
- 5.Howlader N, Noone AM, Krapcho M, et al. editors. SEER Cancer Statistics Review, 1975-2008. Bethesda: National Cancer Institute; 2011. [Google Scholar]
- 6.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. 10.3322/caac.20107 [DOI] [PubMed] [Google Scholar]
- 7.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2095-128. 10.1016/S0140-6736(12)61728-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sagerup CM, Småstuen M, Johannesen TB, et al. Sex-specific trends in lung cancer incidence and survival: a population study of 40,118 cases. Thorax 2011;66:301-7. 10.1136/thx.2010.151621 [DOI] [PubMed] [Google Scholar]
- 9.Fu JB, Kau TY, Severson RK, et al. Lung cancer in women: analysis of the national Surveillance, Epidemiology, and End Results database. Chest 2005;127:768-77. 10.1378/chest.127.3.768 [DOI] [PubMed] [Google Scholar]
- 10.Nagy-Mignotte H, Guillem P, Vesin A, et al. Primary lung adenocarcinoma: characteristics by smoking habit and sex. Eur Respir J 2011;38:1412-9. 10.1183/09031936.00191710 [DOI] [PubMed] [Google Scholar]
- 11.Nordquist LT, Simon GR, Cantor A, et al. Improved survival in never-smokers vs current smokers with primary adenocarcinoma of the lung. Chest 2004;126:347-51. 10.1378/chest.126.2.347 [DOI] [PubMed] [Google Scholar]
- 12.Wheatley-Price P, Blackhall F, Lee SM, et al. The influence of sex and histology on outcomes in non-small-cell lung cancer: a pooled analysis of five randomized trials. Ann Oncol 2010;21:2023-8. 10.1093/annonc/mdq067 [DOI] [PubMed] [Google Scholar]
- 13.Paralkar VR, Li T, Langer CJ. Population characteristics and prognostic factors in metastatic non-small-cell lung cancer: a Fox Chase Cancer Center retrospective. Clin Lung Cancer 2008;9:116-21. 10.3816/CLC.2008.n.018 [DOI] [PubMed] [Google Scholar]
- 14.Jeremic B, Milicic B, Dagovic A, et al. Pretreatment clinical prognostic factors in patients with stage IV non-small cell lung cancer (NSCLC) treated with chemotherapy. J Cancer Res Clin Oncol 2003;129:114-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tibaldi C, Vasile E, Bernardini I, et al. Baseline elevated leukocyte count in peripheral blood is associated with poor survival in patients with advanced non-small cell lung cancer: a prognostic model. J Cancer Res Clin Oncol 2008;134:1143-9. 10.1007/s00432-008-0378-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Denehy L, Hornsby WE, Herndon JE, 2nd, et al. Prognostic validation of the body mass index, airflow obstruction, dyspnea, and exercise capacity (BODE) index in inoperable non-small-cell lung cancer. J Thorac Oncol 2013;8:1545-50. 10.1097/JTO.0000000000000032 [DOI] [PubMed] [Google Scholar]
- 17.Espinosa E, Feliu J, Zamora P, et al. Serum albumin and other prognostic factors related to response and survival in patients with advanced non-small cell lung cancer. Lung Cancer 1995;12:67-76. 10.1016/0169-5002(95)00407-R [DOI] [PubMed] [Google Scholar]
- 18.Yildirim M, Yildiz M, Duman E, et al. Prognostic importance of the nutritional status and systemic inflammatory response in non-small cell lung cancer. J BUON 2013;18:728-32. [PubMed] [Google Scholar]
- 19.O'Connell JP, Kris MG, Gralla RJ, et al. Frequency and prognostic importance of pretreatment clinical characteristics in patients with advanced non-small-cell lung cancer treated with combination chemotherapy. J Clin Oncol 1986;4:1604-14. [DOI] [PubMed] [Google Scholar]
- 20.Dahlberg SE, Schiller JH, Bonomi PB, et al. Body mass index and its association with clinical outcomes for advanced non-small-cell lung cancer patients enrolled on Eastern Cooperative Oncology Group clinical trials. J Thorac Oncol 2013;8:1121-7. 10.1097/JTO.0b013e31829cf942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miron L, Bosanceanu M, Filimon R, et al. Clinical-epidemiological study on advanced non-small cell lung cancer. Rev Med Chir Soc Med Nat Iasi 2014;118:492-6. [PubMed] [Google Scholar]
- 22.Charloux A, Quoix E, Wolkove N, et al. The increasing incidence of lung adenocarcinoma: reality or artefact? A review of the epidemiology of lung adenocarcinoma. Int J Epidemiol 1997;26:14-23. 10.1093/ije/26.1.14 [DOI] [PubMed] [Google Scholar]
- 23.Fasano M, Della Corte CM, Papaccio F, et al. Pulmonary large-cell neuroendocrine carcinoma: from epidemiology to therapy. J Thorac Oncol 2015;10:1133-41. 10.1097/JTO.0000000000000589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iyoda A, Hiroshima K, Nakatani Y, et al. Pulmonary large cell neuroendocrine carcinoma: its place in the spectrum of pulmonary carcinoma. Ann Thorac Surg 2007;84:702-7. 10.1016/j.athoracsur.2007.03.093 [DOI] [PubMed] [Google Scholar]
- 25.Hage R, Seldenrijk K, de Bruin P, et al. Pulmonary large-cell neuroendocrine carcinoma (LCNEC). Eur J Cardiothorac Surg 2003;23:457-60. 10.1016/S1010-7940(02)00837-0 [DOI] [PubMed] [Google Scholar]
- 26.Kimura M, Naito T, Kenmotsu H, et al. Prognostic impact of cancer cachexia in patients with advanced non-small cell lung cancer. Support Care Cancer 2015;23:1699-708. 10.1007/s00520-014-2534-3 [DOI] [PubMed] [Google Scholar]
- 27.Crvenkova S, Pesevska M. Important prognostic factors for the long-term survival in non-small cell lung cancer patients treated with combination of chemotherapy and conformal radiotherapy. J BUON 2015;20:775-81. [PubMed] [Google Scholar]
- 28.Babacan NA, Yucel B, Kilickap S, et al. Lung cancer in women: a single institution experience with 50 patients. Asian Pac J Cancer Prev 2014;15:151-4. 10.7314/APJCP.2014.15.1.151 [DOI] [PubMed] [Google Scholar]
- 29.Ross PJ, Ashley S, Norton A, et al. Do patients with weight loss have a worse outcome when undergoing chemotherapy for lung cancers? Br J Cancer 2004;90:1905-11. 10.1038/sj.bjc.6601781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Topkan E, Parlak C, Selek U. Impact of weight change during the course of concurrent chemoradiation therapy on outcomes in stage IIIB non-small cell lung cancer patients: retrospective analysis of 425 patients. Int J Radiat Oncol Biol Phys 2013;87:697-704. 10.1016/j.ijrobp.2013.07.033 [DOI] [PubMed] [Google Scholar]
- 31.Belani CP, Eckardt J. Development of docetaxel in advanced non-small-cell lung cancer. Lung Cancer 2004;46 Suppl 2:S3-11. 10.1016/S0169-5002(04)80036-9 [DOI] [PubMed] [Google Scholar]
- 32.Liu T, Wu H, Zhuang X, et al. A meta-analysis of platinum plus docetaxel or vinorelbine in the first-line treatment of advanced non-small cell lung cancer Zhongguo Fei Ai Za Zhi 2014;17:327-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu N, He J, Zhang S, et al. A meta-analysis of platinum plus taxanes regimen on treating advanced non-small cell lung cancer. Zhongguo Fei Ai Za Zhi 2009;12:868-74. [DOI] [PubMed] [Google Scholar]
- 34.Lou N, Yang J, Yan H, et al. Efficacies of gefitinib versus paclitaxel/carboplatin for patients with advanced pulmonary adenocarcinoma. Zhonghua Yi Xue Za Zhi 2014;94:2337-41. [PubMed] [Google Scholar]
- 35.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. 10.1056/NEJMoa0810699 [DOI] [PubMed] [Google Scholar]
- 36.Wu YL, Chu DT, Han B, et al. Phase III, randomized, open-label, first-line study in Asia of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer: evaluation of patients recruited from mainland China. Asia Pac J Clin Oncol 2012;8:232-43. 10.1111/j.1743-7563.2012.01518.x [DOI] [PubMed] [Google Scholar]
- 37.Delbaldo C, Michiels S, Rolland E, et al. WITHDRAWN: Second or third additional chemotherapy drug for non-small cell lung cancer in patients with advanced disease. Cochrane Database Syst Rev 2012;4:CD004569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paz-Ares L, Bálint B, de Boer RH, et al. A randomized phase 2 study of paclitaxel and carboplatin with or without conatumumab for first-line treatment of advanced non-small-cell lung cancer. J Thorac Oncol 2013;8:329-37. 10.1097/JTO.0b013e31827ce554 [DOI] [PubMed] [Google Scholar]
- 39.Hainsworth JD, Burris HA, 3rd, Morrissey LH, et al. Paclitaxel, carboplatin, and vinorelbine in the treatment of advanced non-small cell lung cancer: a phase II trial of the Minnie Pearl Cancer Research Network. Cancer J 2000;6:151-6. [PubMed] [Google Scholar]
- 40.Zhu J, Sharma DB, Gray SW, et al. Carboplatin and paclitaxel with vs without bevacizumab in older patients with advanced non-small cell lung cancer. JAMA 2012;307:1593-601. 10.1001/jama.2012.454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shimizu R, Fujimoto D, Kato R, et al. The safety and efficacy of paclitaxel and carboplatin with or without bevacizumab for treating patients with advanced nonsquamous non-small cell lung cancer with interstitial lung disease. Cancer Chemother Pharmacol 2014;74:1159-66. 10.1007/s00280-014-2590-x [DOI] [PubMed] [Google Scholar]
- 42.Zhou C, Wu YL, Chen G, et al. BEYOND: a randomized, double-blind, placebo-controlled, multicenter, phase III study of first-line carboplatin/paclitaxel plus bevacizumab or placebo in Chinese patients with advanced or recurrent nonsquamous non-small-cell lung cancer. J Clin Oncol 2015;33:2197-204. 10.1200/JCO.2014.59.4424 [DOI] [PubMed] [Google Scholar]
- 43.Mac Manus MP, Matthews JP, Wada M, et al. Unexpected long-term survival after low-dose palliative radiotherapy for non-small cell lung cancer. Cancer 2006;106:1110-6. 10.1002/cncr.21704 [DOI] [PubMed] [Google Scholar]
- 44.Quddus AM, Kerr GR, Price A, et al. Long-term survival in patients with non-small cell lung cancer treated with palliative radiotherapy. Clin Oncol (R Coll Radiol) 2001;13:95-8. [DOI] [PubMed] [Google Scholar]
- 45.Fairchild A, Harris K, Barnes E, et al. Palliative thoracic radiotherapy for lung cancer: a systematic review. J Clin Oncol 2008;26:4001-11. 10.1200/JCO.2007.15.3312 [DOI] [PubMed] [Google Scholar]
- 46.Stevens R, Macbeth F, Toy E, et al. Palliative radiotherapy regimens for patients with thoracic symptoms from non-small cell lung cancer. Cochrane Database Syst Rev 2015;1:CD002143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ma JT, Zheng JH, Han CB, et al. Meta-analysis comparing higher and lower dose radiotherapy for palliation in locally advanced lung cancer. Cancer Sci 2014;105:1015-22. 10.1111/cas.12466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Walasek T, Sas-Korczyńska B, Dąbrowski T, et al. Palliative thoracic radiotherapy for patients with advanced non-small cell lung cancer and poor performance status. Lung Cancer 2015;87:130-5. 10.1016/j.lungcan.2014.11.015 [DOI] [PubMed] [Google Scholar]
- 49.Strand TE, Brunsvig PF, Johannessen DC, et al. Potentially curative radiotherapy for non-small-cell lung cancer in Norway: a population-based study of survival. Int J Radiat Oncol Biol Phys 2011;80:133-41. 10.1016/j.ijrobp.2010.01.050 [DOI] [PubMed] [Google Scholar]
- 50.Strøm HH, Bremnes RM, Sundstrøm SH, et al. Concurrent palliative chemoradiation leads to survival and quality of life benefits in poor prognosis stage III non-small-cell lung cancer: a randomised trial by the Norwegian Lung Cancer Study Group. Br J Cancer 2013;109:1467-75. 10.1038/bjc.2013.466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ishii S, Takeda Y, Hirano S, et al. Survival-related clinical factors of patients with advanced non-small cell lung cancer after 2000. Gan To Kagaku Ryoho 2011;38:405-10. [PubMed] [Google Scholar]
- 52.Hendriks LE, Derks JL, Postmus PE, et al. Single organ metastatic disease and local disease status, prognostic factors for overall survival in stage IV non-small cell lung cancer: Results from a population-based study. Eur J Cancer 2015;51:2534-44. 10.1016/j.ejca.2015.08.008 [DOI] [PubMed] [Google Scholar]
- 53.Hsu CL, Chen JH, Chen KY, et al. Advanced non-small cell lung cancer in the elderly: the impact of age and comorbidities on treatment modalities and patient prognosis. J Geriatr Oncol 2015;6:38-45. 10.1016/j.jgo.2014.09.178 [DOI] [PubMed] [Google Scholar]
- 54.Janssen-Heijnen ML, Smulders S, Lemmens VE, et al. Effect of comorbidity on the treatment and prognosis of elderly patients with non-small cell lung cancer. Thorax 2004;59:602-7. 10.1136/thx.2003.018044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Firat S, Byhardt RW, Gore E. Comorbidity and Karnofksy performance score are independent prognostic factors in stage III non-small-cell lung cancer: an institutional analysis of patients treated on four RTOG studies. Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys 2002;54:357-64. 10.1016/S0360-3016(02)02939-5 [DOI] [PubMed] [Google Scholar]
- 56.Sandler BJ, Wang Z, Hancock JG, et al. Gender, age, and comorbidity status predict improved survival with adjuvant chemotherapy following lobectomy for non-small cell lung cancers larger than 4 cm. Ann Surg Oncol 2015. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 57.Tas F, Ciftci R, Kilic L, et al. Age is a prognostic factor affecting survival in lung cancer patients. Oncol Lett 2013;6:1507-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pallis AG, Gridelli C. Is age a negative prognostic factor for the treatment of advanced/metastatic non-small-cell lung cancer? Cancer Treat Rev 2010;36:436-41. 10.1016/j.ctrv.2009.12.013 [DOI] [PubMed] [Google Scholar]
- 59.Chermiti Ben Abdallah F, Ben Ali G, Sadok Boudaya M, et al. Treatment and prognosis of advanced stage non-small-cell lung cancer. Rev Mal Respir 2014;31:214-20. 10.1016/j.rmr.2013.09.002 [DOI] [PubMed] [Google Scholar]